Abstract
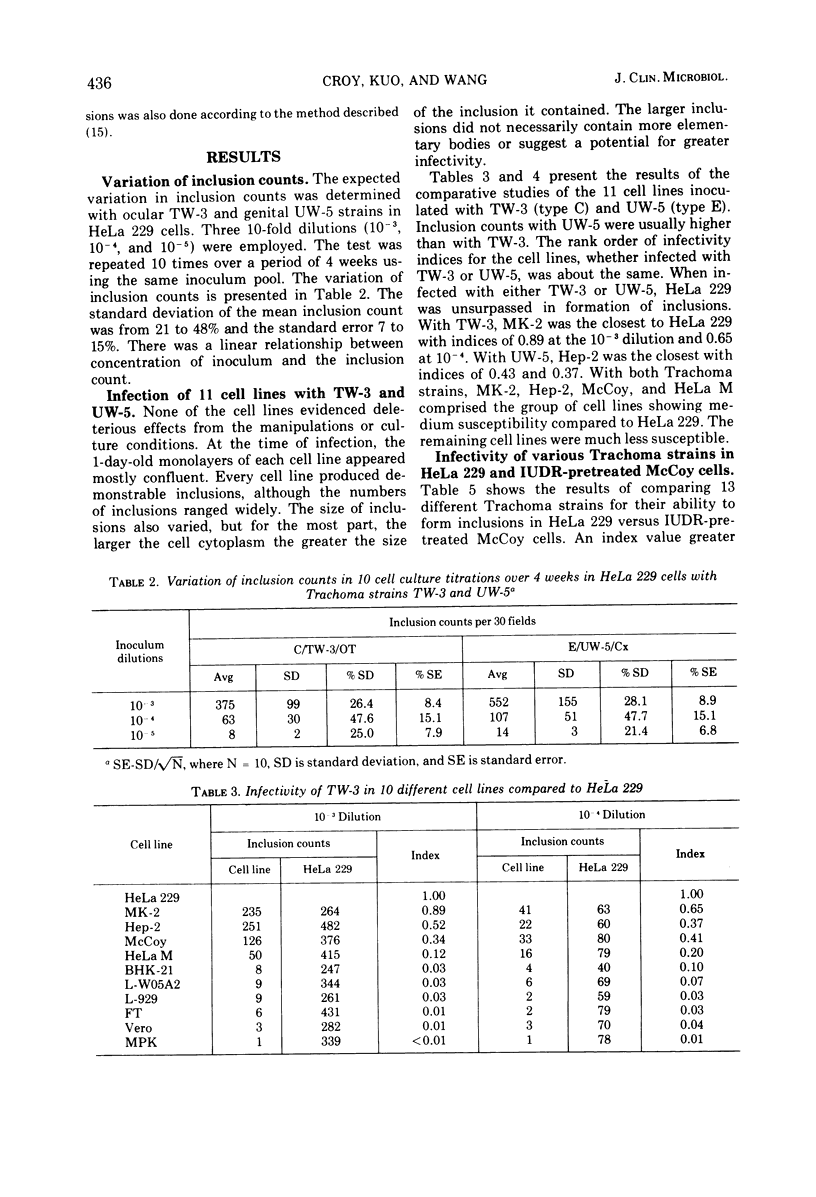
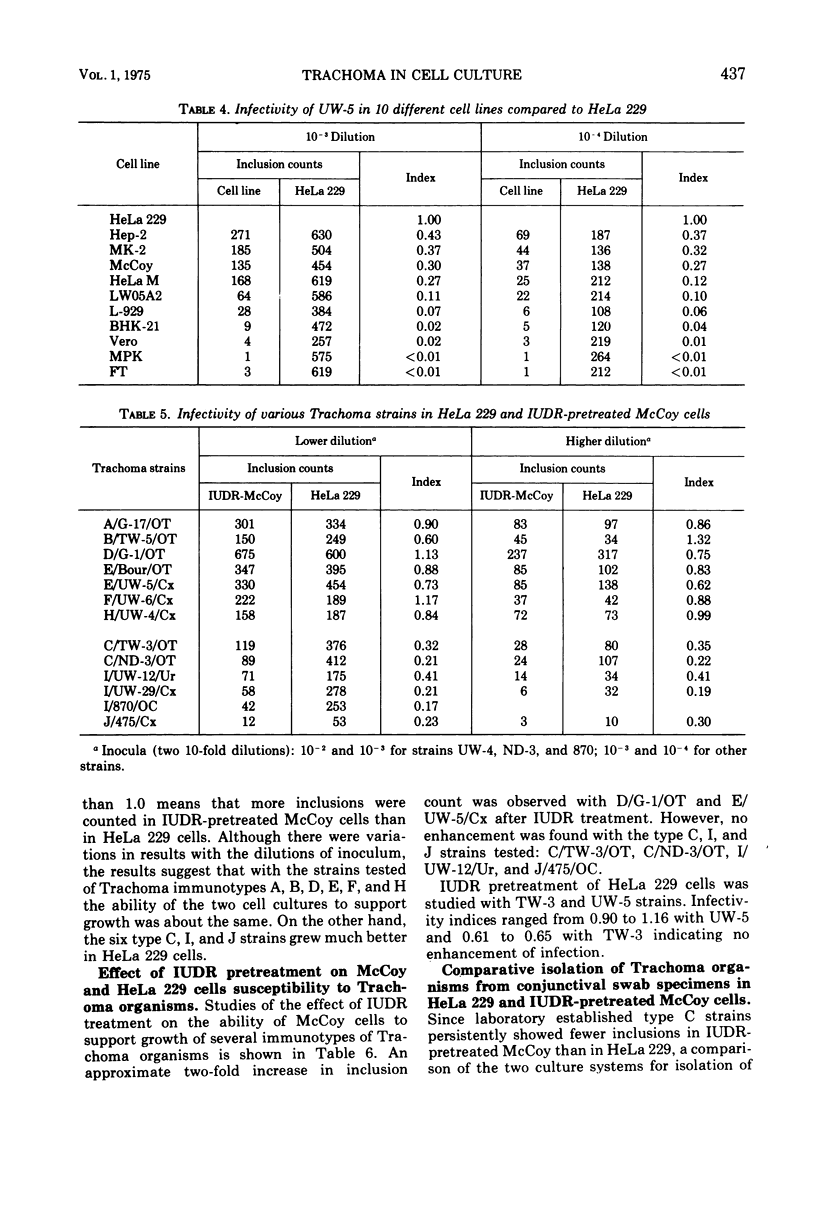
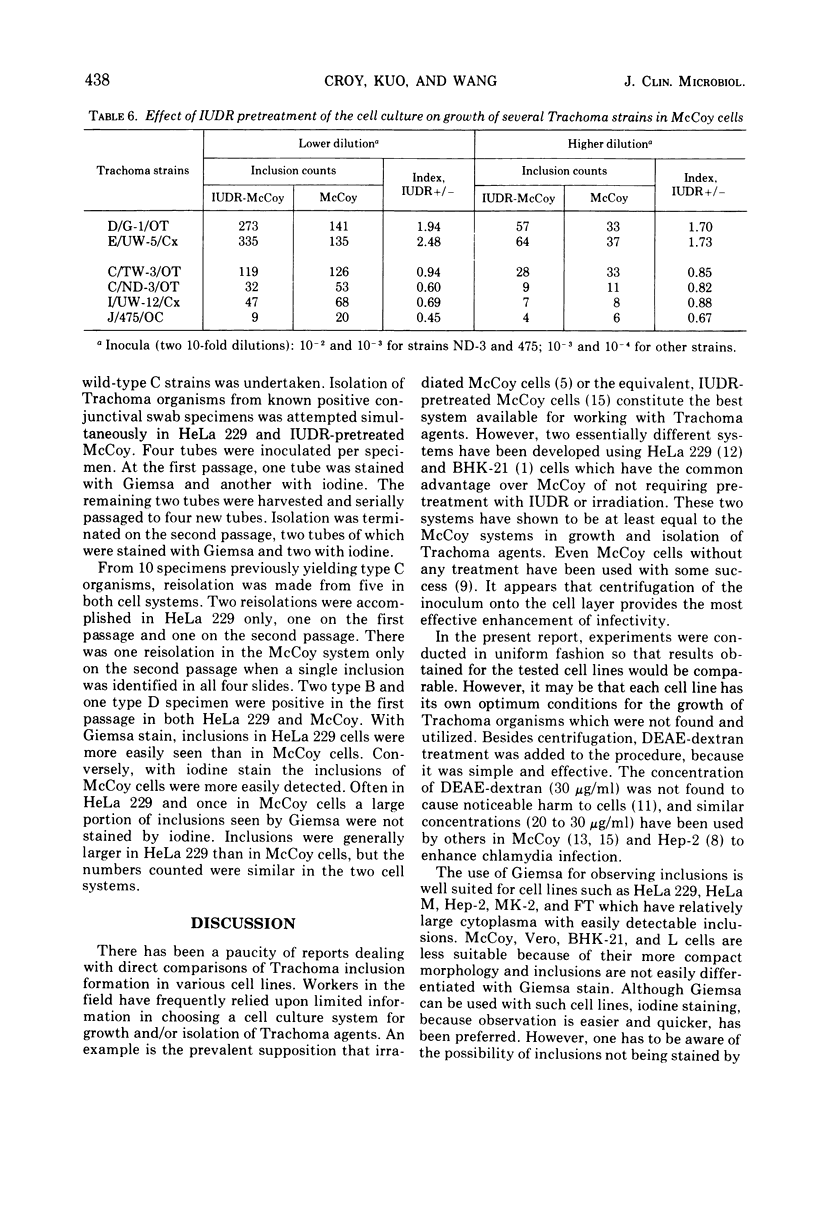
Eleven mammalian cell lines, HeLa 229, HeLa M, Hep-2, FT, BHK-21, Vero, MK-2, MPK, L-WO5A2, McCoy, and L-929, were tested for their susceptibility to infection with Trachoma strains TW-3 (type C, ocular origin) and UW-5 (type E, genital origin). All the cell layers were pretreated with diethylaminoethyl-dextran before inoculation of the organisms, and the inocula were centrifuged onto the cell layers. HeLa 229 was found to be the most sensitive to infection as determined by inclusion counts. The next most susceptible were cell lines MK-2, Hep-2, McCoy, and HeLa M, in that order. Infectivity in these cells ranged from 89 to 12% of that observed in HeLa 229. The remaining cell lines, BHK-21, L-WO5A2, L-929, Vero, MPK, and FT, were much less susceptible with infectivity less than 10% that of HeLa 229. HeLa 229 cells and 5-iodo-2'-deoxyuridine-pretreated McCoy cells have been used most extensively with Trachoma organisms in our laboratories. Infectivity in these two cell culture systems, both pretreated with diethylaminoethyl-dextran, was compared using 13 Trachoma strains of both ocular and genital origins of different immunotypes. The two systems performed similarly except with two type C, three type I, and one type J strains, With the type C, I and J strains tested, considerably fewer inclusions were found in 5-iodo-2'-deoxyuridine-pretreated McCoy than in HeLa 229 because inclusion formation of these strains in McCoy cells was not enhanced by 5-iodo-2'-deoxyuridine pretreatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blyth W. A., Taverne J. Cultivation of TRIC agents: a comparison between the use of BHK-21 and irradiated McCoy cells. J Hyg (Lond) 1974 Feb;72(1):121–128. doi: 10.1017/s0022172400023287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURNESS G., GRAHAM D. M., REEVE P. The titration of trachoma and inclusion blennorrhoea viruses in cell cultures. J Gen Microbiol. 1960 Dec;23:613–619. doi: 10.1099/00221287-23-3-613. [DOI] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- Gordon F. B., Dressler H. R., Quan A. L., McQuilkin W. T., Thomas J. I. Effect of ionizing irradiation on susceptibility of McCoy cell cultures to Chlamydia trachomatis. Appl Microbiol. 1972 Jan;23(1):123–129. doi: 10.1128/am.23.1.123-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Gale J. L., Yeh L. J., Yang C. Y. Pathogenesis and immunology of trachoma. Trans Assoc Am Physicians. 1972;85:203–211. [PubMed] [Google Scholar]
- Harrison M. J. Enhancing effect of DEAE-Dextran on inclusion counts of an ovine Chlamydia (Bedsonia) in cell culture. Aust J Exp Biol Med Sci. 1970 Apr;48(2):207–213. doi: 10.1038/icb.1970.20. [DOI] [PubMed] [Google Scholar]
- Hobson D., Johnson F. W., Rees E., Tait I. A. Simplified method for diagnosis of genital and ocular infections with Chlamydia. Lancet. 1974 Sep 7;2(7880):555–556. doi: 10.1016/s0140-6736(74)91879-0. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M. The continuous passage of agents of trachoma in cell culture. I. Characteristics of TW-3 and Bour strains of trachoma cultivated in serial passage in HeLa 229 cells. J Infect Dis. 1966 Jun;116(3):390–399. doi: 10.1093/infdis/116.3.390. [DOI] [PubMed] [Google Scholar]
- Kuo C., Wang S., Grayston J. T. Differentiation of TRIC and LGV organisms based on enhancement of infectivity by DEAE-dextran in cell culture. J Infect Dis. 1972 Mar;125(3):313–317. doi: 10.1093/infdis/125.3.313. [DOI] [PubMed] [Google Scholar]
- Kuo C., Wang S., Wentworth B. B., Grayston J. T. Primary isolation of TRIC organisms in HeLa 229 cells treated with DEAE-dextran. J Infect Dis. 1972 Jun;125(6):665–668. doi: 10.1093/infdis/125.6.665. [DOI] [PubMed] [Google Scholar]
- Rota T. R., Nichols R. L. Chlamydin trachomatis in cell culture. I. Comparison of efficiencies of infection in several chemically defined media, at various pH and temperature values, and after exposure to diethylaminoethyl-dextran. Appl Microbiol. 1973 Oct;26(4):560–565. doi: 10.1128/am.26.4.560-565.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Gale J. L. Three new immunologic types of trachoma-inclusion conjunctivitis organisms. J Immunol. 1973 Mar;110(3):873–879. [PubMed] [Google Scholar]
- Wentworth B. B., Alexander E. R. Isolation of Chlamydia trachomatis by use of 5-iodo-2-deoxyuridine-treated cells. Appl Microbiol. 1974 May;27(5):912–916. doi: 10.1128/am.27.5.912-916.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


