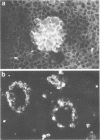
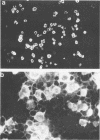
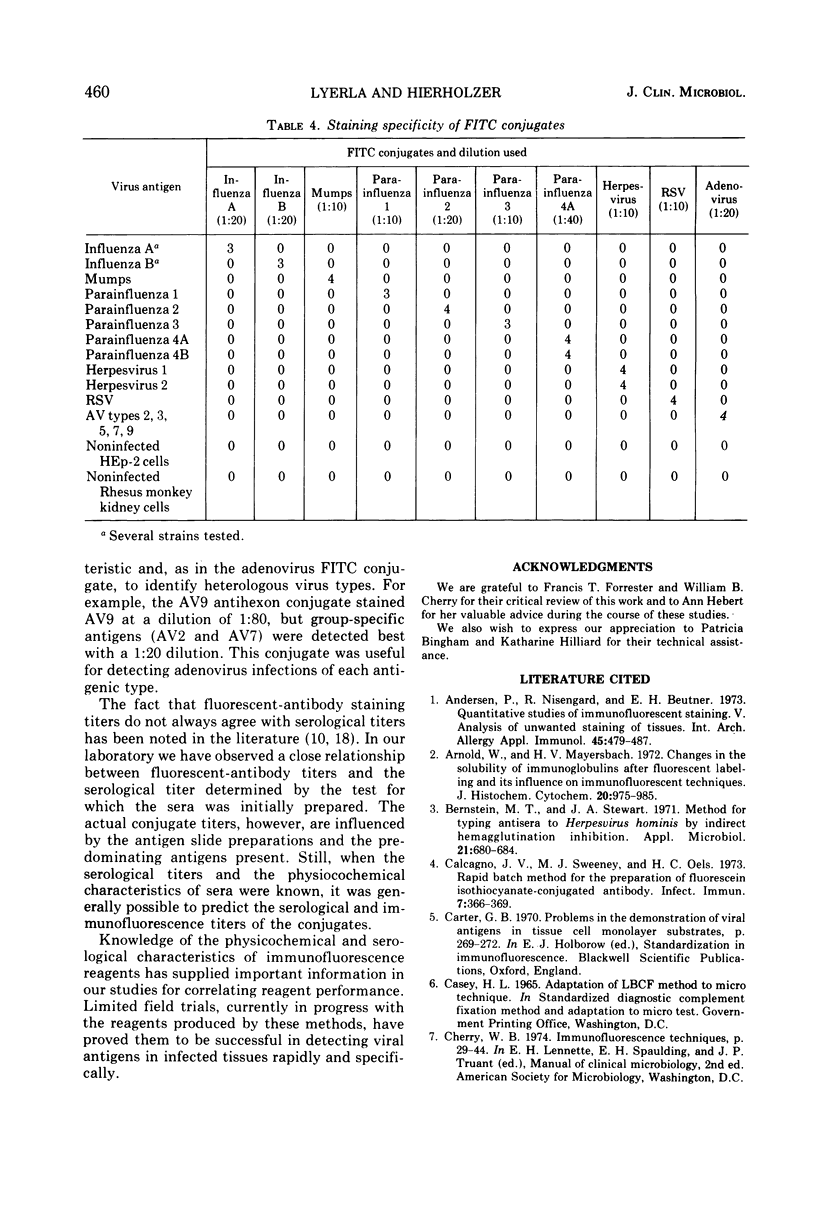
Abstract
Fluorescein-isothiocyanate (FITC) conjugates were prepared by improved methods from standard reference antisera to influenza A and B, mumps, parainfluenza 1, 2, 3, and 4, herpesvirus, respiratory syncytial virus, and adenovirus hexon. The antisera, prepared in a variety of animals, were fractionated three times with selected optimal concentrations of ammonium sulfate and yielded gamma globulins of adequate purity for conjugation with FITC. Conjugates containing optimal fluorescein-to-protein ratios of between 5 and 10 were produced in 2 h by dialysis labeling. Serological titers of each antiserum and conjugate were determined by complement fixation, hemagglutination-inhibition, serum neutralization, and indirect hemagglutination tests where appropriate. When corrected for dilution, the serological titers of the FITC conjugates were identical to those of the starting antisera. The fluorescent-antibody staining titers correlated well with one of the serological parameters of the original serum. The conjugates stained homologous antigens specifically and were free of nonspecific staining at the working dilution. Undesired staining of host cells which was a problem with some of the conjugates produced from sera containing cellular antibodies was removed by absorption with packed cells. These physicochemical and serological findings were then used as a guide in preparing high quality reagents for fluorescent-antibody identification of respiratory viruses.
Full text
PDF

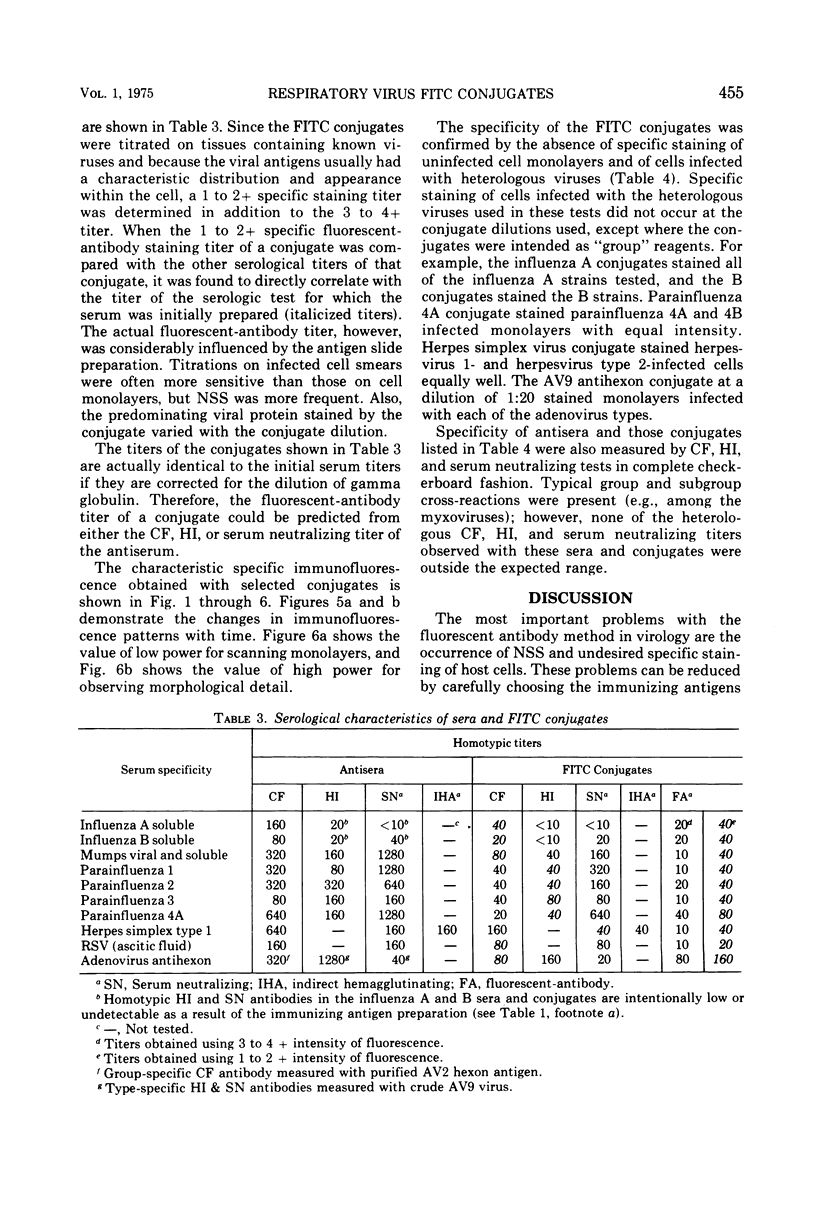
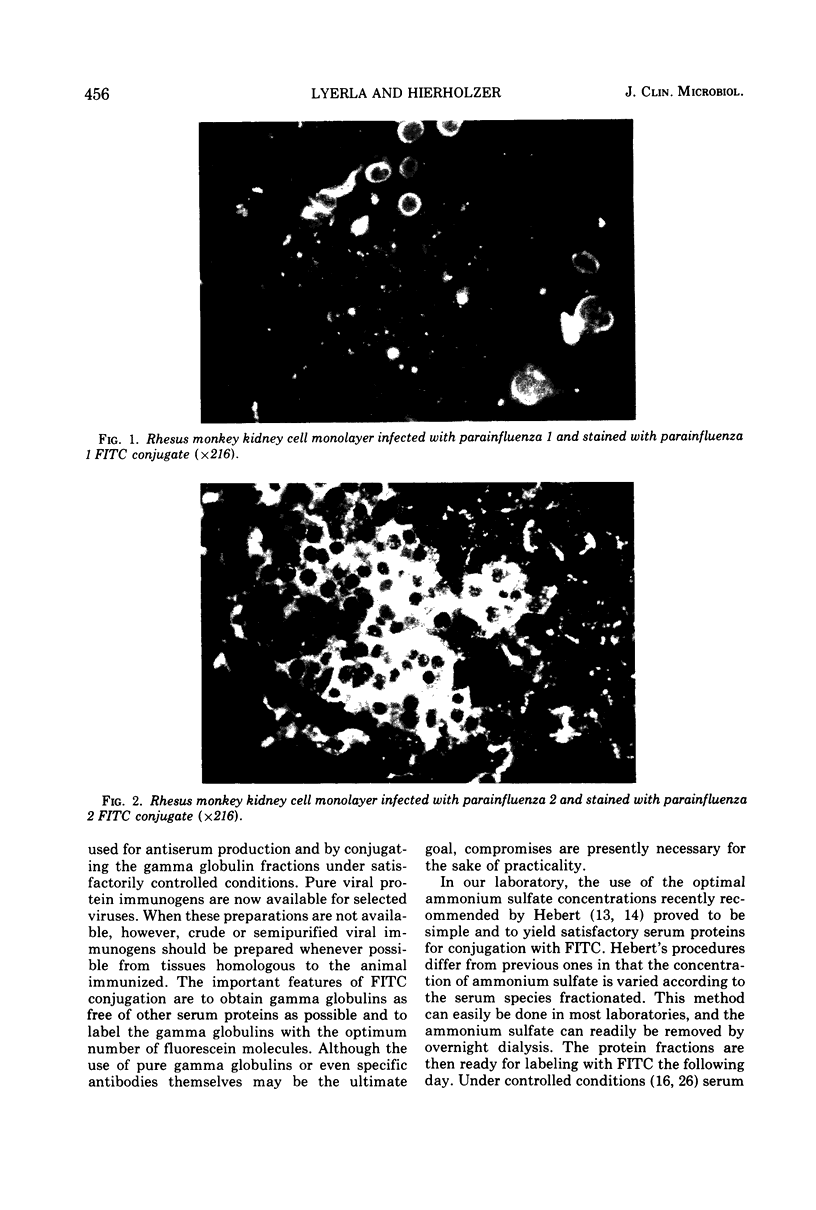
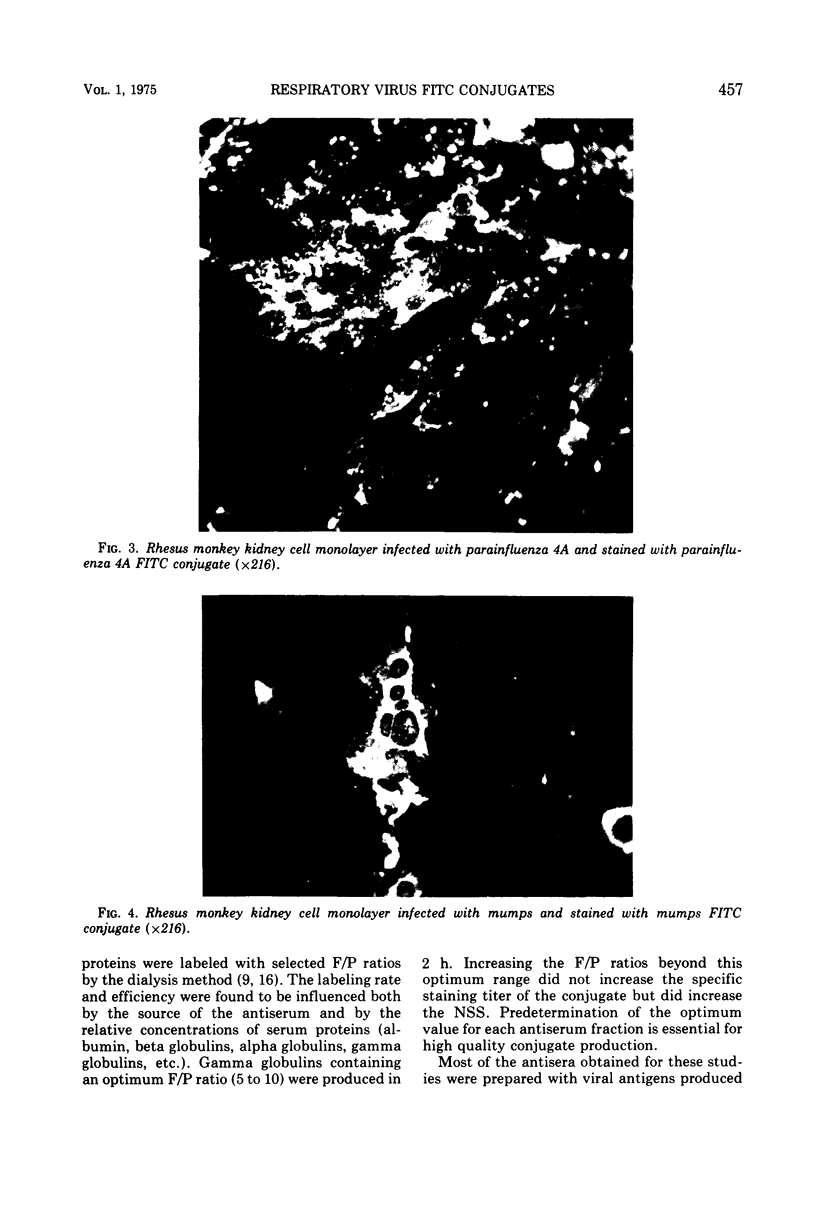
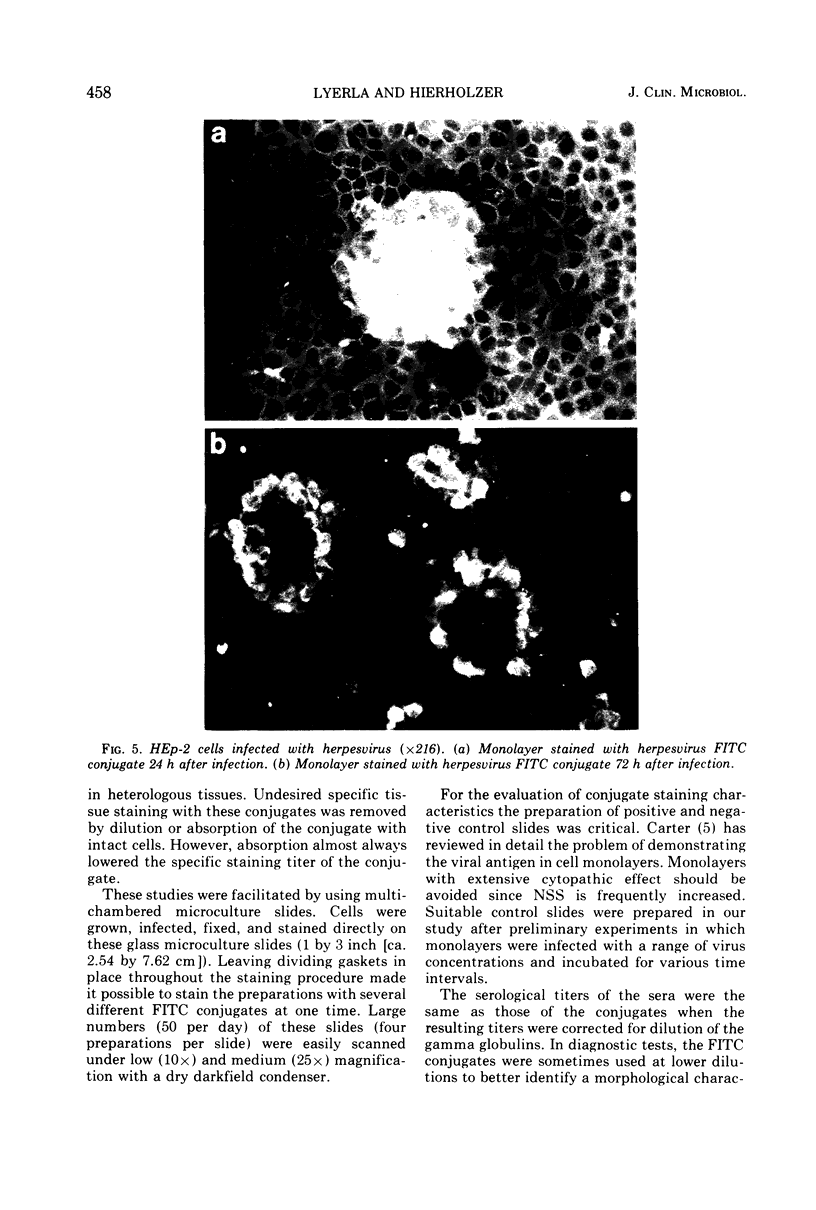


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Nisengard R., Beutner E. H. Quantitative studies of immunofluorescent staining. V. Analysis of unwanted staining of tissues. Int Arch Allergy Appl Immunol. 1973;45(4):479–487. doi: 10.1159/000231095. [DOI] [PubMed] [Google Scholar]
- Arnold W., von Mayersbach H. Changes in the solubility of immunoglobulins after fluorescent labeling and its influence on immunofluorescent techniques. J Histochem Cytochem. 1972 Dec;20(12):975–985. doi: 10.1177/20.12.975. [DOI] [PubMed] [Google Scholar]
- Bernstein M. T., Stewart J. A. Method for typing antisera to Herpesvirus hominis by indirect hemagglutination inhibition. Appl Microbiol. 1971 Apr;21(4):680–684. doi: 10.1128/am.21.4.680-684.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- Calcagno J. V., Sweeney M. J., Oels H. C. Rapid batch method for the preparation of fluorescein isothiocyanate-conjugated antibody. Infect Immun. 1973 Mar;7(3):366–369. doi: 10.1128/iai.7.3.366-369.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio D., Williams S., Dick E. C. Rapid detection and identification of respiratory viruses by direct immunofluorescence. Appl Microbiol. 1970 Aug;20(2):233–239. doi: 10.1128/am.20.2.233-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEDMON R. E., HOLMES A. W., DEINHARDT F. PREPARATION OF FLUORESCEIN ISOTHIOCYANATE-LABELED GAMMA-GLOBULIN BY DIALYSIS, GEL FILTRATION, AND IONEXCHANGE CHROMATOGRAPHY IN COMBINATION. J Bacteriol. 1965 Mar;89:734–739. doi: 10.1128/jb.89.3.734-739.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN G., SPALDING B. H., HUNT W. B., Jr Studies on fluorescent antibody staining II. Inhibition by sub- optimally conjugated antibody globulins. Proc Soc Exp Biol Med. 1962 Nov;111:416–421. doi: 10.3181/00379727-111-27810. [DOI] [PubMed] [Google Scholar]
- Hebert G. A. Ammonium sulfate fractionation of sera: mouse, hamster, guinea pig, monkey, chimpanzee, swine, chicken, and cattle. Appl Microbiol. 1974 Feb;27(2):389–393. doi: 10.1128/am.27.2.389-393.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert G. A., Pelham P. L., Pittman B. Determination of the optimal ammonium sulfate concentration for the fractionation of rabbit, sheep, horse, and goat antisera. Appl Microbiol. 1973 Jan;25(1):26–36. doi: 10.1128/am.25.1.26-36.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert G. A., Pittman B., Cherry W. B. The definition and application of evaluation techniques as a guide for the improvement of fluorescent antibody reagents. Ann N Y Acad Sci. 1971 Jun 21;177:54–69. doi: 10.1111/j.1749-6632.1971.tb35033.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Baumgarten E. M. Miniature tissue culture technique with a modified plastic microplate. Appl Microbiol. 1972 Dec;24(6):999–1000. doi: 10.1128/am.24.6.999-1000.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Dowdle W. R. Immunological basis of the adenovirus 8-9 cross-reaction. J Virol. 1970 Dec;6(6):782–787. doi: 10.1128/jvi.6.6.782-787.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T., Hall E. C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969 Nov;18(5):824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T. Standardized viral hemagglutination and hemagglutination-inhibition tests. I. Standardization of erythrocyte suspensions. Appl Microbiol. 1969 Nov;18(5):816–823. doi: 10.1128/am.18.5.816-823.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore G. E., Dowdle W. R. Antigenic characterization of parainfluenza 4A and 4B by the hemagglutination-inhibition test and distribution of HI antibody in human sera. Am J Epidemiol. 1970 Mar;91(3):308–316. doi: 10.1093/oxfordjournals.aje.a121141. [DOI] [PubMed] [Google Scholar]
- Klugerman M. R. Chemical and physical variables affecting the properties of fluorescein isothiocyanate and its protein conjugates. J Immunol. 1965 Dec;95(6):1165–1173. doi: 10.21236/ad0459385. [DOI] [PubMed] [Google Scholar]
- MARSHALL J. D., EVELAND W. C., SMITH C. W. Superiority of fluorescein isothiocyanate (Riggs) for fluorescent-antibody technic with a modification of its application. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):898–900. doi: 10.3181/00379727-98-24222. [DOI] [PubMed] [Google Scholar]
- MCKINNEY R. M., SPILLANE J. T., PEARCE G. W. FACTORS AFFECTING THE RATE OF REACTION OF FLUORESCEIN ISOTHIOCYANATE WITH SERUM PROTEINS. J Immunol. 1964 Aug;93:232–242. [PubMed] [Google Scholar]
- MCKINNEY R. M., SPILLANE J. T., PEARCE G. W. FLUORESCEIN DIACETATE AS A REFERENCE COLOR STANDARD IN FLUORESCENT ANTIBODY STUDIES. Anal Biochem. 1964 Dec;9:474–476. doi: 10.1016/0003-2697(64)90208-8. [DOI] [PubMed] [Google Scholar]
- McLure A. R., MacFarlane D. E., Sommerville R. G. An intersecting anti-serum pool system for the immunofluorescent identification of respiratory viruses. Arch Gesamte Virusforsch. 1972;37(1):6–11. doi: 10.1007/BF01241145. [DOI] [PubMed] [Google Scholar]
- Peacock M., Burgdorfer W., Ormsbee R. A. Rapid fluorescent-antibody conjugation procedure. Infect Immun. 1971 Feb;3(2):355–357. doi: 10.1128/iai.3.2.355-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGGS J. L., LOH P. C., EVELAND W. C. A simple fractionation method for preparation of fluorescein-labeled gamma globulin. Proc Soc Exp Biol Med. 1960 Dec;105:655–658. doi: 10.3181/00379727-105-26207. [DOI] [PubMed] [Google Scholar]
- Sommerville R. G. Rapid method for the preparation of replicate microslide tissue culture to facilitate immunofluorescent identification of unknown virus isolates. J Clin Pathol. 1967 Mar;20(2):212–214. doi: 10.1136/jcp.20.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville R. G. The production of fluorescent antibody reagents for virus diagnosis in the albino mouse. II. Coupling of mouse immune globulin with fluorescein isothiocyanate (FITC). Arch Gesamte Virusforsch. 1967;20(4):452–458. doi: 10.1007/BF01275226. [DOI] [PubMed] [Google Scholar]
- Spendlove R. S. Optimal labeling of antibody with fluorescein isothiocyanate. Proc Soc Exp Biol Med. 1966 Jun;122(2):580–583. doi: 10.3181/00379727-122-31196. [DOI] [PubMed] [Google Scholar]
- Stevens T. D., Watkins H. M. Rapid identification of viruses by indirect immunofluorescence: standardization and use of antiserum pool to nine respiratory viruses. Appl Microbiol. 1969 Mar;17(3):384–393. doi: 10.1128/am.17.3.384-393.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B. T., Thompson S. H., Goldstein G. Fluorescent antibody staining. 3. Preparation of fluorescein-isothiocyanate-labeled antibodies. J Immunol. 1965 Aug;95(2):225–229. [PubMed] [Google Scholar]