Abstract
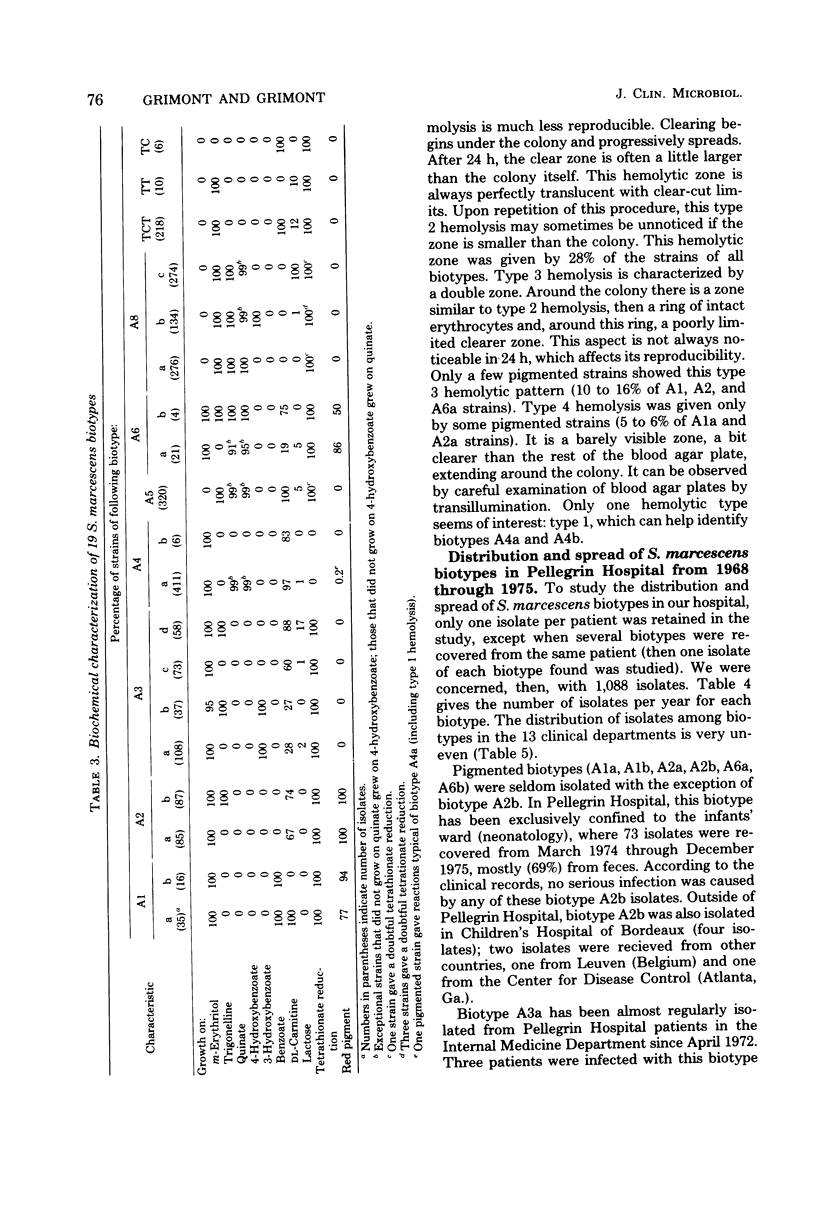
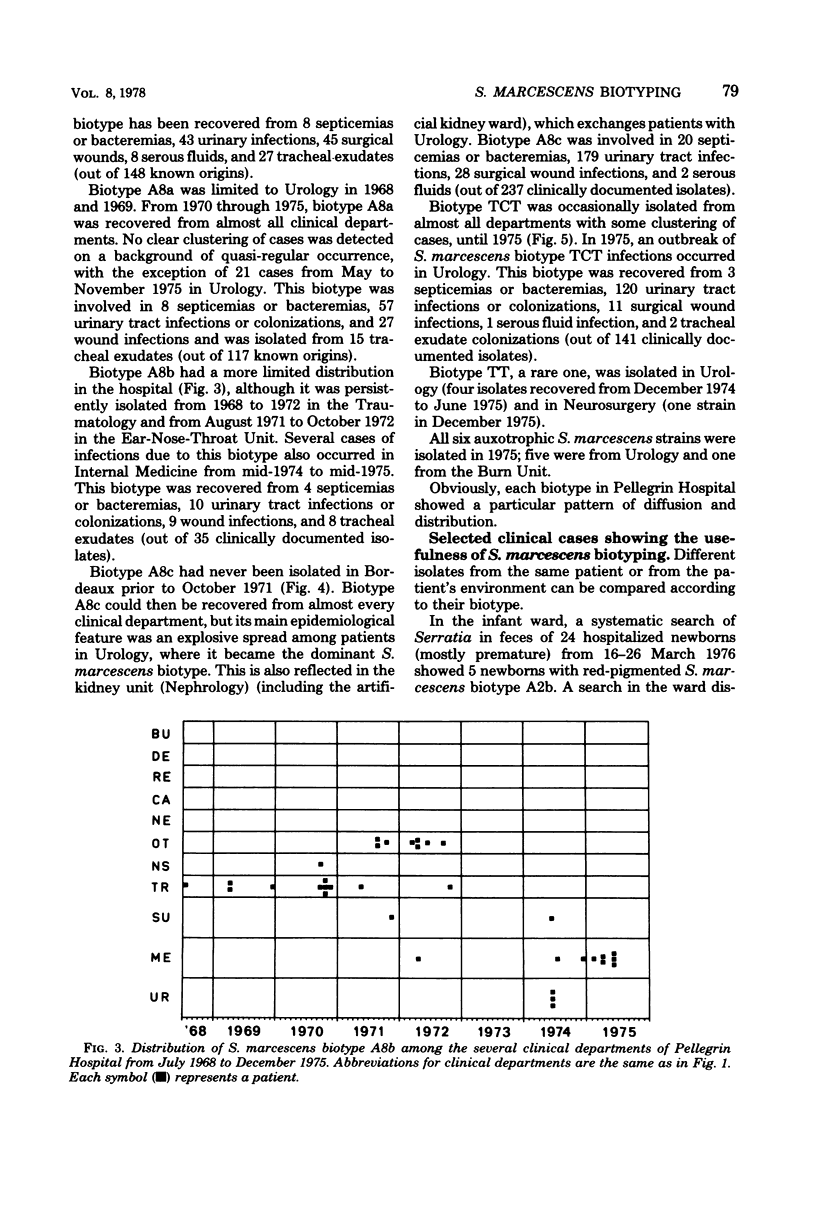
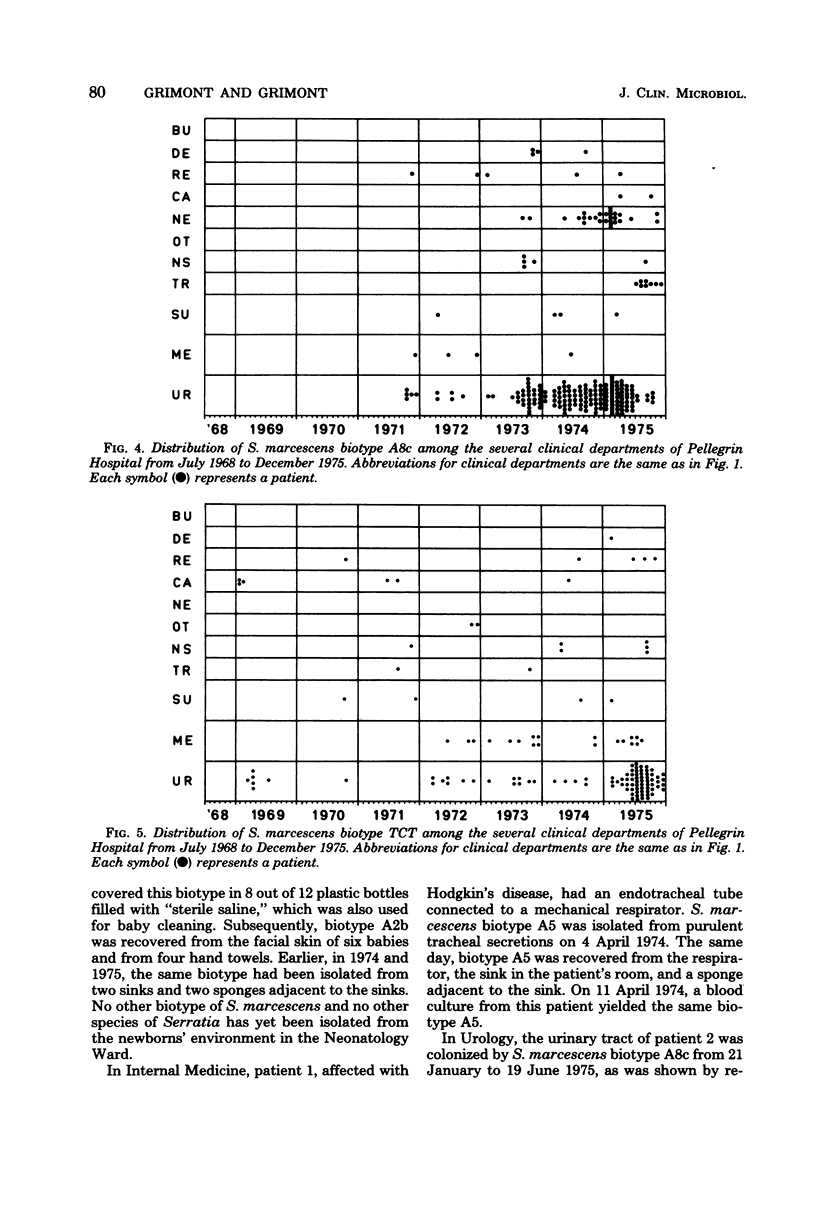
A Serratia marcescens biotyping system using eight carbon sources (benzoate, DL-carnitine, m-erythritol, 3-hydroxybenzoate, 4-hydroxybenzoate, lactose, D-quinate, and trigonelline), a tetrathionate reduction test, production of prodigiosin, and horse blood hemolysis was derived from a recent numerical taxonomic study (Grimont et al., J. Gen. Microbiol. 98:39-66, 1977). A total of 98.6% of 2,210 isolates from various sources could be assigned to 1 of 19 biotypes. Distribution and spread of 1,088 S. marcescens isolates throughout 13 clinical departments of Pellegrin Hospital (Bordeaux, France) were studied from 1968 through 1975. Except for one that colonized the intestinal tract of newborns, the six pigmented biotypes were seldom isolated. Each of the 13 nonpigmented biotypes showed a particular pattern of distribution and spread. The usefulness of S. marcescens biotyping was shown by relating several isolates recovered from patients and their inanimate environment and by pointing out the possible existence of infections or colonizations by two unrelated biotypes. S. marcescens strains isolated from the natural environment (water) are usually pigmented, and their biotypes are uncommon in hospitals. Biotyping can, therefore, be of help in epidemiological and ecological surveys.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler D. A., Lobregat C. M., Gavan T. L. Reproducibility of the analytab (API 20E) system. J Clin Microbiol. 1975 Oct;2(4):322–326. doi: 10.1128/jcm.2.4.322-326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. R., WOODWARD J. M. Some relationships of the somatic antigens of a group of Serratia marcescens cultures. Can J Microbiol. 1957 Jun;3(4):591–597. doi: 10.1139/m57-064. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Davis B. R., Hickman F. W., Presley D. B., Bodey G. P., Negut M., Bobo R. A. Detection of Serratia outbreaks in hospital. Lancet. 1976 Aug 28;2(7983):455–459. doi: 10.1016/s0140-6736(76)92539-3. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd Epidemiological differentiation of Serratia marcescens: typing by bacteriocin production. Appl Microbiol. 1972 Feb;23(2):218–225. doi: 10.1128/am.23.2.218-225.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd Epidemiological differentiation of Serratia marcescens: typing by bacteriocin sensitivity. Appl Microbiol. 1972 Feb;23(2):226–231. doi: 10.1128/am.23.2.226-231.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont P. A., Grimont F., De Rosnay H. L. Taxonomy of the genus Serratia. J Gen Microbiol. 1977 Jan;98(1):39–66. doi: 10.1099/00221287-98-1-39. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ MARCHANT R., OYARCE ROJAS P., ARCAYA O. [The red diaper syndrome. Serratia marcescens infection]. Rev Chil Pediatr. 1960 Jul;31:335–339. [PubMed] [Google Scholar]
- Hamilton R. L., Brown W. J. Bacteriophage typing of clinically isolated Serratia marcescens. Appl Microbiol. 1972 Dec;24(6):899–906. doi: 10.1128/am.24.6.899-906.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E., Ellner P. D. Distribution of Serratia species in clinical specimens. Appl Microbiol. 1974 Sep;28(3):513–514. doi: 10.1128/am.28.3.513-514.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Minor L., Chippaux M., Pichinoty F., Coynault C., Piéchaud M. Méthodes simples permettant de rechercher la tétrathionate-réductase en cultures liquides ou sur colonies isolées. Ann Inst Pasteur (Paris) 1970 Dec;119(6):733–737. [PubMed] [Google Scholar]
- Le Minor S., Pigache F. Etude antigénique de souches de Serratia marcescens isolées en France. I--Antigènes H: individualisation de six nouveaux facteurs H. Ann Microbiol (Paris) 1977 Aug-Sep;128(2):207–214. [PubMed] [Google Scholar]
- McCormack R. C., Kunin C. M. Control of a single source nursery epidemic due to Serratia marcescens. Pediatrics. 1966 May;37(5):750–755. [PubMed] [Google Scholar]
- Neguţ M., Davis B. R., Washington J. A., 2nd Biochemical and serological characteristics of Serratia marcescens isolated from various clinical and environmental sources. Arch Roum Pathol Exp Microbiol. 1975 Jan-Jun;34(1-2):33–39. [PubMed] [Google Scholar]
- Roemisch E., Kocka F. E. Comparison of methods for differentiating among Serratia marcescens isolated from clinical specimens. Am J Clin Pathol. 1976 Jul;66(1):96–100. doi: 10.1093/ajcp/66.1.96. [DOI] [PubMed] [Google Scholar]
- Rubin S. J., Brock S., Chamberland M., Lyons R. W. Combined serotyping and biotyping of Serratia marcescens. J Clin Microbiol. 1976 Jun;3(6):582–585. doi: 10.1128/jcm.3.6.582-585.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Alford R. H., Anderson R., Farmer J. J., 3rd, Melly M. A., Schaffner W. An outbreak of nosocomial infection due to multiply resistant Serratia marcescens: evidence of interhospital spread. J Infect Dis. 1976 Aug;134(2):181–188. doi: 10.1093/infdis/134.2.181. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Grimont P. A., Grimont F., Starr P. B. Caprylate-thallous agar medium for selectively isolating Serratia and its utility in the clinical laboratory. J Clin Microbiol. 1976 Sep;4(3):270–276. doi: 10.1128/jcm.4.3.270-276.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H. Continued surveillance of Serratia marcescens infections by bacteriocin typing: investigation of two outbreaks of cross-infection in an intensive care unit. Appl Microbiol. 1972 May;23(5):982–985. doi: 10.1128/am.23.5.982-985.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H., Kleber I. Continued epidemiological surveillance of Serratia marcescens infections by bacteriocin typing, with particular reference to strains isolated at Erlangen. Zentralbl Bakteriol Orig A. 1974;229(3):372–382. [PubMed] [Google Scholar]
- Traub W. H., Kleber I. Serotyping of Serratia marcescens: evaluation of Le Minor's H-immobilization test and description of three new flagellar H antigens. J Clin Microbiol. 1977 Feb;5(2):115–121. doi: 10.1128/jcm.5.2.115-121.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H., Raymond E. A. Epidemiological surveillance of Serratia marcescens infections by bacteriocin typing. Appl Microbiol. 1971 Dec;22(6):1058–1063. doi: 10.1128/am.22.6.1058-1063.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H., Raymond E. A., Startsman T. S. Bacteriocin (Marcescin) typing of clinical isolates of Serratia marcescens. Appl Microbiol. 1971 May;21(5):837–840. doi: 10.1128/am.21.5.837-840.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véron M. Nutrition et taxonomie des Enterobacteriaceae et bactéries voisines. I. Méthode d'étude des auxanogrammes. Ann Microbiol (Paris) 1975 Apr;126(3):267–274. [PubMed] [Google Scholar]
- WAISMAN H. A., STONE W. H. The presence of Serratia marcescens as the predominating organism in the intestinal tract of the newborn; the occurrence of the red diaper syndrome. Pediatrics. 1958 Jan;21(1):8–12. [PubMed] [Google Scholar]
- Wilfert J. N., Barrett F. F., Ewing W. H., Finland M., Kass E. H. Serratia marcescens: biochemical, serological, and epidemiological characteristics and antibiotic susceptibility of strains isolated at Boston City Hospital. Appl Microbiol. 1970 Feb;19(2):345–352. doi: 10.1128/am.19.2.345-352.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Graevenitz A. The role of opportunistic bacteria in human disease. Annu Rev Microbiol. 1977;31:447–471. doi: 10.1146/annurev.mi.31.100177.002311. [DOI] [PubMed] [Google Scholar]


