Abstract
Rat adult hippocampal progenitor cells (AHPCs) are self-renewing, multipotent neural progenitors that have the ability to differentiate into neurons and glia. Previously, we demonstrated that co-culture of AHPCs with postnatal day two, type 1 cortical astrocytes on laminin-coated micropatterned polymer substrates facilitates selective neuronal differentiation of the AHPCs 1. Under this condition, multi-dimensional cell-cell and/or cell-extracellular matrix interactions, as well as possible soluble factors released from astrocytes provided spatial and temporal control selectively enhancing neuronal differentiation and neurite alignment on topographically different regions of the same substrate. To investigate the potential role of astrocyte-derived soluble factors as cues involved in neuronal differentiation, a non-contact co-culture system was used. Under control conditions, approximately 14% of the AHPCs were immunoreactive (IR) for the neuronal marker, class III β-tubulin (TUJ1-IR). When co-cultured in physical contact with astrocytes, neuronal differentiation increased significantly to about 25%, consistent with our previous results. Moreover, under non-contact co-culture conditions using Transwell insert cultures, neuronal differentiation was dramatically increased to approximately 64%. Furthermore, neurite outgrowth from neuronal cell bodies was considerably greater on the patterned substrate, compared to the non-patterned planar substrate under non-contact co-culture conditions. Taken together, our results demonstrate that astrocyte-derived soluble factors provide cues for specific neuronal differentiation of AHPCs cultured on micropatterned substrates. In addition, a suppressive influence on neuronal differentiation appears to be mediated by contact with co-cultured astrocytes. These results provide important insights into mechanisms for controlling neural progenitor/stem cell differentiation and facilitate development of strategies for CNS repair.
Keywords: adult neural stem cells, laminin, coculture techniques, micropatterning, cell differentiation, nerve regeneration
Introduction
During development of the central nervous system (CNS), reciprocal interactions between neural stem cells and their milieu are important for regulating and coordinating a variety of inter- and intra-cellular processes, such as proliferation, differentiation, migration, and cell survival. These developmental events also impact tissue organization and matrix remodeling 2-5. The local microenvironment of multipotent neural stem/progenitor cells (NPCs), referred to as the neural stem cell ‘niche’, have a profound influence on the fate of the NPCs 6-10. Extracellular matrix (ECM) components have been known to regulate the differentiation of NPCs 9,11-15. Moreover, numerous studies have demonstrated that astrocytes surrounding NPCs play a critical role in mediating neurogenesis 1,16,17. In addition, astrocyte-derived signals have been reported to regulate the structural formation and functional plasticity of synapses in developing and adult CNS 18-20. Our previous results suggested that the enriched astrocytes enhance neuronal differentiation of NPCs isolated from adult rat hippocampus (adult hippocampal progenitor cells, AHPCs) 1. In addition, the synergistic combination of spatial control from three-dimensional (3-D) micropatterned polystyrene substrates coated with the ECM molecule, laminin, along with the biological influence of the astrocytes aligned in the direction of the patterned substrate provided guidance cues for promoting neuronal differentiation of the AHPCs 1. Based on these results we proposed that, in the multi-dimensional environment, the astrocytes present discrete cues to the overlying AHPCs involving contact-mediated or release of soluble factors, or a combination of both. These factors may include specific molecules known to mediate cellular mechanisms that regulate cell growth, development, maturation and communication among the cells 21-24. The soluble factors released by astrocytes may be spatially restricted due to the topography of the micropatterned polystyrene substrate as well as the physically aligned astrocytes within the microgrooves. The astrocyte-derived factors may be locally constrained in the microgrooves and could potentially influence AHPC differentiation. It is possible that the stem cell niche has been mimicked in vitro through the presentation of an optimal combination of signals necessary for neuronal differentiation of the AHPCs.
In the present study, we have investigated the factors responsible for selective neuronal differentiation of AHPCs within the micropatterned multi-dimensional environment. Non-contact co-cultures were established using Transwell® semi-porous membrane inserts to separate the astrocytes from AHPCs cultured on micropatterned substrates but in the same well. In this culture system, the membrane inserts prohibit the physical interaction between the two types of cells but permit exchange of soluble factors between the cells. In an effort to identify the optimal combination of signals creating biological and spatial control over AHPC differentiation, we examined whether the factors responsible for the selective differentiation in the co-cultures were contact-mediated or soluble (or both) and possible roles of the factors.
Materials and Methods
Micropatterned substrate fabrication
Micropatterned polystyrene (PS) substrates were prepared as described in the previous study 1,25. The pattern dimensions used were 16/13/4 μm [groove width/groove spacing (or mesa width)/groove depth] and substrate thickness was approximately 50-70 μm. The micropatterned/non-patterned PS substrates were washed in deionized water, sterilized with 70% ethanol and used to construct cell growth chambers as described previously 25. The PS substrates were coated with poly-L-lysine (PLL, 100 μg/ml; Sigma, St. Louis, MO) solution and mouse-derived laminin (LAM, 10 μg/ml; R&D systems, Inc., Minneapolis, MN) diluted in Earle’s Balanced Salt Solution (EBSS; Gibco, Grand Island, NY) before plating cells.
Astroglial cell isolation and purification
All animal procedures were conducted in accordance with and had the approval of the Iowa State University Committee on Animal Care. Astrocytes were obtained from cerebral cortex of two day old Sprague-Dawley rats as previously described 25. Dissected and dissociated cells were grown in modified minimal essential culture medium (MMEM; Gibco) containing minimum essential medium (MEM; Gibco) supplemented with 40 mM glucose, 2 mM L-glutamine, 1 mM sodium pyruvate and 14 mM sodium bicarbonate, penicillin (100 IU/ml) and streptomycin (100 μg/ml) with 10% v/v fetal bovine serum (FBS; HyClone, Logan, UT), pH 7.35. The cells were cultured in an incubator (37°C, 5% CO2 / 95% humidified air atmosphere) until being confluent in 25 cm2 tissue culture flasks (T-25; Falcon), and screened with an anti-GFAP antibody (see Immunocytochemistry and Antibodies below) before use to ensure purification of an enriched population of type-1 astrocytes. Greater than 95% of the cells in these astrocyte cultures were immunoreactive for the GFAP antibody and no immunostaining for oligodendrocyte (RIP immunoreactivity) or neuronal (TUJ1 immunoreactivity) cell-types were observed. The cultures were not passaged more than 5 times.
Adult hippocampal progenitor cell culture
Adult hippocampal progenitor cells (AHPCs; a gift from Dr. F. H. Gage, La Jolla, CA) were originally isolated from the brains of adult Fischer 344 rats and the expanded cultures from single clones were infected with retrovirus to express enhanced green fluorescent protein (GFP) as reported by Palmer and colleagues 26. The AHPCs were cultured as described previously 1,25. Briefly, AHPCs were maintained in 75 cm2 tissue culture flasks (T-75; Fisher Scientific, Pittsburgh, PA) coated with 10 μg/ml of poly-L-ornithine (Sigma) and 5 μg/ml of LAM. The AHPCs were propagated in complete medium containing Dulbecco’s modified Eagle’s medium/Ham’s F-12 (DMEM/F-12, 1:1; Omega Scientific, Tarzana, CA) supplemented with N2 (Gibco BRL, Gaithersburg, MD), 20 ng/ml basic fibroblast growth factor (human recombinant bFGF; Promega Corporation, Madison, WI), and 2.5 mM L-glutamine (Gibco BRL, Gaithersburg, MD). For in vitro analysis, the AHPCs were detached using 0.05% Trypsin-EDTA (Gibco BRL, Gaithersburg, MD), harvested , and plated onto the micropatterned/non-micropatterned PS substrates coated with PLL (100 μg/ml) and LAM (10 μg/ml in EBSS) (PS-LAM substrates) and maintained in appropriate culture media.
Co-culture of astrocytes and AHPCs
Astrocyte-AHPC co-cultures were established as described previously 1. Briefly, 1.5 × 104 cells/cm2 of AHPCs were plated on top of an astrocyte monolayer and the co-cultures were maintained in a mixed medium (referred to as co-culture medium, CCM) that consisted of astrocyte MMEM without FBS in a 1:1 mixture with AHPC differentiation medium (AHPC complete medium excluding bFGF). Contact co-cultures, as well as control cultures (AHPCs alone and astrocytes alone), were grown for 6 days and then fixed in 4% paraformaldehyde in 0.1 M PO4 buffer, pH 7.4 for immunocytochemical analysis.
To co-culture AHPCs with the astrocytes without physical contact, purified astrocytes were seeded onto 0.4 μm semi-porous polyester membrane of Transwell® inserts inside 6-well plates (Corning, Inc., Corning, NY) at the same initial plating density, and cultured in MMEM including 10% FBS. After 2 days, the MMEM inside the inserts was replaced with CCM following rinses with EBSS and incubated for 4 hours at 37°C. Meanwhile, AHPCs were prepared separately. 1.5 × 104 cells/cm2 of AHPCs were plated on PS-LAM substrates and allowed to attach for 1 hour before removing the o-ring. The PS-LAM substrate on which AHPCs were plated was placed at the bottom of each well and additional CCM was added to the wells. Astrocytes on the insert membrane were then placed inside the well. AHPC-astrocyte non-contact co-cultures were terminated after 6 days and fixed in 4% paraformaldehyde for further analysis.
To obtain astrocyte-conditioned CCM, purified astrocytes were cultured in T-25 flasks in MMEM including 10% FBS at similar seeding densities to those plated on the insert membrane. After 2 days, the MMEM was replaced by CCM after rinses with EBSS. After 2 days of further growth, the CCM conditioned by astrocytes was collected, centrifuged to remove any debris and then used to feed AHPC alone cultures without astrocytes. The cultures were fed every 24 hours.
Immunocytochemistry and Antibodies
AHPCs cultured on PS-LAM substrates were processed for immunocytochemistry as described previously 25. Briefly, fixed cells were incubated in blocking solution containing 5% normal donkey serum, 0.4% bovine serum albumin (BSA; Sigma), and 0.2% Triton X-100 (Fisher Scientific), followed by incubation with primary antibodies overnight at 4°C. After rinsing in phosphate-buffered saline (PBS; 137 mM NaCl, 2.68 mM KCl, 8.1 mM Na2HPO4, 1.47 mM KH2PO4, pH 7.4), cells were incubated in the appropriate biotinylated secondary antibodies, followed by incubation with streptavidin-conjugated Cy3 (Jackson ImmunoResearch, West Grove, PA). Cell nuclei were stained with 4’, 6-diamidino-2-phenylindole, dilactate (DAPI), diluted at 1:2000 in PBS. Preparations were then mounted onto microscope slides using an antifade mounting medium (GelMount; Biomeda Corp., Foster City, CA).
To identify differentiated neurons, antibodies against class III β-tubulin (TUJ1, mouse monoclonal IgG; R&D systems, Inc.) or against microtubule-associated protein 2ab (MAP2ab, mouse monoclonal IgG; Sigma) were used at a dilution of 1:750. To identify differentiated glial cells, anti-receptor interacting protein (RIP, mouse monoclonal IgG; Developmental Studies Hybridoma Bank) diluted at 1:1,600 for oligodendrocytes 17,27 and anti-glial fibrillary acidic protein (GFAP, mouse monoclonal IgG; ICN, Costa Mesa, CA) diluted at 1:500 for astrocytes were used. Biotinylated donkey anti-mouse secondary antibody (Jackson ImmunoResearch) and Cy3-conjugated streptavidin were diluted at 1:500 and 1:15,000 in PBS, respectively. Negative controls were performed in parallel by omission of the primary and/or secondary antibodies. No antibody labeling was observed in these controls.
Quantitative analysis of immunocytochemistry
Following immunocytochemical procedures, the preparations were imaged using a Nikon Eclipse (Nikon Corp., Melville, NY) inverted microscope equipped with standard epifluorescence illumination and digital camera controlled by MetaMorph software (Universal Imaging Corporation, West Chester, PA). A total of 12 microscopic fields (0.24 mm2/field) from each micropatterned/non-patterned PS-LAM substrate, 6 fields from micropatterned half and 6 fields from non-patterned half, were randomly taken. To calculate the percentage of AHPCs immunoreactive (IR) for anti-TUJ1, MAP2ab, RIP or GFAP, the number of GFP-expressing and phenotypic marker-IR AHPCs were divided by the total number of cells (DAPI-stained nuclei).
Neurite outgrowth assay
The images (400 X magnification) of AHPCs co-cultured and immunolabeled against TUJ1 were analyzed. From the images, 21 to 25 TUJ1-IR AHPCs per condition were analyzed using MetaMorph software. The length of major neurites emanating from each cell body was measured. To calculate the average neurite length per cell, total length of the major neurites of each cell, measuring from the edge of the cell body to the tip of the growth cone, was divided by the number of primary neurites. Statistical analysis was performed using GraphPad PRISM (ver. 3.0). All tests were two-tailed tests and p-values less than an alpha of 0.05 were considered significantly different.
Statistical analyses
Statistical analyses were performed on the percentage of the AHPCs IR for each antibody of interest. Since each field was a sub-sample of that half of the substrate, the means of the 6 fields were calculated for use in the analysis so that each field would receive equal weight. The experiment followed a split plot design since the treatment (co-culture or control) was applied to the entire substrate and both micropatterned and non-patterned halves were within one substrate. Due to this design, there were two random effects in the model, (1) for the whole plot effect, consisting of the error term for the treatment and (2) for the split plot effect including the pattern effect and the interaction between the treatment and pattern. The whole plot experimental unit was the entire PS-LAM substrate (approximately 1 cm2 in area) while the split plot experimental unit was the half of the substrate that was either patterned or non-patterned. For each primary antibody (anti-TUJ1, MAP2ab and RIP), N=12 for the whole plot analysis and N=24 for the split plot analysis. Mixed model analysis was performed on the means using the PROC MIXED procedure in SAS. All tests performed were two-sided tests and p-values less than an alpha value of 0.05 were considered significant. Analysis of the residuals was performed and there was no evidence of assumption violations.
Results
To delineate between contact-mediated and soluble neuronal inducing activities associated with the astrocytes, the AHPCs were differentiated in parallel under four different culture conditions: (1) AHPCs cultured alone in CCM (AHPCs alone), (2) AHPCs co-cultured in physical contact with astrocytes (contact co-culture), (3) AHPCs cultured alone but in astrocyte-conditioned CCM (conditioned CCM), and (4) AHPCs co-cultured with astrocytes in non-contact co-culture conditions (non-contact co-culture).
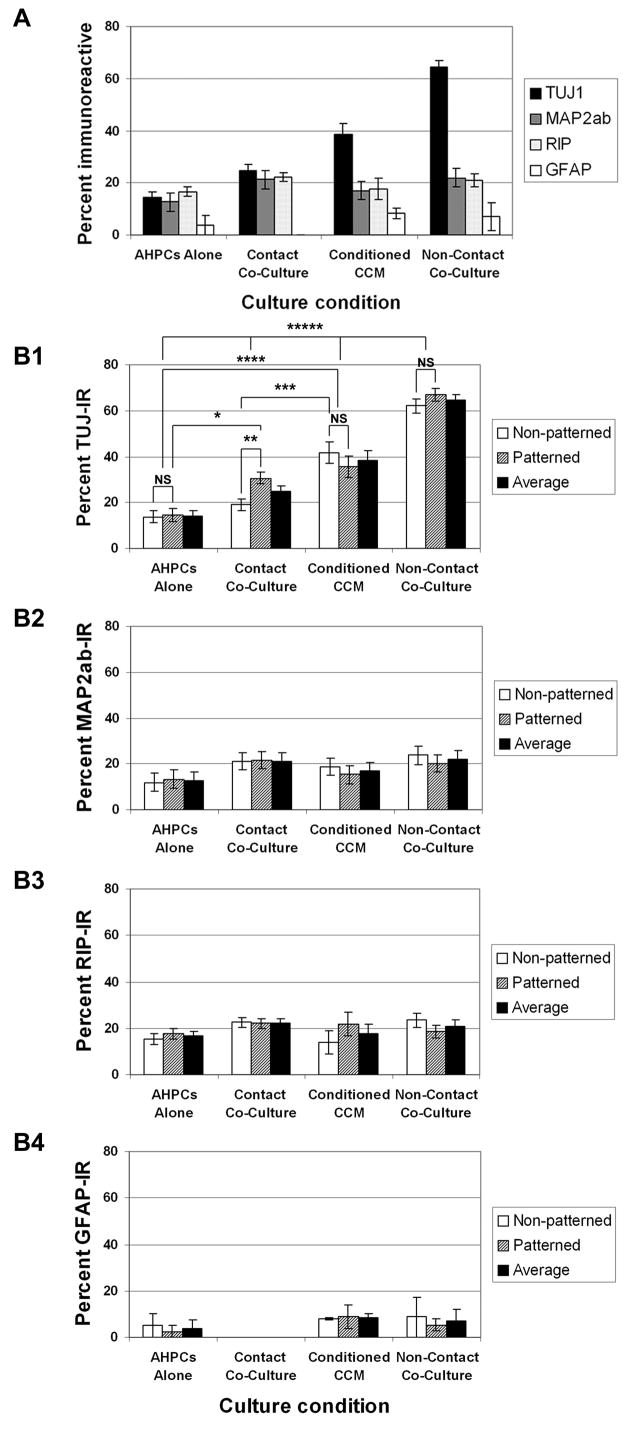
When AHPCs were cultured alone, approximately 14% of the AHPCs were TUJ1-IR, 13% MAP2ab-IR, 17% RIP-IR and 4% GFAP-IR averaging across the entire substrate (Fig. 1 A). In addition, comparison between patterned and non-patterned surfaces of PS-LAM substrates revealed no statistically significant differences in phenotypic differentiation of AHPCs cultured alone (Fig. 1 B1-B4). When co-cultured in physical contact with astrocytes, approximately 25% of the AHPCs were TUJ1-IR, 21% MAP2ab-IR and 22% RIP-IR (Fig. 1 A). Under these contact co-culture conditions, significantly more AHPCs were immunoreactive for the TUJ1 antibody compared to the AHPCs cultured alone (AHPC alone: 14% versus contact co-culture: 25%; Fig. 1 A). Furthermore, under the contact co-culture conditions, significantly more AHPCs were immunolabeled with the TUJ1 antibodies when co-cultured on the patterned side of the substrate compared to the non-patterned side (patterned surface: 31% versus non-patterned surface: 19%; α = 0.05; p < 0.0001) (Fig. 1 B1). However, no statistically significant differences in AHPC differentiation were observed between the patterned and non-patterned sides of the substrates when performing our analysis for the other phenotypic markers (MAP2ab, RIP and GFAP shown in Fig. 1 B2, B3 and B4, respectively). These results were consistent with our previous study 1.
Figure 1.
Differentiation of AHPCs under the four different culture conditions - AHPCs alone, contact co-culture, conditioned CCM and non-contact co-culture. (A) Average percentages ± SEM of phenotypic marker-IR AHPCs, TUJ1 and MAP2ab for neurons and RIP and GFAP for oligodendrocytes and astrocytes, respectively. All phenotypic marker- IR cells were averaged over both patterned and non-patterned surfaces. (B) Quantification of phenotypic marker-IR AHPCs on patterned versus non-patterned substrates. The percentages of TUJ1-IR (B1; asterisks, *, statistically significant difference when p ≤ 0.001; NS, no statistically significant differences), MAP2ab-IR (B2), RIP-IR (B3) and GFAP-IR (B4) AHPCs on patterned and non-patterned substrates in different conditions. N (number of parallel experiments) = 3 or 4. For the other phenotypic markers (MAP2ab and RIP), under all culture conditions, no statistically significant differences in AHPC differentiation were observed between the patterned and non-patterned sides of the substrates. A statistical analysis was not performed on GFAP-IR cells as the data were obtained from two independent experiments and little differences were observed in the average number of GFAP-IR cells for the various experimental conditions.
When AHPCs were cultured alone, but in astrocyte-conditioned CCM, we observed a significant increase in the percentage of AHPCs immunolabeled with the TUJ1 antibody (39% TUJ1-IR) in comparison to AHPCs alone and AHPCs in contact co-culture on the non-patterned surfaces (α = 0.05; p ≤ 0.0006) (Fig. 1 B1). These results suggest that the astrocytes secrete soluble factors that induce neuronal differentiation of the AHPCs. Moreover, this activity appeared to be neurogenic since there was no significant change in the percentage of RIP-IR in the AHPCs when compared to the contact co-cultured AHPCs (Fig. 1 B3). To further explore this neurogenic activity, AHPCs were co-cultured with astrocytes under non-contact co-culture conditions. Under these conditions, we observed a dramatic increase in the percentage of AHPCs immunolabeled with the TUJ1 antibody (64%), illustrating an even greater neuronal induction activity for the soluble astrocyte-derived factor (Fig. 1 A). This level of TUJ1 differentiation was significantly greater compared to TUJ1 expression in the other three parallel culture conditions (α = 0.05; p ≤ 0.001). However, no statistically significant differences in AHPC differentiation were observed between the patterned and non-patterned sides of the substrate in astrocyte-conditioned CCM or non-contact co-cultures for any of the phenotypic markers (TUJ1, MAP2ab, RIP or GFAP; Fig. 1 B1-B4). These results provide strong evidence that astrocytes secrete soluble factors that facilitate the differentiation of the AHPCs towards a neuronal fate. This differentiating activity of the soluble factor(s) secreted from the astrocytes appears to be specifically neurogenic, since no significant effect was observed on the differentiation of RIP- or GFAP-IR cells. In addition, the soluble factor(s) might be short-lived, since the proportion of TUJ1-IR cells cultured in the astrocyte-conditioned CCM (39%) was lower than the proportion of TUJ1-IR cells cultured under non-contact co-culture conditions (64%).
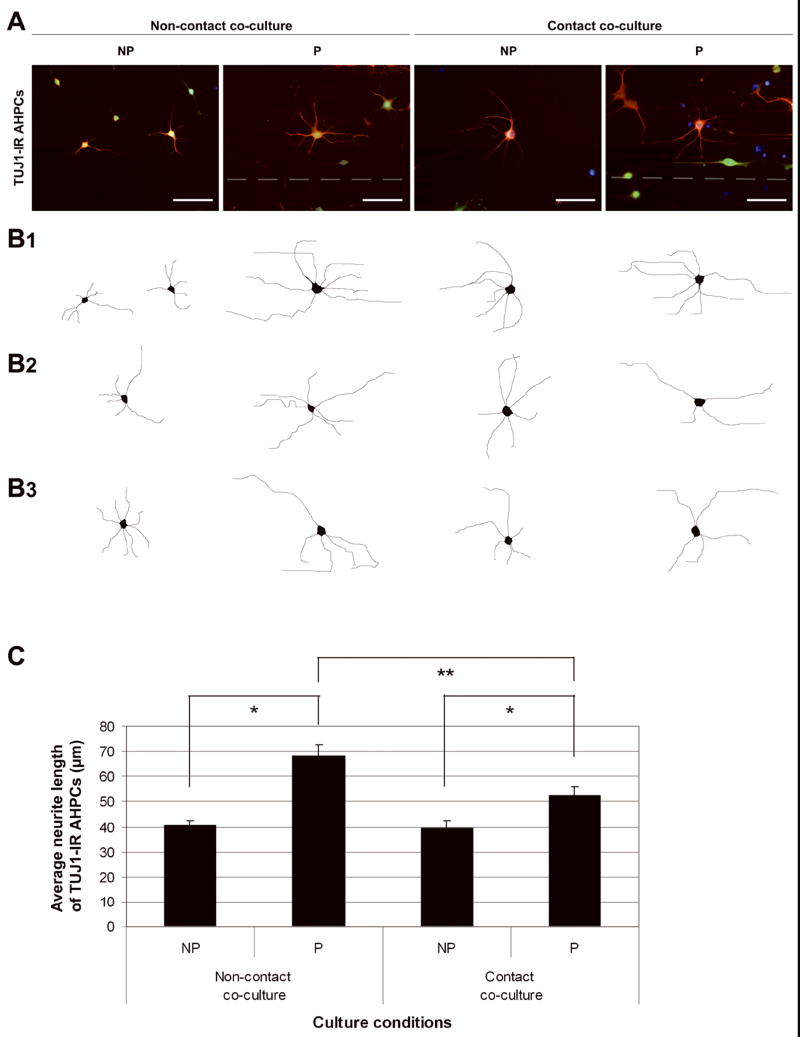
Morphologically, TUJ1-IR AHPCs were observed to possess longer processes under non-contact co-culture condition compared to those in the contact co-cultures. To investigate this possible morphological difference, we performed a quantitative analysis measuring the length of primary major neurites emanating from the cell bodies of TUJ1-IR AHPCs. Under both co-culture conditions, the average length of primary processes oriented in the direction of the grooves were significantly longer on the micropatterned side compared to that on the non-patterned side [Fig. 2; 68.25 μm on patterned surface vs. 40.73 μm on non-patterned surface under non-contact co-culture (α = 0.05; p < 0.0001); 52.60 μm on patterned surface vs. 39.53 μm on non-patterned surface under contact co-culture (α = 0.05; p = 0.0043)]. When comparing the morphology of the AHPCs cultured on the patterned sides under both co-culture conditions, the average length of neurites of the AHPCs in non-contact co-cultures was significantly longer than that in contact co-cultures [Fig. 2; non-contact co-culture vs. contact co-culture (α = 0.05; p = 0.006)] or in the AHPCs alone cultures (data not shown).
Figure 2.
Morphological differences between TUJ1-IR AHPCs cultured with astrocytes with physical contact or without contact, on non-patterned (NP) or patterned (P) PS-LAM substrates. (A) Representative fluorescence images of TUJ1-IR AHPCs cultured in non-contact co-culture conditions or contact co-culture conditions on the NP or P side of the PS-LAM substrates. These are merged images created by the superimposition of TUJ1-IR (red), GFP-expression (green) and DAPI nuclear counterstain (blue) fluorescent images. Dotted white lines indicate the location of a groove on the micropatterned substrate. Scale bar = 50 μm. (B) Line drawing reconstructions of individual TUJ1-IR AHPCs under the different culture conditions illustrating the major primary neurites emanating from the cell body. (B1) Reconstructions of TUJ1-IR AHPCs in (A) for each condition. (B2, B3) Two additional examples of line drawing reconstructions of TUJ1-IR cells. (C) Average primary neurite lengths for TUJ1-IR AHPCs growing under the respective culture conditions. Values are the averaged neurite lengths of TUJ1-IR GFP-expressing AHPCs, mean ± SEM. Asterisks indicate statistical difference (p < 0.05, t test).
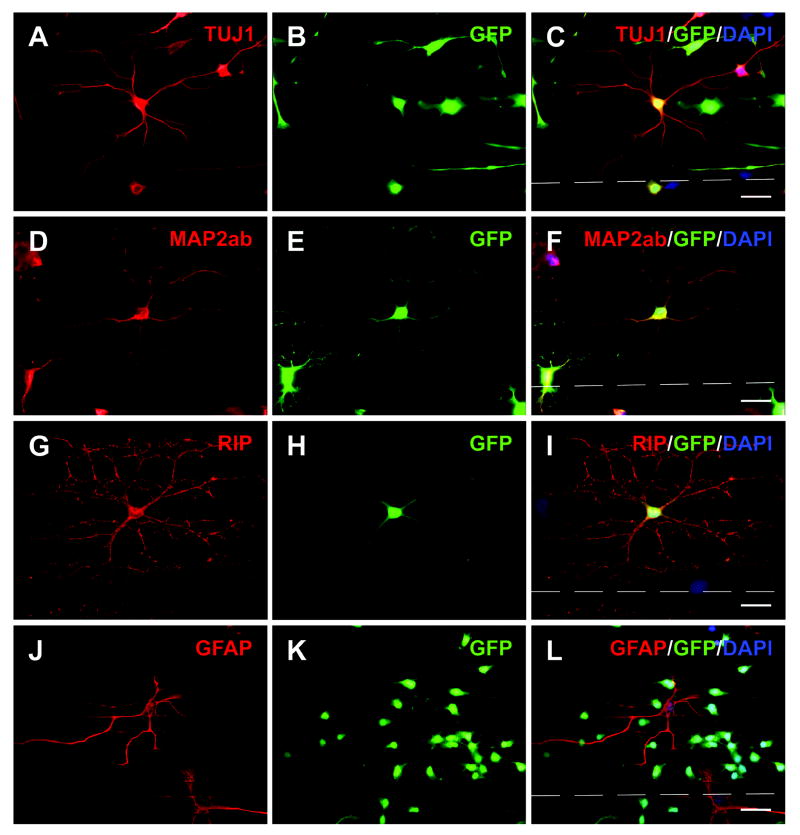
On the patterned substrates, RIP-IR cells elaborated extensive processes weaving intricately inside the grooves and along the mesas of the substrate (Fig. 3 G-I). On the non-patterned substrates, extensive radial outgrowth from RIP-IR cells was consistently observed (Fig. 4 G-I). GFAP-IR cells differentiating on the patterned side of the PS-LAM substrate often elaborated filamentous processes oriented in the direction of the pattern (Fig. 3 J-L). GFAP-IR cells differentiating on the non-patterned side of the substrate usually displayed flattened morphologies with large nuclei (Fig. 4 J-L).
Figure 3.
Differentiation of AHPCs co-cultured with astrocytes on patterned substrates in the non-contact condition. Fluorescence images of TUJ1-IR (A), MAP2ab-IR (D) RIP-IR (G) and GFAP-IR (J) GFP-expressing AHPCs (B, E, H and K, respectively) were merged with DAPI nuclei counterstaining (C, F, I and L, respectively). In non-contact co-cultures, on the patterned surfaces, directed neuritic extension from the neuronal cell body was observed with prominently longer and elaborated processes in the direction of the grooves than on the non-patterned surfaces (TUJ1-IR, A-C and MAP2ab, D-F). RIP-IR cells elaborated extensive processes weaving intricately inside the grooves and along the mesas of the substrate (G-I). GFAP-IR cells displayed flattened morphologies with large nuclei and in many cases, processes strongly immunoreactive for GFAP (J-L). Dotted white lines indicate the location of a groove on the micropatterned substrate. Scale bar = 20 μm.
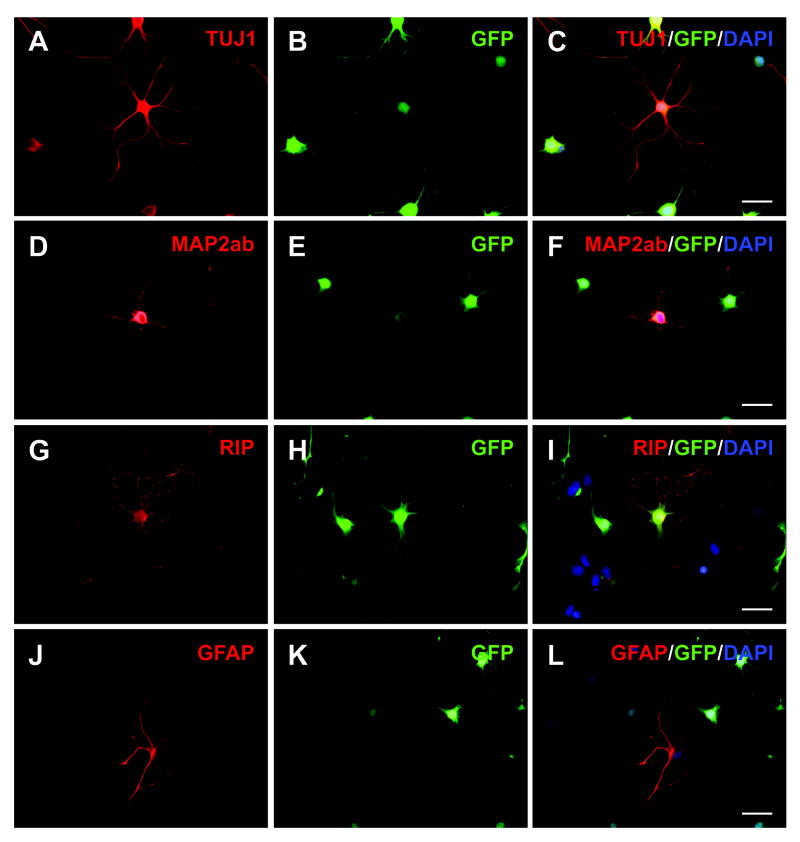
Figure 4.
Differentiation of AHPCs co-cultured with astrocytes on non-patterned, planar substrates in the non-contact condition. Fluorescence images of TUJ1-IR (A, B), MAP2ab-IR (D, E), RIP-IR (G, H) and GFAP-IR (J, K) GFP-expressing AHPCs were merged with DAPI counterstaining (C, F, I, and L, respectively). In general, immunoreactive AHPCs displayed radially directed processes when cultured on non-patterned, planar substrates. Scale bar = 20 μm.
Discussion
There are multiple factors in the microenvironment directly surrounding cells that can induce and maintain their functional stability. Elucidating which factors are involved and how they interact with cells is extremely helpful in understanding the intra- or inter-cellular mechanisms occurring with the in vivo microenvironment. Micropatterned 3-D constructs have been used to study the effects of the microenvironment on cell growth and differentiation 28,29. Microfabrication technology has been applied to the design of substrates having specific architectures for such purposes 30. Culture environments incorporating these substrates are designed to encourage isolated cells to function as they would in their in vivo microenvironments or niches. Our previous study demonstrated an enhancement of selective neuronal differentiation of AHPCs co-cultured with astrocytes on PS-LAM substrates 1. These results provided evidence that physical, chemical and biological cues supplied by the surrounding microenvironment could influence the differentiation of the AHPCs by providing spatial and temporal control through soluble factors and/or contact-mediated cellular mechanisms which can influence CNS development and function 12,22. In this study, we investigated the factors which play an important role in selective neuronal differentiation and the directed outgrowth of AHPCs using a non-contact co-culture system on micropatterned PS-LAM substrates.
Effect of guidance cues on AHPC differentiation and outgrowth
In contact co-cultures, we observed a significantly greater percentage of TUJ1-IR cells on the patterned side of the substrate compared to the non-patterned side, supporting our previous findings that 3-D micropatterned PS substrates can enhance neuronal differentiation of AHPCs 1-3. In addition, on the patterned side of the substrate, the neurites of TUJ1-IR AHPCs were oriented along the direction of the pattern provided by the 3-D environment and they were significantly longer than neurites on the non-patterned side. Under the non-contact co-culture condition, elongation of the neurites oriented in the direction of the grooves were also enhanced on the patterned surfaces, although there was no significant difference in TUJ1 immunoreactivity when cultured on the patterned compared to the non-patterned surfaces. These findings demonstrate that the astrocyte-derived factors in combination with 3-D micropatterned construct coated with purified ECM (laminin) play a role in promoting elongation of the neurites emanating from the differentiated AHPCs. Interestingly, between the two co-culture systems, in the non-contact co-culture, about 2.5 times greater proportion of the AHPCs were TUJ1-IR compared to that in contact co-cultures while there were no significant differences in the percentage of RIP- or GFAP-IR cells. This result provides strong evidence in support of soluble factor(s) being released from astrocytes co-cultured with AHPCs and that the factor(s) specifically promotes neuronal differentiation. In addition, via a contact-mediated mechanism, astrocyte-associated factors likely provide an activity that suppresses neuronal differentiation of AHPCs 22,32-34. To confirm that astrocyte-derived soluble factors enhance neuronal differentiation of AHPCs, we cultured the AHPCs alone (in the absence of astrocytes) but in astrocyte-conditioned CCM which presumably includes the factors released from astrocytes. When the AHPCs were cultured in the astrocyte-conditioned CCM, TUJ1 immunoreactivity was increased approximately 3-fold compared to when cultured in normal CCM. Furthermore, there were significantly more TUJ1-IR AHPCs, on the non-patterned side of the substrate, in astrocyte-conditioned CCM compared to the contact co-culture condition. These results strongly support our hypothesis that astrocyte-derived soluble factors selectively stimulate neuronal differentiation of AHPCs which can be modulated by a contact-mediated suppressive mechanism.
During in vitro differentiation, no significant differences in the percentage of MAP2ab-IR AHPCs were observed among the different culture conditions. It is possible that astrocyte-derived factors selectively affect axonogenesis and outgrowth of AHPCs, rather than dendritic development, since the TUJ1 (class III β-tubulin) antibody is commonly used as an axonal marker in young neurons 35-37 and the MAP2ab (microtubule-associated protein) antibody as a dendritic marker in more mature neurons 38,39. During neuronal development, multiple environmental cues can influence the formation and arborization of neuronal processes 40-42. Although we observed no significant differences in MAP2ab-IR across the different conditions, our results revealed a dramatic increase in TUJ1-IR under co-culture conditions and in astrocyte-conditioned CCM in comparison to the AHPC alone cultures. These results suggest that the astrocyte-derived soluble factors may play a critical role in neurite extension and axonal development during early neuronal differentiation.
Microenvironmental regulation of AHPC outgrowth and differentiation
Our results have demonstrated that the 3-D microenvironment can provide physical and/or molecular cues to the micropatterned PS-LAM substrate in the co-culture conditions and, thus, influence neuronal differentiation of AHPCs. However, in the non-contact co-culture condition, as well as in the astrocyte-conditioned CCM, no significant difference of TUJ1 immunoreactivity was observed between the patterned and non-patterned surfaces. It is possible that the AHPCs are in competition with the astrocytes for the released soluble factors. These factors, secreted from astrocytes, in contact co-culture condition, may concentrate within the grooves where astrocytes are aligned, but they may presumably be taken up by the AHPCs that are proximal to the astrocytes or could influence the astrocytes themselves through an autocrine signaling system. Since many of the factors are short-lived 43, it may be unlikely that the factors would have time to accumulate or diffuse across the substrate. However, in the non-contact co-culture, there may be no direct competition among AHPCs and astrocytes for the released factors. Thus it is possible that the AHPCs are directly and immediately affected by the soluble cues originating from the astrocytes. In contact co-culture, on the patterned surface, simple diffusion of the soluble factors may be less obstructed by the aligned astrocytes than on the non-patterned surface. Thus, a greater proportion of the AHPCs on the patterned surface might be able to differentiate into neuronal cells. However, in the non-contact co-culture condition, diffusion may not be constrained by co-cultured astrocytes because the factors secreted from astrocytes would be released directly into the media and be presented to the AHPCs on both patterned and non-patterned surfaces immediately upon release. The astrocyte-derived factors, thus, are uniformly presented to all AHPCs on the substrates, whether or not the surface is micropatterned. These factors appear to be critical cues for maximum effect on neuronal differentiation and outgrowth of AHPCs.
Extracellular transport processes play critical roles in cellular morphogenesis. During development, diffusion of morphogens or substances that assign different cell fates or their localization at different concentrations specifies many patterns of cell and tissue organization 44. Morphogen transport from a localized site forms microgradients through unknown mechanisms which might be simple diffusion or more elaborate mechanisms 45. It is not yet known how diffusion may be controlling outgrowth and differentiation in our co-culture systems and which factors are released from astrocytes to modulate the neuronal differentiation of the neural progenitor cells. Further studies need to be undertaken to distinctly characterize the mechanism(s) behind this enhanced effect and to elucidate the specific soluble factor(s) involved. The controlled in vitro biological model introduced in this study can potentially provide new insights into diffusion mechanisms that govern cell fates in vivo during development.
Conclusion
The results of this study have demonstrated that astrocyte-derived soluble factors specifically promote neuronal differentiation of adult hippocampal neural progenitor cells (AHPCs). A non-contact co-culture system using semi-porous polyester membrane inserts was established to study a potential role of soluble factors derived from neonatal cortical astrocytes in selective neuronal differentiation of AHPCs. In comparison to control cultures (AHPCs cultured alone and AHPCs co-cultured in physical contact with astrocytes), neuronal differentiation was dramatically increased when AHPCs were co-cultured with astrocytes under non-contact co-culture conditions. These results suggest that astrocyte-derived soluble factors provide cues for enhancing neuronal differentiation of AHPCs cultured on PS-LAM substrates. Furthermore, under non-contact co-culture conditions, neurite length was significantly greater when compared with contact co-culture conditions. In addition, neurite outgrowth on the patterned side of the substrates was considerably greater compared to the non-patterned, planar surface of the PS-LAM substrates. Our results demonstrate that astrocyte-derived soluble factors facilitate neuronal differentiation of the AHPCs, in combination with multiple microenvironmental cues, such as 3-D micropatterned PS substrate and purified ECM molecules (laminin). These results have important implications for developing strategies to promote neuronal differentiation from NPCs and for stem cell-mediated repair of the CNS.
Acknowledgments
Financial support provided by The Glaucoma Foundation, New York to DSS and from the National Institutes of Health to SKM and DSS are gratefully acknowledged (NIGMS 1 RO1 GM072005). The authors would like to thank Dr. Fred H. Gage at the Salk Institute for the gift of the AHPCs. The authors are also grateful to Dr. Robert T. Doyle at the Roy J. Carver Laboratory for Ultrahigh Resolution Biological Microscopy, Department of Genetics, Development & Cell Biology at Iowa State University (ISU) for his helpful advice and suggestions. The authors would also like to thank Christopher C. Blong for his help with substrate preparation and Drs. H. Levine, M. Nilsen-Hamilton and M. Smiley for helpful discussions.
Financial support provided by The National Institutes of Health to SKM and DSS is acknowledged (NIGMS 1 RO1 GM072005).
References
- 1.Recknor JB, Sakaguchi DS, Mallapragada SK. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials. 2006;27(22):4098–108. doi: 10.1016/j.biomaterials.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Bokel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 2002;3(3):311–21. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson E, Fassler R. Insights into extracellular matrix functions from mutant mouse models. Exp Cell Res. 2000;261(1):52–68. doi: 10.1006/excr.2000.5042. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. Cell adhesion: old and new questions. Trends Cell Biol. 1999;9(12):M33–7. [PubMed] [Google Scholar]
- 5.Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14(5):526–32. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Conover JC, Notti RQ. The neural stem cell niche. Cell Tissue Res. 2008;331(1):211–24. doi: 10.1007/s00441-007-0503-6. [DOI] [PubMed] [Google Scholar]
- 7.Garcion E, Halilagic A, Faissner A, ffrench-Constant C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131(14):3423–32. doi: 10.1242/dev.01202. [DOI] [PubMed] [Google Scholar]
- 8.Soen Y, Mori A, Palmer TD, Brown PO. Exploring the regulation of human neural precursor cell differentiation using arrays of signaling microenvironments. Mol Syst Biol. 2006;2:37. doi: 10.1038/msb4100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287(5457):1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 10.Wurmser AE, Palmer TD, Gage FH. Neuroscience. Cellular interactions in the stem cell niche. Science. 2004;304(5675):1253–5. doi: 10.1126/science.1099344. [DOI] [PubMed] [Google Scholar]
- 11.Campos LS. Neurospheres: insights into neural stem cell biology. J Neurosci Res. 2004;78(6):761–9. doi: 10.1002/jnr.20333. [DOI] [PubMed] [Google Scholar]
- 12.Czyz J, Wobus A. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68(4-5):167–74. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- 13.Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res. 2006;83(5):845–56. doi: 10.1002/jnr.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leone DP, Relvas JB, Campos LS, Hemmi S, Brakebusch C, Fassler R, Ffrench-Constant C, Suter U. Regulation of neural progenitor proliferation and survival by beta1 integrins. J Cell Sci. 2005;118(Pt 12):2589–99. doi: 10.1242/jcs.02396. [DOI] [PubMed] [Google Scholar]
- 15.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19(10):971–4. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 16.Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96(13):7526–31. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 18.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–33. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47(3):209–16. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- 20.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291(5504):657–61. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 21.Neugebauer KM, Tomaselli KJ, Lilien J, Reichardt LF. N-cadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro. J Cell Biol. 1988;107(3):1177–87. doi: 10.1083/jcb.107.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell EM, Meiners S, DiProspero NA, Geller HM. Mechanisms of astrocyte-directed neurite guidance. Cell Tissue Res. 1997;290(2):385–93. doi: 10.1007/s004410050945. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi DS, Moeller JF, Coffman CR, Gallenson N, Harris WA. Growth cone interactions with a glial cell line from embryonic Xenopus retina. Dev Biol. 1989;134(1):158–74. doi: 10.1016/0012-1606(89)90086-9. [DOI] [PubMed] [Google Scholar]
- 24.Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988;1(1):33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- 25.Recknor JB, Recknor JC, Sakaguchi DS, Mallapragada SK. Oriented astroglial cell growth on micropatterned polystyrene substrates. Biomaterials. 2004;25(14):2753–67. doi: 10.1016/j.biomaterials.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 26.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8(6):389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 27.Friedman B, Hockfield S, Black JA, Woodruff KA, Waxman SG. In situ demonstration of mature oligodendrocytes and their processes: an immunocytochemical study with a new monoclonal antibody, rip. Glia. 1989;2(5):380–90. doi: 10.1002/glia.440020510. [DOI] [PubMed] [Google Scholar]
- 28.Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, Gerbasio D, Keene DR, Ambrosio L, Netti PA. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26(31):6194–207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Yim EK, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313(9):1820–9. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog. 1998;14(3):356–63. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- 31.Recknor JB, Sakaguchi DS, Mallapragada SK. Growth and differentiation of astrocytes and neural progenitor cells on micropatterned polymer films. Ann N Y Acad Sci. 2005;1049:24–7. doi: 10.1196/annals.1334.004. [DOI] [PubMed] [Google Scholar]
- 32.Liesi P, Silver J. Is astrocyte laminin involved in axon guidance in the mammalian CNS? Dev Biol. 1988;130(2):774–85. doi: 10.1016/0012-1606(88)90366-1. [DOI] [PubMed] [Google Scholar]
- 33.Meiners S, Powell EM, Geller HM. A distinct subset of tenascin/CS-6-PG-rich astrocytes restricts neuronal growth in vitro. J Neurosci. 1995;15(12):8096–108. doi: 10.1523/JNEUROSCI.15-12-08096.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell EM, Geller HM. Dissection of astrocyte-mediated cues in neuronal guidance and process extension. Glia. 1999;26(1):73–83. doi: 10.1002/(sici)1098-1136(199903)26:1<73::aid-glia8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 35.Joshi HC, Cleveland DW. Differential utilization of beta-tubulin isotypes in differentiating neurites. J Cell Biol. 1989;109(2):663–73. doi: 10.1083/jcb.109.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee MK, Rebhun LI, Frankfurter A. Posttranslational modification of class III beta-tubulin. Proc Natl Acad Sci U S A. 1990;87(18):7195–9. doi: 10.1073/pnas.87.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17(2):118–32. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee A, Roach MC, Trcka P, Luduena RF. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of beta-tubulin. J Biol Chem. 1990;265(3):1794–9. [PubMed] [Google Scholar]
- 39.Huber G, Matus A. Differences in the cellular distributions of two microtubule-associated proteins, MAP1 and MAP2, in rat brain. J Neurosci. 1984;4(1):151–60. doi: 10.1523/JNEUROSCI.04-01-00151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watabe-Uchida M, Govek EE, Van Aelst L. Regulators of Rho GTPases in neuronal development. J Neurosci. 2006;26(42):10633–5. doi: 10.1523/JNEUROSCI.4084-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller FD, Kaplan DR. Signaling mechanisms underlying dendrite formation. Curr Opin Neurobiol. 2003;13(3):391–8. doi: 10.1016/s0959-4388(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 42.Stirling RV, Dunlop SA. The dance of the growth cones--where to next? Trends Neurosci. 1995;18(2):111–5. [PubMed] [Google Scholar]
- 43.Muller HW, Junghans U, Kappler J. Astroglial neurotrophic and neurite-promoting factors. Pharmacol Ther. 1995;65(1):1–18. doi: 10.1016/0163-7258(94)00047-7. [DOI] [PubMed] [Google Scholar]
- 44.Swartz MA. Signaling in morphogenesis: transport cues in morphogenesis. Curr Opin Biotechnol. 2003;14(5):547–50. doi: 10.1016/j.copbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Lander AD, Nie Q, Wan FY. Do morphogen gradients arise by diffusion? Dev Cell. 2002;2(6):785–96. doi: 10.1016/s1534-5807(02)00179-x. [DOI] [PubMed] [Google Scholar]