Abstract
Anandamide has been characterized as both an endocannabinoid and endovanilloid. Consistent with its actions as an endovanilloid, previous studies have demonstrated that anandamide can excite primary sensory neurons in vitro via transient receptor potential vanilloid type one (TRPV1) receptors. In the present study, we sought to determine if anandamide excited cutaneous C nociceptors in vivo and if this excitation correlated with nocifensive behaviors. Using teased-fiber electrophysiological methods in the rat, C nociceptors isolated from the tibial nerve with receptive fields (RFs) on the plantar surface of the hindpaw were studied. Injection of anandamide into the RF dose-dependently excited nociceptors at doses of 10 and 100 μg. The TRPV1 receptor antagonists, capsazepine or SB 366791, were applied to the RF to determine if excitation by anandamide was mediated through TRPV1 receptors. Intraplantar injection of either capsazepine (10 μg) or SB 366791 (3 μg) attenuated the excitation produced by 100 μg anandamide. We also determined whether excitation of C nociceptors by anandamide was associated with nocifensive behaviors. Intraplantar injection of 100 μg anandamide produced nocifensive behaviors that were attenuated by pre-treatment with either capsazepine or SB 366791. Furthermore, we determined if intraplantar injection of anandamide altered withdrawal responses to radiant heat. Neither intraplantar injection of anandamide or vehicle produced antinociception or hyperalgesia to radiant heat. Our results indicate that anandamide excited cutaneous C nociceptors and produced nocifensive behaviors via activation of TRPV1 receptors.
Keywords: cannabinoids, TRPV1 receptors, nocifensive behaviors, primary afferent electrophysiology
1. Introduction
Anandamide (AEA) is a membrane-derived fatty acid amide and was the first identified endogenous cannabinoid receptor agonist, or endocannabinoid (Devane et al., 1992). Currently, two receptors for cannabinoids have been isolated and cloned, cannabinoid one (CB1) and cannabinoid two (CB2) receptors (Matsuda et al., 1990; Munro et al., 1993), both being G-protein coupled receptors localized to various neuronal and non-neuronal tissues. CB1 receptors are most commonly expressed on neurons, and activation of these receptors has been shown to be inhibitory by decreasing calcium channel conductance and increasing potassium channel conductance (for review see Howlett et al., 2004; Demuth and Molleman, 2006). AEA has affinity for both CB1 (Devane et al., 1992) and CB2 (Felder et al., 1995; Slipetz et al., 1995) receptors, with slightly higher affinity for CB1 receptors. Previous studies in laboratory animals have demonstrated that systemic administration of anandamide produces typical cannabimimetic effects such as hypothermia, hypolocomotion, catalepsy, and antinociception (Fride and, Mechoulam 1993; Smith et al., 1994) primarily through activation of CB1 receptors (Wise et al., 2007). Additionally, peripheral administration of anandamide attenuates formalin-evoked nociception (Calignano et al., 1998; Guindon et al., 2006) and hyperalgesia following inflammation (Richardson et al., 1998) and nerve injury (Guindon and Beaulieu, 2006) through activation of peripheral CB1 receptors.
In contrast to these inhibitory actions through CB1 receptors, AEA has also been identified as an endogenous ligand for the transient receptor potential vanilloid type one (TRPV1) receptor, and is part of a growing class of endovanilloids (Melck et al., 1999; Zygmunt et al., 1999; Smart et al., 2000). The TRPV1 receptor is a non-selective cationic channel that is activated by capsaicin (Caterina et al., 1997), resiniferatoxin (Szallasi et al., 1999), protons (Caterina et al., 1997; Tominaga et al., 1998), and noxious heat (Caterina et al., 1997). Unlike its inhibitory actions via cannabinoid receptors, high concentrations of AEA excite isolated nociceptive dorsal root ganglion neurons through activation of TRPV1 receptors resulting in depolarizing inward current, increased intracellular calcium, and release of calcitonin-gene related peptide (CGRP) (Tognetto et al., 2001; Olah et al., 2001; Jerman et al., 2002; Ahluwalia et al., 2003; Fischbach et al., 2007). Similar excitatory effects were observed for isolated nociceptive trigeminal ganglion neurons (Roberts et al., 2002; Price et al., 2004). Additional studies demonstrated that AEA excited bronchopulmonary (Lin and Lee, 2002; Kollarik and Undem, 2004; Lee et al., 2005), mesenteric (Zygmunt et al., 1999), and articular (Gauldie et al., 2001) C fibers through interactions with TRPV1 receptors.
Although in vitro studies have demonstrated that AEA can excite dorsal root ganglion neurons and visceral C fibers, it is not known whether AEA excites cutaneous nociceptors in vivo. Therefore, the aim of the present study was to determine if local injections of anandamide into the hindpaw excited cutaneous C nociceptors in vivo, and if so, whether activation of C nociceptors by AEA produced nocifensive behaviors.
Results
2.1 General properties of C nociceptors
A total of 91 cutaneous C nociceptors with mechanical RFs located on the plantar surface of the hindpaw were studied. Examples of conduction latency and responses to noxious pinch and heat are shown for a single nociceptor in figure 1. The mean conduction velocity of all C nociceptors was 0.66±0.01 m/s (range of 0.43 - 1.5 m/s), the mean mechanical threshold was 104.3±9.0 mN (range of 24.1 - 674.2 mN), and the mean heat response threshold was 47.1±0.5°C (range of 41 - 51°C). Of the 91 C nociceptors studied, 40 (44%) responded to heat and mechanical stimuli (CMH nociceptors), 37 (41%) were excited by only mechanical stimuli (CM nociceptors), 8 (9%) responded to mechanical and cold stimuli (CMC nociceptors) and 6 (6%) exhibited responses to heat, mechanical, and cold stimuli (CMHC nociceptors). None of the C nociceptors had ongoing activity prior to any drug injection.
Figure 1.
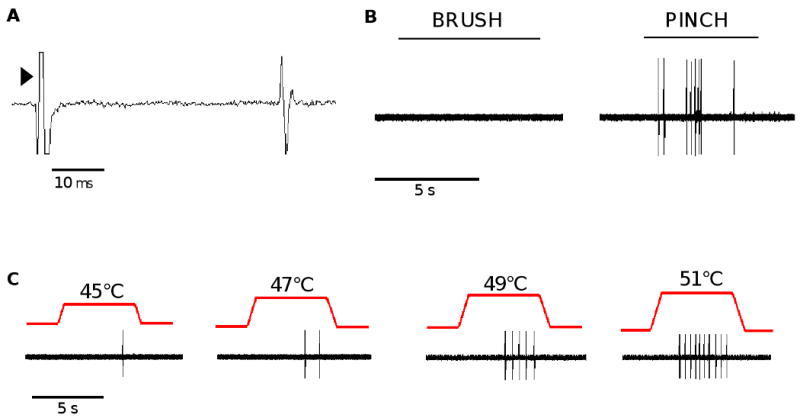
Functional classification of cutaneous C nociceptors. (A) An example of a conduction latency trace for a single C nociceptor illustrating the latency of action potential discharge following electrical stimulation at the RF (arrow head). (B) An example of a response of a C nociceptor evoked by application of a cotton swab brushed across the RF followed by lifting the skin and pinching the RF with a pair of forceps. (C) Responses of a C nociceptor to increasing intensities of heat stimuli applied to the RF.
2.2 Responses of C nociceptors to injection of vehicle
Injection of vehicle (Tocrisolve™:saline) into the RFs excited 4 of 10 C nociceptors, probably due to mechanical distention of the RF (Hilliges et al., 2002). Overall, injection of vehicle evoked 13.5±2.5 impulses with a mean discharge rate of 2.9±0.8 Hz. Importantly, responses only occurred during injection and no post-injection impulses were evoked once the needle was removed from the skin. An example of a typical response to vehicle for a single C nociceptor is displayed in Figure 2a.
Figure 2.
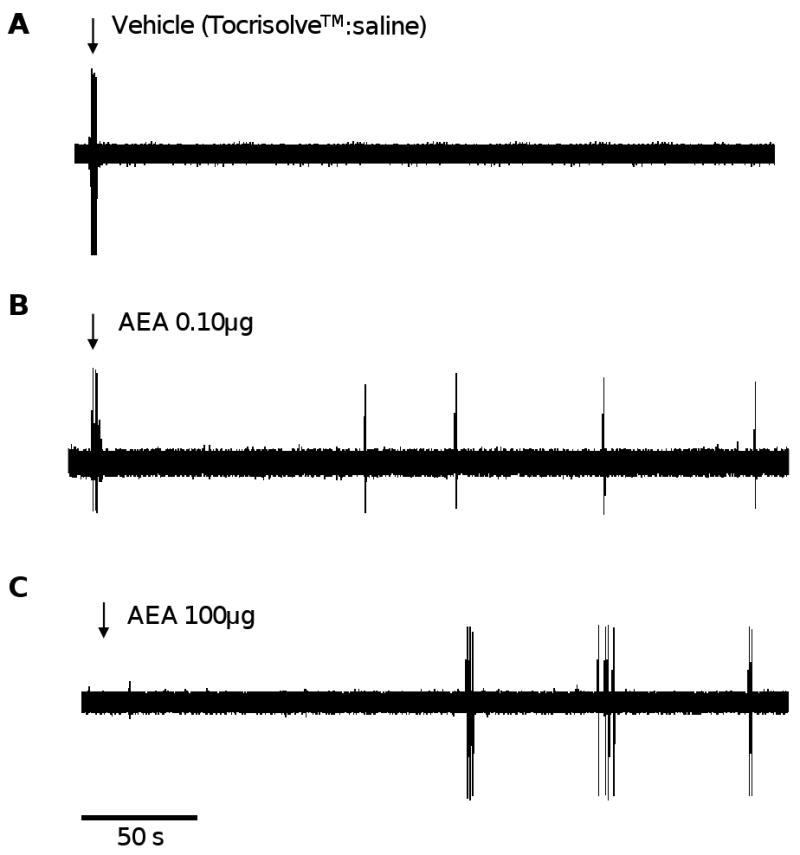
Responses of cutaneous C nociceptors to vehicle or AEA. (A) An example of a response of a single cutaneous C nociceptor to injection of vehicle (Tocrisolve™:saline). (B) An example of excitation produced by injection of 100 ng AEA. (C) An example of the response evoked by injection of 100 μg AEA. Downward arrow designates the time of injection.
2.3 Dose-dependent excitation of C nociceptors by anandamide
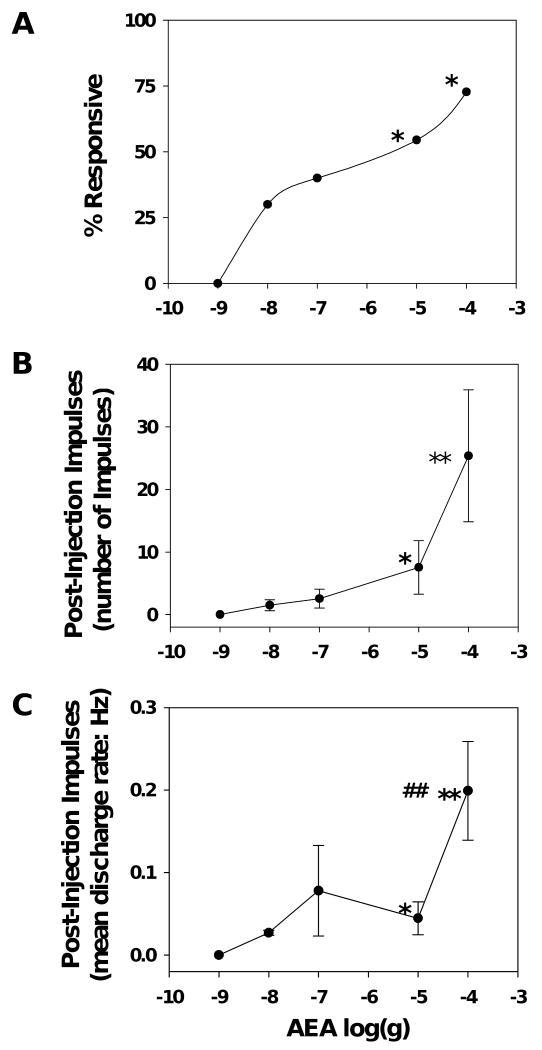
Injection of AEA at doses from 0.001 to 100 μg into the RF excited 24 out of 52 C nociceptors (n=10 - 11 per dose). Injection responses did not vary as a function of AEA dose and the combined injection response produced by all doses of AEA was 10.3±1.8 impulses with a mean discharge rate of 3.0±0.1 Hz. This was similar to the proportion and magnitude of injection responses produced by vehicle. However, unlike vehicle, injection of AEA at doses greater than 0.001 μg evoked responses after the needle was withdrawn from the skin (post-injection impulses). Examples of post-injection impulses evoked by injection of AEA at doses of 0.10 μg and 100 μg are shown for individual C nociceptors in figure 2b and 2c, respectively. The proportion of nociceptors that exhibited post-injection impulses, as well as the number of impulses and discharge rate, were greater after AEA doses of 10 and 100 μg as compared to vehicle (p<0.05; figure 3). Post-injection discharge rates produced by 100 μg AEA were greater than those produced by 10 μg AEA (p<0.01; figure 3). The latency to onset and the duration of post-injection impulse firing did not differ between the doses. The mean latency to onset of post-injection impulses was 60.3±2.8 s and the mean duration of response was 133.6±4.1 s across all doses. The proportion of C nociceptors excited by AEA was not related to functional subtype of C nociceptor. Overall, these data demonstrate that peripheral administration of AEA excited cutaneous C nociceptors in a dose-dependent manner.
Figure 3.
Dose-dependent excitation of cutaneous C nociceptors by AEA. (A) The percent of C nociceptors responsive to each dose of AEA. (B) The mean number of post-injection impulses evoked by each dose of AEA. (C) The mean discharge rate of post-injection impulses evoked by AEA. * indicates a significant difference from vehicle (p<0.05). ** indicates a significant difference from vehicle (p<0.01). ## indicates a significant difference from 10 μg AEA (p<0.01). n=10-11 per group.
Excitation of cutaneous nociceptors by AEA appears to be restricted primarily to C nociceptors since in a small sample of mechanosensitive Aδ nociceptors studied, none were excited by 100 μg AEA (n=5) or vehicle (n=5) (data not shown).
2.4 Attenuation of AEA-evoked responses by TRPV1 receptor antagonists
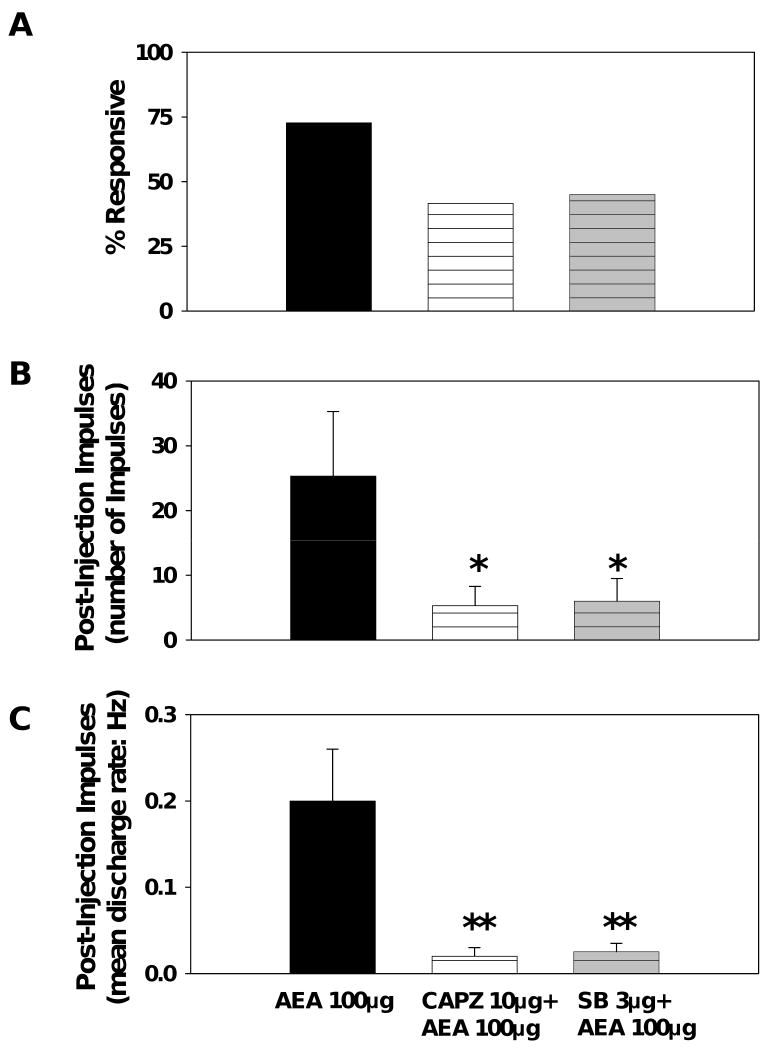
To determine whether excitation of nociceptors by AEA occurred through a TRPV1-dependent mechanism, either capsazepine or SB 366791, both competitive TRPV1 receptor antagonists, was injected into the RF 5 minutes prior to 100 μg AEA. Injection of capsazepine (10 μg), SB 366791 (3 μg), or vehicle (ethanol:saline) produced injection responses that did not differ in either proportion or magnitude from each other or AEA and its vehicle (data not shown). Capsazepine, SB 366791, or vehicle did not produce any post-injection impulses. Pre-treatment with either capsazepine (10 μg) or SB 366791 (3 μg) attenuated the excitation produced by 100 μg AEA. Pre-treatment with either capsazepine or SB 366791 attenuated the number of post-injection impulses (Figure 4b) and the mean discharge rates (Figure 4c) evoked by AEA. These data suggest that excitation of C nociceptors by AEA occurs, at least in part, via TRPV1 receptors.
Figure 4.
Capsazepine attenuates excitation of C nociceptors evoked by AEA. (A) The change in the percent of C nociceptors responsive to 100 μg AEA following pre-treatment with capsazepine (10 μg). Capsazepine produced a significant decrease in both the number of post-injection impulses (B) and the mean discharge rate of post-injection impulses (C) evoked by injection of 100 μg AEA. * indicates a significant difference from 100 μg AEA (p<0.05). ** indicates a significant difference from 100 μg AEA (p<0.01). n=11-12 per group.
2.5 Nocifensive behaviors produced by AEA
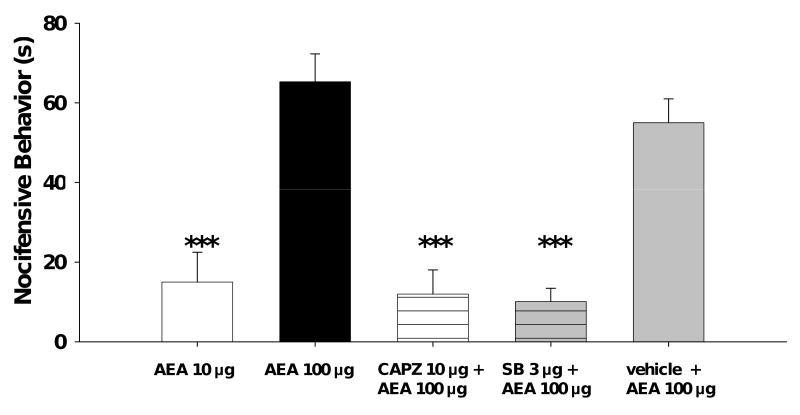
Since AEA excited cutaneous nociceptors, we determined if intraplantar injection of AEA also evoked nocifensive behaviors. Rats received a single intraplantar injection of 10 or 100 μg AEA, or vehicle, and the onset and duration of nocifensive behaviors were recorded. AEA, but not vehicle, produced nocifensive behaviors. Doses of 10 and 100 μg produced nocifensive behaviors that lasted for a duration of 15.0±7.5 s and 65.3±7.0 s, respectively (p<0.001; figure 5). To determine if the nocifensive behaviors produced by AEA were mediated by TRPV1 receptors, rats received an intraplantar injection of capsazepine (10 μg), SB 366791 (3 μg), or vehicle prior to injection of 100 μg AEA. Capsazepine, SB 366791, or vehicle did not produce any nocifensive behaviors (data not shown). However, nocifensive behaviors produced by AEA were attenuated following pre-treatment with capsazepine or SB 366791, but not vehicle (figure 5). The onset of nocifensive behavior produced by AEA did not differ between the groups, and the mean onset of nocifensive behavior across all groups was 63.5±5.9 s. These data are consistent with our electrophysiological studies and suggest that nocifensive behaviors produced by AEA occur, at least in part, through activation of TRPV1 receptors.
Figure 5.
Nocifensive behavior following intraplantar injection of AEA is attenuated by capsazepine. The duration of nocifensive behavior (s) was quantified following injection of AEA at doses of 10 or 100 μg alone or 100 μg AEA following pre-treatment with capsazepine (10 μg) or vehicle. CAPZ: capsazepine. SB: SB 366791. Vehicle: vehicle for capsazepine and SB 366791. NOTE: neither anandamide's vehicle (Tocrisolve:saline), CAPZ/SB's vehicle (ethanol:saline), CAPZ alone, or SB alone evoked nocifensive behaviors. *** indicates a significant difference from 100 μg AEA (p<0.001). n=6-10 per group.
2.6 Withdrawal responses to radiant heat following AEA
Since other TRPV1 receptor agonists produce nocifensive behaviors and hyperalgesia to heat, we determined whether AEA also produced heat hyperalgesia. Paw withdrawal latencies from radiant heat were determined before and after intraplantar injection of vehicle or 100 μg AEA (n=6-7 per group). Vehicle produced a small decrease in the paw withdrawal latencies from 10.7±0.6 s to a peak decrease of 8.3±0.5 s at 30 minutes after injection (p<0.05). AEA produced a similar decrease in paw withdrawal latency. Mean withdrawal latencies decreased from 10.9±0.8 s before injection to a peak decrease of 8.4±0.8 s at 30 minutes after injection (p<0.05), which did not differ at any time point from the vehicle treated group (data not shown). Withdrawal latencies returned to baseline values by 60 minutes after injection of AEA or vehicle. These data suggest that intraplantar injection of AEA does not produce either hyperalgesia or antinociception to radiant heat.
3. Discussion
The present study demonstrated that intraplantar injection of AEA produced dose-dependent excitation of cutaneous C nociceptors which was attenuated by pre-treatment with TRPV1 receptor antagonists capsazepine and SB 366791. Similarly, intraplantar injection of AEA produced nocifensive behaviors that were also attenuated by either capsazepine or SB 366791. Unlike other TRPV1 receptor agonists, intraplantar injection of AEA alone did not alter withdrawal responses to radiant heat. Together, these results demonstrate that AEA excites cutaneous C nociceptors and produces nocifensive behaviors, in part, through activation of TRPV1 receptors.
3.1 Activation of TRPV1 receptors by AEA
In previous studies conducted in vitro, AEA excited isolated DRG neurons (Tognetto et al., 2001; Olah et al., 2001; Jerman et al., 2002; Ahluwalia et al., 2003; Fischback et al., 2007). The present study extends these observations and show that cutaneous C nociceptors in vivo are also excited by AEA. The doses of AEA used here to excite cutaneous nociceptors were similar to those used to excite bronchopulmonary (Lin and Lee, 2002; Kollarik and Undem, 2004; Lee et al., 2005), mesenteric (Zygmunt et al., 1999), and articular (Gauldie et al., 2001) C fibers.
Excitation of C nociceptors by AEA was attenuated by pre-treatment with the TRPV1 receptor antagonists capsazepine and SB 366791, consistent with earlier studies in vitro (Tognetto et al., 2001; Olah et al., 2001; Jerman et al., 2002; Ahluwalia et al., 2003; Fischback et al., 2007). Despite the higher affinity and selectivity of SB 366791 for TRPV1 receptors compared to capsazepine, both attenuated the excitation produced by AEA to a similar degree. The results of the present study are in agreement with and extend earlier studies demonstrating that AEA can excite nociceptive dorsal root ganglion neurons through TRPV1 receptors. Interestingly, it synthetic cannabinoid receptor agonists, such as arachidonyl-2-chloroethylamide (ACEA), WIN 55,212-2, and AM1241 can also activate TRP channels (Price et al., 2004: Jeske et al., 2006; Akopian et al., 2008).
Although our results demonstrate that excitation of cutaneous C nociceptors by AEA is at least partly mediated by activation of TRPV1 receptors, some nociceptors were still excited by AEA after pre-treatment with capsazepine or SB 366791. This suggests that other mechanisms in addition to TRPV1 receptors may be involved in the excitation of nociceptors by AEA. In a previous study it was found that AEA activated TRPV4 receptors following metabolism by cytochrome P450 epoxygenase (Watanabe et al., 2003). Additional studies have shown that other enzymes can oxidize AEA, with unknown actions on nociceptors (for review see Burstein et al., 2000; Woodward et al., 2008).
Although responses of C nociceptors to capsaicin were not determined in the present study, prior studies have clearly documented the excitatory effects of capsaicin on cutaneous C nociceptors in rats (Ren et al., 2005), non-human primates (Baumann et al., 1991) and humans (LaMotte et al., 1992). Intradermal injection of capsaicin produces excitation of C nociceptors immediately following injection that lasts for several minutes (Baumann et al., 1991; LaMotte et al., 1992; Ren et al., 2005). The excitation of C nociceptors following intradermal injection of capsaicin is consistent with the sensation of burning pain in humans (Simone et al., 1989; LaMotte et al., 1992) and nocifensive behaviors exhibited by rats (Gilchrist et al., 1996). In contrast to capsaicin, AEA did not produce hyperalgesia to radiant heat and had a longer onset (≈ 60 s) to excitation of C nociceptors. The decrease in paw withdrawal latencies likely resulted from irritation produced by injection, and we have also observed increased mechanical sensitivity following intraplantar injections (Potenzieri et al., 2008). Although administration of AEA into the RFs of C nociceptors evoked responses during injection, this likely represents a non-specific effect since the magnitude of these responses did not differ between doses of AEA, antagonists, or vehicles.
3.2 Nocifensive behaviors produced by intraplantar injection of AEA
The excitation of cutaneous C nociceptors by AEA likely underlies the nocifensive behaviors evoked by AEA. A strong correlation was found between the onset of nociceptor excitation and the onset of nocifensive behavior after administration of 100 μg AEA. The mean onset of nocifensive behaviors after injection was 64.6±9.4 s, which was similar to the onset of C nociceptor excitation (60.3±2.8 s). Although the duration of excitation of cutaneous C nociceptors by AEA did not differ between the doses used, the magnitude of excitation was greater at higher doses. This increased magnitude of excitation likely contributes to the nocifensive behaviors produced by the higher doses of AEA. In a similar study, nocifensive behaviors following intraplantar injection of ATP in rats also correlated with the magnitude of C nociceptor excitation by ATP (Hamilton et al., 2001). The decrease in both nociceptor excitation and the attenuation of nocifensive behaviors by pre-treatment with capsazepine or SB 366791 also demonstrates the importance of C nociceptors to the nocifensive behaviors following AEA. Prior studies have demonstrated that both capsazepine and SB 366791 can also attenuate nocifensive behaviors evoked by intraplantar injection of capsaicin (Andrade et al., 2008).
Although intraplantar injection of 100 μg AEA produced nocifensive behaviors, it did not alter withdrawal latencies to radiant heat. A similar result was recently reported in which intrathecal administration of 100 μg AEA produced a temporary pro-nociceptive effect described as “vocalization and excitation” without subsequent hyperalgesia to heat (Horvath et al., 2008). This lack of hyperalgesia to heat following AEA differs from that of other endogenous TRPV1 agonists, such as N-arachidonoyldopamine (NADA) (Huang et al., 2002) and N-oleoyldopamine (OLDA) (Chu et al., 2003). The lack of hyperalgesia to heat after AEA may be related to its lower efficacy or its partial agonist activity at rat TRPV1 receptors (Zygmunt et al., 1999; Sprague et al., 2001; Smart et al., 2001; Jerman et al., 2002).
Peripheral administration of AEA does not always produce nocifensive behaviors. For example, application of NADA, but not AEA at doses of 61 to 608 μg, to the cornea of rats elicited nocifensive behavior (Price et al., 2004). This observation is somewhat surprising given the dose-dependent activation of trigeminal ganglion neurons by AEA (Price et al., 2004). In humans, local application of 30 mM AEA into the skin of the forearm produced vasodilatation without sensations of pain (Movahed et al., 2005). The absence of pain sensation following injection of AEA could be related to the route of administration, since AEA was applied to the superficial skin using a lancet (Movahed et al., 2005), whereas in the present study AEA was administered via subcutaneous injection.
Peripheral injection of AEA has traditionally been shown to produce analgesia in rodent pain models (Richardson et al., 1998; Guindon and Beaulieu, 2006; Guindon et al., 2006). This is a dose-dependent effect since the analgesic doses of AEA used in those studies were all in nanogram range and much lower than those needed to excite nociceptors in the present study. These results are consistent with a concentration-dependent, biphasic model for AEA, where at low concentrations AEA acts as an agonist at CB1 receptors to inhibit nociceptive transmission, whereas higher concentrations produce excitation of nociceptors via activation of TRPV1 receptors (Tognetto et al., 2001; Ahluwalia et al., 2003). Thus, it is possible that AEA can produce peripherally-mediated analgesia at low doses without activating C nociceptors, but produce nocifensive behaviors at higher doses.
3.3 Physiological implications for excitation of C nociceptors by AEA
Previous studies have demonstrated that AEA is found in the skin of rodents at low levels (Felder et al., 1996; Calignano et al., 1998; Beaulieu et al., 2000). Therefore, it is unlikely that the concentrations of AEA which excite cutaneous C nociceptors in this study exist under basal conditions. However, under certain conditions the effect of AEA on TRPV1 receptors may be potentiated. For example, the affinity of AEA for TRPV1 receptors increased following activation of PKA (de Petrocellis et al., 2001) and PKC (Vellani et al., 2001), during acidification (pH ≤ 6), and after exposure to inflammatory mediators (Singh Tahim et al., 2005). Thus, under pathological conditions, endogenous AEA may activate TRPV1 receptors. In addition, concentrations of AEA have been shown to increase during cystitis and contractions of the urinary bladder by AEA via TRPV1 receptors were greatly enhanced during cystitis, suggesting that AEA contributes to this pathological state (Harrison et al., 2003; Dinis et al., 2004; Saitoh et al., 2007). During inflammatory conditions, it is possible that elevated concentrations of AEA could serve to promote nociceptive signaling by activating nociceptors via TRPV1 receptors. Further studies are needed to determine the contribution of endogenous AEA to the excitation and sensitization of nociceptors following injury and inflammation.
3.4 Summary
The present study demonstrated that AEA excited cutaneous C nociceptors dose-dependently and produced correlative nocifensive behaviors. Excitation of C nociceptors and nocifensive behaviors produced by AEA were attenuated by pre-treatment with TRPV1 receptor antagonists capsazepine or SB 366791, suggesting that excitation by AEA occurs, at least in part, through activation of TRPV1 receptors. Additional studies are needed to determine the functional roles of AEA in modulating nociceptor activity under normal and pathological conditions.
4. Experimental Procedures
4.1 Subjects
A total of 130 adult, male, Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing 280-350 g were used in this study. Animals were housed on a 12-hour light/dark schedule and allowed ad libitum access to food and water. All animal procedures were approved by the Animal Care Committee at the University of Minnesota, and experiments were conducted according to the guidelines established by the International Association for the Study of Pain.
4.2 Drug preparations and administration
Anandamide (N-(2-Hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide), the TRPV1 receptor antagonists capsazepine (N-[2-(4-Chlorophenyl)ethyl]-1,3,4,5-tetrahydro-7,8-dihy droxy-2H-2-benzazepine-2-carbothioamide) and SB 366791 (4′-Chloro-3-methoxycinnamanilide), and Tocrisolve™100 were acquired from Tocris Bioscience (Ellisville, MO). AEA was supplied pre-dissolved in Tocrisolve™100 (5 μg/μl). Capsazepine and SB 366791 were both prepared in a stock solution of 100% ethanol (20 μg/μl). Capsazepine is a vanilloid receptor antagonist with an IC50 ≈ 230 - 600 nM (Dickenson and Dray, 1991; Seabrook et al., 2002). SB 366791 is a selective TRPV1 receptor antagonist with an IC50 ≈ 6 nM (Gunthorpe et al., 2004). The doses of capsazepine (26.5 nmol) and SB 366791 (10.4 nmol) used here were similar, but slightly higher than doses (1 nmol each) used to attenuate capsaicin-evoked nocifensive behaviors in a previous study (Andrade et al. 2008). All drugs were diluted to their final concentrations in sterile isotonic saline and were administered by subcutaneous injection into the plantar surface of the hindpaw in a total volume of 20 μl using a 0.3 ml insulin syringe. The vehicle used corresponded to the highest dose of AEA (100 μg).
4.3 Electrophysiological recording
All animals were initially anesthetized by intramuscular injection of ketamine (100 mg/kg) and xylazine (45 mg/kg). A catheter was placed into the external jugular vein to provide supplemental anesthesia with sodium pentobarbital (10 mg/kg per hour) to maintain areflexia. Core body temperature was maintained at 37°C using a feedback-controlled heating pad (Harvard Apparatus, Holliston, MA). Animals were euthanized at the end of each experiment with an overdose of sodium pentobarbital.
Electrophysiological recordings were made from identified cutaneous afferent fibers from the left tibial nerve using a teased-fiber approach. The tibial nerve was dissected from the surrounding tissue and the overlaying skin was sewn to a metal ring to form a pool that was filled with warm mineral oil. The tibial nerve was placed onto a mirror platform for fine dissection with sharpened Dumont # 5 forceps (Fine Science Tools, Foster City, CA). Teased fibers were placed onto a tungsten electrode and action potentials were recorded extracellularly. Action potentials were amplified, audio monitored, displayed on an oscilloscope, and stored on a PC computer for data analysis. Only fibers with clearly discriminated single unitary action potentials (units) were studied. Responses of individual units were analyzed off-line using a customized data analysis program (LabVIEW, version 5.1; National Instruments, Austin, TX).
4.4 Identification of Afferent Units
Afferent units were found by mechanically stimulating the plantar surface of the hindpaw with the experimenter's finger or by stimulation with calibrated von Frey monofilaments. Once a single unit was identified, the location of its mechanical receptive field (RF) was identified with von Frey filaments and marked on the skin using a felt-tipped pen.
4.5 Conduction Velocity
The conduction velocity was determined by electrically stimulating the skin with pin electrodes inserted just outside the RF. Electrical stimuli consisted of 200-μs pulses delivered at a rate of 0.5 Hz. Units were excited at 1.5× their electrical threshold and the conduction latency was measured from the time of the electrical stimulus artifact to the evoked action potential. Conduction distance was determined by measuring the distance from the RF to the recording electrode. Conduction velocity (m/s) was calculated by dividing conduction distance by conduction latency.
4.6 Functional Classification of Nociceptors
Units were classified functionally according to their responses to mechanical, heat, and cold stimuli applied to the RF. Mechanical stimuli used to classify units included light brushing with the tip of a cotton swab, mildly pinching with a pair of forceps, and application of von Frey filaments. Mechanical response thresholds were determined using a series of calibrated von Frey monofilaments and defined as the smallest force (mN) that reliably evoked at least one impulse. Heat stimuli were delivered using a feedback-controlled Peltier device (Yale Electronics, New Haven, CT) with a contact area of 1 cm2. Heat response thresholds were determined by stimulating the RF for 5 s using an ascending series of heat stimuli from 35 - 51°C with 2°C intervals and an interstimulus interval of 120 s. Heat response thresholds were defined as the temperature (°C) to evoke at least one impulse. A unit was considered heat-responsive if it responded with at least one impulse to a stimulus temperature of 51°C or less. A unit was classified as cold responsive if it discharged at least one impulse in response to placement of a small piece of ice on its receptive field for 20 s. Units were classified as nociceptors if they exhibited a slowly adapting response to noxious pinch but not to light touch (Leem et al., 1993). Units were further classified as Aδ nociceptors if they had a conduction velocity between 2.5 -25.0 m/s and as C nociceptors if they has a conduction velocity < 2.5 m/s.
4.7 Nociceptor responses to intraplantar injection
Following the classification of a unit as a nociceptor, activity over 300 s in the absence of stimulation was recorded to determine if the unit exhibited ongoing activity. Drug was administered by inserting the needle outside the unit's RF and the injectate was observed as a bleb of fluid centered within the RF. Ongoing activity was also recorded during and for 300 s after injection. Responses were separated into the response during injection (injection responses), and responses that occurred after injection of drug and withdrawal of the needle from the skin (post-injection responses). Injection and post-injection responses are indicated as both the evoked number of impulses and mean discharge rate (Hz). A unit was considered responsive to drug if it discharged ≥ 2 post-injection impulses. Only one nociceptor was studied per animal.
4.8 Nocifensive behaviors
After three consecutive days of acclimation to the testing environment, animals were briefly restrained in a cloth towel and received an intraplantar injection of AEA or vehicle into one hindpaw. Animals were then placed under a clear plastic cage (23×3×13 cm3) on an elevated wire mesh platform for observation of nocifensive behaviors. Nocifensive behaviors consisted of spontaneous lifting, flinching, and licking of the hindpaw. Immediately following injection, a timer was started and the latency to onset (s) and the duration (s) of nocifensive behaviors was recorded over a 5 minute period. The experimenter was blinded to the identity of drug given.
4.9 Withdrawal responses to radiant heat
Paw withdrawal latency (s) was determined according to a method similar to that described by Hargreaves et al., (1988). Withdrawal responses to radiant heat stimuli were determined using a custom built device that uses an encased 50W bulb to deliver a radiant heat source. Rats were placed under a clear plastic cage on a 3-mm thick glass plate that was elevated to allow maneuvering of the radiant heat source beneath it. Rats were acclimated to the testing environment for at least 15 min prior to stimulation. The heat source was positioned such that the focused beam of radiant heat (8 mm diameter) was applied to the mid-plantar surface. Withdrawal latencies to the nearest 0.1 s were measured automatically by use of a photocell that terminated each trial and stopped the timer upon withdrawal of the hindpaw. A 19 s cutoff was imposed to prevent tissue damage. Four stimuli were applied to each hindpaw, alternating between paws, with an interstimulus interval of at least 60 s. Withdrawal latency for each paw was defined as the average of the last three trials. Withdrawal latencies were obtained daily for each hindpaw during a training period of 3 consecutive days. Hyperalgesia and analgesia to heat were defined as a decrease or an increase in paw withdrawal latency, respectively. The experimenter was blinded to the identity of drug given.
4.10 Data analyses
All data are presented as mean±S.E.M. Injection responses produced by vehicles, AEA, and antagonists; dose-response relationships of anandamide on the number of post-injection impulses and mean discharge rates; and between group comparisons on the duration of nocifensive behaviors and paw withdrawal latencies to radiant heat were all made using one-way ANOVA followed by un-paired t-tests with the Bonferroni correction for multiple comparisons. Analysis of the proportions of nociceptors exhibiting injection and post-injection impulses following vehicles, AEA, and antagonists were compared using the Chi-square test followed by pair-wise comparisons using the Fisher Exact test. The effect of antagonists on the number of post-injection impulses and mean discharge rate and were made using un-paired t-tests. The effect of antagonist pre-treatment on the proportion of C nociceptors exhibiting post-injection impulses was determined using the Fisher exact test. All statistical analyses were performed using Sigma Stat software (Systat Software, San Jose, CA). A probability value <0.05 was considered significant.
Acknowledgments
The authors thank Dr. Virginia S. Seybold for critically reading an early manuscript of this paper. This work was supported by grants from the National Institutes of Health (CA91007 and DA011471 to DAS). CP was supported by training grants from the National Institute on Drug Abuse (5T32- DA007234 and 1F31-DA024541). TB was supported by a training grant from the National Institute of Dental and Craniofacial Research (T32-DE007288).
Footnotes
Classification of Terms: none
Section: Sensory Systems, Pain modulation: pharmacology
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. European Journal of Neuroscience. 2003;17:2611–2618. doi: 10.1046/j.1460-9568.2003.02703.x. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. Journal of Neuroscience. 2008;28:1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade EL, Luiz AP, Ferreira J, Calixto JB. Pronociceptive response elicited by TRPA1 receptor activation in mice. Neuroscience. 2008;152:511–520. doi: 10.1016/j.neuroscience.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. Journal of Neurophysiology. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Beaulieu1 P, Bisogno1 T, Punwar S, Farquhar-Smith WP, Ambrosino G, Di Marzo V, Rice AS. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. European Journal of Pharmacology. 2002;396:85–92. doi: 10.1016/s0014-2999(00)00226-0. [DOI] [PubMed] [Google Scholar]
- Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediators. 2000;61:29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–624. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, Walker JM. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. Journal of Biological Chemistry. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, Creminon C, Davis JB, Geppetti P, Di Marzo V. The vanilloid receptor (VR1)-mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. Journal of Neurochemistry. 2001;77:1660–1663. doi: 10.1046/j.1471-4159.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signaling. Life Sciences. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Dray A. Selective antagonism of capsaicin by capsazepine: evidence for a spinal receptor site in capsaicin-induced antinociception. British Journal of Pharmacology. 1991;104:1045–1049. doi: 10.1111/j.1476-5381.1991.tb12547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis P, Charrua A, Avelino A, Yaqoob M, Bevan S, Nagy I, Cruz F. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. Journal of Neuroscience. 2004;24:11253–11263. doi: 10.1523/JNEUROSCI.2657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Molecular Pharmacology. 1995;48:443–450. [PubMed] [Google Scholar]
- Felder CC, Nielsen A, Briley EM, Palkovits M, Priller J, Axelrod J, Nguyen DN, Richardson JM, Riggin RM, Koppel GA, Paul SM, Becker GW. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Letters. 1996;393:231–235. doi: 10.1016/0014-5793(96)00891-5. [DOI] [PubMed] [Google Scholar]
- Fischbach T, Greffrath W, Nawrath H, Treede RD. Effects of anandamide and noxious heat on intracellular calcium concentration in nociceptive drg neurons of rats. Journal of Neurophysiology. 2007;98:929–938. doi: 10.1152/jn.01096.2006. [DOI] [PubMed] [Google Scholar]
- Fride E, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. European Journal of Pharmacology. 1993;231:313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Gauldie SD, McQueen DS, Pertwee R, Chessell IP. Anandamide activates peripheral nociceptors in normal and arthritic rat knew joints. British Journal of Pharmacology. 2001;132:617–621. doi: 10.1038/sj.bjp.0703890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- Guindon J, Beaulieu P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxib and their combination in a model of neuropathic pain. Neuropharmacology. 2006;50:814–823. doi: 10.1016/j.neuropharm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Guindon J, De Lean A, Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 2006;121:85–93. doi: 10.1016/j.pain.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Luis Hannan S, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. Journal of Physiology. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harrison S, De Petrocellis L, Trevisani M, Benvenuti F, Bifulco M, Geppetti P, Di Marzo V. Capsaicin-like effects of N-arachidonoyl-dopamine in the isolated guinea pig bronchi and urinary bladder. European Journal of Pharmacology. 2003;475:107–104. doi: 10.1016/s0014-2999(03)02114-9. [DOI] [PubMed] [Google Scholar]
- Hilliges M, Weidner C, Schmelz M, Schmidt R, Ørstavik K, Torebjörk E, Handwerker H. ATP responses in human C nociceptors. Pain. 2002;98:59–68. doi: 10.1016/s0304-3959(01)00469-9. [DOI] [PubMed] [Google Scholar]
- Horvath G, Kekesi G, Nagy E, Benedek G. The role of TRPV1 receptors in the antinociceptive effect of anandamide at spinal level. Pain. 2008;134:277–284. doi: 10.1016/j.pain.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47:345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proceedings of National Academy of Sciences USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman JC, Gray J, Brough SJ, Ooi L, Owen D, Davis JB, Smart D. Comparison of effects of anandamide at recombinant and endogenous rat vanilloid receptors. British Journal of Anesthesia. 2002;89:882–887. doi: 10.1093/bja/aef281. [DOI] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. Journal of Biological Chemistry. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1-/- mice. Journal of Physiology. 2004;555:115–123. doi: 10.1113/jphysiol.2003.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjörk HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. Journal of Physiology. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Weinreich D, Undem BJ. Effect of olvanil and anandamide on vagal C-fiber subtypes in guinea pig lung. British Journal of Pharmacology. 2005;146:596–603. doi: 10.1038/sj.bjp.0706339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Lee LY. Stimulation of pulmonary vagal C-fibers by anandamide in anaesthetized rats: role of vanilloid type 1 receptors. Journal of Physiology. 2002;539:947–955. doi: 10.1113/jphysiol.2001.013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Loliat SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Melck D, Bisogno T, De Petrocellis L, Chuang H, Julius D, Bifulco M, Di Marzo V. Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochemical and Biophysical Research Communications. 1999;262:275–284. doi: 10.1006/bbrc.1999.1105. [DOI] [PubMed] [Google Scholar]
- Movahed P, Evilevitch V, Andersson TL, Jönsson BA, Wollmer P, Zygmunt PM, Högestätt ED. Vascular effects of anandamide and N-acylvanillylamines in the human forearm and skin microcirculation. British Journal of Pharmacology. 2005;146:171–179. doi: 10.1038/sj.bjp.0706313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murro S, Thomas KL, Abu-Sharr M. Molecular characterization of a peripheral cannabinoid receptor. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Olah Z, Karai L, Iadarola MJ. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. Journal of Biological Chemistry. 2001;276:31163–31170. doi: 10.1074/jbc.M101607200. [DOI] [PubMed] [Google Scholar]
- Potenzieri C, Brink TS, Pacharinsak C, Simone DA. Cannabinoid modulation of Aδ nociceptors during inflammation. Journal of Neurophysiology. 2008;100:2794–2806. doi: 10.1152/jn.90809.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. British Journal of Pharmacology. 2004;141:1118–1130. doi: 10.1038/sj.bjp.0705711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Zou X, Fang L, Lin Q. Sympathetic modulation of activity in Adelta- and C-primary nociceptive afferents after intradermal injection of capsaicin in rats. Journal of Neurophysiology. 2005;93:365–377. doi: 10.1152/jn.00804.2004. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Christie MJ, Connor M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. British Journal of Pharmacology. 2002;137:421–428. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh C, Kitada C, Uchida W, Chancellor MB, de Groat WC, Yoshimura N. The differential contractile responses to capsaicin and anandamide in muscle strips isolated from the rat urinary bladder. European Journal of Pharmacology. 2007;570:182–187. doi: 10.1016/j.ejphar.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Sutton KG, Jarolimek W, Hollingworth GJ, Teague S, Webb J, Clark N, Boyce S, Kerby J, Ali Z, Chou M, Middleton R, Kaczorowski G, Jones AB. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. Journal of Pharmacology and Experimental Therapeutics. 2002;303:1052–1060. doi: 10.1124/jpet.102.040394. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Singh Tahim A, Sántha P, Nagy I. Inflammatory mediators convert anandamide into a potent activator of the vanilloid type 1 transient receptor potential receptor in nociceptive primary sensory neurons. Neuroscience. 2005;136:539–548. doi: 10.1016/j.neuroscience.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Slipetz DM, O'Neill GP, Favreau L, Dufresne C, Gallant M, Gareau Y, Guay D, Labelle M, Metters KM. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Molecular Pharmacology. 1995;48:352–361. [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) British Journal of Pharmacology. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Jerman JC, Gunthorpe MJ, Brough SJ, Ranson J, Cairns W, Hayes PD, Randall AD, Davis JB. Characterisation using FLIPR of human vanilloid VR1 receptor pharmacology. European Journal of Pharmacology. 2001;417:51–58. doi: 10.1016/s0014-2999(01)00901-3. [DOI] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. Journal of Pharmacology and Experimental Therapeutics. 1994;270:219–227. [PubMed] [Google Scholar]
- Sprague J, Harrison C, Rowbotham DJ, Smart D, Lambert DG. Temperature-dependent activation of recombinant rat vanilloid VR1 receptors expressed in HEK293 cells by capsaicin and anandamide. European Journal of Pharmacology. 2001;423:121–125. doi: 10.1016/s0014-2999(01)01123-2. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Szabó T, Bíró T, Modarres S, Blumberg PM, Krause JE, Cortright DN, Appendino G. Resiniferatoxin-type phorboid vanilloids display capsaicin-like selectivity at native vanilloid receptors on rat DRG neurons and at the cloned vanilloid receptor VR1. British Journal of Pharmacology. 1999;128:428–434. doi: 10.1038/sj.bjp.0702810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetto M, Amadesi S, Harrison S, Creminon C, Trevisani M, Carreras M, Matera M, Geppetti P, Bianchi A. Anandamide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. Journal of Neuroscience. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. Journal of Physiology. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Wise LE, Shelton CC, Cravatt BF, Martin BR, Lichtman AH. Assessment of anandamide's pharmacological effects in mice deficient of both fatty acid amide hydrolase and cannabinoid CB1 receptors. European Journal of Pharmacology. 2007;557:44–48. doi: 10.1016/j.ejphar.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Liang Y, Krauss AH. Prostamides (prostaglandin-ethanolamides) and their pharmacology. British Journal of Pharmacology. 2008;153:410–419. doi: 10.1038/sj.bjp.0707434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Anderson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors in sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]