Abstract
Free radical-induced lipid peroxidation has been implicated in a number of human diseases including atherosclerosis, cancer, and neurodegenerative diseases. F2-Isoprostanes (IsoPs) are isomers of prostaglandin PGF2α that are generated in vivo from the free radical-initiated peroxidation of arachidonic acid independent of cyclooxygenase enzymes. Since the discovery of the IsoPs in the early 1990s, a large body of evidence has been accumulated to indicate that quantification of these F2-IsoPs represents the most reliable biomarker to assess oxidative stress in vivo. A variety of analytical approaches have been developed for the quantification of these novel compounds; these methods include mass spectrometry (MS) detection coupled to gas chromatography (GC) or liquid chromatography (LC) separation, and detection using immunological approaches. This article summarizes our current methodology to quantify F2-IsoPs in biological fluids and tissues using GC-MS. This method includes solid phase extraction (SPE), thin layer chromatography (TLC) purification, chemical derivatization, and MS detection using negative ion chemical ionization (NICI) coupled with GC. The protocol described herein has been optimized and validated to provide the best sensitivity and selectivity for quantification of F2-IsoPs from a variety of biological sources.
Introduction
Free radical-induced autooxidation of polyunsaturated fatty acids (PUFAs) has been linked to numerous human disorders, including atherosclerosis, cancer, neurodegenerative diseases, and aging [1–5]. Isoprostanes (IsoPs) are a series of prostaglandin (PG)-like compounds that are formed non-enzymatically in vivo via the peroxidation of arachidonic acid by a free radical-initiated mechanism [6–8]. An important aspect of the formation of IsoPs is that they are generated in situ from arachidonic acid esterified in phospholipids and are subsequently released by phospholipases, such as platelet-activating factor (PAF) acetylhydrolases [9]. On the other hand, the PGs are generated from the oxidation of free arachidonic acid by cyclooxygenases (COXs) [10]. Over the past two decades, quantification of F2-IsoPs, isomers of PGF2α, has emerged as one of the most accurate approaches to assess oxidant injury in vivo [11, 12]. In fact, in the Biomarkers of Oxidative Stress Study (BOSS) sponsored by the Nation Institute of Health (NIH), F2-IsoPs were shown to be the most accurate biomarker to assess in vivo oxidant stress status when compared against other well-known biomarkers [13]. In addition to F2-IsoPs, D2/E2-IsoPs which contain E/D prostane rings analogous to COX-derived PGD2 and PGE2, and isomers of thromboxane, termed isothromboxanes (IsoTxs), are also generated in significant amounts in vivo [14, 15]. Some IsoPs exert potent biological activity by acting as ligands for either plasma membrane bound PG receptors or for nuclear receptors and thus are likely also mediators of oxidant injury [16–18].
F2-IsoPs are stable and robust molecules that are detectable in all human tissues and biological fluids analyzed, including plasma, urine, bronchoalveolar lavage (BAL) fluid, cerebrospinal fluid (CSF), and bile. The quantification of F2-IsoPs in urine and plasma is most convenient and least invasive. Several methods have been developed to analyze F2-IsoPs; these include chromatographic separation involving solid-phase extraction (SPE) or affinity chromatography with or without thin layer chromatography (TLC) followed by final determination by gas chromatography - mass spectrometry (GC–MS), liquid chromatography (LC)-MS, or enzyme immunoassay[19–24]. GC - negative ion chemical ionization mass spectrometric (GC/NICI-MS) employing stable isotope dilution is the preferred method for the quantification of F2-IsoPs and several alternative GC-MS assays have been developed by different investigators including FitzGerald and colleagues [25]. For quantification purpose, we measure 15-F2t-IsoP (also known as 8-Iso PGF2α), and other F2-IsoPs that co-elute with this compound. The advantages of GC-MS include the high resolution of GC separation on fused silica capillary columns and the specificity and sensitivity of MS, which yield quantitative results in the low picogram range [26, 27].
A substantial body of evidence indicates that measurement of F2-IsoPs in body fluids provides a reliable approach to assess lipid peroxidation in vivo. Based on available data, quantification of these compounds in either plasma or urine is representative of their endogenous production and thus gives a highly precise and accurate index of in vivo oxidant stress [24, 28, 29]. Normal levels of F2-IsoPs in healthy humans have been defined [28–30]. Defining these levels is particularly important in that it allows for an assessment of the effects of diseases on endogenous oxidant tone and allows for the determination of the extent to which various therapeutic interventions affect levels of oxidant stress. Elevations of IsoPs in human body fluids and tissues have been found in diverse array of human disorders, including atherosclerosis, diabetes, obesity, cigarette smoking, neurodegenerative diseases, and many others [31]. Further, treatments for some of these conditions, including antioxidant supplementation, antidiabetic treatments, cessation of smoking, and even weight loss, have been shown to decrease production of F2-IsoPs [32].
Principles
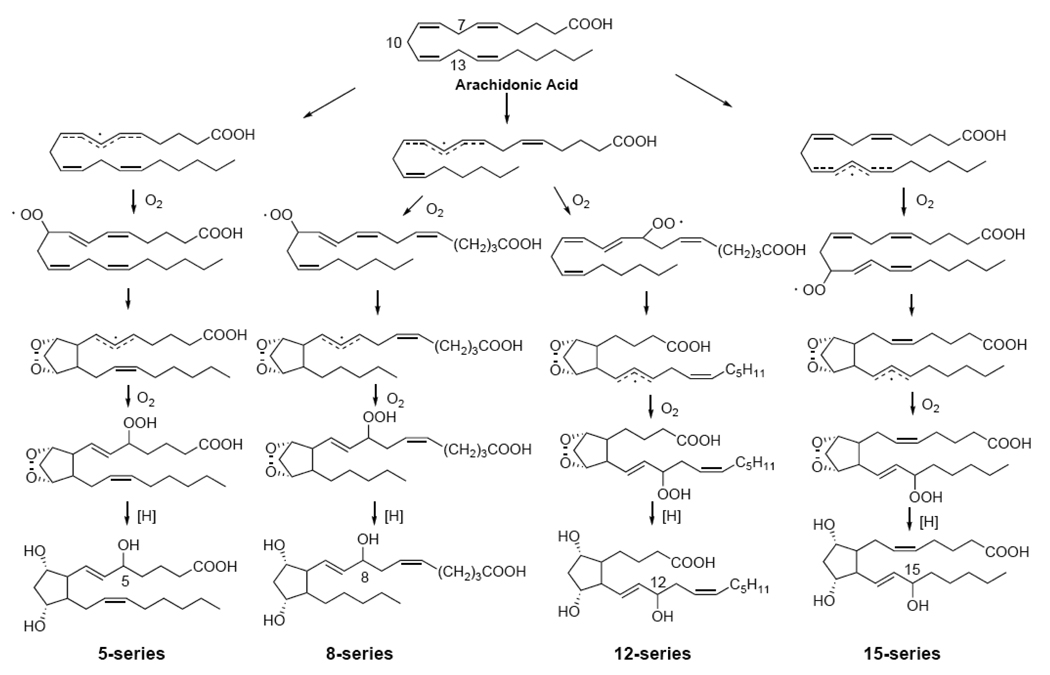
As has been mentioned, F2-IsoPs are formed non-enzymatically as a result of the free radical-mediated peroxidation of arachidonic acid. The mechanism outlined in Fig. 1 is based on the generation of bicyclic endoperoxide intermediates [33]. Arachidonic acid initially undergoes hydrogen atom abstraction by a free radical at the three bisallylic positions of C7, C10, or C13 to yield a delocalized pentadienyl carbon-centered radical. Subsequently, there is an insertion of molecular oxygen to yield peroxyl radicals. These peroxyl radicals undergo further 5-exo cyclization, followed by the addition of another molecule of oxygen to yield the bicyclic endoperoxide (PGG2-like) [34]. These intermediates are then reduced to form F2-IsoPs. Based on this mechanism, four F2-IsoP regioisomers are generated. These are named the 5-, 8-, 12-, and 15-series F2-IsoPs, based on the location of the side chain hydroxyl group in the final product [35, 36]. Each series contains sixteen possible diastereomers and a total of sixty four stereoisomers of F2-IsoPs can be generated.
Figure 1.
Mechanism of formation of F2-Isoprostanes. Four regioisomers are formed each consisting of 16 stereoisomers.
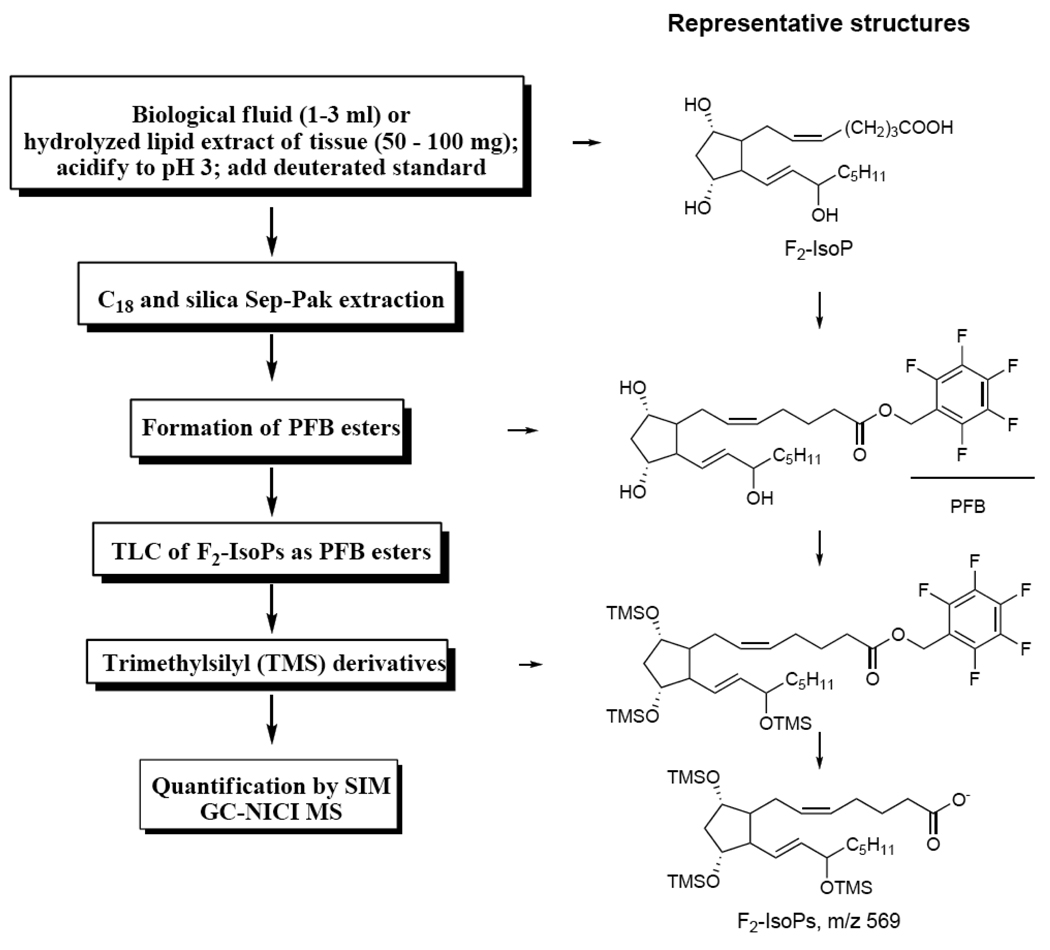
The F2-IsoPs are initially formed esterified to phospholipids after the reduction of the bicyclic endoperoxides and the GC-MS can only analyze the derivatives of free F2-IsoPs. A derivatization protocol has been developed to make the samples suitable for GC-MS analysis (Fig. 2). Both esterified and free F2-IsoPs can be measured. To analyze the esterified F2-IsoPs in tissue samples, the F2-IsoPs are first released under basic condition. After addition of deuterated internal standard, solid phase extraction is carried out using both C18 and silica Sep-Pak to remove the contaminants. Pentafluorobenzyl (PFB) moiety is then introduced to the molecule to enhance the sensitivity of detection using electron capture chemical ionization technique [37]. The last step is to cap the hydroxyl groups by trimethylsilyl (TMS) derivatization. Analysis of the derivatives of F2-IsoPs and the internal standard is carried out using selective ion monitoring (SIM) techniques; the ions monitored are m/z 569 for F2-IsoPs and m/z 573 for the internal standard respectively. The detailed procedures for the purification and derivatization steps will be described in the Protocol section.
Figure 2.
Outline of the procedures used for the extraction, purification, derivatization, and mass spectrometric analysis of F2-IsoPs from biological sources. The representative chemical structures for each derivatization step are also included.
Materials
Deuterated international standard [2H4]-15-F2t-IsoP (8-iso-PGF2α-d4) (Cayman Chemical, cat. no. 316351)
TLC standard, PGF2α methyl ester (Cayman Chemical, cat. no. 16011)
Butylated hydroxytoluene (BHT) (Sigma-Aldrich, cat. no. B1378)
Triphenylphosphine (PPh3) (Sigma-Aldrich, cat. no. 93090 Fluka).
Pentafluorobenzyl bromide (PFBB) (Sigma-Aldrich, cat. no. 10105-2)
N,N′-Diisopropylethylamine (DIPE) (Sigma-Aldrich, cat. no. D3887)
N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) (Supelco., cat. no. 33084)
Dimethyformamide (DMF) (Sigma-Aldrich, cat. no. 6407) (store over calcium hydride to remove water)
Undecane (Sigma-Aldrich) (store over calcium hydride to prevent water accumulation)
Ultrapure water (triply distilled or its equivalent)
Methanol
Chloroform (containing ethanol as a preservative)
Ethyl acetate
Heptane
Acetonitrile
Ethanol
HCl (American Chemical Society certified or equivalent grade)
Sodium chloride
Potassium hydroxide pellets
Anhydrous sodium sulfate
10% Phosphomolybdic acid in ethanol (Sigma-Aldrich, cat. no. P4869)
Nitrogen gas
Stock Solutions
pH 3 water (0.001N HCl): Mix 20 ml 1N hydrochloric acid with 20 L of water
Folch solution: Combine 2 volumes of chloroform with 1 volume of methanol. Dissolve BHT and PPh3 in solution to make a final concentration of 0.005% BHT (wt/vol) and 0.25 mg/ml PPh3. The solution is stored at 4°C in the dark in a brown bottle to prevent light degradation.
PFBB solution: Dilute PFBB to 10% (vol/vol) in dry acetonitrile. Store over calcium hydride to keep solution free of water.
DIPE solution: Dilute DIPE to 10% (vol/vol) in acetonitrile. Store over calcium hydride to keep solution free of water.
TLC standard, PGF2α methyl ester: Dissolve 1 mg of PGF2α methyl ester in 1 ml of chloroform/methanol (3:2, vol/vol) to make a 1 mg/ml solution and stored at −20°C.
Instrumentation
Blade homogenizer-PTA 10S generator (Brinkmann Instruments, Westbury, NY)
C-18 solid-phase extraction (or Sep-Pak) cartridges; each cartridge contains 500 mg of C-18 (Waters, cat. no. WAT036575)
Silica solid-phase extraction (or Sep-Pak) cartridges; each cartridge contains 500 mg of silica (Waters, cat. no. WAT036580)
TLC plates: LK6D silica, marked lanes, glass backed (Whatman, cat. no. WC486562IV)
Capillary GC column (DB-1701, Agilent, cat. no. 21512067)
GC-MS with capabilities for NICI-MS
TLC plate preparation: Prewash all TLC plates with a solution of ethyl acetate and ethanol (90:10, vol/vol). One hour before using plates, drying at 90 °C oven for 10 min and then cool in a desiccator before sample application.
GC-MS setup: For quantification of F2-IsoPs by GC-MS, we routinely use an Agilent 5973 mass spectrometer with a computer interface, but other mass spectrometers can be utilized. The F2-IsoPs are separated on a 15-meter DB1701 fused silica capillary column that gives good separation of F2-IsoPs compared to other columns. The column temperature is programmed from 190 °C to 300 °C at 20 °C per minute. Methane is used as the reagent gas, and helium is used as the carrier gas for NICI. The ion source temperature is set at 200 °C. The ion monitored for endogenous F2-IsoPs is the carboxylate anion m/z 569 (M-181, loss of CH2C6F5). The corresponding carboxylate anion for the deuterated internal standard is m/z 573. The sensitivity of the mass spectrometer is checked every day by injecting a standard consisting of 40 pg each PGF2α and [2H4]-15-F2t-IsoP. Note that PGF2α elutes at a sufficiently different retention time from the F2-IsoPs quantified using this procedure. Therefore, this COX-derived PGF2α does not interfere with the signal of the F2-IsoPs.
Protocol
General precautions
Arachidonic acid in biological samples is susceptible to free radical oxidation and thus prevention of the ex vivo oxidation is crucial in the processing and storage of the samples especially plasma and tissue samples that contain abundant arachidonic acid. For the best results, samples should be flash frozen in liquid nitrogen immediately upon collection and not thawed until analysis. BHT and PPh3 are added to the Folch solution to prevent ex vivo oxidation during the extraction of lipids.
1. Extraction and Hydrolysis of F2-IsoP–Containing Phospholipids in Tissues and Biological Fluids
Levels of F2-IsoPs in a variety of biological sources can be measured as an accurate index of in vivo oxidant stress status. Although free or esterified F2-IsoPs (bound) can be quantified in plasma, we found no advantage in measuring total (free + bound) rather than free F2-IsoPs. Thus the present method measures only free F2-IsoPs. For urine sample, 24-h urine collection represents a reliable probe for the oxidant stress and only free F2-IsoPs is measured. For the tissue samples, the majority of the F2-IsoPs are esterified on phospholipids and basic hydrolysis is necessary to release these compounds after extraction of the total lipids from the tissue homogenates.
(A) Tissue samples
Weigh 50 to 100 mg of tissue and add to 20 ml of ice-cold Folch solution. (Note: Polypropylene tubes are recommended because polystyrene tubes are not resistant to chloroform.)
Homogenized tissue with a blade homogenizer for 30 s or until fully homogenized.
Flush tube with nitrogen or argon and allow the sealed solution to stand at room temperature for 1 h, shaking occasionally.
Add 4 ml of aqueous NaCl (0.9%) and vortex vigorously for 1 min.
Centrifuged at 800×g for 10 min at room temperature to separate the aqueous and organic phases. After centrifugation, the upper aqueous layer is discarded and the lower organic layer is carefully separated from the intermediate semisolid protein layer.
Transfer the organic phase containing the extracted lipids to a 50 ml centrifuge tube and evaporated to dryness under a stream of N2.
Add 2 ml of methanol containing BHT (0.005%) and an equal volume of aqueous KOH (15%). The mixture is then vortexed and flushed with nitrogen before the tube is capped.
Incubate the mixture at 37°C for 30 min to effectively hydrolyze and release free F2-IsoPs.
Acidify the mixture to pH 3 with 1 N HCl and diluted to a final volume of at least 40 ml with pH 3 water. (Note: Dilution of the methanol in the solution with water to 5% or less is necessary to ensure proper column extraction of F2-IsoPs in the subsequent purification procedure.)
(B) Biological fluids
2–3 ml of plasma is added in 7 ml of pH 3 water or 0.25 ml of urine is added in 10 ml of pH 3 water and acidified to pH 3 with 1N HCl.
2. Purification, Derivatization, and Quantification of Free F2-IsoPs
Add accurately 1 ng of the deuterated internal standard [2H4]-15-F2t-IsoP to the sample mixture and vortex. (Note: an accurate syringe is recommended rather than disposal pipette.)
Apply the mixture to C18 Sep-Pak column preconditioned with 5 ml methanol and 5 ml pH 3 water. (Note: Sample solution should be pushed through at a flow of 1–2 ml/min and a steady stream is not recommended.)
Wash the column sequentially with 10 ml pH 3 water and 10 ml of heptane.
Elute the samples from the cartridge with 10 ml of ethyl acetate/heptane (50:50, v/v).
Dry the solution over using about 5 g of anhydrous Na2SO4 for 1 minute to remove water.
Apply the solution to a silica Sep-Pak that was prewashed with 5 ml of ethyl acetate.
Wash the cartridge with 5 ml of ethyl acetate followed by elution of the F2-IsoPs with 5 ml of ethyl acetate/methanol (50:50 v/v).
Evaporate the ethyl acetate/methanol eluant under a stream of nitrogen and add 40 µl of 10% PFBB in acetonitrile and 20 µl of 10% DIPE in acetonitrile to the residue. (Note: PFBB is a potent lachrymator and carry out the experiments in a well-ventilated fume hood).
Vortex and incubate the mixture at 37°C for 20 min.
Add 97 ml of chloroform and 3 ml of ethanol to a TLC tank and place TLC paper in the tank. Allow the tank to equilibrate for 30 minute.
Evaporate the incubation mixture thoroughly under nitrogen and reconstitute it in 50 µl chloroform/methanol (3:2, v/v).
Apply each sample mixture to a separate lane of a prewashed TLC plate. Approximately 2 to 5 µg of the methyl ester of PGF2α is applied on a separate TLC plate.
Place the TLC plates in the tank after the solvent is dry. Remove the plates from the tank after the solvent reaches about 13 cm on the TLC plate.
Visualize the TLC standard plate by spraying with a 10% solution of phosphomolybdic acid in ethanol followed by heating. (Note: DO NOT SPRAY THE SAMPLE PLATES.)
Scrape the silica from the sample plates in the region of the TLC standard 1 cm above and 1 cm below the standard. (Note: the Rf for PGF2α methyl ester should be about 0.15, i.e. the band for the standard is about 2 cm above the original spot.)
Place the scraped silica from each sample into separate microcentrifuge tubes and extract the sample with 1 ml ethyl acetate.
Vortex the mixture vigorously and centrifuge the sample in a benchtop centrifuge at 13,000 r.p.m for 5 minutes.
Carefully remove the solution and place it in a second microcentrifuge tube and evaporate the solvent.
Add 20 µl of BSTFA and 7 µl of dry DMF to the residue and incubate the mixture at 37°C for 20 minutes.
Dry the sample and dissolve it in 20 µl of dry undecane. Transfer the sample to an autosampler vial for GC-MS analysis.
Calculations and Expected Results
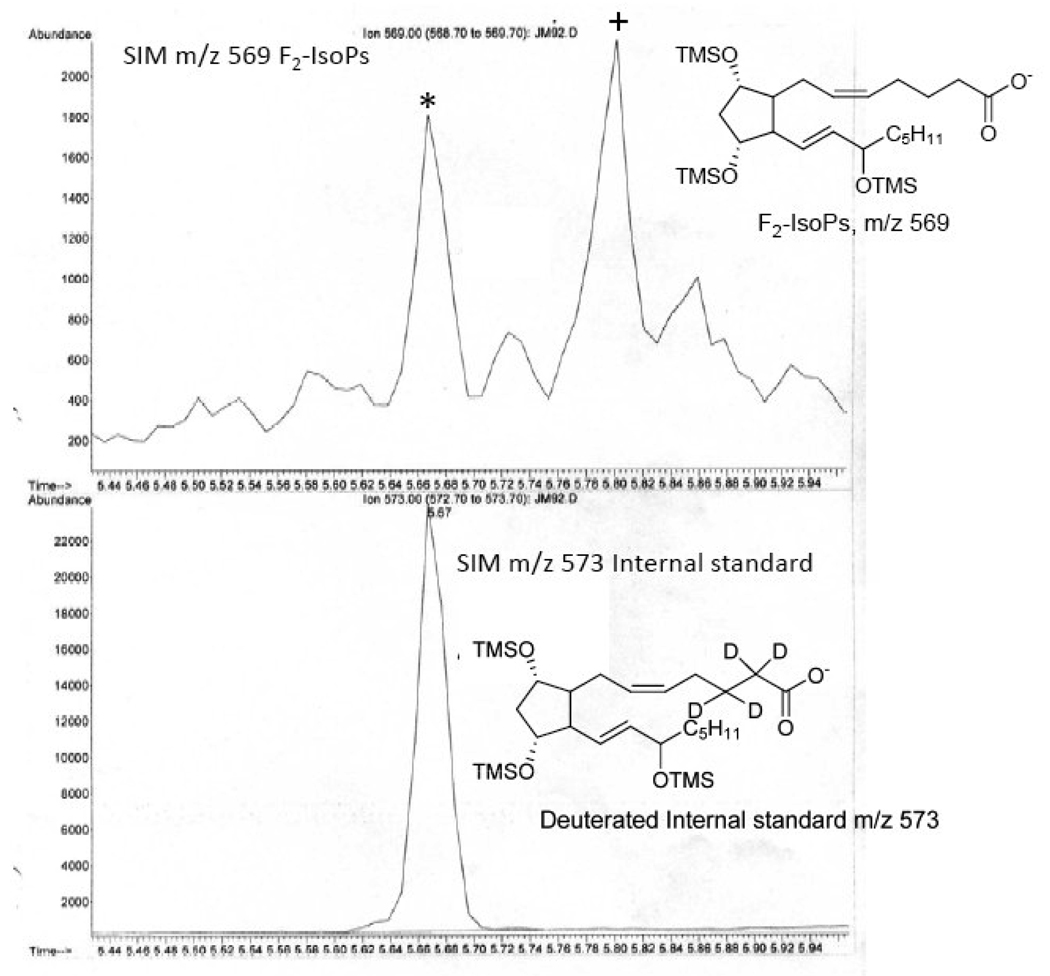
A representative chromatogram obtained from the analysis of F2-IsoPs in mouse liver is shown in Figure 3. For quantification purposes, we compare the height of the peak containing the derivatized 15-F2t-IsoP (m/z 569, peak labeled with * in Fig. 3) with the height of the deuterated internal standard peak (m/z 573). Levels of F2-IsoPs in plasma are reported in picograms or nanograms per milliliter, whereas levels in tissues are reported in nanograms per gram of tissue. Levels of F2-IsoPs in urine are normalized to creatinine clearance and reported as nanograms per milligrams of creatinine. It should be noted that the peak denoted with “+” in Figure 3 may also contain the COX product PGF2α when free F2-IsoPs are analyzed in biological fluids, such as plasma, CSF or urine. However, caution should be exercised regarding the enzymatic origin of this peak; our studies show that majority of this peak in human urine is actually derived from the free radical pathway [38]. Furthermore, this peak is also present when the esterified F2-IsoPs are measured and it can only be derived from the free radical pathway in this case.
Figure 3.
Analysis of F2-IsoPs in liver obtained from a mouse. The m/z 573 ion current chromatogram represent the [2H4] 8-iso-PGF2a internal standard. The m/z 569 ion current chromatogram represents endogenous F2-IsoPs.The peak in the upper chromatogram represented by the star (*) is the one routinely used for quantification of the F2-IsoPs.The peak represented by the plus (+) can be comprised of both F2-IsoPs and COX-derived PGF2α.
Caveats
GC-MS method offers excellent sensitivity and selectivity for F2-IsoPs. However, the labor intensive purification and derivatization steps that are necessary for the GC-MS analysis make it difficult to improve the throughput of the assay. Secondly, experienced and trained personnel are required to set up and operate the rather expensive instrument. In addition the GC-MS method, a number of LC-MS methods for F2-IsoPs analysis have been developed [39–41]. As has been mentioned, F2-IsoPs consist a mixture of four series of regioisomers and each has sixteen diastereomers. These different stereoisomers may be formed and metabolized differently; thus the levels of these individual stereoisomers can be altered under various pathophysiological conditions [38, 40]. GC-MS methods quantify all possible F2-IsoPs stereoisomers while LC-MS methods permit to separate and identify selected regioisomers and diastereomers of F2-IsoPs. The other advantage of LC-MS methods is that the sample preparation for analysis is simpler than that for GC-MS because no derivatization of the molecule is required. In addition to analyze the free F2-IsoPs, we recently also developed LC-MS method to analyze the intact esterified F2-IsoPs on different phospholipid head groups [42]. However, a concern with these LC-MS assays relates to the limits of detection in biological fluids that are often higher than those employing GC-MS [29].
Enzyme immunoassay has also been developed to quantify IsoPs using antibodies which generated against some selected isomers of F2-IsoPs, such as 8-iso-15(R)-PGF2α [43]. Although mass spectrometric methods of F2-IsoP quantification are considered the best methods for analysis, immunoassays have expanded research in this area due to their low cost and relative ease of use [28, 29]. However, multistep sample purification is still necessary, and questions remain as to the specificity of the antibody. Studies comparing GC-MS and enzyme immunoassay assays of the same samples have demonstrated that the values from the assays are correlated but not identical [44, 45].
The measurement of free F2-IsoPs in urine can be confounded by the potential contribution of local F2-IsoP production in the kidney, although the extent to which this occurs is unclear. In light of this issue, we have identified the primary urinary metabolite of 15-F2t-IsoP to be 2,3-dinor-5,6-dihydro-15-F2t-IsoP, and we have developed a highly sensitive and accurate mass spectrometric assay to quantify this molecule [46]. Thus, the quantification of 2, 3-dinor-5,6-dihydro-15-F2t-IsoP might represent a truly noninvasive, time-integrated measurement of systemic oxidation status that can be applied to living subjects.
Acknowledgements
This work is supported by NIH grants DK48831, GM15431, and ES31125. This manuscript is dedicated to the memory of Dr. Jason Morrow.
Abbreviations
- AA
arachidonic acid
- BAL
bronchoalveolar lavage
- BHT
butylated hydroxyl toluene
- BOSS
Biomarkers of Oxidative Stress Study
- BSTFA
N,O-bis(trimethylsilyl)-trifluoroacetamide
- COX
cyclooxygenase
- DMF
dimethylformamide
- DIPE
diisopropylethylamine
- GC
gas chromatography
- IsoPs
isoprostanes
- IsoTx
isothromboxane
- LC
liquid chromatography
- MS
mass spectrometry
- NICI
negative ion chemical ionization
- NIH
National Institute of Health
- PAF
platelet-activating factor
- PFB
pentafluorobenzyl
- PFBB
pentafluorobenzyl bromide
- PGs
prostaglandins
- PPh3
triphenylphosphine
- PUFA
polyunsaturated fatty acid
- SIM
selective ion monitoring
- SPE
solid phase extraction
- TLC
thin layer chromatography
- TMS
trimethylsilyl
- Tx
thromboxanes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chisolm GM, Steinberg D. The Oxidative Modification Hypothesis of Atherosclerosis: An Overview. Free Radic. Biol. Med. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 2.Montine KS, Quinn JF, Zhang J, Fessel JP, Roberts I L Jackson, Morrow LD, Montine TJ. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem. Phys. Lipids. 2004;128:117–124. doi: 10.1016/j.chemphyslip.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Tsimikas S. In Vivo Markers of Oxidative Stress and Therapeutic Interventions. Am. J. Cardiol. 2008;101:S34–S42. doi: 10.1016/j.amjcard.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized Phospholipids, Lp(a) Lipoprotein, and Coronary Artery Disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 5.Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005;338:668–676. doi: 10.1016/j.bbrc.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 6.Morrow JD, Hill E, Burk RF, Nammour TM, Badr KF, Roberts LJ., Jr A Series of Prostaglandin-F2-Like Compounds Are Produced in vivo in Humans by a Noncyclooxygenase, Free radical-Catalyzed Mechanism. Proc. Natl. Acad. Sci. USA. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milne GL, Yin H, Morrow JD. Human Biochemistry of the Isoprostane Pathway. J. Biol. Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts LJ, II, Milne GL. Isoprostanes. J. Lipid Res. 2009;50:S219–S223. doi: 10.1194/jlr.R800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stafforini DM, Sheller JR, Blackwell TS, Sapirstein A, Yull FE, McIntyre TM, Bonventre JV, Prescott SM, Roberts LJ., II Release of Free F2-isoprostanes from Esterified Phospholipids Is Catalyzed by Intracellular and Plasma Platelet-activating Factor Acetylhydrolases. J. Biol. Chem. 2006;281:4616–4623. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- 10.Funk CD. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 11.Roberts LJ, Jr, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 12.Rokach J, Khanapure SP, Hwang SW, Adiyama M, Lawson JA, FitzGerald GA. The Isoprostanes: A Perspective. Prostaglandins. 1997;54:823–851. doi: 10.1016/s0090-6980(97)00183-4. [DOI] [PubMed] [Google Scholar]
- 13.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, FitzGerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, II, LJ R, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Thiel DHV, Wellner D, Walter PB, Tome KB, Mason RP, Barrett JC. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Morrow J, Minton T, Mukundan C, Campbell M, Zackert W, Daniel V, Badr K, Blair I, Roberts L., 2nd Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J. Biol. Chem. 1994;269:4317–4326. [PubMed] [Google Scholar]
- 15.Morrow JD, Awad JA, Wu A, Zackert WE, Daniel VC, Roberts LJ., II Nonenzymatic Free Radical-catalyzed Generation of Thromboxane-like Compounds (Isothromboxanes) in Vivo. J. Biol. Chem. 1996;271:23185–23190. doi: 10.1074/jbc.271.38.23185. [DOI] [PubMed] [Google Scholar]
- 16.Longmire AW, Roberts LJ, Morrow JD. Actions of the E2-isoprostane, 8-ISO-PGE2, on the platelet thromboxane/ endoperoxide receptor in humans and rats: Additional evidence for the existence of a unique isoprostane receptor. Prostaglandins. 1994;48:247–256. doi: 10.1016/0090-6980(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Nammour TM, Fukunaga M, Ebert J, Morrow JD, Roberts LJ, Hoover RL, Badr KF. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J. Clin. Invest. 1992;90:136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou X, Roberts I, Jackson L, Gobeil J, Fernand, Taber D, Kanai K, Abran D, Brault S, Checchin D. Isomer-specific contractile effects of a series of synthetic F2-isoprostanes on retinal and cerebral microvasculature. Free Radic. Biol. Med. 2004;36:163–172. doi: 10.1016/j.freeradbiomed.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Morrow JD, Harris TM, Roberts LJ., Jr Noncyclooxygenase Oxidative Formation of a Series of Novel Prostaglandins - Analytical Ramfications for Measurement of Eicosanoids. Anal. Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD, Roberts LJ, Lester P. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 21.Mori TA, Croft KD, Puddey IB, Beilin LJ. An Improved Method for the Measurement of Urinary and Plasma F2-Isoprostanes Using Gas Chromatography-Mass Spectrometry. Anal. Biochem. 1999;268:117–125. doi: 10.1006/abio.1998.3037. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida Y, Hayakawa M, Habuchi Y, Itoh N, Niki E. Evaluation of Lipophilic Antioxidant Efficacy in Vivo by the Biomarkers Hydroxyoctadecadienoic Acid and Isoprostane. Lipids. 2007;42:463–472. doi: 10.1007/s11745-007-3043-7. [DOI] [PubMed] [Google Scholar]
- 23.Yan W, Byrd GD, Ogden MW. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J. Lipid Res. 2007;48:1607–1617. doi: 10.1194/jlr.M700097-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Lee C-YJ, Huang SH, Jenner AM, Halliwell B. Measurement of F2-isoprostanes, hydroxyeicosatetraenoic products, and oxysterols from a single plasma sample. Free Radic. Biol. Med. 2008;44:1314–1322. doi: 10.1016/j.freeradbiomed.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Pratico D, Barry OP, Lawson JA, Adiyaman M, Hwang S-W, Khanapure SP, Iuliano L, Rokach J, FitzGerald GA. IPF2alpha -I: An index of lipid peroxidation in humans. Proc. Natl. Acad. Sci. USA. 1998;95:3449–3454. doi: 10.1073/pnas.95.7.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow JD. Quantification of Isoprostanes as Indices of Oxidant Stress and the Risk of Atherosclerosis in Humans. Arterisocl. Thromb. Vas. Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 27.Nourooz-zadeh J. Key issues in F2-isoprostane analysis. Biochem. Soc. Trans. 2008;36:1060–1065. doi: 10.1042/BST0361060. [DOI] [PubMed] [Google Scholar]
- 28.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protocols. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 29.Milne GL, Yin H, Brooks JD, Sanchez S, L. Jackson Roberts I, Morrow JD. Quantification of F2-Isoprostanes in Biological Fluids and Tissues as a Measure of Oxidant Stress. Methods Enzymol. 2007;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 30.Fam SS, Morrow JD. The Isoprostanes: Unique Products of Arachidonic Acid Oxidation-A Review. Curr. Med. Chem. 2003;10:1723–1740. doi: 10.2174/0929867033457115. [DOI] [PubMed] [Google Scholar]
- 31.Yin H, Musiek ES, Morrow JD. Quantification of Isoprostanes as an index of oxidative stress: A update. J. Biol. Sci. 2006;6:469–479. [Google Scholar]
- 32.Roberts LJ, II, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic. Biol. Med. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin H, Porter NA. New Insights Regarding the Autoxidation of Polyunsaturated Fatty Acids. Antioxid. Redox Signal. 2005;7:170–184. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- 34.Yin H, Havrilla CM, Morrow JD, Porter NA. Formation of Isoprostane Bicyclic Endoperoxides from the Autoxidation of Cholesteryl Arachidonate. J. Am. Chem. Soc. 2002;124:7745–7754. doi: 10.1021/ja0201092. [DOI] [PubMed] [Google Scholar]
- 35.Yin H, Havrilla CM, Gao L, Morrow JD, Porter NA. Mechanisms for the Formation of Isoprostane Endoperoxides from Arachidonic Acid: "Dioxetane" Intermediate or beta-Fragmentation of Peroxyl Radicals? J. Biol. Chem. 2003;278:16720–16725. doi: 10.1074/jbc.M300604200. [DOI] [PubMed] [Google Scholar]
- 36.Yin H, Morrow JD, Porter NA. Identification of a Novel Class of Endoperoxides from Arachidonate Autoxidation. J. Biol. Chem. 2004;279:3766–3776. doi: 10.1074/jbc.M307137200. [DOI] [PubMed] [Google Scholar]
- 37.Singh G, Gutierrez A, Xu K, Blair IA. Liquid Chromatography/Electron Capture Atmospheric Pressure Chemical Ionization/Mass Spectrometry: Analysis of Pentafluorobenzyl Derivatives of Biomolecules and Drugs in the Attomole Range. Anal. Chem. 2000;72:3007–3013. doi: 10.1021/ac000374a. [DOI] [PubMed] [Google Scholar]
- 38.Yin H, Gao L, Tai H-H, Murphey LJ, Porter NA, Morrow JD. Urinary Prostaglandin F2α Is Generated from the Isoprostane Pathway and Not the Cyclooxygenase in Humans. J. Biol. Chem. 2007;282:329–336. doi: 10.1074/jbc.M608975200. [DOI] [PubMed] [Google Scholar]
- 39.Taylor AW, Bruno RS, Frei B, Traber MG. Benefits of prolonged gradient separation for high-performance liquid chromatography-tandem mass spectrometry quantitation of plasma total 15-series F2-isoprostanes. Anal. Biochem. 2006;350:41–51. doi: 10.1016/j.ab.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Lawson JA, Reilly M, Adiyaman M, Hwang S-W, Rokach J, FitzGerald GA. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F2-isoprostanes in human urine. Proc. Natl. Acad. Sci. USA. 1999;96:13381–13386. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin H, Porter NA, Morrow JD. Separation and identification of F2-isoprostane regioisomers and diastereomers by novel liquid chromatographic/mass spectrometric methods. J. Chromatogr. B. 2005;827:157–164. doi: 10.1016/j.jchromb.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 42.Yin H, Cox BE, Liu W, Porter NA, Morrow JD, Milne GL. Identification of intact oxidation products of glycerophospholipids in vitro and in vivo using negative ion electrospray iontrap mass spectrometry. J. Mass Spectrom. 2009;44:672–680. doi: 10.1002/jms.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basu S. Radioimmunosassay of 8-iso-prostaglandin F2αan index for oxidative injury via free radical catalysed lipid peroxidation. Prostaglandins Leukot. Essent. Fatty Acids. 1998;58:319–325. doi: 10.1016/s0952-3278(98)90042-4. [DOI] [PubMed] [Google Scholar]
- 44.Proudfoot J, Barden A, Mori TA, Burke V, Croft KD, Beilin LJ, Puddey IB. Measurement of Urinary F2-Isoprostanes as Markers of in Vivo Lipid Peroxidation--A Comparison of Enzyme Immunoassay with Gas Chromatography/Mass Spectrometry. Anal. Biochem. 1999;272:209–215. doi: 10.1006/abio.1999.4187. [DOI] [PubMed] [Google Scholar]
- 45.Bessard J, Cracowski J-L, Stanke-Labesque F, Bessard G. Determination of isoprostaglandin F2α type III in human urine by gas chromatography-electronic impact mass spectrometry. Comparison with enzyme immunoassay. J. Chromatogr. B. 2001;754:333–343. doi: 10.1016/s0378-4347(00)00621-6. [DOI] [PubMed] [Google Scholar]
- 46.Morales CR, Terry ES, Zackert WE, Montine TJ, Morrow JD. Improved assay for the quantification of the major urinary metabolite of the isoprostane 15-F2t-Isoprostane (8-iso-PGF2α) by a stable isotope dilution mass spectrometric assay. Clin. Chim. Acta. 2001;314:93–99. doi: 10.1016/s0009-8981(01)00637-4. [DOI] [PubMed] [Google Scholar]