Abstract
Internalization and trafficking of cell-surface membrane receptors and proteins into subcellular compartments is mediated by specific short-sequence signal motifs, which are usually located within the cytoplasmic domains of these receptor and protein molecules. The signals usually consist of short linear amino acid sequences, which are recognized by adaptor coat proteins along the endocytic and sorting pathways. The complex arrays of signals and recognition proteins ensure the dynamic movement, accurate trafficking, and designated distribution of transmembrane receptors and ligands into intracellular compartments, particularly to the endosomal-lysosomal system. This review summarizes the new information and concepts, integrating them with the current and established views of endocytosis, intracellular trafficking, and sorting of membrane receptors and proteins. Particular emphasis has been given to the functional roles of short-sequence signal motifs responsible for the itinerary and destination of membrane receptors and proteins moving into the subcellular compartments. The specific characteristics and functions of short-sequence motifs, including various tyrosine-based, dileucine-type, and other short-sequence signals in the trafficking and sorting of membrane receptors and membrane proteins are presented and discussed.
Keywords: Membrane receptors, short sequence signals, endocytosis, intracellular trafficking receptor sorting, receptor recycling, endosomes, lysosomes
2. INTRODUCTION
Early and recent studies have demonstrated that ligand-receptor complexes are internalized through the organized cellular endocytic pathways (1–9). It is thought that most ligand-receptor complexes are concentrated in the clathrin-coated pits of the plasma membrane, which are cleaved off to produce clathrin-coated vesicles (10–12). The vesicles rapidly lose their coats, a change that facilitates their fusion with early endosomes, which are maintained at a slightly acidic pH and function as platforms for many ligand-receptor complexes. The bound ligand-receptor complexes are delivered to the lysosomes, where both ligand and receptor are degraded (6, 8, 13, 14). Under certain conditions, however, the ligand is delivered to lysosomes for degradation, while the receptor returns to the plasma membrane to bind new ligand for additional rounds of internalization. This process is referred to as retroendocytosis (8, 13, 15, 16). Alternatively, some endosomal and lysosomal proteins traffic through the plasma membrane to their main destinations to maintain a steady-state condition. In addition, endosomes and/or lysosomes can be accessed through the biosynthetic pathway, where newly synthesized acidic hydrolases with attached mannose-6-phosphate groups can bind to mannose-6-phosphate receptors in the trans-Golgi network (TGN), and be transported via an intracellular route to endosomes (17, 18). Eventually, the receptor is destined to reach either lysosomes or the plasma membrane.
The proper and directed operation of the endocytic pathway of membrane receptors and their bound ligand requires numerous important and critical sorting decisions along the endocytic trafficking itinerary. At the plasma membrane, receptors may either remain at the cell surface or be rapidly internalized into coated pits and vesicles (1, 19, 20). Subsequently, the receptors can proceed and be delivered to the lysosomes or may recycle back to the plasma membrane. Alternatively, involving the TGN, the receptors could be destined to go to endosomes or the plasma membrane. This complex array of routing and trafficking decisions is directed by a set of sorting-signal sequence motifs in the cytoplasmic domains of the receptor molecules, which recognize the routing pathways and deliver receptors to their intended locations in the intracellular compartments.
Numerous studies have demonstrated the role of carboxyl-terminus domain of various membrane receptors and membrane proteins in mediating the adaptive changes, which accelerate their trafficking and sorting from the cell surface into the intracellular compartments (4, 5, 7, 21–25). It is believed that the carboxyl-terminal tail of a number of membrane receptors and proteins is absolutely required for signaling and plays important roles in meditating internalization, sorting, down-regulation and desensitization processes. Recently, the short sequence signal motifs located in the carboxyl-terminal domain and their roles in mediating the molecular mechanisms of receptor endocytosis and trafficking have received considerable importance. However, more studies are needed to reveal the new signal motifs of membrane receptors and membrane proteins, which direct internalization, trafficking, and sorting events. This review focuses on the characteristic features and specific functions of short sequence signal motifs involved in trafficking and intracellular routing of membrane receptors or membrane proteins, which are either targeted to endosomes, lysosomes, and/or related subcellular organelles or recycled back to the plasma membrane.
3. MODULATION OF RECEPTOR TRAFFICKING AND SEQUESTRATION BY SHORT-SEQUENCE SIGNAL MOTIFS
Trafficking of membrane receptors or membrane proteins between cellular organelles is mediated through the membrane-bound vesicular structures. It has been demonstrated that the targeting and sorting of individual receptor and membrane protein is directed by their intrinsic sequence-based signal motifs in the endocytic and secretory pathways (3, 4, 7, 26–30). It is envisioned that the receptor-mediated endocytosis of various ligand-receptor complexes may involve sequential sorting steps through which ligand-receptor complexes and membrane proteins could eventually be degraded, recycled back to the cell surface, or released into the cell exterior (Fig. 1). Many of these events may take place sequentially. The first step would be the noncovalent binding of ligand to cell-surface receptor proteins. Subsequently, the receptors, through some intrinsic affinity or aggregation itinerary, travel via the endocytic vesicles and delivered to the intended subcellular compartments (6, 8, 13, 26, 31, 32). During the sequential processes of endocytosis of ligand-receptor complexes via the intracellular sorting pathway, the ligand-receptor complexes may proceed according to one or more possibilities. The majority of ligand-receptor complexes are targeted to the lysosomes, leading to degradation of the ligand-receptor complexes. During the endocytic process of certain ligand-receptor complexes, the acidic pH of endosomes induces dissociation of ligand from the receptor. However, in certain instances, ligand is dissociated from the receptor in acidic vesicles referred to as endosomes, subsequently a population of receptor molecules may recycle back to the plasma membrane and ligand is transported to the lysosomes (13). The recycling of endocytosed receptors back to the plasma membrane may occur simultaneously with the process leading to the endosome-dependent pathway. The release of intact ligand may occur through the process of retroendocytosis which involves the fusion of receptor containing vesicles with plasma membrane. The release of intact ligand by retro-endocytotic pathway has been exemplified for various hormones including epidermal growth factor (33), insulin (34) transferrin (18, 35, 36), and atrial natriuretic peptide (ANP) (13). .
Figure 1.
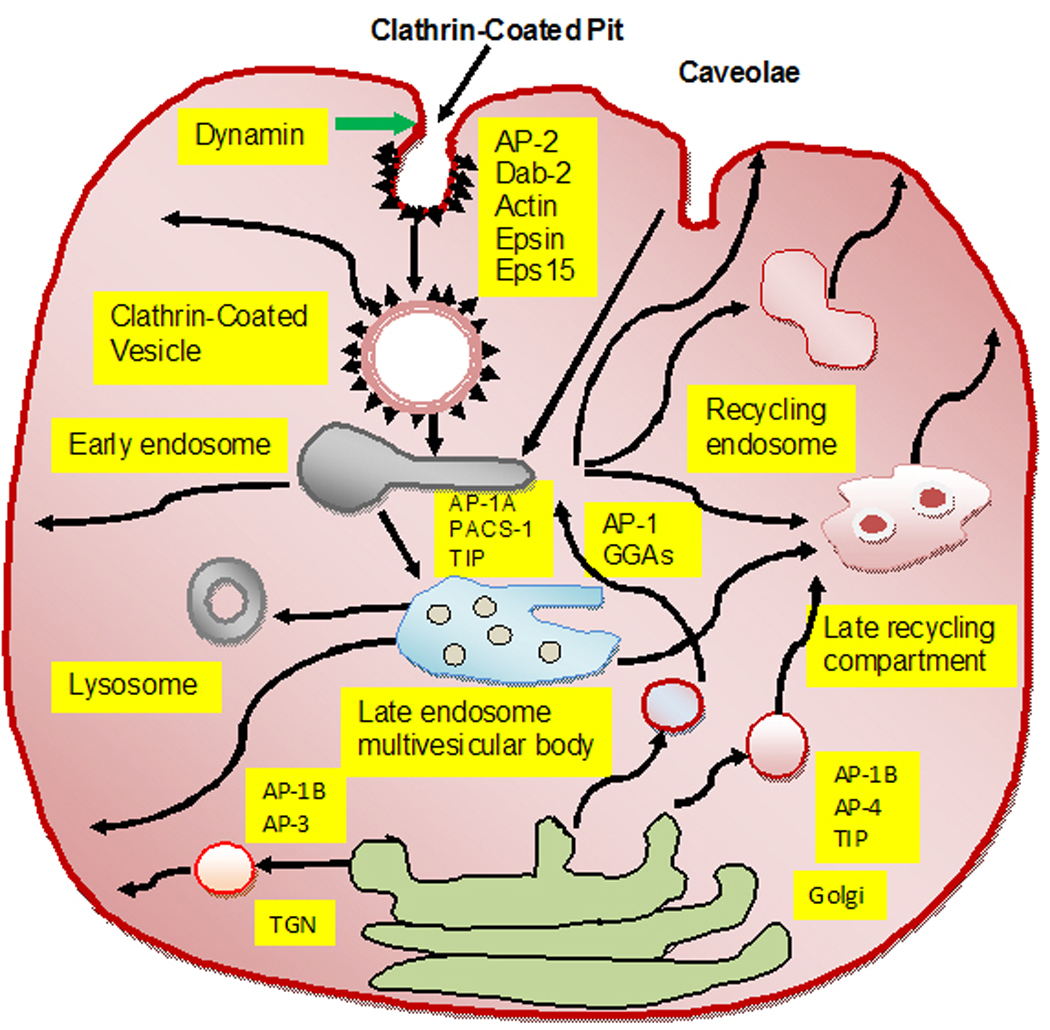
Diagrammatic representation of intracellular pathways of receptor-mediated endocytosis and trafficking: The ligand binding to specific cell surface receptor leads to a selective recruitment of ligand-receptor complexes into clathrin-coated pits. The coated pit represents a small area of the plasma membrane, which invaginates and pinch-off into vesicle in the cytosol. Coated pits and vesicles also trigger the recruitment of adapter proteins for example AP-2 and other interacting protein molecules. The caveolin pathway also internalizes the cargo complex, independent of clathrin-coated pits and the surface of caveolae is coated by caveolin. Both clathrin- and caveolin-dependent routes require dynamin protein to achieve the fission of the membrane invaginations and vesicle internalization. The ligand-receptor complexes within the cargo entering via the clathrin and caveolin pathways are usually directed to early endosomes. From the endosomes, the receptors and ligands are sorted to various subcellular locations, where the internalized molecules are either routed to degradative compartments such as the late endosomes, and/or lysosomes, or recycled to the plasma membrane via recycling endosomes. The recycled molecules can participate in several rounds of endocytosis. Alternatively, the internalized cargo molecules may be sequestered in endosomes for a longer period of time and continue to spark signaling events. Some early and late endosomes also contain membrane structures in the lumen, which are referred to as multi-vesicular bodies (MVBs). The endosome and lysosome system can also transmit and receive cargo from trans-Golgi network (TGN) involving vesicular intermediate carriers. The key proteins involved in the trafficking of molecules at different locations have been indicated such as AP-1, AP-1A, AP-1B, AP-2, AP-4, Dab-1, Esp15, GGA, PACS-1, and TIP.
Internalization of macromolecules is usually carried out by two main structural devices formed on the plasma membrane, which include clathrin-coated vesicles and caveolae. The clathrin-mediated endocytosis is regarded as an established mechanism for the internalization of cargo as well as a large number of membrane receptors and proteins from the cell surface (4, 8, 9, 37–39). Moreover, the clathrin-dependent cargo usually contain a short sequence motif such as [D/E]XXXL[L/I] and YXXQ, recognized by adaptor protein-2 (AP-2) and may contain Asn-Pro-X-Tyr sequence (NPXY) motifs, which are recognized by the accessory clathrin adaptor proteins (4, 40). A clathrin-containing coated pit is formed by deepening invagination and separation from the plasma membrane and yields a clathrin-coated vesicle. The vesicle is clipped by the constriction of the vesicle neck involving the mechanochemical force generated by dynamin (41). On the other hand, caveolae are assembled by the protein caveolin, which is constituted in the endoplasmic reticulum and then travels to the plasma membrane. Caveolae participate in the internalization of various types of macromolecules and also function as docking sites for the assembly of intracellular signaling networks (42–44). A number of cargo molecules are known to be internalized involving caveolae such as transforming growth factor-beta receptor (TGF-β R), ubiquitinated epidermal growth factor receptor, integrins, adenosine receptors, and glutamate transporter (45–49). The functioning of caveolae largely depends on the cluster structure of membrane lipids including cholesterol and gangliosides and such partitioning of plasma membrane facilitates the cargo internalization via caveolae (50). Similar to clathrin-mediated internalization, caveolae-dependent endocytic mechanism also requires dynamin (51–53). Although, there is only limited similarity between clathrin-mediated and caveolae-dependent internalization mechanisms, the Src-dependent phosphorylation of dynamin on Tyr231 and Tyr597 residues enhances dynamin assembly, which facilitates both clathrin- and caveolae- dependent internalization (53–55).
The short functional signal motifs have been instrumental in our understanding of the endocytosis and intracellular trafficking of various forms of transmembrane receptor-ligand complexes from the plasma membrane into intracellular compartments. To date, most of the endosomal-lysosomal signals which have been characterized are contained within the cytoplasmic domains of membrane receptor proteins. The signal motifs usually constitute short linear arrays of amino acids, which are degenerate motifs of four to seven amino-acid residues (4, 7). Among them, two or three amino acid residues usually are critical to the functional characteristics of that particular sequence-signal motif. The critical functional residues are most likely to be bulky hydrophobic amino acids. However, it has been suggested that the charged amino acid residues are important determinants of specificity and exhibit functional significance in the endocytosis and molecular trafficking of membrane receptors and membrane proteins (4, 7). Interestingly, high performance algorithms for the identification of short sequences should provide an extremely useful tool to search through the genome databases for new signal motifs involved in the internalization and trafficking of membrane receptors and proteins (56).
Earlier studies indicated that two major classes of endosomal-lysosomal trafficking and sorting signals are tyrosine-based and dileucine-based signals (3). However, signals based on the charged amino acid residues, as well as acidic residues, also have critical functions in the intracellular trafficking and sequestration of membrane receptors (57). The hallmark characteristic of endocytic and trafficking signals, which specifies and distinguishes these signals from other sequence motifs is their presence in the cytoplasmic domains, particularly the carboxyl-terminal regions of transmembrane receptors. However, not all signal motifs are short peptide sequences. In certain circumstances, the trafficking and sorting determinants seem to be folded structures and the critical amino acid residues are not particularly colinear. A compilation of membrane receptors and membrane proteins with known signal sequence motifs is presented in Table 1. However, much work remains to be accomplished to identify new motifs for internalization, trafficking, and intracellular distribution of several types of membrane receptors and proteins.
Table 1.
A number of important short-sequence motifs required for internalization and trafficking of various membrane receptors and membrane proteins
| Membrane Receptor/Protein | Signal Motifs | |
|---|---|---|
| Three-Letter Amino Acid Code |
Single-Letter Amino Acid Code |
|
| 1. Beta-adrenergic receptor | Asp-Ser-Leu-Leu | DSLL |
| 2. Beta-amyloid precursor protein | Tyr-Glu-Asn-Pro-Thr-Tyr | YENPTY |
| 3. CD-Mannose-6-phosphate receptor | Tyr-Lys-Tyr-Ser-Lys-Val | YKYSKV |
| 4. CD44 | His-Leu-Val-Asn-Lys | HLVNK |
| 5. CI-Mannose-6-phosphate receptor | Tyr-Ser-Lys-Val | YSKV |
| 6. Guanylyl cyclase/ natriuretic peptide receptor-A | Gly-Asp-Ala-Tyr | GDAY |
| 7. GLUT4 | Phe-Gln-Gln-Ile | FQQI |
| 8. Igp-A/lamp-1 | Gly-Tyr-Gln-Thr-Ile | GYQTI |
| 9. Igp-B/lamp-2 | Gly-Tyr-Glu-Gln-Phe | GYEQF |
| 10. LDL receptor | Phe-Asp-Asn-Pro-Val-Tyr | FDNPVY |
| 11. LH receptor | Gly-Thr-Ala-Leu-Leu | GTALL |
| 12. Lysosomal acid phosphatase | Gly-Tyr-Arg-His-Val | GYRHV |
| 13. Mannose phosphate receptor | Phe-Glu-Asn-Thr-Leu-Tyr | FENTLY |
| 14. Polymeric Ig receptor | Tyr-Ser-Ala-Phe | YSAF |
| 15. P2x receptor (ATP-gated ion channel) | Tyr-Glu-Gln-Gly-Leu | YEQGL |
| 16. TGN38 | Tyr-Gln-Arg-Leu | YQRL |
| 17. Transferrin receptor | Tyr-Thr-Arg-Phe | YTRF |
| 18. T-cell receptor (CD3) | Tyr-Gln-Pro-Leu | YQPL |
| 19. Fc receptor | Leu-Leu | LL |
| 20. MHC class II invariant chain | Leu-Ile | LI |
The degenerate short tetra or hexa peptide sequence motifs usually contain tyrosine or phenylalanine residues, followed by hydrophobic or aromatic residues. Some sequences also contain acidic residues in conjunction with required tyrosine. Certain membrane receptors make use of the dileucine-type of signal motifs, which are essential for internalization and trafficking of membrane receptors and membrane proteins into subcellular compartments. GLUT4, glucose transporter 4; LDL, low density lipoprotein; LH, leutinizing hormone; TGN, Trans-Golgi network
3.1. Characteristic features and roles of NPXY-type signal-sequence motifs
In large part, the NPXY signal motifs mediate rapid internalization of membrane proteins, including members of LDL receptors, beta-1 integrin, megalin, and beta-amyloid precursor protein families, as well as receptor tyrosine kinases. These include members of the insulin receptor and epidermal growth factor receptor family, as well as neurotrophin receptors (4, 58–61). The replacement of Asn, Pro, or Tyr residues with Ala in the LDL receptor largely abolishes the receptor internalization. However, the replacement of Tyr with Phe has no discernible effect on the internalization process. At its discovery, the low-density lipoprotein (LDL) mutant receptor was shown to have normal ligand binding characteristics; however, its defective receptor internalization pointed to a critical function of the cytoplasmic domain in overall receptor endocytosis and trafficking (58, 62). Those initial studies were instrumental in identification of the FXNPXY sequence motif as the first recognized trafficking and sequestration signal of membrane receptors and proteins. The substitution of a cysteine residue for a tyrosine residue in the NPXY motif of the cytoplasmic domain of the LDL receptor in a patient with familial hypercholesterolemia rapidly abrogated the internalization of this receptor protein (62). Later, it was demonstrated that in the NPXY sequence motif, the critical residue is tyrosine (58). It has been suggested that hypercholesterolemia results from a tyrosine-phenylalanine mutation in the NPXY motif of LDL receptor (23, 58, 62).
Genetic defects in the autosomal recessive hypercholesterolemia (ARH) gene on chromosome 1 have been mapped in patients with defects in either LDL receptor or the proteinaceous component of LDL particles that binds the receptor. These patients show remarkable level of circulating LDL-cholesterol components in an autosomal dominant manner (63, 64). Evidence suggests that ARH displays the intrinsic ability to interact with endocytic motif of LDL receptor as well as with the components of clathrin and AP-2 in the internalization process (65–67). On the other hand, it has also been indicated that ARH may not be solely responsible for LDL delivery in all cell and tissue types. The adaptor protein disabled-2 (Dab 2) and ARH have been shown to exhibit remarkable similarities with regard to structure-function relationship (67, 68). The Dab 2 participates as an intermediate adaptor for LDL internalization; however, both Dab 2 and ARH might be functionally redundant (65–67). The amino-terminal phosphotyrosine binding (PTB) domain of both ARH and Dab 2 physically binds and interacts with NPXY sequence of LDL receptor as well as with membrane-bound phosphatidyl inositol 4, 5-bisphosphate (PtdIns (4,5) P2), which allows cargo recognition at the cell surface (65, 67, 69–71). Since, ARH and Dab 2 bind to clathrin, AP-2 adaptor, PtdIns (4, 5) P2, and the NPXY sequence, both ARH and Dab 2 are generally termed as clathrin-associated sorting proteins (CLAPs). The existence of AP −2, ARH, Dab 2, transferrin, and LDL receptor in the varying clathrin structures has challenged the presence of specialized clathrin coats for the segregated internalization involving the cargo-specific coat assembly at the cell surface (12, 66, 72). Later studies showed that mutation of tyrosine to phenylalanine in the NPXY motif makes the LDL receptor unrecognizable by Dab-2, which interacts with AP-2 in clathrin-mediated endocytosis of this receptor protein (73).
The NPXY motifs recruit clathrin and adaptor protein molecules and act as cargo recognition motifs for their delivery to endosomes and lysosomes (4, 23, 67). Several studies have shown that NPXY motifs initially recruit AP-2 at the plasma membrane and activate the mu2 subunit of AP-2, after which the beta-2 subunit of AP-2 binds clathrin at the cell surface, leading to clathrin-mediated endocytosis (4, 23, 27, 40, 74–76). The NPXY motifs have also been shown to recruit and interact with the phosphotyrosine domain of the adaptor protein Dab-2 (73, 75). Interesting, Dab-2 directly interacts with the NPXY motifs and leads to clathrin-mediated endocytosis by activating clathrin and AP-2 (23, 68, 77, 78). The AP-2 is composed of four subunits (alpha, beta2, mu2, and sigma2), which interact with NPXY motifs of membrane receptors and membrane proteins in the process of internalization (4, 79). In essence, the NPXY minimal signal sequence is shared by a large number of proteins containing this motif. However, in certain circumstances, the NPXY motif by itself is not sufficient for internalization. For example, in some members of the LDL receptor family, phenylalanine and aspartic acid residues are required before asparagine and provide a functional sequence motif of FDNPVY for efficient internalization and trafficking processes (58). Nevertheless, the LDL receptor and other membrane proteins that contain NPXY signal motifs are internalized via clathrin-coated pits (80, 81). The FDNPVY sequence motif binds to components of the clathrin coat and within this context, the NPXY residues adopt a beta-turn structure (82). Furthermore, chimeric insertion of the FDNPVY sequence into the transferrin receptor leads to rapid internalization, whereas insertion of only the NPVY sequence does not enhance endocytosis of this receptor protein (83). Similarly, endocytosis of beta-amyloid precursor protein is directed by a longer sequence motif, GYENPTY, in which the first tyrosine residue seems to have a more critical role in internalization events (84). Thus, either Phe or Tyr residue at this same position seem to have a critical function in conjunction with the NPXY motif.
Membrane proteins such as megalin and LDL receptor-related protein-1 (LRP-1) contain more than one copy of the NPXY motif (4, 23). Earlier studies indicated that the FDNPVY sequence motif binds to purified AP-2 and that this binding is dependent on the Phe and Tyr residues of the FDNPVY motif (74). Most NPXY signal motifs are located in the cytoplasmic domain within 40–500 amino-acid residues. AP-2 and similar adaptor proteins are located between the clathrin lattice and the plasma membrane, which are predicted to be in an appropriate position to interact with cytoplasmic signal motifs (4, 23). Because of its position in the cytoplasmic domain, the FDNPVY signal motif of the LDL receptor may extend only 30–40Å from the transmembrane spanning region (85). However, the terminal domain of clathrin is located almost 100 Å from the plasma membrane. Intriguingly, the NPXY motif is required for endocytosis, although the extent of internalization of a protein that contains this motif in the cytosolic intracellular domain does not depend on the context of sequence of the NPXY motif (7). It has been reported that the mutations in the NPXY motif disrupted the interaction between the cytoplasmic tail of the LDL receptor and sorting nexin17 (SNX17), which contains FERM-like region (protein4.1, Ezrin, radixin, moesin) (67, 86). These authors suggested that SNX17 functions in the internalization and cellular trafficking of LDL receptor through interaction with the NPXY signal motif located in its cytoplasmic carboxyl-terminal domain. On the other hand, it also has been reported that NPXY motif in the LDL receptor did not mediate its interaction with SNX17 (87).
Previous studies have suggested that NPXY signals are not directly recognized by AP-2, however, these signals interact with proteins containing the phosphotyrosine-binding (PTB) domain, also known as phosphotyrosine-interacting (PTI) domain (23, 73, 88). The PTB domain recruits adaptor proteins such as insulin receptor substrate-1 (IRS-1) or Shc for interaction with NPXY motifs (73, 89–93). In these circumstances, a tyrosine residue usually functions as a phosphorylated amino acid. It has also been indicated that PTB domain-containing proteins show potentially greater selectivity for NPXY signal motifs (73, 88). Several studies have demonstrated that proteins containing PTB domains such as Dab-1 and Dab-2 participate in LDL receptor internalization (94–98). Both Dab-1 and Dab-2 directly bind FXNPXY sequence motifs located in various members of the LDL receptor family (73, 75, 99). However, overexpression of the PTB domain of either Dab-1 or Dab-2 impedes internalization of LDL receptor, leading to the accumulation of receptor on the cell surface (68, 100). Another intriguing finding was that genetic disruption of Dab-2 and LDL receptor family member megalin produced proteinuria in null mutant (-/-) mice as compared with wild-type (+/+) littermates (68, 101). Since megalin functions in protein re-absorption in renal proximal tubules, the in vivo results implicated the role of Dab-2 in the endocytosis of megalin (68, 102–104). Both Dab-1 and Dab-2 contain signal motifs, which bind to clathrin and AP-2 at the carboxyl-terminal to their PTB domain (65, 67, 73). Similarly, Grb-2 has been shown to facilitate the internalization of epidermal growth factor receptor protein (105). Nevertheless, further studies are needed to determine more definitive functions of Dab-1and Dab-2 proteins and their interaction with NPXY motif in the internalization of membrane receptors and membrane proteins. It has been shown that NPXY motifs in the carboxyl-terminal domain of beta-5 integrin act as the molecular switch for distinct biological processes of integrin activation, endocytosis, and sorting (4, 61, 106, 107) The NPXY motifs in the cytosolic tail of beta-1 integrin also function in the recruitment of adaptor proteins and clathrin for endocytosis and act as sorting signals for internalized cargo assembly (59).
3.2. Characteristic features and roles of GDXY-type signal-sequence motif
Recently, we found that the tetrameric sequence Gly920-Asp921-Ala922-Tyr923 (GDAY) sequence motif in the carboxyl terminal-domain of guanylyl cyclase/natriuretic peptide receptor-A (GC-A/NPRA) serves as an internalization signal for endocytosis of NPRA (57). The residues Gly920 and Tyr923 constitute the important elements in the GDAY internalization signal motif. However, it is thought that the residue Asp921 provides an acidic environment for efficient signaling of GDAY motif during the GC-A/NPRA receptor internalization process. It was found that single mutation Asp921 to alanine did not have a major effect on receptor internalization, but did significantly attenuate the recycling of internalized receptors back to the plasma membrane (57). On the other hand, mutation of Gly920 and Tyr923 to alanine inhibited the internalization of NPRA, although these residues had no discernible effect on the recycling of this receptor protein. These findings suggested that the tyrosine-based GDAY sequence motif modulates the early internalization of NPRA, whereas aspartic acid residue in the GDAY motif seems to mediate recycling or later sorting of this receptor protein. If that is so, two overlapping motifs within the GDAY sequence in the carboxyl-terminal domain of NPRA exert different but specific effects on endocytosis and subsequent trafficking of this receptor protein. However, more studies are needed to define the dual role of the GDAY motif in the events involved in the internalization, sequestration, and recycling of NPRA (57, 108).
It is conceivable that the GDAY motif has a dual function in receptor internalization into the cell interior and in subsequent recycling of internalized receptor back to the plasma membrane. The mutations of Gly920 and Tyr923 to alanine in NPRA cDNA attenuated the internalization of mutant receptors by almost 50% as compared with wild-type receptor (57). However, the mutation of Asp921 to alanine had only a minimal effect on the internalization of NPRA (Fig. 2). Thus it seems possible that the regulation of receptor internalization relies largely on residues Gly920 and Tyr923 in the carboxyl-terminal domain of NPRA. Interestingly, on the other hand, mutation of Asp921 to alanine significantly attenuated the recycling of internalized receptor back to the plasma membrane. These findings suggested that the role of the NPRA cytoplasmic tail is important, being comparable to the thyrotropin-stimulating hormone receptor, in that its disruption attenuates receptor internalization (109). The point and deletion mutations within the carboxyl-terminal region of NPRA have a major effect on internalization and recycling of this receptor protein (57, 110). To test whether similar amounts of mutant receptors were available on the cell surface, HEK-293 cells expressing either wild-type or GDAY/AAAA mutant NPRA were allowed to bind 125I-ANP for 1 h at 4°C. After washing the free ligand, the cell surface bound 125I-ANP radioactivity was determined. The results showed that the GDAY/AAAA mutant receptor displayed a dose-response curve similar to that of wild-type receptor (Fig. 3). The recombinant HEK-293 cells harboring both wild-type and GDAY/AAAA mutant receptors expressed 1.87×106±0.038×106 and 1.89×106±0.036×106 receptor sites/cell, respectively. The binding affinity was comparable in both cell lines, with a Kd=2.4×10−8±0.025×10−8 M. The independent HEK-293 recombinant cell lines containing wild-type, G920A, D921A, Y923A or GDAY/AAAA receptors were utilized for the functional studies, and each clonal cell line yielded comparable receptor binding characteristics (57).
Figure 2.
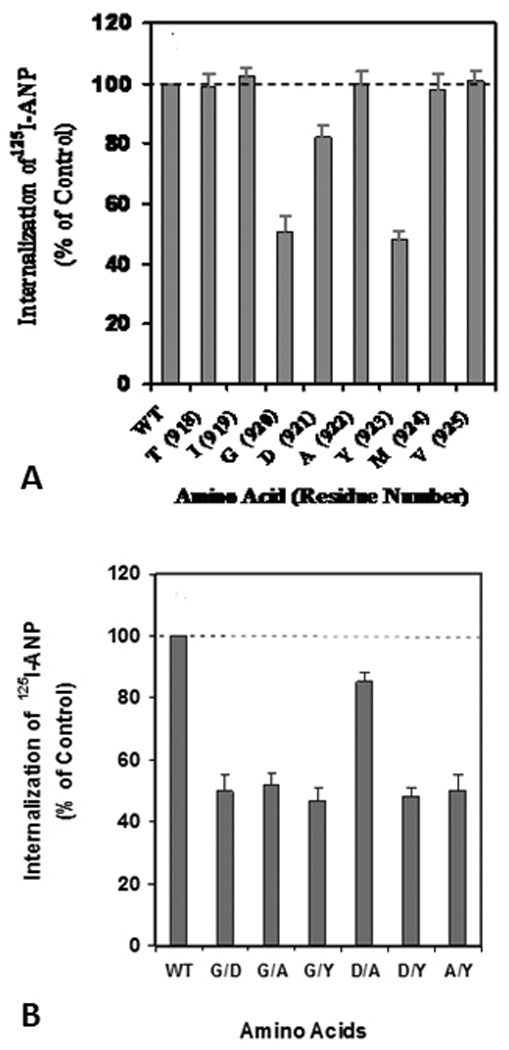
Requirements of GDAY sequence for Internalization of guanylyl cyclase/natriuretic peptide in HEK-293 Cells: Mutagenesis of Gly-Asp-Ala-Tyr (GDAY) sequence of guanylyl cyclase/natriuretic peptide receptor-A (GCA/NPRA) and expression of both wild-type and GDAY/AAAA mutant receptors in human embryonic kidney-293 (HEK-293) cells. (A) Alanine substitutions at amino acid residues 918–925. (B) Alanine substitutions indicating two residues in different combinations in GDAY motif. Confluent HEK-293 cells expressing either wild-type or mutant receptors were washed twice with 2 ml of assay medium (Dulbecco’s modified Eagle’s medium containing 0.1% bovine serum albumin) and then exposed to 125I-ANP at 4˚C for 1 h in the absence or presence of unlabeled ANP. After which, the cells were washed four times with assay medium and reincubated in 2 ml of fresh medium at 37˚C. After 10 min internalization and incubation period, the internalization of ligand-receptor complexes was quantified. Figure has been reproduced from the previous publication and has been adapted with the permission (57).
Figure 3.
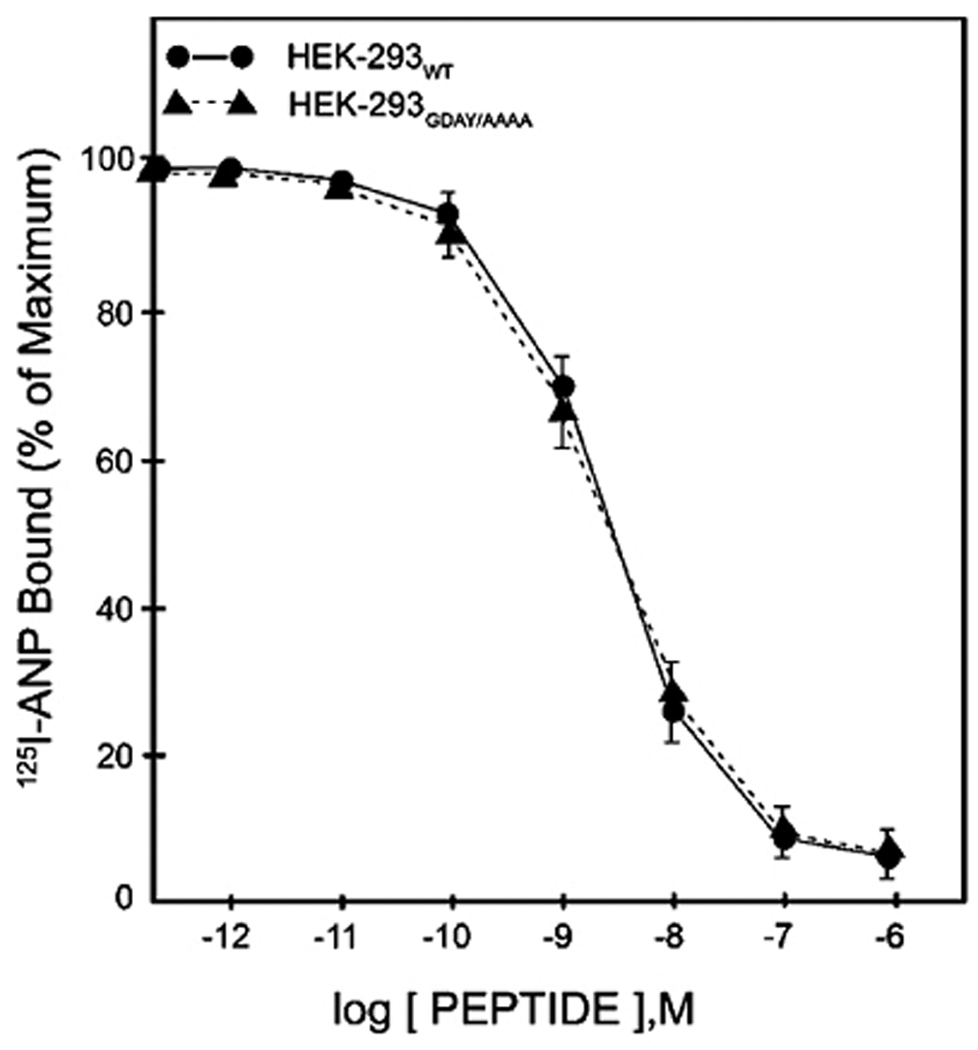
Competition binding of 125I-ANP in HEK-293 cells expressing wild-type or GDAY/AAAA mutant receptors: Confluent HEK-293 cells were cultured in 6 cm2 dishes and incubated in 2 ml of assay medium with 1 nM 125I-ANP and increasing concentrations of unlabelled ANP at 4 °C for 1 h. Cells were washed four times, each with 2 ml of assay medium to remove the unbound radioligand, and then dissolved in 1 M NaOH. Specific 125I-ANP radioactivity was determined in the solubilized cell extract and the binding curves are derived from the specific binding data. The non-specific binding was determined by using 100-fold excess molar concentrations of unlabelled ANP. The Kd values and receptor densities (Bmax) were determined from the binding competition and by Scatchard analysis of the 125I-ANP binding data. Figure has been reproduced from the previous publication and has been adapted with the permission (57).
Earlier, it was demonstrated that the GC-A/NPRA, an important receptor for ANP and brain natriuretic peptides (BNP), is internalized and redistributed in a ligand-dependent manner into subcellular compartments (111–116). As shown in Fig. 4, ligand-dependent endocytosis and sequestration of NPRA involves a series of sequential sorting steps through which ligand-receptor complexes could eventually be degraded, receptor recycled back to the plasma membrane, and intact ligand released into the cell exterior (110, 113, 115). The recycling of endocytosed receptor to the plasma membrane and the release of intact ANP into the cell exterior occur simultaneously with the processes leading to degradation of the majority of ligand-receptor complexes into lysosomes, which seems to be controlled by receptor sequences in the carboxyl-terminal domain of GC-A/NPRA (57, 110). Furthermore, a return in 125I-ANP binding in trypsin-treated cells indicated that 125I-ANP binding in trypsinized cells is due to recycling of NPRA (57, 115). It should be noted, however, that, as compared with cells expressing wild-type NPRA, HEK-293 cells expressing Gly920/A and Tyr923/A mutant receptors exhibited a decrease of almost 35% in 125I-ANP-binding after trypsin treatment. On the other hand, cells expressing Asp921/A or triple mutant GDAY/AAAA receptor showed a more than 60% decrease in 125I-ANP binding after trypsin treatment as compared with wild-type receptor (57). These earlier results provided the evidence that, after trypsin treatment, the greater return of 125I-ANP binding in recombinant wild-type HEK-293 cells expressing wild-type receptors is due to a large population of internalized receptors and subsequent recycling to the plasma membrane as compared with cells expressing GDAY mutant receptors. Also, after exposure of HEK-293 cells to ANP for 60 min at 37˚C, the 125I-ANP binding sites were decreased by almost 68% in cells expressing wild-type NPRA, but only 30%–38% in cells expressing Gly920/A, Tyr923/A and GDAY/AAAA mutant receptors (57). Those findings provided direct evidence that treatment of cells with unlabeled ANP accelerated the disappearance of surface receptors, indicating that ANP-dependent down-regulation of NPRA may involve the internalization of ligand-receptor complexes and that GDAY mutant receptor is relatively more resistant to ligand-mediated down-regulation.
Figure 4.
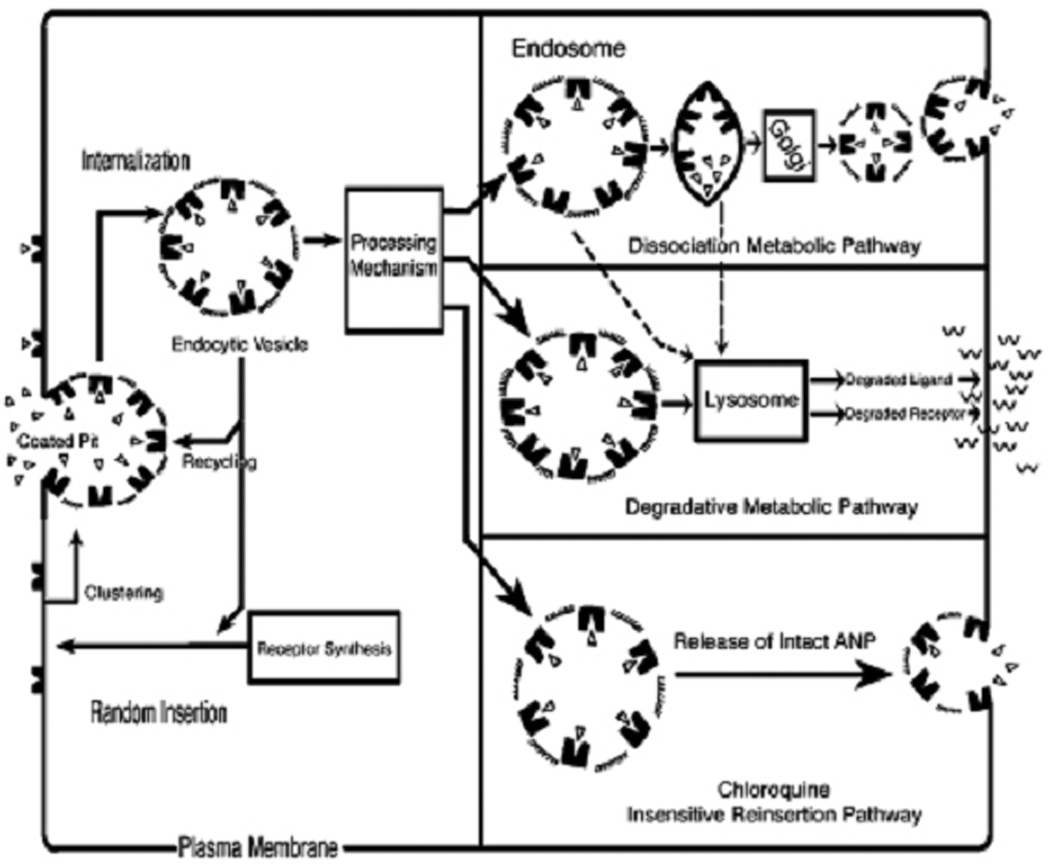
Schematic representation of internalization, recycling and intracellular degradation of membrane receptors in intact cells: The schematic diagram shown postulates the stoichimetric kinetics of internalization, subcellular sequestration, recycling, and ultimately metabolic turnover of ligand-receptor complexes from cell surface to cell interior and back to the plasma membrane. The scheme depicts that after synthesis: a) the receptor is inserted in the plasma membrane, b) the ligand-receptor complex enters the cell via coated pits, and c) the complex is processed intracellularly through endosome, lysosome, and/or chloroquine-insensitive pathways. Sorting of bound ligand-receptor complexes into the intracellular compartments may occur by i) lysosomal degradative metabolic pathway, ii) endosomal dissociation metabolic pathway, and/or iii) release through the chloroquine-insensitive pathway. The cardiac hormone atrial natriuretic peptide (ANP) and its biologically active membrane receptor have been exemplified. Figure has been reproduced from the previous publication and has been adapted with the permission (13).
Interestingly, guanylyl cyclase-B/natriuretic peptide receptor-B (GC-B/NPRB) has also been shown to be internalized and recycled in hippocampus neurons and C6 glioma cells cultures (117). These authors suggested that the trafficking of GC-B/NPRB occurs in the ligand-dependent manner in response to C-type natriuretic peptide (CNP) stimulation. The trafficking of NPRB has been suggested to involve a clathrin-dependent mechanisms. Our recent work has suggested that the internalization of GC-A/NPRA involves clathrin-dependent pathways (118). The process of receptor internalization is severely diminished by inhibitors of clathrin proteins such as chlorpromazine and monodensyl cadaverine. However, interaction of the GDAY motif in GC-A/NPRA with clathrin adaptor proteins remains to be established.
The internalization of platelet-activating factor is also regulated by a putative motif, DPXXY (119), and that of type-2 vasopressin receptor by the NPXXY sequence (120). Similarly, it has been suggested that the YXXL motif functions in endocytosis of LDL receptor-related protein (121). A common feature of these internalization signal motifs, including NPXY and GDAY, is the presence of a tyrosine residue at the end of the tetrapeptide sequence (57, 119). Moreover, tyrosine residues in the mannose-6-phosphate receptor and in the influenza virus hemagglutinin are also involved in endocytosis, even though they are not present in the context of NPXY or YXRF consensus sequence motifs. Therefore, if a universal internalization signal exists, it may not be based on a universal amino acid sequence (4, 23, 119, 122, 123). It has been suggested that the critical characteristics of all these sequences might be their specification of a particular conformation, such as a tight beta-turn in protein structure (124). It also has been suggested that the Gly920 residue constitutes the important element in conjunction with the Tyr923 residue of the GDAY internalization signal (57). Similarly, the Gly950-Pro951-Leu952-Tyr953 motif has been implicated in internalization of the insulin receptor, in which Gly950 and Tyr953 residues have been shown to be critical components (125). These authors suggested that a common internalization motif consists of four amino acids with an aromatic residue, specifically a tyrosine in the fourth position. It has also been proposed that tyrosine recognition signals form a small surface loop (126), but one differing in structure from that proposed by others in terms of the positioning of tyrosine in the loop structure (4, 83). Earlier studies have provided direct evidence not only that the NPXY sequence in the LDL receptor forms a beta-turn structure, but that the peptides containing the NPXY motif assume a reverse-turn conformation with Tyr in the fourth position of the turn (127). The substitution of Tyr with residues known to be inactive in endocytosis resulted in disruption of the beta-turn conformation. A similar approach was used to obtain evidence that the PPGY sequence of the acid phosphatase in the cytoplasmic tail forms a type 1 beta turn with the Tyr in the fourth position (128). All of these studies indicated that the presence of Tyr in the fourth position of internalization signals is critical for receptor endocytosis.
Similarly, seven transmembrane G-protein-linked receptors have a homologous motif, NPXXY. Mutation of this sequence to NPXXA resulted in a complete loss of agonist-induced receptor sequestration (129, 130). The conserved tyrosine was required for internalization of vasopressin receptor; the NPXXY sequence represented a general sequestration motif for seven transmembrane G-protein-linked receptors (120). However, there are certain exceptions to this rule, for example, the sequence motif YXXF (Tyr-X-Arg-Phe) in which the critical Tyr residue is included at the amino-terminal first position, and provides general consensus of YXXphi motifs for internalization of mannose-6-phosphate receptors, insulin-like growth factor receptors, and transferrin receptors (4, 124, 126).
3.3. Characteristic features and functions of YXXphi-type signal-sequence motifs
The tyrosine-based YXXphi signal motifs, which are found in the carboxyl-terminus domains of various membrane receptors and membrane proteins, have been extensively characterized, which participate in the internalization and trafficking processes. The YXXphi sorting signals direct the targeting of integral membrane proteins by interacting with the mu1, mu2, mu3, and mu4 subunits of adaptor protein complexes AP-1, AP-2, AP-3, and AP-4, respectively (4, 7, 76, 131–134). The YXXphi motif was initially identified as a clathrin-dependent sorting signal in the cytoplasmic tail of the transferrin receptor (83, 124, 135). In the YXXphi signal motifs, Y is the tyrosine residue, X is any amino acid residues, and phi is an amino acid residue with large bulky hydrophobic side chains. Many membrane receptors and membrane proteins contain YXXphi signal motifs, which are used more broadly than are the NPXY motifs. The YXXphi signal sequences have dual specificity, such as an endocytic functional motif and direct trafficking within the endosomal and/or secretory pathways (4, 7, 23, 136–138).
YXXphi motifs are present in the cytoplasmic domains of several transmembrane receptors and proteins such as transferrin and asialoglycoprotein receptors. The tetrapeptide sequence YXXphi is the minimal signal motif, which provides trafficking and sorting information to transmembrane receptor proteins (135). In this tetrapeptide sequence motif, the Y residue is critical for the signal. In most conditions, it cannot even be substituted for by other aromatic amino acid residues, since the phenolic hydroxyl group of the tyrosine is essential for generating the endocytic and trafficking signal. The two X residues are also considered to contribute to the specificity and potency of the signals for the endocytic and sorting events. The phi position in the tetrapeptide sequence is thought to accommodate a varying number of amino acid residues containing bulky hydrophobic side chains (139, 140). It is also thought that the identity of amino acid residues at the phi position confers the specificities and properties of the trafficking and sorting signals (139). In certain instances, the YXXphi signal motifs also direct lysosomal sorting and contain acidic residues at the X position (140). Furthermore, this tetrameric YXXphi motif may also involve a glycine residue preceding the tyrosine residue (YGXphi). Substituting alanine in place of glycine interferes with lysosomal targeting, but does not affect endocytosis (141). The YXXphi signal motifs with internalization specificity are usually located within 10–40 amino acid residues from the transmembrane spanning domain, but not at the carboxyl-terminus of the endocytotic receptors and other membrane proteins (142). Furthermore, the tetrameric YXXphi motifs for lysosomal targeting are usually located at six to eight amino acid residues from the transmembrane spanning domain at the carboxyl-terminal of the membrane receptors and proteins (4, 142).
Thus, it needs to be emphasized that the tetrameric YXXphi signal motifs participate in both receptor internalization and trafficking, as well as lysosomal targeting of the membrane receptor and proteins (140, 143, 144). Originally, it was suggested that YXXphi signals are recognized and interact with AP-1 and AP-2 complexes (132, 145). Thus, it is thought that most YXXphi signal motifs, characterized thus far function largely as internalization signals. The AP-2 interacts with tetrameric YXXphi signal motif and signifies its effect in the internalization process (142). It has been demonstrated that mutations in X residues, which substantially decrease the interaction with AP-2, have little effect on the internalization process (146). However, both tyrosine and phi residues are important in internalization and trafficking events (147). The previous findings have indicated that the presence of a glycine residue before the critical tyrosine residue of YXXphi signals helps to recognize the lysosomal membrane proteins (148). It has been suggested that the YSGL motif interacts with currently unknown intracellular proteins and governs constitutive internalization of chemokine-CXCR3 receptor protein (149). These results have been supported by the findings that YXXphi signals such as the YKKL motif within the carboxyl-terminal of other G-protein-coupled receptors such as the protease-activated receptor-1 (PAR-1), governs the constitutive internalization in a clathrin- and dynamin -dependent manner, although independently of the beta-arrestin system (76, 150).
3.4. Characteristic features and roles of dileucine-dependent signal-sequence motifs
Dileucine (LL)-based sequence motifs are known to have a broad range of functions in the endocytosis and trafficking of various membrane receptors and membrane proteins (4, 151–154). The dileucine motif is also recognized by AP complexes similar to tyrosine-based motifs such as NPXY and YXXphi signal motifs, which control the trafficking of various membrane proteins involving both endocytic and secretory pathways. Early studies using deletion analysis determined that the CD3-gamma chain of the T-cell antigen receptor confers on its ability to be rapidly internalized and delivered to the lysosomes (151). A segment of the receptor containing DKphiTLL sequence was found to be critical for both internalization and delivery of receptor to the lysosomes. Nevertheless, both leucine residues as well as other amino acid residues were dispensable for the function of DKphiTLL motif in endocytosis and delivery to lysosomes (151). It has also been shown that the deletion of LLHV and HLLPM amino acid sequences from the carboxyl-terminal regions of mannose-6-phosphate receptors impaired their trafficking from TGN to endosomes (153, 155).
The LL motif is characterized by four to seven amino acid residues, which precede dileucine residues. The dileucine residues usually are preceded by a polar residue and a negatively charged amino acid residue, which may be aspartic acid, glutamic acid, or phosphoserine. The LL sequence motif is usually context-dependent and it is characteristically surrounded by polar and/or charged amino acid residues. However, a particular sequence before dileucine residues is not absolutely required. Although dileucine motifs with acidic amino acid residues are constitutively active, those LL motifs that contain serine residue are activated by phosphorylation (152). In certain circumstances, some membrane receptors or membrane proteins contain more than one dileucine motif; one of the leucine residues can be substituted for tyrosine-based signal motifs. The LL signal motifs also have a broad range of functions and several transmembrane proteins contain dileucine-based sorting signals (4, 153, 156). In CD3 and mannose-6-phosphate receptors, dileucine signals correspond to a distinct class of LL signal that includes [DE] XXXL[LI] and DXXLL motifs. Like YXXphi motifs, the [DE]XXXL[LI] signals are critical in rapid internalization and targeting to endosomal-lysosomal compartments. These features of dileucine-type signal motifs suggest that they can be recognized at the plasma membrane as well as in the intracellular locations (24, 154, 157).
Interestingly, dileucine motifs usually function in the regulation of protein trafficking in both endocytosis and the secretory pathway (158, 159). It has also been shown that LL motifs in the cytoplasmic tail of GABA receptor can act at the level of the TGN to control the expression of receptor on the cell surface (159). The LL motif present in the amino-terminus region of glucose transporter 8 (GLUT 8) has been suggested to regulate intracellular sequestration in various cell systems (160–163). In those early studies, mutation of the dileucine motif showed increased expression of Glut 8 on the cell surface. The endocytosis of GLUT 8 by co-expression of the dominant-negative mutant of dynamin, enhanced the accumulation of transporter on the cell surface and GLUT 8 constantly recycled between distinct intracellular vesicles and the cell surface via the dynamin-dependent pathway (162). These results suggested that amino-terminus dileucine motifs in Glut 8 transporter constitute a docking site that is responsible for endocytic processes.
In fact, the dileucine- and tyrosine-based motifs have been indicated as endocytic signals for various membrane proteins and receptors (4, 160, 164). Both LL- and tyrosine-based signals interact with clathrin-associated adaptor protein complexes (APs), which help recruit membrane proteins into clathrin-coated pits and finally into clathrin-coated vesicles (79). During endocytosis, AP-2 plays a central function in the formation of clathrin-coated vesicles. However, the recognition of LL-based signals by adaptor proteins has still not been well characterized. The earlier studies have shown that certain dileucine motifs bind to mu subunits of APs, but that some [DE] XXXL[LI] motifs interact with the gamma and sigma1 subunits of AP-1 , as well as with delta and sigma 3 subunits of AP-3 (165–167). On the other hand, certain LL-based signals may be recognized by additional adaptor proteins such as a group of clathrin-binding monomeric adaptor proteins (4). Recent studies have demonstrated that the LL-based sorting motif in GLUT 8 transporter protein interacts with the beta2-adaptin subunit of AP-2 clathrin adaptor protein complexes (160). The recruitment of GLUT 8 within the endocytic machinery seem to be enhanced by direct interaction of the dileucine sequence motif with beta 2-adaptin, and the endocytosis could be the main step at which the GLUT 8 transporter is regulated. Similarly, a splice variant of high-density lipoprotein (HDL) receptor, which is known as a scavenger receptor II (SR-BII), is internalized via clathrin-containing endocytic vesicles (168). The SR-BII receptors contain the dileucine motif in the carboxyl-terminal domain, which acts as an endocytic signaling motif for internalization and sequestration of HDL receptor proteins into intracellular compartments.
The putative dileucine motif of SR-BII seems to be somewhat unusual, as it carries a serine residue after the dileucine repeat motif. This motif lacks the preceding acidic amino acid residues conforming to the well-defined consensus dileucine motifs [DE]XXXL(LI) and DXXLL (168). Also, numerous studies have found that the original variant of the HDL receptor, SR-BI, has a function in the endocytosis of HDL, but one involving a nonvesicular mechanism (169–172). Furthermore, dileucine-based signal motifs have been identified in various other vesicular transport membrane proteins such as the vesicular acetylcholine transporter (VAchT), vesicular GLUT 1 (VGLUT1), and tyrosinase (154, 173, 174). In particular, the dileucine-based motif has been shown to be sufficient for both internalization and synaptic vesicle targeting during VAchT trafficking and sorting (154).
The dileucine signal motif [D/E]XXXL[L/I] has been shown to function in the internalization of various membrane receptors and membrane protein molecules (4, 166). The acidic amino acid residues at position −4 and/or −5 from the first invariant leucine residue are considered to be critical in endosomal as well as lysosomal targeting. However, the second leucine residue can be substituted with an isoleucine residue without a significant loss in targeting function. The dileucine DXXLL signal motifs participate in the recycling of membrane proteins between the TGN and endosomes (4). In addition, DXXLI motifs have been implicated in sorting of mannose-6-phosphate receptors from the TGN to the endosomal system. The retrograde transport from endosomes to the TGN is regulated by acidic clusters involving different sequences and retromer complex (4, 175, 176). Nevertheless, some transmembrane proteins contain [D/E]XXXL[L/I] motif and/or YXXphi signal, both of which have been shown to function in internalization and subcellular trafficking events. Several [D/E]XXXL [L/I] signal motifs interact and/or bind to mu adaptins as well as beta subunits of APs (55, 165, 167, 177, 178). Previously, it was indicated that the complexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition sit (179, 180). Interestingly, a recent crystallographic study has demonstrated that the AP-2 adaptor core binds with the dileucine motif of CD4, and has suggested that the interaction between a dileucine-motif-containing-protein molecule and AP-2 complex is dynamic in nature (181). Interestingly, The [D/E]XXXL[L/I] motifs also bind to AP-1 and AP-3, and might be activated by posttranslational modification such as phosphorylation and/or activation of accessory adaptor protein molecules (166, 182, 183). Furthermore, the dileucine motifs have also been implicated to function in AP-2-mediated lysosomal targeting of membrane receptors (184).
The DXXLL-based sorting motifs present in the carboxyl-terminal tail of mannose-6-phosphate receptors are recognized by ADP-ribosylation-factor (ARF)-dependent clathrin adaptor proteins, usually referred to as GGAs (Golgi-localizing, gamma-adaptor homology domain, ARF-binding protein). The GGAs are a family of proteins involved in protein trafficking from the Golgi to the endosomes and/or lysosomes (4, 185–188). It has been documented that GGAs display a critical function in packaging of mannose-6-phosphate receptors into clathrin-coated vesicls in the TGN, which could be regulated by the phosphorylation state of GGAs (131, 186, 189). Thus, to ascertain the function of dileucine-based signals in relation to [D/E]XXXL [L/I] or DXXXL motifs, it should be emphasized that the former are recognized by heterotetrameric adaptor complexes such as AP-1, AP-2, AP-3, and AP-4, which later bind GGA adaptor proteins (4, 147, 148, 190–192).
3.5. Characteristic features and functions of other trafficking motifs
In addition to the motifs mentioned above, some other interesting short-sequence motifs play important roles in the internalization and trafficking processes of several membrane receptors and membrane proteins. Beta-amyloid precursor protein (beta-APP) contains a YENPTY sequence motif near the carboxyl-terminus, which directs its internalization (193, 194). After endocytosis, beta-APP is delivered to endosomes and a population of internalized molecules is recycled back to the cell surface. At the same time a fraction of the beta-APP molecules is also delivered to the lysosomes for eventual degradation. The internalization and intracellular sequestration of the newly synthesized glucose transporter 4 (GLUT4) is regulated by both the FQQI and SLL motifs (195). However, after the initial internalization process from the plasma membrane to the intracellular compartments, the SLL signal sequence does not seem to be involved in the intracellular routing of the GLUT4 into the specific cellular compartments. It has been reported that the amino-terminal FQQI motif is required for internalization of GLUT4; however, other studies have suggested that a combination of additional motifs is also required (196–200). On the other hand, several other studies have indicated that SLL motifs along with acidic cluster sequence in the carboxyl-terminal domain are required for both endocytosis and intracellular sequestration of GLUT4 in insulin-responsive storage compartment (201–203).
The ionotropic P2X4 receptor contains a non-canonical tyrosine-based motif YXXGL, which is important for internalization and sorting of this receptor protein (204, 205). Site directed mutagenesis experiments have revealed that mutation of YXXGL motif in the receptor caused reduction in the internalization and a dramatic increase in the expression of receptor on the cell surface (205). A five-amino acid residue motif, which is characterized by GTALL sequence in the carboxyl-terminus tail of leutinizing hormone (LH) receptor directs the ligand-receptor complexes from a degradative to recycling pathway (206). The GTALL motif also shows sequence homology to carboxyl-terminus tetrapeptide sequence motif DSLL, which has been suggested to participate in the internalization of β-adrenergic receptors (207). Using site-directed mutagenesis, later it was suggested that only the first two amino acid residues Gly and Thr of the GTALL motif seem to be important for the recycling of the internalized LH receptor (208). The VXXSL motif has also been shown to be necessary for the expression of voltage-gated K+ channels (209). The deletion of the VXXSL motif may contribute to genetic defects in expression of K+ channels. The site-directed mutagenesis studies have shown that the crucial motif sequence may constitute [V/L]XXSL and thus leucine residue can be replaced by valine. It was noted that inverting the sequence of VXXSL motif or replacing the identified residues with alanine can disrupt the surface expression of the channels (209).
Interestingly, the Gag proteins from human immunodeficiency virus type 1 (HIV-1) as well as other retroviruses contain short sequence motifs, which promote the interaction with multi-vesicular bodies (MVBs) and directly interface with the endocytic machinery. HIV-1 Gag contains IL signal, which directs the internalization of Gag into the intracellular compartments (210). The mutation of Ile and Leu residues to alanine blocked the internalization of Gag protein. Those previous studies indicated that trafficking of Gag occurs from the plasma membrane into the MVBs, and also interacts with endosomal proteins (210–212).
The transmembrane glycoprotein CD44 is largely localized in the basolateral domain of polarized epithelial cells and functions as a principal receptor for matrix components such as glycosaminoglycans (213–215). The basolateral localization of CD44 is modulated by its carboxyl-terminal domain and the truncation of the carboxyl tail results in the localization of this protein in the apical membrane (214, 216, 217). The carboxyl-terminal domain of CD44 is highly conserved and is required for its localization in the basolateral membrane (218). Site-directed mutagenesis experiments revealed that a minimum of 5 amino acid residue sequence, HLVNK, plays a critical role in the correct localization of CD44 in the plasma membrane (217). These authors suggested that HLVNK sequence motif plays a dual role either as a TGN sorting signal for the localization of CD44 in the basolateral membrane or as an internalization motif necessary for the transcytosis of CD44 in the apical membrane. Nevertheless, transcytosis usually requires the recruitment of membrane proteins into the coated pits; however, it has been previously suggested that CD44 does not seem to be internalized via coated pits either in the basolateral or apical sub-domains of the plasma membrane (219).
4. CONCLUSIONS AND PROSPECTIVE VIEW
In the recent years, much has been learned about the function and mechanisms of endocytic pathways responsible for the trafficking and molecular sorting of membrane receptors and their ligands into intracellular compartments. Substantial evidence supports the notion that the expression and cellular activity of membrane receptors is accomplished by the insertion of receptors into the plasma membrane, ligand binding, and the movement of ligand-receptor complexes through the endocytic pathway via clathrin-coated pits, clathrin-independent pathways, and/or caveolae into the subcellular compartments. Assessment of the stoichiometric distribution of the membrane receptors and proteins from the cell surface to the intracellular compartments has provided the definitive means of directly determining the dynamics of translocation, trafficking, and redistribution of biologically active receptor molecules. In this dynamic process, the membrane receptors with bound ligand or activated membrane proteins are usually rapidly internalized and delivered to the endosomes. Within the endosomes, the trafficking molecules are either directed to lysosomes, where the ligand-receptor complexes or membrane proteins are degraded, or recycled back to the plasma membrane, where the receptors can bind new ligand. It has been established that specific short-sequence motifs located in the intracellular cytoplasmic domain of various membrane receptors or membrane proteins govern and promote the internalization, trafficking, and/or sorting into the subcellular compartments.
This review delineates and provides the current concept of the function of various specific short-sequence signal motifs, which inherently control the endocytosis, trafficking, and sorting processes of many membrane receptors or membrane protein molecules. These processes include the interaction of ligand-receptor complexes, which can govern the rate at which the receptor traverses the intracellular compartments; the intrinsic regulation of receptor or protein, which must be adequately processed for appropriate routing in various subcellular compartments; and protein-protein interactions during the intracellular trafficking. As a result, the efficient internalization and subsequent intracellular sorting of cell-surface transmembrane receptors and other membrane proteins is mediated by specific signal motifs, which usually are present in the cytoplasmic domains of targeted molecules. The internalization and sorting signal motifs usually are short linear sequences of amino acids, which are recognized by various interacting proteins and direct itinerant routing protein molecules to facilitate endocytic and/or biosynthetic products to their intended final destinations. The internalization and sorting-signal motifs include tyrosine-based sequences such as NPXY, GDXY and YXXphi, dileucine-based motifs (LL and LL/I), as well as single amino acid-specific sequences most often located in the carboxyl-terminal domains of the targeting membrane receptors or membrane protein molecules.
The specific routing path followed by membrane receptors or membrane proteins may be cell- and tissue- specific. The cellular regulation and expression of targeted protein molecules involve intracellular trafficking and movement into subcellular compartments. However, we still do not fully understand the cellular pathways and routing of receptor trafficking, as well as the rate at which these proteins traverse the distance into the cell interior, which influences the specificity and sensitivity of the cells to ligand-receptor complexes. Similarly, the actions of specific receptor biosynthesis and subcellular assembly responsible for receptor trafficking and function remain to be determined. It is particularly important that we continue to define the function of short-sequence motifs in biosynthetic cargo assemblies and the regulatory actions of these assemblies in receptor endocytosis, trafficking, sequestration, desensitization/inactivation, down-regulation, and metabolic degradation of specific membrane receptors or membrane proteins. Indeed, the function of short-sequence signal motifs in protein-protein interactions among all the trafficking pathways of specific membrane receptors and proteins remains to be established.
ACKNOWLEDGMENTS
I gratefully acknowledge the contributions of numerous investigators in this field whose work I have not been able to include in this short review. I thank my wife Kamala Pandey for her assistance in the preparation of this manuscript. I also thank many individuals in my laboratory and their immense contribution is gratefully acknowledged. I offer special thanks to Dr. Bharat B. Aggarwal, Department of Experimental Therapeutics and Cytokine Research Laboratory at MD Anderson Cancer Center, Houston, TX and Dr. Susan L. Hamilton, Department of Molecular Physiology and Biophysics at Baylor College of Medicine, Houston, TX, who made their facilities available to us during our displacement due to Hurricane Katrina. The work in the author’s laboratory was supported by grants from the National Institutes of Health (HL 57531 and HL 62147).
Abbreviations
- AP
adaptor protein
- PTB domain
phosphotyrosine-binding domain
- PTI domain
phosphotyrosine-interacting domain
- GC-A
guanylyl cyclase-A
- NPRA
natriuretic peptide receptor-A
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- GC-B
guanylyl cyclase-B
- NPRB
natriuretic peptide receptor-B
- CNP
C-type natriuretic peptide, ARF, ADP (adenosine diphosphate) –ribosylation factor
- GGA
Golgi-localizing, gamma-adaptor homology domain
- ARF
binding protein
- TGN
trans-Golgi network
REFERENCES
- 1.Goldstein JL, Anderson RG, Brown MS. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- 2.Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135(2):341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonifacino JS, Dell'Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145(5):923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 5.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 6.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19(4):436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- 8.Sorkin A, Von Zastrow M. Signal transduction endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3(8):600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 9.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 10.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4(5):409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 11.Gagliardi M, Piddini E, Vincent JP. Endocytosis: a positive or a negative influence on Wnt signalling? Traffic. 2008;9(1):1–9. doi: 10.1111/j.1600-0854.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Zhao X, Puertollano R, Bonifacino JS, Eisenberg E, Greene LE. Adaptor and clathrin exchange at the plasma membrane and trans-Golgi network. Mol Biol Cell. 2003;14(2):516–528. doi: 10.1091/mbc.E02-06-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey KN, Nguyen HT, Sharma GD, Shi SJ, Kriegel AM. Ligand-regulated internalization, trafficking, and down-regulation of guanylyl cyclase/atrial natriuretic peptide receptor-A in human embryonic kidney 293 cells. J Biol Chem. 2002;277(7):4618–4627. doi: 10.1074/jbc.M106436200. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Wang L, Zheng J, Anderson JL, Toews ML. Identification of distinct carboxyl-terminal domains mediating internalization and down-regulation of the hamster alpha(1B)- adrenergic receptor. Mol Pharmacol. 2000;57(4):687–694. doi: 10.1124/mol.57.4.687. [DOI] [PubMed] [Google Scholar]
- 15.Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11(3):165–180. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- 16.Marshall S. Degradative processing of internalized insulin in isolated adipocytes. J Biol Chem. 1985;260(25):13517–13523. [PubMed] [Google Scholar]
- 17.McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16(4):379–391. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37(3):789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- 19.Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6(2):157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 20.Sorkina T, Huang F, Beguinot L, Sorkin A. Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalization of epidermal growth factor receptor. J Biol Chem. 2002;277(30):27433–27441. doi: 10.1074/jbc.M201595200. [DOI] [PubMed] [Google Scholar]
- 21.Brothers SP, Janovick JA, Maya-Nunez G, Cornea A, Han XB, Conn PM. Conserved mammalian gonadotropin-releasing hormone receptor carboxyl terminal amino acids regulate ligand binding, effector coupling and internalization. Mol Cell Endocrinol. 2002;190(1–2):19. doi: 10.1016/s0303-7207(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 22.Kraft K, Olbrich H, Majoul I, Mack M, Proudfoot A, Oppermann M. Characterization of sequence determinants within the carboxyl-terminal domain of chemokine receptor CCR5 that regulate signaling and receptor internalization. J Biol Chem. 2001;276(37):34408–34418. doi: 10.1074/jbc.M102782200. [DOI] [PubMed] [Google Scholar]
- 23.Stolt PC, Bock HH. Modulation of lipoprotein receptor functions by intracellular adaptor proteins. Cell Signal. 2006;18(10):1560–1571. doi: 10.1016/j.cellsig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Miranda KC, Khromykh T, Christy P, Le TL, Gottardi CJ, Yap AS, Stow JL, Teasdale RD. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem. 2001;276(25):22565–22572. doi: 10.1074/jbc.M101907200. [DOI] [PubMed] [Google Scholar]
- 25.Pandey KN. Biology of natriuretic peptides and their receptors. Peptides. 2005;26(6):901–932. doi: 10.1016/j.peptides.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Dupre DJ, Hebert TE. Biosynthesis and trafficking of seven transmembrane receptor signalling complexes. Cell Signal. 2006;18(10):1549–1559. doi: 10.1016/j.cellsig.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Owen DJ, Vallis Y, Pearse BM, McMahon HT, Evans PR. The structure and function of the beta 2-adaptin appendage domain. Embo J. 2000;19(16):4216–4227. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takei K, Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol. 2001;11(9):385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhausen T. Single-handed recognition of a sorting traffic motif by the GGA proteins. Nat Struct Biol. 2002;9(4):241–244. doi: 10.1038/nsb0402-241. [DOI] [PubMed] [Google Scholar]
- 30.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436(7047):78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 31.Marchese A, Chen C, Kim YM, Benovic JL. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci. 2003;28(7):369–376. doi: 10.1016/S0968-0004(03)00134-8. [DOI] [PubMed] [Google Scholar]
- 32.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122(5):735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Burwen SJ, Barker ME, Goldman IS, Hradek GT, Raper SE, Jones AL. Transport of epidermal growth factor by rat liver: evidence for a nonlysosomal pathway. J Cell Biol. 1984;99(4 Pt 1):1259–1265. doi: 10.1083/jcb.99.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renston RH, Jones AL, Christiansen WD, Hradek GT, Underdown BJ. Evidence for a vesicular transport mechanism in hepatocytes for biliary secretion of immunoglobulin A. Science. 1980;208(4449):1276–1278. doi: 10.1126/science.7375938. [DOI] [PubMed] [Google Scholar]
- 35.Lamb JE, Ray F, Ward JH, Kushner JP, Kaplan J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J Biol Chem. 1983;258(14):8751–8758. [PubMed] [Google Scholar]
- 36.Tietze C, Schlesinger P, Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1(3):161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- 38.Antonescu CN, Foti M, Sauvonnet N, Klip A. Ready, set, internalize: mechanisms and regulation of GLUT4 endocytosis. Biosci Rep. 2009;29(1):1–11. doi: 10.1042/BSR20080105. [DOI] [PubMed] [Google Scholar]
- 39.Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19(4):417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163(2):203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauvonnet N, Dujeancourt A, Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of gammac cytokine receptor. J Cell Biol. 2005;168(1):155–163. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foti M, Porcheron G, Fournier M, Maeder C, Carpentier JL. The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2007;104(4):1242–1247. doi: 10.1073/pnas.0610523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8(3):185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 44.Richter T, Floetenmeyer M, Ferguson C, Galea J, Goh J, Lindsay MR, Morgan GP, Marsh BJ, Parton RG. High-resolution 3D quantitative analysis of caveolar ultrastructure and caveola-cytoskeleton interactions. Traffic. 2008;9(6):893–909. doi: 10.1111/j.1600-0854.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- 45.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5(5):410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 46.Gines S, Ciruela F, Burgueno J, Casado V, Canela EI, Mallol J, Lluis C, Franco R. Involvement of caveolin in ligand-induced recruitment and internalization of A(1) adenosine receptor and adenosine deaminase in an epithelial cell line. Mol Pharmacol. 2001;59(5):1314–1323. [PubMed] [Google Scholar]
- 47.Gonzalez MI, Krizman-Genda E, Robinson MB. Caveolin-1 regulates the delivery and endocytosis of the glutamate transporter, excitatory amino acid carrier 1. J Biol Chem. 2007;282(41):29855–29865. doi: 10.1074/jbc.M704738200. [DOI] [PubMed] [Google Scholar]
- 48.Sharma DK, Brown JC, Cheng Z, Holicky EL, Marks DL, Pagano RE. The glycosphingolipid, lactosylceramide, regulates beta1-integrin clustering and endocytosis. Cancer Res. 2005;65(18):8233–8241. doi: 10.1158/0008-5472.CAN-05-0803. [DOI] [PubMed] [Google Scholar]
- 49.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, P. Fiore PDi, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102(8):2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choudhury A, Marks DL, Proctor KM, Gould GW, Pagano RE. Regulation of caveolar endocytosis by syntaxin 6-dependent delivery of membrane components to the cell surface. Nat Cell Biol. 2006;8(4):317–328. doi: 10.1038/ncb1380. [DOI] [PubMed] [Google Scholar]
- 51.Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141(1):85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141(1):101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279(19):20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 54.Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274(3):1185–1188. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 55.Yao D, Ehrlich M, Henis YI, Leof EB. Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit. Mol Biol Cell. 2002;13(11):4001–4012. doi: 10.1091/mbc.02-07-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajasekaran S, Balla S, Huang CH, Thapar V, Gryk M, Maciejewski M, Schiller M. High-performance exact algorithms for motif search. J Clin Monit Comput. 2005;19(4–5):319–328. doi: 10.1007/s10877-005-0677-y. [DOI] [PubMed] [Google Scholar]
- 57.Pandey KN, Nguyen HT, Garg R, Khurana ML, Fink J. Internalization and trafficking of guanylyl (guanylate) cyclase/natriuretic peptide receptor A is regulated by an acidic tyrosine-based cytoplasmic motif GDAY. Biochem J. 2005;388(Pt 1):103–113. doi: 10.1042/BJ20041250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265(6):3116–3123. [PubMed] [Google Scholar]
- 59.Maginnis MS, Mainou BA, Derdowski A, Johnson EM, Zent R, Dermody TS. NPXY motifs in the beta1 integrin cytoplasmic tail are required for functional reovirus entry. J Virol. 2008;82(7):3181–3191. doi: 10.1128/JVI.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol. 2005;345(1):1–20. doi: 10.1016/j.jmb.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 61.Singh S, D'Mello V, van Bergen en Henegouwen P, Birge RB. A NPxY-independent beta5 integrin activation signal regulates phagocytosis of apoptotic cells. Biochem Biophys Res Commun. 2007;364(3):540–548. doi: 10.1016/j.bbrc.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis CG, Lehrman MA, Russell DW, Anderson RG, Brown MS, Goldstein JL. The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell. 1986;45(1):15–24. doi: 10.1016/0092-8674(86)90533-7. [DOI] [PubMed] [Google Scholar]
- 63.Al-Kateb H, Bahring S, Hoffmann K, Strauch K, Busjahn A, Nurnberg G, Jouma M, Bautz EK, Dresel HA, Luft FC. Mutation in the ARH gene and a chromosome 13q locus influence cholesterol levels in a new form of digenic-recessive familial hypercholesterolemia. Circ Res. 2002;90(9):951–958. doi: 10.1161/01.res.0000018002.43041.08. [DOI] [PubMed] [Google Scholar]
- 64.Norman D, Sun XM, Bourbon M, Knight BL, Naoumova RP, Soutar AK. Characterization of a novel cellular defect in patients with phenotypic homozygous familial hypercholesterolemia. J Clin Invest. 1999;104(5):619–628. doi: 10.1172/JCI6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He G, Gupta S, Yi M, Michaely P, Hobbs HH, Cohen JC. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J Biol Chem. 2002;277(46):44044–44049. doi: 10.1074/jbc.M208539200. [DOI] [PubMed] [Google Scholar]
- 66.Keyel PA, Mishra SK, Roth R, Heuser JE, Watkins SC, Traub LM. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol Biol Cell. 2006;17(10):4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. Embo J. 2002;21(18):4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris SM, Tallquist MD, Rock CO, Cooper JA. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. Embo J. 2002;21(7):1555–1564. doi: 10.1093/emboj/21.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mishra SK, Keyel PA, Edeling MA, Dupin AL, Owen DJ, Traub LM. Functional dissection of an AP-2 beta2 appendage-binding sequence within the autosomal recessive hypercholesterolemia protein. J Biol Chem. 2005;280(19):19270–19280. doi: 10.1074/jbc.M501029200. [DOI] [PubMed] [Google Scholar]
- 70.Stolt PC, Jeon H, Song HK, Herz J, Eck MJ, Blacklow SC. Origins of peptide selectivity and phosphoinositide binding revealed by structures of disabled-1 PTB domain complexes. Structure. 2003;11(5):569–579. doi: 10.1016/s0969-2126(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 71.Yun M, Keshvara L, Park CG, Zhang YM, Dickerson JB, Zheng J, Rock CO, Curran T, Park HW. Crystal structures of the Dab homology domains of mouse disabled 1 and 2. J Biol Chem. 2003;278(38):36572–36581. doi: 10.1074/jbc.M304384200. [DOI] [PubMed] [Google Scholar]
- 72.Yim YI, Scarselletta S, Zang F, Wu X, Lee DW, Kang YS, Eisenberg E, Greene LE. Exchange of clathrin, AP2 and epsin on clathrin-coated pits in permeabilized tissue culture cells. J Cell Sci. 2005;118(Pt 11):2405–2413. doi: 10.1242/jcs.02356. [DOI] [PubMed] [Google Scholar]
- 73.Morris SM, Cooper JA. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2001;2(2):111–123. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- 74.Boll W, Rapoport I, Brunner C, Modis Y, Prehn S, Kirchhausen T. The mu2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and YppO sorting signals at distinct sites. Traffic. 2002;3(8):590–600. doi: 10.1034/j.1600-0854.2002.30808.x. [DOI] [PubMed] [Google Scholar]
- 75.Oleinikov AV, Zhao J, Makker SP. Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem J. 2000;347(Pt 3):613–621. [PMC free article] [PubMed] [Google Scholar]
- 76.Paing MM, Johnston CA, Siderovski DP, Trejo J. Clathrin adaptor AP2 regulates thrombin receptor constitutive internalization and endothelial cell resensitization. Mol Cell Biol. 2006;26(8):3231–3242. doi: 10.1128/MCB.26.8.3231-3242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nesterov A, Carter RE, Sorkina T, Gill GN, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 1999;18(9):2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124(4):729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 80.Anderson RG, Brown MS, Goldstein JL. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977;10(3):351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- 81.Roth TF, Cutting JA, Atlas SB. Protein transport: a selective membrane mechanism. J Supramol Struct. 1976;4(4):527–548. doi: 10.1002/jss.400040413. [DOI] [PubMed] [Google Scholar]
- 82.Kibbey RG, Rizo J, Gierasch LM, Anderson RG. The LDL receptor clustering motif interacts with the clathrin terminal domain in a reverse turn conformation. J Cell Biol. 1998;142(1):59–67. doi: 10.1083/jcb.142.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collawn JF, Kuhn LA, Liu LF, Tainer JA, Trowbridge IS. Transplanted LDL and mannose-6-phosphate receptor internalization signals promote high-efficiency endocytosis of the transferrin receptor. Embo J. 1991;10(11):3247–3253. doi: 10.1002/j.1460-2075.1991.tb04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274(27):18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- 85.Musacchio A, Smith CJ, Roseman AM, Harrison SC, Kirchhausen T, Pearse BM. Functional organization of clathrin in coats: combining electron cryomicroscopy and X-ray crystallography. Mol Cell. 1999;3(6):761–770. doi: 10.1016/s1097-2765(01)80008-3. [DOI] [PubMed] [Google Scholar]
- 86.Burden JJ, Sun XM, Garcia AB, Soutar AK. Sorting motifs in the intracellular domain of the low density lipoprotein receptor interact with a novel domain of sorting nexin-17. J Biol Chem. 2004;279(16):16237–16245. doi: 10.1074/jbc.M313689200. [DOI] [PubMed] [Google Scholar]
- 87.Stockinger W, Sailler B, Strasser V, Recheis B, Fasching D, Kahr L, Schneider WJ, Nimpf J. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. Embo J. 2002;21(16):4259–4267. doi: 10.1093/emboj/cdf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Howell BW, Lanier LM, Frank R, Gertler FB, Cooper JA. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol. 1999;19(7):5179–5188. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isakoff SJ, Yu YP, Su YC, Blaikie P, Yajnik V, Rose E, Weidner KM, Sachs M, Margolis B, Skolnik EY. Interaction between the phosphotyrosine binding domain of Shc and the insulin receptor is required for Shc phosphorylation by insulin in vivo. J Biol Chem. 1996;271(8):3959–3962. doi: 10.1074/jbc.271.8.3959. [DOI] [PubMed] [Google Scholar]
- 90.Laminet AA, Apell G, Conroy L, Kavanaugh WM. Affinity, specificity, and kinetics of the interaction of the SHC phosphotyrosine binding domain with asparagine-X-X-phosphotyrosine motifs of growth factor receptors. J Biol Chem. 1996;271(1):264–269. doi: 10.1074/jbc.271.1.264. [DOI] [PubMed] [Google Scholar]
- 91.Sakaguchi K, Okabayashi Y, Kasuga M. Shc mediates ligand-induced internalization of epidermal growth factor receptors. Biochem Biophys Res Commun. 2001;282(5):1154–1160. doi: 10.1006/bbrc.2001.4680. [DOI] [PubMed] [Google Scholar]
- 92.Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416(6877):183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- 93.Waterman H, Katz M, Rubin C, Shtiegman K, Lavi S, Elson A, Jovin T, Yarden Y. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J. 2002;21(3):303–313. doi: 10.1093/emboj/21.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Homayouni R, Rice DS, Sheldon M, Curran T. Disabled-1 binds to the cytoplasmic domain of amyloid precursor-like protein 1. J Neurosci. 1999;19(17):7507–7515. doi: 10.1523/JNEUROSCI.19-17-07507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Howell BW, Gertler FB, Cooper JA. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 1997;16(1):121–132. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sheldon M, Rice DS, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell BW, Cooper JA, Goldowitz D, Curran T. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 1997;389(6652):730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- 97.Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273(50):33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- 98.Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- 99.Mishra SK, Watkins SC, Traub LM. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc Natl Acad Sci U S A. 2002;99(25):16099–16104. doi: 10.1073/pnas.252630799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275(33):25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 101.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 102.Maurer ME, Cooper JA. Endocytosis of megalin by visceral endoderm cells requires the Dab2 adaptor protein. J Cell Sci. 2005;118(Pt 22):5345–5355. doi: 10.1242/jcs.02650. [DOI] [PubMed] [Google Scholar]
- 103.Verroust PJ, Kozyraki R. The roles of cubilin and megalin, two multiligand receptors, in proximal tubule function: possible implication in the progression of renal disease. Curr Opin Nephrol Hypertens. 2001;10(1):33–38. doi: 10.1097/00041552-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 104.Yang DH, Smith ER, Roland IH, Sheng Z, He J, Martin WD, Hamilton TC, Lambeth JD, Xu XX. Disabled-2 is essential for endodermal cell positioning and structure formation during mouse embryogenesis. Dev Biol. 2002;251(1):27–44. doi: 10.1006/dbio.2002.0810. [DOI] [PubMed] [Google Scholar]
- 105.Jiang X, Huang F, Marusyk A, Sorkin A. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol Biol Cell. 2003;14(3):858–870. doi: 10.1091/mbc.E02-08-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17(5):509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 107.Liddington RC, Ginsberg MH. Integrin activation takes shape. J Cell Biol. 2002;158(5):833–839. doi: 10.1083/jcb.200206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pandey KN. Emerging roles of antriuretic peptides and their receptors in pathophysiology of hypertension and cardiovascular regulation. J. Am. Soc. Hypert. 2008;2(4):210–226. doi: 10.1016/j.jash.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nussenzveig DR, Heinflink M, Gershengorn MC. Agonist-stimulated internalization of the thyrotropin-releasing hormone receptor is dependent on two domains in the receptor carboxyl terminus. J Biol Chem. 1993;268(4):2389–2392. [PubMed] [Google Scholar]
- 110.Pandey KN, Kumar R, Li M, Nguyen H. Functional domains and expression of truncated atrial natriuretic peptide receptor-A: the carboxyl-terminal regions direct the receptor internalization and sequestration in COS-7 cells. Mol Pharmacol. 2000;57(2):259–267. [PubMed] [Google Scholar]
- 111.Rathinavelu A, Isom GE. Differential internalization and processing of atrial-natriuretic-factor B and C receptor in PC12 cells. Biochem J. 1991;276(Pt 2):493–497. doi: 10.1042/bj2760493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pandey KN, Inagami T, Misono KS. Atrial natriuretic factor receptor on cultured Leydig tumor cells: ligand binding and photoaffinity labeling. Biochemistry. 1986;25(26):8467–8472. doi: 10.1021/bi00374a022. [DOI] [PubMed] [Google Scholar]
- 113.Pandey KN. Stoichiometric analysis of internalization, recycling, and redistribution of photoaffinity-labeled guanylate cyclase/atrial natriuretic factor receptors in cultured murine Leydig tumor cells. J Biol Chem. 1993;268(6):4382–4390. [PubMed] [Google Scholar]
- 114.Pandey KN. Dynamics of internalization and sequestration of guanylyl cyclase/atrial natriuretic peptide receptor-A. Can J Physiol Pharmacol. 2001;79(8):631–639. [PubMed] [Google Scholar]
- 115.Pandey KN. Intracellular trafficking and metabolic turnover of ligand-bound guanylyl cyclase/atrial natriuretic peptide receptor-A into subcellular compartments. Mol Cell Biochem. 2002;230(1–2):61–72. [PubMed] [Google Scholar]
- 116.Pandey KN. Internalization and trafficking of guanylyl cyclase/natriuretic peptide receptor-A. Peptides. 2005;26(6):985–1000. doi: 10.1016/j.peptides.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 117.Brackmann M, Schuchmann S, Anand R, Braunewell KH. Neuronal Ca2+ sensor protein VILIP-1 affects cGMP signalling of guanylyl cyclase B by regulating clathrin-dependent receptor recycling in hippocampal neurons. J Cell Sci. 2005;118(Pt 11):2495–2505. doi: 10.1242/jcs.02376. [DOI] [PubMed] [Google Scholar]
- 118.Somanna NK, Arise KK, Pandey KN. Analysis of natriuretic peptide receptor A Internalization by ribonucleic acid interference. J Am Investig Med. 2007;55:S262. [Google Scholar]
- 119.Chen Z, Dupre DJ, Gouill CLe, Rola-Pleszczynski M, Stankova J. Agonist-induced internalization of the platelet-activating factor receptor is dependent on arrestins but independent of G-protein activation. Role of the C terminus and the (D/N)PXXY motif. J Biol Chem. 2002;277(9):7356–7362. doi: 10.1074/jbc.M110058200. [DOI] [PubMed] [Google Scholar]
- 120.Bouley R, Sun TX, Chenard M, McLaughlin M, McKee M, Lin HY, Brown D, Ausiello DA. Functional role of the NPxxY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells. Am J Physiol Cell Physiol. 2003;285(4):C750–C762. doi: 10.1152/ajpcell.00477.2002. [DOI] [PubMed] [Google Scholar]
- 121.Li Y, Marzolo MP, Kerkhof Pvan, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275(22):17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 122.Thies RS, Webster NJ, McClain DA. A domain of the insulin receptor required for endocytosis in rat fibroblasts. J Biol Chem. 1990;265(17):10132–10137. [PubMed] [Google Scholar]
- 123.Lazarovits J, Roth M. A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell. 1988;53(5):743–752. doi: 10.1016/0092-8674(88)90092-x. [DOI] [PubMed] [Google Scholar]
- 124.Collawn JF, Stangel M, Kuhn LA, Esekogwu V, Jing SQ, Trowbridge IS, Tainer JA. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- 125.Rajagopalan M, Neidigh JL, McClain DA. Amino acid sequences Gly-Pro-Leu-Tyr and Asn-Pro-Glu-Tyr in the submembranous domain of the insulin receptor are required for normal endocytosis. J Biol Chem. 1991;266(34):23068–23073. [PubMed] [Google Scholar]
- 126.Ktistakis NT, Thomas D, Roth MG. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990;111(4):1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bansal A, Gierasch LM. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991;67(6):1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- 128.Eberle W, Sander C, Klaus W, Schmidt B, von Figura K, Peters C. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a beta turn. Cell. 1991;67(6):1203–1209. doi: 10.1016/0092-8674(91)90296-b. [DOI] [PubMed] [Google Scholar]
- 129.Gabilondo AM, Krasel C, Lohse MJ. Mutations of Tyr326 in the beta 2-adrenoceptor disrupt multiple receptor functions. Eur J Pharmacol. 1996;307(2):243–250. doi: 10.1016/0014-2999(96)00247-6. [DOI] [PubMed] [Google Scholar]
- 130.Barak LS, Tiberi M, Freedman NJ, Kwatra MM, Lefkowitz RJ, Caron MG. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated beta 2-adrenergic receptor sequestration. J Biol Chem. 1994;269(4):2790–2795. [PubMed] [Google Scholar]
- 131.Hirst J, Lindsay MR, Robinson MS. GGAs: roles of the different domains and comparison with AP-1 and clathrin. Mol Biol Cell. 2001;12(11):3573–3588. doi: 10.1091/mbc.12.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269(5232):1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 133.Sorkin A, Di Fiore PP, Carpenter G. The carboxyl terminus of epidermal growth factor receptor/erbB-2 chimerae is internalization impaired. Oncogene. 1993;8(11):3021–3028. [PubMed] [Google Scholar]
- 134.Parent JL, Labrecque P, Driss Rochdi M, Benovic JL. Role of the differentially spliced carboxyl terminus in thromboxane A2 receptor trafficking: identification of a distinct motif for tonic internalization. J Biol Chem. 2001;276(10):7079–7085. doi: 10.1074/jbc.M009375200. [DOI] [PubMed] [Google Scholar]
- 135.Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 136.Marks MS, Roche PA, Donselaar Evan, Woodruff L, Peters PJ, Bonifacino JS. A lysosomal targeting signal in the cytoplasmic tail of the beta chain directs HLA-DM to MHC class II compartments. J Cell Biol. 1995;131(2):351–369. doi: 10.1083/jcb.131.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kirchhausen T, Pines J, Toldo L, Lafont F. Membranes and sorting. Membrane permeability. Curr Opin Cell Biol. 1997;9(4):473. doi: 10.1016/s0955-0674(97)80020-8. [DOI] [PubMed] [Google Scholar]
- 138.Marks MS, Ohno H, Kirchnausen T, Bonracino JS. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7(3):124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 139.Gough NR, Zweifel ME, Martinez-Augustin O, Aguilar RC, Bonifacino JS, Fambrough DM. Utilization of the indirect lysosome targeting pathway by lysosome-associated membrane proteins (LAMPs) is influenced largely by the C-terminal residue of their GYXXphi targeting signals. J Cell Sci. 1999;112(Pt 23):4257–4269. doi: 10.1242/jcs.112.23.4257. [DOI] [PubMed] [Google Scholar]
- 140.Rous BA, Reaves BJ, Ihrke G, Briggs JA, Gray SR, Stephens DJ, Banting G, Luzio JP. Role of adaptor complex AP-3 in targeting wild-type and mutated CD63 to lysosomes. Mol Biol Cell. 2002;13(3):1071–1082. doi: 10.1091/mbc.01-08-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harter C, Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992;117(2):311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rohrer J, Schweizer A, Russell D, Kornfeld S. The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J Cell Biol. 1996;132(4):565–576. doi: 10.1083/jcb.132.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Madrid R, Maout SLe, Barrault MB, Janvier K, Benichou S, Merot J. Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. Embo J. 2001;20(24):7008–7021. doi: 10.1093/emboj/20.24.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Williams MA, Fukuda M. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol. 1990;111(3):955–966. doi: 10.1083/jcb.111.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. Embo J. 1996;15(21):5789–5795. [PMC free article] [PubMed] [Google Scholar]
- 146.Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271(46):29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- 147.Aguilar RC, Boehm M, Gorshkova I, Crouch RJ, Tomita K, Saito T, Ohno H, Bonifacino JS. Signal-binding specificity of the mu4 subunit of the adaptor protein complex AP-4. J Biol Chem. 2001;276(16):13145–13152. doi: 10.1074/jbc.M010591200. [DOI] [PubMed] [Google Scholar]
- 148.Boehm M, Bonifacino JS. Adaptins: the final recount. Mol Biol Cell. 2001;12(10):2907–2920. doi: 10.1091/mbc.12.10.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meiser A, Mueller A, Wise EL, McDonagh EM, Petit SJ, Saran N, Clark PC, Williams TJ, Pease JE. The chemokine receptor CXCR3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. J Immunol. 2008;180(10):6713–6724. doi: 10.4049/jimmunol.180.10.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Paing MM, Temple BR, Trejo J. A tyrosine-based sorting signal regulates intracellular trafficking of protease-activated receptor-1: multiple regulatory mechanisms for agonist-induced G protein-coupled receptor internalization. J Biol Chem. 2004;279(21):21938–21947. doi: 10.1074/jbc.M401672200. [DOI] [PubMed] [Google Scholar]
- 151.Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69(7):1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 152.Geisler C, Dietrich J, Nielsen BL, Kastrup J, Lauritsen JP, Odum N, Christensen MD. Leucine-based receptor sorting motifs are dependent on the spacing relative to the plasma membrane. J Biol Chem. 1998;273(33):21316–21323. doi: 10.1074/jbc.273.33.21316. [DOI] [PubMed] [Google Scholar]
- 153.Johnson KF, Kornfeld S. A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function. J Biol Chem. 1992;267(24):17110–17115. [PubMed] [Google Scholar]
- 154.Colgan L, Liu H, Huang SY, Liu YJ. Dileucine motif is sufficient for internalization and synaptic vesicle targeting of vesicular acetylcholine transporter. Traffic. 2007;8(5):512–522. doi: 10.1111/j.1600-0854.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- 155.Johnson KF, Kornfeld S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J Cell Biol. 1992;119(2):249–257. doi: 10.1083/jcb.119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen HJ, Yuan J, Lobel P. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J Biol Chem. 1997;272(11):7003–7012. doi: 10.1074/jbc.272.11.7003. [DOI] [PubMed] [Google Scholar]
- 157.Matter K, Yamamoto EM, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126(4):991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:559–609. doi: 10.1146/annurev.pharmtox.44.101802.121558. [DOI] [PubMed] [Google Scholar]
- 159.Restituito S, Couve A, Bawagan H, Jourdain S, Pangalos MN, Calver AR, Freeman KB, Moss SJ. Multiple motifs regulate the trafficking of GABA(B) receptors at distinct checkpoints within the secretory pathway. Mol Cell Neurosci. 2005;28(4):747–756. doi: 10.1016/j.mcn.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 160.Schmidt U, Briese S, Leicht K, Schurmann A, Joost HG, Al-Hasani H. Endocytosis of the glucose transporter GLUT8 is mediated by interaction of a dileucine motif with the beta2-adaptin subunit of the AP-2 adaptor complex. J Cell Sci. 2006;119(Pt 11):2321–2331. doi: 10.1242/jcs.02943. [DOI] [PubMed] [Google Scholar]
- 161.Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem. 2000;275(7):4607–4612. doi: 10.1074/jbc.275.7.4607. [DOI] [PubMed] [Google Scholar]
- 162.Lisinski I, Schurmann A, Joost HG, Cushman SW, Al-Hasani H. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem J. 2001;358(Pt 2):517–522. doi: 10.1042/0264-6021:3580517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shin BC, McKnight RA, Devaskar SU. Glucose transporter GLUT8 translocation in neurons is not insulin responsive. J Neurosci Res. 2004;75(6):835–844. doi: 10.1002/jnr.20054. [DOI] [PubMed] [Google Scholar]
- 164.Schmidt SL, Correa PL, Tolentino JC, Manhaes AC, Felix RM, Azevedo JC, Barbirato GB, Mendes MH, Boechat Y, Cabral H, Schmidt GJ, Dohmann HF, Mesquita CT. Value of combining activated brain FDG-PET and cardiac MIBG for the differential diagnosis of dementia: differentiation of dementia with Lewy bodies and Alzheimer disease when the diagnoses based on clinical and neuroimaging criteria are difficult. Clin Nucl Med. 2008;33(6):398–401. doi: 10.1097/RLU.0b013e3181708244. [DOI] [PubMed] [Google Scholar]
- 165.Hofmann MW, Honing S, Rodionov D, Dobberstein B, von Figura K, Bakke O. The leucine-based sorting motifs in the cytoplasmic domain of the invariant chain are recognized by the clathrin adaptors AP1 and AP2 and their medium chains. J Biol Chem. 1999;274(51):36153–36158. doi: 10.1074/jbc.274.51.36153. [DOI] [PubMed] [Google Scholar]
- 166.Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol. 2003;163(6):1281–1290. doi: 10.1083/jcb.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rodionov DG, Bakke O. Medium chains of adaptor complexes AP-1 and AP-2 recognize leucine-based sorting signals from the invariant chain. J Biol Chem. 1998;273(11):6005–6008. doi: 10.1074/jbc.273.11.6005. [DOI] [PubMed] [Google Scholar]
- 168.Eckhardt ER, Cai L, Shetty S, Zhao Z, Szanto A, Webb NR, Van der Westhuyzen DR. High density lipoprotein endocytosis by scavenger receptor SR-BII is clathrin-dependent and requires a carboxyl-terminal dileucine motif. J Biol Chem. 2006;281(7):4348–4353. doi: 10.1074/jbc.M513154200. [DOI] [PubMed] [Google Scholar]
- 169.Nieland TJ, Ehrlich M, Krieger M, Kirchhausen T. Endocytosis is not required for the selective lipid uptake mediated by murine SR-BI. Biochim Biophys Acta. 2005;1734(1):44–51. doi: 10.1016/j.bbalip.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 170.Silver DL, Wang N, Xiao X, Tall AR. High density lipoprotein (HDL) particle uptake mediated by scavenger receptor class B type 1 results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J Biol Chem. 2001;276(27):25287–25293. doi: 10.1074/jbc.M101726200. [DOI] [PubMed] [Google Scholar]
- 171.Wustner D. Mathematical analysis of hepatic high density lipoprotein transport based on quantitative imaging data. J Biol Chem. 2005;280(8):6766–6779. doi: 10.1074/jbc.M413238200. [DOI] [PubMed] [Google Scholar]
- 172.Wustner D, Mondal M, Huang A, Maxfield FR. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J Lipid Res. 2004;45(3):427–437. doi: 10.1194/jlr.M300440-JLR200. [DOI] [PubMed] [Google Scholar]
- 173.Gois SDe, Jeanclos E, Morris M, Grewal S, Varoqui H, Erickson JD. Identification of endophilins 1 and 3 as selective binding partners for VGLUT1 and their co-localization in neocortical glutamatergic synapses: implications for vesicular glutamate transporter trafficking and excitatory vesicle formation. Cell Mol Neurobiol. 2006;26(4–6):679–693. doi: 10.1007/s10571-006-9054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51(1):71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 175.Crump CM, Xiang Y, Thomas L, Gu F, Austin C, Tooze SA, Thomas G. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 2001;20(9):2191–2201. doi: 10.1093/emboj/20.9.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94(2):205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 177.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109(4):523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- 178.Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. Embo J. 1998;17(8):2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chaudhuri R, Lindwasser OW, Smith WJ, Hurley JH, Bonifacino JS. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J Virol. 2007;81(8):3877–3890. doi: 10.1128/JVI.02725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Doray B, Lee I, Knisely J, Bu G, Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell. 2007;18(5):1887–1896. doi: 10.1091/mbc.E07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kelly BT, McCoy AJ, Spate K, Miller SE, Evans PR, Honing S, Owen DJ. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456(7224):976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Conner SD, Schmid SL. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol. 2002;156(5):921–929. doi: 10.1083/jcb.200108123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ricotta D, Conner SD, Schmid SL, von Figura K, Honing S. Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol. 2002;156(5):791–795. doi: 10.1083/jcb.200111068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang F, Jiang X, Sorkin A. Tyrosine phosphorylation of the beta2 subunit of clathrin adaptor complex AP-2 reveals the role of a di-leucine motif in the epidermal growth factor receptor trafficking. J Biol Chem. 2003;278(44):43411–43417. doi: 10.1074/jbc.M306072200. [DOI] [PubMed] [Google Scholar]
- 185.Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001;20(9):2180–2190. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Doray B, Bruns K, Ghosh P, Kornfeld SA. Autoinhibition of the ligand-binding site of GGA1/3 VHS domains by an internal acidic cluster-dileucine motif. Proc Natl Acad Sci U S A. 2002;99(12):8072–8077. doi: 10.1073/pnas.082235699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bonifacino JS, Jackson CL. Endosome-specific localization and function of the ARF activator GNOM. Cell. 2003;112(2):141–142. doi: 10.1016/s0092-8674(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 188.Puertollano R, van der Wel NN, Greene LE, Eisenberg E, Peters PJ, Bonifacino JS. Morphology and dynamics of clathrin/GGA1-coated carriers budding from the trans-Golgi network. Mol Biol Cell. 2003;14(4):1545–1557. doi: 10.1091/mbc.02-07-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292(5522):1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- 190.Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol. 2001;13(4):444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 191.Bonifacino JS. The GGA proteins: adaptors on the move. Nat Rev Mol Cell Biol. 2004;5(1):23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- 192.Tsacoumangos A, Kil SJ, Ma L, Sonnichsen FD, Carlin C. A novel dileucine lysosomal-sorting-signal mediates intracellular EGF-receptor retention independently of protein ubiquitylation. J Cell Sci. 2005;118(Pt 17):3959–3971. doi: 10.1242/jcs.02527. [DOI] [PubMed] [Google Scholar]
- 193.Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269(26):17386–17389. [PubMed] [Google Scholar]
- 194.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283(44):29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Capilla E, Suzuki N, Pessin JE, Hou JC. The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized (gamma)-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment. Mol Endocrinol. 2007;21(12):3087–3099. doi: 10.1210/me.2006-0476. [DOI] [PubMed] [Google Scholar]
- 196.Garippa RJ, Judge TW, James DE, McGraw TE. The amino terminus of GLUT4 functions as an internalization motif but not an intracellular retention signal when substituted for the transferrin receptor cytoplasmic domain. J Cell Biol. 1994;124(5):705–715. doi: 10.1083/jcb.124.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Melvin DR, Marsh BJ, Walmsley AR, James DE, Gould GW. Analysis of amino and carboxy terminal GLUT-4 targeting motifs in 3T3-L1 adipocytes using an endosomal ablation technique. Biochemistry. 1999;38(5):1456–1462. doi: 10.1021/bi980988y. [DOI] [PubMed] [Google Scholar]
- 198.Palacios S, Lalioti V, Martinez-Arca S, Chattopadhyay S, Sandoval IV. Recycling of the insulin-sensitive glucose transporter GLUT4. Access of surface internalized GLUT4 molecules to the perinuclear storage compartment is mediated by the Phe5-Gln6-Gln7-Ile8 motif. J Biol Chem. 2001;276(5):3371–3383. doi: 10.1074/jbc.M006739200. [DOI] [PubMed] [Google Scholar]
- 199.Piper RC, Tai C, Kulesza P, Pang S, Warnock D, Baenziger J, Slot JW, Geuze HJ, Puri C, James DE. GLUT-4 NH2 terminus contains a phenylalanine-based targeting motif that regulates intracellular sequestration. J Cell Biol. 1993;121(6):1221–1232. doi: 10.1083/jcb.121.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Al-Hasani H, Kunamneni RK, Dawson K, Hinck CS, Muller-Wieland D, Cushman SW. Roles of the N- and C-termini of GLUT4 in endocytosis. J Cell Sci. 2002;115(Pt 1):131–140. doi: 10.1242/jcs.115.1.131. [DOI] [PubMed] [Google Scholar]
- 201.Martinez-Arca S, Lalioti VS, Sandoval IV. Intracellular targeting and retention of the glucose transporter GLUT4 by the perinuclear storage compartment involves distinct carboxyl-tail motifs. J Cell Sci. 2000;113(Pt 10):1705–1715. doi: 10.1242/jcs.113.10.1705. [DOI] [PubMed] [Google Scholar]
- 202.Shewan AM, Marsh BJ, Melvin DR, Martin S, Gould GW, James DE. The cytosolic C-terminus of the glucose transporter GLUT4 contains an acidic cluster endosomal targeting motif distal to the dileucine signal. Biochem J. 2000;350(Pt 1):99–107. [PMC free article] [PubMed] [Google Scholar]
- 203.Shewan AM, van Dam EM, Martin S, Luen TB, Hong W, Bryant NJ, James DE. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. Mol Biol Cell. 2003;14(3):973–986. doi: 10.1091/mbc.E02-06-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem. 2006;281(22):15044–15049. doi: 10.1074/jbc.M600442200. [DOI] [PubMed] [Google Scholar]
- 205.Royle SJ, Bobanovic LK, Murrell-Lagnado RD. Identification of a non-canonical tyrosine-based endocytic motif in an ionotropic receptor. J Biol Chem. 2002;277(38):35378–35385. doi: 10.1074/jbc.M204844200. [DOI] [PubMed] [Google Scholar]
- 206.Kishi M, Liu X, Hirakawa T, Reczek D, Bretscher A, Ascoli M. Identification of two distinct structural motifs that, when added to the C-terminal tail of the rat LH receptor, redirect the internalized hormone-receptor complex from a degradation to a recycling pathway. Mol Endocrinol. 2001;15(9):1624–1635. doi: 10.1210/mend.15.9.0698. [DOI] [PubMed] [Google Scholar]
- 207.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401(6750):286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 208.Galet C, Min L, Narayanan R, Kishi M, Weigel NL, Ascoli M. Identification of a transferable two-amino-acid motif (GT) present in the C-terminal tail of the human lutropin receptor that redirects internalized G protein-coupled receptors from a degradation to a recycling pathway. Mol Endocrinol. 2003;17(3):411–422. doi: 10.1210/me.2002-0161. [DOI] [PubMed] [Google Scholar]
- 209.Levitan ES, Takimoto K. Surface expression of Kv1 voltage-gated K+ channels is governed by a C-terminal motif. Trends Cardiovasc Med. 2000;10(7):317–320. doi: 10.1016/s1050-1738(01)00078-0. [DOI] [PubMed] [Google Scholar]
- 210.Lindwasser OW, Resh MD. Human immunodeficiency virus type 1 Gag contains a dileucine-like motif that regulates association with multivesicular bodies. J Virol. 2004;78(11):6013–6023. doi: 10.1128/JVI.78.11.6013-6023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lindwasser OW, Resh MD. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J Virol. 2001;75(17):7913–7924. doi: 10.1128/JVI.75.17.7913-7924.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A. 2001;98(24):13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 214.Neame SJ, Isacke CM. The cytoplasmic tail of CD44 is required for basolateral localization in epithelial MDCK cells but does not mediate association with the detergent-insoluble cytoskeleton of fibroblasts. J Cell Biol. 1993;121(6):1299–1310. doi: 10.1083/jcb.121.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994;6(5):726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 216.Gouyer V, Leteurtre E, Delmotte P, Steelant WF, Krzewinski-Recchi MA, Zanetta JP, Lesuffleur T, Trugnan G, Delannoy P, Huet G. Differential effect of GalNAcalpha-O-bn on intracellular trafficking in enterocytic HT-29 and Caco-2 cells: correlation with the glycosyltransferase expression pattern. J Cell Sci. 2001;114(Pt 8):1455–1471. doi: 10.1242/jcs.114.8.1455. [DOI] [PubMed] [Google Scholar]
- 217.Sheikh H, Isacke CM. A di-hydrophobic Leu-Val motif regulates the basolateral localization of CD44 in polarized Madin-Darby canine kidney epithelial cells. J Biol Chem. 1996;271(21):12185–12190. doi: 10.1074/jbc.271.21.12185. [DOI] [PubMed] [Google Scholar]
- 218.Isacke CM. The role of the cytoplasmic domain in regulating CD44 function. J Cell Sci. 1994;107(Pt 9):2353–2359. doi: 10.1242/jcs.107.9.2353. [DOI] [PubMed] [Google Scholar]
- 219.Bretscher MS, Thomson JN, Pearse BM. Coated pits act as molecular filters. Proc Natl Acad Sci U S A. 1980;77(7):4156–4159. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]


