Abstract
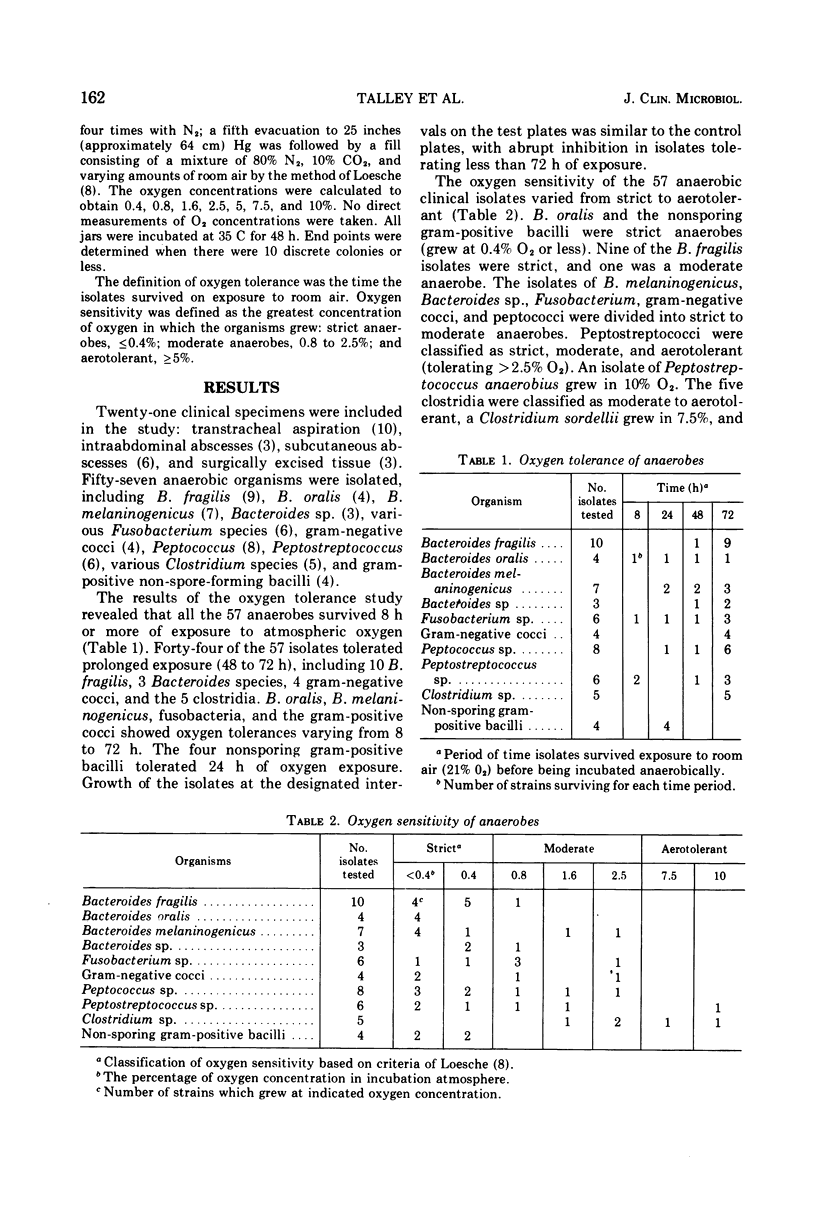
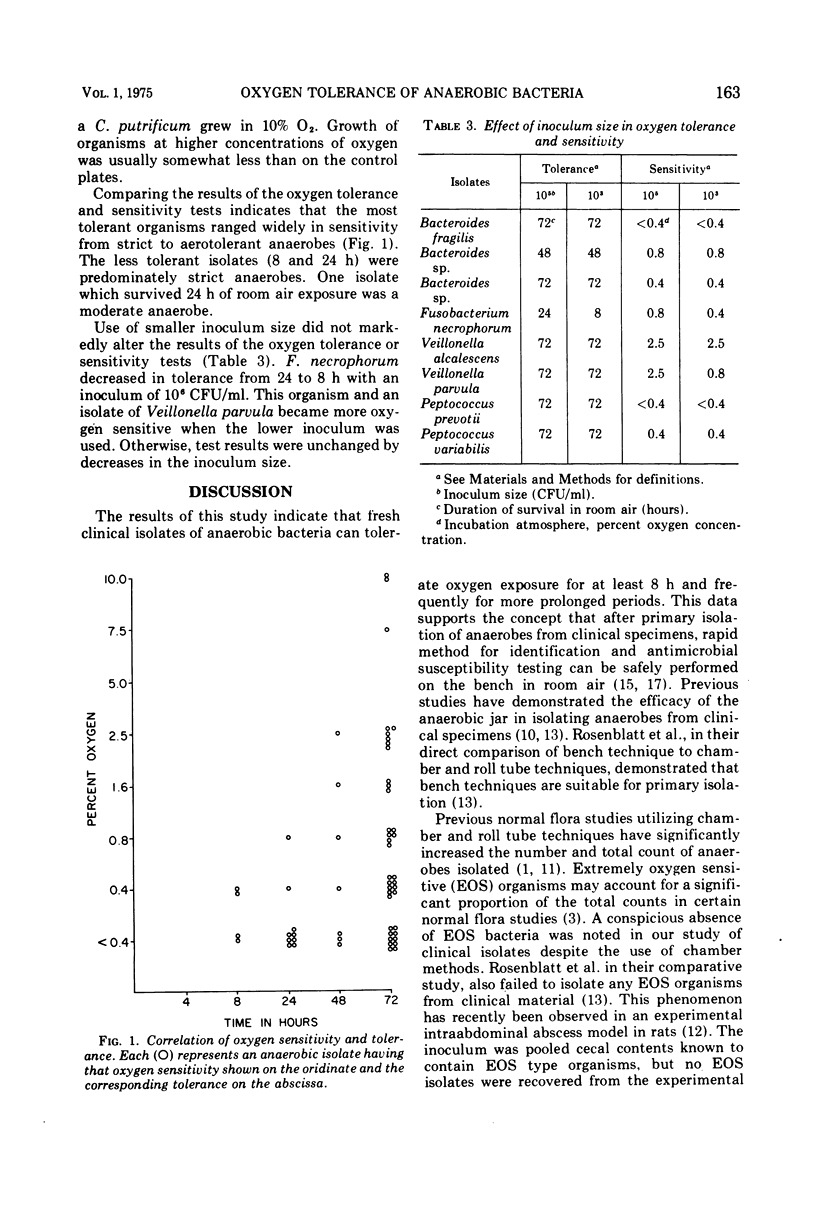
The oxygen tolerance and sensitivity of 57 freshly isolated anaerobic bacteria from clinical specimens was studied. All the organisms tolerated 8 h or more of exposure to oxygen in room air. Growth of the isolates in increasing oxygen concentrations demonstrated that the 57 isolates varied in oxygen sensitivity from strict to aerotolerant anaerobes. Comparison of the oxygen tolerance and sensitivity showed that the most tolerant organisms (best survival after prolonged exposure) included anaerobes capable of growth at only 0.4% or less O2 (strict) as well as those able to grow in as much as 10% O2. The least tolerant were predominately strict anaerobes. Decrease in the inoculum size from a concentration of 10(8) to 10(6) colony-forming units per ml had only a minor effect. The data indicate that the brief oxygen exposure with bench techniques in clinical laboratories would not be deleterious to the anaerobic bacteria present in clinical specimens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attebery H. R., Finegold S. M. Combined screw-cap and rubber-stopper closure for Hungate tubes (pre-reduced anaerobically sterilized roll tubes and liquid media). Appl Microbiol. 1969 Oct;18(4):558–561. doi: 10.1128/am.18.4.558-561.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Hill G. B., Osterhout S. Experimental effects of hyperbaric oxgen on selected clostridial species. I. In-vitro studies. J Infect Dis. 1972 Jan;125(1):17–25. doi: 10.1093/infdis/125.1.17. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J. Isolation and indentification of anaerobic bacteria in the clinical laboratory. A 2-year experience. Mayo Clin Proc. 1974 May;49(5):300–308. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. E., Fallon A., Finegold S. M. Comparison of methods for isolation of anaerobic bacteria from clinical specimens. Appl Microbiol. 1973 Jan;25(1):77–85. doi: 10.1128/am.25.1.77-85.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr S. E., Thompson F. S., Dowell V. R., Jr, Balows A. Micromethod system for identification of anaerobic bacteria. Appl Microbiol. 1973 May;25(5):713–717. doi: 10.1128/am.25.5.713-717.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y. Y., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria. I. Susceptibility of Bacteroides fragilis to tetracycline. Appl Microbiol. 1972 Feb;23(2):268–275. doi: 10.1128/am.23.2.268-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


