Abstract
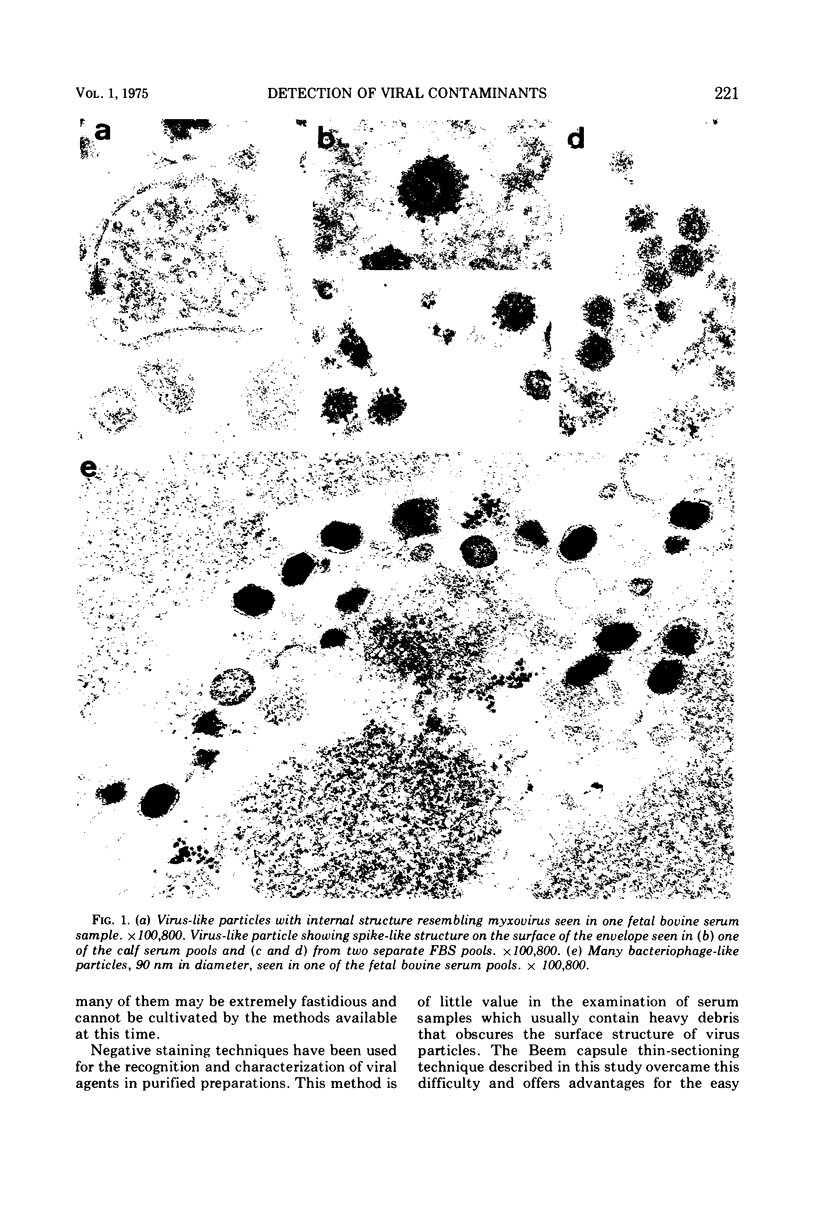
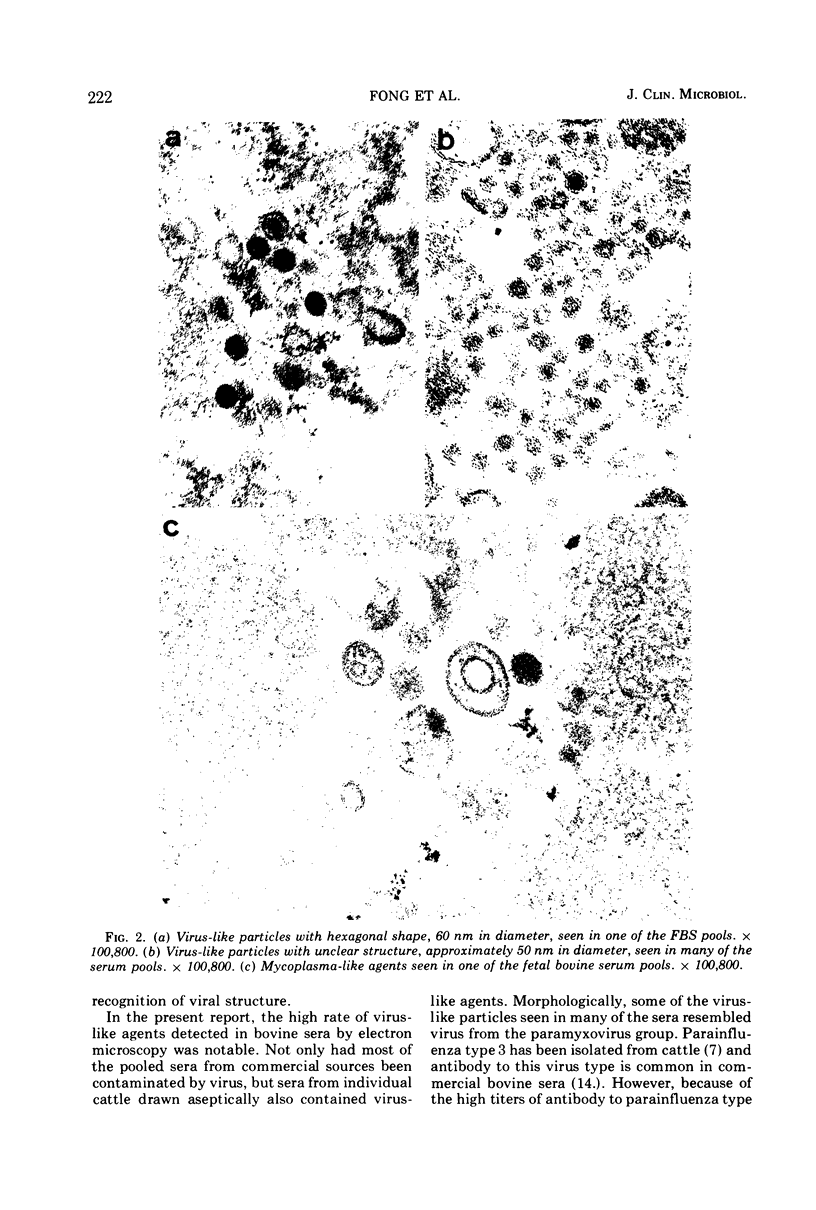
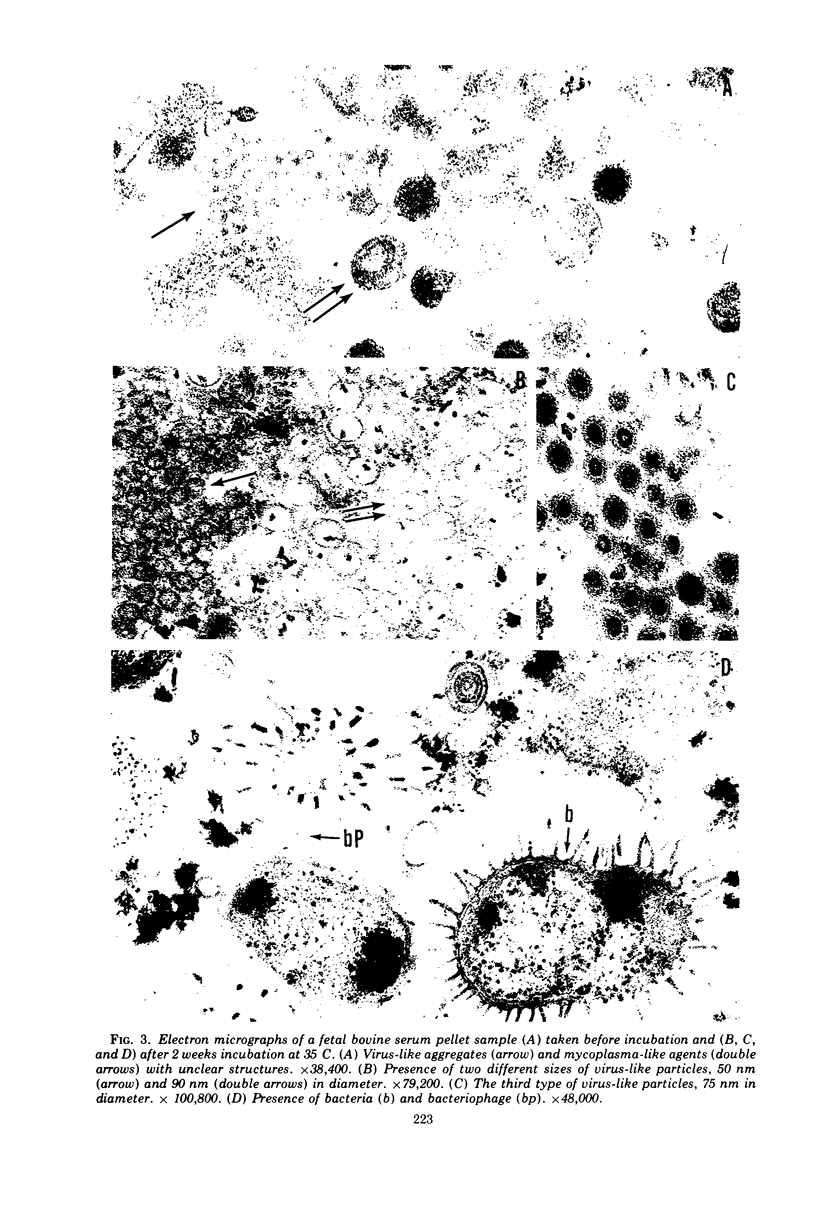
A total of 25 lots of bovine serum samples were pelleted in Beem capsules for thin sectioning and were examined by electron microscopy. These included 17 lots of fetal bovine serum pools and five lots of calf serum pools obtained from commercial sources, and three lots of adult bovine serum from local dairy farms. Virus-like particles, 50 to 300 nm in diameter, were detected in 17 of 25 (68%) of the sera. Five of 25 serum samples showed the presence of mycoplasma-like agents. Incubation of bovine serum at 35 C for 1 or 2 weeks appeared to destroy some of these agents, but in certain instances it enhanced bacteria and bacteriophage contaminants. The advantages of electron microscopy using the thin-sectioning technique for detection of microbial contamination in bovine sera are illustrated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barile M. F., Kern J. Isolation of Mycoplasma arginini from commercial bovine sera and its implication in contaminated cell cultures. Proc Soc Exp Biol Med. 1971 Nov;138(2):432–437. doi: 10.3181/00379727-138-35913. [DOI] [PubMed] [Google Scholar]
- Boone C. W., Mantel N., Caruso T. D., Jr, Kazam E., Stevenson R. E. Quality control studies on fetal bovine serum used in tissue culture. In Vitro. 1971 Nov-Dec;7(3):174–189. doi: 10.1007/BF02617963. [DOI] [PubMed] [Google Scholar]
- Chu F. C., Johnson J. B., Orr H. C., Probst P. G., Petricciani J. C. Bacterial virus contamination of fetal bovine sera. In Vitro. 1973 Jul-Aug;9(1):31–34. doi: 10.1007/BF02615986. [DOI] [PubMed] [Google Scholar]
- Fogh J., Holmgren N. B., Ludovici P. P. A review of cell culture contaminations. In Vitro. 1971 Jul-Aug;7(1):26–41. doi: 10.1007/BF02619002. [DOI] [PubMed] [Google Scholar]
- Fong C. K., Hsiung G. D. Development of an equine herpesvirus in two cell culture systems: light and electron microscopy. Infect Immun. 1972 Nov;6(5):865–876. doi: 10.1128/iai.6.5.865-876.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff A. J., Rimer V., Gaeta L. Gamma-globulin foetal bovine sera: significance in virology. Nature. 1967 May 20;214(5090):805–806. doi: 10.1038/214805b0. [DOI] [PubMed] [Google Scholar]
- Kniazeff A. J. Viruses infecting cattle and their role as endogenous contaminants of cell cultures. Natl Cancer Inst Monogr. 1968 Dec;29:123–132. [PubMed] [Google Scholar]
- Merril C. R., Friedman T. B., Attallah A. F., Geier M. R., Krell K., Yarkin R. Isolation of bacteriophages from commercial sera. In Vitro. 1972 Sep-Oct;8(2):91–93. doi: 10.1007/BF02615965. [DOI] [PubMed] [Google Scholar]
- Molander C. W., Kniazeff A. J., Boone C. W., Paley A., Imagawa D. T. Isolation and characterization of viruses from fetal calf serum. In Vitro. 1971 Nov-Dec;7(3):168–173. doi: 10.1007/BF02617962. [DOI] [PubMed] [Google Scholar]
- Petricciani J. C., Chu F. C., Johnson J. B., Meyer H. M., Jr Bacteriophages in live virus vaccines. Proc Soc Exp Biol Med. 1973 Dec;144(3):789–792. doi: 10.3181/00379727-144-37683. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Gehle W. D. Pelleting virus-infected cells for thin-section electron microscopy. Proc Soc Exp Biol Med. 1969 Apr;130(4):1117–1119. doi: 10.3181/00379727-130-33731. [DOI] [PubMed] [Google Scholar]
- Swack N. S., Fong C. K., Hsiung G. D., Gross P. A. Methods for the detection of viruses in bovine serum. J Clin Microbiol. 1975 Feb;1(2):212–218. doi: 10.1128/jcm.1.2.212-218.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]