Abstract
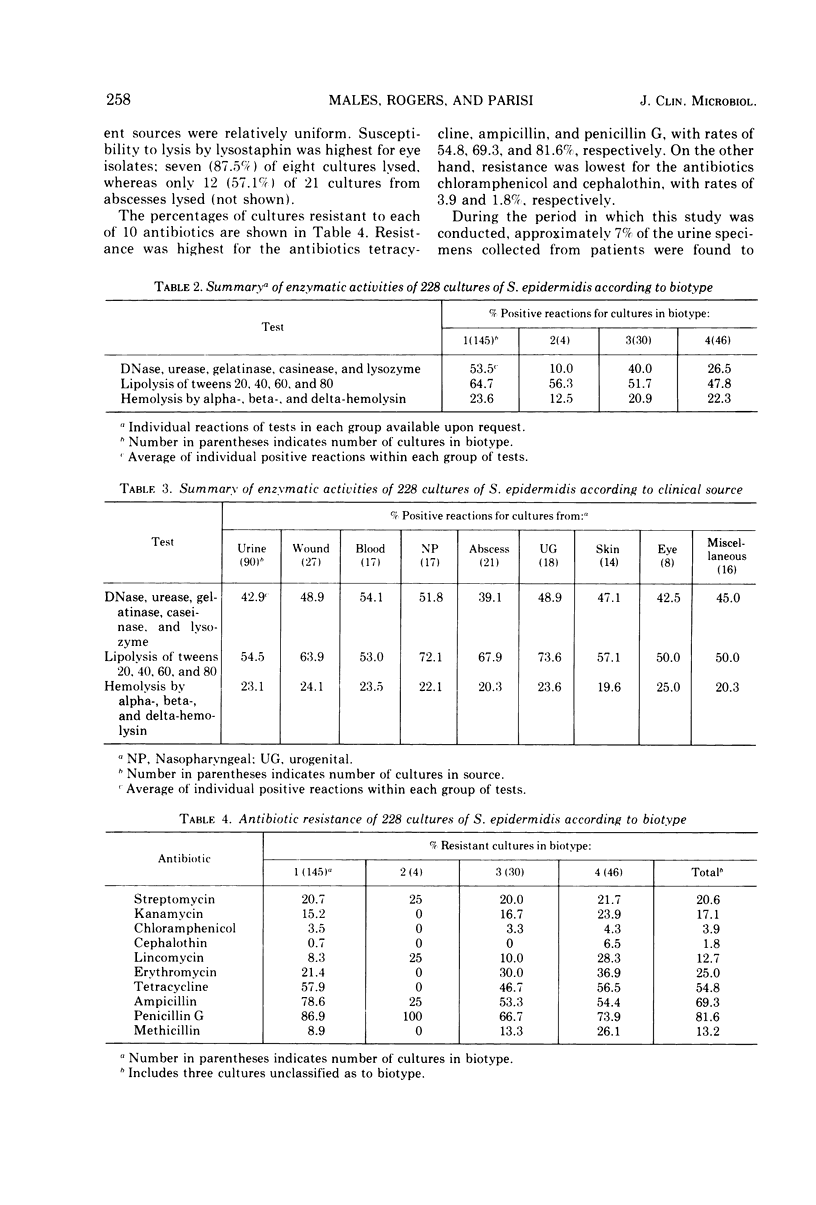
The biotyping scheme of Baird-Parker was applied to cultures of Staphylococcus epidermidis from patients. In all, 63.6% of 228 cultures belonged to biotype 1, followed by biotypes 4, 3, and 2 in decreasing order of incidence. When classified according to clinical source of isolation, cultures of S. epidermidis were most frequently isolated from urine, with 39.5% of 228 cultures from this source. Each of the four biotypes was distributed throughout all nine catagories of clinical sources. The production of virulence factors was based on the results of three groups of tests: (i) deoxyribonuclease, urease, gelatinase, caseinase, and lysozyme production; (ii) lipolytic activity on the tweens; and (iii) hemolysin production. Enzymatic activity was highest for organisms in biotypes 1, followed by biotypes 3, 4, and 2 in decreasing order. Of the 228 cultures, 76.3% were lysed by lysostaphin. Resistance to antibiotics was highest for tetracycline, ampicillin, and penicillin, with rates of 54.8, 69.3, and 81.6%, respectively. The role of S. epidermidis as an etiological agent was studied by analyzing the laboratory and clinical data of 80 patients selected at random with bacteriuric S. epidermidis. Organisms in biotype 1 were most commonly associated with urinary tract infection. The significance of certain biotypes of S. epidermidis as opportunistic pathogens among compromised hosts in a hospital environment is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alder V. G., Gillespie W. A., Mitchell R. G., Rosendal K. The lipolytic activity of Micrococcaceae from human and animal sources. J Med Microbiol. 1973 May;6(2):147–154. doi: 10.1099/00222615-6-2-147. [DOI] [PubMed] [Google Scholar]
- BAIRD-PARKER A. C. A classification of micrococci and staphylococci based on physiological and biochemical tests. J Gen Microbiol. 1963 Mar;30:409–427. doi: 10.1099/00221287-30-3-409. [DOI] [PubMed] [Google Scholar]
- Bailey R. R. Significance of coagulase-negative Staphylococcus in urine. J Infect Dis. 1973 Feb;127(2):179–182. doi: 10.1093/infdis/127.2.179. [DOI] [PubMed] [Google Scholar]
- Baird-Parker A. C. The basis for the present classification of staphylococci and micrococci. Ann N Y Acad Sci. 1974 Jul 31;236(0):7–14. doi: 10.1111/j.1749-6632.1974.tb41478.x. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Holt R. J. Lysozyme production by staphylococci and micrococci. J Med Microbiol. 1971 Aug;4(3):375–379. doi: 10.1099/00222615-4-3-375. [DOI] [PubMed] [Google Scholar]
- Kleck J. L., Donahue J. A. Production of thermostable hemolysin by cultures of Staphylococcus epidermidis. J Infect Dis. 1968 Jun;118(3):317–323. doi: 10.1093/infdis/118.3.317. [DOI] [PubMed] [Google Scholar]
- Lachica R. V., Hoeprich P. D., Genigeorgis C. Nuclease production and lysostaphin susceptibility of Staphylococcus aureus and other catalase-positive cocci. Appl Microbiol. 1971 May;21(5):823–826. doi: 10.1128/am.21.5.823-826.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsik F. J., Parisi J. T. Significance of Staphylococcus epidermidis in the clinical laboratory. Appl Microbiol. 1973 Jan;25(1):11–14. doi: 10.1128/am.25.1.11-14.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew B. H., Rosenblum E. D. Transduction of tetracycline resistance in Staphylococcus epidermidis. Antimicrob Agents Chemother. 1972 Jun;1(6):508–511. doi: 10.1128/aac.1.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. G. Classification of Staphylococcus albus strains isolated from the urinary tract. J Clin Pathol. 1968 Jan;21(1):93–96. doi: 10.1136/jcp.21.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi J. T., Talbot H. W., Jr Improved rapid plate method for the isolation of bacteriophages from lysogenic bacteria. Appl Microbiol. 1974 Sep;28(3):503–504. doi: 10.1128/am.28.3.503-504.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulverer G., Damen G., Neugebauer M. Antibiotic resistance of Staphylococcus albus. Med Microbiol Immunol. 1972;158(1):32–43. doi: 10.1007/BF02122006. [DOI] [PubMed] [Google Scholar]
- Reisner R. M., Puhvel M. Lipolytic activity of Staphylococcus albus. J Invest Dermatol. 1969 Jul;53(1):1–7. [PubMed] [Google Scholar]
- Shalita A. R., Rosenthal S. A. Tetracycline-resistant staphylococci in acne vulgaris. Acta Derm Venereol. 1972;52(1):64–66. [PubMed] [Google Scholar]
- Speller D. C., Mitchell R. G. Coagulase-negative staphylococci causing endocarditis after cardiac surgery. J Clin Pathol. 1973 Jul;26(7):517–522. doi: 10.1136/jcp.26.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart C. A., Van Stratum E., Rustigian R. Further Studies on Urease Production by Proteus and Related Organisms. J Bacteriol. 1945 May;49(5):437–444. doi: 10.1128/jb.49.5.437-444.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Van Boven C. P., Winkler K. C. Phage-typing of coagulase-negative staphylococci. J Med Microbiol. 1972 Feb;5(1):9–19. doi: 10.1099/00222615-5-1-9. [DOI] [PubMed] [Google Scholar]


