Abstract
Diabetic nephropathy is the leading cause of chronic renal failure. Myofibroblasts play a major role in the synthesis and secretion of extracellular matrix in diabetic renal fibrosis. Increasing evidence suggests that endothelial cells may undergo endothelial-myofibroblast transition under physiological and pathophysiological circumstances. Therefore, this study investigates whether endothelial-myofibroblast transition occurs and contributes to the development of diabetic renal interstitial fibrosis. Diabetes was induced by administration of streptozotocin to Tie2-Cre;LoxP-EGFP mice, an endothelial lineage-traceable mouse line generated by crossbreeding B6.Cg-Tg(Tek-cre)12F1v/J mice with B6.Cg-Tg(ACTB-Bgeo/GFP)21Lbe/J mice. The endothelial-myofibroblast transition was also studied in MMECs (a mouse pancreatic microvascular endothelial cell line) and primary cultures of CD31+/EYFP− (enhanced yellow fluorescent protein) endothelial cells isolated from adult normal α-smooth muscle actin promoter-driven-EYFP (α-SMA/EYFP) mouse kidneys. Confocal microscopy demonstrated that 10.4 ± 4.2 and 23.5 ± 7.4% of renal interstitial myofibroblasts (α-SMA+) in 1- and 6-month streptozotocin-induced diabetic kidneys were of endothelial origin (EGFP+/α-SMA+ cells), compared with just 0.2 ± 0.1% of myofibroblasts in vehicle-treated Tie2-Cre;LoxP-EGFP mice (P < 0.01). Confocal microscopy and real-time PCR showed that transforming growth factor (TGF)-β1 induced de novo expression of α-SMA and loss of expression of VE-cadherin and CD31 in MMECs and primary cultures of renal endothelial cells in a time- and dose-dependent fashion. These findings demonstrate that the endothelial-myofibroblast transition occurs and contributes to the early development and progression of diabetic renal interstitial fibrosis and suggest that the endothelial-myofibroblast transition may be a therapeutic target.
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease in the Western world. Approximately 25 to 35% of patients with type 1 diabetes1 and 5 to 10% of patients with type II diabetes2 develop DN. Glomerulosclerosis and interstitial fibrosis are the key morphological features of DN, and both correlate well with the development and progression of renal disease.3 Myofibroblasts play a major role in the synthesis and secretion of extracellular matrix in the development and progression of renal fibrosis. In DN, cells expressing α-smooth muscle actin (α-SMA), the putative marker of myofibroblasts, are located primarily in the renal interstitium and to a lesser extent in glomeruli in association with mesangial proliferation.4 The number of myofibroblasts is inversely correlated with renal function in DN.5
Importantly however, the origin of myofibroblasts in DN remains unclear. It is generally believed that myofibroblasts may be derived from resident fibroblasts, epithelial cells through the epithelial-myofibroblast transition, mesangial cells, or bone marrow-derived cells. Interestingly, increasing evidence suggests that endothelial cells may undergo endothelial-myofibroblast transition (EndoMT) under physiological and pathophysiological circumstances6,7 and thereby give rise to myofibroblasts. Arciniegas et al8 demonstrated that transforming growth factor (TGF)-β1 can induce aortic endothelial cells to differentiate into α-SMA+ cells in vitro, suggesting a novel role for TGF-β1 in atherogenesis. Moreover, embryonic endothelial cells have been shown to transdifferentiate into mesenchymal cells expressing α-SMA in vitro and in vivo,9 and vascular endothelium-derived cells contain α-SMA in restenosis,10 inflammation, and hypertension,11 suggesting that myofibroblasts may be of endothelial origin.
The involvement of TGF-β1 in renal fibrosis, including DN, has been the subject of extensive investigation.12 TGF-β1 exerts its biological effects by signaling through TGF-β type II and type I receptors,13 and their downstream effectors, R-Smads (Smad2 and Smad3). TGF-β/Smad2/3 signaling pathways are activated in human DN14 and diabetic mouse kidneys.15,16 Smad3-null mice are resistant to streptozocin (STZ)-induced DN.17 It remains largely unknown whether TGF-β1 can induce EndoMT in microvascular endothelial cells in DN, one of the major microvascular complications of diabetes and whether Smad3 plays a pivotal role in the process of TGF-β1-induced EndoMT.
In this study we investigated whether EndoMT occurs and contributes to the development of renal interstitial fibrosis in STZ-induced DN in an endothelial lineage-traceable mouse line, the Tie2-Cre;LoxP-EGFP mouse. We also assess whether a specific inhibitor for Smad3 (SIS3)18 can inhibit TGF-β-induced EndoMT in a mouse microvascular endothelial cell line (MMECs).
Materials and Methods
Animals
Tie2-Cre;LoxP-EGFP mice were generated by crossbreeding B6.Cg-Tg(Tek-cre)12F1v/J mice (stock number 004128, The Jackson Laboratory, Bar Harbor, Maine)19 with B6.Cg-Tg(ACTB-Bgeo/GFP)21Lbe/J mice (stock number 004178, The Jackson Laboratory).20 Diabetes was induced in Tie2-Cre;LoxP-EGFP mice (n = 20) at 8 weeks of age by the intraperitoneal administration of 50 μg/g STZ (Sigma-Aldrich, St. Louis, MO) for 5 consecutive days. Control Tie2-Cre;LoxP-EGFP mice (n = 6) received daily intraperitoneal injections of 0.1 M sodium citrate buffer (pH 4.5) for 5 days. Biochemical parameters and renal histology were assessed 4 weeks and 6 months after the onset of diabetes. Urine in bladder was obtained for urinary albumin excretion when the mice were sacrificed. The albumin/creatinine ratio was measured with Albuwell M and Creatinine Companion (Exocell, Philadelphia, PA).
α-SMA/EYFP (enhanced yellow fluorescent protein) mice were kindly provided by Dr. James Lessard (Cincinnati Children’s Hospital Medical Centre, Cincinnati, Ohio). In α-SMA/EYFP mice, EYFP expression is driven by the α-SMA promoter/enhancer and is expressed not only in smooth muscle cells but also in renal myofibroblasts. All experiments were performed with the approval of a Monash University Animal Ethics Committee, which adheres to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Histology and Immunofluorescence Microscopy
The following antibodies were used for immunofluorescence: rat anti-CD31 (1:100, catalog number 550274, BD Biosciences, San Diego, CA); mouse anti-α-SMA conjugated with cyanine 3 (1:2000, catalog number C6198, Sigma-Aldrich); rat anti-CD31 conjugated with allophycocyanin (1:100, catalog number CBL1337P, Millipore, Temecula, CA); rat anti-VE-cadherin (1:50, catalog number 14-1441, eBioscience, San Diego; CA), and goat anti-rat Alexa Fluor 647 conjugate (1:2000, Invitrogen, Carlsbad, CA). Sections were analyzed with an Olympus Fluoview 1000 confocal microscope (Olympus, Tokyo, Japan), FV10-ASW software (version 1.7, Olympus), an oil UPLFL 60X objective (numerical aperture 1.25, Olympus) at × 2, × 3, or × 6 digital zoom and step size of 1 μm if Z-series confocal microscopic analysis was applied. Channels were acquired sequentially. Contrast and brightness of the images were further adjusted in ImageJ (http://rsbweb.nih.gov/ij).
Quantitation of Myofibroblasts of Endothelial Cell Origin
Enhanced green fluorescent protein (EGFP)-positive/α-SMA+ cells were counted in glomeruli and cortex. Twenty randomly selected glomeruli and five cortical fields were analyzed at ×600 magnification in each of five sections from each kidney. The numbers of endothelial cell-origin myofibroblasts per glomerular cross section (EGFP+/α-SMA+ cells/glomerular cross section) and per mm2 of cortex (excluding glomeruli) (EGFP+/α-SMA+ cells/mm2 of cortex) were determined, as well as the percentage of α-SMA+ cells that also expressed EGFP (EGFP+/α-SMA+ cells).
MMEC Culture
MMECs were grown in a 5% CO2 atmosphere at 37°C in Dulbecco’s modified Eagle’s medium (Life Technologies BRL, Gaithersburg, MD) containing 5% fetal bovine serum in six-well plastic plates or eight-chamber glass slides (Nunc, Naperville, IL). Recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) was added at concentrations of 0, 0.1, and 0.5 ng/ml to the cell cultures for 7 days in chamber slides and 3, 6, and 12 hours in six-well plates. In blocking studies, MMECs were cultured with 0.5 ng/ml TGF-β1, 0.5 ng/ml TGF-β1 + dimethyl sulfoxide, or 0.5 ng/ml TGF-β1 + 2 μmol/L SIS3 for 7 days in eight-chamber glass slides and 12 hours in six-well plates.
Isolation and Culture of Renal CD31+/ EYFP− Cells
Single-cell suspensions from α-SMA/EYFP mouse kidneys were labeled with rat anti-CD31 allophycocyanin (1:50). Renal CD31+/EYFP− cells were sorted using FACSDiva (BD Biosciences). Dead cells were excluded by a combination of scatter gates and 4,6-diamidino-2-phenylindole (DAPI) staining. Cells were plated at 0.5 × 106 cells/cm2 in human fibronectin-coated eight-well chamber slides (BD Biosciences). Cells were incubated in an EGM-2 Bullet kit system (Clonetics, San Diego, CA) with 5% fetal bovine serum. At the time of stimulation, TGF-β1 was added at concentrations of 0, 0.1, and 0.5 ng/ml for 7 days.
RNA Extraction and Real-Time PCR
Total RNA from cultured endothelial cells was isolated, and RT-PCR and real-time PCR were performed with an RT-PCR kit (Invitrogen) and SYBR Green PCR Reagents (Sigma-Aldrich). Primers were as follows: mouse CD31, 5′-AGGCTTGCATAGAGCTCCAG-3′ and 5′-TTCTTGGTTTCCAGCTATGG-3′; α-SMA, 5′-CTGACAGAGGCACCACTGAA-3′ and 5′-GAAATAGCCAAGCTCAG-3′; mouse glyceraldehyde-3-phosphate dehydrogenase, 5′-CAGATCCACAACGGATATATTGGG-3′ and 5′-CATGACAACTTTGGCATTGTGG-3′. Reaction specificity was confirmed by electrophoresis analysis of products before real-time PCR, and bands of expected size were detected. Ratios for CD31/glyceraldehyde-3-phosphate dehydrogenase and α-SMA/glyceraldehyde-3-phosphate dehydrogenase were calculated for each sample and expressed as the mean ± SD.
Statistical Analysis
Data are presented as means ± SD; statistical analyses was performed using one-way analysis of variance with GraphPad Prism 3.0 or two-way analysis of variance if appropriate (GraphPad Software, Inc., San Diego, CA). Post-test Tukey’s analysis was used when appropriate. A P < 0.05 was considered statistically significant.
Results
Establishment of an Endothelial Lineage-Traceable Mouse Line (Tie2-Cre;LoxP-EGFP Mouse)
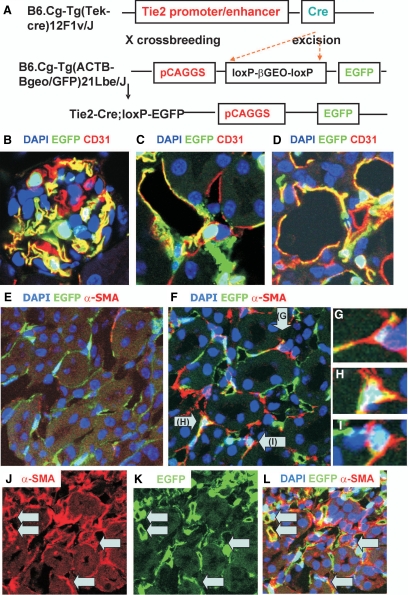
EndoMT is a process whereby endothelial cells lose their endothelial cell markers and acquire myofibroblast markers. To trace endothelial cells and their derivatives, we have generated an endothelial lineage-traceable mouse line (Tie2-Cre;LoxP-EGFP mice) through the crossbreeding of B6.Cg-Tg(Tek-cre)12F1v/J mice with B6.Cg-Tg(ACTB-Bgeo/GFP)21Lbe/J mice. Tie2 is an endothelial cell marker. In Tie2-Cre mice, Cre recombinase is under the direction of the Tie2 promoter/enhancer, which has been shown to provide uniform expression in endothelial cells during embryogenesis and adulthood.21 In Tie2-Cre;LoxP-EGFP mice, EGFP is expressed by a strong promoter (pCAGGS) on Cre-mediated excision of a loxP stop cassette. Therefore, in this mouse, EGFP is expressed in endothelial cells as well as in cells of endothelial origin, despite any subsequent phenotypic changes (Figure 1A). To verify that EGFP-positive cells are endothelial cells, CD31, an endothelial cell marker, was used for immunostaining kidneys of Tie2-Cre;LoxP-EGFP mice. Confocal microscopy demonstrated that approximately 55 ± 15% of endothelial cells expressed EGFP, whereas 97.3 ± 1.6% of EGFP-positive cells were endothelial cells in vehicle-treated kidneys (Figure 1, B–D, Supplemental Figure S1, see http://ajp.amjpathol.org).
Figure 1.
A: Gene constructs in Tie2-Cre;LoxP-EGFP mice. The LoxP-EGFP transgene consists of the strong pCAGGS promoter, directing expression of a loxP-flanked βgeo (lacZ/neomycin-resistance) fusion gene followed by the coding sequence of EGFP. EGFP is expressed after Cre excision. B–D: Confocal images of a normal glomerulus (B) and cortical (C) and medullary (D) peritubular capillaries in Tie2-Cre;LoxP-EGFP mouse kidneys. Confocal microscopy demonstrates EGFP+/α-SMA+ cells in 6-month vehicle-injected kidney (E) and in 1-month (F) and 6-month (J–L) STZ-induced DN. DAPI, blue; EGFP, green; CD31 and α-SMA, red. Yellow indicates colocalization. F: Arrows indicate EGFP+/α-SMA+ cells. Three EGFP+/α-SMA+ double-labeled cells are enlarged in insets (G–I) from F. J–L: Arrows indicate EGFP+/α-SMA+ cells in 6-month STZ-induced DN. Original magnification: ×1800 (B–D); ×600 (E–F); ×3600 (G–I); ×600 (J–L).
EndoMT Contributes to the Early Development of Renal Interstitial Fibrosis in STZ-Induced DN in Tie2-Cre;LoxP-EGFP Mice
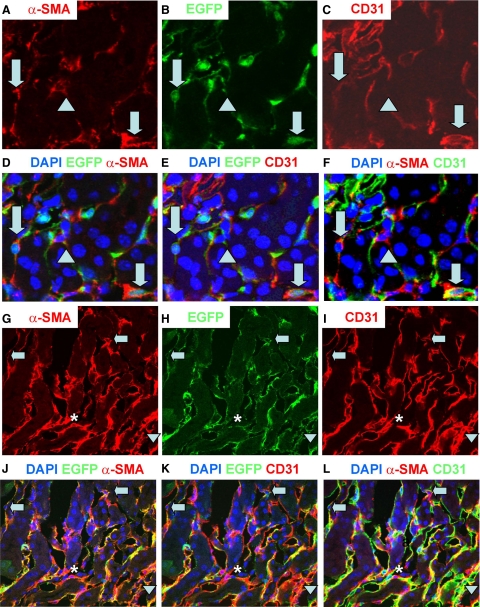
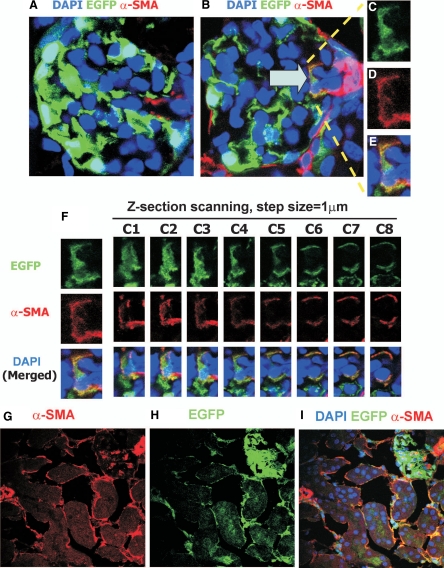
To investigate the contribution of EndoMT to the early development of diabetic renal interstitial fibrosis, diabetes was induced in Tie2-Cre;LoxP-EGFP mice by administration of STZ. By 1 month after induction of diabetes, confocal microscopy demonstrated that the number of renal interstitial myofibroblasts and the expression of collagen type IV in tubulointerstitium (Supplemental Figure S2, see http://ajp.amjpathol.org) were significantly increased in STZ-induced DN compared with those in vehicle-treated kidneys, suggesting the early development of renal interstitial fibrosis. Confocal microscopy also showed that the number of EGFP+/α-SMA+ cells in the interstitium and the percentage of α-SMA+ cells in the interstitium that were EGFP+/α-SMA+ were dramatically higher in STZ-induced DN than in vehicle-treated kidneys in Tie2-Cre;LoxP-EGFP mice (28.1 ± 10.5/ versus 1.4 ± 1.1/mm2, p < 0.01; 10.4 ± 4.2 versus 0.2 ± 0.1%, p < 0.01) (Figure 1, E–I). Further analysis showed that 80% of EGFP+/α-SMA+ cells were CD31-positive, whereas 20% were CD31-negative (Figure 2, A–F). In vehicle-treated kidneys, 97% of EGFP+ cells were CD31+ (Supplemental Figure S1, see http://ajp.amjpathol.org), suggesting that some endothelial-origin myofibroblasts may lose expression of this endothelial marker. By 1 month after induction of diabetes, there was no significant difference in urine albumin excretion (the ratio of urine albumin to creatinine) between vehicle-treated and STZ-induced DN groups (40.4 ± 8.6 versus 70.54 ± 37.7 μg/mg, p > 0.05), suggesting that early EndoMT is independent of albuminuria. The EGFP+/α-SMA+ cells in glomeruli were located in afferent and efferent arterioles (Figure 3, A–F), but the number of such cells was very low (0.2 ± 0.1/glomerular cross section). These findings suggest that EndoMT occurs in the early STZ-induced diabetic kidney and contributes to the early development of diabetic renal interstitial fibrosis. By 6 months after induction of diabetes in Tie2-Cre;LoxP-EGFP mice, confocal microscopy demonstrated that the number of EGFP+/α-SMA+ cells in the interstitium and the percentage of α-SMA+ cells in the interstitium that were EGFP+/α-SMA+ further increased to 76.3 ± 21.8/mm2 and 23.5 ± 7.4%, respectively (Figure 1, J–L, Figure 2, G–L, and Figure 3, G–I), suggesting the contribution of endothelial-origin myofibroblasts to interstitial fibrosis in the development and progression of DN.
Figure 2.
Confocal microscopy demonstrates α-SMA (red, A and G), EGFP (green, B and H), CD31 (red, C and I), overlapping (DAPI, blue, D–F and J–L) in 1-month (A–F) and 6-month (G–L) STZ-induced DN. Arrows indicate α-SMA+/EGFP+/CD31+ cells; arrowheads indicate α-SMA+/EGFP+/CD31− cells and asterisks indicate an α-SMA+/EGFP−/CD31− cell. Original magnification: A through D, ×1200; G through L, X600.
Figure 3.
EndoMT occurs in glomeruli of Tie2-Cre;LoxP-EGFP mice with 1-month DN (A–F). Confocal microscopy demonstrates DAPI (blue), EGFP (green), and α-SMA (red) in normal kidney (A) and 1-month STZ-induced DN (B) in glomeruli. In B arrow indicates an EGFP+/α-SMA+ cell in an afferent/efferent arteriole in mouse with DN. The EGFP+/α-SMA+ cell is enlarged in insets (C–E). F: Z-sections of the EGFP+/α-SMA+ cell in B. Step size = 1 μm. Confocal microscopy demonstrates α-SMA (red, G), EGFP (green, H), and DAPI (blue, merged image, I) in 6-month STZ-induced DN. Original magnification: ×1800 (A and B); ×3600 (C–F); ×600 (G–I).
TGF-β1 Induces EndoMT in Vitro
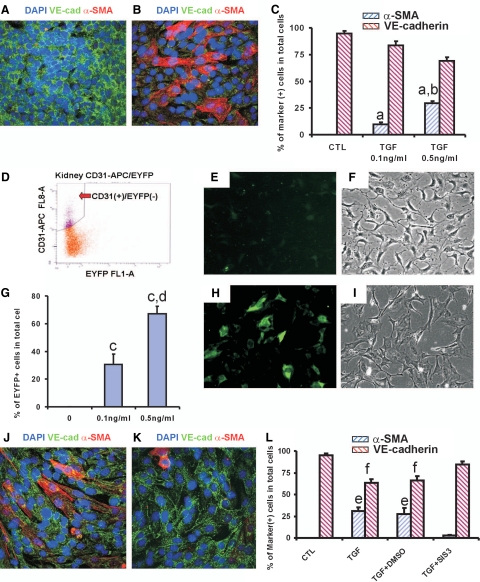
TGF-β1 plays a pivotal role in the development and progression of renal fibrosis. To investigate whether TGF-β1 can induce EndoMT in vitro, MMECs were cultured in the presence of TGF-β1. Confocal microscopy (Figure 4, A–C) and real-time PCR (Supplemental Figure S3, A and B, see http://ajp.amjpathol.org) demonstrated that TGF-β1 induces de novo expression of α-SMA and loss of expression of the endothelial cell markers VE-cadherin (Figure 4, A–C) and CD31 (Supplemental Figure S3, A and B, see http://ajp.amjpathol.org) in a dose- and time-dependent fashion.
Figure 4.
TGF-β1 induces EndoMT in vitro. A and B: Confocal microscopy demonstrates DAPI (blue), VE-cadherin (VE-cad, green), and α-SMA (red) in MMECs cultured with 0.5 ng/ml TGF-β1 (B) or without TGF-β1 (A) for 7 days. Original magnification, ×600. C: Quantitation of VE-cadherin+ and α-SMA+ cells. One-way analysis of variance: a, versus control (CTL), P < 0.05; b, versus 0.1 ng/ml TGF-β1, P < 0.05; c, versus control or 0.1 ng/ml TGF-β1, P < 0.05. D: Isolation of CD31+/EYFP− endothelial cells from α-SMA/EYFP mouse kidneys by fluorescence-activated cell sorting. APC, allophycocyanin. Epifluorescent (E and H) and bright-field (F and I) microscopy demonstrates that 0.5 ng/ml TGF-β1 induces expression of EYFP (H and I). Original magnification, ×400. G: Quantitation of percentages of EYFP+ cells in total cells. One-way analysis of variance: d, versus control, P < 0.05; e, versus control or 0.1 ng/ml TGF-β1, respectively, P < 0.05. J and K: Confocal microscopy demonstrates DAPI (blue), VE-cadherin (green), and α-SMA (red) in MMECs with TGF-β1 + dimethylsulfoxide (DMSO) (J) or TGF-β1 + 2 μmol/L SIS3 (K). L: Percentages of VE-cadherin+ and α-SMA+ cells in control, 0.5 ng/ml TGF-β1, 0.5 ng/ml TGF-β1 + DMSO, and 0.5 ng/ml TGF-β1 + 2 μmol/L SIS3. One-way analysis of variance: f, versus control or TGF-β1 + SIS3, P < 0.05; g, versus control or TGF-β1 + SIS3, respectively, P < 0.05.
Next, we investigated whether TGF-β1 can induce EndoMT in primary cultures of renal endothelial cells. To exclude the possibility that these cultures were contaminated with small numbers of mesenchymal cells, fluorescence-activated cell sorting was used to select CD31+/EYFP− cells from normal adult α-SMA/EYFP mouse kidneys (Figure 4D). Seven days after culture with TGF-β1, epifluorescent microscopy demonstrated that renal endothelial cells also express EYFP in a dose-dependent fashion (Figure 4, E–I). To investigate whether blockade of the TGF-β1/Smad3 signaling pathway can inhibit TGF-β1-induced EndoMT in MMECs, a specific inhibitor for Smad3, SIS3, was used. As expected, confocal microscopy (Figure 4, J–L) and real-time PCR (Supplemental Figure S4, A and B, see http://ajp.amjpathol.org) demonstrated that culture in the presence of SIS3 abrogated TGF-β1-induced EndoMT in MMECs. Taken together, these data demonstrate that TGF-β1 induces EndoMT in vitro, and blockade of TGF-β1/Smad3 signaling abolishes TGF-β1-mediated EndoMT.
Discussion
It is generally believed that podocytes and mesangial cells are the major cellular mediators in DN. In this study, we used an endothelial-lineage traceable mouse line, the Tie2-Cre;LoxP-EGFP mouse, to investigate the role of endothelial cells in the development of DN. Our study clearly demonstrated that the number of endothelial-origin myofibroblasts as well as the percentage of myofibroblasts of endothelial origin was significantly higher in STZ-induced DN than normal kidneys. These findings suggest that endothelial cells may undergo EndoMT and thereby contribute to the initiation of interstitial fibrosis in the development of DN. Our study also demonstrated the existence of endothelial-origin myofibroblasts in afferent/efferent arterioles in glomeruli of mice with STZ-induced DN, although in a very low number, suggesting that endothelial-origin myofibroblasts not only contribute to the early development of interstitial fibrosis but also possibly to glomerulosclerosis.
An increasing number of studies have demonstrated associations between microalbuminuria and endothelial dysfunction in both type I and type II diabetes.22,23,24,25 Stehouwer et al25 demonstrated that endothelial dysfunction precedes development of microalbuminuria in insulin-dependent diabetes mellitus. Recent studies from several groups have shown that endothelial nitric oxide synthase deficiency in both type I and type II diabetic mouse models is associated with the develop of lesions similar to those observed in human diabetic renal disease.26,27,28 Taken together, the above studies suggest that endothelial dysfunction may play a pivotal role in the pathogenesis of DN. Our study showed that by 1 month after STZ-induced diabetes, renal endothelial cells underwent EndoMT and contributed to the accumulation of renal myofibroblasts. EndoMT occurred independently of albuminuria, suggesting that renal endothelial cells may play a role in the initiation of renal interstitial fibrosis through the process of EndoMT.
Recently, Zeisberg et al29 analyzed kidneys 6 months after injection of STZ in CD1 mice. They demonstrated that approximately 40% of all fibroblast-specific protein 1-positive cells and 50% of the α-SMA-positive cells in STZ kidneys were also CD31 positive, reasoning that these fibroblasts are probably of endothelial origin. In our experiments, we studied EndoMT in an endothelial lineage-traceable mouse line (the Tie2-Cre;LoxP-EGFP mouse) in 1-month STZ-induced diabetic kidneys, demonstrating that EndoMT occurs and contributes to early development of diabetic renal interstitial fibrosis. We also demonstrated EndoMT in MMECs and primary cultures of renal endothelial cells. Our results, together with those of Zeisberg et al29, strongly indicate the existence of endothelial-origin myofibroblasts/fibroblasts in diabetic nephropathy and suggest that EndoMT may be a pathway leading to interstitial fibrosis in DN. Further studies are needed to confirm whether blockade of this pathway can retard or even reverse the development and progression of diabetic renal interstitial fibrosis.
It is clear that TGF-β1/Smad signaling plays an important role in the pathogenesis of DN. The TGF-β1-induced epithelial-myofibroblast transition has been extensively explored. Smad3-null mice are protected from tubulointerstitial fibrosis induced by unilateral ureteral obstruction30 and are resistant to STZ-induced renal fibrosis. Culture of primary renal tubular epithelial cells from wild-type or Smad3-null mice demonstrates that the Smad3 pathway is essential for the TGF-β1-induced epithelial-myofibroblast transition and autoinduction of TGF-β1.30 Our findings demonstrated that a specific inhibitor for Smad3, SIS3, abrogates TGF-β1-induced EndoMT in MMECs and renal endothelial cells. This suggests that Smad3 is a potential target for inhibition of EndoMT and that the therapeutic potential of SIS3 warrants further investigation in animal models of DN.
In the present study, only 55% of endothelial cells expressed EGFP. The activation of EGFP requires the deletion of loxP sites. The latter is determined by the activity of Cre recombinase, which is driven by the Tie2 promoter. The incomplete expression of EGFP in endothelial cells under the control of the Tie2 promoter fragment may be due to site-specific effects such as close proximity to the centromere,31 condensation of chromatin,32,33 transgene orientation, or the methylation state of the inserted genes.34 More than 97% of EGFP-positive cells expressed CD31 whereas fewer than 3% of EGFP+ cells were CD31−. These latter cells may be tubular epithelial cells, fibroblasts in the tubulointerstitial compartment, podocytes or mesangial cells in the glomerulus, or bone marrow-derived cells. The mechanisms causing this nonspecific expression are largely unknown,35 but may include developmental staging, other environmental factors, and unspecified genetic interactions affecting the activity of the Tie2 promoter. However, the fewer than 3% unspecific EGFP+/CD31− cells do not influence our results or conclusions.
The present study demonstrated that EndoMT contributes to the early development and progression of renal interstitial fibrosis in STZ-induced diabetic renal disease. Inhibition or reversal of EndoMT may be a potential target for the treatment and prevention of DN.
Supplementary Material
Acknowledgments
The authors wish to thank Monash MicroImaging Facility at Monash University for confocal imaging. We also thank Professor James L. Lessard for providing the α-SMA/EYFP mouse line.
Footnotes
Address reprint requests to Jinhua Li, M.D., Ph.D., Department of Anatomy and Developmental Biology, Monash University, Clayton, Victoria 3800, Australia. E-mail: jinhua.li@med.monash.edu.au.
See related Commentary on page 1371
Supported by Kidney Health Australia and the National Health and Medical Research Council (NHMRC) of Australia. J.L. is the recipient of a NHMRC Peter Doherty Postdoctoral Fellowship.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Rossing P, Rossing K, Jacobsen P, Parving HH. Unchanged incidence of diabetic nephropathy in IDDM patients. Diabetes. 1995;44:739–743. doi: 10.2337/diab.44.7.739. [DOI] [PubMed] [Google Scholar]
- Lewis JB. Diabetic nephropathy in patients with type II diabetes. Geriatr Nephrol Urol. 1999;9:167–175. doi: 10.1023/a:1008378600115. [DOI] [PubMed] [Google Scholar]
- Cooper ME, Jandeleit-Dahm K, Thomas MC. Targets to retard the progression of diabetic nephropathy. Kidney Int. 2005;68:1439–1445. doi: 10.1111/j.1523-1755.2005.00555.x. [DOI] [PubMed] [Google Scholar]
- Essawy M, Soylemezoglu O, Muchaneta-Kubara EC, Shortland J, Brown CB, el Nahas AM. Myofibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant. 1997;12:43–50. doi: 10.1093/ndt/12.1.43. [DOI] [PubMed] [Google Scholar]
- Pedagogos E, Hewitson T, Fraser I, Nicholls K, Becker G. Myofibroblasts and arteriolar sclerosis in human diabetic nephropathy. Am J Kidney Dis. 1997;29:912–918. doi: 10.1016/s0272-6386(97)90466-2. [DOI] [PubMed] [Google Scholar]
- Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B, Durand RE, Hoodless PA, Karsan A. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res. 2004;74:910–917. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- Arciniegas E, Sutton AB, Allen TD, Schor AM. Transforming growth factor β1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992;103(Pt 2):521–529. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res. 1997;80:444–451. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- Beranek JT. Vascular endothelium-derived cells containing smooth muscle actin are present in restenosis. Lab Invest. 1995;72:771. [PubMed] [Google Scholar]
- Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Zimmerman CM, Padgett RW. Transforming growth factor B signaling mediators and regulators. Gene. 2000;249:17–30. doi: 10.1016/s0378-1119(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Li JH, Huang XR, Zhu HJ, Oldfield M, Cooper M, Truong LD, Johnson RJ, Lan HY. Advanced glycation end products activate Smad signaling via TGF-β-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J. 2004;18:176–178. doi: 10.1096/fj.02-1117fje. [DOI] [PubMed] [Google Scholar]
- Isono M, Chen S, Hong SW, Iglesias-de la Cruz MC, Ziyadeh FN. Smad pathway is activated in the diabetic mouse kidney and Smad3 mediates TGF-β-induced fibronectin in mesangial cells. Biochem Biophys Res Commun. 2002;296:1356–1365. doi: 10.1016/s0006-291x(02)02084-3. [DOI] [PubMed] [Google Scholar]
- Hong SW, Isono M, Chen S, Iglesias-De La Cruz MC, Han DC, Ziyadeh FN. Increased glomerular and tubular expression of transforming growth factor-β1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am J Pathol. 2001;158:1653–1663. doi: 10.1016/s0002-9440(10)64121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Maezawa Y, Yokote K, Joh K, Kobayashi K, Kawamura H, Nishimura M, Roberts AB, Saito Y, Mori S. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem Biophys Res Commun. 2003;305:1002–1007. doi: 10.1016/s0006-291x(03)00885-4. [DOI] [PubMed] [Google Scholar]
- Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-β1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–55. [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992;340:319–323. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- Dogra G, Rich L, Stanton K, Watts GF. Endothelium-dependent and independent vasodilation studies at normoglycaemia in type I diabetes mellitus with and without microalbuminuria. Diabetologia. 2001;44:593–601. doi: 10.1007/s001250051665. [DOI] [PubMed] [Google Scholar]
- Chan WB, Chan NN, Lai CW, So WY, Lo MK, Lee KF, Chow CC, Metreweli C, Chan JC. Vascular defect beyond the endothelium in type II diabetic patients with overt nephropathy and moderate renal insufficiency. Kidney Int. 2006;70:711–716. doi: 10.1038/sj.ki.5001652. [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Fischer HR, van Kuijk AW, Polak BC, Donker AJ. Endothelial dysfunction precedes development of microalbuminuria in IDDM. Diabetes. 1995;44:561–564. doi: 10.2337/diab.44.5.561. [DOI] [PubMed] [Google Scholar]
- Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–2669. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, Harris RC, Takahashi T. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol. 2007;170:1473–1484. doi: 10.2353/ajpath.2007.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ, Croker B. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie KW, Lee M, Fantes JA, Graham E, Clark AJ, Springbett A, Lathe R, McClenaghan M. Variegated transgene expression in mouse mammary gland is determined by the transgene integration locus. Proc Natl Acad Sci USA. 1996;93:6659–6664. doi: 10.1073/pnas.93.13.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick D, Sutherland H, Robertson G, Whitelaw E. Variegated expression of a globin transgene correlates with chromatin accessibility but not methylation status. Nucleic Acids Res. 1996;24:4902–4909. doi: 10.1093/nar/24.24.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman IL, Kadulin SG, Razin SV. Transgenic animals in medicine: integration and expression of foreign genes, theoretical and applied aspects. Med Sci Monit. 2004;10:RA274-RA285. [PubMed] [Google Scholar]
- Feng YQ, Lorincz MC, Fiering S, Greally JM, Bouhassira EE. Position effects are influenced by the orientation of a transgene with respect to flanking chromatin. Mol Cell Biol. 2001;21:298–309. doi: 10.1128/MCB.21.1.298-309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Glaubitz M, Kuhlow D, Thierbach R, Birringer M, Steinberg P, Pfeiffer AF, Ristow M. Variable expression of Cre recombinase transgenes precludes reliable prediction of tissue-specific gene disruption by tail-biopsy genotyping. PLoS ONE. 2007;2:e1013. doi: 10.1371/journal.pone.0001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.