Abstract
Central sensitization represents an enhancement in the function of neurons and circuits in nociceptive pathways caused by increases in membrane excitability and synaptic efficacy as well as to reduced inhibition and is a manifestation of the remarkable plasticity of the somatosensory nervous system in response to activity, inflammation, and neural injury. The net effect of central sensitization is to recruit previously subthreshold synaptic inputs to nociceptive neurons, generating an increased or augmented action potential output: a state of facilitation, potentiation, augmentation, or amplification. Central sensitization is responsible for many of the temporal, spatial, and threshold changes in pain sensibility in acute and chronic clinical pain settings and exemplifies the fundamental contribution of the central nervous system to the generation of pain hypersensitivity. Because central sensitization results from changes in the properties of neurons in the central nervous system, the pain is no longer coupled, as acute nociceptive pain is, to the presence, intensity, or duration of noxious peripheral stimuli. Instead, central sensitization produces pain hypersensitivity by changing the sensory response elicited by normal inputs, including those that usually evoke innocuous sensations.
Perspective
In this article, we review the major triggers that initiate and maintain central sensitization in healthy individuals in response to nociceptor input and in patients with inflammatory and neuropathic pain, emphasizing the fundamental contribution and multiple mechanisms of synaptic plasticity caused by changes in the density, nature, and properties of ionotropic and metabotropic glutamate receptors.
Keywords: Central sensitization, inflammatory pain, neuropathic pain, scaffolding protein, heterosynaptic facilitation
Acute nociceptive pain is that physiological sensation of hurt that results from the activation of nociceptive pathways by peripheral stimuli of sufficient intensity to lead to or to threaten tissue damage (noxious stimuli).374 Nociception, the detection of noxious stimuli,282 is a protective process that helps prevent injury by generating both a reflex withdrawal from the stimulus and as a sensation so unpleasant that it results in complex behavioral strategies to avoid further contact with such stimuli. An additional important phenomenon that further enhances this protective function is the sensitization of the nociceptive system that occurs after repeated or particularly intense noxious stimuli, so that the threshold for its activation falls and responses to subsequent inputs are amplified.132,376,380 In the absence of ongoing tissue injury, this state of heightened sensitivity returns over time to the normal baseline, where high-intensity stimuli are again required to initiate nociceptive pain; the phenomenon is long lasting but not permanent. The nociceptor-induced sensitization of the somatosensory system is adaptive in that it makes the system hyperalert in conditions in which a risk of further damage is high, for example, immediately after exposure to an intense or damaging stimulus. This sensitization is the expression of use-dependent synaptic plasticity triggered in the central nervous system (CNS) by the nociceptor input and was the first example of central sensitization, discovered 26 years ago.369 Since then, we have learned that a number of different forms of functional, chemical, and structural plasticity can sensitize the central nociceptive system to produce pain hypersensitivity under both normal and pathological circumstances, some of which are persistent.
In many clinical syndromes, pain is no longer protective. The pain in these situations arises spontaneously, can be elicited by normally innocuous stimuli (allodynia), is exaggerated and prolonged in response to noxious stimuli (hyperalgesia), and spreads beyond the site of injury (secondary hyperalgesia). Central sensitization has provided a mechanistic explanation for many of the temporal, spatial, and threshold changes in pain sensibility in acute and chronic clinical pain settings and has highlighted the fundamental contribution of changes in the CNS to the generation of abnormal pain sensitivity. Although phenomenologically central sensitization may appear to be comparable to peripheral sensitization, it differs substantially, both in terms of the molecular mechanisms responsible and its manifestation. Peripheral sensitization represents a reduction in threshold and an amplification in the responsiveness of nociceptors that occurs when the peripheral terminals of these high-threshold primary sensory neurons are exposed to inflammatory mediators and damaged tissue46,105,124,242 and, in consequence, is restricted to the site of tissue injury.124 Although peripheral sensitization certainly contributes to the sensitization of the nociceptive system and thereby to inflammatory pain hypersensitivity at inflamed sites (primary hyperalgesia), it nevertheless represents a form of pain elicited by activation of nociceptors, albeit one with a lower threshold due to the increased peripheral transduction sensitivity, and generally requires ongoing peripheral pathology for its maintenance. Peripheral sensitization appears to play a major role in altered heat but not mechanical sensitivity, which is a major feature of central sensitization.
Central sensitization, in contrast to peripheral sensitization, co-opts novel inputs to nociceptive pathways including those that do not normally drive them, such as large low-threshold mechanoreceptor myelinated fibers to produce Aβ fiber–mediated pain.376 It also produces pain hypersensitivity in noninflamed tissue by changing the sensory response elicited by normal inputs and increases pain sensitivity long after the initiating cause may have disappeared and when no peripheral pathology may be present. Because central sensitization results from changes in the properties of neurons in the CNS, the pain is no longer coupled, as acute nociceptive pain is, to the presence, intensity, or duration of particular peripheral stimuli. Instead, central sensitization represents an abnormal state of responsiveness or increased gain of the nociceptive system. The pain is effectively generated as a consequence of changes within the CNS that then alter how it responds to sensory inputs, rather than reflecting the presence of peripheral noxious stimuli. In this respect, central sensitization represents a major functional shift in the somatosensory system from high-threshold nociception to low-threshold pain hypersensitivity. We all experience pain as arising from “out there,” and, in consequence, imagine that it is actually triggered by noxious stimuli where we feel the pain. Central sensitization reveals, however, that this in many cases is a sensory illusion; specific alterations in the CNS can result in painful sensations occurring in the absence of either peripheral pathology or noxious stimuli, and the target for treatment in these situations must be the CNS not the periphery.
Central sensitization corresponds to an enhancement in the functional status of neurons and circuits in nociceptive pathways throughout the neuraxis caused by increases in membrane excitability, synaptic efficacy, or a reduced inhibition. The net effect is that previously subthreshold synaptic inputs are recruited to generate an increased or augmented action potential output, a state of facilitation, potentiation, or amplification. The reason that these cellular changes alter the system so profoundly is that normally only a small fraction of the synaptic inputs to dorsal horn neurons contribute to their action potential output.373 Nociceptive-specific neurons, for example, although dominated by large monosynaptic and polysynaptic synaptic potentials from nociceptors in their receptive field, typically also have small-amplitude synaptic inputs from low-threshold afferents and from nociceptor inputs outside their receptive fields, which constitute a subliminal fringe that normally does not drive the output of the cells (Fig 1). Recruiting these subthreshold inputs to the output of a neuron markedly alters its receptive field properties, with profound changes in receptive field threshold, spatial, and temporal properties (Fig 2). This provides an opportunity for rapid functional plasticity that can be revealed experimentally by increasing the excitability of the neuron or by blocking inhibitory transmitters. After administration of GABA or glycine receptor antagonists, for example, Aβ inputs are recruited to neurons in the superficial dorsal horn,17 and pain-like behavior can be elicited by movement of just a few hairs.289 The receptive field of somatosensory neurons are, therefore, not fixed or hard wired, but are instead highly malleable. This malleability or plasticity is the substrate for the functional effects of central sensitization, and the means is a change in synaptic efficacy.
Figure 1.
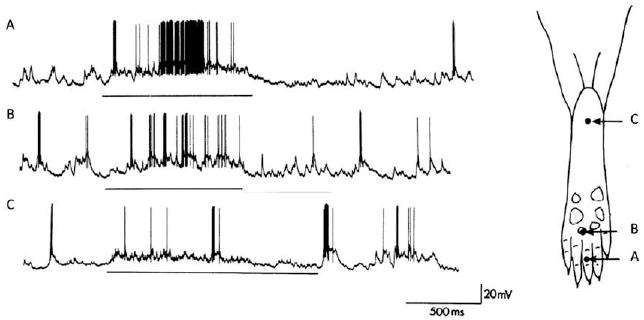
Subthreshold synaptic inputs. The substrate for receptive field plasticity. Intracellular in vivo recordings from a nociceptive-specific rat dorsal horn neuron revealing subthreshold synaptic inputs. The output of somatosensory neurons is determined by those peripheral sensory inputs that produce sufficiently large-amplitude monosynaptic and polysynaptic potentials to evoke an action potential discharge (A and B). This constitutes the receptive field or firing zone of the neuron. However, stimuli outside the receptive field can evoke synaptic inputs that are too small normally to produce action potential outputs (C), and this constitutes a subliminal fringe or low-probability firing fringe, which can be recruited if synaptic efficacy is increased, to expand and change the receptive field. In this particular neuron, a standard pinch stimulus applied to points A, B, and C evoked only action potentials at points A and B but clear subthreshold synaptic inputs at C. Modified from Reference 365.
Figure 2.
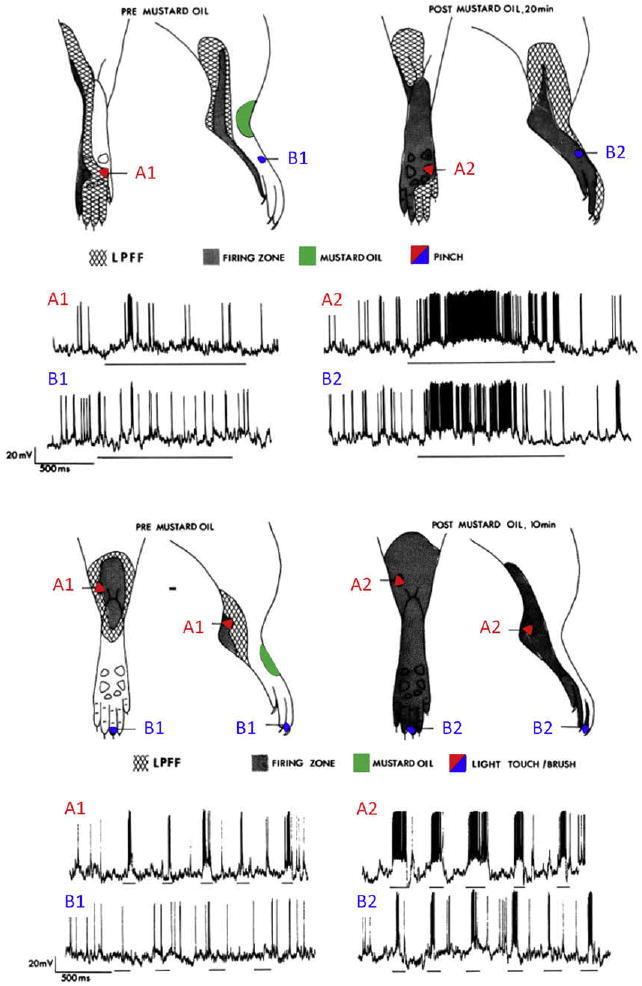
Expansion of receptor fields during central sensitization. Recruiting subthreshold synaptic inputs to the output of a nociceptive-specific neuron can markedly alter its receptive field properties, producing changes in receptive field threshold and spatial extent. When neurons in the dorsal horn spinal cord are subject to activity-dependent central sensitization, they exhibit some or all the following: development of or increases in spontaneous activity, a reduction in threshold for activation by peripheral stimuli, increased responses to suprathreshold stimulation, and enlargement of their receptive fields. The examples in this figure of intracellular recordings of rat dorsal horn neurons show the cutaneous receptive fields before central sensitization (pre mustard oil) and after the induction of central sensitization (post mustard oil) and indicate how subthreshold nociceptive (pinch, top) and low-threshold (brush, bottom) inputs in the low-probability firing fringe (LPFF) are recruited by central sensitization. Central sensitization was produced by topical application of mustard oil, which generates a brief burst of activity in TRPA1-expressing nociceptors and resulted in the neural equivalent of secondary hyperalgesia (top) and tactile allodynia (bottom). Note that the mustard oil (conditioning input) was applied to a different area (in red) from the test pinch or brush inputs (in blue), so that the changes observed are due to hetero-synaptic facilitation. Modified from Reference 364.
When neurons in the dorsal horn spinal cord are subject to central sensitization, they exhibit some or all the following: development of or increases in spontaneous activity, a reduction in the threshold for activation by peripheral stimuli, increased responses to suprathreshold stimulation, and an enlargement of their receptive fields (Fig 2). Several features appear particular to central sensitization: conversion of nociceptive-specific neurons to wide-dynamic neurons that now respond to both innocuous and noxious stimuli, progressive increases in the responses elicited by a standard series of repeated innocuous stimuli (temporal windup), an expansion of the spatial extent of their input, and changes that outlast an initiating trigger.132,368,369,372,376 These electrophysiological changes correlate remarkably with the development in human experimental subjects after a noxious conditioning input of allodynia (particularly dynamic tactile or brush-evoked allodynia), the temporal summation of repeated low-intensity stimuli from an innocuous sensation to pain, with “afterpain” on cessation of the stimulus, and widespread secondary hyperalgesia. These changes can be elicited in human volunteers by noxious stimulation of the skin (as with topical or intradermal capsaicin or repeated heat stimuli340) and in the gastrointestinal tract by exposure to low pH solutions.272
Central sensitization contributes to neuropathic37 and inflammatory pain,26,274,395 migraine,35 and irritable bowel syndrome.253 In these patients, it is involved in producing abnormal responsiveness to noxious and innocuous stimuli and a spread of tenderness beyond lesion sites. Central sensitization may also play a fundamental role in the abnormal and widespread pain sensitivity in patients with fibromyalgia.6,68,301-303 Given the major role of central sensitization in the generation of clinical pain hypersensitivity, it is essential that we understand the triggers and mechanisms responsible for the induction and maintenance of the switch in the somatosensory system from the physiological state, in which the sensory experiences evoked by low-intensity stimuli (innocuous sensations) and noxious stimuli (pain) are quite distinct and separate, to a dysfunctional hypersensitive system in which this discrimination is lost.
The Discovery of Central Sensitization
The first evidence for a central component to acute pain hypersensitivity was provided in 1983.369 Electrophysiological recordings from single biceps femoris α-motoneuron axons were used to measure the output of the nociceptive system, in this case the flexor reflex withdrawal response elicited by noxious stimuli (Fig 3). These recordings revealed, as expected, that under normal conditions there was no spontaneous activity in the motor neurons and that their activation required a noxious mechanical or thermal stimulus to the skin. These neurons had high-threshold nociceptive-specific receptive fields restricted to the toes or hind paw, in keeping with their activation only as part of the flexion withdrawal reflex. After repeated peripheral noxious heat stimuli sufficient to generate mild inflammation of the hind paw, however, an increased excitability of the motor neurons was detected that lasted for several hours and included a reduction in threshold and enlargement of the cutaneous receptive fields. The flexor motor neurons were now no longer nociceptive-specific but could be activated by low-intensity (innocuous) peripheral inputs such as light touch.369 Three experiments showed that this change in receptive field properties was due to alterations in the CNS and not the periphery. First, electric stimulation of Aβ sensory fibers began to elicit responses in the motor neurons after the conditioning noxious heat stimuli, whereas these inputs elicited no response before. Second, a local anesthetic block of the site of the peripheral injury did not result in collapse of the expanded receptive fields: The change was autonomous once it was triggered by the peripheral input. Finally, the hypersensitivity produced by the noxious heat could be mimicked in extent and duration by a brief 20-second low-frequency electrical stimulation of the sural nerve only at C-fiber strength, which produced changes lasting for tens of minutes. The interpretation of all these data was that noxious heat stimulation, by activating C-fiber nociceptors, had induced a central plasticity of the nociceptive system, which was thereafter capable of responding to stimuli outside of the injury area and to low-threshold afferents that previously did not activate the nociceptive system. This led to the articulation of a more general hypothesis that brief trains of nociceptor C-fiber input could trigger or condition a long-lasting sensitization of the nociceptive system (an effect termed central sensitization) by producing activity-dependent changes in the functional properties of neurons in the dorsal horn of the spinal cord and that this contributed both to postinjury flexor reflex and pain hypersensitivity.
Figure 3.
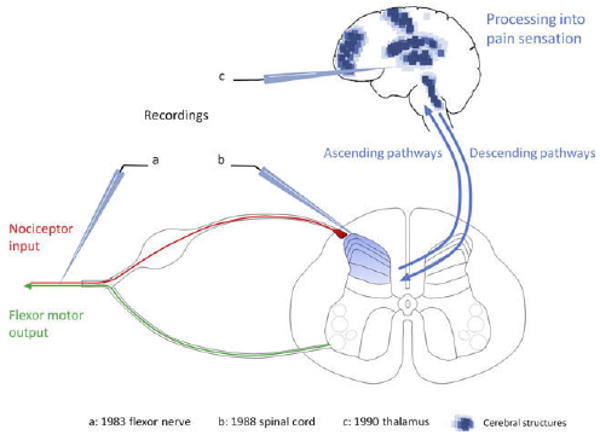
Schematic representation of the structures exhibiting central sensitization. The first evidence for central sensitization was generated in 1983 by revealing injury-induced changes in the cutaneous receptive field properties of flexor motor neurons as an integrated measure of the functional plasticity in the spinal cord. A conditioning noxious stimulus resulted in long-lasting reductions in the threshold and an expansion of the receptive field of the motor neurons that was shown to be centrally generated. Essentially identical changes were then described in lamina I and V neurons in the dorsal horn of the spinal cord (b) as well as in spinal nucleus pars caudalis (Sp5c), thalamus (c), amygdala, and anterior cingulate cortex. Imaging techniques have revealed several brain structures in human subjects that exhibit changes compatible with central sensitization (blue dots).
Before the discovery of central sensitization, the receptive field properties of dorsal horn neurons was thought to be fixed by the geometry of their dendrites relative to the central terminals of sensory axons.33 Although plasticity of the receptive fields of dorsal horn neurons had been shown to occur after peripheral nerve injury, this was thought to be due to a loss of presynaptic inhibition increasing synaptic input from silent or ineffective synapses and not to plasticity in dorsal horn neurons.71 After the first demonstration of central sensitization in flexor motor neurons, essentially identical changes were soon described in many studies in lamina I and V neurons in the dorsal horn of the spinal cord55,79,173,176,192,287,365,372 (Fig 3) as well as in spinal nucleus pars caudalis (Sp5c),36 thalamus78 (Fig 3), amygdala,219,220 and anterior cingulate cortex.364 More recently, functional magnetic resonance imaging, positron emission tomography, and magnetoencephalography have revealed in human subjects that several other brain structures implicated in pain (parabrachial nucleus, periaqueductal gray [PAG], superior colliculus, prefrontal cortex) also exhibit changes compatible with increases in excitability corresponding to central sensitization187,209,212,244,283 (Fig 3).
Activity-Dependent Central Sensitization
The original description of central sensitization referred to an activity- or use-dependent form of functional synaptic plasticity that resulted in pain hypersensitivity after an intense noxious stimulus. This plasticity was triggered by the activity evoked in dorsal horn neurons by input from C-nociceptors, as after repeated heat stimuli above 49°C,369 electrical stimulation of C-fibers (1 Hz for 10 to 20 seconds),358 and chemical activation of nociceptors by irritant compounds such as allyl isothiocyanate (mustard oil)372 and formalin, which both act through the TRPA1 channel135,199 as well as capsaicin, which activates TRPV1 channels.158 To induce central sensitization, the noxious stimulus must be intense, repeated, and sustained. Input from many fibers is required over tens of seconds; a single stimulus, such as a pinch, is insufficient. Peripheral tissue injury is not necessary, although the degree of noxious stimulation that produces frank tissue injury almost always induces central sensitization, so that the phenomenon is very prominent after post-traumatic or surgical injury. Interestingly, nociceptor afferents innervating muscles or joints produce a longer-lasting central sensitization than those that innervate skin.358
Once the phenomenon had been shown to be robust, easily activated, and detected in both preclinical and human subjects, the issue then was what molecular mechanisms were responsible. The first major mechanistic insight was that the induction and maintenance of acute activity-dependent central sensitization was dependent on NMDA receptors,379 revealing a key involvement of glutamate and its receptors. We now appreciate from 2 decades of investigation by many labs that central sensitization comprises 2 temporal phases, each with specific mechanisms. The early phosphorylation-dependent and transcription-independent phase results mainly from rapid changes in glutamate receptor and ion channel properties.376 The later, longer-lasting, transcription-dependent phase drives synthesis of the new proteins responsible for the longer-lasting form of central sensitization observed in several pathological conditions.376 We will review the current understanding of these mechanisms.
Triggers of Activity-Dependent Central Sensitization
Glutamate, the fast transmitter of primary afferent neurons, binds to several receptors on postsynaptic neurons in the dorsal horn of spinal cord, including ionotropic amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), N-methyl-D-Aspartate (NMDA), and Kainate (KA) receptors and several metabotropic (G-protein coupled) glutamate receptor subtypes (mGluR). In the superficial laminae of the dorsal horn, AMPAR and NMDAR are present in virtually every synapse and are arranged in a mosaic-like manner, whereas mGluRs sit at the extremities of the postsynaptic density zone (PSD).10,11,15,247 At the subunit level, NMDAR is a tetramer that contains 2 low-affinity glycine-binding NR1 subunits and 2 subunits from the 6 different NR2A-D or NR3A/B subunits.305 The most common NMDA complexes in the dorsal horn are composed of NR1-NR2A/B subunits.202,215,243 AMPAR is also a tetramer, and its most abundant subunits in the dorsal horn of the spinal cord are the calcium (Ca2+)-permeable subunits GluR1 and GluR3 and the non–Ca2+-permeable GluR2 subunit.252 In basal conditions, inhibitory interneurons appear to preferentially express GluR1, whereas the excitatory neurons appear to express mostly GluR2.145,338 The AMPAR complex can also be a GluR1/GluR2 heteromer,11 in which case the receptor displays mainly GluR2 properties.233 A subpopulation of lamina I neurons lacking the NK1 receptor and expressing the GluR4 (Ca2+-permeable) subunit has been identified.248 The mGluR family is composed of 8 receptors that form 3 groups, based on their sequence similarities and their coupling with specific Gα-proteins.54 Group I mGluRs (mGluR1 and 5) are coupled with Gαq-proteins (whose activation causes an increase of [Ca2+]i), whereas group II (mGluR 2 and 3) and group III (mGluR4, 6, 7 and 8) are coupled with Gαi/o-proteins. All mGluRs except for mGluR6 and 8 are expressed in the spinal cord,346 whereas only mGluR6 appears not to be expressed by primary afferent neurons.39 In addition, a lamina-specific pattern of expression has been characterized for mGluR1α (lamina V), mGluR5 (laminas I-II),10,247 and mGluR2/3 (lamina II inner),12 suggesting precise and distinct physiological roles for the different subtypes.
Activation of NMDAR is an essential step in both initiating and maintaining activity-dependent central sensitization as its blockade by noncompetitive (MK801) or competitive (D-CPP) NMDAR antagonists prevent and reverse the hyperexcitability of nociceptive neurons induced by nociceptor conditioning inputs184,379 and conditional deletion of NR1 abolishes NMDA synaptic inputs and acute activity-dependent central sensitization.300 NMDAR is both a trigger and effector of central sensitization. Under normal conditions, the NMDAR channel is blocked in a voltage-dependent manner by a magnesium (Mg2+) ion sitting in the receptor pore.198 Sustained release by nociceptors of glutamate and the neuropeptides substance P and CGRP leads to sufficient membrane depolarization to force Mg2+ to leave the NMDAR pore, whereupon glutamate binding to the receptor generates an inward current.198 Removal of this voltage-dependent block is a major mechanism for rapidly boosting synaptic efficacy and allows entry of Ca2+ into the neuron, which then activates numerous intracellular pathways that then contribute to the maintenance of central sensitization. In addition to the critical role of NMDAR in increasing the excitability nociceptive neurons, activation of group I mGluRs by glutamate also appear important for the development of central sensitization. Although these receptors do not participate to basal nociception,221,392 their activation is necessary for activity-dependent central sensitization mediated by C-fibers.14,67,165,296,392,393 In contrast, activation of group II-III mGluRs is associated with a reduction of capsaicin-induced central sensitization.296
Substance P (SP), which is co-released with glutamate by unmyelinated peptidergic nociceptors, is also involved in the generation of central sensitization.8,146,183,192,365 Substance P binds to the neurokinin-1 (NK1) G-protein–coupled receptor, which is expressed by spinothalamic, spinoparabrachial, and spino-PAG neurons101 and causes a long-lasting membrane depolarization,112 and contributes to the temporal summation of C-fiber–evoked synaptic potentials80,388,389 as well as to intracellular signaling. Ablation of NK1-positive neurons in the spinal cord leads to a reduction in capsaicin-evoked central sensitization, confirming the importance of projecting neurons expressing the substance P receptor in this phenomenon.146,192 Calcitonin gene-related peptide (CGRP), also synthesized by small diameter sensory neurons, potentiates the effects of SP367 and participates in central sensitization through postsynaptic CGRP1 receptors, which activate PKA and PKC.307,308 CGRP also enhances release of brain-derived neurotrophic factor (BDNF) from trigeminal nociceptors,34 which may contribute to its involvement in migraine and other primary headaches.82,103
BDNF is a neurotrophic factor and synaptic modulator that is synthesized by nociceptor neurons and released into the spinal cord404 in an activity-dependent manner,19 where it also has a role in the production of central sensitization.113,144,330 On binding to its high-affinity trkB receptor, BDNF enhances NMDAR-mediated C-fiber–evoked responses144 and causes activation of several signaling pathways in spinothalamic track neurons, including ERK141,245,292 and PKC.293
The inflammatory kinin bradykinin is produced in the spinal cord in response to intense peripheral noxious stimuli and acts through its Gq-coupled B2 receptor, which is expressed by dorsal horn neurons41,359 and boosts synaptic strength by activating PKC, PKA, and ERK.152 ERK is also activated by a serotoninergic (5-HT) descending input involving the ionotropic 5-HT3 receptor143,310,313,396 and possibly the 5-HT7 GS-coupled receptor.29 Fig 4 summarizes the key known synaptic triggers of central sensitization.
Figure 4.
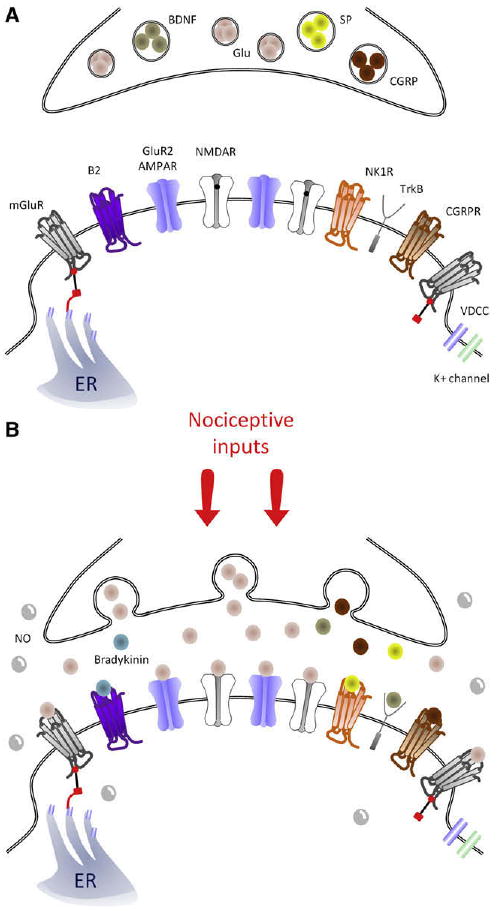
Central sensitization triggers: Schematic representation of key synaptic triggers of central sensitization. (A), Model of the synapse between the central terminal of a nociceptor and a lamina I neuron under control, basal conditions. mGluR receptors sit at the extremities of the synapse and are linked to the endoplasmic reticulum (ER). Note that NMDAR channels are blocked by Mg2+ in the pore (black dot). After a barrage of activity in the nociceptor (B), the primary afferent presynaptic terminal releases glutamate that binds to AMPAR, NMDAR (now without Mg2+), and mGluR, as well as substance P, CGRP, and BDNF, which bind to NK1, CGRP1, and TrkB receptors, respectively. B2 receptors are also activated by spinally produced bradykinin. NO is produced by several cell types in the spinal cord and can act presynaptically and postsynaptically.
Signaling Pathways and Activity-Dependent Central Sensitization
An increase in intracellular Ca2+ beyond a certain threshold level appears to be the key trigger for initiating activity-dependent central sensitization. Calcium influx through NMDAR appears to be particularly prominent in the induction phase but can also occur through calcium-permeable AMPARs,159,355 voltage-gated calcium channels,52,376 as well as from release from intracellular microsomal stores in response to activation of several metabotropic receptors106,182 (Fig 5, A through C). Why is the calcium-induced activation of intracellular kinases so important? The reason is that ionotropic NMDA and AMPA glutamate receptors can be phosphorylated on several key residues located on their C-terminus,40,42 and this post-translational modification changes their activity as well as their trafficking to or from the membrane,40,161 which with similar post-translational changes in other ion channels, produces the functional changes that manifest as central sensitization (Fig 6, A through C). AMPAR subunit GluR1 residue Ser831 is phosphorylated by protein kinase C (PKC) and calcium-calmodulin-dependent protein kinase II (CaM-KII); Ser845 is the phosphorylation target of PKA, and GluR2 has 1 main site for phosphorylation by PKC, on Ser880.40 For the NR1 subunit of NMDAR, PKC phosphorylates Ser896, whereas PKA has 2 potential phosphorylation sites on Ser890 and Ser897.168,333 NMDAR conductance properties are modified by phosphorylation of tyrosine residues located on the NR2A and NR2B subunits at 3 potential sites (Tyr1292, 1325, or 1387 for NR2A and Tyr1252, 1336, or 1472 for NR2B), through activation of nonreceptor tyrosine kinases such as Src or Fyn.42,106,107,268
Figure 5.
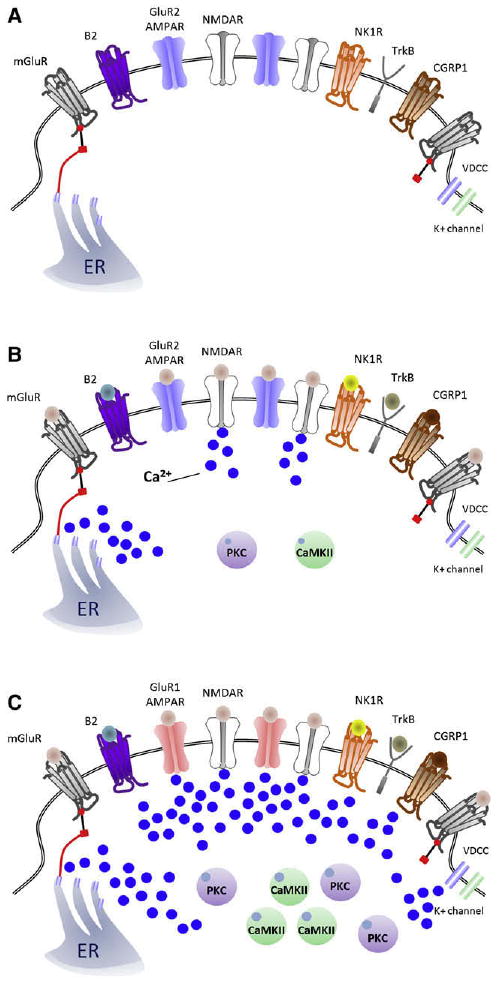
Sources of Ca2+ in the synapse of nociceptive neurons for inducing central sensitization. (A), Model of a nociceptor–dorsal horn neuron synapse under control, nonactivated conditions. After nociceptor input (B), activation of NMDAR and mGluR result in a rapid increase of [Ca2+]i that activates PKC and CaMKII, 2 major effectors of central sensitization. (C), Representation of a synapse during peripheral inflammation-induced central sensitization, where there is a shift from GluR2/3 to GluR1-containing AMPARs that enables, along with voltage-dependent calcium channels and NMDAR, entry of Ca2+ and which, together with activation of the G-coupled MGluR, NK1, B2, and CGRP1 receptors, which release intracellular Ca2+ stores, recruits PKC and CaMKII, strengthening the excitatory synapse.
Figure 6.
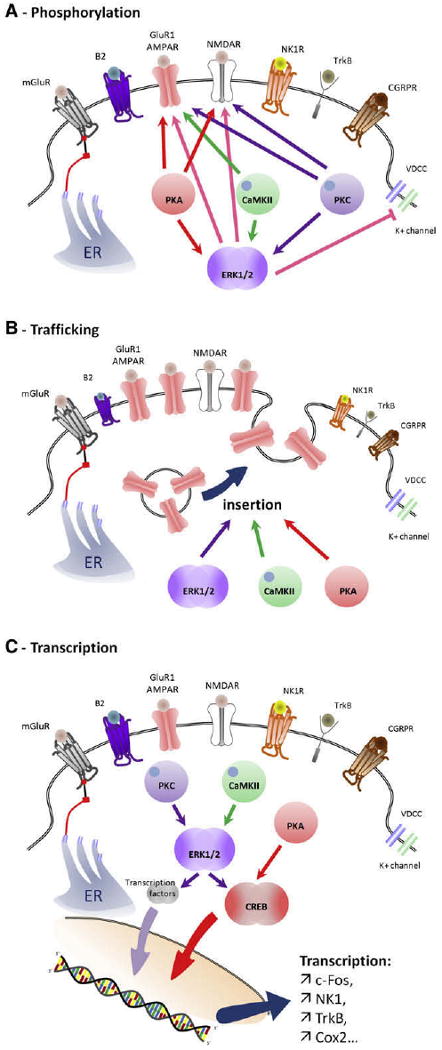
Contribution of PKC, CaMKII, PKA, and ERK activation to central sensitization. (A), Phosphorylation by PKC, CaM-KII, PKA, and ERK cause changes in the threshold and activation kinetics of NMDA and AMPA receptors, boosting synaptic efficacy. ERK also produces a decrease in K+ currents through phosphorylation of Kv4.2 channels, increasing membrane excitability. (B), PKA, CaMKII, and ERK promote recruitment of GluR1-containing AMPAR to the membrane from vesicles stored under the synapse. (C), Transcriptional changes mediated by activation of CREB and other transcription factors driving expression of genes including c-Fos, NK1, TrkB, and Cox-2, to produce a long-lasting strengthening of the synapse.
Stimulation of AMPAR and group I mGluRs89,106,118,281 participate with NMDAR in the activation of the intracellular pathways that sustain central sensitization. These include the PLC/PKC pathway, through opening of Ca2+ channels on the endoplasmic reticulum,85,391 the phosphatidylinositol-3-kinase (PI3 K) pathway,246 and the mitogen-activated protein kinase (MAPK) pathway that involves the extracellular signal-regulated kinases (ERK1 and ERK2), which are 44- and 42-kDa Ser/Thr kinases, respectively, with 90% sequence identity, and the cAMP response element binding protein (CREB).130,138,141,357,363 One way that ERK and CREB are activated is through an elevation in intracellular Ca2+ sufficient to drive a calmodulin-induced stimulation of adenylyl cyclases 1 and 8, whose cAMP production in turn activates PKA and subsequent cascade(s).363
The activation of ERK by phosphorylation is regulated by a core signaling module that consists of an apical MAPK kinase kinase (MAP3 K), a MAPK kinase (MEK or MKK), and the downstream ERK. Many different signaling pathways can induce ERK activation in addition to its canonical ras/raf pathway, and this can be readily detected immunohistochemically in the dorsal horn within minutes of peripheral noxious stimuli130 (Fig 7). The presence of phosphorylated ERK reveals the anatomical distribution of those neurons whose intracellular signaling has been activated by the nociceptor input and are presumably undergoing the synaptic changes that constitute central sensitization120,131,141,152 (Fig 7). In the spinal cord, ERK is only activated in neurons in response to intense peripheral noxious stimulation, effectively identical to those stimuli that induce central sensitization,130,363 suggesting that ERK phosphorylation is a better marker of the neural plasticity that mediates central sensitization than c-Fos, which can be activated by low threshold stimuli that do not induce central sensitization.126 Because the ERK pathway is composed of successive protein kinases, each of which can be activated by several different signaling pathways,131 most of the triggers of central sensitization such as NMDAR, group I mGluR, TrkB, NK1, or CGRP1 converge to activate ERK120,130,131,171 (Fig 8). Once activated, ERK produces translational and post-translational effects that participate in the maintenance of central sensitization in spinal cord neurons.131,387 The post-translational effects include an increase of NMDAR function through phosphorylation of its NR1 subunit152,293 (Fig 6, A), recruitment of AMPAR to the membrane93,254 (Fig 6, B) leading to an increase in AMPAR, and NMDAR currents boosting synaptic efficacy.152 Furthermore, ERK produces a decrease in K+ currents through phosphorylation of the residue Ser616 of Kv4.2 channels leading to an increase in membrane excitability118,119 (Fig 6, A). Transcriptional changes are mediated by activation of CREB as well as other transcription factors, which drives expression of several genes including c-Fos, NK1, TrkB, and Cox-2131 (Fig 6, C). Inhibition of ERK activation using inhibitors of MEK reduces behavioral measures of activity-dependent central sensitization.137,142
Figure 7.
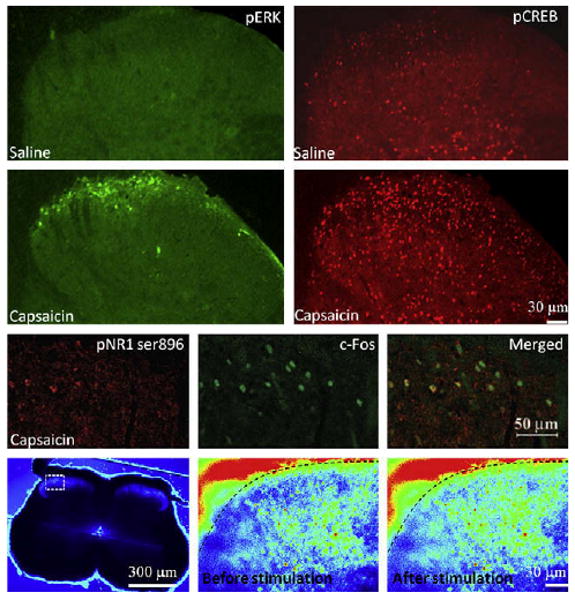
Key effectors of central sensitization in the dorsal horn. Subcutaneous injection of capsaicin, a potent inducer of central sensitization, causes rapid activation of ERK and CREB (upper panels) as well as a PKC-induced phosphorylation of the NR1 subunit of NMDAR in c-Fos–positive neurons of the superficial laminas of the spinal cord (middle panels). ERK activation participates in transcriptional and post-translational changes, CREB activation promotes transcription of several genes involved in central sensitization. NR1 phosphorylation by PKC increases NMDAR activity. ERK and PKC are activated through the increase of intracellular calcium that occurs during stimulation of nociceptive fibers (lower panels). Signals are shown in pseudocolor from blue (weak intensity) to red (strong intensity). ERK and CREB staining are from Reference 141; NR1 phosphorylation staining from Reference 32, and calcium imaging from Reference 179.
Figure 8.
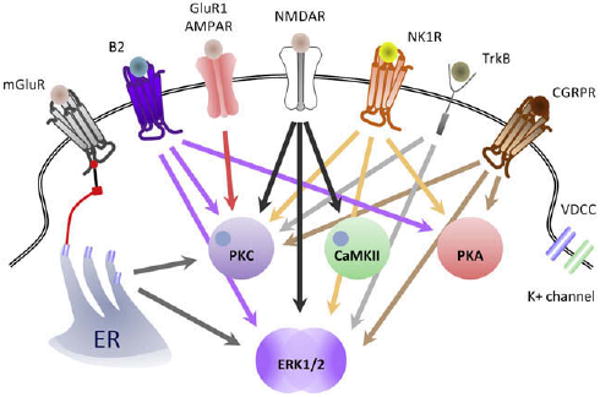
Key intracellular pathways contributing to the generation of central sensitization. NMDAR activation causes activation of PKC, CaMKII, and ERK (black arrows); GluR1-containing AMPAR activate PKC (red arrow); NK1 and CGRP1 receptors activate PKC, PKA, and ERK (orange and brown arrows, respectively); TrkB s activates of PKC and ERK (purple arrows); and mGluR, via release of Ca2+ from microsomal stores, activates PKC and ERK (gray arrows). Note that most of the triggers of central sensitization: Activation of NMDAR, mGluR, TrkB, NK1, CGRP1, or B2 converge to activate ERK.
Nitric oxide (NO) synthesized by either neuronal or inducible NO synthases in the dorsal horn381 also has a role in central sensitization.381-383 Potential mechanisms for NO actions include the cGMP synthesis cascade, nitrosylation of membrane channels, ADP-ribosylation, and production of reactive species.64,278 The NO-cGMP pathway involves soluble guanylate cyclase, which is expressed by NK1-positive spinothalamic neurons, as well as inhibitory interneurons.73 Fig 8 summarizes key intracellular pathways whose activation contributes to the generation of central sensitization.
Effectors of Activity-Dependent Central Sensitization
AMPAR and NMDAR phosphorylation during central sensitization increases the activity/density of these receptors, leading to postsynaptic hyperexcitability.32,86-88,134,178,345,394,407,408 The first phase of central sensitization is a fast augmentation of excitatory glutamatergic synapses in the superficial dorsal horn that strengthens nociceptive transmission and recruits non-nociceptive input to the pathway. This is achieved by phosphorylation of numerous receptor and ion channel targets that lead to changes in threshold, channel kinetics, and voltage dependence, as well as a modification in the trafficking of the receptors to the synapse (Fig 6, A and B). On noxious stimulation, PKA phosphorylates GluR1 subunits,87,88,214 leading to an insertion of these receptors into the synapse84,93 and thereby an increase in synaptic strength.20 Phosphorylation of GluR1-containing AMPAR by PKC and CaMKII also increases the excitability of nociceptive neurons.40,88
NR1 phosphorylation by PKA408 or PKC32,407 participates in the development of hypersensitivity97,262,345 by increasing the response of NMDARs to glutamate.44,259 Phosphorylation of the NR2B subunit of NMDAR, mediated through Src activation, increases the opening of the receptor channel268 and prevents endocytosis of activated receptors by disrupting the binding site of AP-2, a protein involved in clathrin-coated endocytosic vesicle formation.42 Decoupling Src interaction with NMDAR blocks NR2B phosphorylation and reduces formalin-induced and inflammatory pain without altering basal nociceptive pain.178
Activation of PKC contributes to hyperexcitability in nociceptive neurons by several different pathways. First, PKC reduces the Mg2+ block of NMDAR and increases the probability of channel opening, facilitating the activated state of NMDAR.44 Second, activation of PKC decreases inhibitory transmission at the segmental level by reducing GABA and glycine tonic inhibition174 and the descending inhibition driven from the PAG.175 Disinhibition, mediated by whatever means, leaves dorsal horn neurons more susceptible to activation by excitatory inputs including non-nociceptive A-fibers, and is 1 of the major mechanisms triggering and maintaining central sensitization.17,289,341,390 Finally, PKC contributes with PKA to the activation of ERK in a manner that requires their coactivation and is triggered by the central release of bradykinin.152
Activation of guanylate cyclase seems to be the major way that NO contributes to the induction of sensitization275,322,403 through increases in neuronal excitability and a reduction in inhibition,173,176,275 although an NO-mediated activation of ADP-ribosyltransferase may participate in the maintenance of central sensitization.403
Global Features of Activity-Dependent Central Sensitization
The key features of acute activity-dependent central sensitization are that it is induced with a short latency (seconds) by intense, repeated, or sustained nociceptor inputs and typically lasts for tens of minutes to several hours in the absence of further nociceptor input. It generally requires activation of NMDA receptors for its induction, and these receptors contribute to its maintenance. Nevertheless, as reviewed above, multiple different triggers can contribute to the establishment of this form of central sensitization: glutamate acting on NMDAR, but also on AMPAR and mGluR, the neuropeptides substance P and CGRP, the kinin bradykinin, as well as BDNF and NO (Fig 4). The reason so many different transmitters, modulators, and their receptors are involved is that it is not their specific action that is important but rather that they are released directly from or induced in response to nociceptor afferent activity, and each can separately or together initiate the activation of those multiple intracellular signaling pathways that lead to the establishment of hyperexcitability in dorsal horn neurons (Figs 6 and 8). In other words, there are many parallel inputs to dorsal horn neurons that can independently or cooperatively initiate central sensitization. Elevation in intracellular calcium, by whatever means, is 1 major trigger, activating multiple calcium-dependent kinases that act on receptors and ion channels to increase synaptic efficacy (Figs 5 and 6). Many central sensitization-inducing inputs also activate ERK, and this MAPK appears to have a pivotal role, contributing to increases in AMPA and NMDA currents as well as reducing potassium currents (Fig 6, A). However, even this kinase may not be essential. Other kinases such as PKC, PKA, and Src can, independent of ERK, also modulate ionotropic receptors to lead to an increase in synaptic efficacy (Fig 6, A).
What has become clear is that there is no single defining molecular mechanism of central sensitization but rather that it is a general phenomenon, one that produces distinct changes in somatosensory processing but nevertheless can be mediated by several different processes that, in response to nociceptor input, can (1) increase membrane excitability, (2) facilitate synaptic strength, or (3) decrease inhibitory influences in dorsal horn neurons. Similarly, the effectors of this plasticity are multiple: changes in the threshold and activation kinetics of NMDA and AMPA receptors and in their trafficking to the membrane, alterations in ion channels to increase inward currents and reduce outward currents, and reductions in the release or activity of GABA and glycine (Fig 9). These changes produce dramatic alterations in function. However, they are usually relatively short-lasting and reversible. Phosphatases will dephosphorylate receptors and ion channels resetting their activity to baseline levels, trafficking to the membrane will reverse by endocytosis, and, with time, the increased gain of the nociceptive neurons will fade, at least in the absence of any further triggering inputs.186,400-402 Different, transcription-dependent changes are required for longer-lasting effects, and these generally do not occur in response only to nociceptor activity but are the consequence of peripheral inflammation and nerve injury (see below). Activity-dependent central sensitization, even though it increases pain sensitivity, is in most situations an adaptive mechanism. Unlike nociceptive pain, which warns of potential damage in response to noxious stimuli, central sensitization creates a situation in which pain is elicited by innocuous stimuli. This change is protective, because it helps healing by limiting use of an injured body part until the injury is fully repaired. Central sensitization becomes pathological, however, if inflammation persists, as with rheumatoid arthritis, in which no healing occurs, and in situations in which central sensitization becomes autonomous and is maintained in the absence of active peripheral pathology. Central sensitization represents not only a state in which pain can be triggered by less intense inputs but in which the central sensitization itself can be maintained by a lower level or different kind of input. Ongoing activity in C-fibers, even at levels that do not elicit central sensitization in basal conditions, is sufficient to maintain central sensitization once it has been induced for prolonged periods (days).153 Furthermore, although nociceptor input is required to trigger central sensitization, phenotypic changes in myelinated fibers after inflammation and nerve injury can enable these afferents to acquire the capacity to generate central sensitization (see later).
Figure 9.
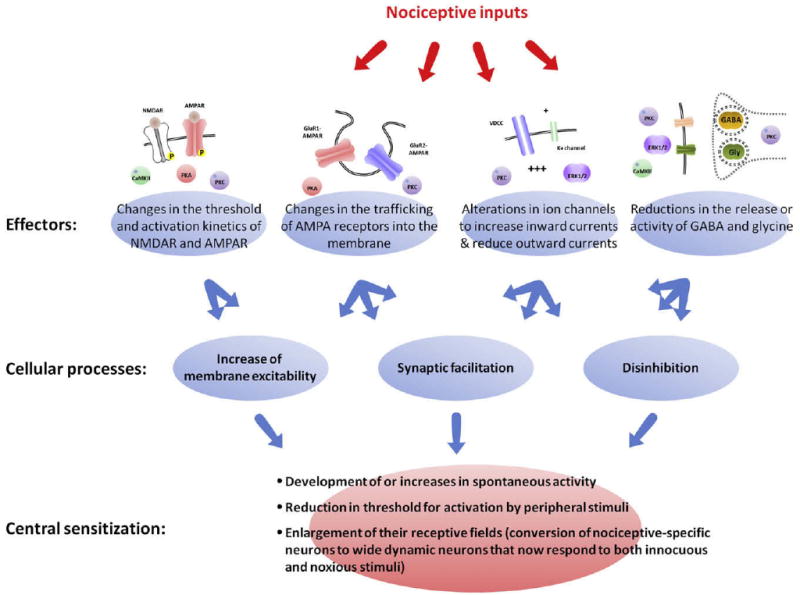
Multiple cellular processes lead to central sensitization. Central sensitization is not defined by activation of a single molecular pathway but rather represents the altered functional status of nociceptive neurons. During central sensitization, these neurons display 1 or all of the following: i, development of or an increase in spontaneous activity; ii, reduction in threshold for activation; and iii, enlargement of nociceptive neuron receptive fields. These characteristics can be produced by several different cellular processes including increases in membrane excitability, a facilitation of synaptic strength, and decreases in inhibitory transmission (disinhibition). Similarly, these mechanisms can be driven by different molecular effectors including PKA, PKC, CaMKII, and ERK1/2. These kinases participate in changes in the threshold and activation kinetics of NMDA and AMPA receptors and in their trafficking to the membrane, cause alterations in ion channels that increase inward currents and reduce outward currents, and reduce the release or activity of GABA and glycine.
Activity-Dependent Central Sensitization and Synaptic Plasticity
That the activity-dependent synaptic plasticity in the dorsal horn responsible for central sensitization is reversible differs from the permanent activity-dependent synaptic change in the cortex that leads to long-term memory, long-term potentiation (LTP), in which the efficacy only of activated synapses is changed. Synaptic changes with some resemblance to cortical LTP do occur in the spinal cord, that is, a form of homosynaptic potentiation. However, the major synaptic alteration underlying activity-dependent central sensitization is heterosynaptic potentiation, in which activity in 1 set of synapses enhances activity in nonactivated synapses, typically by “sensitizing” the entire neuron, something that never occurs with cortical LTP.
Homosynaptic potentiation is a type of use-dependent facilitation of a synapse evoked by activation of that same synapse (Fig 10, A). Classic LTP in the CA1 region of the hippocampus is formally defined as input-specific homosynaptic facilitation27,164,384 and is dependent on NMDAR activation, Ca2+ influx, and activation of Ca2+-dependent intracellular signaling pathways, notably the CaMKII pathway.164 Although the increased Ca2+ is relatively widespread in neurons after tetanic conditioning stimulation of afferents,128,182,260 only the stimulated synapse is potentiated.164,260 The development of LTP by 2 independent synapses using asynchronous pairing stimulation has been described in the hippocampus, but, once again, only conditioned synapses are potentiated.123
Figure 10.
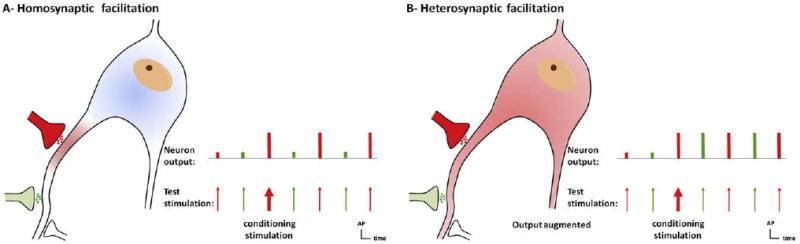
Homo synaptic and heterosynaptic facilitation. (A), Homosynaptic potentiation is a form of use-dependent facilitation of a synapse evoked by activation of that same synapse (in red). A nonconditioned synapse (green) is not potentiated. This type of potentiation is commonly called long-term potentiation (LTP). LTP-like homosynaptic potentiation can contribute to primary hyperalgesia. (B), Heterosynaptic facilitation represents a form of activity-dependent facilitation in which activity in 1 set of synapses (conditioning input, red) augments subsequent activity in other, nonactivated groups of synapses (test input, green). Heterosynaptic potentiation is responsible for the major sensory manifestations of use-dependent central sensitization: pain in response to low-threshold afferents (allodynia) and spread of pain sensitivity to noninjured areas (secondary hyperalgesia).
One form of homosynaptic facilitation in spinal cord neurons is windup, in which the action potential discharge elicited by a low-frequency (0.5 to 5 Hz) train of identical C-fiber strength stimuli gets larger on each successive stimulus200 (Fig 11). Windup is the result of the activation of NK1 and CGRP1 receptors after release of substance P and CGRP from peptidergic nociceptors to produce a cumulative membrane depolarization from the temporal summation of slow synaptic potentials.290 This then enables activation of NMDAR by removal of the Mg2+ block, further boosting the responses in a nonlinear fashion63,72,331,379 (Fig 11). The stimuli that induce windup (repeated C-fiber stimulation) can lead to central sensitization,379 and, although windup is often considered to be an aspect of central sensitization, it is instead the reflection of activity-dependent excitability increases in neurons during a nociceptor conditioning paradigm rather than changes that follow such inputs, which is when central sensitization manifests. Windup disappears within tens of seconds of the end of the stimulus train as the membrane potential returns to its normal resting level (Fig 11).
Figure 11.
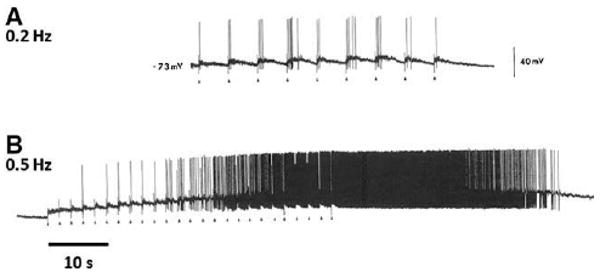
Action potential windup. Windup is the consequence of a cumulative membrane depolarization resulting from the temporal summation of slow synaptic potentials. Under normal conditions, low-frequency stimulations of C-fibers (0.2 Hz) cause steady neuronal discharges (A) as the membrane potential has sufficient time to return to resting potential between stimuli. At a frequency of 0.5 Hz or higher, activation of NK1 and CGRP1 receptors by release of substance P and CGRP from peptidergic nociceptors produces a cumulative increase in membrane depolarization. This then enables activation of NMDAR by removal of the voltage-dependent Mg2+ block, further boosting the responses in a non-linear fashion (B). Windup disappears within tens of seconds of the end of the stimulus train as the membrane potential returns to its normal resting level (B). Modified from Reference 323.
Another form of homosynaptic facilitation occurs in NK1-positive lamina I neurons in the dorsal horn neuron. This has been termed LTP, although, unlike classic hippocampal LTP, this form of homosynaptic facilitation appears not to be persistent, or at least the functional effects on pain sensitivity are not permanent instead lasting, like central sensitization for a few hours, with no evident change equivalent to long-term memory. Perhaps, therefore, to avoid confusion with cortical plasticity, the term LTP should be avoided for homosynaptic potentiation in the spinal cord because the changes in the dorsal horn are long-lasting (hours) rather than long-term (persistent). The original description of this long-lasting homosynaptic potentiation in the dorsal horn referred to an activity-dependent facilitation of excitatory postsynaptic currents in spinoparabrachial neurons in response to high-frequency (tetanic burst; 100 Hz) stimulation of C-fibers.127,177,271 The physiological relevance of this phenomenon was questionable because C-fibers do not fire at such high frequencies. Conditioning C-fiber stimulation at a low frequency (2 Hz) was subsequently shown also to elicit a long-lasting homosynaptic potentiation in lamina I spino-PAG neurons but not in spinoparabrachial neurons.128 This low-frequency potentiation is dependent on elevations in Ca2+, which activates PLC, PKC, CaMKII, and NOS.128 Capsaicin and formalin injection also evoke a homosynaptic long-lasting potentiation, as manifested by an enhancement of C-fiber–evoked synaptic potentials after the capsaicin/formalin evoked conditioning input.128 Capsaicin is, of course, also a potent inducer of activity-dependent central sensitization,294,340,383 characterized by the production of secondary hyperalgesia and tactile allodynia. However, both of these particular forms of pain hypersensitivity reflect heterosynaptic and not homosynaptic facilitation. Indeed, heterosynaptic facilitation characterizes most major changes in the receptive field properties of neurons and in pain sensitivity, in preclinical models, and human subjects368,369,376 (Fig 10, B).
Interestingly, healthy human subjects receiving high-frequency stimulation of C-fibers exhibit increased pain in the stimulated region, quite possibly caused by homosynaptic facilitation, but also show evidence of heterosynaptic facilitation, as manifested by dynamic mechanical allodynia in adjacent nonstimulated areas.149 The combination of the homosynaptic potentiation of conditioning nociceptor inputs and the heterosynaptic facilitation of nonconditioned fibers in the nociceptive pathway constitutes central sensitization.
Heterosynaptic facilitation represents a form of activity-dependent facilitation where activity in 1 set of synapses (the conditioning input) augments subsequent activity in another nonactivated group of synapses (the test input) (Fig 10, B). For homosynaptic potentiation, the test and conditioning inputs are the same; for heterosynaptic facilitation they are different. “LTP”-like phenomena in spinobrachial neurons can only account for the augmentation of the same C-fiber inputs that evoked the facilitation and cannot contribute to either secondary hyperalgesia or tactile allodynia. Repeated nociceptor input, such as that generated by capsaicin, will simultaneously generate both a potentiation of the activated C-fiber synapses (homosynaptic), and, unlike LTP in the hippocampus, also a potentiation of neighboring nonactivated synapses (heterosynaptic). It seems likely, therefore, that long-lasting potentiation in projecting dorsal horn neurons is simply a restricted aspect of the general widespread changes induced in these neurons by nociceptor activity.
Heterosynaptic potentiation appears to dominate the functional sensory manifestations of use-dependent central sensitization. After injection of capsaicin, for example, the thresholds of sensory fibers innervating the area surrounding the injection site are not modified,23,157,340,365 but pain hypersensitivity in these areas is prominent and depends on centrally mediated heterosynaptic facilitation. The same argument holds for the activation of pain in response to tactile stimulation or Aβ fiber inputs during central sensitization. It is no surprise, then, that spinal “LTP” shares major mechanisms with central sensitization (NMDAR, Ca2+, kinases, and NO) because it is very likely that the phenomenon of central sensitization includes both homosynaptic and heterosynaptic facilitations triggered by the same process; the major difference is that heterosynaptic potentiation results from the spread of signaling from the conditioning synapse to other synapses in the neuron182,270,371 (Fig 10, B). Homosynaptic changes will contribute with peripheral sensitization to primary hyperalgesia,128,270 whereas heterosynaptic facilitation alone is responsible for secondary hyperalgesia and allodynia.
Although several different forms of LTP have been characterized in the hippocampus,188,224,384 “spreading” or heterosynaptic LTP has not been reported, even though release of Ca2+ from intracellular stores can cause a spread of long-term depression (LTD) to neighboring, unstimulated synapses.227 What, then, is responsible for heterosynaptic facilitation in dorsal horn neurons? Two major candidates are the activation of mGluRs and NO. mGluRs are coupled to the Ca2+ channels of the endoplasmic reticulum85 and play an important role in central sensitization.7 Consequently, the release of intracellular Ca2+ in spinal cord neurons on mGluR activation may participate in spreading facilitation from conditioned synapses to neighboring test synapses. NO is also a major effector of spinal cord neuronal plasticity211,309 and diffuses rapidly from the site of its production to produce multiple effects at a distance via its downstream signaling pathways, and in this way may contribute to the heterosynaptic facilitation characteristic of central sensitization. It is certainly likely that these and other “spreading” signals cooperate to produce the widespread synaptic facilitation so characteristic to central sensitization. Scaffolding proteins play a major role in the addressing of specific kinases to the synapse and represent another potential mechanism for widespread synaptic facilitation. A recent study has shown that in the hippocampus, CaMKII activation is restricted to the synaptic bouton of a conditioned synapse, thus only allowing homosynaptic facilitation at that specific site.164 It is likely in the dorsal horn that CaMKII activation will be much more widespread and indeed the dendrites of dorsal horn neurons lack synaptic boutons.
Central Sensitization in Pathological Settings
In addition to its role in rapidly and reversibly sensitizing the nociceptive system by activity-dependent changes in synaptic strength and excitability, central sensitization also contributes to the longer-lasting and sometimes persistent pain hypersensitivity present in pathological situations involving inflammation and damage to the nervous system. The molecular and cellular mechanisms involved include some that are also responsible for activity-dependent central sensitization and others that are unique to either inflammation or nerve injury. NMDAR,47,193,255,280,315 AMPAR,180,239 group I mGluR,7,65,75,92,102,118,221,288,392,405 group II-III mGluR,45,104,194,207,286,398 BDNF,144,179,190,230 SP and CGRP,2,4,163 NO,50,316 and bradykinin241 have all been shown to contribute both to the development of central sensitization and to pain hypersensitivity in inflammatory and neuropathic pain models.
Inflammatory Pain
Peripheral inflammation induces a phenotypic switch in primary sensory neurons that comprises a change in their neurochemical character and properties due to alterations in transcription and translation. We will only discuss here those changes that relate specifically to central sensitization by virtue of changes in the synaptic input produced by the afferents and will not review the major changes that also alter peripheral transduction sensitivity and membrane excitability (peripheral sensitization), although of course, anything that increases nociceptor afferent input will also indirectly lead to increased central sensitization. Large DRG neurons begin, unlike in their naive condition, to express SP and BDNF when their peripheral terminals are exposed to inflammatory signals and nerve growth factor (NGF).191,223 Consequently, activation of the myelinated fibers by low-intensity innocuous stimuli now releases these neuropeptides in the spinal cord, and conditioning stimulation of the afferents acquires the capacity to generate central sensitization, something they normally cannot do185,190,223 (Fig 12). After peripheral inflammation, Aβ-mediated synaptic input to superficial dorsal horn neurons is substantially increased from the very low levels found in noninflamed animals.16 TrkA-expressing nociceptors, instead of a phenotypic switch, begin to express higher levels of neuropeptides and other NGF-dependent proteins as a result of exposure to the increased NGF produced by inflammation.370,375
Figure 12.
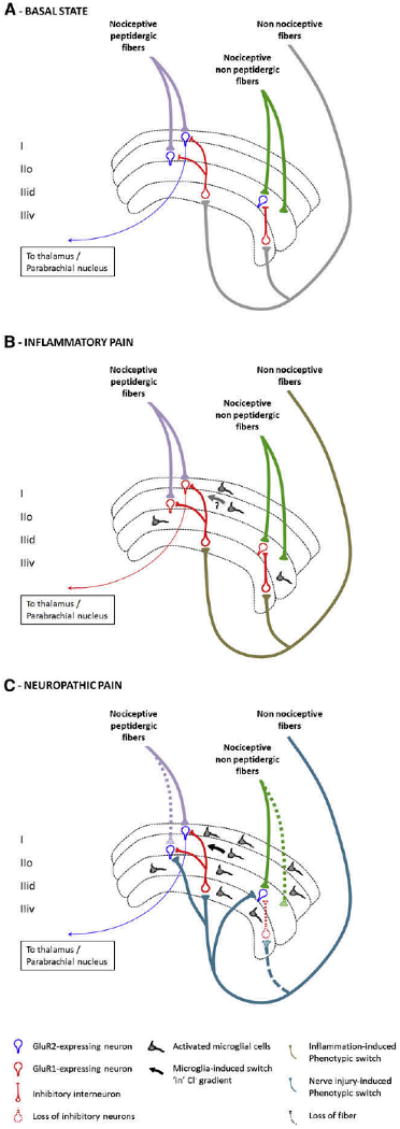
Central sensitization in pathological settings. A, Representation of the superficial lamina of the dorsal horn of the spinal cord. Nociceptive peptidergic fibers contact lamina I and II outer (I and IIo) neurons that express GluR2-containing AMPAR (in blue). Some of these neurons project to the thalamus, parabrachial nucleus, and PAG. Nociceptive nonpeptidergic fibers contact neurons in dorsal lamina II inner (IIid), which also express GluR2-containing AMPAR. Non-nociceptive large fibers contact deeper laminae but also send collaterals to inhibitory interneurons in the ventral part of lamina II (IIiv).222 B, Alterations in the superficial lamina in inflammatory pain. Because large DRG neurons begin to express SP and BDNF, stimulation of these afferents acquires the capacity to generate central sensitization. Neurons now express GluR1-containing AMPAR at their synapse (in red), resulting in an increase of Ca2+ influx on their activation. Some spinal cord microglial cells are activated and release factors that contribute to the development of central sensitization by enhancing excitatory and reducing inhibitory currents. C, Representation of superficial lamina in neuropathic pain states. After peripheral nerve injury, there is a loss of C-fiber central terminals and the sprouting of myelinated A-β fibers from deep to superficial lamina. Injured sensory neurons in the dorsal root ganglion exhibit a change in transcription that alters their membrane properties, growth, and transmission. Large fibers begin to express substance P, BDNF, and the synthetic enzymes for tetrahydrobiopterin, an essential cofactor for NOS and can drive central sensitization. Recruitment and activation of microglial cells is an essential step in the development of pain after nerve injury and to trigger central sensitization by releasing proinflammatory cytokines that increase neuronal excitability and BDNF that induces a switch in Cl- gradients, resulting in a loss of inhibition. Loss of inhibition is also caused by excitotoxic apoptosis of inhibitory interneurons.
A critical central pathway for the generation of inflammatory pain hypersensitivity involves induction of cyclooxygenase-2 (Cox-2) in dorsal horn neurons, to drive production of prostaglandin E2 (PGE2).269,349 PGE2 binds to its EP2 GPCR on dorsal horn neurons to potentiate AM-PAR and NMDAR currents,152 activate nonselective cation channels,18 and reduce in inhibitory glycinergic neurotransmission by blocking glycinergic receptors with α3 subunits9,111,152,213 (Fig 12). PGE2 also acts on EP4 receptors on presynaptic terminals to increase transmitter release.350 The importance of the central neuronal induction of COX-2 to inflammatory hyperalgesia is revealed by conditional deletion of COX-2 only in neurons, which results in the retention of peripheral inflammation and heat hyperalgesia but an almost complete loss of mechanical pain hypersensitivity.350
Under normal conditions, microglia are the only immunocompetent cells of the nervous system66,362 and constantly probe or survey the CNS parenchyma to maintain homeostasis.62,225 After peripheral inflammation, some spinal cord microglial cells change their shape, function, and chemical expression.115,258,311,312 In particular, p38 MAPK is activated311,312 and leads to the synthesis and release of pro-inflammatory cytokines,115,258 among which, IL-1β and TNF-α contribute to the development of central sensitization by enhancing excitatory and reducing inhibitory currents and by activating induction of COX-2142,269 (Fig 12).
Neurons in the superficial lamina of the dorsal horn usually display a GluR2 AMPAR phenotype (ie, are Ca2+-impermeable)252; however, peripheral inflammation triggers a shift from GluR2/3 to GluR1-containing AMPARs at the membrane159,238,355 (see “PSD Proteins and AMPAR Recycling and Subunit Switch,” below). Under these conditions, activation of AMPAR elicits entry of Ca2+, which can then participate in the activation of the signaling pathways that drive central sensitization. Ca2+-permeable AMPARs appear to be a major source of the [Ca2+]i increase in inflammatory pain, generating as much Ca2+ influx as with NMDAR activation.182 The functional state of NMDAR is also modified in response to peripheral inflammation, with phosphorylation of NR2B subunits by Src resulting in increased activity of the receptors106,107,178 and in their maintenance at the synapse.42 Finally, peripheral inflammation also promotes group I mGluR insertion into the membrane (mGluR5) and closer to the synapse (mGluR1), thereby further clustering these receptors at the synapse.247
Neuropathic Pain
After peripheral nerve injury, damaged and nondamaged A- and C-fibers begin to generate spontaneous action potentials. Because these do not arise from the peripheral terminal, it is a form of ectopic input.70,74 Such input in C-fibers can initiate and then maintain activity-dependent central sensitization in the dorsal horn.154 However, because of chemical and structural changes in A fibers,56,229,386 input in these afferents can also begin to drive central sensitization.69 Injured, and to a much lesser extent, noninjured sensory neurons in the dorsal root ganglion exhibit a massive change in transcription that alter their membrane properties, growth, and transmitter function.56,231,232,386 These changes are much greater than those that occur in response to peripheral inflammation, where only a few tens of transcripts are altered in the DRG195 and involve altered expression of about 1000 transcripts, including ion channels, receptors, transmitters, and the molecular machinery necessary for axon regeneration.56,261 Among the many changes, large fibers begin to express new transmitters and neuromodulators including substance P and BDNF and the synthetic enzymes for tetrahydrobiopterin, an essential cofactor for NOS. Stimulation of non-nociceptive fibers now triggers release of factors that can drive central sensitization.24,56,91,229,327,386
Structural changes also contribute to altered synaptic function. Peripheral nerve injury leads to a transganglionic degeneration of C-fiber terminals in lamina II.13,136 This loss of presynaptic input, together with the triggering of increases in the intrinsic axonal growth capacity as part of the regenerative response of the injured neurons, provides an opportunity and the molecular means for myelinated A-β fibers to sprout from laminae III-IV into laminae I-II and make contact with nociceptive-specific neurons.166,191,285,377,378 The original experiments describing the sprouting phenomenon were conducted using cholera toxin B subunit as a selective tracer for A-fibers as well as single axonal label with HRP. The selectivity of this toxin after peripheral nerve injury is somewhat controversial.125,339 Nevertheless, immunostaining for c-Fos activation and electrophysio-logical recordings have clearly established that peripheral nerve injury causes large myelinated fibers to begin to drive nociceptive neurons in superficial lamina.24,151,235,366
A reduction in the synthesis, release, or activity of inhibitory transmitters leads to a state of disinhibition, whose net functional effects are very similar to that produced by increases in synaptic strength of excitatory synapses and in membrane excitability.289,341,390 In neuropathic pain states, there is substantial disinhibition in the superficial dorsal horn with loss of GABAergic and a reduction in glycinergic inhibitory currents210 that can be attributed, at least in part, to apoptosis of inhibitory interneurons.277 This neuronal death appears to be the result of an NMDAR-induced excitotoxicity that develops over time rather than to the large amount of glutamate released centrally at the time of nerve injury.277 One laboratory failed to find significant loss of neurons or of GA-BAergic content in the dorsal horn of neuropathic pain animal models.249-251 The reasons for this discrepancy are not clear but may reflect technical differences in how the studies were performed. Interestingly, the reduction in glycinergic neurotransmission caused by the activation of EP2 receptors after peripheral inflammation does not appear to operate after nerve injury, further indicating that some inflammatory and neuropathic pain mechanisms differ.117
Another mechanism contributing to the reduction in segmental inhibition in a subpopulation of lamina I neurons in the spinal cord after nerve injury is dependent on BDNF effects on an anion transporter, changing anion gradients across neuronal membrane to alter the inhibitory efficacy of GABA. Under normal conditions, the intracellular concentrations of Cl- are maintained by the opposed effects of Cl--cotransporter K+-Cl- exporter 2 channels (KCC2) and Na+-K+-Cl- exporter 1 channels (NKCC1). KCC2 drives Cl- ions out of the cells (along with K+) and NKCC1 is responsible for an influx of K+, Na+, and Cl- into the cells. The net effect of these 2 co-transporters is a steady-state Cl- concentration gradient in which opening of Cl- channels (such as GABAA receptors) causes entry of Cl- into the neuron and hyperpolarizes the neurons. After peripheral nerve injury, BDNF released by activated microglial cells results in a reduction of KCC2 expression in a subset of neurons in the superficial lamina of the dorsal horn.57,58,203 Consequently, activation of GABAA receptors by GABA result in a diminution or absence of Cl- entry into the cell and thus a disinhibition of these nociceptive neurons57,58,179,203 (Fig 12). As after peripheral inflammation, there is also an increase in descending excitatory controls from the RVM in the brainstem after peripheral nerve injury, as well as a reduction of descending inhibitory controls.60,95,351,356
Peripheral nerve injury causes a massive activation of, and change in, glial cells in the spinal cord as well as infiltration of peripheral immune-competent cells, notably macrophages and T-cells.38,317,360 The extent and duration of the changes in microglia and astrocytes is much greater than in response to peripheral inflammation. Activated microglia produce and release trophic factors, neurotransmitters, cytokines, and reactive oxygen species263,361 and appear to play an essential step in the development of pain after nerve injury by triggering central sensitization through their interaction with neurons.133,160,162,201,206,256,257,352 Numerous signals trigger microglial activation and recruitment, including ATP and NO,62,81,225 cytokines, and chemokines, some of which are released by injured sensory neurons and others by microglial cells themselves or by astrocytes and T-cells.1,3,66,76,204,348,361,362 Release of cytokines by microglia increases neuronal excitability through activation of ERK and CREB.131,142 Activated microglia also release BDNF and NO,58,116 promoting segmental disinhibition.57 Finally, microglia can also provoke neuronal death by producing ROS, pro-apoptotic cytokines such as TNF,121 and by a diminished glutamate uptake.48,326,332 T-cells produce specific cytokines such as IFN-γ, which reduce GABAergic currents in the dorsal horn354 through activation of IFN-γ receptors353 and also activate and recruit microglia. Astrocytes also become activated after peripheral nerve injury,98,131,205 with a slower onset and more prolonged time course than microglia, and may play more of a role in the maintenance of neuropathic pain hypersensitivity than microglia.94,399,406 What seems clear is that multiple different mechanisms operate after nerve injury to increase excitability and reduce inhibition.
Scaffolding Proteins, Synaptic Plasticity, and Central Sensitization During Inflammation and After Nerve Injury
The proteins that make up the PSD can drive a major functional reorganization of synapses, modifying post-synaptic efficacy by altering receptor density at the membrane and producing switches from Ca2+-impermeable to Ca2+-permeable AMPARs (Fig 13). The PSD is not simply a structural landmark of the synapse but contains elements essential both for the formation of the synapse and for changes in its properties. Absence of scaffolding proteins or specific disruption of their binding sites results in a dramatic reduction in synaptic plasticity because the proteins contribute both to transcriptional and post-translational events. They initiate signaling cascades that lead to the activation of transcription factors, traffic newly synthesized receptors to the PSD, and “address” kinases and phosphatases to specific receptors in a stimulus-dependent manner. Although the involvement of the PSD in synaptic plasticity in the cortex is much better established than in the spinal cord, there is increasing evidence for a major role for the PSD in changing synaptic efficacy in response to peripheral inflammation and nerve injury.
Figure 13.
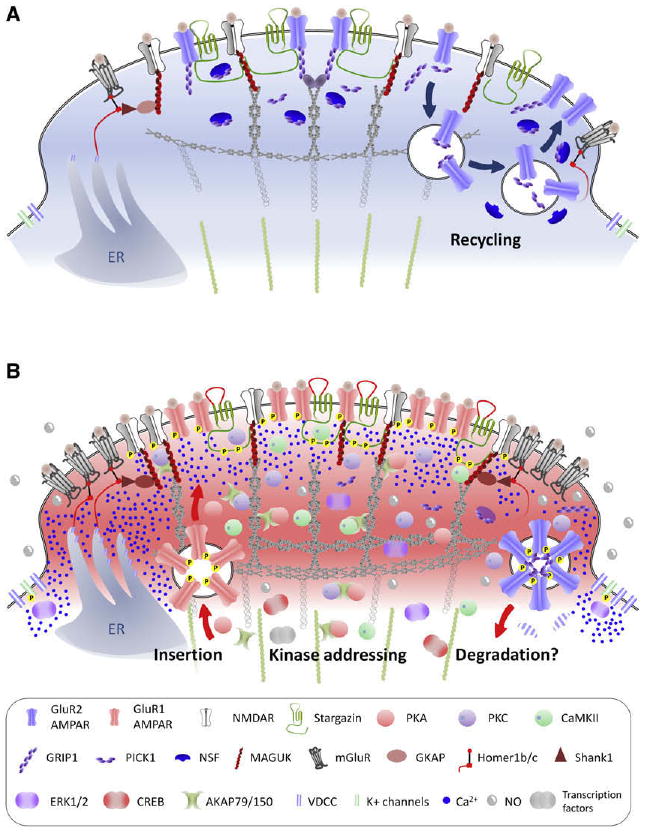
Role of scaffolding proteins in central sensitization. Representation of the post synaptic density (PSD) region of a synapse of a nociceptive neuron in the superficial lamina of the spinal cord under basal conditions (A) and during inflammatory pain (B). The proteins that make up the PSD can drive a major functional reorganization of synapses, modifying postsynaptic efficacy by altering receptor density at the membrane and producing switches from Ca2+-impermeable to Ca2+-permeable AMPARs. In addition, scaffolding proteins mediate the “addressing” of kinases to the receptors, thereby increasing their activity. Under normal conditions (A), neurons mostly express GluR2-containing AMPAR that are anchored to the PSD via GRIP-1. NMDAR are recycled in an activity-dependent manner via PICK-1 and NSF. During inflammation (B), there is an endocytosis of GluR2-containing AMPAR (initiated by PKC phosphorylation of GluR2) along with a loss of NSF that prevents their reinsertion into the synapse. GluR1-containing AMPAR are expressed at the membrane, where their activity is increased by phosphorylation with PKA as well as by the ectodomain of stargazin, whose C-terminus segment is phosphorylated by PKC and CaMKII. MAGUK can participate to increase glutamate receptors density at the synapse, and phosphorylated stargazin promotes AMPAR and NMDAR clustering. Scaffolding proteins such as AKAP79/150 and the MAGUKs also “address” kinases to specific synaptic position at the right time to phosphorylate AMPAR and NMDAR subunits, thereby increasing their activity. The Homer-Shank1-GKAP-MAGUK complex couples mGluR and NMDAR to intrasomal Ca2+ stores, so that activation of glutamate receptors leads to high levels of Ca2+ in the PSD that activate PKC and CaMKII, also recruited to the PSD by scaffolding proteins. PKC, CaMKII, and other kinases converge to activate ERK, which reduce K+ channels activity and promote transcriptional activity.
The PSD consists of cytoskeletal proteins, signaling molecules, membrane receptors, and scaffolding proteins.234 Scaffolding proteins are families of proteins characterized by their ability to interact with numerous partners, and these proteins form the dense molecular structure of the postsynaptic component of the synapse. A particularly abundant component of the PSD are proteins containing a specific peptidergic domain called PDZ, which is named after the protein in which the sequence was first identified (postsynaptic density protein 95 [PSD-95]/discs large/zonula occludens 1). This family of proteins includes, among hundreds of members, the 4 membrane-associated guanylate kinases (MAGUK): PSD-95, PSD-93, synapse associated protein (SAP)-97, SAP-102. The MAGUKs represent the most abundant scaffolding protein family in the PSD234 and are characterized by 3 PDZ domains, an Src homology region (SH3) domain, and a guanylate kinase-like (GK) domain,148 making them central elements of the synapse scaffold. The prime binding protein for the MAGUK family is the NMDAR subunit NR2,155 but it also binds to the transmembrane AMPAR regulatory proteins (TARPs),43 nonreceptor tyrosine kinases,329 nNOS,30,31 GKAP,217 and AKAP79/150.53 MAGUKs can be seen as the functional scaffold of the PSD and are essential for the structural integrity of synapses but also modulate the insertion of glutamate receptors into the synapse and physically bring together key enzymes to the PSD.59,148,167,197,265 Knock-down of PSD-95 and PSD-93, as well as targeted mutagenesis of the residues required for their protein:protein interaction, both prevent and reduce central sensitization in normal conditions321 as well as in inflammatory319,323,397 and neuropathic pain models99,320,323,397 but do not alter nociception or locomotor functions.319-321,323
Another member of the PDZ family, stargazin, is a 4-transmembrane domain protein whose putative secondary structure is close to the Ca2+-channel γ subunit, and was named γ2.170 Stargazin however, does not play an important role in neuronal Ca2+ channels170 but is instead highly concentrated in the PSD and co-immunoprecipitates with GluR1, 2, and 4.43,335 The protein is a major AMPAR partner, along with the 4 other isoforms, γ3, γ4, γ7, and γ8,140,335 which form the TARP subgroup.335 Stargazin traffics AMPAR from the endoplasmic reticulum to the extrasynaptic membrane.43,334 Once stargazin and AMPAR are addressed to the extrasynaptic membrane, their recruitment to the synapse requires interaction of the C-terminus segment of stargazin with PSD-95.21,276 Activity-dependent phosphorylation of stargazin by PKC and CaMKII342 produces a massive insertion of AMPAR into the membrane,337 whereas stargazin's dephosphorylation by PP1 or PP2B reduces the number of AMPAR at the synapse.337 In addition, via the interaction between stargazin's ectodomain and the glutamate binding region of AMPAR, stargazin modulates the activity of AMPAR by slowing channel deactivation and desensitization and increasing the affinity of the receptors for glutamate,49,334,336 thereby potentiating synaptic strength. Disruption of stargazin in the spinal cord inhibits the second phase of formalin-induced pain and reduces the heat hyperalgesia caused by intraplantar CFA injection.318 Recently, cornichon homolog 2 (CNIH-2) and cornichon homolog 3 (CNIH-3) have been found to be novel partner proteins for AMPAR in the CNS.279 Cornichon proteins bind to GluR1-4 AM-PAR and, as for stargazin, they promote AMPAR surface expression and slow their deactivation kinetics.279 Because their expression in the CNS is estimated to be in 70% of neurons, and because cornichon and stargazin appear to be mutually exclusive,279 the determination of their presence in spinal cord neurons and their role in central sensitization is something that needs to be investigated.
EphrinB-ephBR receptor interactions participate in NMDAR clustering at the PSD.90 EphBRs are receptor tyrosine kinases expressed by postsynaptic neurons, whereas EphrinB is anchored to the presynaptic membrane.156 The kinase activity of EphBR is not required for the initial clustering of NMDAR at the synapse but is essential for their maintenance.61 Stimulation of EphBR potentiates NMDAR-induced Ca2+ influx and the phosphorylation of CREB through the activation of the nonreceptor tyrosine kinase src314 and recruits CaM-KII to the synapse236 to increase NMDAR activity.314 Activation of EphBR in the spinal cord induces thermal hyperalgesia (but not allodynia),22,299 without modifying nociception.22 Inhibition of EphRB prevents or reverses inflammatory22,291 and neuropathic150,299 pain and prevents establishment of NMDAR-induced spinal cord “LTP.”299 More specifically, targeted disruption of the coupling between EphRB-activated Src and NR2B also prevents the development of central sensitization without altering basal nociceptive transmission.178 In addition, sustained nociceptive activity leads to an upregulation and reorganization of presynaptic EphB increasing EphB-EphRB interaction.22,298,299
The nonreceptor tyrosine kinase family also includes Fyn, which phosphorylates NR2 subunits268 and binds to PSD-93 to phosphorylate NR2A/B273 and could play a role in the maintenance of neuropathic pain.5
NMDA receptors are structurally connected with group I metabotropic glutamate receptors through a complex composed of PSD-95, GKAP, Shank1, and Homer1b/c. GKAP binds to the GK domain of PSD-95147,217 and recruits Shank1 to the PSD via their PDZ domain.216,264 Shank1 then binds to the EVH1 domain of homer1b/c.343 Homer1b/c proteins have a coiled-coil structure in their C-terminus region that enables them to form homotetramers or heterotetramers.284 This assembly of Homers leaves 3 available EVH1 domains that can bind several other targets such as group I mGluRs (but not group 2 or 3 mGluRs),28 inositol triphosphate receptors (IP3R), or the actin cytoskeleton.284,344 The interaction between Homer1b and IP3R and between Shank1 and Homer1b/c controls local Ca2+ release from the endoplasmic reticulum upon mGluRs activation.266,267 Homer1a, a short isoform of Homer1b/c that lacks the coiled-coil structure, is an immediate early gene activated on neuronal activity and participates in remodeling synapses in an activity-dependent manner.129 Homer1a is upregulated in dorsal horn neurons after the subcutaneous injection of formalin or CFA324 as well as transiently after peripheral nerve injury.208 Factors responsible for Homer1a activation include NMDAR, ERK1/2 and Src.208,324 Knock-down of Homer1a increases pain-like behaviors specific to central sensitization and not those associated with peripheral sensitization,324 whereas overexpression reduced inflammatory pain-like behavior without altering basal nociception.324 Homer1a probably causes a disruption of the clustering properties of Homer1b/c protein and of Ca2+ release on NMDAR or mGluR activation.324 In contrast, homer1b activation leads to an increase of AMPAR activity after the stimulation of mGluR, whereas activation of Homer1a inhibits this.347 In addition, Homer1b/c proteins could play an important role in clustering group 1 mGluRs at the synapse after CFA injection,247 an effect that is reduced by Homer1a overexpression.385 Homer proteins are, therefore, convergent factors that potentially link major glutamate receptors with Ca2+ stores in neurons, and the balance between Homer1b/c and Homer1a may play an important role in the development and maintenance of central sensitization.
PSD Proteins and AMPAR Recycling and Subunit Switch
Stargazin may be important in creating the switch from GluR2- to GluR1-containing AMPAR in response to peripheral inflammation159,355 by specifically addressing GluR1-containing AMPARs to the synapse and then strengthening their activity via its ectodomain.334 Peripheral inflammation leads via PKA to a phosphorylation of Ser831 and Ser845 on GluR1 in the spinal cord,180 which, in association with CaMKII activation, promotes a transfer of GluR1-containing AMPARs to the membrane,84 where they are maintained by synaptic activity.189
GluR2-containing AMPARs are associated with high affinity to GRIP-1, which clusters the receptors at the synapse,77,114 whereas PICK-1 is a critical element for activity-dependent endocytosis of the receptor.51,96,226 NMDAR activation leads to a PKC-mediated phosphorylation of GluR2 at Ser880,172,238 which decreases GluR2's affinity for GRIP-1, whereas the increase in intracellular Ca2+ recruits PICK-1 to the synapse,51,108,238 where it reduces the clustering of GluR2-containing AM-PAR51,172,196,238,304,328 (Fig 14). On endocytosis, vesicles can either be inserted back into the synapse under the action of NSF,122,226,228,297 which disrupts the [GluR2-PICK-1] complex,109 or be maintained out of the synapse through PICK-1.172 During inflammation, NSF expression is reduced in the spinal cord,139 thereby preventing GluR2-containing AMPAR reinsertion into the synapse.
Figure 14.
Synaptic scaffolding proteins and the switch to GluR1-containing AMPAR after peripheral inflammation. Under basal conditions, GluR2-containing AMPARs are associated in the synapse with stargazin and GRIP-1. The C-terminus of stargazin binds to PSD-95 (1). Peripheral inflammation leads to glutamate release from nociceptors and AMPA and NMDA receptor activation. NMDAR activation leads to a Ca2+ influx that activates PKC (2). Activated PKC phosphorylates GluR2-containing AMPARs at ser880, which leads to a loss of affinity for stargazin and GRIP-1, allowing PICK-1 to bind to the receptor (3). The GluR2-containing AMPAR and PICK-1 complex undergo endocytosis, whereas NSF is downregulated, preventing reinsertion of GluR2-containing AMPAR into the synapse. Meanwhile, peripheral inflammation also leads to activation of PKA that promotes GluR1-containing AMPAR to be inserted into the membrane (4). Because GluR2-containing AMPARs are internalized and the Ca2+-permeable GluR1-containing AMPAR are inserted into the membrane, there is a switch from GluR2- to GluR1-containing AMPAR (5 and 6). Increased [Ca2+]i causes an activation of PKC and CaMKII, which phosphorylate the C-terminus of stargazin, increasing its affinity for PSD-95 (CaMKII) and modifying its ectodomain (PKC), which causes increased GluR1 affinity for glutamate and single-channel conductance and a higher channel opening probability, further increasing Ca2+ influx (7).
The net effect of these complex changes is an increase in GluR1 and a decrease in GluR2 containing AMPAR at the synapse (Fig 14). Once inserted into the synapse, GluR1-containing AMPAR remain there for as long as there is glutamate release,83,189 and their activation is potentially increased by stargazin's ectodomain, thereby further promoting the Ca2+-dependent pathways required for maintenance of central sensitization. In addition, the phosphorylation of stargazin's C-terminus domain by PKC and CaMKII342 increases its affinity for PSD-95, thereby re-enforcing the clustering of AMPAR with NMDAR at the synapse.276
After peripheral nerve injury, GluR2 and GRIP-1 are up-regulated in dorsal horn neurons,100,110 whereas PICK-1 expression is not modified and NSF is downregulated.100 Peptides that disrupt the binding of GRIP-1 or NSF with GluR2 partially decrease neuropathic pain-like behaviors.100 PICK-1 is also required for Gi/o-coupled mGluR7 trafficking to the membrane.306 Activation of mGluR7 can block P/Q-type Ca2+ channels240 and reduces the pain caused by injection of capsaicin218 or peripheral nerve injury.237 The upregulation of GRIP-1 but not of PICK-1 in neuropathic pain models would promote AM-PAR maintenance at the synapse but not that of mGluR7, resulting in increased excitability in these cells.
Finally, the A kinase-anchoring protein 79/150 (AKAP79/150) binds to PSD-9553 and is a scaffold for protein kinases and phosphatases,53,169 specifically trafficking enzymes within the PSD to increase (kinases) or reduce (phosphatases) synaptic transmission. When AKAP79/150 recruits PKA181,295 or PKC325 in the PSD, it promotes insertion of new AMPAR to the membrane (via PKA) as well as increasing their activity (via PKC). In contrast, recruitment of PP2B25,169 triggers AMPAR endocytosis.25 AKAP79/150 may function therefore as a “master switch” of central sensitization by promoting phosphorylation or dephosphorylation of stargazin and AMPAR.
Conclusion
Before central sensitization was discovered, there were 2 major models of pain. The first was that it was a labeled-line system, in which specific “pain pathways” were activated only by particular peripheral “pain stimuli” and that the amplitude and duration of pain was determined solely by the intensity and timing of these inputs. The second model evoked “gate controls” in the CNS, which, by opening or closing, enabled or prevented pain. Neither model envisaged, however, that pain may arise as a result of changes in the properties of neurons in the CNS: central sensitization. We now appreciate that there are indeed specific nociceptive pathways and that these are subject to complex facilitating and inhibitory controls; both models were in part correct. We also know though, that changes in the functional properties of the neurons in these pathways are sufficient to reduce pain threshold, increase the magnitude and duration of responses to noxious input, and permit normally innocuous inputs to generate pain sensations. Pain is not then simply a reflection of peripheral inputs or pathology but is also a dynamic reflection of central neuronal plasticity. The plasticity profoundly alters sensitivity to an extent that it is a -major contributor to many clinical pain syndromes and represents a major target for therapeutic intervention. The past 26 years have seen enormous advances both in our appreciation of the nature and manifestations of central sensitization and in its underlying molecular mechanisms. We have great insight into what triggers can induce central sensitization, through which signaling pathways and by means of which effectors. The complexity is daunting because the essence of central sensitization is a constantly changing mosaic of alterations in membrane excitability, reductions in inhibitory transmission, and increases in synaptic efficacy, mediated by many converging and diverging molecular players on a background of phenotypic switches and structural alterations. Nevertheless, enormous progress has been made in dissecting out where, when, and how the plasticity occurs, although clearly, more is still waiting to be learned.
Acknowledgments
Supported by the National Institutes of Health and the Fondation pour la Recherche Médicale.
Footnotes
Editor's Note: This article is 1 in a series of invited Critical Review articles designed to celebrate The Journal of Pain's 10th year anniversary of publication.
References
- 1.Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- 3.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17:8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Yamamoto T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–1454. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- 6.Ablin J, Neumann L, Buskila D. Pathogenesis of fibromyalgia: A review. Joint Bone Spine. 2008;75:273–279. doi: 10.1016/j.jbspin.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Adwanikar H, Karim F, Gereau RW. Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain. 2004;111:125–135. doi: 10.1016/j.pain.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Afrah AW, Fiska A, Gjerstad J, Gustafsson H, Tjolsen A, Olgart L, Stiller CO, Hole K, Brodin E. Spinal substance P release in vivo during the induction of long-term potentiation in dorsal horn neurons. Pain. 2002;96:49–55. doi: 10.1016/s0304-3959(01)00414-6. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE(2) selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2002;5:34–40. doi: 10.1038/nn778. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez FJ, Villalba RM, Carr PA, Grandes P, Somohano PM. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J Comp Neurol. 2000;422:464–487. doi: 10.1002/1096-9861(20000703)422:3<464::aid-cne11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Antal M, Fukazawa Y, Eordogh M, Muszil D, Molnar E, Itakura M, Takahashi M, Shigemoto R. Numbers, densities, and colocalization of AMPA- and NMDA-type glutamate receptors at individual synapses in the superficial spinal dorsal horn of rats. J Neurosci. 2008;28:9692–9701. doi: 10.1523/JNEUROSCI.1551-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronica E, Catania MV, Geurts J, Yankaya B, Troost D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: Upregulation in reactive astrocytes. Neuroscience. 2001;105:509–520. doi: 10.1016/s0306-4522(01)00181-6. [DOI] [PubMed] [Google Scholar]
- 13.Arvidsson J, Ygge J, Grant G. Cell loss in lumbar dorsal root ganglia and transganglionic degeneration after sciatic nerve resection in the rat. Brain Res. 1986;373:15–21. doi: 10.1016/0006-8993(86)90310-0. [DOI] [PubMed] [Google Scholar]
- 14.Azkue JJ, Liu XG, Zimmermann M, Sandkuhler J. Induction of long-term potentiation of C fibre-evoked spinal field potentials requires recruitment of group I, but not group II/III metabotropic glutamate receptors. Pain. 2003;106:373–379. doi: 10.1016/j.pain.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Azkue JJ, Mateos JM, Elezgarai I, Benitez R, Osorio A, Diez J, Bilbao A, Bidaurrazaga A, Grandes P. The metabotropic glutamate receptor subtype mGluR 2/3 is located at extrasynaptic loci in rat spinal dorsal horn synapses. Neurosci Lett. 2000;287:236–238. doi: 10.1016/s0304-3940(00)01189-7. [DOI] [PubMed] [Google Scholar]
- 16.Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Abeta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci. 1999;19:859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 18.Baba H, Kohno T, Moore KA, Woolf CJ. Direct activation of rat spinal dorsal horn neurons by prostaglandin E2. J Neurosci. 2001;21:1750–1756. doi: 10.1523/JNEUROSCI.21-05-01750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Battaglia AA, Sehayek K, Grist J, McMahon SB, Gavazzi I. EphB receptors and ephrin-B ligands regulate spinal sensory connectivity and modulate pain processing. Nat Neurosci. 2003;6:339–340. doi: 10.1038/nn1034. [DOI] [PubMed] [Google Scholar]
- 23.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: The search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 24.Bester H, Beggs S, Woolf CJ. Changes in tactile stimuli-induced behavior and c-Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve crush. J Comp Neurol. 2000;428:45–61. doi: 10.1002/1096-9861(20001204)428:1<45::aid-cne5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya S, Biou V, Xu W, Schluter O, Malenka RC. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat Neurosci. 2009;12:172–181. doi: 10.1038/nn.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bliddal H, Danneskiold-Samsoe B. Chronic widespread pain in the spectrum of rheumatological diseases. Best Pract Res Clin Rheumatol. 2007;21:391–402. doi: 10.1016/j.berh.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 28.Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: A protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 29.Brenchat A, Romero L, Garcia M, Pujol M, Burgueno J, Torrens A, Hamon M, Baeyens JM, Buschmann H, Zamanillo D, Vela JM. 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain. 2009;141:239–247. doi: 10.1016/j.pain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 31.Brenman JE, Christopherson KS, Craven SE, McGee AW, Bredt DS. Cloning and characterization of postsynaptic density 93, a nitric oxide synthase interacting protein. J Neurosci. 1996;16:7407–7415. doi: 10.1523/JNEUROSCI.16-23-07407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- 33.Brown AG. Neuronal organization in the dorsal horn of the spinal cord. Acta Morphol Hung. 1983;31:87–99. [PubMed] [Google Scholar]
- 34.Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 2006;99:1338–1350. doi: 10.1111/j.1471-4159.2006.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: A race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- 36.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 37.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol. 2008;38:448–458. doi: 10.1002/eji.200737485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501:780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho AL, Duarte CB, Carvalho AP. Regulation of AMPA receptors by phosphorylation. Neurochem Res. 2000;25:1245–1255. doi: 10.1023/a:1007644128886. [DOI] [PubMed] [Google Scholar]
- 41.Chapman V, Dickenson AH. The spinal and peripheral roles of bradykinin and prostaglandins in nociceptive processing in the rat. Eur J Pharmacol. 1992;219:427–433. doi: 10.1016/0014-2999(92)90484-l. [DOI] [PubMed] [Google Scholar]
- 42.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 45.Chen SR, Pan HL. Distinct roles of group III metabotropic glutamate receptors in control of nociception and dorsal horn neurons in normal and nerve-injured Rats. J Pharmacol Exp Ther. 2005;312:120–126. doi: 10.1124/jpet.104.073817. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Tanner K, Levine JD. Mechanical sensitization of cutaneous C-fiber nociceptors by prostaglandin E2 in the rat. Neurosci Lett. 1999;267:105–108. doi: 10.1016/s0304-3940(99)00345-6. [DOI] [PubMed] [Google Scholar]
- 47.Cheng HT, Suzuki M, Hegarty DM, Xu Q, Weyerbacher AR, South SM, Ohata M, Inturrisi CE. Inflammatory pain-induced signaling events following a conditional deletion of the N-methyl-D-aspartate receptor in spinal cord dorsal horn. Neuroscience. 2008;155:948–958. doi: 10.1016/j.neuroscience.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho CH, St Gelais F, Zhang W, Tomita S, Howe JR. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 50.Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX. Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund's adjuvant-induced persistent pain. Pain. 2005;119:113–123. doi: 10.1016/j.pain.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 51.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coderre TJ, Melzack R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3671–3675. doi: 10.1523/JNEUROSCI.12-09-03671.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 54.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 55.Cook AJ, Woolf CJ, Wall PD. Prolonged C-fibre mediated facilitation of the flexion reflex in the rat is not due to changes in afferent terminal or motoneurone excitability. Neurosci Lett. 1986;70:91–96. doi: 10.1016/0304-3940(86)90443-x. [DOI] [PubMed] [Google Scholar]
- 56.Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 58.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 59.Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O'Dell TJ, Grant SG. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27:2673–2682. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 61.Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 62.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 63.Davies SN, Lodge D. Evidence for involvement of N-methylaspartate receptors in ‘wind-up’ of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- 64.Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol. 2001;41:203–236. doi: 10.1146/annurev.pharmtox.41.1.203. [DOI] [PubMed] [Google Scholar]
- 65.de Novellis V, Siniscalco D, Galderisi U, Fuccio C, Nolano M, Santoro L, Cascino A, Roth KA, Rossi F, Maione S. Blockade of glutamate mGlu5 receptors in a rat model of neuropathic pain prevents early over-expression of pro-apoptotic genes and morphological changes in dorsal horn lamina II. Neuropharmacology. 2004;46:468–479. doi: 10.1016/j.neuropharm.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 66.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 67.Derjean D, Bertrand S, Le Masson G, Landry M, Morisset V, Nagy F. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat Neurosci. 2003;6:274–281. doi: 10.1038/nn1016. [DOI] [PubMed] [Google Scholar]
- 68.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420–1429. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 69.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196:115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 70.Devor M, Seltzer Z. Pathophysiology of Damaged Nerves in Relation to Chronic Pain. Edinburg, UK: Churchill Livingstone; 1999. [Google Scholar]
- 71.Devor M, Wall PD. Reorganisation of spinal cord sensory map after peripheral nerve injury. Nature. 1978;276:75–76. doi: 10.1038/276075a0. [DOI] [PubMed] [Google Scholar]
- 72.Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 73.Ding JD, Weinberg RJ. Localization of soluble guanylyl cyclase in the superficial dorsal horn. J Comp Neurol. 2006;495:668–678. doi: 10.1002/cne.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dogrul A, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR[5]) antagonist, in experimental neuropathic pain in rats. Neurosci Lett. 2000;292:115–118. doi: 10.1016/s0304-3940(00)01458-0. [DOI] [PubMed] [Google Scholar]
- 76.Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem. 2008;107:50–60. doi: 10.1111/j.1471-4159.2008.05566.x. [DOI] [PubMed] [Google Scholar]
- 77.Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: A synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 78.Dostrovsky JO, Guilbaud G. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain. 1990;40:93–104. doi: 10.1016/0304-3959(90)91056-O. [DOI] [PubMed] [Google Scholar]
- 79.Dougherty PM, Willis WD. Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capsaicin-induced sensitization in the monkey. J Neurosci. 1992;12:883–894. doi: 10.1523/JNEUROSCI.12-03-00883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dougherty PM, Willis WD. Enhancement of spinothalamic neuron responses to chemical and mechanical stimuli following combined micro-iontophoretic application of N-methyl-D-aspartic acid and substance P. Pain. 1991;47:85–93. doi: 10.1016/0304-3959(91)90015-P. [DOI] [PubMed] [Google Scholar]
- 81.Duan Y, Sahley CL, Muller KJ. ATP and NO dually control migration of microglia to nerve lesions. Dev Neurobiol. 2009;69:60–72. doi: 10.1002/dneu.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edvinsson L. CGRP-receptor antagonism in migraine treatment. Lancet. 2008;372:2089–2090. doi: 10.1016/S0140-6736(08)61710-9. [DOI] [PubMed] [Google Scholar]
- 83.Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 85.Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23:80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- 86.Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fang L, Wu J, Lin Q, Willis WD. Protein kinases regulate the phosphorylation of the GluR1 subunit of AMPA receptors of spinal cord in rats following noxious stimulation. Brain Res Mol Brain Res. 2003;118:160–165. doi: 10.1016/j.molbrainres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 88.Fang L, Wu J, Zhang X, Lin Q, Willis WD. Increased phosphorylation of the GluR1 subunit of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor in rats following intradermal injection of capsaicin. Neuro-science. 2003;122:237–245. doi: 10.1016/s0306-4522(03)00526-8. [DOI] [PubMed] [Google Scholar]
- 89.Ferguson AR, Bolding KA, Huie JR, Hook MA, Santillano DR, Miranda RC, Grau JW. Group I metabotropic glutamate receptors control metaplasticity of spinal cord learning through a protein kinase C-dependent mechanism. J Neurosci. 2008;28:11939–11949. doi: 10.1523/JNEUROSCI.3098-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fox MA, Umemori H. Seeking long-term relationship: Axon and target communicate to organize synaptic differentiation. J Neurochem. 2006;97:1215–1231. doi: 10.1111/j.1471-4159.2006.03834.x. [DOI] [PubMed] [Google Scholar]
- 91.Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fundytus ME, Osborne MG, Henry JL, Coderre TJ, Dray A. Antisense oligonucleotide knockdown of mGluR1 alleviates hyperalgesia and allodynia associated with chronic inflammation. Pharmacol Biochem Behav. 2002;73:401–410. doi: 10.1016/s0091-3057(02)00831-6. [DOI] [PubMed] [Google Scholar]
- 93.Galan A, Laird JM, Cervero F. In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain. 2004;112:315–323. doi: 10.1016/j.pain.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 94.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gardell LR, Vanderah TW, Gardell SE, Wang R, Ossipov MH, Lai J, Porreca F. Enhanced evoked excitatory transmitter release in experimental neuropathy requires descending facilitation. J Neurosci. 2003;23:8370–8379. doi: 10.1523/JNEUROSCI.23-23-08370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 97.Garraway SM, Xu Q, Inturrisi CE. siRNA-mediated knockdown of the NR1 subunit gene of the NMDA receptor attenuates formalin-induced pain behaviors in adult rats. J Pain. 2009;10:380–390. doi: 10.1016/j.jpain.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garrison CJ, Dougherty PM, Carlton SM. GFAP expression in lumbar spinal cord of naive and neuropathic rats treated with MK-801. Exp Neurol. 1994;129:237–243. doi: 10.1006/exnr.1994.1165. [DOI] [PubMed] [Google Scholar]
- 99.Garry EM, Moss A, Delaney A, O'Neill F, Blakemore J, Bowen J, Husi H, Mitchell R, Grant SG, Fleetwood-Walker SM. Neuropathic sensitization of behavioral reflexes and spinal NMDA receptor/CaM kinase II interactions are disrupted in PSD-95 mutant mice. Curr Biol. 2003;13:321–328. doi: 10.1016/s0960-9822(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 100.Garry EM, Moss A, Rosie R, Delaney A, Mitchell R, Fleetwood-Walker SM. Specific involvement in neuropathic pain of AMPA receptors and adapter proteins for the GluR2 subunit. Mol Cell Neurosci. 2003;24:10–22. doi: 10.1016/s1044-7431(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 101.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 102.Giles PA, Trezise DJ, King AE. Differential activation of protein kinases in the dorsal horn in vitro of normal and inflamed rats by group I metabotropic glutamate receptor subtypes. Neuropharmacology. 2007;53:58–70. doi: 10.1016/j.neuropharm.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 103.Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2007;13:39–44. doi: 10.1016/j.molmed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 104.Goudet C, Chapuy E, Alloui A, Acher F, Pin JP, Eschalier A. Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain. 2008;137:112–124. doi: 10.1016/j.pain.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 105.Guenther S, Reeh PW, Kress M. Rises in [Ca2+]i mediate capsaicin- and proton-induced heat sensitization of rat primary nociceptive neurons. Eur J Neurosci. 1999;11:3143–3150. doi: 10.1046/j.1460-9568.1999.00734.x. [DOI] [PubMed] [Google Scholar]
- 106.Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci. 2002;22:6208–6217. doi: 10.1523/JNEUROSCI.22-14-06208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 2005;24:3266–3278. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hanley JG, Khatri L, Hanson PI, Ziff EB. NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron. 2002;34:53–67. doi: 10.1016/s0896-6273(02)00638-4. [DOI] [PubMed] [Google Scholar]
- 110.Harris JA, Corsi M, Quartaroli M, Arban R, Bentivoglio M. Upregulation of spinal glutamate receptors in chronic pain. Neuroscience. 1996;74:7–12. doi: 10.1016/0306-4522(96)00196-0. [DOI] [PubMed] [Google Scholar]
- 111.Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schutz B, Abo-Salem OM, Zimmer A, Poisbeau P, Welzl H, Wolfer DP, Betz H, Zeilhofer HU, Muller U. GlyR alpha3: An essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 112.Henry JL. Effects of substance P on functionally identified units in cat spinal cord. Brain Res. 1976;114:439–451. doi: 10.1016/0006-8993(76)90965-3. [DOI] [PubMed] [Google Scholar]
- 113.Heppenstall PA, Lewin GR. BDNF but not NT-4 is required for normal flexion reflex plasticity and function. Proc Natl Acad Sci U S A. 2001;98:8107–8112. doi: 10.1073/pnas.141015098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hirai H. Modification of AMPA receptor clustering regulates cerebellar synaptic plasticity. Neurosci Res. 2001;39:261–267. doi: 10.1016/s0168-0102(00)00237-6. [DOI] [PubMed] [Google Scholar]
- 115.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 116.Horvath RJ, Nutile-McMenemy N, Alkaitis MS, Deleo JA. Differential migration, LPS-induced cytokine, chemokine, and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. J Neurochem. 2008;107:557–569. doi: 10.1111/j.1471-4159.2008.05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hosl K, Reinold H, Harvey RJ, Muller U, Narumiya S, Zeilhofer HU. Spinal prostaglandin E receptors of the EP2 subtype and the glycine receptor alpha3 subunit, which mediate central inflammatory hyperalgesia, do not contribute to pain after peripheral nerve injury or formalin injection. Pain. 2006;126:46–53. doi: 10.1016/j.pain.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 118.Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RW. Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci. 2007;27:13181–13191. doi: 10.1523/JNEUROSCI.0269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RW. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 120.Hu HJ, Gereau RW. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons, II: Modulation of neuronal excitability. J Neurophysiol. 2003;90:1680–1688. doi: 10.1152/jn.00341.2003. [DOI] [PubMed] [Google Scholar]
- 121.Huang Y, Erdmann N, Peng H, Zhao Y, Zheng J. The role of TNF related apoptosis-inducing ligand in neurodegenerative diseases. Cell Mol Immunol. 2005;2:113–122. [PubMed] [Google Scholar]
- 122.Huang Y, Man HY, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. S-ni-trosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron. 2005;46:533–540. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 123.Huang YY, Pittenger C, Kandel ER. A form of long-lasting, learning-related synaptic plasticity in the hippocampus induced by heterosynaptic low-frequency pairing. Proc Natl Acad Sci U S A. 2004;101:859–864. doi: 10.1073/pnas.2237201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hucho T, Levine JD. Signaling pathways in sensitization: Toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 125.Hughes DI, Scott DT, Todd AJ, Riddell JS. Lack of evidence for sprouting of Abeta afferents into the superficial laminas of the spinal cord dorsal horn after nerve section. J Neurosci. 2003;23:9491–9499. doi: 10.1523/JNEUROSCI.23-29-09491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 127.Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- 128.Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 129.Inoue Y, Udo H, Inokuchi K, Sugiyama H. Homer1a regulates the activity-induced remodeling of synaptic structures in cultured hippocampal neurons. Neuroscience. 2007;150:841–852. doi: 10.1016/j.neuroscience.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 130.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 131.Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 133.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jones TL, Sorkin LS. Activated PKA and PKC, but not CaMKIIalpha, are required for AMPA/Kainate-mediated pain behavior in the thermal stimulus model. Pain. 2005;117:259–270. doi: 10.1016/j.pain.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 135.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 136.Kapadia SE, LaMotte CC. Deafferentation-induced alterations in the rat dorsal horn, I: Comparison of peripheral nerve injury vs. rhizotomy effects on presynaptic, postsynaptic, and glial processes J Comp Neurol. 1987;266:183–197. doi: 10.1002/cne.902660205. [DOI] [PubMed] [Google Scholar]
- 137.Karim F, Hu HJ, Adwanikar H, Kaplan D, Gereau RW. Impaired inflammatory pain and thermal hyperalgesia in mice expressing neuron-specific dominant negative mitogen activated protein kinase kinase (MEK) Mol Pain. 2006;2:2. doi: 10.1186/1744-8069-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karim F, Wang CC, Gereau RW. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Katano T, Furue H, Okuda-Ashitaka E, Tagaya M, Watanabe M, Yoshimura M, Ito S. N-ethylmaleimide-sensitive fusion protein (NSF) is involved in central sensitization in the spinal cord through GluR2 subunit composition switch after inflammation. Eur J Neurosci. 2008;27:3161–3170. doi: 10.1111/j.1460-9568.2008.06293.x. [DOI] [PubMed] [Google Scholar]
- 140.Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS. New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kayser V, Elfassi IE, Aubel B, Melfort M, Julius D, Gingrich JA, Hamon M, Bourgoin S. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A-/-, 5-HT1B-/-, 5-HT2A-/-, 5-HT3A-/- and 5-HTT-/-knock-out male mice. Pain. 2007;130:235–248. doi: 10.1016/j.pain.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 144.Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kerr RC, Maxwell DJ, Todd AJ. GluR1 and GluR2/3 subunits of the AMPA-type glutamate receptor are associated with particular types of neurone in laminae I-III of the spinal dorsal horn of the rat. Eur J Neurosci. 1998;10:324–333. doi: 10.1046/j.1460-9568.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- 146.Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci. 2002;22:9086–9098. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim E, Naisbitt S, Hsueh YP, Rao A, Rothschild A, Craig AM, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 149.Klein T, Stahn S, Magerl W, Treede RD. The role of heterosynaptic facilitation in long-term potentiation (LTP) of human pain sensation. Pain. 2008 July 3; doi: 10.1016/j.pain.2008.06.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 150.Kobayashi H, Kitamura T, Sekiguchi M, Homma MK, Kabuyama Y, Konno S, Kikuchi S, Homma Y. Involvement of EphB1 receptor/EphrinB2 ligand in neuropathic pain. Spine. 2007;32:1592–1598. doi: 10.1097/BRS.0b013e318074d46a. [DOI] [PubMed] [Google Scholar]
- 151.Kohno T, Moore KA, Baba H, Woolf CJ. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol. 2003;548:131–138. doi: 10.1113/jphysiol.2002.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koltzenburg M, Lundberg LE, Torebjork HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51:207–219. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- 154.Koltzenburg M, Wahren LK, Torebjork HE. Dynamic changes of mechanical hyperalgesia in neuropathic pain states and healthy subjects depend on the ongoing activity of unmyelinated nociceptive afferents. Pflugers Arch. 1992;420:R452. [Google Scholar]
- 155.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 156.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 157.LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 159.Larsson M, Broman J. Translocation of GluR1-containing AMPA receptors to a spinal nociceptive synapse during acute noxious stimulation. J Neurosci. 2008;28:7084–7090. doi: 10.1523/JNEUROSCI.5749-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Latremoliere A, Mauborgne A, Masson J, Bourgoin S, Kayser V, Hamon M, Pohl M. Differential implication of proinflammatory cytokine interleukin-6 in the development of cephalic versus extracephalic neuropathic pain in rats. J Neurosci. 2008;28:8489–8501. doi: 10.1523/JNEUROSCI.2552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 162.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 163.Lee SE, Kim JH. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci Res. 2007;58:245–249. doi: 10.1016/j.neures.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 164.Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lefebvre C, Fisher K, Cahill CM, Coderre TJ. Evidence that DHPG-induced nociception depends on glutamate release from primary afferent C-fibres. Neuroreport. 2000;11:1631–1635. doi: 10.1097/00001756-200006050-00007. [DOI] [PubMed] [Google Scholar]
- 166.Lekan HA, Carlton SM, Coggeshall RE. Sprouting of A beta fibers into lamina II of the rat dorsal horn in peripheral neuropathy. Neurosci Lett. 1996;208:147–150. doi: 10.1016/0304-3940(96)12566-0. [DOI] [PubMed] [Google Scholar]
- 167.Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- 168.Leonard AS, Hell JW. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D-aspartate receptors at different sites. J Biol Chem. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- 169.Lester LB, Faux MC, Nauert JB, Scott JD. Targeted protein kinase A and PP-2B regulate insulin secretion through reversible phosphorylation. Endocrinology. 2001;142:1218–1227. doi: 10.1210/endo.142.3.8023. [DOI] [PubMed] [Google Scholar]
- 170.Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 171.Lever IJ, Pezet S, McMahon SB, Malcangio M. The signaling components of sensory fiber transmission involved in the activation of ERK MAP kinase in the mouse dorsal horn. Mol Cell Neurosci. 2003;24:259–270. doi: 10.1016/s1044-7431(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 172.Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin Q, Palecek J, Paleckova V, Peng YB, Wu J, Cui M, Willis WD. Nitric oxide mediates the central sensitization of primate spinothalamic tract neurons. J Neurophysiol. 1999;81:1075–1085. doi: 10.1152/jn.1999.81.3.1075. [DOI] [PubMed] [Google Scholar]
- 174.Lin Q, Peng YB, Willis WD. Inhibition of primate spinothalamic tract neurons by spinal glycine and GABA is reduced during central sensitization. J Neurophysiol. 1996;76:1005–1014. doi: 10.1152/jn.1996.76.2.1005. [DOI] [PubMed] [Google Scholar]
- 175.Lin Q, Peng YB, Willis WD. Possible role of protein kinase C in the sensitization of primate spinothalamic tract neurons. J Neurosci. 1996;16:3026–3034. doi: 10.1523/JNEUROSCI.16-09-03026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin Q, Peng YB, Wu J, Willis WD. Involvement of cGMP in nociceptive processing by and sensitization of spinothalamic neurons in primates. J Neurosci. 1997;17:3293–3302. doi: 10.1523/JNEUROSCI.17-09-03293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu XG, Sandkuhler J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-D-aspartic acid receptor blockage. Neurosci Lett. 1995;191:43–46. doi: 10.1016/0304-3940(95)11553-0. [DOI] [PubMed] [Google Scholar]
- 178.Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325–1332. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lu VB, Biggs JE, Stebbing MJ, Balasubramanyan S, Todd KG, Lai AY, Colmers WF, Dawbarn D, Ballanyi K, Smith PA. Brain-derived neurotrophic factor drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J Physiol. 2009;587(Pt 5):1013–1032. doi: 10.1113/jphysiol.2008.166306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu Y, Sun YN, Wu X, Sun Q, Liu FY, Xing GG, Wan Y. Role of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor subunit GluR1 in spinal dorsal horn in inflammatory nociception and neuropathic nociception in rat. Brain Res. 2008;1200:19–26. doi: 10.1016/j.brainres.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 181.Lu Y, Zhang M, Lim IA, Hall DD, Allen M, Medvedeva Y, McKnight GS, Usachev YM, Hell JW. AKAP150-anchored PKA activity is important for LTD during its induction phase. J Physiol. 2008;586:4155–4164. doi: 10.1113/jphysiol.2008.151662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Luo C, Seeburg PH, Sprengel R, Kuner R. Activity-dependent potentiation of calcium signals in spinal sensory networks in inflammatory pain states. Pain. 2008;140:358–367. doi: 10.1016/j.pain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 183.Ma QP, Woolf CJ. Involvement of neurokinin receptors in the induction but not the maintenance of mechanical allodynia in rat flexor motoneurones. J Physiol. 1995;486(Pt 3):769–777. doi: 10.1113/jphysiol.1995.sp020852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ma QP, Woolf CJ. Noxious stimuli induce an N-methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: Implications for the treatment of mechanical allodynia. Pain. 1995;61:383–390. doi: 10.1016/0304-3959(94)00195-K. [DOI] [PubMed] [Google Scholar]
- 185.Ma QP, Woolf CJ. Progressive tactile hypersensitivity: An inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- 186.Maeda T, Hamabe W, Gao Y, Fukazawa Y, Kumamoto K, Ozaki M, Kishioka S. Morphine has an antinociceptive effect through activation of the okadaic-acid-sensitive Ser/Thr protein phosphatases PP 2 A and PP5 estimated by tail-pinch test in mice. Brain Res. 2005;1056:191–199. doi: 10.1016/j.brainres.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 187.Maihofner C, Jesberger F, Seifert F, Kaltenhauser M. Cortical processing of mechanical hyperalgesia: A MEG study. Eur J Pain. 2009 April 3; doi: 10.1016/j.ejpain.2009.02.007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 188.Malenka RC, Bear Mf. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 189.Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci U S A. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: Peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mannion RJ, Doubell TP, Coggeshall RE, Woolf CJ. Collateral sprouting of uninjured primary afferent A-fibers into the superficial dorsal horn of the adult rat spinal cord after topical capsaicin treatment to the sciatic nerve. J Neurosci. 1996;16:5189–5195. doi: 10.1523/JNEUROSCI.16-16-05189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 193.Mao J, Price DD, Hayes RL, Lu J, Mayer DJ, Frenk H. Intrathecal treatment with dextrorphan or ketamine potently reduces pain-related behaviors in a rat model of peripheral mononeuropathy. Brain Res. 1993;605:164–168. doi: 10.1016/0006-8993(93)91368-3. [DOI] [PubMed] [Google Scholar]
- 194.Marabese I, de Novellis V, Palazzo E, Scafuro MA, Vita D, Rossi F, Maione S. Effects of (S)-3,4-DCPG, an mGlu8 receptor agonist, on inflammatory and neuropathic pain in mice. Neuropharmacology. 2007;52:253–262. doi: 10.1016/j.neuropharm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 195.Maratou K, Wallace VC, Hasnie FS, Okuse K, Hosseini R, Jina N, Blackbeard J, Pheby T, Orengo C, Dickenson AH, McMahon SB, Rice AS. Comparison of dorsal root ganglion gene expression in rat models of traumatic and HIV-associated neuropathic pain. Eur J Pain. 2009;13:387–398. doi: 10.1016/j.ejpain.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- 197.Mauceri D, Gardoni F, Marcello E, Di Luca M. Dual role of CaMKII-dependent SAP97 phosphorylation in mediating trafficking and insertion of NMDA receptor subunit NR2A. J Neurochem. 2007;100:1032–1046. doi: 10.1111/j.1471-4159.2006.04267.x. [DOI] [PubMed] [Google Scholar]
- 198.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 199.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- 201.Meunier A, Latremoliere A, Dominguez E, Mauborgne A, Philippe S, Hamon M, Mallet J, Benoliel JJ, Pohl M. Lentiviral-mediated targeted NF-kappaB blockade in dorsal spinal cord glia attenuates sciatic nerve injury-induced neuropathic pain in the rat. Mol Ther. 2007;15:687–697. doi: 10.1038/sj.mt.6300107. [DOI] [PubMed] [Google Scholar]
- 202.Mi R, Sia GM, Rosen K, Tang X, Moghekar A, Black JL, McEnery M, Huganir RL, O'Brien RJ. AMPA receptor-dependent clustering of synaptic NMDA receptors is mediated by Stargazin and NR2A/B in spinal neurons and hippocampal interneurons. Neuron. 2004;44:335–349. doi: 10.1016/j.neuron.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 203.Miletic G, Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain. 2008;137:532–539. doi: 10.1016/j.pain.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Milligan ED, Sloane EM, Watkins LR. Glia in pathological pain: A role for fractalkine. J Neuroimmunol. 2008;198:113–120. doi: 10.1016/j.jneuroim.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O'Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 207.Minami T, Nishihara I, Uda R, Ito S, Hyodo M, Hayaishi O. Involvement of glutamate receptors in allodynia induced by prostaglandins E2 and F2 alpha injected into conscious mice. Pain. 1994;57:225–231. doi: 10.1016/0304-3959(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 208.Miyabe T, Miletic G, Miletic V. Loose ligation of the sciatic nerve in rats elicits transient up-regulation of Homer1a gene expression in the spinal dorsal horn. Neurosci Lett. 2006;398:296–299. doi: 10.1016/j.neulet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 209.Mohr C, Leyendecker S, Mangels I, Machner B, Sander T, Helmchen C. Central representation of cold-evoked pain relief in capsaicin induced pain: An event-related fMRI study. Pain. 2008;139:416–430. doi: 10.1016/j.pain.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 210.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moreno-Lopez B, Gonzalez-Forero D. Nitric oxide and synaptic dynamics in the adult brain: Physiopathological aspects. Rev Neurosci. 2006;17:309–357. doi: 10.1515/revneuro.2006.17.3.309. [DOI] [PubMed] [Google Scholar]
- 212.Moylan Governo RJ, Morris PG, Prior MJ, Marsden CA, Chapman V. Capsaicin-evoked brain activation and central sensitization in anaesthetised rats: A functional magnetic resonance imaging study. Pain. 2006;126:35–45. doi: 10.1016/j.pain.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 213.Muller F, Heinke B, Sandkuhler J. Reduction of glycine receptor-mediated miniature inhibitory postsynaptic currents in rat spinal lamina I neurons after peripheral inflammation. Neuroscience. 2003;122:799–805. doi: 10.1016/j.neuroscience.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 214.Nagy GG, Al-Ayyan M, Andrew D, Fukaya M, Watanabe M, Todd AJ. Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-unmasking method. J Neurosci. 2004;24:5766–5777. doi: 10.1523/JNEUROSCI.1237-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nagy GG, Watanabe M, Fukaya M, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004;20:3301–3312. doi: 10.1111/j.1460-9568.2004.03798.x. [DOI] [PubMed] [Google Scholar]
- 216.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 217.Naisbitt S, Kim E, Weinberg RJ, Rao A, Yang FC, Craig AM, Sheng M. Characterization of guanylate kinase-associated protein, a postsynaptic density protein at excitatory synapses that interacts directly with postsynaptic density-95/synapse-associated protein 90. J Neurosci. 1997;17:5687–5696. doi: 10.1523/JNEUROSCI.17-15-05687.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Neugebauer V, Chen PS, Willis WD. Groups II and III metabotropic glutamate receptors differentially modulate brief and prolonged nociception in primate STT cells. J Neurophysiol. 2000;84:2998–3009. doi: 10.1152/jn.2000.84.6.2998. [DOI] [PubMed] [Google Scholar]
- 219.Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol. 2003;89:716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- 220.Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: Differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Neugebauer V, Lucke T, Schaible HG. Requirement of metabotropic glutamate receptors for the generation of inflammation-evoked hyperexcitability in rat spinal cord neurons. Eur J Neurosci. 1994;6:1179–1186. doi: 10.1111/j.1460-9568.1994.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 222.Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKC gamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 224.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 225.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 226.Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 227.Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- 228.Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- 229.Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K. Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J Neurosci. 1995;15:7633–7643. doi: 10.1523/JNEUROSCI.15-11-07633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55:1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 231.Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci. 2004;20:2881–2895. doi: 10.1111/j.1460-9568.2004.03754.x. [DOI] [PubMed] [Google Scholar]
- 232.Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci. 2003;23:4117–4126. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci. 2005;8:853–854. doi: 10.1038/nn1476. [DOI] [PubMed] [Google Scholar]
- 234.Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci. 2007;34:503–518. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 235.Okamoto M, Baba H, Goldstein PA, Higashi H, Shimoji K, Yoshimura M. Functional reorganization of sensory pathways in the rat spinal dorsal horn following peripheral nerve injury. J Physiol. 2001;532:241–250. doi: 10.1111/j.1469-7793.2001.0241g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulin-dependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- 237.Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 2008;139:117–126. doi: 10.1016/j.pain.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 238.Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009;29:3206–3219. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Park JS, Yaster M, Guan X, Xu JT, Shih MH, Guan Y, Raja SN, Tao YX. Role of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund's adjuvant-induced inflammatory pain. Mol Pain. 2008;4:67. doi: 10.1186/1744-8069-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Perroy J, Gutierrez GJ, Coulon V, Bockaert J, Pin JP, Fagni L. The C terminus of the metabotropic glutamate receptor subtypes 2 and 7 specifies the receptor signaling pathways. J Biol Chem. 2001;276:45800–45805. doi: 10.1074/jbc.M106876200. [DOI] [PubMed] [Google Scholar]
- 241.Petcu M, Dias JP, Ongali B, Thibault G, Neugebauer W, Couture R. Role of kinin B1 and B2 receptors in a rat model of neuropathic pain. Int Immunopharmacol. 2008;8:188–196. doi: 10.1016/j.intimp.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 242.Petho G, Derow A, Reeh PW. Bradykinin-induced nociceptor sensitization to heat is mediated by cyclooxygenase products in isolated rat skin. Eur J Neurosci. 2001;14:210–218. doi: 10.1046/j.0953-816x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 243.Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultra-structural localization patterns similar to those of NR1. J Neurosci. 1994;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 245.Pezet S, Malcangio M, Lever IJ, Perkinton MS, Thompson SW, Williams RJ, McMahon SB. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol Cell Neurosci. 2002;21:684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- 246.Pezet S, Marchand F, D'Mello R, Grist J, Clark AK, Malcangio M, Dickenson AH, Williams RJ, McMahon SB. Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions. J Neurosci. 2008;28:4261–4270. doi: 10.1523/JNEUROSCI.5392-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pitcher MH, Ribeiro-da-Silva A, Coderre TJ. Effects of inflammation on the ultrastructural localization of spinal cord dorsal horn group I metabotropic glutamate receptors. J Comp Neurol. 2007;505:412–423. doi: 10.1002/cne.21506. [DOI] [PubMed] [Google Scholar]
- 248.Polgar E, Al-Khater KM, Shehab S, Watanabe M, Todd AJ. Large projection neurons in lamina I of the rat spinal cord that lack the neurokinin 1 receptor are densely innervated by VGLUT2-containing axons and possess GluR4-containing AMPA receptors. J Neurosci. 2008;28:13150–13160. doi: 10.1523/JNEUROSCI.4053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Polgar E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I-III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111:144–150. doi: 10.1016/j.pain.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 250.Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 251.Polgar E, Todd AJ. Tactile allodynia can occur in the spared nerve injury model in the rat without selective loss of GABA or GABA(A) receptors from synapses in laminae I-II of the ipsilateral spinal dorsal horn. Neuroscience. 2008;156:193–202. doi: 10.1016/j.neuroscience.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Polgar E, Watanabe M, Hartmann B, Grant SG, Todd AJ. Expression of AMPA receptor subunits at synapses in laminae I-III of the rodent spinal dorsal horn. Mol Pain. 2008;4:5. doi: 10.1186/1744-8069-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7:529–535. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 254.Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Qu XX, Cai J, Li MJ, Chi YN, Liao FF, Liu FY, Wan Y, Han JS, Xing GG. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol. 2009;215:298–307. doi: 10.1016/j.expneurol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 256.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 257.Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: Mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104:655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- 258.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 259.Raman IM, Tong G, Jahr CE. Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron. 1996;16:415–421. doi: 10.1016/s0896-6273(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 260.Regehr WG, Tank DW. Postsynaptic NMDA receptor-mediated calcium accumulation in hippocampal CA1 pyramidal cell dendrites. Nature. 1990;345:807–810. doi: 10.1038/345807a0. [DOI] [PubMed] [Google Scholar]
- 261.Rodriguez Parkitna J, Korostynski M, Kaminska-Chowaniec D, Obara I, Mika J, Przewlocka B. Przewlocki R: Comparison of gene expression profiles in neuropathic and inflammatory pain. J Physiol Pharmacol. 2006;57:401–414. [PubMed] [Google Scholar]
- 262.Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, Beitz AJ, Lee JH. Depletion of capsaicin-sensitive afferents prevents lamina-dependent increases in spinal N-methyl-D-aspartate receptor subunit 1 expression and phosphorylation associated with thermal hyperalgesia in neuropathic rats. Eur J Pain. 2008;12:552–563. doi: 10.1016/j.ejpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 263.Romero-Sandoval EA, Horvath RJ, DeLeo JA. Neuroimmune interactions and pain: Focus on glial-modulating targets. Curr Opin Investig Drugs. 2008;9:726–734. [PMC free article] [PubMed] [Google Scholar]
- 264.Romorini S, Piccoli G, Jiang M, Grossano P, Tonna N, Passafaro M, Zhang M, Sala C. A functional role of postsynaptic density-95-guanylate kinase-associated protein complex in regulating Shank assembly and stability to synapses. J Neurosci. 2004;24:9391–9404. doi: 10.1523/JNEUROSCI.3314-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci. 2003;23:4567–4576. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 267.Sala C, Roussignol G, Meldolesi J, Fagni L. Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J Neurosci. 2005;25:4587–4592. doi: 10.1523/JNEUROSCI.4822-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Salter MW, Kalia LV. Src kinases: A hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 269.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 270.Sandkuhler J. Understanding LTP in pain pathways. Mol Pain. 2007;3:9. doi: 10.1186/1744-8069-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sandkuhler J, Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci. 1998;10:2476–2480. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- 272.Sarkar S, Aziz Q, Woolf CJ, Hobson AR, Thompson DG. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet. 2000;356:1154–1159. doi: 10.1016/S0140-6736(00)02758-6. [DOI] [PubMed] [Google Scholar]
- 273.Sato Y, Tao YX, Su Q, Johns RA. Post-synaptic density-93 mediates tyrosine-phosphorylation of the N-methyl-d-aspartate receptors. Neuroscience. 2008;153:700–708. doi: 10.1016/j.neuroscience.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schaible HG, Schmelz M, Tegeder I. Pathophysiology and treatment of pain in joint disease. Adv Drug Deliv Rev. 2006;58:323–342. doi: 10.1016/j.addr.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 275.Schmidtko A, Gao W, Konig P, Heine S, Motterlini R, Ruth P, Schlossmann J, Koesling D, Niederberger E, Tegeder I, Friebe A, Geisslinger G. cGMP produced by NO-sensitive guanylyl cyclase essentially contributes to inflammatory and neuropathic pain by using targets different from cGMP-dependent protein kinase I. J Neurosci. 2008;28:8568–8576. doi: 10.1523/JNEUROSCI.2128-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138:514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, Klocker N. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- 280.Seltzer Z, Cohn S, Ginzburg R, Beilin B. Modulation of neuropathic pain behavior in rats by spinal disinhibition and NMDA receptor blockade of injury discharge. Pain. 1991;45:69–75. doi: 10.1016/0304-3959(91)90166-U. [DOI] [PubMed] [Google Scholar]
- 281.Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006;51:623–630. doi: 10.1016/j.neuropharm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 282.Sherrington CS. Observations on the scratch-reflex in the spinal dog. J Physiol. 1906;34:1–50. doi: 10.1113/jphysiol.1906.sp001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shih YY, Chiang YC, Chen JC, Huang CH, Chen YY, Liu RS, Chang C, Jaw FS. Brain nociceptive imaging in rats using (18)f-fluorodeoxyglucose small-animal positron emission tomography. Neuroscience. 2008;155:1221–1226. doi: 10.1016/j.neuroscience.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 284.Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Shortland P, Kinman E, Molander C. Sprouting of A-fibre primary afferents into lamina II in two rat models of neuropathic pain. Eur J Pain. 1997;1:215–227. doi: 10.1016/s1090-3801(97)90107-5. [DOI] [PubMed] [Google Scholar]
- 286.Simmons RM, Webster AA, Kalra AB, Iyengar S. Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol Biochem Behav. 2002;73:419–427. doi: 10.1016/s0091-3057(02)00849-3. [DOI] [PubMed] [Google Scholar]
- 287.Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: Central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- 288.Siniscalco D, Giordano C, Fuccio C, Luongo L, Ferraraccio F, Rossi F, de Novellis V, Roth KA, Maione S. Involvement of subtype 1 metabotropic glutamate receptors in apoptosis and caspase-7 over-expression in spinal cord of neuropathic rats. Pharmacol Res. 2008;57:223–233. doi: 10.1016/j.phrs.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: Disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 290.Sivilotti LG, Thompson SW, Woolf CJ. Rate of rise of the cumulative depolarization evoked by repetitive stimulation of small-caliber afferents is a predictor of action potential windup in rat spinal neurons in vitro. J Neurophysiol. 1993;69:1621–1631. doi: 10.1152/jn.1993.69.5.1621. [DOI] [PubMed] [Google Scholar]
- 291.Slack S, Battaglia A, Cibert-Goton V, Gavazzi I. EphrinB2 induces tyrosine phosphorylation of NR2B via Src-family kinases during inflammatory hyperalgesia. Neuroscience. 2008;156:175–183. doi: 10.1016/j.neuroscience.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Slack SE, Grist J, Mac Q, McMahon SB, Pezet S. TrkB expression and phospho-ERK activation by brain-derived neurotrophic factor in rat spinothalamic tract neurons. J Comp Neurol. 2005;489:59–68. doi: 10.1002/cne.20606. [DOI] [PubMed] [Google Scholar]
- 293.Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci. 2004;20:1769–1778. doi: 10.1111/j.1460-9568.2004.03656.x. [DOI] [PubMed] [Google Scholar]
- 294.Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD. Inhibitors of G-proteins and protein kinases reduce the sensitization to mechanical stimulation and the desensitization to heat of spinothalamic tract neurons induced by intradermal injection of capsaicin in the primate. Exp Brain Res. 1997;115:15–24. doi: 10.1007/pl00005675. [DOI] [PubMed] [Google Scholar]
- 295.Snyder EM, Colledge M, Crozier RA, Chen WS, Scott JD, Bear MF. Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J Biol Chem. 2005;280:16962–16968. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Soliman AC, Yu JS, Coderre TJ. mGlu and NMDA receptor contributions to capsaicin-induced thermal and mechanical hypersensitivity. Neuropharmacology. 2005;48:325–332. doi: 10.1016/j.neuropharm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 297.Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 298.Song XJ, Cao JL, Li HC, Zheng JH, Song XS, Xiong LZ. Up-regulation and redistribution of ephrinB and EphB receptor in dorsal root ganglion and spinal dorsal horn neurons after peripheral nerve injury and dorsal rhizotomy. Eur J Pain. 2008;12:1031–1039. doi: 10.1016/j.ejpain.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 299.Song XJ, Zheng JH, Cao JL, Liu WT, Song XS, Huang ZJ. EphrinB-EphB receptor signaling contributes to neuropathic pain by regulating neural excitability and spinal synaptic plasticity in rats. Pain. 2008;139:168–180. doi: 10.1016/j.pain.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 300.South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulavsky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002;4:299–305. doi: 10.1007/s11926-002-0038-5. [DOI] [PubMed] [Google Scholar]
- 302.Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–323. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 305.Stephenson FA, Cousins SL, Kenny AV. Assembly and forward trafficking of NMDA receptors (Review) Mol Membr Biol. 2008;25:311–320. doi: 10.1080/09687680801971367. [DOI] [PubMed] [Google Scholar]
- 306.Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain CJ, Roche KW. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron. 2008;58:736–748. doi: 10.1016/j.neuron.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/s0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 308.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 309.Susswein AJ, Katzoff A, Miller N, Hurwitz I. Nitric oxide and memory. Neuroscientist. 2004;10:153–162. doi: 10.1177/1073858403261226. [DOI] [PubMed] [Google Scholar]
- 310.Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- 311.Svensson CI, Hua XY, Protter AA, Powell HC, Yaksh TL. Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport. 2003;14:1153–1157. doi: 10.1097/00001756-200306110-00010. [DOI] [PubMed] [Google Scholar]
- 312.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 313.Svensson CI, Tran TK, Fitzsimmons B, Yaksh TL, Hua XY. Descending serotonergic facilitation of spinal ERK activation and pain behavior. FEBS Lett. 2006;580:6629–6634. doi: 10.1016/j.febslet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- 315.Tal M, Bennett GJ. Neuropathic pain sensations are differentially sensitive to dextrorphan. Neuroreport. 1994;5:1438–1440. doi: 10.1097/00001756-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 316.Tanabe M, Nagatani Y, Saitoh K, Takasu K, Ono H. Pharmacological assessments of nitric oxide synthase isoforms and downstream diversity of NO signaling in the maintenance of thermal and mechanical hypersensitivity after peripheral nerve injury in mice. Neuropharmacology. 2009;56:702–708. doi: 10.1016/j.neuropharm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 317.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tao F, Skinner J, Su Q, Johns RA. New role for spinal Stargazin in alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor-mediated pain sensitization after inflammation. J Neurosci Res. 2006;84:867–873. doi: 10.1002/jnr.20973. [DOI] [PubMed] [Google Scholar]
- 319.Tao F, Su Q, Johns RA. Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice. Mol Ther. 2008;16:1776–1782. doi: 10.1038/mt.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tao F, Tao YX, Gonzalez JA, Fang M, Mao P, Johns RA. Knockdown of PSD-95/SAP90 delays the development of neuropathic pain in rats. Neuroreport. 2001;12:3251–3255. doi: 10.1097/00001756-200110290-00022. [DOI] [PubMed] [Google Scholar]
- 321.Tao YX, Huang YZ, Mei L, Johns RA. Expression of PSD-95/SAP90 is critical for N-methyl-D-aspartate receptor-mediated thermal hyperalgesia in the spinal cord. Neuroscience. 2000;98:201–206. doi: 10.1016/s0306-4522(00)00193-7. [DOI] [PubMed] [Google Scholar]
- 322.Tao YX, Johns RA. Activation and up-regulation of spinal cord nitric oxide receptor, soluble guanylate cyclase, after formalin injection into the rat hind paw. Neuroscience. 2002;112:439–446. doi: 10.1016/s0306-4522(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 323.Tao YX, Rumbaugh G, Wang GD, Petralia RS, Zhao C, Kauer FW, Tao F, Zhuo M, Wenthold RJ, Raja SN, Huganir RL, Bredt DS, Johns RA. Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci. 2003;23:6703–6712. doi: 10.1523/JNEUROSCI.23-17-06703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Tappe A, Klugmann M, Luo C, Hirlinger D, Agarwal N, Benrath J, Ehrengruber MU, During MJ, Kuner R. Synaptic scaffolding protein Homer1a protects against chronic inflammatory pain. Nat Med. 2006;12:677–681. doi: 10.1038/nm1406. [DOI] [PubMed] [Google Scholar]
- 325.Tavalin SJ. AKAP79 selectively enhances protein kinase C regulation of GluR1 at a Ca2+-calmodulin-dependent protein kinase II/protein kinase C site. J Biol Chem. 2008;283:11445–11452. doi: 10.1074/jbc.M709253200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Tawfik VL, Regan MR, Haenggeli C, Lacroix-Fralish ML, Nutile-McMenemy N, Perez N, Rothstein JD, DeLeo JA. Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection. Neuroscience. 2008;152:1086–1092. doi: 10.1016/j.neuroscience.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, Wu T, Allchorne A, Diatchenko L, Binshtok AM, Goldman D, Adolph J, Sama S, Atlas SJ, Carlezon WA, Parsegian A, Lotsch J, Fillingim RB, Maixner W, Geisslinger G, Max MB, Woolf CJ. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 328.Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, McBain CJ, Isaac JT. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci U S A. 1999;96:435–440. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, McMahon SB. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci U S A. 1999;96:7714–7718. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Thompson SW, King AE, Woolf CJ. Activity-dependent changes in rat ventral horn neurons in vitro: Summation of prolonged afferent evoked postsynaptic depolarizations produce a d-2-amino-5-phosphonovaleric acid sensitive windup. Eur J Neurosci. 1990;2:638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 332.Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- 333.Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 334.Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- 335.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology. 2007;52:87–91. doi: 10.1016/j.neuropharm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 337.Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 338.Tong CK, MacDermott AB. Both Ca2+-permeable and -impermeable AMPA receptors contribute to primary synaptic drive onto rat dorsal horn neurons. J Physiol. 2006;575:133–144. doi: 10.1113/jphysiol.2006.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Tong YG, Wang HF, Ju G, Grant G, Hokfelt T, Zhang X. Increased uptake and transport of cholera toxin B-subunit in dorsal root ganglion neurons after peripheral axotomy: possible implications for sensory sprouting. J Comp Neurol. 1999;404:143–158. [PubMed] [Google Scholar]
- 340.Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Tsui J, Malenka RC. Substrate localization creates specificity in calcium/calmodulin-dependent protein kinase II signaling at synapses. 2006;281:13794–13804. doi: 10.1074/jbc.M600966200. [DOI] [PubMed] [Google Scholar]
- 343.Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 344.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 345.Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006;399:85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 346.Valerio A, Paterlini M, Boifava M, Memo M, Spano P. Metabotropic glutamate receptor mRNA expression in rat spinal cord. Neuroreport. 1997;8:2695–2699. doi: 10.1097/00001756-199708180-00012. [DOI] [PubMed] [Google Scholar]
- 347.Van Keuren-Jensen K, Cline HT. Visual experience regulates metabotropic glutamate receptor-mediated plasticity of AMPA receptor synaptic transmission by homer1a induction. J Neurosci. 2006;26:7575–7580. doi: 10.1523/JNEUROSCI.5083-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.van Rossum D, Hanisch UK. Microglia. Metab Brain Dis. 2004;19:393–411. doi: 10.1023/b:mebr.0000043984.73063.d8. [DOI] [PubMed] [Google Scholar]
- 349.Vardeh D, Wang D, Costigan M, Lazarus M, Saper CB, Woolf CJ, Fitzgerald GA, Samad TA. COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J Clin Invest. 2009;119:287–294. doi: 10.1172/JCI37098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Vasko MR, Campbell WB, Waite KJ. Prostaglandin E2 enhances bradykinin-stimulated release of neuropeptides from rat sensory neurons in culture. J Neurosci. 1994;14:4987–4997. doi: 10.1523/JNEUROSCI.14-08-04987.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 352.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 353.Vikman K, Robertson B, Grant G, Liljeborg A, Kristensson K. Interferon-gamma receptors are expressed at synapses in the rat superficial dorsal horn and lateral spinal nucleus. J Neurocytol. 1998;27:749–759. doi: 10.1023/a:1006903002044. [DOI] [PubMed] [Google Scholar]
- 354.Vikman KS, Hill RH, Backstrom E, Robertson B, Kristensson K. Interferon-gamma induces characteristics of central sensitization in spinal dorsal horn neurons in vitro. Pain. 2003;106:241–251. doi: 10.1016/S0304-3959(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 355.Vikman KS, Rycroft BK, Christie MJ. Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol. 2008;586:515–527. doi: 10.1113/jphysiol.2007.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Vogel C, Mossner R, Gerlach M, Heinemann T, Murphy DL, Riederer P, Lesch KP, Sommer C. Absence of thermal hyperalgesia in serotonin transporter-deficient mice. J Neurosci. 2003;23:708–715. doi: 10.1523/JNEUROSCI.23-02-00708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Walker SM, Meredith-Middleton J, Lickiss T, Moss A, Fitzgerald M. Primary and secondary hyperalgesia can be differentiated by postnatal age and ERK activation in the spinal dorsal horn of the rat pup. Pain. 2007;128:157–168. doi: 10.1016/j.pain.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 358.Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wang H, Kohno T, Amaya F, Brenner GJ, Ito N, Allchorne A, Ji RR, Woolf CJ. Bradykinin produces pain hypersensitivity by potentiating spinal cord glutamatergic synaptic transmission. J Neurosci. 2005;25:7986–7992. doi: 10.1523/JNEUROSCI.2393-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: Implications of immune activation for pain control and increasing the efficacy of opioids”. Brain Res Rev. 2007;56:148–169. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Watkins LR, Maier SF. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 362.Watkins LR, Milligan ED, Maier SF. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 363.Wei F, Vadakkan KI, Toyoda H, Wu LJ, Zhao MG, Xu H, Shum FW, Jia YH, Zhuo M. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci. 2006;26:851–861. doi: 10.1523/JNEUROSCI.3292-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol. 2001;532:823–833. doi: 10.1111/j.1469-7793.2001.0823e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Willis WD. Long-term potentiation in spinothalamic neurons. Brain Res Brain Res Rev. 2002;40:202–214. doi: 10.1016/s0165-0173(02)00202-3. [DOI] [PubMed] [Google Scholar]
- 366.Woodbury CJ, Kullmann FA, McIlwrath SL, Koerber HR. Identity of myelinated cutaneous sensory neurons projecting to nocireceptive laminae following nerve injury in adult mice. J Comp Neurol. 2008;508:500–509. doi: 10.1002/cne.21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Woolf C, Wiesenfeld-Hallin Z. Substance P and calcitonin gene-related peptide synergistically modulate the gain of the nociceptive flexor withdrawal reflex in the rat. Neurosci Lett. 1986;66:226–230. doi: 10.1016/0304-3940(86)90195-3. [DOI] [PubMed] [Google Scholar]
- 368.Woolf CJ. Central sensitization: Uncovering the relation between pain and plasticity. Anesthesiology. 2007;106:864–867. doi: 10.1097/01.anes.0000264769.87038.55. [DOI] [PubMed] [Google Scholar]
- 369.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 370.Woolf CJ. Phenotypic modification of primary sensory neurons: The role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441–448. doi: 10.1098/rstb.1996.0040. [DOI] [PubMed] [Google Scholar]
- 371.Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–108. [PubMed] [Google Scholar]
- 372.Woolf CJ, King AE. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci. 1990;10:2717–2726. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Woolf CJ, King AE. Subthreshold components of the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat lumbar spinal cord. J Neurophysiol. 1989;62:907–916. doi: 10.1152/jn.1989.62.4.907. [DOI] [PubMed] [Google Scholar]
- 374.Woolf CJ, Ma Q. Nociceptors: Noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 375.Woolf CJ, Ma QP, Allchorne A, Poole S. Peripheral cell types contributing to the hyperalgesic action of nerve growth factor in inflammation. J Neurosci. 1996;16:2716–2723. doi: 10.1523/JNEUROSCI.16-08-02716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 377.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 378.Woolf CJ, Shortland P, Reynolds M, Ridings J, Doubell T, Coggeshall RE. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J Comp Neurol. 1995;360:121–134. doi: 10.1002/cne.903600109. [DOI] [PubMed] [Google Scholar]
- 379.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation: Implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 380.Woolf CJ, Walters ET. Common patterns of plasticity contributing to nociceptive sensitization in mammals and Aplysia. Trends Neurosci. 1991;14:74–78. doi: 10.1016/0166-2236(91)90024-o. [DOI] [PubMed] [Google Scholar]
- 381.Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain. 2001;94:47–58. doi: 10.1016/S0304-3959(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 382.Wu J, Lin Q, Lu Y, Willis WD, Westlund KN. Changes in nitric oxide synthase isoforms in the spinal cord of rat following induction of chronic arthritis. Exp Brain Res. 1998;118:457–465. doi: 10.1007/s002210050302. [DOI] [PubMed] [Google Scholar]
- 383.Wu J, Lin Q, McAdoo DJ, Willis WD. Nitric oxide contributes to central sensitization following intradermal injection of capsaicin. Neuroreport. 1998;9:589–592. doi: 10.1097/00001756-199803090-00005. [DOI] [PubMed] [Google Scholar]
- 384.Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- 385.Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 386.Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Xu Q, Garraway SM, Weyerbacher AR, Shin SJ, Inturrisi CE. Activation of the neuronal extracellular signal-regulated kinase 2 in the spinal cord dorsal horn is required for complete Freund's adjuvant-induced pain hypersensitivity. J Neurosci. 2008;28:14087–14096. doi: 10.1523/JNEUROSCI.2406-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Xu XJ, Dalsgaard CJ, Wiesenfeld-Hallin Z. Intrathecal CP-96,345 blocks reflex facilitation induced in rats by substance P and C-fiber-conditioning stimulation. Eur J Pharmacol. 1992;216:337–344. doi: 10.1016/0014-2999(92)90428-7. [DOI] [PubMed] [Google Scholar]
- 389.Xu XJ, Dalsgaard CJ, Wiesenfeld-Hallin Z. Spinal substance P and N-methyl-D-aspartate receptors are coactivated in the induction of central sensitization of the nociceptive flexor reflex. Neuroscience. 1992;51:641–648. doi: 10.1016/0306-4522(92)90303-j. [DOI] [PubMed] [Google Scholar]
- 390.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: Effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 391.Yashpal K, Fisher K, Chabot JG, Coderre TJ. Differential effects of NMDA and group I mGluR antagonists on both nociception and spinal cord protein kinase C translocation in the formalin test and a model of neuropathic pain in rats. Pain. 2001;94:17–29. doi: 10.1016/S0304-3959(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 392.Young MR, Fleetwood-Walker SM, Dickinson T, Black-burn-Munro G, Sparrow H, Birch PJ, Bountra C. Behavioural and electrophysiological evidence supporting a role for group I metabotropic glutamate receptors in the mediation of nociceptive inputs to the rat spinal cord. Brain Res. 1997;777:161–169. [PubMed] [Google Scholar]
- 393.Young MR, Fleetwood-Walker SM, Mitchell R, Munro FE. Evidence for a role of metabotropic glutamate receptors in sustained nociceptive inputs to rat dorsal horn neurons. Neuropharmacology. 1994;33:141–144. doi: 10.1016/0028-3908(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 394.Yu XM, Salter MW. Src, a molecular switch governing gain control of synaptic transmission mediated by N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1999;96:7697–7704. doi: 10.1073/pnas.96.14.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract Res Clin Rheumatol. 2007;21:481–497. doi: 10.1016/j.berh.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 396.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Zhang B, Tao F, Liaw WJ, Bredt DS, Johns RA, Tao YX. Effect of knock down of spinal cord PSD-93/chapsin-110 on persistent pain induced by complete Freund's adjuvant and peripheral nerve injury. Pain. 2003;106:187–196. doi: 10.1016/j.pain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 398.Zhang HM, Chen SR, Pan HL. Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain. Neuroscience. 2009;158:875–884. doi: 10.1016/j.neuroscience.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- 400.Zhang X, Wu J, Fang L, Willis WD. The effects of protein phosphatase inhibitors on the duration of central sensitization of rat dorsal horn neurons following injection of capsaicin. Mol Pain. 2006;2:23. doi: 10.1186/1744-8069-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Zhang X, Wu J, Lei Y, Fang L, Willis WD. Protein phosphatase 2A regulates central sensitization in the spinal cord of rats following intradermal injection of capsaicin. Mol Pain. 2006;2:9. doi: 10.1186/1744-8069-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Zhang X, Wu J, Lei Y, Fang L, Willis WD. Protein phosphatase modulates the phosphorylation of spinal cord NMDA receptors in rats following intradermal injection of capsaicin. Brain Res Mol Brain Res. 2005;138:264–272. doi: 10.1016/j.molbrainres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 403.Zhang XC, Zhang YQ, Zhao ZQ. Different roles of two nitric oxide activated pathways in spinal long-term potentiation of C-fiber-evoked field potentials. Neuropharmacology. 2006;50:748–754. doi: 10.1016/j.neuropharm.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 404.Zhou XF, Rush RA. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience. 1996;74:945–953. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]
- 405.Zhu CZ, Wilson SG, Mikusa JP, Wismer CT, Gauvin DM, Lynch JJ, 3rd, Wade CL, Decker MW, Honore P. Assessing the role of metabotropic glutamate receptor 5 in multiple nociceptive modalities. Eur J Pharmacol. 2004;506:107–118. doi: 10.1016/j.ejphar.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 406.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 407.Zou X, Lin Q, Willis WD. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Res. 2004;1020:95–105. doi: 10.1016/j.brainres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 408.Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience. 2002;115:775–786. doi: 10.1016/s0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]


