Abstract
We targeted LYN, a src-tyosine kinase involved in B cell activation, in case-control association studies using populations of European American, African American and Korean subjects. Our combined European-derived population, consisting of 2463 independent cases and 3131 unrelated controls, demonstrates significant association with rs6983130 in a female-only analysis with 2254 cases and 2228 controls (p=1.1 × 10−4, OR=0.81 (95% CI: 0.73 – 0.90)). This SNP is located in the 5′ UTR within the first intron near the transcription initiation site of LYN. Additional SNPs upstream of the first exon also show weak and sporadic association in subsets of the total European American population. Multivariate logistic regression analysis implicates rs6983130 as a protective factor for SLE susceptibility when anti-dsDNA, anti-chromatin, anti-52 kDa Ro or anti-Sm autoantibody status were used as covariates. Subset analysis of the European American female cases by ACR classification criteria reveals a reduction in the risk of hematologic disorder with rs6983130 compared to cases without hematologic disorders (p=1.5 × 10−3, OR=0.75 (95% C.I.=0.62-0.89)). None of the 90 SNPs tested demonstrate significant association with SLE in the African American or Korean populations. These results support an association of LYN with European-derived individuals with SLE, especially within autoantibody or clinical subsets.
Keywords: systemic lupus erythematosus, association, LYN, SNP
Introduction
Systemic lupus erythematosus [SLE (OMIM 152700)] is a complex autoimmune disease distinguished by a loss of tolerance to self antigens, deposition of immune complexes, and tissue inflammation and destruction. Clinical manifestations variably include arthralgia, arthritis, rashes, alopecia, serositis, leucopenia, thrombocytopenia, and renal involvement1. There is a striking predisposition for females to develop SLE (especially during childbearing years) that crosses ancestral and geographic boundaries, with an overall female:male ratio of ~9:1 2, 3.The prevalence of SLE is approximately 2 to 4-fold higher in non-Caucasian compared to Caucasian populations4-6. These ancestral- and sex-specific differences in SLE prevalence are likely due to a complex interaction of many factors including ancestral-specific differences in genetic susceptibility to SLE, environmental exposures, and hormonal influences. Genetic linkage, association and epidemiologic studies have established a genetic contribution to SLE susceptibility7-9.
Hyperactive B cells are a well established cellular phenotype associated with individuals with SLE. There are likely many different factors that lead to this hyper-responsive B cell phenotype. In the recent genome wide association studies (GWAS) of SLE, two B cell signaling molecules, BLK and BANK110, 11 were found to be associated with SLE. These data suggest that aberrant regulation of B cell signaling may be one mechanism for generating hyper-responsive B cells, which might lead to aberrant B cell development, selection and ultimately influence the production of autoantibodies.
LYN expression is decreased in lupus patients compared to controls12 and functional differences in LYN ubiquitination13 have been associated with SLE risk. In mice, Lyn deficiency is one of the few src family PTK deficiencies that affect BCR signaling14-19. Altered Lyn expression or activity leads to altered B cell function, hyperresponsiveness to BCR stimulation and lupus-like autoimmune disease14, 16, 20-23. These cumulative studies suggest that LYN plays a role in fine-tuning the immune response and alterations of LYN may contribute to autoimmunity.
Recently, a genome-wide association study conducted on European-derived female lupus subjects identified a genetic association between LYN and SLE24. In that study, two SNPs, rs7829816 and rs2667978, showed significant association in some of the cohorts tested, but failed to consistently replicate in all cohorts. However, the overall combined association for these SNPs did reach genome-wide significance (p=5.4 × 10−9, OR=0.77 (95% C.I. 0.70-0.84))24. In the current study, we have extensively fine mapped the entire LYN gene in multiple racial populations to attempt confirmation of this genetic association and to further identify clinical or autoantibody subsets associated with LYN alleles.
Results
Individual Population Analysis
In this study, 90 SNPs from the 5′UTR (3 SNPs) and introns (87 SNPs) of LYN were genotyped. At the time this experiment was performed, no known non-synonymous SNPs in coding regions of the LYN gene were known. In addition, SNPs from LYN which were previously suggested to be associated with SLE24, were genotyped. Genotyping was carried out in three separate, but overlapping collaborative experiments in European-American and European-derived populations. Two additional populations, one African-American and one Korean, were also genotyped for these SNPs (Table 1 and Supplemental Table 1s online). The subjects in the European populations (sets 1-3) partially overlap with those reported in the genome wide association study recently reported24. For set 1 from this paper, 21% of the cases and controls were also in the GWAS phase (set 1 and set 2) of the previous study done by SLEGEN24. The subjects used in set 2 from this paper overlapped with or related to 14% of the subjects in the GWAS and confirmation phase (set 3 and set 4) of the previous study by SLEGEN24. For set 3a from this paper, 77% of the cases and 63% of the control subjects were either identical or related to individuals in the previous study by SLEGEN24. Finally, set 4 from this study overlapped with 67% of the individuals in the previous study24. Only two SNPs, out of the 90 tested here, overlap with previous studies. We also subdivided the European subjects based on gender and country of origin to uncover possible enrichment in one gender or possible influence of population substructure on the association results. To ensure no population substructure effects influenced these results we also used, where appropriate, principle components calculated using the 20,506 SNPs analyzed in the overall collaborative project on the European trimmed population as covariates in our association analysis to correct for any residual substructure.
Table 1.
Demographics of Analysis Datasets
| Cases | Controls | ||||
|---|---|---|---|---|---|
| Population Description | male | female | male | female | |
| Set 1 | European American Female Only | - | 532 | - | 868 |
| Set 2 | European American Female Only (173 cases and 334 controls overlap with set 1) |
- | 507 | - | 549 |
| Set 3A | European American, Female + Male (210 cases and 561 controls overlap with set 1 and 346 cases and 415 controls overlap with set 2 above) |
106 | 1153 | 608 | 1499 |
| Set 3B | European American (set 3A, Female only) | - | 1153 | - | 1499 |
| Set 3C | European American (set 3A, Male only) | 106 | - | 608 | - |
| Set 4 | European-derived, Female + Male (partial overlap with Sets 1,2, 3A above) |
197 | 1763 | 899 | 1908 |
| Set 5 | European-derived, Female (no overlap with any other sets.) |
- | 1400 | - | 1595 |
| Set 6 | African American, Female + Male. | 40 | 542 | 236 | 514 |
| Set 7 | Korean, Female + Male | 40 | 621 | 51 | 730 |
Set 1 represents an initial screening population where 13 SNPs in LYN were tested for association in a female, European-American case-control study. While no SNPs reached significance based on a Bonferroni corrected p-value of 0.004, multiple SNPs clearly trended towards significance (Supplemental Table 2a-i online). SNPs rs10095917 and rs12334430, which bracket the most significant SNP (rs7829816) reported in the published SLE GWAS24 yielded p-values of 0.006 and 0.007 with OR=0.77 (95% CI: 0.64-0.93) and OR=0.76 (95% CI: 0.63-0.93) respectively. Set 2 represents the second experiment which tested 5 SNPs included in Set 1 and 12 additional SNPs covering the promoter, 3′UTR and SNPs flanking those typed in Set 1. This particular population contained only female subjects and was not independent of Set 1. None of the SNPs tested in Set 2, even those previously identified, were significantly associated with SLE. However, this may be because Set 2 had 344 fewer people than set 1 and only included 507 people from Set 1.
In the third collaborative genotyping experiment, 90 SNPs spanning the entire LYN gene were genotyped in European-derived subjects, in addition to large African-American (582 cases and 750 controls) and Korean (661 cases and 781 controls) populations. The analysis of European-American Set 3 was performed on all subjects (set 3a), females only (set 3b), and males only (set 3c). The strongest LYN association in the whole European-American dataset was observed with rs6983130, a SNP located in the 5′ UTR within the first intron near the transcription initiation site (PCA corrected p=0.002, OR=0.79 (95% CI: 0.68-0.92)). When removing the males from the analysis, a female-only analysis strengthened the association with rs6983130 (PCA corrected p=0.00017, OR=0.75 (95% CI: 0.64-0.87)), suggesting that there may be a slight gender influence at this particular SNP. Set 4 contains the EA subjects in set 3A plus 1404 subjects from the UK and Sweden. Results from these separate analyses are shown in Supplemental Table 2a-i. It is interesting to note that the addition of the 1404 European samples did not significantly affect the results observed when analyzing set 3a alone.
Two of the SNPs in this region and one adjacent SNP were typed in an independent European-derived population of 1400 female cases and 1595 female controls (set 5). None of the SNPs typed in this confirmation population showed significance. Similarly, none of the LYN SNPs typed in the African-American (set 6) or Korean populations (set 7) demonstrated association with SLE.
Overall Association Summary
Since our results were obtained from overlapping experiments, both at the sample and typed SNP level, we combined our European population analysis to obtain the maximum independent association for each individual SNP as shown in Supplemental Table 2i online. The number of independent cases and controls used to assess association for each SNP is appropriately indicated. A summary of the maximum independent association for the four European subsets based on gender and country of origin is shown in Table 2. Interestingly, rs6983130 continued to be significant in all European-derived populations. rs6474026, which is 254 bp downstream of rs6983130 (r2=0.966 and D’ =0.993, see Supplemental Figure 1s online), was most strongly associated with the European female subset (p=0.005, OR=0.86 (95% CI: 0.77-0.96)) but also demonstrated association (p<0.02) with the other three European subsets. It is also of interest, that only in the combined analyses containing the 1404 European-derived subjects along with the European-American sets 1-3, does one observe association with the previously reported rs7829816 24, with the peak in the female only European-derived subset (p=0.0028, OR=0.85 (95% CI: 0.76-0.95), Table 2 and Supplemental Tables 2a-i online).
Table 2.
LYN association results from a sample containing the maximum number of independent European-derived Females
| SNP | BP | P | OR (95% CI) | # cases/# ctrls |
|---|---|---|---|---|
| rs6983130 | 56955793 | 0.000111 | 0.81 (0.73 - 0.90) | 2254/2228 |
| rs6474026 | 56956047 | 0.005326 | 0.86 (0.77 - 0.96) | 2174/2025 |
| rs4551325 | 56985745 | 0.005079 | 0.88 (0.80 - 0.96) | 2156/1999 |
| rs1397976 | 56987939 | 0.006796 | 0.88 (0.80 - 0.97) | 2156/1999 |
| rs10095917 | 56992814 | 0.019 | 0.89 (0.80 - 0.98) | 2254/2228 |
| rs6985030 | 57007430 | 0.009595 | 0.85 (0.76 - 0.96) | 2156/1999 |
| rs7829816 | 57011940 | 0.002802 | 0.85 (0.76 - 0.95) | 2174/2025 |
| rs2100245 | 57012654 | 0.004572 | 0.86 (0.77 - 0.95) | 2174/2025 |
| rs16922459 | 57016726 | 0.005438 | 0.85 (0.75 - 0.95) | 2156/1999 |
| rs16922463 | 57024223 | 0.0045 | 0.85 (0.77 - 0.95) | 2156/1999 |
| rs7813271 | 57025705 | 0.005987 | 0.86 (0.78 - 0.96) | 2254/2228 |
| rs2668021 | 57036101 | 0.02379 | 0.90 (0.81 - 0.99) | 2254/2228 |
| rs2719266 | 57037257 | 0.08279 | 0.93 (0.85 - 1.01) | 2174/2025 |
| rs2719265 | 57037831 | 0.09851 | 0.93 (0.85 - 1.01) | 2174/2025 |
| rs907425 | 57038845 | 0.09692 | 0.93 (0.85 - 1.01) | 2156/1999 |
| rs1877301 | 57039144 | 0.07897 | 0.93 (0.85 - 1.01) | 2174/2025 |
| rs868541 | 57039314 | 0.07557 | 0.92 (0.85 - 1.01) | 2156/1999 |
| rs2667985 | 57042912 | 0.1078 | 0.90 (0.79 - 1.02) | 2156/1999 |
| rs1027987 | 57044737 | 0.07922 | 0.93 (0.85 - 1.01) | 2156/1999 |
| rs1027986 | 57045025 | 0.07968 | 0.93 (0.85 - 1.01) | 2156/1999 |
| rs907423 | 57046172 | 0.073 | 0.92 (0.85 - 1.01) | 2156/1999 |
| rs2719232 | 57048738 | 0.07238 | 0.92 (0.85 - 1.01) | 2156/1999 |
| rs16922508 | 57054784 | 0.04299 | 0.90 (0.82 - 1.00) | 2156/1999 |
| rs7828483 | 57055442 | 0.03213 | 0.89 (0.81 - 0.99) | 2156/1999 |
| rs2667978 | 57060505 | 0.01596 | 0.88 (0.79 - 0.98) | 2174/2025 |
| rs4061077 | 57065996 | 0.01504 | 0.89 (0.81 - 0.98) | 2246/2226 |
| rs9650314 | 57066164 | 0.02607 | 0.89 (0.80 - 0.99) | 2156/1999 |
| rs16920192 | 57066580 | 0.03147 | 0.90 (0.81 - 0.99) | 2156/1999 |
| rs1912818 | 57066875 | 0.02036 | 0.89 (0.81 - 0.98) | 2254/2228 |
| rs2719252 | 57067406 | 0.02872 | 0.89 (0.81 - 0.99) | 2156/1999 |
| rs2719253 | 57067720 | 0.03252 | 0.90 (0.81 - 0.99) | 2156/1999 |
| rs2667972 | 57069215 | 0.02367 | 0.88 (0.79 - 0.98) | 2156/1999 |
| rs2719243 | 57069530 | 0.03252 | 0.90 (0.81 - 0.99) | 2156/1999 |
| rs2719242 | 57069786 | 0.02633 | 0.89 (0.81 - 0.99) | 2156/1999 |
| rs4738466 | 57073255 | 0.1214 | 0.90 (0.79 - 1.03) | 2156/1999 |
Abbreviations: SNP: single nucleotide polymorphism, BP (base pairs), P (chi-square p-value), OR (odds ratio), 95% CI (95% confidence interval),.
Significant p-values (p<0.00071 based on Bonferroni correction) are highlighted in bold.
Stratification by ACR criteria
Significant association was also observed when European-American female lupus cases (Set 3B) were further stratified based upon the presence of ACR clinical criteria (Table 3 and Supplemental Table 3a-e online). rs6983130 again produced the strongest association when lupus cases were stratified by hematological disorders. The OR without stratification was 0.79 (95% CI: 0.69-0.91) while the OR=0.75 (95% CI: 0.62-0.89) in female subjects with hematological disorders. Interestingly, several SNPs (rs2667985, rs4738466, and rs7824121) were more strongly associated when lupus cases were stratified based on discoid rash (Table 2).
Table 3.
Stratification of Lupus Female Cases based on ACR Criteria
| No Stratification (1545/1593) |
Discoid Rash (147/1593) | Hematalogical Disorders (666/1593) |
||||
|---|---|---|---|---|---|---|
| SNP | P | OR (95% C.I.) | P | OR (95% C.I.) | P | OR (95% C.I.) |
| rs6983130 | 0.00067 | 0.79 (0.69-0.91) | 0.18 | 0.8 (0.57-1.11) | 0.0015 | 0.75 (0.62-0.89) |
| rs2667985 | 0.073 | 0.87 (0.75-1.01) | 0.0022 | 0.5 (0.32-0.79) | 0.11 | 0.85 (0.7-1.04) |
| rs4738466 | 0.037 | 0.85 (0.74-0.99) | 0.0062 | 0.55 (0.36-0.85) | 0.14 | 0.86 (0.71-1.05) |
| rs7824121 | 0.063 | 0.87 (0.75-1.01) | 0.0084 | 0.57 (0.37-0.87) | 0.18 | 0.88 (0.72-1.06) |
Abbreviation: OR, Odds Ratio; 95% CI, 95% Confidence Interval.
The numbers in parenthesis in the headings represent the (# cases meeting the tested criteria/total # controls).
The p-value and odds ratio improvements are shown in bold.
Autoantibodies as Covariate Factors
A logistic regression analysis evaluating the effect of autoantibody specificities as a covariate upon European-derived female patient (Set 3B) associations for 90 SNPs typed in LYN was performed. Supplementary Table 5s shows the frequencies of the autoantibody positive and negative female cases and controls as assessed by the BioPlex 2200 ANA assay. All autoantibodies were significantly correlated with case status. Table 4 and Supplemental Table 4a-d online summarize the logistic regression analysis of association with autoantibody status as a covariate factor. Of the 10 lupus specific autoantibodies tested, anti-dsDNA, anti-chromatin, anti-Sm and anti-52 kDa Ro each strengthened the association of rs6983130 with SLE when used as covariates in the analyses. While the p-values do not reach significance when correcting for multiple testing, there is clearly a trend towards significance with a clear enhancement of the ORs, especially for anti-chromatin (p=0.0097, OR=0.59 (95% CI: 0.39-0.88)) compared to the analysis without applying the covariate (p=0.096, OR=0.75 (95% CI: 0.53-1.05)). The SLE GWAS identified SNP (rs7829816) was not significantly associated with any of the autoantibodies when used as covariates in the analysis.
Table 4.
Summary results from a logistic regression association analysis in 301 female cases and 298 controls with autoantibodies as covariates
| SNP | No covariate | dsDNA | Chrom | Sm | RNP 68kD | Ro 52kD/SSA52 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | OR (95% C.I.) | P | OR (95% C.I.) | P | OR (95% C.I.) | P | OR (95% C.I.) | P | OR (95% C.I.) | P | OR (95% C.I.) | |
| rs6983130 | 0.096 | 0.75 (0.53-1.05) | 0.042 | 0.68 (0.46-0.99) | 0.0097 | 0.59 (0.39-0.88) | 0.035 | 0.67 (0.47-0.97) | 0.089 | 0.74 (0.52-1.05) | 0.045 | 0.69 (0.47-0.99) |
| rs6474026 | 0.10 | 0.79 (0.59-1.05) | 0.052 | 0.73 (0.53-1) | 0.012 | 0.65 (0.46-0.91) | 0.036 | 0.72 (0.53-0.98) | 0.11 | 0.79 (0.59-1.06) | 0.075 | 0.76 (0.56-1.03) |
| rs2100245 | 0.073 | 0.77 (0.58-1.03) | 0.14 | 0.79 (0.59-1.08) | 0.035 | 0.71 (0.51-0.98) | 0.027 | 0.71 (0.52-0.96) | 0.039 | 0.73 (0.55-0.98) | 0.067 | 0.75 (0.56-1.02) |
| rs16922459 | 0.10 | 0.77 (0.57-1.05) | 0.14 | 0.78 (0.56-1.08) | 0.051 | 0.7 (0.49-1) | 0.039 | 0.7 (0.51-0.98) | 0.10 | 0.77 (0.56-1.05) | 0.07 | 0.74 (0.53-1.03) |
| rs7828483 | 0.068 | 0.74 (0.53-1.02) | 0.076 | 0.73 (0.51-1.03) | 0.047 | 0.69 (0.48-0.99) | 0.029 | 0.68 (0.48-0.96) | 0.043 | 0.71 (0.51-0.99) | 0.027 | 0.68 (0.48-0.96) |
Abbreviation: OR: Odds Ratio; 95% CI: 95% Confidence Interval.
The significant increases with covariate in analysis are shown as bold and italicized.
Discussion
Our study is the largest to date to examine the possible genetic association of LYN with SLE in multiple large populations of different ancestries (European-derived, African American and Korean). While our study does replicate a previously observed association with rs782981624, data from our study suggests that this association is not a dominant lupus effect.
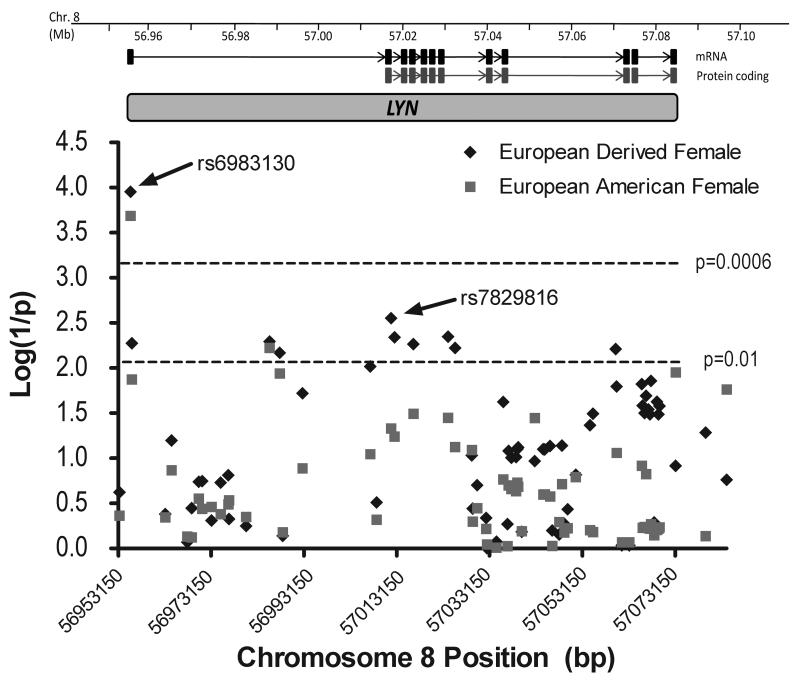
The strongest and most consistent association found in this study was at rs6983130, which is within the first intron at the 5′ end near the primary transcription initiation site (Figure 1). This SNP showed the strongest association in the European-American female population (Set 3B). There did appear to be a strong gender influence with this SNP when analyzing only female subjects. In addition, rs6983130 demonstrated the strongest association in the ACR subset analysis with hematologic manifestations. rs6983130 also showed associations with autoantibodies strongly associated with the development of SLE, specifically anti-dsDNA, anti-chromatin, anti-52 kDa Ro and anti-Sm when one used autoantibody positivity as a covariate in a logistic regression analysis.
Figure 1.
Combined case-control association analysis results. Chromosome 8 genomic organization with LYN mRNA and protein coding exon positions are indicated. The graph depicts the association results, represented as log(1/P-value), for the maximal # of independent cases and controls in the various subsets as indicated. All association data for all populations are presented in Supplementary Table 2i online.
While the associations uncovered in LYN do not meet a strict genome wide association significance level, associations at rs6983130 (p=0.00017, OR=0.75 (95% C.I.=0.64-0.87) in the European-American females (Set3B) do meet a stringent application of the Bonferroni correction for multiple testing and the permutation testing p-value (100,000 permutations) was 0.00018 which demonstrates that the association at this particular SNP is significant. LYN represents a gene with a small effect in the etiology of SLE and may interact with other SLE associated genes.
This study has evaluated polymorphisms in the LYN gene located approximately 2 kb upstream of the transcription initiation site through to the 3′UTR. The linkage disequilibrium observed in this region suggests that very small, tightly associated blocks are found throughout the large first intron (Supplemental Figure 1s). Two additional, much larger blocks span the coding region of the gene. Two of the three polymorphisms in a related src-family tyrosine kinase, BLK, recently described as being strongly associated with SLE from three separate SLE GWAS10, 24, 25, are found far upstream of the BLK proximal promoter. As the genomic structure of LYN is remarkably similar to that of BLK, it is possible that by analogy that untyped SNPs further upstream of the LYN promoter may drive the observed LYN association. Future studies aimed at more extensive SNP genotyping upstream of those typed here are warranted.
Methods
DNA samples
Genomic DNA samples were obtained from 2199 unrelated SLE patients of European-decent and 2740 controls from the Lupus Family Registry and Repository (LFRR) at the Oklahoma Medical Research Foundation (OMRF), the PROFILE Study Group coordinating center at the University of Alabama Birmingham and the five collaborating PROFILE centers at the University of Alabama Birmingham, Johns Hopkins University, Northwestern University, University of Texas Health Center Houston, and the University of Puerto Rico Medical Center Campus, as well as other individual collaborators at the OMRF, the Medical University of South Carolina, Feinstein Institute for Medical Research in New York, the United Kingdom and Sweden (Table 1 and Supplemental Table 1s online).
All SLE patients met at least 4 of the 11 revised SLE classification criteria of the American College of Rheumatology (ACR)26, 27. DNA was isolated from biological specimens provided from each participant after obtaining informed consent as approved by the recruiting site Institutional Review Boards or ethical committees.
Genotyping
We genotyped 90 SNPs spanning the LYN gene including known potentially functional SNPs and SNPs from predicted haplotype blocks in multiple ancestries. Genotyping was carried out in three separate multi-investigator collaborative experiments. The first experiment corresponding to Set 1 (Table 1) included 1538 user-selected SNPs genotyped on a custom Illumina BeadStation array using Golden Gate assay technology. In this first study, only 14 SNPs were typed in LYN. The second collaborative experiment (Set 2, Table 1) consisted of two separate Illumina BeadXpress custom genotyping arrays where 96 and 384 user-selected SNPs were genotyped. In the second study, only 18 SNPs were typed in LYN. The third experiment was a large, multiuser experiment where 20,506 individual investigator SNPs were genotyped in a large study containing European-derived (2165 European-derived SLE patients and 2902 healthy controls), African-American (582 SLE patients and 750 healthy controls) and Korean (661 SLE patients and 781 healthy controls) populations. The Illumina BeadStation using iSelect assay technology was used to obtain genotypes from these populations. In this third study, only 73 SNPs were typed in the LYN gene. Data from Sets 3A-C, 4, 6 and 7 (Table 1) correspond to the genotyping obtained from this third custom genotyping experiment. Finally, data for Set 5 (Table 1) corresponds to Illumina BeadXpress genotyping of a European-derived replication population in Dr. Alarcón-Riquelme’s laboratory in Sweden. The demographics of this population are outlined in Table 1 and Supplemental Table 1s data online.
Quality control of genotyping
Genotype data were only used from samples with a call rate greater than 90% of the SNPs screened (98.05% of the samples). The average call rate for all samples was 97.18%. Only genotype data from SNPs with a call frequency greater than 90% in the samples tested and an Illumina GeneTrain score greater than 0.7 (96.74% of all SNPs screened) were used for analysis.
Single SNP analysis
Case-control associations and Hardy-Weinberg Proportions were calculated using PLINK28. Only SNPs with minor allele frequencies (MAF) >0.01 and Hardy Weinberg Proportions in the controls p>0.001 were used for the analysis. The allelic frequencies were calculated for each SNP and case-control associations were analyzed by standard Pearson’s Chi-square test. P values were considered statistically significant only if they met the experiment specific significance level to account for multiple testing using the Bonferroni method. For data in Set 1 this cutoff was be p<0.004, in Set 2 the cutoff was p<0.003 and in Sets 3A-C, 4, 6, and 7 the cutoff was p<0.0006. Odds ratios (OR) and 95% confidence intervals (CIs) were calculated for each SNP using logistic regression. In addition, for the data in Sets 3 and 4, principle component values derived as outlined below were used as covariates in the association analysis to correct for possible residual European population substructure.
Population Stratification Analysis
The European-derived samples used in Sets 3 and 4 were part of a collaborative study where population substructure parameters were defined using a principle component analysis (PCA) performed on 20,506 SNPs24, 29. Four principal components were initially identified that explained a total of ~60% of the observed genetic variation and allowed identification of outliers from the European cluster. Before outlier removal, the estimated inflation factor (λ) was 1.84. After removal of outliers, the inflation factor was 1.12 indicating that these cleaned data should have a very small population substructure effect on our results. In addition, after trimming of outliers, another round of PCA was performed and five newly calculated PCA values were used as covariates in the the subsequent association analysis to correct for any residual European population substructure effects. No additional outliers were identified using the new PCA values, which produced a final inflation factor of 1.15.
Logistic Regression Analysis using BioPlex2200 Normalized Intensity as Covariates
The BioPlex 2200 (Bio-Rad, Hercules, CA) is a high throughput automated serological analysis unit that utilizes multiplex bead technology for antibody detection. The BioPlex results are reported on a scale from 0-8. This scale is set relative to calibrator positive and negative control samples provided by the manufacturer. The defined positive cut-off value for each assay is then set to 1.0, with Factor XIII index greater than 0.2 as serum validation control. However, dsDNA is reported in IU/mL with a positive cut-off of 10.0 IU/mL. Ten of 13 autoantibodies commonly associated with lupus (dsDNA, chromatin, ribosomal P, 60kD Ro (SS-A 60), 52kD Ro (SS-A 52), La (SS-B), Sm, Sm/RNP complex, nRNP A, and nRNP 68) were evaluated using BioPlex 2200 in the stored serum from 301 patients and 298 controls of the independent European female population. Autoantibody levels above the threshold were considered positive and denoted as 1, whereas the negative samples were denoted as 0 in the dichotomous covariate data set. Each autoantibody was entered individually into the logistic regression model as a covariate. The p-value and odds ratios with 95% confidence interval of the logistic model were calculated using PLINK28.
Association with LYN in Lupus Specific Subsets Defined by ACR Criteria
To assess the potential role of LYN in SLE development and disease etiology, cases were stratified based on the presence of the 11 ACR clinical criteria and associations were analyzed comparing the stratified lupus patients to all 1594 unrelated European-derived controls using PLINK28. The ACR clinical criteria information was obtained from the Lupus Family Registry Repository (LFRR) and individual investigators.
Supplementary Material
Acknowledgements
The authors thank the participants, both patients and controls, who graciously agreed to take part in these studies by donating samples to the various collections, including the Lupus Family Registry and Repository (LFRR: http://lupus.omrf.org), PROFILE, BIOLUPUS, and many other individual or multicenter collaborator initiated collections. We also thank the recruitment and technical teams at each of the sample procurement sites for their important contributions. We thank the Wake Forest University Health Sciences Center for Public Health Genomics for support of the data analysis efforts of our Wake Forest University collaborators. Finally, we thank the various funding sources as outlined on the title page for their continued support for the collection of samples and the conduct of this research.
Members of BIOLUPUS who have provided samples to this study are: Peter Junker, Ann Voss and Helle Laustrup (Odense, Denmark), Bernard Lawerys and Fredric Houssieau (Louvain, Belgium), Carlos Vasconcelos and Berta Martins Da Silva (Porto, Portugal), Carmen Gutierrez and Ana Suárez (Oviedo, Spain), Torsten Witte (Hannover, Germany), Sandra D’Alfonso, Sergio Migliaresi, Mauro Galeazzi and Gian Domenico Sebastiani (Novara, Naples, Siena and Rome, Italy), Bernardo Pons-Estel and the members of GENLES (Rosario, Argentina), and Emoke Endreffy (Szeged, Hungary). Peter K Gregersen from the Feinstein Institute of Medical Research and Jorge R Oksenberg from the University of California at San Francisco graciously provided controls used in this study. Members of PROFILE who have provided samples to this study are Graciela S Alarcón, Elizabeth E Brown, Robert P Kimberly, Jeffery C Edberg and Gerald McGwin, Jr. (Univ. Alabama Birmingham, Birmingham, AL, USA), Rosalind Ramsey-Goldman (Northwestern University Feinberg School of Medicine, Chicago, IL, USA), John D Reveille (Univ. Texas Health Sci. Ctr., Houston, TX, USA), Luis M Vilá (University of Puerto Rico Medical Sciences Campus, San Juan, PR) and Michelle A Petri (Johns Hopkins Hospital, Baltimore, MD, USA).
Support: This project was funded by National Institutes of Health RR020143 (JMG and JBH), RR015577 (JMG, JBH, JAJ), NIAID-DAIT-BAA-05-11 (JMG and JAJ), AI031584 (JBH, JMG, JAJ), AR053483 (JMG, SKN and JAJ), AR48940 (JBH, JAJ), AI063622 (SKN), Kirkland Scholar awards (JBH and JAJ), AR049084 (SKN, JBH, RPK, RRG, JDR, MAP, LMV, GSA, JCE, GMcG Jr.), AR42460 (JBH), AR12253 (JBH), AR62277 (JBH), AI24717 (JBH), AI063274 (PMG), AR052125 (PMG), AR043247 (KLM), DEO15223 (JBH), Alliance for Lupus Research (JBH), the US Department of Veterans Affairs (JBH), Swedish Research Council (MEAR), the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea. (A010252, A080588) (SCB), the Torsten & Ragnar Söderbergs Foundation (MEAR), the Swedish Foundation Against Rheumatism (MEAR), the Gustaf Vth-80th-Year Foundation (MEAR), Plan Nacional de I+D, Spain (SAF06-00398) (JM), the Junta de Andalucía, grupo CTS-180 (JM) and OHRS award # HR08-037 from the Oklahoma Center for the Advancement of Science & Technology (JMG). Dr. Harley has received consulting fees, speaking fees, and/or director’s fees from Bio-Rad Laboratories, Merck, UCB Inc., ImmunoVision Inc., IVAX Diagnostics and JK Autoimmunity and owns stock or stock options in IVAX Diagnostics.
Footnotes
Supplementary information is available at the Genes & Immunity’s website.
References
- [1].Lahita RG. Systemic lupus erythematosus. 4th ed Academic Press; New York: 2004. [Google Scholar]
- [2].Fessel WJ. Systemic lupus erythematosus in the community. Incidence, prevalence, outcome, and first symptoms; the high prevalence in black women. Arch Intern Med. 1974 Dec;134(6):1027–35. [PubMed] [Google Scholar]
- [3].Siegel M, Lee S. The epidemiology of systemic lupus erythematosus. Semin Arthritis Rheum. 1973;3:1–54. doi: 10.1016/0049-0172(73)90034-6. [DOI] [PubMed] [Google Scholar]
- [4].Hart HH, Grigor RR, Caughey DE. Ethnic difference in the prevalence of systemic lupus erythematosus. Ann Rhuem Dis. 1983;42:529–32. doi: 10.1136/ard.42.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Michet CJ, Mc Kenna CH, Elveback CR, Kaslow RA, Kurland LT. Epidemiology of systemic lupus erythematosus and other connective tissue diseases in Rochester, Minnesota 1950 through 1979. Mayo Clin Proc. 1985;60:105–13. doi: 10.1016/s0025-6196(12)60294-8. [DOI] [PubMed] [Google Scholar]
- [6].Serdula MK, Rhoads GG. Frequency of systemic lupus erythematosus in different ethnic groups in Hawaii. Arthritis Rheum. 1979;22(4):328–33. [Google Scholar]
- [7].Harley JB, Kelly JA, Kaufman KM. Unraveling the genetics of systemic lupus erythematosus. Springer Semin Immunopathol. 2006 Oct;28(2):119–30. doi: 10.1007/s00281-006-0040-5. [DOI] [PubMed] [Google Scholar]
- [8].Sestak AL, Nath SK, Harley JB. Genetics of systemic lupus erythematosus: how far have we come? Rheum Dis Clin North Am. 2005 May;31(2):223–44. v. doi: 10.1016/j.rdc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- [9].Nath SK, Kilpatrick J, Harley JB. Genetics of human systemic lupus erythematosus: the emerging picture. Curr Opin Immunol. 2004 Dec;16(6):794–800. doi: 10.1016/j.coi.2004.09.007. [DOI] [PubMed] [Google Scholar]
- [10].Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, et al. Association of Systemic Lupus Erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med. 2008 Jan 20; doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- [11].Kozyrev SV, Abelson AK, Wojcik J, Zaghlool A, Reddy MV Linga, Sanchez E, et al. Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nat Genet. 2008 Feb;40(2):211–6. doi: 10.1038/ng.79. [DOI] [PubMed] [Google Scholar]
- [12].Liossis SN, Solomou EE, Dimopoulos MA, Panayiotidis P, Mavrikakis MM, Sfikakis PP. B-cell kinase lyn deficiency in patients with systemic lupus erythematosus. J Investig Med. 2001 Mar;49(2):157–65. doi: 10.2310/6650.2001.34042. [DOI] [PubMed] [Google Scholar]
- [13].Flores-Borja F, Kabouridis PS, Jury EC, Isenberg DA, Mageed RA. Decreased Lyn expression and translocation to lipid raft signaling domains in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2005 Dec;52(12):3955–65. doi: 10.1002/art.21416. [DOI] [PubMed] [Google Scholar]
- [14].Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995 Oct 20;83(2):301–11. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- [15].Chan VW, Lowell CA, DeFranco AL. Defective negative regulation of antigen receptor signaling in Lyn-deficient B lymphocytes. Curr Biol. 1998 May 7;8(10):545–53. doi: 10.1016/s0960-9822(98)70223-4. [DOI] [PubMed] [Google Scholar]
- [16].Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, et al. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995 Nov;3(5):549–60. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- [17].DeFranco AL, Chan VW, Lowell CA. Positive and negative roles of the tyrosine kinase Lyn in B cell function. Semin Immunol. 1998 Aug;10(4):299–307. doi: 10.1006/smim.1998.0122. [DOI] [PubMed] [Google Scholar]
- [18].Sillman AL, Monroe JG. Surface IgM-stimulated proliferation, inositol phospholipid hydrolysis, Ca2+ flux, and tyrosine phosphorylation are not altered in B cells from p59fyn-1-mice. J Leukoc Biol. 1994 Dec;56(6):812–6. doi: 10.1002/jlb.56.6.812. [DOI] [PubMed] [Google Scholar]
- [19].Texido G, Su IH, Mecklenbrauker I, Saijo K, Malek SN, Desiderio S, et al. The B-cell-specific Src-family kinase Blk is dispensable for B-cell development and activation. Mol Cell Biol. 2000 Feb;20(4):1227–33. doi: 10.1128/mcb.20.4.1227-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang J, Koizumi T, Watanabe T. Altered antigen receptor signaling and impaired Fas-mediated apoptosis of B cells in Lyn-deficient mice. J Exp Med. 1996 Sep 1;184(3):831–8. doi: 10.1084/jem.184.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, et al. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998 Apr;8(4):497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- [22].Hibbs ML, Harder KW, Armes J, Kountouri N, Quilici C, Casagranda F, et al. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J Exp Med. 2002 Dec 16;196(12):1593–604. doi: 10.1084/jem.20020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dorner T, Lipsky PE. Signalling pathways in B cells: implications for autoimmunity. Curr Top Microbiol Immunol. 2006;305:213–40. doi: 10.1007/3-540-29714-6_11. [DOI] [PubMed] [Google Scholar]
- [24].Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008 Feb;40(2):204–10. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Graham RR, Cotsapas C, Davies L, Hackett R, Lessard CJ, Leon JM, et al. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nat Genet. 2008 Aug 1; doi: 10.1038/ng.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- [27].Hochberg MC, Wallace Dj HBH. The epidemiology of systemic lupus erythematosus. In: Anonymous, editor. Dubois,Äô Lupus Erythematosus. Williams and Wilkins; Baltimore: 1997. pp. 49–65. [Google Scholar]
- [28].Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007 Sep;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006 Aug;38(8):904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.