Abstract
Background
We implemented a controlled randomized trial that compared two interventions -provision of written resource navigation information (enhanced usual care (EUC)) versus written information plus patient navigation (TPN) aimed at improving adjuvant treatment adherence and follow-up among 487 low-income, predominantly Latinas with breast or gynecologic cancer.
Methods
Women are randomized to either TPN or EUC. We assess chemotherapy, radiation and hormone therapy and follow-up over 12 months. Breast and gynecologic patients are analyzed separately.
Results
Overall adherence rates were (87-94%) and there were no significant differences between TPN and EUC groups. Among women with breast cancer, 90% of EUC and 88% of TPN patients completed chemotherapy (14% EUC-26% TPN had delayed completion), 2% EUC-4% TPN failed to complete, and 8% EUC-7% TPN refused, chemotherapy. Radiation treatment adherence was similar between groups –90% completed (40% EUC-42% TPN delayed completion); in both groups, 2% failed completion and 8% refused radiation. Among gynecologic patients, 87% EUC-94% TPN completed chemotherapy (41% EUC-31% TPN with delays), 7% EUC-6% TPN failed completion and 6% EUC refused chemotherapy; 87% EUC-84% TPN completed radiation (51% EUC-42% TPN with delays), 5% EUC-8% TPN failed completion and 8% EUC-5% TPN refused radiation.
Conclusions
Treatment adherence across randomized groups was notably higher than reported in previous studies, suggesting that active telephone patient navigation or written resource informational materials may facilitate adherence among low-income, predominantly Hispanic women. Adherence may have also been facilitated by federal-state Breast and Cervical Cancer treatment funding.
Keywords: Adjuvant Cancer Treatment Adherence, Patient Navigation, Low-Income Hispanics
Despite declines in overall cancer-related mortality,1 disparity in cancer survival among racial/ethnic minorities remains significant.2-6 It is, in part, attributable to structural constraints and contextual factors that restrict access to health care.2,7 Low-income and minority women are less likely to receive adjuvant treatment for breast and gynecologic cancer,8-14 and more likely to terminate their treatment prematurely and to have higher mortality rates.15-18 However, in contrast to research on interventions to improve screening and abnormal follow-up,19-24 few studies have tested interventions to improve adjuvant treatment adherence among lower socio-economic populations.25-29
Previous studies have found low-income minorities experienced poorer radiation treatment adherence for cervical cancer when compared with national rates (16% vs. 63%), a high rate of treatment interruptions (64%) and discontinuation of treatment for non-medical reasons (20%);30 noncompliance with hematologic oral self-administered medication more than 70% of the time (measured by drug serum levels); and missed appointments more than 30% of the time.31,32 An intervention trial on low-income patients with hematologic malignancies found that control patients were noncompliant 73.1% and fully compliant 21% of the time. After controlling for all other variables, disease severity, compliance with allopurinol, and an educational intervention were associated with significantly better adherence and survival rates.33 Predominantly indigent, minority patients with early breast cancer were less compliant with a standard breast-conservation and radiation therapy program than that reported in clinical trials.34 Suggesting a positive effect on adherence from access to treatment insurance, a SEER-Medicare database of 24,510 patients documented high rates of completion (87%) of 25 or more radiation therapy sessions (although with greater non-completion rates among Black women with mastectomy),35 while analysis of 3,193 Medicare patients with colon cancer found that 78.2% completed the prescribed course of chemotherapy.36
Our controlled randomized trial compared patient adherence to adjuvant cancer treatment and post-treatment follow-up among 487 low-income predominantly Latino women with breast or gynecologic cancer, randomized to a structured patient navigation (TPN) or to modestly enhanced usual care (EUC). To better understand the hypothesized role of socio-economic and culturally mediated attitudes and socio-emotional support in adherence, we conducted semi-structured qualitative interviews with 29 women from both study groups.
METHODS
The IMPAACT (Improving Patient Access and Adherence to Cancer Treatment) trial and the qualitative study were approved by USC Health Sciences IRB. We recruited women with breast or gynecologic cancer to test a culturally tailored patient navigation model that was previously found to be effective in improving abnormal screen follow-up.24 The TPN model combined interactive health education (decisional support), counseling (emotional support), written care site and community service navigation information, and navigator active assistance to facilitate access and adherence to adjuvant treatment. Patients age ≥18 were recruited in oncology clinics at an urban public safety net medical center if they had a primary diagnosis of breast (Stage 0-III) or gynecologic FIGO 0-4B cancer. We excluded palliative care patients.
Study Participants
Of 596 eligible patients, we enrolled 487 (82%) from June 2002 to July 2004 with no statistically significant differences between enrolled and non-enrolled by age, ethnicity, or cancer stage. We randomly assigned patients to IMPAACT TPNervention (n=248) or to EUC (n=239).
Study Design
IMPAACT is integrated with oncology care provided under “real world” service delivery conditions and is designed to enhance standard care through quality improvement enhancements provided by a bilingual, bicultural Patient Navigator (PN) and MSW team or distribution of written materials. There was no “only usual care” group based on previous trials of showing patient navigation to improve screening and abnormal screen adherence.19-24
EUC patients received site standard oncology care, financial department facilitation of receipt of Medi-Cal Treatment Funding and supportive services available to all cancer patients plus an English or Spanish listing of community resources (e.g., mental health services, cancer support groups), and medical center social work, financial, and transportation and childcare resources services and a patient and family member educational pamphlet on depression and cancer at baseline. These written materials were not routinely provided as part of usual care in this public sector safety net care system.
TPN is tailored to improve treatment and follow-up access and adherence by influencing predisposing (knowledge/attitudes), reinforcing (social support/cues to action), and enabling (barrier reduction skill) consistent with the Health Belief Model37 and Socio-cultural Explanatory Theory.38,39 TPN provides: an initial structured telephone interview assessing adherence barriers; health education, problem-solving, and self-management support; and applies a structured adherence risk algorithm to assign service intensity: Level 1 service (6 and 12 month follow-up call); Level 2 (telephone or in-person navigation services); Level 3 (MSW brief depression or anxiety counseling and/or referral).
Measures
Demographic and Clinical Characteristics
Patient demographics, cancer site, stage, and treatment phase at study enrollment came from medical records or baseline interviews. Among women reporting pain, further assessment was made using the Brief Pain Inventory (BPI). 40-41 Patients self-reported functional status using the Karnofsky Performance Status Scale (KPSS), an 11-point rating scale which ranges from normal functioning (10) to death (0).42 The Functional Assessment of Cancer Therapy Scale – General (FACT-G), 43-44 a valid and reliable 27-item Health Related Quality of Life (HRQL) assessed physical, functional, social/family, and emotional well-being. Co-morbid health conditions came from patient self-report. PHQ-9 assessed major depressive disorder and45-46 the Brief Symptom Inventory to assess anxiety. 47 Experienced abstractors collected adherence data from all available charts (n=444, 91.2% of study enrollees). Adherence to external beam radiation or IV-chemotherapy is defined as completed as scheduled (CAsSched), completed but delayed (C-Delay) due to missed treatment appointments, did not complete (DNComplete) or declined; unless the interruption was physician prescribed or resulted from machine breakdown. Rreceipt of hormone and anti-depressant medication was obtained from site pharmacy records. We randomly recruited qualitative study patients from 164 women following completion of the 6-month interview; 29 provided written consent (15 TPN and 14 EUC, 24 Hispanic). We tape-recorded individual in-person or telephone guided interviews (11 in English, 18 in Spanish).
Statistical Analysis
Mean, standard error and percentage were used to describe the general characteristics and distributions of predicting and outcome variables. Logistic Regression and Polytomous Logistic Regression models test intervention effects on treatment adherence. We assess breast and gynecologic patients separately using SAS, version 9.1 (SAS Institute, Cary, North Carolina). Qualitative Transcript Data were coded and analyzed using standard methodology of “Coding Consensus, Co-occurrence, and Comparison”48-51
RESULTS
Study Enrollment and Attrition
Consort Figure 1 details enrollment and attrition over 12 months. Attrition rates do not vary significantly between groups and reasons for attrition are similar between groups. Baseline characteristics were compared between patients who had completed outcome measures at two follow-up waves (61%) and non-completers at any follow-up wave (39%). Patients lost to follow-up are more likely to be 8.9% US born vs 63% foreign born, p=0.019), less than 10 years in US (68.7% vs. 80.6%, p=0.003), and to have gynecologic cancer (65.6% vs 42%, p<0.001).
Figure 1.
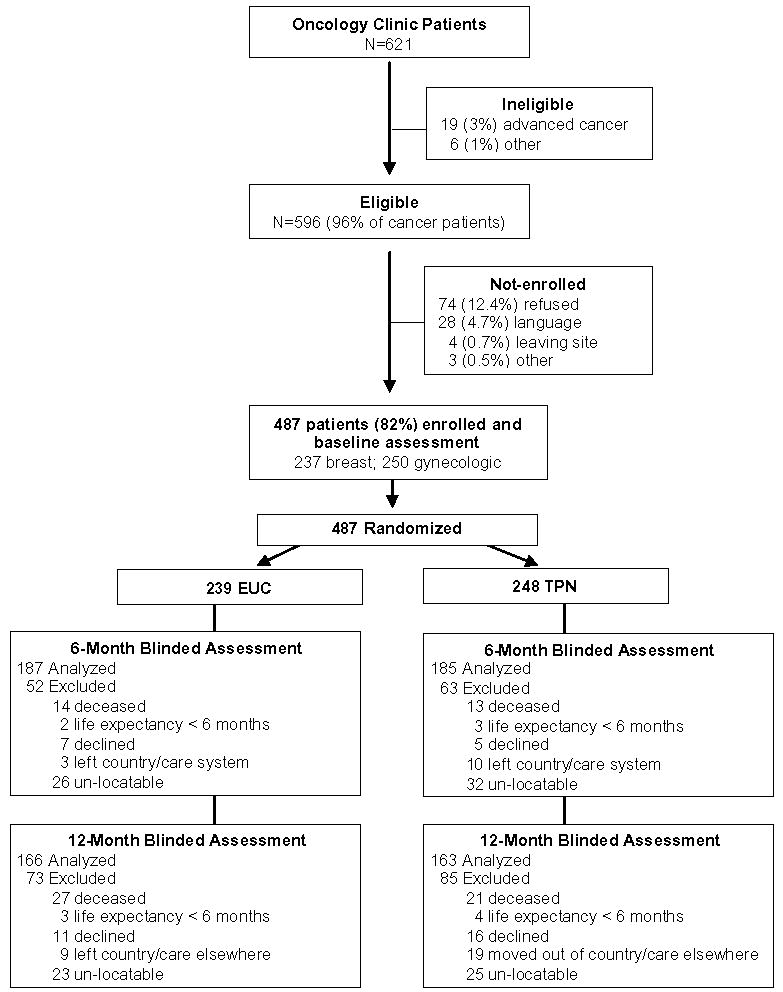
IMPAACT Consort Flowchart
Sample Characteristics
Patients are predominantly Latina, Spanish-speaking, foreign born, unemployed and with Medi-Cal Cancer Treatment Funding or local government medical insurance at enrollment (Table 1). There were no significant differences between study groups at baseline on key characteristics including cancer treatment phase (prior to treatment, active cancer treatment, or follow-up care), age, ethnicity, cancer stage, self-reported KPSS, major depression, or severe pain between study groups.
Table 1.
Patient Demographic and Clinical Characteristics at Baseline
| Breast Cancer | Gynecologic Cancer | |||||
|---|---|---|---|---|---|---|
| EUC N=114 | TPN N=123 | EUC N=125 | TPN N=125 | |||
| n(%) | n(%) | p | n(%) | n(%) | p | |
| Age ≥ 50 y | 63(55%) | 69(56%) | 0.90 | 65(52%) | 63(50%) | 0.80 |
| Hispanic | 80(70%) | 89(72%) | 0.71 | 98(78%) | 103(82%) | 0.43 |
| English speaking | 30(28%) | 32(28%) | 0.94 | 34(28%0 | 28(23%) | 0.40 |
| Foreign Born | 96(84%) | 109(89%) | 0.32 | 108(86%) | 109(87%) | 0.85 |
| In US ≥ 10 y | 67(72%) | 84(82%) | 0.09 | 73(69%) | 70(65%) | 0.59 |
| Less than high school education | 58(51%) | 64(53%) | 0.71 | 78(63%) | 66(53%) | 0.11 |
| Unemployed | 95(83%) | 89(72%) | 0.04 | 101(81%) | 111(89%) | 0.08 |
| Unmarried | 60(53%) | 68(55%) | 0.68 | 78(62%) | 83(66%) | 0.51 |
| Health Insurance/Benefits | ||||||
| Medi-Cal/Medicare | 39(34%) | 40(33%) | 0.95 | 57(46%) | 58(46%) | 0.89 |
| Local government | 41(36%) | 47(38%) | 35(28%) | 32(26%) | ||
| Other | 7(6%) | 9(7%) | 2(2%) | 1(1%) | ||
| None | 27(24%) | 27(22%) | 31(25%) | 34(27%) | ||
| Cancer Stage | ||||||
| Stage 0, 1, 2, unstaged | 93(82%) | 103(84%) | 0.66 | 73(58%) | 74(59%) | 0.90 |
| Stage 3, 4, recurrent | 21(18%) | 20(16%) | 52(42%) | 51(41%) | ||
| Cancer Treatment Phase | ||||||
| Prior to treatment | 50(44%) | 68(55%) | 0.19 | 27(22%) | 23(18%) | 0.80 |
| Active treatment | 59(52%) | 52(42%) | 72(58%) | 74(59%) | ||
| Follow-up | 5(4%) | 3(2%) | 26(21%) | 28(22%) | ||
| Comorbid medical condition | 65(57%) | 66(54%) | 0.60 | 69(55%) | 76(61%) | 0.37 |
| Depression | ||||||
| Major Depression (PHQ-9 ≥10) + Dysthymia | 21 (18%) | 21 (17%) | 0.99 | 8 (6%) | 8 (6%) | 0.92 |
| Major Depression | 15 (13%) | 15 (12%) | 14 (11%) | 11 (9%) | ||
| Dysthymia | 18 (16%) | 20 (16%) | 15 (12%) | 17 (14%) | ||
| Anxiety (BSI score ≥14) | 7(20%) | 2(6%) | 0.08 | 7(11%) | 9(14%) | 0.59 |
| Reporting Pain | 48(42%) | 47(39%) | 0.58 | 69(55%) | 69(55%) | 1.00 |
| (BPI score ≥ 7) | 7(20%) | 2(6%) | 0.08 | 7(11%) | 9(14%) | 0.59 |
| Mean(SE) | Mean(SE) | Mean(SE) | Mean(SE) | |||
| KPSS | 6.8(0.2) | 7.1(0.2) | 0.27 | 6.3(0.2) | 6.7(0.2) | 0.09 |
| Functional Well-Being | 14.1(0.5) | 14.3(0.5) | 0.87 | 14.5(0.5) | 14.8(0.5) | 0.70 |
| Emotional Well-Being | 15.4(0.5) | 14.8(0.5) | 0.44 | 16.5(0.4) | 16.7(0.4) | 0.81 |
| Physical Well-Being | 21.2(0.6) | 22.7(0.5) | 0.04 | 19.5(0.5) | 19.0(0.6) | 0.49 |
| Social-Family Well-Being | 17.0(0.5) | 16.9(0.5) | 0.87 | 19.3(0.6) | 19.0(0.6) | 0.72 |
Survival and Quality-of-Life Outcomes
Logistic regression models with control of baseline scores and cancer stage find no significant effect on survival or quality-of-life improvement except for a significant improvement in emotional well-being in gynecologic EUC patients at the end of 12 months (OR=2.72, 95% CI: 1.25-5.9, p=0.01).
Treatment Adherence
Treatment profiles did not vary significantly between TPN and EUC (Table 2). The majority of patients received chemotherapy. Breast cancer patients received hormone therapy after acute treatment. Overall adjuvant treatment adherence rates were notably high and there were no significant differences between study groups (Table 3).
Table 2.
Adjuvant Treatment over 12 months
| Breast Cancer | Gynecologic Cancer | |||
|---|---|---|---|---|
| EUC | TPN | EUC | TPN | |
| Chemotherapy and Radiation* | ||||
| • Received chemotherapy + RT | 33 (32%) | 30 (27%) | 25 (22%) | 26 (22%) |
| • Only chemotherapy | 25 (24%) | 33 (30%) | 24 (21%) | 25 (22%) |
| • Only RT | 13 (13%) | 17 (15%) | 9 (8%) | 8 (7%) |
| Hormone Therapy ** | 71 (62%) | 82 (67%) | n/a | n/a |
| Pain Medication | 89 (78%) | 84 (68%) | 103 (82%) | 108 (86%) |
| Antidepressants | 25 (22%) | 28 (23%) | 15 (12%) | 11 (9%) |
| End of trial | ||||
| • Follow-up | 58 (56%) | 61 (55%) | 55 (48%) | 58 (50%) |
| • Continuing chemotherapy/RT | 8 (8%) | 9 (8%) | 4 (3%) | 9 (8%) |
| • hospice care | 1 (1%) | 1 (1%) | ||
| • Deceased | 5 (5%) | 6 (5%) | 20 (17%) | 14 (12%) |
| • Receiving care elsewhere | 4 (4%) | 3 (3%) | 2 (2%) | 4 (3%) |
| • Lost follow-up | 28 (27%) | 31 (28%) | 33 (29%) | 30 (26%) |
444 patient medical charts reviewed (103 breast UC, 110 breast TPN, 115 gynecologic UC and 116 gynecologic TPN)
Hormone therapy was not mutual exclusive from chemotherapy and/or radiation therapy
Table 3.
Treatment Adherence between TPN and EUC Groups
| Breast | Gynecologic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n(%) |
OR | 95% CI | p | n(%) |
OR | 95% CI | p | |||
| EUC | TPN | EUC | TPN | |||||||
| Chemotherapy | ||||||||||
| NC/Declined | 6 (10%) | 8 (12%) | 1 | 7 (13%) | 3 (6%) | 1 | ||||
| C-Delay | 9 (14%) | 18 (26%) | 1.50 | (0.40 - 5.65) | 0.55 | 22 (41%) | 16 (31%) | 1.70 | (0.38 - 7.59) | 0.49 |
| CAsSched | 48 (76%) | 42 (62%) | 0.66 | (0.21-2.05) | 0.47 | 25 (46%) | 33 (63%) | 3.08 | (0.72-13.12) | 0.13 |
| RT | ||||||||||
| NC/Declined | 5 (10%) | 5 (10%) | 1 | 5 (13%) | 5 (13%) | 1 | ||||
| C-Delay | 20 (40%) | 21 (42%) | 1.05 | (0.26-4.19) | 0.94 | 20 (51%) | 16 (42%) | 0.80 | (0.2-3.25) | 0.76 |
| CAsSched | 25 (50%) | 24 (48%) | 0.96 | (0.25-3.74) | 0.95 | 14 (36%) | 17 (45%) | 1.21 | (0.29-5.06) | 0.79 |
| Follow-up | ||||||||||
| Non-adherent | 32 (31%) | 36 (33%) | 1 | 44 (38%) | 40 (34%) | 1 | ||||
| Adherent | 71 (69%) | 74 (67%) | 0.93 | (0.52-1.65) | 0.80 | 71 (62%) | 76 (66%) | 1.18 | (0.69-2.01) | 0.55 |
CAsSched: completed as scheduled; C-Delay: completed but delayed; NC/Declined: not completed or declined.
Among women with breast cancer, 76% EUC-62% TPN completed chemotherapy as scheduled (CAsSched), while an additional 15% EUC-26% TPN completed with delays (C-Delay) (10% EUC-22% TPN attributable to toxicity or other medical reasons (ToxDelay), 5% EUC-4% TPN to non-medical reasons (OthDelay)). Only 1 EUC and 3 TPN patients did not complete (DNComplete) and 5 patients in each study group declined chemo treatment. Adherence to radiation treatment was similar between breast cancer EUC and TPN groups (50% CAsSched, 40% C-Delay, 2% DNComplete and 8% declined).
Among gynecologic patients, 46% CAsSched, 28% ToxDelay, 13% OthDelay, 7% DNComplete, 6% declined in EUC and 63% CAsSched, 21% ToxDelay, 10% OthDelay, 6% DNComplete in TPN. About 87% gynecologic patients in each study group completed radiation treatment, including C-Delay 51% EUC-42% TPN. Only 2 EUC and 3 TPN patients failed to complete, and 3 EUC and 2 TPN refused, radiation treatment.
Controlling for study group using Polytomous logistic regression models, gynecologic patients with advanced cancer (stage 3, 4 or recurrent) had significantly higher odds of being non-adherent to chemotherapy (p<0.01). Cancer stage was not associated with radiation adherence for gynecologic patients, or either treatment adherence for breast patients. In addition, there were no significant differences in adherence rates when comparing patients’ baseline status (i.e., prior-to-initiating treatment versus during or post acute treatment) between study groups.
Nearly 1/3 of breast and more than 1/3 of gynecologic patients in each study group were not fully adherent to follow-up appointments, having missed at least one scheduled follow-up appointment or failing to return to clinic. Site pharmacy records indicated that 44 patients obtained tamoxifen or arimidex, with overall 59% adherence and no significant difference in rates between study groups. Of 27 patients receiving antidepressants, adherence rates, defined as obtaining the prescribed number of refills, were significantly better among TPN patients - 67% (10 out of 15) versus 25% among EUC patients (3 out of 12), p=0.03.
Adherence, Mortality and Quality-of-Life Outcomes
There are no significant interactions between study group and adherence (CAsSched or Not CAsSched) for either mortality or quality of life outcomes. Therefore, combined data from TPN and EUC is used to evaluate the associations between timely adherence and death rate and quality of life FACT-G subscales (Table 4). Gynecologic patients who failed to complete the prescribed chemotherapy regimen as scheduled had a higher death rate at 12 months (29.17% vs 10.34%, p=0.01). FACT-G subscale means do not vary between timely and not timely adherence groups in regression models controlled by baseline scores and cancer stage. Patient costs of care worries are similar between TPN and EUC groups. Polytomous logistic regression models compare odds of adherence to treatment between patients with or without reported cost concerns at either 6 or 12 months. Adherence is not significantly associated with cost concerns in either breast or gynecologic patients.
Table 4.
Quality-of-Life and Adherence
| Breast | Gynecologic | |||||
|---|---|---|---|---|---|---|
| Not CAsSched | CAsSched | p | Not CAsSched | CAsSched | p | |
| Chemotherapy | ||||||
| Death (n (%)) | 4 (9.76%) | 4 (4.44%) | 0.15 | 14 (29.17%) | 6 (10.34%) | 0.01 |
| FACT-G (Mean (SE)) | ||||||
| • Functional | 15.56 (1.07) | 17.04 (0.64) | 0.24 | 13.91 (1.21) | 16.58 (0.96) | 0.10 |
| • Emotional | 17.85 (0.80) | 18.31 (0.49) | 0.63 | 17.78 (1.04) | 17.58 (0.82) | 0.89 |
| • Physical | 21.29 (0.88) | 22.56 (0.54) | 0.22 | 19.76 (1.07) | 22.06 (0.84) | 0.11 |
| • Social-Family | 18.96 (0.97) | 18.40 (0.59) | 0.62 | 17.65 (1.28) | 18.70 (1.01) | 0.53 |
| Radiation Therapy | ||||||
| Death (n (%)) | 2 (3.92%) | 3 (6.12%) | 0.31 | 2 (4.35%) | 4 (12.90%) | 0.13 |
| FACT-G (Mean (SE)) | ||||||
| • Functional | 16.12 (0.88) | 17.54 (0.86) | 0.26 | 15.70 (1.06) | 16.38 (1.49) | 0.71 |
| • Emotional | 17.96 (0.63) | 18.38 (0.61) | 0.64 | 18.35 (0.87) | 17.11 (1.26) | 0.43 |
| • Physical | 22.07 (0.78) | 22.60 (0.76) | 0.64 | 21.08 (0.94) | 22.84 (1.32) | 0.29 |
| • Social-Family | 18.45 (0.84) | 18.86 (0.82) | 0.73 | 18.73 (1.05) | 15.59 (1.46) | 0.09 |
Abbreviation: CAsSched=completed as scheduled
Qualitative Study Results
Information collected from the semi-structured interviews provided additional insight on economic, cultural and systems barriers to adherence. Although all women in this study were receiving care in a public safety net system, lack of insurance to pay for treatment and related out-of pocket costs were cited as barriers to care by 21% of women interviewed. In contrast, women also cited strong efforts on their part to be adherent to prescribed treatment and follow-up appointments; and attributed their desire to adhere to treatment to several factors, including respect for the advice of caring physicians and family members and a strong desire to survive.
DISCUSSION
The high rate of adjuvant treatment adherence by women in both TPN and EUC groups is striking, and is in contrast to previous adherence intervention trials among low-income minority women described above.30-33 Thus, this study raises key questions about what degree of patient navigation assistance is needed to improve cancer treatment adherence in safety net care systems. In this study, informational material on community resources and depression and cancer, and patient navigation to facilitate receipt of financial and supportive services may have facilitated treatment adherence.
Social structural factors (e.g. lack of health insurance, out-of-pocket treatment, lost wages and transportation costs) may also affect treatment adherence.52 Thus, relatively high adherence rates across study groups might be partly attributable to the study site routine referral to the California Cancer Treatment Fund (under the federal-state Breast and Cervical Cancer Prevention and Treatment Act’s funding of poor women) implemented within Medi-Cal in January, 2002 (prior to the initiation of study recruitment). The similarity of adherence rates in our trial with studies of Medicare insured patients35-36 suggests that available treatment funding for this population is likely to facilitate treatment participation.
Study limitations include patient attrition, demographic and cancer site differences between adherent and non-adherent, lack of medical charts for all patients, relatively small numbers in subgroup analyses raise questions about treatment adherence among patients lost to follow-up. Moreover, in a previous longitudinal analysis of this study trial population, socio-economic stress was significantly associated with depression and poorer quality-of-life over time.53 However, brief intervention for patients meeting criteria for major clinical depression (referral for brief counseling followed by referral to a mental health provider) did not result in significant differences in receipt of antidepressants for patients who reported major depressive symptoms. In contrast, two randomized controlled trial of intensive collaborative care management that included antidepressant medication and/or psychotherapy plus relapse prevention and maintenance care significantly improved major depression over 12 months in a similar low-income minority population54-55 and in a population in Scotland.56
Conclusion
This study’s high adjuvant treatment adherence results raise the probability that a treatment funding program and routine efforts to ensure that eligible patients receive the funding plus either telephone delivered navigational information and reminders or written informational materials (in this case in Spanish when preferred) may facilitate cancer treatment adherence among underserved low-income Hispanic women in safety net programs. Further research,57 including an ongoing multi-site study58 on patient navigation community practice models, may provide additional answers to questions of efficacy for specific populations as well as comparative cost data and degree of effectiveness of diverse navigational programs. Evaluation of national and state data on the effects of publicly financed treatment funding will also add to our understanding.
Acknowledgments
This research was supported by the National Cancer Institute RO1 CA94827 (K. Ell, PI).
References
- 1.Lenzer J. US cancer mortality falls for the first time. BMJ. 2006;332:444–447. doi: 10.1136/bmj.332.7539.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackman DJ, Masi CM. Racial and ethnic disparities in breast cancer mortality: are we doing enough to address the root causes? J Clin Oncol. 2006;24:2170–2178. doi: 10.1200/JCO.2005.05.4734. [DOI] [PubMed] [Google Scholar]
- 3.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002) Cancer Epidemiol Biomarkers Prev. 2006;15:792–797. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 4.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer model of health disparities: understanding mortality differences in minority populations. J Clin Oncol. 2006;24:2179–2187. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 5.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 6.Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003;97:2853–2860. doi: 10.1002/cncr.11411. [DOI] [PubMed] [Google Scholar]
- 7.Bigby J, Holmes MD. Disparities across the breast cancer continuum. Cancer Causes Control. 2005;16:35–44. doi: 10.1007/s10552-004-1263-1. [DOI] [PubMed] [Google Scholar]
- 8.Bickell NA, LePar F, Wang JJ, Leventhal H. Lost opportunities: physicians’ reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25:2516–2521. doi: 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 9.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 10.Du Xianglin L, Gor BJ. Racial disparities and trends in radiation therapy after breast-conserving surgery for early-stage breast cancer in women 1992 to 2002. Ethn Dis. 2007;17:122–128. [PMC free article] [PubMed] [Google Scholar]
- 11.Joslyn SA. Racial differences in treatment and survival from early-stage breast carcinoma. Cancer. 2002;95:1759–1766. doi: 10.1002/cncr.10827. [DOI] [PubMed] [Google Scholar]
- 12.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch TPNern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Naeim A, Hurria A, Leake B, Maly RC. Do age and ethnicity predict breast cancer treatment received? A cross-sectional urban population based study. Breast cancer treatment: age and ethnicity. Crit Rev Oncol Hematol. 2006;59:234–242. doi: 10.1016/j.critrevonc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 15.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 16.Tammemagi CM. Racial/ethnic disparities in breast and gynecologic cancer treatment and outcomes. Cur Opinion Obs Gyn. 2007;19:31–36. doi: 10.1097/GCO.0b013e3280117cf8. [DOI] [PubMed] [Google Scholar]
- 17.Byers TE, Wolf HJ, Bauer MS, et al. The impact of socioeconomic status on survival after cancer in the United States. Cancer. 2008;5:241–246. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Ferrante J, Won BR, Hammeed M. Barriers to adequate follow-up during adjuvant therapy may be important factors in the worse outcome for Black women after breast cancer treatment. J Surg Onc. 2008;6:26–29. doi: 10.1186/1477-7819-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas BC, Nandamohan TI, Nair MK, Pandey M. Screening for distress can predict loss of follow-up and treatment in cancer patients: results of development and validation of the Distress Inventory for Cancer Version 2. Psychooncology. 2008 doi: 10.1002/pon.1422. online. [DOI] [PubMed] [Google Scholar]
- 20.Kirk MC, Hudis CA. Insight into barriers against optimal adherence to oral hormonal therapy in women with breast cancer. Clin Breast Cancer. 2008;8:155–161. doi: 10.3816/CBC.2008.n.016. [DOI] [PubMed] [Google Scholar]
- 21.Magai C, Consedine N, Neugut AI, Hershman DL. Common psychosocial factors underlying breast cancer screening and breast cancer treatment adherence: a conceptual review and synthesis. J Women’s Health. 2007;16:11–23. doi: 10.1089/jwh.2006.0024. [DOI] [PubMed] [Google Scholar]
- 22.Vargas RB, Ryan GW, Jackson CA, Rodriquez R, Freeman HP. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer. 2008;113:426–433. doi: 10.1002/cncr.23547. [DOI] [PubMed] [Google Scholar]
- 23.Gabram SG, Lund MJ, Gardner BS, et al. Effects of an outreach and internal navigation program on breast cancer diagnosis in an urban cancer center with a large African-American population. Cancer. 2008;113:602–607. doi: 10.1002/cncr.23568. [DOI] [PubMed] [Google Scholar]
- 24.Ell K, Vourlekis B, Lee P-J, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44:26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Meyerowitz BE, Richardson J, Hudson S, Leedham B. Ethnicity and cancer outcomes: behavioral and psychosocial considerations. Psychol Bul. 1998;123:47–70. doi: 10.1037/0033-2909.123.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Bruner DW, Jones M, Buchanan D, Russo J. Reducing cancer disparities for minorities: a multidisciplinary research agenda to improve patient access to health systems, clinical trials, and effective cancer therapy. J Clin Oncol. 2006;24:2209–2215. doi: 10.1200/JCO.2005.04.8116. [DOI] [PubMed] [Google Scholar]
- 27.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, co-morbidities and demographics? Cancer. 2008;112:171–80. doi: 10.1002/cncr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schleinitz MD, DePalo D, Blume J, Stein M. Can differences in breast cancer utilities explain disparities in breast cancer care? J Gen TPNern Med. 2006;21:1253–1260. doi: 10.1111/j.1525-1497.2006.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Formenti SC, Meyerowitz BE, Ell K, et al. Inadequate adherence to radiotherapy in Latina immigrants with carcinoma of the cervix: Potential impact on disease free survival. Cancer. 1995;75:1135–1140. doi: 10.1002/1097-0142(19950301)75:5<1135::aid-cncr2820750513>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Levine AM, Richardson JL, Marks G, et al. Compliance with oral drug therapy in patients with hematologic malignancy. J Clin Onc. 1987;5:1469–1476. doi: 10.1200/JCO.1987.5.9.1469. [DOI] [PubMed] [Google Scholar]
- 32.Richardson JL, Marks G, Johnson CA, et al. Path model of multidimensional compliance with cancer therapy. Health Psych. 1987;6:183–207. doi: 10.1037//0278-6133.6.3.183. [DOI] [PubMed] [Google Scholar]
- 33.Richardson JL, Shelton DR, Krailo M, Levine AM. The effect of compliance with treatment on survival among patients with hematologic maliganancies. J Clin Oncol. 1990;8:356–364. doi: 10.1200/JCO.1990.8.2.356. [DOI] [PubMed] [Google Scholar]
- 34.Li BD, Brown WA, Ampil F, Burton G, Yu H, McDonald JC. Patient compliance is critical for equivalent clinical outcomes for breast cancer treated by breast-conversation therapy. Ann Surg. 2000;231:883–889. doi: 10.1097/00000658-200006000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srokowski TP, Fang S, Duan Z, et al. Completion of adjuvant radiation therapy among women with breast cancer. Cancer. 2008;113:22–39. doi: 10.1002/cncr.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobie SA, Baldwin LM, Dominitz JA, et al. Completion of therapy by Medicare patients with stage III colon cancer. J National Cancer Inst. 2006;98:610–9. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker M, Drachman R, Kirscht P. A new approach to explaining sick-role behavior in low-income populations. Am J Pub Health. 1974;64:205–216. doi: 10.2105/ajph.64.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernal G, Bonilla J, Bellido C. Ecological validity and cultural sensitivity for outcome research: Issues for the cultural adaptation and development of psychosocial treatments with Hispanics. J Abnormal Psychol. 1995;23:67–82. doi: 10.1007/BF01447045. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz P. The role of culture in psychiatric care. Am J Psychiatry. 1998;155:1763–1765. doi: 10.1176/ajp.155.12.1763. [DOI] [PubMed] [Google Scholar]
- 40.Cleeland CS. Pain assessment in cancer. In: Osaba D, editor. Effect of Cancer on Quality of Life. Boca Raton FL: CRC Press; 1991. pp. 293–305. [Google Scholar]
- 41.Anderson K, Mendoza T, Valero V, et al. Minority cancer patients and their providers: Pain management attitudes and practice. Cancer. 2000;88:1929–1938. [PubMed] [Google Scholar]
- 42.Mor V, Laliberte L, Morris JN, Wiermann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. J Clin Oncol. 1984;2:1170–76. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 43.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E. The Functional Assessment of Cancer Therapy Scale: Development and Validation of the General Measure. J Clin Oncol. 1993;11(3):570–79. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 44.Cella D, Hernandez L, Corona M, et al. Spanish language translation and initial validation of the functional assessment of cancer therapy quality-of-life instrument. Med Care. 1998;36:1407–1417. doi: 10.1097/00005650-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen TPNern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wittkampf KA, Naeije L, Schene AH, et al. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psych. 2007;29:388–395. doi: 10.1016/j.genhosppsych.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 48.Willms DG, Best JA, Taylor DW, et al. A systematic approach for using qualitative methods in primary prevention research. Med Anthropol Q. 1990;4:391–409. [Google Scholar]
- 49.Glaser BG, Strauss AL. The Discovery of Grounded Theory; Strategies for Qualitative Research. Aldine; Chicago: 1967. [Google Scholar]
- 50.Strauss AL, Corbin J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Sage; Thousand Oaks, CA: 1998. [Google Scholar]
- 51.Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2. Sage; Thousand Oaks, CA: 1994. [Google Scholar]
- 52.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcome. CA CANCER J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 53.Ell K, Xie B, Wells A, et al. Economic stress among low-income women with cancer: effects on quality of life. Cancer. 2008;112(3):616–625. doi: 10.1002/cncr.23203. [DOI] [PubMed] [Google Scholar]
- 54.Dwight-Johnson M, Ell K, Lee PJ. Can collaborative care address the needs of low-income Latinas with comorbid depression and cancer? Results from a randomized pilot study. Psychosomatics. 2005;46:224–32. doi: 10.1176/appi.psy.46.3.224. [DOI] [PubMed] [Google Scholar]
- 55.Ell K, Xie B, Quon B, et al. Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol. 2008;26(27):4488–4496. doi: 10.1200/JCO.2008.16.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372:40–48. doi: 10.1016/S0140-6736(08)60991-5. [DOI] [PubMed] [Google Scholar]
- 57.Moore S. Facilitating oral chemotherapy treatment and compliance through patient/family-focused education. Cancer Nurs. 2007;30(2):112–122. doi: 10.1097/01.NCC.0000265009.33053.2d. [DOI] [PubMed] [Google Scholar]
- 58.Wells KJ, Battaglia TA, Dudley DJ, et al. Patient Navigation: state of the art or is it science? Cancer. 2008;113:1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freund KM, Battaglia TA, Calhoun E, et al. National Cancer Institute Patient Navigation Research Program. Cancer. 2008;113:3391–3399. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]


