Abstract
Antimicrobial peptides (AMPs) are multi-functional peptides whose fundamental biological role in vivo has been proposed to be the elimination of pathogenic microorganisms, including Gram-positive and -negative bacteria, fungi, and viruses. Genes encoding these peptides are expressed in a variety of cells in the host, including circulating phagocytic cells and mucosal epithelial cells, demonstrating a wide range of utility in the innate immune system. Expression of these genes is tightly regulated; they are induced by pathogens and cytokines as part of the host defense response, and they can be suppressed by bacterial virulence factors and environmental factors which can lead to increased susceptibility to infection. New research has also cast light on alternative functionalities, including immunomodulatory activities, which are related to their unique structural characteristics. These peptides represent not only an important component of innate host defense against microbial colonization and a link between innate and adaptive immunity, but also form a foundation for the development of new therapeutic agents.
Keywords: Defensin, magainin, innate immunity, membrane disruption
I. Introduction
The innate immune system provides organisms with a rapid, non-specific first line of defense against colonization by pathogenic microorganisms. The components of innate immunity include the barrier function of the skin, reduced pH of the stomach, the sweeping motion of the cilia in the airway to remove inhaled pathogens, and chemical defenses which include host defense peptides. These gene-encoded defense molecules, initially known as antimicrobial peptides (AMPs), are a diverse collection of peptides that participate in several aspects of innate immunity, and may also provide the basis for the design of novel therapeutic agents. Numerous reviews have been written on the diversity of these peptides, their structure and activities in vitro and in vivo, and genetics and gene regulation (see, for example, [1-6]).
While the antibiotic properties of secretions were observed as early as Fleming [7], the presence of broad-spectrum antimicrobial activity in blood cells was first described in the 1950s [8]. Subsequently, it was discovered that phagocytic cells produce intracellular cationic AMPs in response to infection [9], as did innate immune cells in insects [10]). In 1983, Lehrer and Selsted purified two AMPs with three intralinked cysteines from rabbit lung macrophages, which were later named defensins [11]. Subsequent examination of different cell types, including those of the myeloid lineage and epithelium have uncovered a wide variety of AMPs both from mammals as well as amphibians [12] fish [13], insects [14], birds [15], and plants [16]. The varied structural and functional attributes of the hundreds of AMPs identified to date have been reviewed in detail (see, for example [17], and updated information may be obtained at one of several AMP databases ([http://www.bbcm.univ. trieste.it/∼tossi/amsdb.html]; [http://aps.unmc.edu/AP/main.php]; and [http://research.i2r.a-star.edu.sg/Templar/DB/ANTIMIC/] [18]. In this review, after a brief discussion of AMP structure and function, we will attempt to incorporate new information regarding the varied activities of AMPs in mammals, which may help understand their roles in host defense.
II. AMP Structure
Although AMPs are commonly classified by variation in structural characteristics, there are some structural features that AMPs share, including a length of less than 60 amino acids, broad-spectrum antimicrobial activity at physiological conditions, and an overall positive charge (reviewed in [6, 19]). The fundamental structural principle underlying this class of peptides is the ability to adopt an amphipathic shape in which clusters of hydrophobic and hydrophilic amino acids segregate [6]. AMPs can be divided into five sub-categories on the basis of their amino acid composition and structure including anionic peptides, linear amphipathic α-helical, cationic peptides enriched for specific amino acids, peptide fragments, and peptides with cysteines that form intramolecular bonding (reviewed in [1, 20]). Some examples of these classes are shown in Table 1.
Table 1. Classes of AMPs.
| Class | Structure Properties | Examples | Source(s) | Physiological Location | Reference(s) |
|---|---|---|---|---|---|
| Anionic | Negatively charged | Maximin, Dermicidin | Amphibians, humans | Airway epithelia | [1, 21] |
| Linear | Cationic, α-helical | Cecropin, magainin pluerocidin | Amphibians, mammals, insects | skin | [1, 12, 13, 59] |
| Linear, Cationic, enriched | Cationic, enriched in amino acids, extended | Abaecin, Indolicidin, histatins | Insects, mammals | Skin, intestinal epithelium | [1, 31, 63] |
| Peptide fragments | Cationic, β-turn | Lactoferrin, cathelicidins | humans | blood | [1, 25, 26] |
| Charged peptides with cysteine | Cationic, anionic, forms disulfide bonds, possess cysteine | α-, β-, and θ-defensins, protegrin | Birds, reptiles, mammals, plants | ubiquitous | [6, 90, 228] |
The first subgroup is composed of anionic peptides similar to the charged-neutralizing propeptides of larger zymogens. These small peptides are present in surfactant extracts, bronchoalveolar lavage fluid, and airway epithelial cells [1]. Produced in millimolar (mM) quantities they require zinc as a cofactor and display activity against both Gram-positive and -negative bacteria. Examples include dermcidin in humans [21] and maximin H5 from amphibians [22].
The second, and most evolutionarily diverse subgroup contains ∼290 linear cationic α-helical peptides, most of which are less than 40 amino acids in length and possess a three dimensional structure with a kink or hinge in the middle. Although disordered in solution, these molecules adopt an α-helical secondary structure while in contact with membranes [1]. A direct correlation has been discovered between α-helical content and antimicrobial activity. Examples of these peptides include cecropins [23], magainin [12], pleurocidin from the winter flounder [13], and melittin from bee venom [24].
The third group contains approximately 44 peptides all of which are linear in shape and enriched in specific amino acids. Members from this group include 1) bactenecins and 2) proline-arginine-rich peptide (PR-39), both of which are rich in proline (33-49%) and arginine (13-33%); 3) proline rich (38%) abaecin from bees; and 4) indolicidin from cattle, rich in tryptophan [1]. This group lacks cysteine residues, making it very flexible and fluid in solution as well.
The fourth group is comprised of charged peptides that are fragments of larger peptides. These peptides possess antimicrobial activity and are similar in shape and size to other AMPs. For example, lactoferricin, [25] is a peptide derived from the digested N-terminal portion of lactoferrin. Similarly, cathelicidins are peptides which are found at the C-terminus of precursors whose N-termini share a homology with a porcine serine protease known as cathelin [26].
The fifth group is composed of ∼380 members which all contain conserved 6-cysteine residue motifs forming intramolecular disulfide bonds and β-sheets [1]. This is an extremely diverse group of proteins known as defensins [20], which is hypothesized to have originated in prokaryotes [27]. This has diverged into plant defensins, arthropod defensins, and the β- defensins found in higher animals, including birds, reptiles and mammals (reviewed in [28]). At some point in mammalian evolution the α-defensins diverged from the β-defensin family. A third defensin subfamily, the θ-defensins are found only in rhesus monkeys, having apparently evolved from a mutation in α-defensins [29]. Similar to the defensins is a liver-specific peptide, hepcidin, which exhibits both antimicrobial activity and iron-regulatory activity [30]. Hepcidin is also cysteine-rich, with two β-sheets.
Other peptides with specific antimicrobial activities, such as the antifungal histatins [31] and the antiviral zap proteins [32], are characterized by diverse structures, demonstrating the wide variety of antimicrobial host defense peptides. While the exact details of these mechanisms remain unknown a dissection of the physical characteristics of each class of peptide allows us to speculate as to what must occur at each of the specific steps to result in the induction of bacterial killing. AMPs are traditionally broken into four structural classes: linear, beta-sheet, loop, and extended structures [33]. There are some peptides that do not fit any of these categories and there are some that only display this type of secondary structure while aggregated or interacting with membranes; e.g. the bovine neutrophil peptide indolicidin is unstructured in aqueous environments but adopts a boat-like conformation after interacting with membranes [34]. Human defensins share several distinct structural folds that are unique when compared to the rest of the known AMPs. The structural frame of the molecules is formed by triple stranded antiparallel beta-sheet, restrained by three disulfide bridges [35, 36], commonly referred to as the defensin-like fold [37]. The structure of HNP3 is identified as its archetype [38]. Even the cyclic θ-defensins, retrocyclin-1 and RTD1, posses a similar beta-hairpin tertiary structures with the internal disulfide bonding [39, 40].
Despite the structural conservations found among human defensins the triple stranded antiparallel beta-sheet is the most conserved feature. One very notable physical characteristic of this feature is a beta-bulge located in the middle of the second beta-strand [41]. This bulge, which accentuates a twist for this strand, initiates a beta-hairpin between the second and third strands of the antiparallel beta-sheet [42]. Studies have confirmed that due to backbone torsion angles the energetically significant placement of the glycine residue in the G16X17C18 (according to HNP2 numbering) is the only natural structure for the beta-bulge motif [43]. The Gly residue has been successfully substituted with the use of a D-amino acid [43]. Named the GXC motif this structure is conserved in all known alpha- and beta-defensins.
While AMPs share many conserved structural characteristics the intermolecular differences can vary dramatically, especially amongst the defensin family. This is most prominent when the molecular surfaces are compared as the inherent variability is a direct result of differences in the physical composition of the proteins (i.e. length, amino acids, distribution of charged residues) [35]. Wieprecht et al. showed a relationship between overall hydrophobicity and lipid affinity for magainin analogs [44]. They found that the ability of hydrophobicity to modulate membrane activity increased with decreasing electrostatic peptide-lipid interactions and an increasing role of hydrophobic interactions [44]. Structure function studies using amino acid substitution have shown the varied impact of the addition or subtraction of overall positive charge from the antimicrobial ability of the molecules [45-47]. Szyk and collaborators surveyed the arrangement of charged residues in regions of α-defensins in the amino and carboxy termini and found little difference in the distribution of electrostatic potential over the remainder of the molecule [48]. Although the surface properties of defensins are potentially at the core of their biological importance elucidation of the subtleties of their mechanism may be too monumental of a task at this time.
III. Spectrum of Antimicrobial Activity
From their initial isolations and characterizations, AMPs, especially those from higher organisms, were observed to exhibit a broad-spectrum of activities against microorganisms, including Gram-positive and –negative bacteria (reviewed in [1]), fungi (reviewed in [49]), mycoplasma [50], and viruses (reviewed in [51]). Antimicrobial activity for bacteria and fungi, usually presented as Minimal Inhibitory Concentration (MIC), is in the 1-50μg/ml range for most peptides against a wide variety of Gram-positive and – negative bacteria as well as fungi such as Candida albicans. As multiple forms of a peptide were identified in single species, such as β-defensins in human for example, it was observed that different peptides exhibited species-specific and strain-specific activity. Table 2 shows a sample of some peptides and their spectrum of activity as well as the variability between strains of microbe.
Table 2. Susceptibilities of Microbes to AMPs as Reported in the Literature.
| MIC, μg/ml | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Strain | HNP1-3 | hBD1 | hBD2 | hBD3 | LL-37 | Mgn-2 | Reference |
| E. coli | D31 | 62 | 1-7 | [45, 229] | ||||
| ATCC25922 | 9-23 (LD99) | 102 (LD99) | 1-7 | [229, 230] | ||||
| ML-35 | 0.7 | 4-40 | 0.6 | [231] | ||||
| ATCC 11303 | 6 | [232] | ||||||
| DH5α | 0.8 | [233] | ||||||
| P. gingivalis | ATCC33277 | 34.6 | 5.7-31.3 | >125 | [234, 235] | |||
| WA50 | >250 | 50 | >250 | 200-250 | 100 | [235] | ||
| 381 | >250 | [236] | ||||||
| ATCC 49417 | 62.5 | 37.8 | [237] | |||||
| P. aeruginosa | NCTC 6750 | 1 | [238] | |||||
| MR3007 | >250 | 4.7 | [231] | |||||
| PA01 | 10 | 26.5 | 2.25 | [233, 239] | ||||
| ATCC 27853 | 100 | 75 | 25 | 35-70 | [229, 240] | |||
| ATCC17648 | 62-250 | [236] | ||||||
| S. aureus | 29213 | 7-16 (LD90) | 9.5 | 210 | 20 | [147] | ||
| COL | >50 | 10 | 5 | 5 | [241] | |||
| MRSA* | 2.5-25 | 25 | 3-10 | 8-64 | [231, 242] | |||
| ATCC 25923 | 21-52 (LD90) | >26.5 | [230] | |||||
| B. cepacia | ATCC25416 | >250 | >79.1 | [231] | ||||
| ATCC17770 | 6.6 | [239] | ||||||
| S. mutans | ATCC 10449 | 61-123 | [236] | |||||
| NCTC 10449 | 4.1 | [243] | ||||||
| ATCC 25175 | 4.1 | 5 | [235] | |||||
| MT403R | 25 | 25 | [244] | |||||
| LM-7 | 20 | 5 | 1 | 1 | [244] | |||
| C. albicans | OPC76 | >41 | 4.1 | 4.1 | [245] | |||
| FC16 | 9.4 | 3.2 | [235] | |||||
| ATCC 10231 | 3 (IC50) | [246] | ||||||
| ATCC 820 | >250 | >250 | [231] | |||||
varying resistant strains.
In addition to bacteria and fungi, it was also observed that some peptides exhibited inhibitory activity against some viruses [52]. Specifically, this activity was initially limited to enveloped viruses, suggesting a common mechanism for activity between peptides with an affinity for membranes (see below). More recently, however, it has become apparent that some antiviral AMPs are active at multiple steps in viral pathogenesis, including viral entry and replication [53]. The observation of a β-defensin in the viral defense cell known as the plasmacytoid dendritic cell (PDC) further supports their role in the innate defense against viruses [54]. Synthetic peptides based on naturally occurring sequences have been examined for their potential as antiviral drugs, especially against HIV [55].
IV. Mechanism of Action of Antimicrobial Peptides
All known AMPs are imparted with similar physical properties that provide them with their multi-faceted abilities. Their potent antimicrobial activities stem from the possession of a cationic charge due to the presence of multiple lysine, tryptophan, and arginine residues, a large portion of hydrophobic residues (50% or higher), hydrophobicity, and amphipathicity. A number of studies have contributed to defining and understanding the mechanism of action of AMPs [12, 38, 56, 57]. The focus of these experiments has been the interaction of AMPs with membranes using such methods as fluorescent dye release in model membrane systems [58], ion channel formation [59], and methods to measure secondary structure including circular dichroism [60], NMR [61], and neutron diffraction [62]. From these experiments it has been generally accepted that AMP mediated killing of microorganisms typically occurs through membrane permeation, although non-membrane disruptive peptides have also been discovered [56]. These non-membrane disruptive peptides have been shown to affect several internal cellular processes from macromolecular synthesis (i.e. RNA, DNA synthesis) [63] to loss of ATP from actively respiring cells [64]. For example, the lantibiotic mersacidin, an AMP from Gram-positive bacteria that contains the thioether amino acid lanthionine, has been shown to combine with lipid II. This prevents peptidoglycan precursors from becoming polymeric nascent peptidoglycan thus inhibiting cell wall formation. Buforin II, among other AMPs, has been shown to bind to both DNA and RNA from E. coli altering their electrophoretic mobility in vitro [56, 65]. PR-39 and indolicidin are also noteworthy for their unusual ability to bind intracellular targets. Agerberth and colleagues showed that PR-39 antibacterial activity involves binding to the membrane, typical behavior of antimicrobial peptides, but not pore formation [66]. Instead, it has been postulated that a passive uptake mechanism allows PR-39 into the cell and subsequent binding interactions stop protein and DNA synthesis [66]. An alternative perspective is now being considered that as a group, AMPs have specific effects on organisms based on the physical features that vary from peptide to peptide [34]. Below we will discuss the structural variations with respect to antimicrobial killing.
A. Attraction
Regardless of the type of organism or the class of peptide the first step of any peptide mediated function is attraction. This behavior is ultimately governed by the charge and amphipathicity of the peptide. Attraction is presumed to occur when the initial interactions between the cationic peptides first occur via electrostatic interactions with negatively charged moieties on the bacterial membrane. These anionic constituents include lipopolysaccharide (LPS) phosphate groups and anionic lipids in the case of Gram-negative outer membranes and teichoic acids in the case of Gram-positive membranes [67]. Cationic peptides have been shown to possess a higher affinity for LPS in the outer leaflet of the outer membrane in Gram-negative bacteria than do native divalent cations such as Mg2+ and Ca2+ [68]. In contrast, AMPs display lower cytotoxicity to host cells due to the fact that their membranes possess a higher percentage of cholesterol. Further, the introduction of cationic charges to the bacterial surface, or the segregation of anionic phospholipids to the cytoplasmic surface of the membrane can lead to bacterial resistance (reviewed in [69]).
B. Attachment
The distribution of polar and hydrophobic residues can result in pronounced interactions of the peptides with the phospholipid membranes [70]. This physical property leads to the second step, attachment, as the peptides must now traverse the exterior capsular polysaccharides to reach the inner lipid layer [1]. The possession of Arg and Trp residues also plays a key role in membrane insertion as the electrostatic interactions of the Arg side chains with the phosphate head groups serves to stabilize the peptide-membrane interaction [71]. This is enhanced by Trp residues which have a preference for the interfacial regions of lipid bilayers [72]. They are considered hydrophobic due to the possession of an extensive π-electron system of their uncharged side chain [73]. The π-electron system of Trp participates in cation-π binding which is energetically favorable in aqueous solution allowing the residues to be stacked. When stacked perpendicularly this shields Arg residues allowing them to make cation-π interactions with water, thus stabilizing their insertion into the membrane [73].
After AMPs bind to the membrane surface they adopt an energetically favorable secondary structure dictated by the hydrophobicity of the peptide. For example, the majority of α-helical peptides possess relatively constant levels of hydrophobicity along the α-helical axis. This forces them to adopt membrane orientations that are either parallel or perpendicular to the membrane itself [74].
During the peptide-membrane interaction two physically distinct states occur [75]. The first is low peptide/lipid ratios where the defensins first embed parallel to the lipid head groups causing the membrane to stretch [76]. X-ray and neutron diffraction studies have shown that as the peptide/lipid ratios increase pores begin to form when the thinning membrane reaches a fraction of its previous thickness [77]. The second state occurs when high peptide/lipid ratios are experienced, with pores having begun to form in the critically thin membrane, and the peptides orient themselves perpendicularly and insert into the bilayer [78].
C. Models of Insertion
After insertion several models have been developed that explain how AMPs kill organisms through membrane permeation. It is highly likely that these models vary for each class of AMPs and types of microorganisms (an example, using magainin as a model, is shown in Fig. 1). The first model, the carpet model (Fig. 1B), begins with peptide aggregation on the bilayer surface [17]. As the concentration increases it is thought that the peptides intercalate into the membrane in a detergent like manner causing the bilayers to continuously bend so the water core is lined by both the inserted peptides and the lipid head groups [79]. Once a critical threshold concentration has been reached the membrane disintegrates and forms micelles [80]. This model explains the activity of the AMP
Fig. (1). Magainin and mechanisms of purported pore formation.
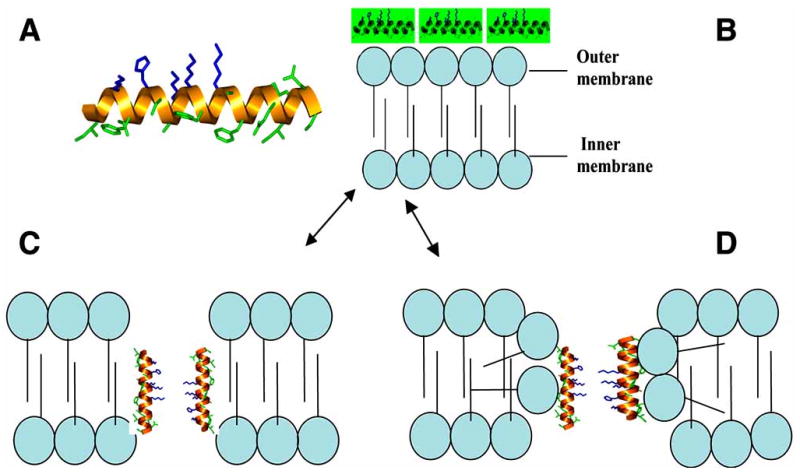
1A. α-helical host defense peptide, magainin. Hydrophobic residues are green, basic residues are blue. 1B. Carpet model as AMP blanket the membrane building up charge differential. 1C. Barrel stave model as AMP permeate the membrane disallowing the flip-flop of phospholipids between the leaflets. 1D. The toroidal pore method that allows the phospholipids membrane to flip flop, caused by bending of the lipids, and segregating them into micelles.
The second model, known as the barrel-stave (Fig. 1C), was based on the activity of magainin-2 and states that the peptides form a bundle in the membrane with a pore in the center, much like a barrel, with the AMPs as the staves [81]. The hydrophobic regions of the peptide interact with the lipid core while the hydrophilic portions face outward [1]. For example, the AMP alamethicin, has been discovered to induce pores that contain between 3-11 helical molecules with inner and outer diameters calculated as ∼1.8 to ∼4.0 nm, respectively [82].
The third model, the toroidal pore model (Fig. 1D), combines the actions of the previous two beginning with aggregation on the membrane surface. The peptides then insert themselves perpendicularly into the membrane and induce the monolayers to continuously bend causing the water core to be lined by both the inserted peptides and lipid head groups [83]. During this action the polar faces of the peptides interact with the polar head groups of the lipids resulting in the formation of a continuous bend that connects the two leaflets of the membrane [81]. This behavior creates toroidal pores in the membrane causing the lipids to form micelles and subsequent membrane disruption [61]. The toroidal pore model differs from the barrel stave model in that the peptides are always associated with the lipid head groups even when perpendicularly inserted into the lipid bilayers [81]. This behavior exemplifies the actions of AMPs such as magainin, protegrin, and melittin [81]. Toroidal pores formed by magainin are thought to contain only 4-7 peptide monomers to ∼90 lipid molecules [84, 85].
Experimental evidence with AMP structural variants has shown that there is clear interplay between the charge and an optimal threshold of hydrophobicity that enhances antimicrobial ability [17, 77, 86]. A recent study by Chen et al. has been found that AMP analogs that are too hydrophobic are more prone to eukaryotic cell damage [86]. They have proposed an explanation based on a membrane discrimination mechanism for AMPs who utilize either the barrel stave in eukaryotic cells or the carpet model in prokaryotic cells [86]. This mechanism is based on the difference in lipid composition between the two types of membranes. It is a well established fact that eukaryotic membranes contain abundant amounts of zwitterionic phospholipids, such as cholesterol and sphingomyelin, and the absence of negatively charged compounds, in contrast to prokaryotic membranes [87]. Since AMPs with higher hydrophobicities penetrate deeper into membranes they would lyse eukaryotic cells though pore formation. On the other hand, since insertion into bacterial cells is not always necessary for peptide mediated killing, less hydrophobic AMPs need only aggregate parallel to the membrane surface to facilitate proper interaction with polar surface areas.
D. Secondary Factors Affecting AMP Activity
The activity of AMPs in vivo has also been found to be enhanced by other mitigating factors such as components of the host environment. For example, the effects of carbonate are independent of any potential changes of AMP, and need not be immediately present in the surrounding media [88]. Instead, the presence of carbonate has been shown to alter bacterial gene expression in vivo corresponding to an enhanced AMP sensitivity phenotype [88].
Studies have also shown that there is a dependence on ionic interactions when AMP activity is evaluated in the presence of monovalent, divalent, or polyanions [45]. Even in the presence of minimal levels of ions, 50 mM of NaCl, suppression of activity has been observed in several types of AMPs including LL-37 and hBDs [45, 88]. This is due to the peptide being blanketed with chloride ions, negating the positive charge of the molecule, thus preventing binding to the microbial membrane. In contrast to their antimicrobial activity, the chemotaxic behavior of defensins is not suppressed by physiological concentrations of salt and proteins, further supporting the notion that AMP activity is dependent on ionic bonding [89]. Unfortunately, there has been little research into modifying the various classes of AMPs and their subtypes in order to make them less susceptible in such environments. While it is recognized that the mammalian host milieu contains a minimal salt concentration, evident by the microbicidal ability of AMPs, any potential chemical alterations to counter act the effect of chloride ions would potentially result in serious structural changes to the peptide. Combined with the high level of entropy needed to displace the chloride ions these changes would subsequently affect other important components of its activity (i.e. charge, hydrophobicity, etc) thus rendering the peptide ineffectual.
V. Resistance to AMPs
One of the driving forces behind the research into AMPs has been the observation that these peptides exhibit broad-spectrum activity, and that bacteria do not appear to develop resistance as easily as with conventional antibiotics [90]. As mentioned above, there is a natural variability among peptides with respect to activity. Some strains are naturally resistant to the activity of some peptides, suggesting that the expression of multiple types of peptides in the same tissue is necessary to obtain a broad-spectrum defense. In general, several natural mechanisms of resistance to AMP activity have been observed. Some bacteria employ secreted proteases which can inactivate the AMP, rendering them insensitive to their activity. These include the gingipains from Porphyromonas gingivalis, which are arginine- and lysine-specific proteases, and can digest highly cationic antimicrobial peptides [91]. Similarly, proteases from Staphylococcus aureus can inactivate LL-37 [92]. While peptides, such as defensins, are characterized by a secondary structure based on multiple disulphide bonds, which make them less sensitive to proteases, such a resistance mechanism has been observe in multiple bacterial species.
As described above, the major initial event in AMP-bacteria interaction is the ionic interaction between the cationic peptide and the anionic envelope of the bacteria. Since these structures are almost universal among bacteria, it forms one of the foundations of the sensitivity of bacteria to AMPs. Regardless, bacterial species have developed mechanisms to modify even these important structures by even partially neutralizing the envelope and inhibiting the interactions with the peptides (reviewed in [93]). For example, genetic changes are found in both Gram-positive bacteria, which modify the teichoic acids, as well as Gram-negative bacteria, which modify the lipid A portion of LPS.
Initial studies demonstrated the difficulty in developing resistance among sensitive bacterial strains [90, 94, 95]. These were carried out using a standard technique, that of growing the bacteria in sub-MIC concentrations for up to 20 passages. The results showed no increase in the MIC at this point, supporting the utility of AMPs to address the growing problem of antibiotic-resistant bacterial strains. However, when a novel method was employed, whereby bacteria were grown for over 700 passages in slowly increasing concentrations of an AMP, a drastically different result was obtained. Here, strains that were previously sensitive to AMPs at 1μg/ml, develop resistance to >500μg/ml [96]. In a separate study, co-challenge of a bacterium, P. gingivalis, with sublethal levels of defensin and either heat or hydrogen peroxide could lead to the development of resistance to defensin [97]. Together, these studies point out that care must be taken before development of AMPs as therapeutics.
VI. Expression of Mammalian AMP Genes
Genes encoding AMPs in mammals are expressed throughout the body, both in circulating cells and epithelium (for reviews, see, for example, [4, 98-100]). The most abundant human AMPs, defensins and cathelicidins, are produced from genes that encode larger precursor proteins, each with a different precursor structure (Fig. 2). These structures lead to gene regulation both at the transcriptional and posttranscriptional levels, to provide controlled expression of these multifunctional molecules. The coordinated transcriptional regulation of AMP genes can lead to multiple AMP expression at a single site, providing a combination of host defense molecules [101, 102]. A recent computational study examined the upstream regions of numerous AMP genes in human, mouse and rat, including defensins and cathelicidins, and has characterized them according to similarities in their transcription factor binding sites [103]. The results support the concept of coordinate transcriptional regulation of AMP genes, to provide the most comprehensive antimicrobial host defense.
Fig. (2). Gene structure for human α-defensins (A), β-defensins (B) and cathelicidin (C).
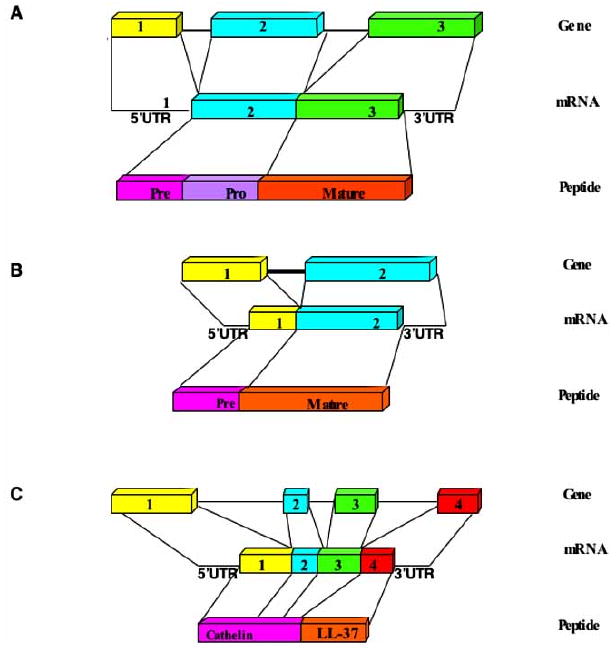
The general structure for each family is shown with exon numbers for the genes, the 5′ and 3′ Untranslated Regions (UTRs) for the mRNA, and the precursor regions for the peptides.
In humans, the α-defensin family includes four peptides found in neutrophils (HNP 1-4) (reviewed in [104]), and two peptides in Paneth cells within the small intestine (HD-5 and –6) (reviewed in [105]), which are differentially expressed. Transcription of HNP1-4 is found in bone marrow and in immature myeolocytes, but stops with maturation of the neutrophil [106]. Precursor α-defensins are produced in promyelocytes, which are then processed to the mature, active forms during granulogenesis [107]. The active peptides are found in primary granules in the neutrophils, where they can then be used as part of the oxygen-independent antibacterial mechanism of these cells. In contrast, intestinal α-defensins are transcribed in the Paneth cells, where the pre-propeptides are translated. Upon removal of the signal peptide during the secretory process, the inactive propeptide is secreted into the lumen of the small intestine, where it is activated by a trypsin-mediated removal of the propiece in humans [108], and by matrilysin in mice [109].
In contrast, the β-defensins are found primarily in epithelial cells, at numerous sites throughout the body, including oral, airway and skin epithelium (reviewed in [2]). As β-defensins lack an acidic propiece [110], their expression is primarily at the level of transcription, producing active peptide. In epithelial cells, human β-defensin 1 (hBD1) is generally transcribed at a constitutive, low level. The mRNAs for other β-defensins, including hBD2, 3 and 4, are found at low levels, but transcription is induced by a variety of factors including microbes and cytokines (reviewed in [2, 4]). Specifically, this includes Toll-like receptor (TLR) agonists such as LPS, and inflammatory mediators such as TNF-α, Interleukin (IL)-1β and IL-17 [111]. More recently, three cytokines, IL-12, 23 and 27 were shown to enhance the IL-1β-mediated induction of hBD2 [112]. Together the multitude of factors that induce β-defensin transcription suggest a complex role for these peptides in innate immunity.
In the airway, which is a generally sterile tissue, the epithelial cells are highly responsive to the presence of microbes, including Gram-positive and –negative bacteria, which recognize the potential pathogens through a TLR pathway, and lead to an NF-κB-mediated induction of β-defensin gene expression [113]. Similarly, induction by IL-17 proceeds through NF-κB as well [114]. In contrast, the oral cavity, which is home to hundreds of bacterial species, expresses β-defensins at several sites, including the gingival epithelium (reviewed in [115]). However, the genes are induced only by a subset of bacteria, and the induction utilizes different pathways, including p38, JNK [116] and NF-κB [117], depending on the species. Even within the NF-κB activation, there are different pathways leading to activation utilized by different microbes [118] This control may be partially responsible for regulating the homoeostatic levels of bacteria at this site.
Some β-defensins have been observed in circulating blood cells, and their expression appears to be similarly regulated. In the viral defensive PDC, hBD1 can be found [54], and appears to be induced by viral challenge (Ryan et al., manuscript in preparation). In macrophages and monocytes, hBD2 is induced by cytokines, including interferon-γ [119]. Both α- and β-defensins have been observed in breast milk [120], including the neutrophil peptides HNP 1-4 as well as the intestinal α-defensins HD-5 and –6. There is some evidence suggesting that these α-defensins may play a role in this tissue in the protection against transmission of HIV through this route [121].
Alongside β-defensins, LL-37 is also expressed in the surface epithelia of conducting airways [122], and in bronchoalveolar lavage fluid (BALF) [123]. Its expression is induced by bacteria and cytokines, similar to β-defensins, confirming the computationally observed similarities in their promoter regions. To demonstrate this peptide's role in airway defense, a complex animal model was used. Specifically, human respiratory epithelial cells were seeded on denuded rat tracheas. These tracheas were implanted in the flanks of nude mice, creating a xenograft [124-126]. These xenografted tracheas secrete hBD-1 and -2 and LL-37 into the airway surface fluid (ASF), which exhibits antibacterial activity. While airway cell cultures from patients with cystic fibrosis (CF) have reduced antibacterial activity, overexpression of LL-37 in xenografts developed from human cells exhibiting the CF defect results in normal antibacterial activity, compared with untransfected cells [124], supporting the role of this peptide in antibacterial host defense.
Similar to the expression pattern in the airway, LL-37 is observed in both healthy gingival epithelium and in neutrophils, with an increase in expression observed in inflamed gingiva [127].
By computational examination of putative promoter sequences, Wang et al. [128] discovered the presence of a Vitamin D Response Element (VDRE) upstream from the cathelicidin antimicrobial peptide (CAMP) gene. This promoter element is recognized by a nuclear receptor (VDR) which heterodimerizes with the retinoid X receptor upon activation by the hormonally active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) (reviewed in [129]). The presence of such a sequence suggested that LL-37 mRNA might be inducible by vitamin D. Further studies demonstrated this to be the case in several cell types, including monocytes and primary keratinocytes, as well as established cell lines such as U937 (a monocyte line), HL-60 (a promyelocyte) and SCC25 (tongue carcinoma) [128, 130]. LL-37 mRNA, protein and antimicrobial activity was also induced in primary cultures of airway epithelium by 1,25(OH)2D3 [131]. Further studies demonstrated that the induction was in response to a VDRE-mediated increase in transcription, which led to the increase in LL-37 mRNA and peptide, as well as an increase in the antimicrobial activity of the cell culture medium [130]. More recently, Liu et al. demonstrated that the activation of TLR 2/1 (a receptor for patterns including those found on Mycobacterium tuberculosis) increased transcription of the VDR and the Vitamin-D1-hydroxylase genes. This led to a VDRE-mediated increase in LL-37 levels in macrophages, and a subsequent increased killing of M. tuberculosis [132]. Together, these studies support the potential use of this less toxic agent such as Vitamin D in the increase in antibacterial activity of tissues to prevent bacterial colonization.
Such a therapeutic modulation may be useful in the oral cavity as well. Preliminary data from our laboratory show that a similar induction of LL-37 by 1,25(OH)2D3 occurs in cultured gingival epithelial cells (Yim et al., manuscript in preparation).
The identification of single nucleotide polymorphisms in the 5′ untranslated region (UTR) of hBD1 that are associated with clinical phenotypes (see below), however, suggest that there may be some level of posttranscriptional regulation as well. And as with the transcriptional regulation, LL-37 peptide expression also appears to be controlled by elements in the 5′ UTR [133].
VII. Mobilization of AMPs by Cells
The changes in mRNA levels for AMPs as described above are often seen as a surrogate for the ability of cells to control microbial growth [134-136]. While the amount of antimicrobial peptide mRNA, or the peptide itself is an important aspect of overall antimicrobial capacity of effector cells, it is necessary to bring the peptide into contact with the microbes in order to kill them. For example, the cellular processes which operate to bring various types of granules and vesicles close to the phagosomes of macrophages and neutrophils, and then to mediate fusion of those organelles with phagosomes containing microbes are tightly regulated [137]. In this case, exposure of the bacteria to defensins within the phagosome allows much higher local concentrations than could be achieved by extracellular secretion, and therefore one would expect it to be a more efficient mechanism for killing potential pathogens. However, neutrophils do not always use granule-phagosome fusion to bring antimicrobial peptides into contact with bacteria. Neutrophils are known to degranulate, expelling several types of granules into the extracellular fluid, including those containing defensins [138, 139]. In addition, since the HNP are extremely protease resistant, one would expect microbes internalized via phagocytosis to be exposed to these defensins as neutrophils undergo apoptosis, and the internal organelles break down and allow mixing of intracellular constituents [140, 141].
Epithelial cells are the primary source of β-defensins and cathelicidin fragments. However, they are not known to be particularly phagocytic. How then can their repertoire of intracellular antimicrobial peptides (β-defensin 1, 2, 3, and cathelicidin for example) be brought into contact with potential pathogens? In the example of the oral cavity, gingival epithelial cells secrete antimicrobial peptides directly into the gingival crevice [142]. This contributes to the constitutive hostility of the crevicular fluid to the growth of pathogens. Similarly, epithelial cell-expressed β-defensins and LL-37 are found in urine [143, 144] and bronchoalveolar lavage fluid [145], potentially contributing to the overall antimicrobial defense at these sites. In the case of the skin, the antimicrobial barrier begins with secretion of antimicrobial peptides from cells at the base of eccrine sweat glands, which then flow with sweat onto the surface of the skin (reviewed in [146]). That surface is composed of the non-vital stratum corneum, which forms a tough, waterproof covering over the vital cells of the epidermis. However, in contrast to other epithelia, the skin is often subject to environmental insult which introduce bacteria through the tough stratum corneum and into the vital cell layers below.
The specific pathways by which antimicrobial peptides are liberated from various epithelial cells, and how those pathways might be regulated becomes important in certain types of antimicrobial responses. For example, recent studies have shown that human keratinocytes are able to rapidly kill S. aureus which come into contact with them [147]. This activity is entirely dependent on mobilization of hBD3 from the cytoplasm onto the bacteria at the plasma membrane. The secretory process depends on contact between the bacteria and the cells, and mobilization of the hBD3 is focal, rather than global. However, in individuals with atopic dermatitis (AD), which is characterized by frequent and persistent colonization of the skin by S. aureus, the ability of keratinocytes to kill S. aureus is impaired [148]. The defect arises not from impaired synthesis of hBD3, and the cytoplasmic complement of this peptide is comparable with that of normal individuals. However, mobilization of the peptide following contact of the bacteria with the keratinocytes of patients with atopic dermatitis is substantially inhibited.
In addition to reduced mobilization of hBD3, bacteria may gain an advantage in the skin of patients with AD due to reduced synthesis of LL-37, and hBD2 [149]. In the cases of LL-37 and hBD2, synthesis is inhibited by the cytokine milieu present in the skin of AD patients, which is richer in Th2 cytokines relative to the skin of normal individuals [150].
Therefore, there are various modes by which antimicrobial peptides are brought into contact with potential pathogens, each one specialized for the anatomical site to be defended against invasion of microbes, and the microbes themselves which are common to those sites: phagosome-lysosome fusion, or phagosome-granule fusion in the cases of macrophages and neutrophils; extracellular secretion into confined extracellular spaces in the cases of gingival epithelial cells secreting into the gingival crevice, or Paneth cells secreting into the base of the intestinal crypts; and keratinocytes utilizing focal secretion onto bacteria bound to the plasma membrane.
VIII. Inhibition of Expression
While initial experiments demonstrated the variety of factors that induced expression of AMPs, suggesting their role in host defense, more recent studies have examined factors that suppress or inhibit AMP expression. Such an inhibition can lead to reduced basal levels of AMPs, or the lack of induced levels, and provide a better environment for pathogen growth. One class of inhibitors can be found in pathogens themselves, as a mechanism to evade AMP activation. This has been observed with Bordetella bronchiseptica, an animal airway pathogen closely related to the etiologic agent for whooping cough in humans, B. pertussis. Wild-type strains of B. bronchiseptica encode a type III secretion factor which, when introduced into host cells can inhibit NF-κB activation [151]. While the LPS from this Gram-negative bacterium can induce β-defensin expression in airway epithelial cells, strains encoding the type III secretion factor inhibit the NF-κB-mediated induction, leading to lower levels of the peptide [113]. While the exact mechanism of this species' inhibition is unknown, other type III secretion factors that inhibit NF-κB activation are known to affect the ubiquitin proteasome system (reviewed in [152]).
Similarly, other pathogens have been observed to inhibit AMP gene expression. These include the inhibition of hBD1 and LL-37 expression in the gut by Shigella spp [153], and of LL-37 in cervical epithelial cells by Neisseria gonorrhea [154]. These both appear to also be inhibiting at the level of transcriptional regulation, although through other potential mechanisms.
Other factors that inhibit TLR-mediated NF-κB induction can similarly suppress the pathogen-mediated induction of AMPs that are regulated by that pathway. Infection of bovine tracheal epithelial cells with one type of Bovine viral diarrhea virus inhibits the subsequent induction of β-defensin gene expression by LPS [155]. This can render the animal more susceptible to bacterial airway infections which are known to be associated with viral infection. We have observed that Herpes Simplex Virus can suppress hBD2 expression in gingival epithelial cells (Ryan et al., manuscript in preparation), which can similarly affect the defense against periodontal pathogens, as some forms of periodontal disease are associated with Herpesvirus infections [156]. These viral-mediated suppressions may also proceed through inhibition of the ubiquitin pathway as was recently demonstrated for the measles-virus inhibition of TLR signaling [157].
As part of a general suppression of the immune system, certain cytokines have also been observed to inhibit AMP gene expression. In cases of atopic dermatitis, where increased levels of IL-4 and IL-13 [149], or IL-10 [158] are observed, there are reduced levels of hBD2 and LL-37. In both of these studies, exogenous addition of these cytokines inhibits the AMP gene expression in keratinocytes, suggesting these molecules regulate AMP gene expression in these pathogenic conditions. Addition of IL-6 and IFN-γ has also been observed to reduce levels of LL-37 in T- and NK-cells [159], suggesting that cytokines play an important role in regulating AMP levels throughout the body.
Exogenous chemicals, both therapeutic and environmental, can also affect AMP gene induction. We observed that air pollutant particles can inhibit the LPS-mediated induction of β-defensin gene expression in airway epithelial cells in a dose-dependent manner, primarily due to vanadium [160]. Such an effect could promote airway infections in highly polluted areas. As with the effect of pathogens, it is due to the inhibition of NF-κB induction. Thus, chemicals that are known to affect this pathway can lead to reduced levels of some AMPs. Specifically, dexamethasone treatment will lead to an inhibition in the induction of β-defensin gene expression through this mechanism [161, 162].
In addition to extrinsic factors, innate deficiencies in AMP gene expression can lead to reduced levels or a reduction in the induction. Three disorders with high levels of periodontal disease have been observed to correlate with lower AMP gene regulation. In one case, gingival epithelial cells from a patient with Localized Aggressive Periodontitis (LAP) failed to induce β-defensin gene expression in response to bacteria, but not IL-1β, while normal cells responded to both [163]. A rare disorder, severe congenital neutropenia (also known as morbus Kostmann), is associated with a complete absence of LL-37, and is characterized by, among other symptoms, chronic periodontitis, and overgrowth with a periodontal pathogen, Aggregatibacter actinomycetemcomitans [164, 165]. Individuals with another genetic disorder, Papillon-Lefevre Syndrome, demonstrate a deficiency in LL-37 and exhibit severe periodontitis. This may be due to a deficiency in serine proteinases that process hCAP-18 to the mature, active LL-37 peptide [166].
Genetic variability within the AMP genes themselves can lead to reduced levels of the peptide, and increased susceptibility to infection. Single nucleotide polymorphisms in the hBD1 gene are associated with chronic obstructive pulmonary disease [167], increased levels of Candida albicans in the oral cavity [168], and increased risk of HIV-1 infection [169, 170], although the causal relationships between the polymorphism and the diseases are unknown.
IX. Antimicrobial Peptides as Immune Regulators
A. Introduction
Most reviews of AMPs up until the year 2000 described these host derived peptides as the body's “natural antibiotics;” i.e., as microbicidal agents that can function rapidly against multiple microbial species at epithelial barriers or during phagocytosis. The early pioneering work by Territo et al. [171], demonstrated for the first time that neutrophil derived α-defensins were chemotactic towards human monocytes. This finding, however, could not be appreciated nor put into context until a number of years later when other laboratories started realizing that AMPs had indeed additional properties related to “cross-talking” with innate and adaptive immunity. This section will endeavor to summarize recent findings that point to AMPs as contributing to immune regulation. As more information is gathered from such findings, it is anticipated that exploiting AMP immune regulatory strategies will become more commonplace as translational options in bolstering the host response without incurring concerns of bacterial resistance. In fact, the first landmark in vivo report using an anti-infective peptide to selectively modulate the innate immune response, was recently published [172]. The authors described the utility of a 13 amino acid nontoxic peptide, IDR-1 (KSRIVPAIPVSLL-NH2), in a mouse model of aggressive bacterial infection. Interestingly, while the peptide showed little antimicrobial activity, it was reported to attenuate pro-inflammatory cytokine production by microbial products, while promoting selective recruitment of monocytes over neutrophils and enhancing and sustaining the levels of monocyte chemokines. While mechanisms for this selective activity still need to be elucidated, results are reminiscent of findings attributed to LL-37 and its anti-inflammatory capacity (see below in B. AMP neutralization of LPS). Overall, this novel study showed for the first time that inflammation can be attenuated in vivo through the use of anti-infective peptides. It is important to state at the outset that works highlighted herein that focus on AMP capacity to regulate epithelial cell proliferation, enhanced wound healing, inhibition/induction of pro-inflammatory cytokines, angiogenesis/anti-angiogenesis, stimulation of chemokine production, chemotaxis of various leukocytes, mast cell degranulation or modulation of host cell gene expression were determined in physiological conditions, not in media of low ionic strength that are often used to determine antimicrobial activity. Therefore, positive outcomes in the presence of serum and physiological salts suggest that results obtained are actually relevant functions.
B. AMP Neutralization of LPS
The ability of AMPs, particularly LL-37, to neutralize endotoxin, was first believed to be due to their cationic and amphipathic capacities to interact with anionic glycolipid LPS [173], as well as their ability to block LPS binding to LPS binding protein, as an initial step in activating immune cells [174]. Further investigation revealed that AMPs can actually inhibit pro-inflammatory responses induced by LPS. LPS- induced genes in macrophages can be suppressed by LL-37 [172]; it directly up-regulates macrophage gene expression, including certain anti-inflammatory genes [175]. Importantly, these observations were reported in whole blood and in low micromolar concentrations of LL-37 [176]. These results suggest that LL-37 has anti-inflammatory properties. Interestingly, while LL-37 was able to inhibit TNFα production in bacteria challenged macrophages [175], polymyxin B, another AMP that inhibits LPS binding to LPS binding protein [174], could not; i.e., suggesting specificity of activity by LL-37 Moreover, LL-37 was found to also induce expression of potent chemokines such as IL-8 and MCP-1 [175]. One could speculate, therefore, that the action of LL-37 in the context of neutralizing endotoxin, may be part of a feedback mechanism intended to limit the induction of septic levels of pro-inflammatory cytokines. By rebalancing an obviously dangerous scenario, LL-37 and other AMPs could then participate in recruiting cells to initiate healing and repair processes.
C. AMP Related Chemotaxis Activity and Associated Receptors
As stated above, the first non-microbicidal related activity attributed to AMPs was that α-defensin human neutrophil peptide 1 (HNP-1) and -2, but not -3, are chemotactic towards human monocytes [171]. Subsequently, these peptides were found to also chemoattract naive (CD4+/CD45RA+) CD4+ and CD8+ T cells, as well as immature dendritic cells (iDC), but not memory (CD4+/CD45RO+) T cells [177]. Later, LL-37 was found to be chemotactic for monocytes, T cells and neutrophils, but not dendritic cells, and that this recruitment was dependent upon the G protein coupled receptor (GPCR) formyl peptide receptor-like 1 (FPRL1) [178-180]. In addition to FPRL1, LL-37 also utilizes the purinergic receptor P2X7 to activate a number of cell types [181-183]. Interestingly, Elssner at al (2004) showed that by transactivating P2X7, LL-37 promotes IL-1β processing and secretion; a result that may enhance inflammatory effectors through synergy between LL-37 and released IL-1β [184].
LL-37 chemoattracts mast cells, but apparently in an FPRL1 receptor independent manner [185], and promotes mast cell activation [186, 187]. hBD1, -2 and -3 were found to recruit memory T cells and iDC via the GPCR CCR6 [188, 189]. HBD-2 can also recruit mast cells [185] and induce mast cell degranulation, prostaglandin D2 production and intracellular Ca2+ mobilization [187]. hBD3 and hBD4 have also been shown to induce mast cell degranulation, prostaglandin D2 production, intracellular Ca2+ mobilization and chemotaxis [190]. Moreover, hBD2 and hBD3 are chemotactic for human neutrophils via CCR6 [191]. Interestingly, hBD3 has been shown to recruit monocytes in an isoform dependent manner; i.e., different disulfide bond motifs chemoattract monocytes to varying degrees [192]. This suggests that oxidative conditions in mucosae of chronic disease could impact conformational outcomes of AMPs during folding, which could then impact their ability to recruit innate and adaptive immune cells.
The specificity of AMPs for receptors and respective outcomes of these interactions is noteworthy, and best exemplified when comparing LL-37 and hBD3. As stated above, LL-37 recruits a number of peripheral blood mononuclear cells through interaction with the GPCR FPRL1. However, we recently showed that hBD3 has no effect on formyl-met-leu-phe (FMLP) receptors, such as FPRL1 [193]. Instead, hBD3 interacts with another GPCR, CXCR4, resulting in antagonism of T cell migration, not promotion of chemotaxis [193]. CXCR4 is an important co-receptor HIV-1 uses to fuse and replicate in CD4+ T cells [194]. We previously showed that hBD3 protects T cells from HIV-1 infection [194] by promoting CXCR4 internalization, without cellular activation [193]. Since CXCR4 also plays an important role in hemopoiesis, neurogenesis, cardiogenesis and angiogenesis, hBD3 or its derivatives offer a new paradigm in immunoregulatory therapeutics and provide the opportunity to enhance future drug design.
D. AMPs can also Direct Chemotaxis, Indirectly
AMPs have been shown to induce a variety of chemokines in epithelial cells, thereby enhancing their own chemotactic capacity and possibly prolonging chemotaxis overall. IL-8 can be produced in epithelial cells upon challenge with either LL-37 or α-defensins [175, 195]. hBD3 and LL-37 can induce chemokines such as monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-3α (MIP-3α; CCL20) and interferon-γ inducible protein-10 (IP-10; CXCL10) in human epidermal keratinocytes [196]. These data, along with information from the previous section, collectively, could be saying that AMPs have a multifaceted role in controlling microbial infections. Aside from their direct antimicrobial activity, AMPs could initially promote leukocyte migration to combat infection, as evidenced by up-regulation of IL-8 and MCP-1, followed at a later point in the inflammatory process by acting as feedback inhibitors to control inflammation by attenuating immune cell activation.
E. AMP Related Epidermal Growth Factor Receptor (EGFR) Interactions
LL-37 can induce lung epithelial cell signaling by transactivating the epidermal growth factor receptor (EGFR). This is apparently carried out in a multi-step fashion, where LL-37 activates membrane-bound metalloproteinases, which then cleave membrane-anchored EGFR-ligands [197], which then activate the cell by interacting with EGFR. Since neutrophils are the major source of LL-37, it is conceivable that infiltrating neutrophils, by releasing LL-37, could contribute to lung epithelial cell signaling. In addition, neutrophil derived MMP-9 and MMP-25 [198, 199] could aid in releasing epithelial membrane bound EGFR ligands and thereby contribute to EGFR activation and cell signaling. These intriguing results may suggest that neutrophils regulate epithelial cell activity in the lungs, and possibly elsewhere, via LL-37. Furthermore, LL-37 can induce keratinocyte migration via heparin-binding-EGF-mediated transactivation of EGFR, and can also promote cell proliferation via EGFR [200]. Importantly, the first in vivo verification of an AMP promoting wound healing was recently demonstrated when adenoviral transfer of LL-37 to excisional wounds in mice promoted re-epithelialization and granulation tissue formation [200]).
Clearly, other AMPs, in conjunction with LL-37, function collectively in possibly promoting wound healing. This is evidenced by the following: (1), epidermal growth factor (EGF), when released in areas of infection, has been shown to induce epithelial cell proliferation and wound healing [201]. Interestingly, both LL-37 [202] and hBD-3 [196] promote epithelial cell migration and proliferation. Sorensen et al. [203] found that additional EGFR ligands, such as insulin growth factor 1 (IGF-1) and transforming growth factor α (TGF-α), induce expression of a host of epithelial cell derived AMPs, including LL-37, hBD3, neutrophil gelatinase-associated lipocalin (NGAL) and secretory leukocyte protease inhibitor (SLPI), suggesting a common EGFR dependent mechanism for AMP induction. Alpha-defensins from human neutrophils also induce airway epithelial cell proliferation in an EGFR independent fashion [204], while wound closure; i.e., epithelial cell migration, appears to require EGFR activation and downstream signaling pathways [205]. In addition, these peptides promote the expression of MUC5B and MUC5AC, two mucins that contribute to regeneration of the epithelium [205]. Therefore, collective AMP induction and activation may work in synergy to support the growth and antimicrobial potential of keratinocytes when endangered through microbial challenges and wounding.
F. Evidence and Implications for AMP Expression in Wounds
Heilborn et al. [206] discovered (1) LL-37 is highly expressed in skin wounds in vivo, reaching highest levels 48 hrs post-injury and declining to lowest levels upon wound closure; (2) it is also detected in the inflammatory infiltrate and in epithelium migrating over the wound bed; (3) blocking antibodies to LL-37 inhibit re-epithelialization in a concentration dependent manner. However, in chronic ulcers, LL-37 expression is very low and is not detected in ulcer edge epithelium [206]. Since angiogenesis is an important component in tissue repair and wound healing, Koczulla et al. [207], investigated the neo-vascularization capacity of LL-37 in in vitro and in vivo models. They found that the peptide activated endothelial cells to proliferate and form vessel-like structures. Interestingly, mice deficient in CRAMP, the mouse equivalent of LL-37, are deficient in wound neo-vascularization [207].
Differential expression of AMPs in human synovial membranes is governed by specific diseases. HBD-3 and/or LL-37 are detected in synovial membrane samples from pyogenic arthritis (PA), osteoarthritis (OA) or rheumatoid arthritis (RA), while bactericidal permeability-increasing protein (BPI), HD5, HD6 and hBD2 are absent from all of these samples [208]. Under inflammatory conditions, hBD3 is induced in PA, LL-37 in RA and both in OA [208]. More recently, cytokines involved in the pathogenesis of OA, TNF-α and IL-1, were shown to induce hBD-3 in cultured chondrocytes and hBD-3 was shown to mediate tissue remodeling in articular cartilage by increasing chondrocyte derived cartilage-degrading matrix- metalloproteases and reducing levels of their endogenous inhibitors [209]. The authors concluded that hBD-3 links host defense mechanisms and inflammation with tissue-remodeling processes in articular cartilage and suggest that hBD-3 is a new factor in the pathogenesis of OA.
G. AMP Activity in Adaptive Immunity
From a series of studies conducted over the last seven years, we can now point to the ability of AMPs to modulate adaptive immune functions. A number of studies have reported that co-administering AMPs with relatively benign antigens results in enhancement of the host's cell mediated and humoral immune responses to these antigens. Co-administering ovalbumin (OVA) with α-defensins HNP1-3 in mice leads to enhanced IgG antibody response to OVA when compared to OVA alone [210]. OVA-specific CD4+ T cells were found to produce elevated cytokine levels as well [210]. These data suggest that the HNPs act as adjuvants. Another study showed enhanced OVA-specific IgG response in mice when OVA was conasally administered with either 1 μg of either HNP-1, hBD-1 or hBD-2 [211]. Furthermore, intraperitoneal administration of a B-cell lymphoma idiotype antigen combined with daily injections of HNPs increased IgG levels to that antigen and augmented resistance to tumor challenge in mice [212]. These findings strongly implicate α-defensins as immune adjuvants that promote T cell-dependent cellular immunity as well as antigen-specific immunoglobulin production.
While the mechanisms for these intriguing outcomes have not been established, we can speculate that AMPs may be modulating lymphocyte responses, modifying cytokine expression during the APC encounter with the antigen, and possibly, as we recently reported with hBD3 [213], causing the maturation of iDCs by inducing co-stimulatory molecules, resulting in more effective antigen presentation and subsequent robust T cell activation. Clearly, these and future studies will lead to an enhanced interest in AMPs and their homologs as immuno-therapeutic candidates to bolster the host's immune response.
Using a DNA-vaccine strategy, Biragyn et al., [214] immunized mice with constructs encoding murine beta defensins or various chemokines fused to non-immunogenic lymphoma antigens, and studied their capacity to deliver antigens to subsets of immune cells in order to elicit antitumor immunity. This elegant study demonstrated that DNA immunization, where the vaccine contained murine defensins or chemokines that chemoattract immature dendritic cells (iDC) via CCR6; i.e., mBD2, MIP3α, but not mature DCs, elicit humoral and protective immunity against lymphoma [214]. The authors speculated that the targeting of iDCs by these specific defensins and chemokines via CCR6 [189], results in increased uptake of antigen and induces the expression of co-stimulatory molecules that have been reported by others to induce a robust immune response against weak immunogens [215-218]. Interestingly, this group showed in the murine model that mBD2, which does not appear to have a human ortholog, can activate murine iDC directly via TLR4 [219]. More recently, we showed that hBD3 induces expression of costimulatory molecules CD40, CD80 and CD86 on human iDCs and monocytes [213], and that hBD3 promotes expression of a pro-inflammatory cytokine profile in APC (Funderburg et al., in preparation). LL-37 has been shown to modulate dendritic cell differentiation by enhancing endocytic capacity, upregulating co-stimulatory molecule expression, enhancing secretion of proinflammatory cytokines and promoting Th1 responses in vitro [220]. Human α-defensins have also been shown to promote expression of costimulatory molecules on lymphocytes [210] as well as the production of proinflammatory cytokines [221]. Chemokines, such as MCP-1, can promote IL-4 production [222] and induce Th2 polarization [223], while macrophage-derived chemokines (MCD; CCL22) selectively chemoattract Th2 cells toward APC [224]. These collective observations lead us to conclude that specific defensin molecules and chemokines, or their active homologs, could one day be used as adjuvants to both target antigen to APC as well as selectively prime for humoral or cellular immune responses in vivo.
X. Conclusions
While initially identified as host defense peptides with broad-spectrum antimicrobial activity, the AMPs described here are now recognized as multifunctional peptides, whose ultimate role or roles in human immunity have yet to be fully defined. Found in both cells of the myeloid lineage as well as on epithelial surfaces, both constitutively expressed and inducible as part of an innate immune response, they are major participants in the host defense against microbial infection. Their numerous other activities, however, suggest that they may carry out several functions. Furthermore, while their development as exogenous antimicrobial therapeutic agents has not been highly successful, their other activities may lend them to provide the foundation for pharmacological use. Alteration of the physical capabilities of AMPs to enhance their activity for therapeutic applicability has reached a precipice. The majority of research has confirmed the importance of both a net charge and hydrophobicity in relation to antimicrobial activity and any attempts to alter one characteristic could potentially ruin the other [45]. For example, mutation experiments with hBD3 demonstrated a higher level of cytotoxicty for variants with increased hydrophobicity but more potent antibacterial effects with a higher positive charge [46].
While they offer a host of potential therapeutic benefits further research needs to be carried out to enhance AMP pharmacokinetics [225]. The continual refinement of peptidomimetic technologies [226, 227], for example, may allow more cost effective solutions to production as well as complex formulation to maximize activity and minimize potential issues of toxicity.
Acknowledgments
Supported by grants from the US Public Health Service, R01 DE14897 and R01 HL67871 (to G.D.), R21 AI52316 (to K.O.K), and R01 DE18276, R01 DE16334, R01 DE17334 and R01 DE15510 (to A.W); and from the Cystic Fibrosis Foundation and Polymedix, Inc. (to G.D.).
Abbreviations
- AMP
Antimicrobial peptide
- ASF
Airway surface fluid
- DC
Dendritic cell
- hBD
Human beta-defensins
- HD
Human defensin
- HNP
Human neutrophil peptide
- JNK
Jun N-terminal kinase
- LPS
Lipopolysaccharide
- VDR
Vitamin D receptor
- VDRE
Vitamin D response element
- TLR
Toll-like receptor
- SNP
Single nucleotide polymorphism
- PDC
Plasmacytoid dendritic cell
- OA
Osteoarthritis
- PA
Pyogenic arthritis
- RA
Rhematoid arthritis
- BPI
Bactericidal permeability increasing protein
- OVA
Ovalbumin
- APC
Antigen presenting cell
References
- 1.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–50. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 2.Diamond G, Laube D, Klein-Patel ME. In: Mammalian Host Defence Peptides. Hancock REW, Devine DA, editors. Vol. 6. Cambridge University Press; UK: Cambridge: 2004. pp. 111–38. [Google Scholar]
- 3.Hancock REW, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–10. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser V, Diamond G. Expression of mammalian defensin genes. J Leukoc Biol. 2000;68:779–84. [PubMed] [Google Scholar]
- 5.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 6.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 7.Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond (Biol) 1922;93:306–17. [Google Scholar]
- 8.Skarks RC, Watson DW. Antimicrobial factors of normal tissues and fluids. Bacteriol Rev. 1957;21:273–94. doi: 10.1128/br.21.4.273-294.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeya HI, Spitznagel JK. Antibacterial and enzymic basic proteins from leukocyte lysosomes: separation and identification. Science. 1963;142:1085–87. doi: 10.1126/science.142.3595.1085. [DOI] [PubMed] [Google Scholar]
- 10.Hultmark D, Steiner H, Rasmuson T, Boman HG. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980;106:7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 11.Selsted ME, Brown DM, DeLange RJ, Lehrer RI. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983;258:14485–9. [PubMed] [Google Scholar]
- 12.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–53. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole AM, Weis P, Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J Biol Chem. 1997;272:12008–13. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- 14.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 15.van Dijk A, Veldhuizen EJ, Haagsman HP. Avian defensins. Vet Immunol Immunopathol. 2008 doi: 10.1016/j.vetimm.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro MS, Fontes W. Plant defense and antimicrobial peptides. Protein Pept Lett. 2005;12:13–8. [PubMed] [Google Scholar]
- 17.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmachary M, Krishnan SP, Koh JL, Khan AM, Seah SH, Tan TW, et al. ANTIMIC: a database of antimicrobial sequences. Nucleic Acids Res. 2004;32:D586–9. doi: 10.1093/nar/gkh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 20.Boman HG. Innate immunity and the normal microflora. Immunol Rev. 2000;173:5–16. doi: 10.1034/j.1600-065x.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- 21.Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–7. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 22.Lai R, Liu H, Hui Lee W, Zhang Y. An anionic antimicrobial peptide from toad Bombina maxima. Biochem Biophys Res Commun. 2002;295:796–9. doi: 10.1016/s0006-291x(02)00762-3. [DOI] [PubMed] [Google Scholar]
- 23.Steiner H, Hultmark D, Engstrom A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–8. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 24.Fennell JF, Shipman WH, Cole LJ. Antibacterial action of melittin, a polypeptide from bee venom. Proc Soc Exp Biol Med. 1968;127:707–10. doi: 10.3181/00379727-127-32779. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy W, Takase M, Wakabayashi H, Kawase K, Tomita M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol. 1992;73:472–9. doi: 10.1111/j.1365-2672.1992.tb05007.x. [DOI] [PubMed] [Google Scholar]
- 26.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 27.Zhu S. Evidence for myxobacterial origin of eukaryotic defensins. Immunogenetics. 2007;59:949–54. doi: 10.1007/s00251-007-0259-x. [DOI] [PubMed] [Google Scholar]
- 28.Wong JH, Xia L, Ng TB. A review of defensins of diverse origins. Curr Protein Pept Sci. 2007;8:446–59. doi: 10.2174/138920307782411446. [DOI] [PubMed] [Google Scholar]
- 29.Selsted ME. Theta-defensins: cyclic antimicrobial peptides produced by binary ligation of truncated alpha-defensins. Curr Protein Pept Sci. 2004;5:365–71. doi: 10.2174/1389203043379459. [DOI] [PubMed] [Google Scholar]
- 30.Ganz T. Hepcidin--a peptide hormone at the interface of innate immunity and iron metabolism. Curr Top Microbiol Immunol. 2006;306:183–98. doi: 10.1007/3-540-29916-5_7. [DOI] [PubMed] [Google Scholar]
- 31.Edgerton M, Koshlukova SE. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv Dent Res. 2000;14:16–21. doi: 10.1177/08959374000140010201. [DOI] [PubMed] [Google Scholar]
- 32.Gao G, Guo X, Goff SP. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science. 2002;297:1703–6. doi: 10.1126/science.1074276. [DOI] [PubMed] [Google Scholar]
- 33.Powers JP, Hancock RE. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–91. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Rozek A, Friedrich CL, Hancock RE. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry. 2000;39:15765–74. [PubMed] [Google Scholar]
- 35.Hoover DM, Chertov O, Lubkowski J. The structure of human beta-defensin-1: new insights into structural properties of beta-defensins. J Biol Chem. 2001;276:39021–6. doi: 10.1074/jbc.M103830200. [DOI] [PubMed] [Google Scholar]
- 36.Hoover DM, Rajashankar KR, Blumenthal R, Puri A, Oppenheim JJ, Chertov O, et al. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J Biol Chem. 2000;275:32911–8. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 37.Sawai MV, Jia HP, Liu L, Aseyev V, Wiencek JM, McCray PB, Jr, et al. The NMR structure of human beta-defensin-2 reveals a novel alpha-helical segment. Biochemistry. 2001;40:3810–6. doi: 10.1021/bi002519d. [DOI] [PubMed] [Google Scholar]
- 38.Hill CP, Yee J, Selsted ME, Eisenberg D. Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science. 1991;251:1481–5. doi: 10.1126/science.2006422. [DOI] [PubMed] [Google Scholar]
- 39.Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 40.Tran D, Tran PA, Tang YQ, Yuan J, Cole T, Selsted ME. Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J Biol Chem. 2002;277:3079–84. doi: 10.1074/jbc.M109117200. [DOI] [PubMed] [Google Scholar]
- 41.Yount NY, Yeaman MR. Multidimensional signatures in antimicrobial peptides. Proc Natl Acad Sci USA. 2004;101:7363–8. doi: 10.1073/pnas.0401567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann GR, Legault P, Selsted ME, Pardi A. Solution structure of bovine neutrophil β-defensin-12: The peptide fold of the β-defensins is identical to that of classical defensins. Biochemistry. 1995;34:13663–71. doi: 10.1021/bi00041a048. [DOI] [PubMed] [Google Scholar]
- 43.Xie C, Prahl A, Ericksen B, Wu Z, Zeng P, Li X, et al. Reconstruction of the conserved beta-bulge in mammalian defensins using D-amino acids. J Biol Chem. 2005;280:32921–9. doi: 10.1074/jbc.M503084200. [DOI] [PubMed] [Google Scholar]
- 44.Wieprecht T, Dathe M, Krause E, Beyermann M, Maloy WL, MacDonald DL, et al. Modulation of membrane activity of amphipathic, antibacterial peptides by slight modifications of the hydrophobic moment. FEBS Lett. 1997;417:135–40. doi: 10.1016/s0014-5793(97)01266-0. [DOI] [PubMed] [Google Scholar]
- 45.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–80. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kluver E, Schulz-Maronde S, Scheid S, Meyer B, Forssmann WG, Adermann K. Structure-activity relation of human beta-defensin 3: influence of disulfide bonds and cysteine substitution on antimicrobial activity and cytotoxicity. Biochemistry. 2005;44:9804–16. doi: 10.1021/bi050272k. [DOI] [PubMed] [Google Scholar]
- 47.Sawai MV, Waring AJ, Kearney WR, McCray PB, Jr, Forsyth WR, Lehrer RI, et al. Impact of single-residue mutations on the structure and function of ovispirin/novispirin antimicrobial peptides. Protein Eng. 2002;15:225–32. doi: 10.1093/protein/15.3.225. [DOI] [PubMed] [Google Scholar]
- 48.Szyk A, Wu Z, Tucker K, Yang D, Lu W, Lubkowski J. Crystal structures of human alpha-defensins HNP4, HD5, and HD6. Protein Sci. 2006;15:2749–60. doi: 10.1110/ps.062336606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aerts AM, Francois IE, Cammue BP, Thevissen K. The mode of antifungal action of plant, insect and human defensins. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-8035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuwano K, Tanaka N, Shimizu T, Kida Y. Antimicrobial activity of inducible human beta defensin-2 against Mycoplasma pneumoniae. Curr Microbiol. 2006;52:435–8. doi: 10.1007/s00284-005-0215-7. [DOI] [PubMed] [Google Scholar]
- 51.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–56. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 52.Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–74. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salvatore M, Garcia-Sastre A, Ruchala P, Lehrer RI, Chang T, Klotman ME. alpha-Defensin inhibits influenza virus replication by cell-mediated mechanism(s) J Infect Dis. 2007;196:835–43. doi: 10.1086/521027. [DOI] [PubMed] [Google Scholar]
- 54.Ryan LK, Diamond G, Amrute S, Feng Z, Weinberg A, Fitzgerald-Bocarsly P. Detection of HBD1 peptide in peripheral blood mononuclear cell subpopulations by intracellular flow cytometry. Peptides. 2003;24:1785–94. doi: 10.1016/j.peptides.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 55.Cole AM, Hong T, Boo LM, Nguyen T, Zhao C, Bristol G, et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci USA. 2002;99:1813–8. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park CB, Yi KS, Matsuzaki K, Kim MS, Kim SC. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc Natl Acad Sci USA. 2000;97:8245–50. doi: 10.1073/pnas.150518097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rice WG, Ganz T, Kinkade JMJ, Selsted ME, Lehrer RI, Parmley RT. Defensin-rich dense granules of human neutrophils. Blood. 1987;70:757–65. [PubMed] [Google Scholar]
- 58.Ladokhin AS, Selsted ME, White SH. Sizing membrane pores in lipid vesicles by leakage of co-encapsulated markers: pore formation by melittin. Biophys J. 1997;72:1762–6. doi: 10.1016/S0006-3495(97)78822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christensen B, Fink J, Merrifield RB, Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci (USA) 1988;85:5072–6. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee MT, Chen FY, Huang HW. Energetics of pore formation induced by membrane active peptides. Biochemistry. 2004;43:3590–9. doi: 10.1021/bi036153r. [DOI] [PubMed] [Google Scholar]
- 61.Bechinger B, Aisenbrey C, Bertani P. The alignment, structure and dynamics of membrane-associated polypeptides by solid-state NMR spectroscopy. Biochim Biophys Acta. 2004;1666:190–204. doi: 10.1016/j.bbamem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Wu Y, He K, Ludtke SJ, Huang HW. X-ray diffraction study of lipid bilayer membranes interacting with amphiphilic helical peptides: diphytanoyl phosphatidylcholine with alamethicin at low concentrations. Biophys J. 1995;68:2361–9. doi: 10.1016/S0006-3495(95)80418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Subbalakshmi C, Sitaram N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol Lett. 1998;160:91–6. doi: 10.1111/j.1574-6968.1998.tb12896.x. [DOI] [PubMed] [Google Scholar]
- 64.Kavanagh K, Dowd S. Histatins: antimicrobial peptides with therapeutic potential. J Pharm Pharmacol. 2004;56:285–9. doi: 10.1211/0022357022971. [DOI] [PubMed] [Google Scholar]
- 65.Brotz H, Bierbaum G, Markus A, Molitor E, Sahl HG. Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism? Antimicrob Agents Chemother. 1995;39:714–9. doi: 10.1128/AAC.39.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agerberth B, Boman A, Andersson M, Jörnvall H, Mutt V, Boma HG. Isolation of three antibacterial peptides from pig intestine: gastric inhibitory polypeptide(7-42), diazepam-binding inhibitor(32-86) and a novel factor, peptide 3910. Eur J Biochem. 1993;216:623–9. doi: 10.1111/j.1432-1033.1993.tb18182.x. [DOI] [PubMed] [Google Scholar]
- 67.Nizet V. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol. 2007;120:13–22. doi: 10.1016/j.jaci.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Scott MG, Yan H, Hancock RE. Biological properties of structurally related alpha-helical cationic antimicrobial peptides. Infect Immun. 1999;67:2005–9. doi: 10.1128/iai.67.4.2005-2009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weidenmaier C, Kristian SA, Peschel A. Bacterial resistance to antimicrobial host defenses--an emerging target for novel antiinfective strategies? Curr Drug Targets. 2003;4:643–9. doi: 10.2174/1389450033490731. [DOI] [PubMed] [Google Scholar]
- 70.Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1529–39. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Lourenzoni MR, Namba AM, Caseli L, Degreve L, Zaniquelli ME. Study of the interaction of human defensins with cell membrane models: relationships between structure and biological activity. J Phys Chem B. 2007;111:11318–29. doi: 10.1021/jp067127g. [DOI] [PubMed] [Google Scholar]
- 72.Yau WM, Wimley WC, Gawrisch K, White SH. The preference of tryptophan for membrane interfaces. Biochemistry. 1998;37:14713–8. doi: 10.1021/bi980809c. [DOI] [PubMed] [Google Scholar]
- 73.Chan DI, Prenner EJ, Vogel HJ. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta. 2006;1758:1184–202. doi: 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 74.Dennison SR, Morton LH, Harris F, Phoenix DA. Antimicrobial properties of a lipid interactive alpha-helical peptide VP1 against Staphylococcus aureus bacteria. Biophys Chem. 2007;129:279–83. doi: 10.1016/j.bpc.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 75.Huang HW. Action of antimicrobial peptides: two-state model. Biochemistry. 2000;39:8347–52. doi: 10.1021/bi000946l. [DOI] [PubMed] [Google Scholar]
- 76.Chen HM, Leung KW, Thakur NN, Tan A, Jack RW. Distinguishing between different pathways of bilayer disruption by the related antimicrobial peptides cecropin B, B1 and B3. Eur J Biochem. 2003;270:911–20. doi: 10.1046/j.1432-1033.2003.03451.x. [DOI] [PubMed] [Google Scholar]
- 77.Lee MT, Hung WC, Chen FY, Huang HW. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci USA. 2008;105:5087–92. doi: 10.1073/pnas.0710625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee DG, Park Y, Jin I, Hahm KS, Lee HH, Moon YH, et al. Structure-antiviral activity relationships of cecropin A-magainin 2 hybrid peptide and its analogues. J Pept Sci. 2004;10:298–303. doi: 10.1002/psc.504. [DOI] [PubMed] [Google Scholar]
- 79.Matsuzaki K, Murase O, Fujii N, Miyajima K. Translocation of a channel-forming antimicrobial peptide, magainin 2, across lipid bilayers by forming a pore. Biochemistry. 1995;34:6521–6. doi: 10.1021/bi00019a033. [DOI] [PubMed] [Google Scholar]
- 80.Matsuzaki K, Murase O, Miyajima K. Kinetics of pore formation by an antimicrobial peptide, magainin 2, in phospholipid bilayers. Biochemistry. 1995;34:12553–9. doi: 10.1021/bi00039a009. [DOI] [PubMed] [Google Scholar]
- 81.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J. 2001;81:1475–85. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spaar A, Munster C, Salditt T. Conformation of peptides in lipid membranes studied by x-ray grazing incidence scattering. Biophys J. 2004;87:396–407. doi: 10.1529/biophysj.104.040667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsuzaki K, Murase O, Fujii N, Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996;35:11361–8. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 84.Matsuzaki K, Sugishita K, Harada M, Fujii N, Miyajima K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim Biophys Acta. 1997;1327:119–30. doi: 10.1016/s0005-2736(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 85.Matsuzaki K, Sugishita K, Ishibe N, Ueha M, Nakata S, Miyajima K, et al. Relationship of membrane curvature to the formation of pores by magainin 2. Biochemistry. 1998;37:11856–63. doi: 10.1021/bi980539y. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother. 2007;51:1398–406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lugtenberg B, Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983;737:51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- 88.Dorschner RA, Lopez-Garcia B, Peschel A, Kraus D, Morikawa K, Nizet V, et al. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. Faseb J. 2006;20:35–42. doi: 10.1096/fj.05-4406com. [DOI] [PubMed] [Google Scholar]
- 89.Pazgier M, Li X, Lu W, Lubkowski J. Human defensins: synthesis and structural properties. Curr Pharm Des. 2007;13:3096–118. doi: 10.2174/138161207782110381. [DOI] [PubMed] [Google Scholar]
- 90.Steinberg DA, Hurst MA, Fujii CA, Kung AH, Ho JF, Cheng FC, et al. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother. 1997;41:1738–42. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Devine DA, Marsh PD, Percival RS, Rangarajan M, Curtis MA. Modulation of antibacterial peptide activity by products of Porphyromonas gingivalis and Prevotella spp. Microbiology. 1999;145(Pt 4):965–71. doi: 10.1099/13500872-145-4-965. [DOI] [PubMed] [Google Scholar]
- 92.Sieprawska-Lupa M, Mydel P, Krawczyk K, Wojcik K, Puklo M, Lupa B, et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother. 2004;48:4673–9. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–36. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 94.Ge Y, MacDonald D, Henry MM, Hait HI, Nelson KA, Lipsky BA, et al. In vitro susceptibility to pexiganan of bacteria isolated from infected diabetic foot ulcers. Diagn Microbiol Infect Dis. 1999;35:45–53. doi: 10.1016/s0732-8893(99)00056-5. [DOI] [PubMed] [Google Scholar]
- 95.Mosca DA, Hurst MA, So W, Viajar BS, Fujii CA, Falla TJ. IB-367, a protegrin peptide with in vitro and in vivo activities against the microflora associated with oral mucositis. Antimicrob Agents Chemother. 2000;44:1803–8. doi: 10.1128/aac.44.7.1803-1808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perron GG, Zasloff M, Bell G. Experimental evolution of resistance to an antimicrobial peptide. Proc Biol Sci. 2006;273:251–6. doi: 10.1098/rspb.2005.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shelburne CE, Coulter WA, Olguin D, Lantz MS, Lopatin DE. Induction of beta-defensin resistance in the oral anaerobe Porphyromonas gingivalis. Antimicrob Agents Chemother. 2005;49:183–7. doi: 10.1128/AAC.49.1.183-187.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laube DM, Yim S, Ryan LK, Kisich KO, Diamond G. Antimicrobial peptides in the airway. Curr Top Microbiol Immunol. 2006;306:153–82. doi: 10.1007/3-540-29916-5_6. [DOI] [PubMed] [Google Scholar]
- 99.O'Neil DA. Regulation of expression of beta-defensins: endogenous enteric peptide antibiotics. Mol Immunol. 2003;40:445–50. doi: 10.1016/s0161-5890(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 100.Selsted ME, Szlarek D, Lehrer RI. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984;45:150–4. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caverly JM, Diamond G, Gallup JM, Brogden KA, Dixon RA, Ackermann MR. Coordinated expression of tracheal antimicrobial peptide and inflammatory-response elements in the lungs of neonatal calves with acute bacterial pneumonia. Infect Immun. 2003;71:2950–5. doi: 10.1128/IAI.71.5.2950-2955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Russell JP, Diamond G, Tarver AP, Scanlin TF, Bevins CL. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect Immun. 1996;64:1565–8. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brahmachary M, Schonbach C, Yang L, Huang E, Tan SL, Chowdhary R, et al. Computational promoter analysis of mouse, rat and human antimicrobial peptide-coding genes. BMC Bioinformatics. 2006;7(Suppl 5):S8. doi: 10.1186/1471-2105-7-S5-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ganz T, Lehrer RI. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–8. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 105.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–9. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 106.Date Y, Nakazato M, Shiomi K, Toshimori H, Kangawa K, Matsuo H, et al. Localization of human neutrophil peptide (HNP) and its messenger RNA in neutrophil series. Ann Hematol. 1994;69:73–7. doi: 10.1007/BF01698485. [DOI] [PubMed] [Google Scholar]
- 107.Harwig SSL, Park ASK, Lehrer RI. Characterization of defensin precursors in mature human neutrophils. Blood. 1992;79:1532–7. [PubMed] [Google Scholar]
- 108.Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3:583–90. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 109.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, YS LB, Stratman JL, et al. Regulation of Intestinal alpha-Defensin Activation by the Metalloproteinase Matrilysin in Innate Host Defense. Science. 1999;286:113–7. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 110.Beckloff N, Diamond G. Computational analysis suggests beta-defensins are processed to mature peptides by signal peptidase. Protein Peptide Lett. 2008;15:536–40. doi: 10.2174/092986608784567618. [DOI] [PubMed] [Google Scholar]
- 111.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–91. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 112.Kanda N, Watanabe S. IL-12, IL-23, and IL-27 enhance human beta-defensin-2 production in human keratinocytes. Eur J Immunol. 2008;38(5):1287–96. doi: 10.1002/eji.200738051. [DOI] [PubMed] [Google Scholar]
- 113.Legarda D, Klein-Patel ME, Yim S, Yuk MH, Diamond G. Suppression of NF-kappaB-mediated beta-defensin gene expression in the mammalian airway by the Bordetella type III secretion system. Cell Microbiol. 2005;7:489–97. doi: 10.1111/j.1462-5822.2004.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kao CY, Kim C, Huang F, Wu R. Requirements for two proximal NF-kappa B binding sites and Ikappa B-zeta in IL-17A-induced human beta -defensin 2 expression by conducting airway Epithelium. J Biol Chem. 2008;283(22):15309–18. doi: 10.1074/jbc.M708289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chung WO, Dommisch H, Yin L, Dale BA. Expression of defensins in gingiva and their role in periodontal health and disease. Curr Pharm Des. 2007;13:3073–83. doi: 10.2174/138161207782110435. [DOI] [PubMed] [Google Scholar]
- 116.Krisanaprakornkit S, Kimball JR, Dale BA. Regulation of human beta-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-kappaB transcription factor family. J Immunol. 2002;168:316–24. doi: 10.4049/jimmunol.168.1.316. [DOI] [PubMed] [Google Scholar]
- 117.Chung WO, Dale BA. Innate immune response of oral and foreskin keratinocytes: utilization of different signaling pathways by various bacterial species. Infect Immun. 2004;72:352–8. doi: 10.1128/IAI.72.1.352-358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chung WO, Dale BA. Differential utilization of nuclear factor-kappaB signaling pathways for gingival epithelial cell responses to oral commensal and pathogenic bacteria. Oral Microbiol Immunol. 2008;23:119–26. doi: 10.1111/j.1399-302X.2007.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duits LA, Ravensbergen B, Rademaker M, Hiemstra PS, Nibbering PH. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology. 2002;106:517–25. doi: 10.1046/j.1365-2567.2002.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Armogida SA, Yannaras NM, Melton AL, Srivastava MD. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc. 2004;25:297–304. [PubMed] [Google Scholar]
- 121.Kuhn L, Trabattoni D, Kankasa C, Semrau K, Kasonde P, Lissoni F, et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr. 2005;39:138–42. [PMC free article] [PubMed] [Google Scholar]
- 122.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agerberth B, Grunewald J, Castanos VE, Olsson B, J H, Wigzell H, et al. Antibacterial Components in Bronchoalveolar Lavage Fluid from Healthy Individuals and Sarcoidosis Patients. Am J Respir Crit Care Med. 1999;160:283–90. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
- 124.Bals R, Weiner DJ, Meegalla RL, Wilson JM. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest. 1999;103:1113–7. doi: 10.1172/JCI6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Engelhardt JF, Yankaskas JR, Wilson JM. In vivo retroviral gene transfer into human bronchial epithelia of xenografts. J Clin Invest. 1992;90:2598–607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–60. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 127.Hosokawa I, Hosokawa Y, Komatsuzawa H, Goncalves RB, Karimbux N, Napimoga MH, et al. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol. 2006;146:218–25. doi: 10.1111/j.1365-2249.2006.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 129.Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 130.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 131.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction Of Cathelicidin In Normal And Cf Bronchial Epithelial Cells By 1,25-Dihydroxyvitamin D3. J Cystic Fibrosis. 2007;6:403–10. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 133.Elloumi HZ, Holland SM. Complex regulation of human cathelicidin gene expression: novel splice variants and 5′UTR negative regulatory element. Mol Immunol. 2008;45:204–17. doi: 10.1016/j.molimm.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Albanesi C, Fairchild HR, Madonna S, Scarponi C, De Pita O, Leung DY, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984–92. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- 135.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 136.Platz J, Beisswenger C, Dalpke A, Koczulla R, Pinkenburg O, Vogelmeier C, et al. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol. 2004;173:1219–23. doi: 10.4049/jimmunol.173.2.1219. [DOI] [PubMed] [Google Scholar]
- 137.Tapper H. The secretion of preformed granules by macrophages and neutrophils. J Leukoc Biol. 1996;59:613–22. doi: 10.1002/jlb.59.5.613. [DOI] [PubMed] [Google Scholar]
- 138.Jan MS, Huang YH, Shieh B, Teng RH, Yan YP, Lee YT, et al. CC chemokines induce neutrophils to chemotaxis, degranulation, and alpha-defensin release. J Acquir Immune Defic Syndr. 2006;41:6–16. doi: 10.1097/01.qai.0000188336.94090.14. [DOI] [PubMed] [Google Scholar]
- 139.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157:1124–31. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 140.Kisich KO, Higgins M, Diamond G, Heifets L. Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect Immun. 2002;70:4591–9. doi: 10.1128/IAI.70.8.4591-4599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177:1864–71. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 142.Diamond DL, Kimball JR, Krisanaprakornkit S, Ganz T, Dale BA. Detection of beta-defensins secreted by human oral epithelial cells. J Immunol Methods. 2001;256:65–76. doi: 10.1016/s0022-1759(01)00442-2. [DOI] [PubMed] [Google Scholar]
- 143.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–41. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 144.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Ganz T. Human β-defensin-1: An antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–42. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, Conway BA, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–6. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hata TR, Gallo RL. Antimicrobial peptides, skin infections, and atopic dermatitis. Semin Cutan Med Surg. 2008;27:144–50. doi: 10.1016/j.sder.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kisich KO, Howell MD, Boguniewicz M, Heizer HR, Watson NU, Leung DY. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J Invest Dermatol. 2007;127:2368–80. doi: 10.1038/sj.jid.5700861. [DOI] [PubMed] [Google Scholar]
- 148.Kisich KO, Carspecken CW, Fieve S, Boguniewicz M, Leung DY. Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human beta-defensin-3. J Allergy Clin Immunol. 2008;122(1):62–8. doi: 10.1016/j.jaci.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 149.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 150.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–9. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 151.Yuk MH, Harvill ET, Cotter PA, Miller JF. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol Microbiol. 2000;35:991–1004. doi: 10.1046/j.1365-2958.2000.01785.x. [DOI] [PubMed] [Google Scholar]
- 152.Angot A, Vergunst A, Genin S, Peeters N. Exploitation of eukaryotic ubiquitin signaling pathways by effectors translocated by bacterial type III and type IV secretion systems. PLoS Pathog. 2007;3:e3. doi: 10.1371/journal.ppat.0030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7:180–5. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 154.Bergman P, Johansson L, Asp V, Plant L, Gudmundsson GH, Jonsson AB, et al. Neisseria gonorrhoeae downregulates expression of the human antimicrobial peptide LL-37. Cell Microbiol. 2005;7:1009–17. doi: 10.1111/j.1462-5822.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 155.Al-Haddawi M, Mitchell GB, Clark ME, Wood RD, Caswell JL. Impairment of innate immune responses of airway epithelium by infection with bovine viral diarrhea virus. Vet Immunol Immunopathol. 2007;116:153–62. doi: 10.1016/j.vetimm.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 156.Contreras A, Umeda M, Chen C, Bakker I, Morrison JL, Slots J. Relationship between herpesviruses and adult periodontitis and periodontopathic bacteria. J Periodontol. 1999;70:478–84. doi: 10.1902/jop.1999.70.5.478. [DOI] [PubMed] [Google Scholar]
- 157.Yokota S, Okabayashi T, Yokosawa N, Fujii N. Measles virus P protein suppresses Toll-like receptor signal through up-regulation of ubiquitin-modifying enzyme A20. FASEB J. 2008;22:74–83. doi: 10.1096/fj.07-8976com. [DOI] [PubMed] [Google Scholar]
- 158.Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, et al. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. J Invest Dermatol. 2005;125:738–45. doi: 10.1111/j.0022-202X.2005.23776.x. [DOI] [PubMed] [Google Scholar]
- 159.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 160.Klein-Patel ME, Diamond G, Boniotto M, Saad S, Ryan LK. Inhibition of beta-Defensin Gene Expression in Airway Epithelial Cells by Low Doses of Residual Oil Fly Ash is Mediated by Vanadium. Toxicol Sci. 2006;92:115–25. doi: 10.1093/toxsci/kfj214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Duits LA, Rademaker M, Ravensbergen B, van Sterkenburg MA, van Strijen E, Hiemstra PS, et al. Inhibition of hBD-3, but not hBD-1 and hBD-2, mRNA expression by corticosteroids. Biochem Biophys Res Commun. 2001;280:522–5. doi: 10.1006/bbrc.2000.4157. [DOI] [PubMed] [Google Scholar]
- 162.Jang BC, Lim KJ, Suh MH, Park JG, Suh SI. Dexamethasone suppresses interleukin-1beta-induced human beta-defensin 2 mRNA expression: involvement of p38 MAPK, JNK, MKP-1, and NF-kappaB transcriptional factor in A549 cells. FEMS Immunol Med Microbiol. 2007;51:171–84. doi: 10.1111/j.1574-695X.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 163.Laube D, Dongari-Bagtzoglou A, Kashleva H, Eskdale J, Gallagher G, Diamond G. Differential regulation of innate immune response genes in gingival epithelial cells stimulated with Aggregatibacter actinomycetemcomitans. J Periodontal Res. 2008;43:116–23. doi: 10.1111/j.1600-0765.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 164.Carlsson G, Wahlin YB, Johansson A, Olsson A, Eriksson T, Claesson R, et al. Periodontal disease in patients from the original Kostmann family with severe congenital neutropenia. J Periodontol. 2006;77:744–51. doi: 10.1902/jop.2006.050191. [DOI] [PubMed] [Google Scholar]
- 165.Putsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–9. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 166.de Haar SF, Hiemstra PS, van Steenbergen MT, Everts V, Beertsen W. Role of polymorphonuclear leukocyte-derived serine proteinases in defense against Actinobacillus actinomycetemcomitans. Infect Immun. 2006;74:5284–91. doi: 10.1128/IAI.02016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Matsushita I, Hasegawa K, Nakata K, Yasuda K, Tokunaga K, Keicho N. Genetic variants of human beta-defensin-1 and chronic obstructive pulmonary disease. Biochem Biophys Res Commun. 2002;291:17–22. doi: 10.1006/bbrc.2002.6395. [DOI] [PubMed] [Google Scholar]
- 168.Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. Single-nucleotide polymorphisms (SNPs) in human beta-defensin 1: high-throughput SNP assays and association with candida carriage in type I diabetics and nondiabetic controls. J Clin Microbiol. 2003;41:90–6. doi: 10.1128/JCM.41.1.90-96.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Braida L, Boniotto M, Pontillo A, Tovo PA, Amoroso A, Crovella S. A single-nucleotide polymorphism in the human beta-defensin 1 gene is associated with HIV-1 infection in Italian children. Aids. 2004;18:1598–600. doi: 10.1097/01.aids.0000131363.82951.fb. [DOI] [PubMed] [Google Scholar]
- 170.Milanese M, Segat L, Pontillo A, Arraes LC, de Lima Filho JL, Crovella S. DEFB1 gene polymorphisms and increased risk of HIV-1 infection in Brazilian children. Aids. 2006;20:1673–5. doi: 10.1097/01.aids.0000238417.05819.40. [DOI] [PubMed] [Google Scholar]
- 171.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–20. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, et al. An anti-infective peptide that selectively modulates the innate immune response. Nat Biotechnol. 2007;25:465–72. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
- 173.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, et al. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells. J Immunol. 2001;167:3329–38. doi: 10.4049/jimmunol.167.6.3329. [DOI] [PubMed] [Google Scholar]
- 174.Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE, Gold MR. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol. 2000;164:549–53. doi: 10.4049/jimmunol.164.2.549. [DOI] [PubMed] [Google Scholar]
- 175.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–91. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 176.Hoshino K, Ogawa K, Hishitani T, Kitazawa R. Influence of heart surgery on magnesium concentrations in pediatric patients. Pediatr Int. 2003;45:39–44. doi: 10.1046/j.1442-200x.2003.01667.x. [DOI] [PubMed] [Google Scholar]
- 177.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- 178.Chertov O, Michiel DF, Xu L, Wang M, Tani K, Murphy WJ, et al. Identification of defensin-1, defensin-2 and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–40. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 179.Yang D, Chen Q, Schmidt AM, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J Leukoc Biol. 2001;69:691–7. [PubMed] [Google Scholar]
- 181.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–94. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 182.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol. 2006;176:3044–52. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- 183.Zuyderduyn S, Ninaber DK, Hiemstra PS, Rabe KF. The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J Allergy Clin Immunol. 2006;117:1328–35. doi: 10.1016/j.jaci.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 184.Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007;179:7684–91. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 185.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C- dependent pathway. Int Immunol. 2002;14:421–6. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 186.Chen X, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, et al. Human cathelicidin LL-37 increases vascular permeability in the skin via mast cell activation, and phosphorylates MAP kinases p38 and ERK in mast cells. J Dermatol Sci. 2006;43:63–6. doi: 10.1016/j.jdermsci.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 187.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–75. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 188.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Identification and Biological Characterization of Mouse beta-defensin 14, the orthologue of human beta-defensin 3. J Biol Chem. 2008;283:5414–9. doi: 10.1074/jbc.M709103200. [DOI] [PubMed] [Google Scholar]
- 189.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 190.Chen X, Niyonsaba F, Ushio H, Hara M, Yokoi H, Matsumoto K, et al. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur J Immunol. 2007;37:434–44. doi: 10.1002/eji.200636379. [DOI] [PubMed] [Google Scholar]
- 191.Niyonsaba F, Ogawa H, Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology. 2004;111:273–81. doi: 10.1111/j.0019-2805.2004.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim JJ, et al. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci USA. 2003;100:8880–5. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Feng Z, Dubyak GR, Lederman MM, Weinberg A. Cutting edge: human beta defensin 3--a novel antagonist of the HIV-1 coreceptor CXCR4. J Immunol. 2006;177:782–6. doi: 10.4049/jimmunol.177.2.782. [DOI] [PubMed] [Google Scholar]
- 194.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. Aids. 2003;17:F39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 195.Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:L888–96. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 196.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 197.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 198.Kang T, Yi J, Guo A, Wang X, Overall CM, Jiang W, et al. Subcellular distribution and cytokine- and chemokine-regulated secretion of leukolysin/MT6-MMP/MMP-25 in neutrophils. J Biol Chem. 2001;276:21960–8. doi: 10.1074/jbc.M007997200. [DOI] [PubMed] [Google Scholar]
- 199.Kjeldsen L, Bainton DF, Sengelov H, Borregaard N. Structural and functional heterogeneity among peroxidase-negative granules in human neutrophils: identification of a distinct gelatinase-containing granule subset by combined immunocytochemistry and subcellular fractionation. Blood. 1993;82:3183–91. [PubMed] [Google Scholar]
- 200.Carretero M, Escamez MJ, Garcia M, Duarte B, Holguin A, Retamosa L, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128:223–36. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 201.Tarnawski A, Szabo IL, Husain SS, Soreghan B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J Physiol Paris. 2001;95:337–44. doi: 10.1016/s0928-4257(01)00046-8. [DOI] [PubMed] [Google Scholar]
- 202.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–8. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 203.Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol. 2003;170:5583–9. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- 204.Aarbiou J, Ertmann M, van Wetering S, van Noort P, Rook D, Rabe KF, et al. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J Leukoc Biol. 2002;72:167–74. [PubMed] [Google Scholar]
- 205.Aarbiou J, Verhoosel RM, Van Wetering S, De Boer WI, Van Krieken JH, Litvinov SV, et al. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am J Respir Cell Mol Biol. 2004;30:193–201. doi: 10.1165/rcmb.2002-0267OC. [DOI] [PubMed] [Google Scholar]
- 206.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–89. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 207.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Paulsen F, Pufe T, Conradi L, Varoga D, Tsokos M, Papendieck J, et al. Antimicrobial peptides are expressed and produced in healthy and inflamed human synovial membranes. J Pathol. 2002;198:369–77. doi: 10.1002/path.1224. [DOI] [PubMed] [Google Scholar]
- 209.Varoga D, Pufe T, Harder J, Schroder JM, Mentlein R, Meyer-Hoffert U, et al. Human beta-defensin 3 mediates tissue remodeling processes in articular cartilage by increasing levels of metalloproteinases and reducing levels of their endogenous inhibitors. Arthritis Rheum. 2005;52:1736–45. doi: 10.1002/art.21090. [DOI] [PubMed] [Google Scholar]
- 210.Lillard JW, Jr, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–6. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brogden KA, Heidari M, Sacco RE, Palmquist D, Guthmiller JM, Johnson GK, et al. Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease. Oral Microbiol Immunol. 2003;18:95–9. doi: 10.1034/j.1399-302x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 212.Tani K, Murphy WJ, Chertov O, Salcedo R, Koh CY, Utsunomiya I, et al. Defensins act as potent adjuvants that promote cellular and humoral immune responses in mice to a lymphoma idiotype and carrier antigens. Int Immunol. 2000;12:691–700. doi: 10.1093/intimm/12.5.691. [DOI] [PubMed] [Google Scholar]
- 213.Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, Harding CV, et al. Human -defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci USA. 2007;104:18631–5. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, Klyushnenkova E, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–53. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 215.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 216.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hodge JW, Rad AN, Grosenbach DW, Sabzevari H, Yafal AG, Gritz L, et al. Enhanced activation of T cells by dendritic cells engineered to hyperexpress a triad of costimulatory molecules. J Natl Cancer Inst. 2000;92:1228–39. doi: 10.1093/jnci/92.15.1228. [DOI] [PubMed] [Google Scholar]
- 218.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–82. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 220.Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172:1146–56. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 221.van Wetering S, Sterk PJ, Rabe KF, Hiemstra PS. Defensins: key players or bystanders in infection, injury, and repair in the lung? J Allergy Clin Immunol. 1999;104:1131–8. doi: 10.1016/s0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- 222.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–36. [PubMed] [Google Scholar]
- 223.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–11. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 224.Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–8. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 225.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–7. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 226.Rotem S, Mor A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamem.2008.10.020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 227.Scott RW, Degrado WF, Tew GN. De novo designed synthetic mimics of antimicrobial peptides. Curr Opin Biotechnol. 2008 doi: 10.1016/j.copbio.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dent Res. 2008;87:915–27. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zasloff M, Martin B, Chen HC. Antimicrobial Activity of Synthetic magainin Peptides and Several Analogues. Proc Natl Acad Sci. 1988;85:910–3. doi: 10.1073/pnas.85.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ericksen B, Wu Z, Lu W, Lehrer RI. Antibacterial activity and specificity of the six human alpha-defensins. Antimicrob Agents Chemother. 2005;49:269–75. doi: 10.1128/AAC.49.1.269-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–14. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta-defensin 3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 233.Travis SM, Anderson NN, Forsyth WR, Espiritu C, Conway BD, Greenberg EP, et al. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun. 2000;68:2748–55. doi: 10.1128/iai.68.5.2748-2755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ji S, Hyun J, Park E, Lee BL, Kim KK, Choi Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res. 2007;42:410–9. doi: 10.1111/j.1600-0765.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 235.Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–9. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wei GX, Campagna AN, Bobek LA. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother. 2006;57:1100–9. doi: 10.1093/jac/dkl120. [DOI] [PubMed] [Google Scholar]
- 237.Ji S, Kim Y, Min BM, Han SH, Choi Y. Innate immune responses of gingival epithelial cells to nonperiodontopathic and periodontopathic bacteria. J Periodontal Res. 2007;42:503–10. doi: 10.1111/j.1600-0765.2007.00974.x. [DOI] [PubMed] [Google Scholar]
- 238.Varkey J, Nagaraj R. Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines. Antimicrob Agents Chemother. 2005;49:4561–6. doi: 10.1128/AAC.49.11.4561-4566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Garcia JR, Jaumann F, Schulz S, Krause A, Rodriguez-Jimenez J, Forssmann U, et al. Identification of a novel, multifunctional beta-defensin (human beta- defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001;306:257–64. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- 240.Huang LC, Jean D, Proske RJ, Reins RY, McDermott AM. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr Eye Res. 2007;32:595–609. doi: 10.1080/02713680701446653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71:3730–9. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Giacometti A, Cirioni O, Del Prete MS, Paggi AM, D'Errico MM, Scalise G. Combination studies between polycationic peptides and clinically used antibiotics against Gram-positive and Gram-negative bacteria. Peptides. 2000;21:1155–60. doi: 10.1016/s0196-9781(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 243.Lundy FT, Nelson J, Lockhart D, Greer B, Harriott P, Marley JJ. Antimicrobial activity of truncated alpha-defensin (human neutrophil peptide (HNP)-1) analogues without disulphide bridges. Mol Immunol. 2008;45:190–3. doi: 10.1016/j.molimm.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 244.Ouhara K, Komatsuzawa H, Yamada S, Shiba H, Fujiwara T, Ohara M, et al. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, {beta}-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55:888–96. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- 245.Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res. 2005;84:445–50. doi: 10.1177/154405910508400509. [DOI] [PubMed] [Google Scholar]
- 246.Helmerhorst EJ, Van't Hof W, Veerman EC, Simoons-Smit I, Nieuw Amerongen AV. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem J. 1997;326(Pt 1):39–45. doi: 10.1042/bj3260039. [DOI] [PMC free article] [PubMed] [Google Scholar]


