Abstract
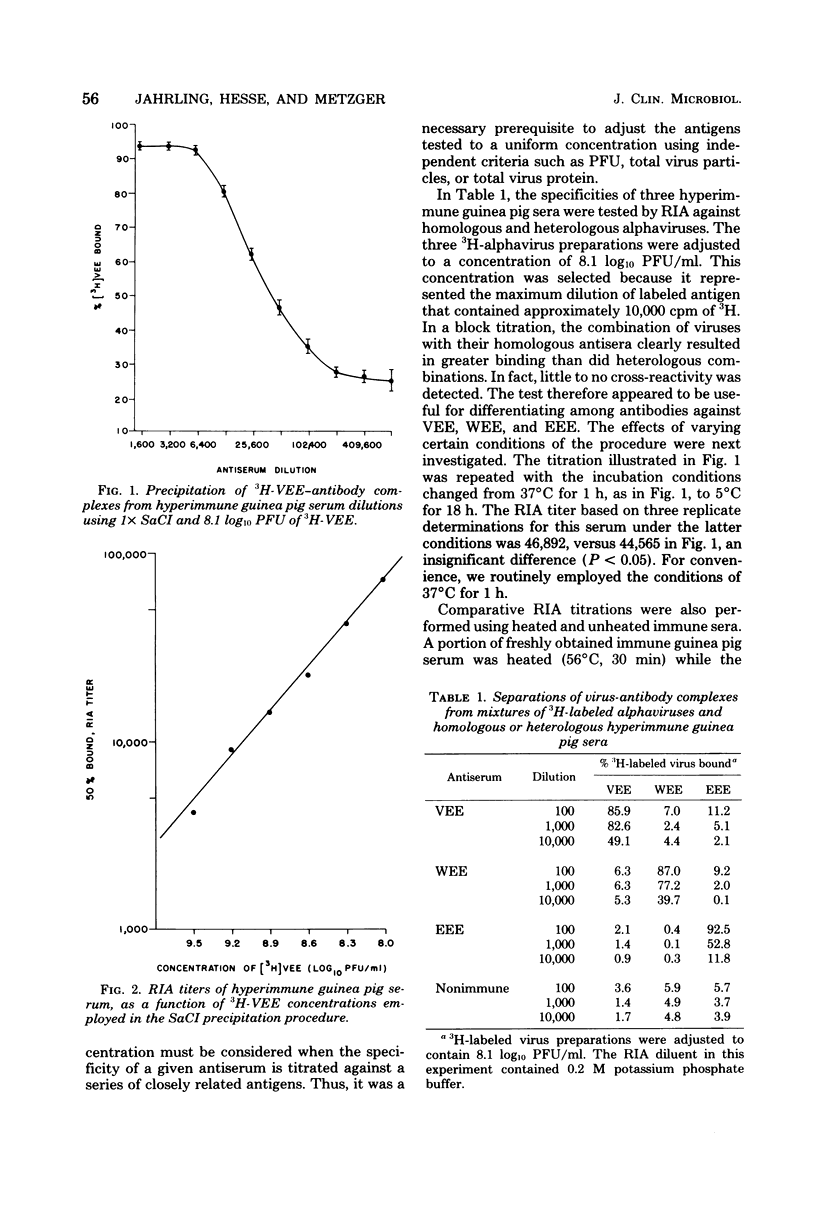
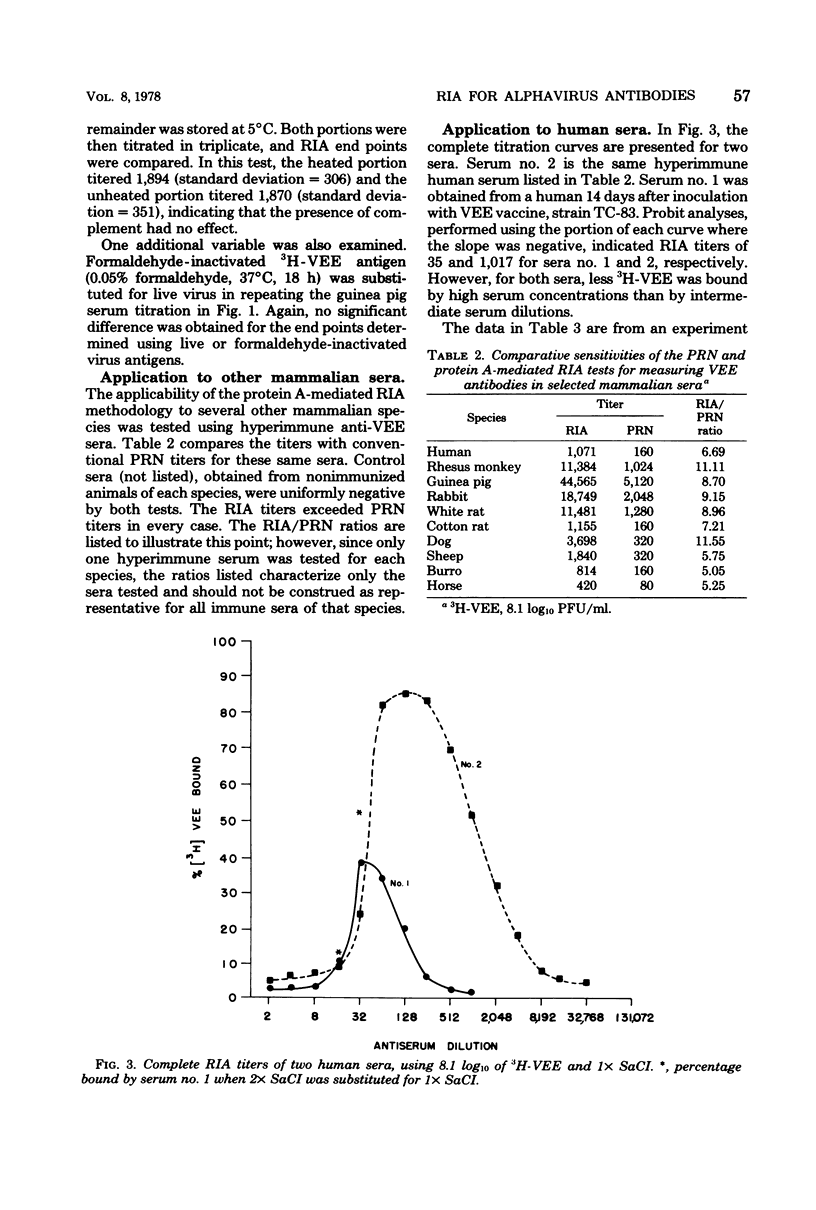
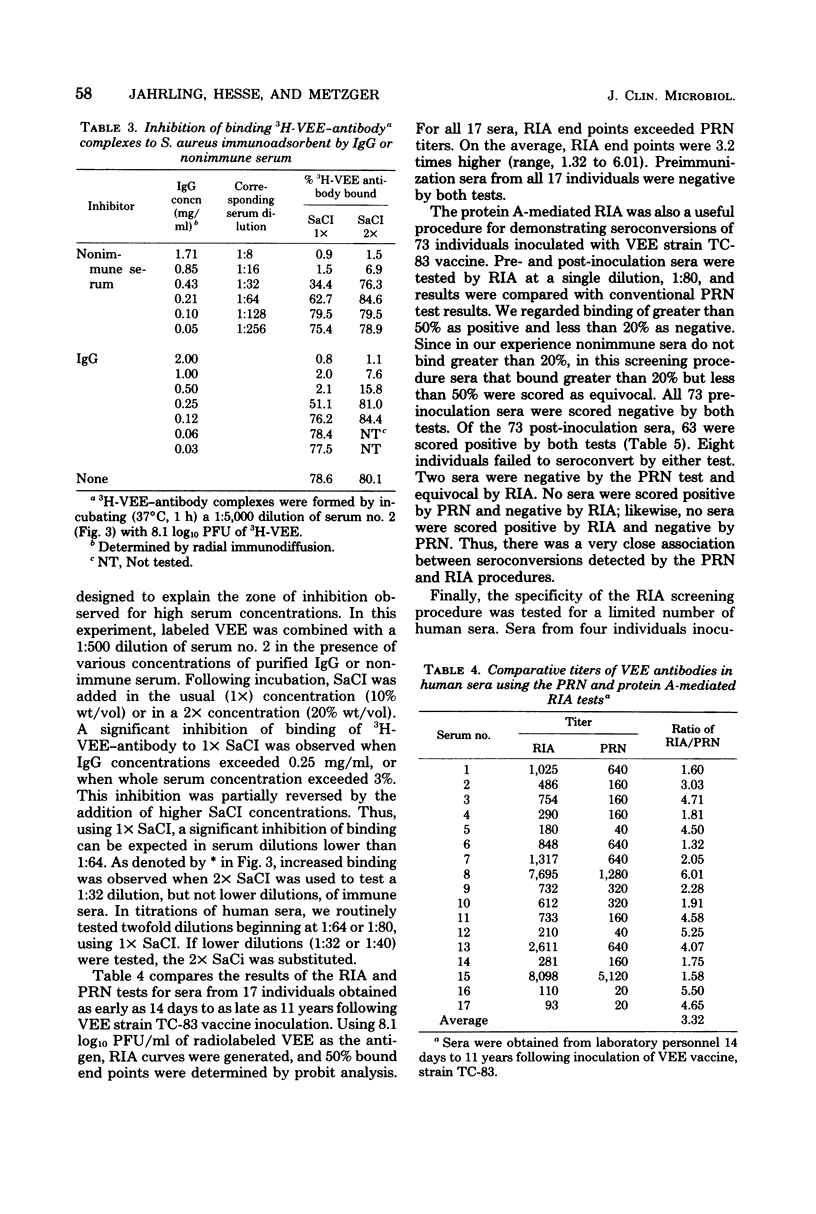
A radioimmunoassay (RIA) procedure is described for measuring antibodies to alphaviruses in human and other mammalian sera. The test employed protein Abearing Staphylococcus aureus as a solid-phase immunoadsorbent for 3H-labeled viruses complexed with immunoglobulin G. Using antibodies produced in humans and guinea pigs, the RIA procedure clearly differentiated among antibodies to Venezuelan, western, and eastern equine encephalomyelitis viruses. Sensitivity of the RIA depended on the concentrations of labeled viruses employed. The dilution of serum that effected binding of 50% of the 3H-labeled virus (determined by probit analysis) was consistently higher than the neutralizing antibody titer determined by a conventional plaque reduction neutralization test using 80% plaque reduction end points. In addition, sera from 73 individuals were screened for seroconversion following live attenuated Venezuelan equine encephalomyelitis virus vaccine (strain TC-83) inoculation, by RIA using a single serum dilution (1:80); results were identical with seroconversions identified by plaque reduction neutralization test. Hyperimmune Venezuelan equine encephalomyelitis virus sera from a number of mammalian species were successfully titrated by RIA; the species tested were human, guinea pig, white rat, rabbit, burro, dog, monkey, sheep, and cotton rat. The protein A-mediated RIA is a rapid, sensitive, specific, and precise serological tool for measuring antibodies to surface antigens of alphaviruses, and should allow the subsequent development of a competitive binding RIA to measure antigenic potency of inactivated alphavirus vaccines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunda M. J., Minden P., Sharpton T. R., McClatchy J. K., Farr R. S. Precipitation of radiolabeled antigen-antibody complexes with protein A-containing Staphylococcus aureus. J Immunol. 1977 Jul;119(1):193–198. [PubMed] [Google Scholar]
- Brunner H., Schaeg W., Brück U., Schummer U., Schiefer H. G. A staphylococcal radioimmunoassay for detection of antibodies to Mycoplasma pneumoniae. Med Microbiol Immunol. 1977 May 18;163(1):25–35. doi: 10.1007/BF02126706. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- Forsgren A. Protein A from Staphylococcus aureus. V. Reaction with guinea pig gamma-globulins. J Immunol. 1968 May;100(5):921–926. [PubMed] [Google Scholar]
- Jahrling P. B., Beall J. L. Chromatographic separations of alphavirus strains by hydroxylapatite. J Clin Microbiol. 1977 Sep;6(3):238–243. doi: 10.1128/jcm.6.3.238-243.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Dendy E., Eddy G. A. Correlates to increased lethality of attenuated Venezuelan encephalitis virus vaccine for immunosuppressed hamsters. Infect Immun. 1974 May;9(5):924–930. doi: 10.1128/iai.9.5.924-930.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Gorelkin L. Selective clearance of a benign clone of Venezuelan equine encephalitis virus from hamster plasma by hepatic reticuloendothelial cells. J Infect Dis. 1975 Dec;132(6):667–676. doi: 10.1093/infdis/132.6.667. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B. Virulence heterogeneity of a predominantly avirulent Western equine encephalitis virus population. J Gen Virol. 1976 Jul;32(1):121–128. doi: 10.1099/0022-1317-32-1-121. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Metzger J. F., Spero L. Production, purification, and chemical characterization of Staphylococcus aureus exfoliative toxin. Infect Immun. 1975 Nov;12(5):1206–1210. doi: 10.1128/iai.12.5.1206-1210.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S., Kronvall G. The use of protein A-containing Staphylococcus aureus as a solid phase anti-IgG reagent in radioimmunoassays as exemplified in the quantitation of alpha-fetoprotein in normal human adult serum. Eur J Immunol. 1974 Jan;4(1):29–33. doi: 10.1002/eji.1830040108. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kronvall G., Frommel D. Definition of staphylococcal protein A reactivity for human immunoglobulin G fragments. Immunochemistry. 1970 Jan;7(1):124–127. doi: 10.1016/0019-2791(70)90036-4. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Seal U. S., Finstad J., Williams R. C., Jr Phylogenetic insight into evolution of mammalian Fc fragment of gamma G globulin using staphylococcal protein A. J Immunol. 1970 Jan;104(1):140–147. [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Lind I., Live I., Mansa B. Variation in staphylococcal protein A reactivity with gamma G-globulins of different species. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(6):673–682. doi: 10.1111/j.1699-0463.1970.tb04357.x. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Heterogeneity of nonimmune immunoglobulin Fc reactivity among gram-positive cocci: description of three major types of receptors for human immunoglobulin G. Infect Immun. 1977 Sep;17(3):475–482. doi: 10.1128/iai.17.3.475-482.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


