Abstract
Neuropathic pain resulting from chronic constriction injury (CCI) is critically linked to sensitization of peripheral nociceptors. Voltage gated sodium channels are major contributors to this state and their expression can be upregulated by nerve growth factor (NGF). We have previously demonstrated that neurotrophin-3 (NT-3) acts antagonistically to NGF in modulation of aspects of CCI-induced changes in trkA-associated nociceptor phenotype and thermal hyperalgesia. Thus, we hypothesized that exposure of neurons to increased levels of NT-3 would reduce expression of Nav1.8 and Nav1.9 in DRG neurons subject to CCI. In adult male rats, Nav1.8 and Nav1.9 mRNAs are expressed at high levels in predominantly small to medium size neurons. One week following CCI, there is reduced incidence of neurons expressing detectable Nav1.8 and Nav1.9 mRNA, but without a significant decline in mean level of neuronal expression, and similar findings observed immunohistochemically. There is also increased accumulation/redistribution of channel protein in the nerve most apparent proximal to the first constriction site. Intrathecal infusion of NT-3 significantly attenuates neuronal expression of Nav1.8 and Nav1.9 mRNA contralateral and most notably, ipsilateral to CCI, with a similar impact on relative protein expression at the level of the neuron and constricted nerve. We also observe reduced expression of the common neurotrophin receptor p75 in response to CCI that is not reversed by NT-3 in small to medium sized neurons and may confer an enhanced ability of NT-3 to signal via trkA, as has been previously shown in other cell types. These findings are consistent with an analgesic role for NT-3.
Keywords: Nav1.8, Nav1.9, DRG, sciatic nerve, CCI, nociceptor, nerve growth factor
Introduction
Neuropathic pain and its associated syndromes - including hyperalgesia and spontaneous pain - have become well characterized over the last decade. Complicit in the development of hyperalgesia is the sensitization of nociceptors, with ectopic discharge of the nociceptor being a contributing factor in spontaneous pain. It is well established that both the activation threshold of a neuron and the potential for spontaneous firing is regulated by sodium channels (Hodgkin and Huxley, 1952; Catterall, 1995). This hyperexcitability of sensory neurons following nerve injury has been associated with altered expression and the redistribution of voltage gated sodium channels to the tips of the injured axons and/or the neuromas (Devor et al., 1989; England et al., 1994, 1996; Amir et al., 1999).
The tetrodotoxin-resistant (TTX-R) sodium channels Nav1.8 and Nav1.9 are proposed to play an active role in the generation of neuropathic pain syndromes. They are associated with nociceptive neurons (Fang et al., 2002; Djourhi et al., 2003), and have been detected primarily in small diameter, but also in medium and large diameter DRG neurons (Akopian et al., 1996; Black et al., 1996; Rush et al., 1998; Cummins et al., 1999; Dib-Hajj et al., 1999; Renganathan et al., 2000; Hong and Wiley, 2006).
The development of hyperalgesia and allodynia in nerve ligation models is reduced with Nav1.8 antisense treatment (Porreca et al., 1999; Lai et al., 2002; Joshi et al., 2006), while ectopic firing of neurons is related to Nav1.8 protein expression (Novakovic et al., 1998; Gold et al., 2003). Evidence of a direct role of Nav1.9 in the development of thermal and mechanical hyperalgesia is lacking in rats with neuropathic pain states (Porecca et al., 1999; Priest et al., 2005). Although still debated (Hillsley et al., 2006), Nav1.9 may play a significant role in the increased excitability of nociceptive axons during inflammation (Herzog et al., 2001; Baker et al., 2003; Rush and Waxman, 2004; Priest et al., 2005).
Reported alterations in the expression of both Nav1.8 and Nav1.9 in response to chronic constriction injury (CCI) are conflicting (Novakovic et al., 1998; Dib-Hajj et al., 1999; Decosterd et al., 2002), and the channels respond differently to inflammation (Tanaka et al., 1998; Black et al., 2004; Gould et al., 2004). This may be due to the complexity of the CCI model which produces preferential axotomy of large sized DRG neurons and exposure of the remaining intact axons to an inflammatory environment (Bennett and Xie, 1988; Kajander and Bennett, 1992) with increased levels of nerve growth factor (NGF) that increase injury-induced expression of these channels (Dib-Hajj et al., 1998; Fjell et al., 1999).
Neurotrophin-3 (NT-3) can prevent the development and maintenance of thermal hyperalgesia, and in general, acts in an antagonistic fashion to NGF in the CCI model of neuropathic pain (Wilson-Gerwing et al., 2005; Wilson-Gerwing and Verge, 2006). This study investigates whether NT-3 is capable of modulating levels of expression of the sodium channel isoforms Nav1.8 and Nav1.9 following CCI in a manner consistent with an analgesic role for NT-3.
Methods
Animal surgery
All animal procedures were conducted in accordance with the National Institutes of Health policy on the use of animals in research and the University of Saskatchewan animal care committee guidelines (protocol 19920164). A total of 32 young adult male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 250–300 g were used. Animals were anesthetized for surgery with sodium pentobarbital (Somnitol, 65 mg/kg; MTC Pharm, Cambridge, Ontario, Canada). Pre- and post-operative (for 24 h) subcutaneous injections of buprenorphine (Temgesic, 0.1–0.2 mg/kg) were given to alleviate any post-operative discomfort. To examine the effect of NT-3 on the expression of Nav1.8 and Nav1.9, 40 rats were used: 17 underwent 7 d unilateral CCI of the sciatic nerve (Bennett and Xie, 1988), 3 received sham CCI surgeries whereby the sciatic nerve was exposed but not ligated, 17 received 7 d unilateral CCI with intrathecal infusion of NT-3 for the duration of the injury, and 3 received 7 d unilateral CCI with sham pump implantation whereby the dorsal roots were exposed, the dura opened as with the CCI + NT-3 procedure, but no pump was implanted.
NT-3 (generously supplied by Regeneron Pharmaceuticals, Tarrytown, NY) was delivered intrathecally for 7 d via mini-osmotic pumps (model 2001; Alza, Cupertino, CA) inserted at the lumbar sacral junction as per Verge et al. (1989a) at a concentration and rate of 600 ng/μl/hr (Karchewski et al., 2002) in a solution of PBS containing rat serum albumin (1 mg/ml), streptomycin (100 U/ml), and penicillin (100 U/ml). This dose of NT-3 was the minimum dose found to selectively reverse injury-associated gene expression in injured trkC-expressing neurons (Verge et al., 1996; Jongsma Wallin et al., 2001; Karchewski et al., 2002). At the conclusion of the experiments, rats were killed, and tissue was dissected and processed for in situ hybridization and/or immunohistochemistry as described below. Previous studies have demonstrated a lack of influence ipsilateral and contralateral to injury when vehicle is infused intrathecally (Verge et al., 1989a; Verge et al., 1995; Jongsma Wallin et al., 2001; Wilson-Gerwing et al., 2005).
In situ hybridization
Deeply anesthetized animals were perfused via the aorta with 0.1 M PBS, pH 7.4, followed by 4% paraformaldehyde in 0.1M PBS. The right and left L4 and L5 DRG were rapidly dissected, postfixed for 1 hour in the same fixative, and cryoprotected in 20% sucrose in 0.1M PBS overnight. Paired experimental and control tissues were mounted in the same cryomold (to ensure processing under identical conditions), covered with OCT compound (Tissue Tek; Miles Laboratories, Elkhart, IN, USA) and frozen in cooled isopentane. Transverse sections were cut at 6 μm on a Micron cryostat (Zeiss, Canada), thaw mounted onto Probe-On+ slides (Fisher Scientific, Edmonton, AB, Canada) and stored with desiccant at − 20 °C until hybridization.
Prior to hybridization, slides were air dried, fixed in 4% paraformaldehyde, and washed in 1X PBS. Sections were then treated with proteinase K (20 μg/ml) containing 10 ml 1M Tris-HCl (pH 7.6), 2 ml 0.5 M EDTA, 200 μl proteinase K stock (20 mg/ml) and 188 μl ddH20, rinsed in 1 X PBS, and post-fixed in 4% paraformaldehyde. Slides were then rinsed and dehydrated in ascending alcohols.
Oligonucleotide probes complementary to and selective for Nav1.8 mRNA [complementary to bases 640–687 (Akopian et al., 1996)], Nav1.9 mRNA [complementary to bases 2811–2858 (Dib-Hajj et al., 1998)] and p75 mRNA [complementary to bases 873–920 (Radeke et al., 1987)] were synthesized (University of Calgary DNA services, Alberta, Canada). The probes were checked against the GenBank database (NIH) to ensure no greater than 60% homology was found to sequences other than the cognate transcript. The probes were labeled at the 3′-end with α-[35S]dATP (New England Nuclear, Boston, MA, USA) using terminal deoxynucleotidyl-transferase (Amersham Pharmacia Biotech, Piscataway, NJ, USA) in a buffer containing 10 mM CoCl2, 1 mM dithiothreitol, 300 mM Tris base and 1.4 M-potassium cacodylate (pH 7.2), and purified through Bio-Spin® Disposable Chromatograph Columns (Bio-Rad laboratories, Hercules, CA, USA) containing 200 mg of NENSORB™ PREP Nucleic Acid Purification Resin (NEN®, Boston, MA, USA). Dithiothreitol was added to a final concentration of 10 nM.
Hybridization was carried out according to published procedures (Dagerlind et al., 1992) on a minimum of 5 slides/probe from each of the experimental and control groupings. Briefly, the sections were hybridized at 43 °C for 14–18 hours in a buffer containing 50% formamide (Sigma Aldrich, Oakville, ON, Canada), 4X SSC (1X SSC – 0.15 M NaCl, 0.015 sodium citrate), 1X Denhart’s solution (0.02% bovine serum albumin and 0.02% Ficoll), 1% sarcosyl (N-laurylsarcosine), 0.02 M phosphate buffer (pH 7.0), 10% dextran sulphate, 500 μg/ml heat-denatured sheared salmon sperm DNA, 200 mM dithiothreitol and 107 cpm/ml of probe. After hybridization, the slides were washed for 4 × 15 mins in 1X SSC at 55°C, dehydrated in ascending alcohols, processed for radioautography as per Karchewski et al., 2002 and exposed for 7 to 10 days before developing in D-19 (Kodak, Rochester, NY, USA).
The specificity of hybridization signal for the Nav 1.8 and Nav 1.9 probes was confirmed as described in Wilson-Gerwing et al., 2005.
Quantification and analysis
All slides from the 16 groupings of 7 d experimental and control animals were analyzed qualitatively and relative changes in hybridization signal from one group to another noted for sections mounted on the same slide to avoid bias due to the variance in hybridization signal observed from slide to slide. Representative slides were selected for further quantitative analysis. These slides had a similar number of neurons in all DRG sections. Photomontages were prepared and individual neurons with a visible nucleus were identified. Using a 40X light objective and a 2X optivar with an interactive computer-assisted image-analysis system (Richardson et al., 1989), the cross-sectional area of individual neurons and the percentage of cytoplasmic area covered by silver grains was measured for each neuron with a visible nucleus in the DRG section. The area per grain was kept constant for all neurons and a correction for grain overlap was made to obtain a parameter linearly related to density of silver grains (Richardson et al., 1989). Software for the image analysis system was Northern Eclipse, Version 7.0 (Empix Imaging, Mississauga, ON, Canada) and supplemented with Microsoft Office Excel 2003 (Microsoft Corporation, Redmond, WA) and Prism 4.0 (Graph Pad Software, San Diego, CA). Cells were considered labeled if they had more than five times background levels of silver grains, as determined by averaging grain densities over defined areas of the neuropil devoid of positively labeled cell bodies. This criterion for determining labeled neuronal profiles correlates well with the identification of labeled versus unlabeled neurons as determined manually using a 63X oil immersion objective.
Analysis was performed in each instance on all neurons with a nucleus present in the section being quantified: for Nav1.8 this represents 12 DRG sections or 2236 neuronal profiles (Intact: n = 3 animals, CCI: n = 3 animals, Intact + NT-3: n = 3 animals, CCI+NT-3: n = 3 animals); while for Nav1.9 this represents 12 DRG sections or 2025 neuronal profiles (Intact: n = 3 animals, CCI: n = 3 animals, Intact + NT-3: n = 3 animals, CCI+NT-3: n = 3 animals). The contralateral intact DRG was used as an intact control as previous research from our lab has demonstrated that CCI does not induce bilateral hyperalgesia (Wilson-Gerwing et al., 2005) and we have not discerned any qualitative differences between sham-operated or naive DRG and contralateral intact DRG with respect to Nav1.8 or Nav1.9 expression.
To determine whether alterations in the mean labeling index were significant, a nonparametric ANOVA test was employed (Kruskal-Wallis Test) since it could not be assumed that our data followed a Gaussian distribution. Following the Kruskal-Wallis test, Dunn’ Multiple Comparison test was used to determine significant differences between specific groups of data (p < 0.001). All statistical calculations were performed using Prism 4.0 (GraphPad Software, San Diego, CA).
Immunohistochemistry
Transverse 10 μm sections were cut on the cryostat, thaw-mounted onto Probe-ON+ slides (Fisher Scientific), and processed for immunohistochemistry. For Nav1.8: sections were washed in 0.1 M PBS, then permeabilized with 0.3% Triton X-100 in 0.1 M PBS for 45 minutes at room temperature. Sections were blocked overnight at 4 °C in 10% goat serum and 0.3% Triton X-100 in 0.1 M PBS, then incubated overnight at 4 °C with rabbit anti-Nav 1.8 Affinity Purified Polyclonal Antibody (1:200; Chemicon International, Temecula, CA, USA) diluted with 10% goat serum and 0.3% Triton X-100 in 0.1 M PBS. Sections were visualized with Alexa Fluor® 488 F(ab′)2 fragment of goat anti-rabbit IgG (H+L) (1:250; Molecular Probes, Eugene, OR, USA) in 2% goat serum in 0.1 M PBS for 2 hours at room temperature. Slides were washed and coverslipped with 50% glycerol/50% PBS. For Nav1.9: sections were washed in 0.1 M PBS, then permeabilized with 0.3% Triton X-100 in 0.1 M PBS for 45 minutes at room temperature. Sections were blocked overnight at 4 °C in 10% goat serum, 3% BSA, and 0.3% Triton X-100 in 0.1 M PBS, then incubated overnight at 4 °C with rabbit anti-Nav1.9 Affinity Purified Polyclonal Antibody (1:100; Chemicon International, Temecula, CA, USA) diluted with 10% goat serum, 3% BSA, and 0.3% Triton X-100 in 0.1 M PBS. Sections were visualized with Alexa Fluor® 546 goat anti-rabbit IgG (H+L), F(ab′)2 fragment conjugate (1:250; Molecular Probes, Eugene, OR, USA) in 2% goat serum for 2 hours at room temperature. Slides were washed and coverslipped with 50% glycerol/50% PBS. Control sections were processed in the same manner, but without the primary antibody. Results were visualized using a Zeiss Axioscope 50 microscope equipped with incident-light fluorescence optics and a digital camera.
Results
NT-3 significantly attenuates neuronal Nav1.8 mRNA expression
Analysis of sections processed for in situ hybridization to detect neuronal expression of Nav1.8 mRNA revealed that in DRG contralateral to CCI (Intact), detectable hybridization signal was observed in 70.3% of neurons analyzed (Table 1). Consistent with previous reports (Akopian et al., 1996; Black et al., 1996; Cummins et al., 1999; Dib-Hajj et al., 1999), we found that expression is observed primarily in small to medium sized (<35 μm in diameter) neurons. These neurons represent 60.7% of all neurons analyzed, while only 9.6% were larger sized (>35 μm in diameter) neurons (Table 1).
Table 1. Effects of CCI +/− NT-3 infusion on numbers of neurons expressing Nav1.8 mRNA.
The above table summarizes the alterations in the incidence (percentage) of Nav1.8 mRNA positive neurons in L5 DRG neurons under various experimental conditions as indicated in the left column. Those neurons < 35 μm are characterized as small to medium in size and those neurons > 35 μm are characterized as medium to large in size. Detectable levels of hybridization signal are all those > 5X background where as moderate to high levels of hybridization signal are those > 20X background. CCI produces a decrease in the percentages of neurons positively labeled for Nav1.8. Infusion of NT-3 further decreases the percentage of Nav1.8 mRNA positive neurons detected most evident ipsilateral to CCI.
| Nav 1.8 | Labeled Neurons (% total population) | Small to medium (<35μm) labeled neurons (% total population) | Large (>35 μm) labeled neurons (% total population) | |||
|---|---|---|---|---|---|---|
| > 5X bkgd | > 20X bkgd | > 5X bkgd | > 20X bkgd | > 5X bkgd | > 20X bkgd | |
| Intact | 70.3 | 42.9 | 60.7 | 38.8 | 9.6 | 4.1 |
| Intact + NT-3 | 52.4 | 28.4 | 40.5 | 24.2 | 11.9 | 4.2 |
| CCI | 55.0 | 34.1 | 50.4 | 31.8 | 4.6 | 2.3 |
| CCI + NT-3 | 33.4 | 12.8 | 28.5 | 10.7 | 4.9 | 2.1 |
Seven days after CCI, there is a reduction in the percentage of DRG neurons expressing detectable levels of Nav1.8 (from 70.3% to 55.0%; Figure 1; Table 1). However, the mean labeling index for these neurons was not significantly changed following CCI (from 18.53 +/− 0.9414 to 17.50 +/− 1.008; p<0.001; Figure 2), suggesting that the neurons that express Nav1.8 do so at slightly higher levels in response to CCI as was apparent for a subpopulation of small to medium size neurons. The decreased incidence of expression, consistent with the findings of Dib-Hajj et al.(1999), occurs primarily in the small to medium diameter neurons (Table 1). The smaller decrease in incidence of expression in the larger cells (presumably those axotomized by CCI) is in agreement with that observed by Decosterd et al. (2002).
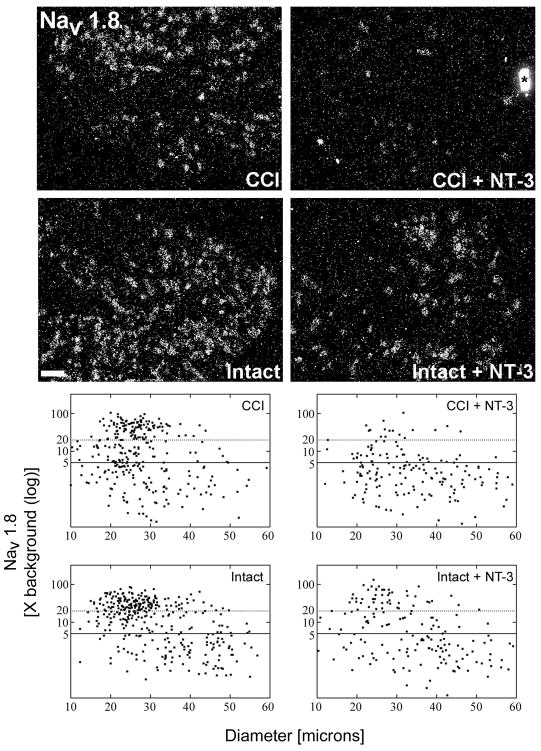
Figure 1. Message levels for the tetrodotoxin resistant sodium channel Nav1.8 are reduced in response to NT-3 treatment.
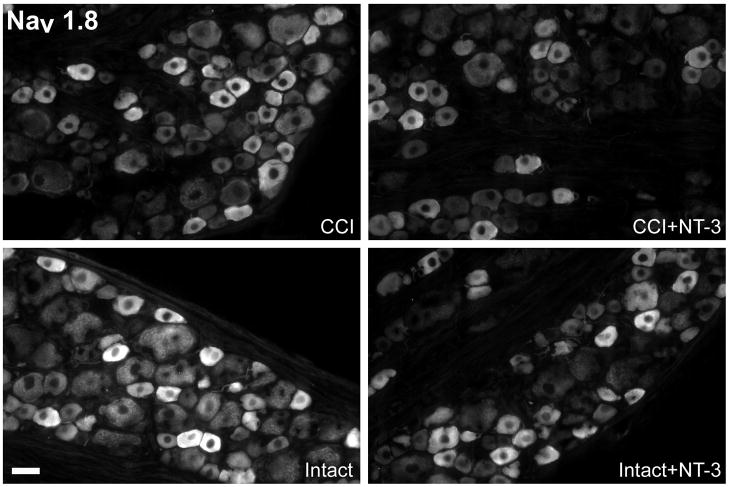
Top: Darkfield photomicrographs of 6 μm thick adult rat L5 DRG sections processed for in situ hybridization to detect Nav1.8 transcripts contralateral (Intact) or ipsilateral to 7d CCI (CCI) and after a 7d unilateral CCI plus intrathecal infusion of 600 ng/μl/hr NT-3 (Intact+NT-3; CCI+NT-3). Scale bar = 100 μm. Note: Treatment with NT-3 dramatically reduces levels of Nav1.8 mRNA following CCI.
Bottom: Representative scatterplots whereby each point represents the labeling index of an individual neuron identified in 6 μm thick sections of L5 DRG processed to detect Nav1.8 mRNA. The relationship between Nav1.8 mRNA labeling intensity (y-axis, log scale) and perikaryal diameter (x-axis) is depicted. Experimental states are indicated at the top right of each graph and are as described above. Labeling refers to the ratio of silver grain density over the neuronal cytoplasm to grain density over areas of the neuropil devoid of positive hybridization signal. Solid lines divide the plots into labeled and unlabeled populations; dotted lines separate lightly labeled from moderate to heavily labeled populations of Nav1.8 –expressing neurons. Note: In DRG contralateral to CCI, Nav1.8 is expressed predominantly in small to medium neurons with some large neurons also expressing detectable levels. CCI results in a slight decrease in the incidence of neurons expressing Nav1.8, while a subpopulation of small and medium size neurons have elevated expression. NT-3 infusion results in a decrease in the levels of detectable Nav1.8 mRNA in DRG both ipsilateral and contralateral to CCI, with a more pronounced effect ipsilateral to injury.
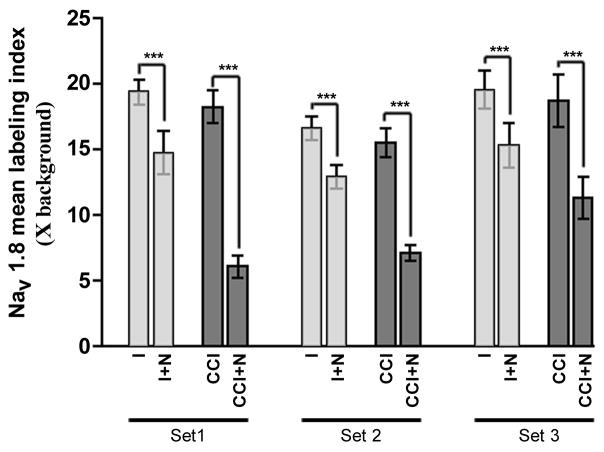
Figure 2. NT-3 treatment significantly reduces mean Nav1.8 mRNA labeling indices.
Graph depicts quantification of relative differences between experimental groups (as indicated) in Nav1.8 mRNA mean labeling indices for all DRG neurons (n=2236) measured in DRG sections processed for in situ hybridization in 3 separate experiments [representing 3 animals having undergone 7 day unilateral CCI [Intact (I) and CCI] and 3 animals having undergone 7 day unilateral CCI with intrathecal infusion of NT-3 [Intact+NT-3 (I+N) and CCI+NT-3 (CCI+N)]. Bars represent the standard error of the mean (s.e.m.). Asterisks indicate significant differences between experimental groups (Kruskal-Wallis test with Dunn’s Multiple Comparison test; *** p<0.001). NT-3 infusion results in a significant decrease in mean Nav1.8 mRNA expression in L5 neurons both ipsilateral and contralateral to CCI.
When NT-3 is infused for the duration of the 7 day CCI, we find that the relative level of expression of Nav1.8 mRNA is significantly decreased both ipsilateral (CCI + NT-3) and contralateral (Intact + NT-3) to CCI as compared to the control DRG (CCI, Intact; Figure 1). In NT-3 infused DRG contralateral to CCI (Intact + NT-3) the percentage of neurons expressing detectable levels of Nav1.8 is reduced from 70.3% to 52.4% (Figure 1; Table 1). The mean labeling index was significantly decreased from 18.53 +/− 0.9414 in the intact state to 14.34 +/− 0.7077 in the NT-3 treated group (p<0.001; Figure 2). A greater attenuation of neuronal Nav1.8 expression was observed in the NT-3 treated DRG ipsilateral to CCI (CCI + NT-3). Again, the incidence of a neuron expressing detectable levels of Nav1.8 mRNA was reduced from 55.0% to 33.4% (Figure 1, Table 1). This was accompanied by a dramatic and significant decrease in the mean labeling index from 17.50 +/− 1.008 to 8.166 +/− 1.598 (p<0.001; Figure 2). The decrease in the relative level of Nav1.8 expression is most apparent in those small to medium sized sensory neurons in both ipsilateral and contralateral DRG (Figure 1; Table 1). There is also a decreased incidence of expression observed in some larger sized neurons albeit to a lesser extent (Figure 1; Table 1).
NT-3 significantly attenuates neuronal Nav1.9 mRNA expression
Analysis of sections processed for in situ hybridization to detect neuronal expression of Nav1.9 mRNA revealed that in DRG contralateral to CCI (Intact), detectable hybridization signal occurred over 72.9% of neurons. As observed for Nav1.8 mRNA, this was localized primarily over small to medium sized neurons representing 59.0% of all neurons analyzed, while only 13.9% of the neurons analyzed were large size neurons that express detectable levels of this message (Figure 3; Table 2). These findings are consistent with those previously described (Black et al., 1996; Cummins et al., 1999; Dib-Hajj et al., 1999).
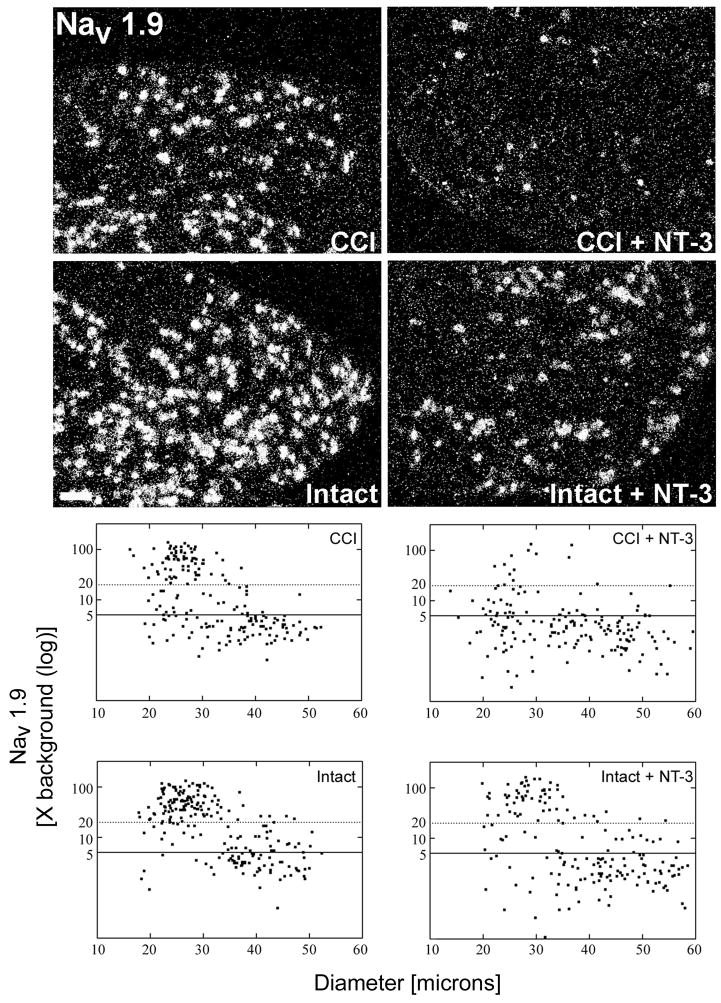
Figure 3. Message levels for the tetrodotoxin resistant sodium channel Nav1.9 are reduced in response to NT-3 treatment.
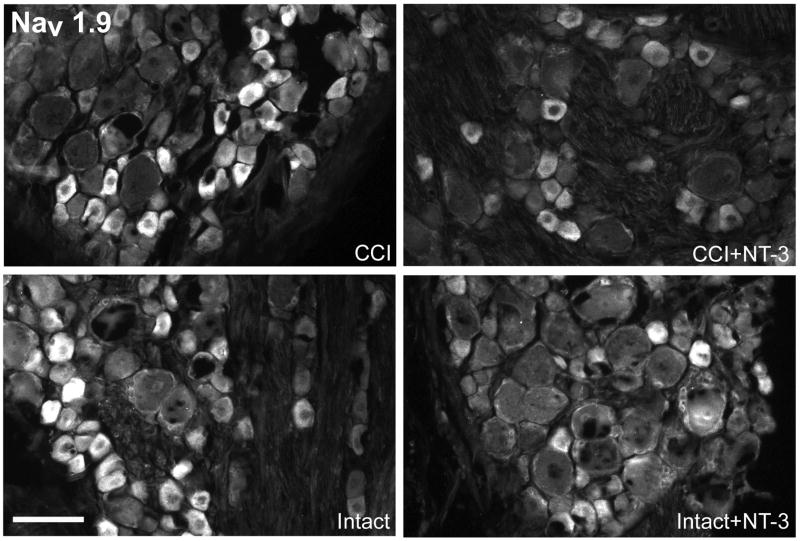
Top: Darkfield photomicrographs of 6 μm thick adult rat L5 DRG sections processed for in situ hybridization to detect Nav1.9 mRNA transcripts contralateral (Intact) or ipsilateral to 7d CCI (CCI) and after a 7d unilateral CCI plus intrathecal infusion of 600 ng/μl/hr NT-3 (Intact+NT-3; CCI+NT-3). Scale bar = 100 μm. Treatment with NT-3 dramatically reduces levels of Nav1.9 mRNA following CCI.
Bottom: Representative scatterplots whereby each point represents the labeling index of an individual neuron identified in 6μm thick sections of L5 DRG processed to detect Nav1.9 mRNA. The relationship between Nav1.9 mRNA labeling intensity (y-axis, log scale) and perikaryal diameter (x-axis) is depicted. Experimental states are indicated at the top right of each graph and are as described above. Labeling refers to the ratio of silver grain density over the neuronal cytoplasm to grain density over areas of the neuropil devoid of positive hybridization signal. Solid lines divide the plots into labeled and unlabeled populations; dotted lines separate lightly labeled from moderate to heavily labeled populations of Nav1.9 –expressing neurons. Note: In DRG contralateral to CCI, Nav1.9 is expressed predominantly in small to medium neurons with a low level of expression detectable in some large neurons. CCI results in a slight decrease in Nav1.9 expression in large size neurons with an increased expression in small to medium size neurons. NT-3 infusion results in a decrease in the levels of detectable Nav1.9 mRNA in DRG both ipsilateral and contralateral to CCI, with a more pronounced effect ipsilateral to injury.
Table 2. Effects of CCI +/− NT-3 infusion on numbers of neurons expressing Nav1.9 mRNA.
The above table summarizes the alterations in the incidence (percentage) of Nav1.9 mRNA positive neurons in L5 DRG under various experimental conditions as indicated in the left column. Those neurons < 35 μm are characterized as small to medium in size and those neurons > 35 μm are characterized as medium to large in size. Detectable levels of hybridization signal are all those > 5X background where as moderate to high levels of hybridization signal are those > 20X background. CCI produces a decrease in the percentages of neurons positively labeled for Nav1.9. Infusion of NT-3 further decreases the percentage of Nav1.9 mRNA positive neurons detected most evident ipsilateral to CCI.
| Nav 1.9 | Labeled Neurons (% total population) | Small to medium (<35μm) labeled neurons (% total population) | Large (>35 μm) labeled neurons (% total population) | |||
|---|---|---|---|---|---|---|
| > 5X bkgd | > 20X bkgd | > 5X bkgd | > 20X bkgd | > 5X bkgd | > 20X bkgd | |
| Intact | 72.9 | 51.7 | 59.0 | 48.7 | 13.9 | 3.0 |
| Intact + NT-3 | 49.4 | 28.5 | 39.7 | 26.1 | 9.7 | 2.4 |
| CCI | 60.9 | 40.0 | 54.8 | 38.6 | 6.1 | 1.4 |
| CCI + NT-3 | 32.3 | 11.6 | 27.4 | 11.1 | 4.9 | 0.5 |
Seven days after CCI, there is a reduction in the percentage of neurons expressing detectable levels of Nav1.9 from 60.9% to 32.3% (Figure 3), with the reduced incidence of expression occurring in both the small to medium and large diameter sensory neurons (Table 2). These findings are consistent with the decreased incidence of expression observed in the small sized DRG neurons observed by Dib-Hajj et al. (1999), and the decrease in the large sized neurons (presumably those axotomized by CCI) reported by Decosterd et al. (2002). The mean labeling index for these neurons, however, was not significantly decreased following CCI (33.29 +/− 7.145 in the intact to 27.23 +/− 6.756 following CCI; p<0.001; Figure 4) and is probably reflective of the increased expression observed in a subpopulation of small to medium size neurons.
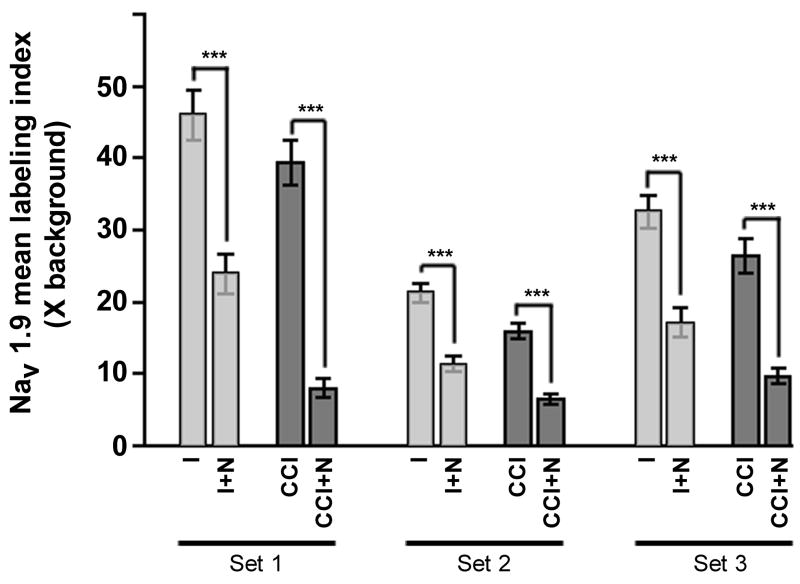
Figure 4. Mean Nav1.9 mRNA labeling indices are significantly decreased following NT-3 treatment.
Graph depicts quantification of relative differences between experimental groups (as indicated) in Nav1.9 mRNA mean labeling indices for all DRG neurons (n=2025) measured in DRG sections processed for in situ hybridization in 3 separate experiments [representing 3 animals having undergone 7 day unilateral CCI [Intact (I) and CCI] and 3 animals having undergone 7 day unilateral CCI with intrathecal infusion of NT-3 [Intact+NT-3 (I+N) and CCI+NT-3 (CCI+N)]. Bars represent the standard error of the mean (s.e.m.). Asterisks indicate significant differences between experimental groups (Kruskal-Wallis test with Dunn’s Multiple Comparison test; *** p<0.001). NT-3 infusion results in a significant decrease in mean Nav1.9 mRNA expression in L5 neurons both ipsilateral and contralateral to CCI.
Consistent with our Nav1.8 mRNA results, NT-3 infusion for the duration of the seven day CCI effected a marked reduction in the incidence and relative levels of Nav1.9 mRNA expression observed in neurons both contralateral (Intact + NT-3) and ipsilateral (CCI + NT-3) to CCI and (Figure 3). In NT-3 infused DRG contralateral to CCI (Intact + NT-3), the mean labeling index was significantly decreased from 33.29 +/− 7.145 in the intact state to 17.52 +/− 3.621 in the NT-3 treated group (p<0.001; Figure 4). There was also a decrease in as the percentage of neurons expressing detectable levels of Nav1.9 from 72.9% to 49.4% (Figure 3; Table 2). A more dramatic attenuation of Nav1.9 expression was observed in the NT-3 treated DRG ipsilateral to CCI (CCI + NT-3). The mean labeling index was significantly decreased with NT-3 treatment from 27.23 +/− 6.756 to 8.080 +/− 0.9123 (p<0.001; Figure 4), while the percentage of neurons expressing detectable levels of Nav1.9 mRNA was also reduced from 60.9% to 32.3% (Table 2). In the NT-3 treated DRG both contralateral (Intact + NT-3) and ipsilateral (CCI + NT-3) to CCI, the decreased levels of expression are most prominent in the small to medium sized neuronal populations.
Relative levels of neuronal Nav1.8 and Nav1.9 protein expression are decreased with NT-3 treatment
To examine whether the ability of NT-3 to attenuate Nav1.8 and Nav1.9 message was reflected with a similar decrease in protein expression of these two ion channels, sections from the same ganglia were processed immunohistochemically to detect either Nav1.8 or Nav1.9 protein. Qualitative analysis of the immunofluorescence revealed that the patterns of protein expression were similar to those observed for mRNA. In the intact state Nav1.8 protein was observed most prominently in the smaller DRG neurons, with some medium to large sized neurons also expressing low to moderate levels (Figure 5). Similarly, Nav1.9 immunofluorescence signal was observed predominantly in the small to medium sized DRG neurons. Relative levels of Nav1.8 protein remain largely unchanged 7 days following CCI (Figure 5). Levels of Nav1.9 protein appear slightly elevated following CCI (Figure 6) with some small neurons expressing Nav1.9 and at higher levels than observed in the intact state.
Figure 5. NT-3 infusion attenuates Nav1.8 protein expression.
Fluorescence photomicrographs depict levels of Nav1.8-like immunoreactivity in 10 μm sections of DRG ipsilateral (CCI) and contralateral to CCI (Intact) L5 DRG with or without immediate intrathecal infusion of NT-3 (CCI+NT-3; Intact+NT-3), as indicated. Scale bar, 60 μm. Note: In the DRG contralateral to CCI (Intact), levels of Nav1.8 protein are highest in small to medium sized dorsal root ganglion neurons. Seven days after CCI, levels of expression have decreased slightly relative to the intact state most evident in the medium sized neurons. Intrathecal infusion of NT-3 at the time of injury also results in markedly reduced levels of Nav1.8 protein expression ipsilateral to CCI.
Figure 6. NT-3 infusion results in decreased expression of Nav1.9 protein.
Fluorescence photomicrographs depict levels of Nav1.9-like immunoreactivity in 10 μm sections of DRG ipsilateral (CCI) and contralateral to CCI (Intact) L5 DRG with or without immediate intrathecal infusion of NT-3 (CCI+NT-3; Intact+NT-3), as indicated. Scale bar, 60 μm. Note: In the DRG contralateral to CCI (Intact), levels of Nav1.9 protein are highest in small sized dorsal root ganglion neurons. Seven days after CCI, levels of expression have decreased relative to the intact state. Intrathecal infusion of NT-3 at the time of injury results in markedly reduced levels of Nav1.9 protein ipsilateral to CCI.
Infusion of NT-3 effected a decrease in the levels of Nav1.8 protein detected in neurons in DRG both contralateral (Intact + NT-3) and ipsilateral to CCI (CCI + NT-3) (Figure 5). On the other hand, infusion of NT-3 had only a modest effect on relative levels of Nav1.9 protein expression in neurons contralateral to CCI (Intact + NT-3) as opposed to the decrease in both the relative levels and the incidence of neurons expressing detectable Nav1.9 protein in DRG ipsilateral to CCI (CCI + NT-3) (Figure 6).
NT-3 effects a decrease in Nav1.8 and Nav1.9 protein in the nerve and neuroma
Following nerve injury, voltage gated sodium channels are highly localized/redistributed to the tips of the injured axons and/or neuromas despite a lower level of expression in the cell body of these neurons (Devor et al., 1989; England et al., 1994, 1996; Amir et al., 1999). Thus, we asked whether the dramatic reduction in sodium channel expression effected by NT-3 in neurons ipsilateral to CCI is also reflected in a reduced localization of these channels to the neuroma at the constriction sites formed by the ligatures, unlike that observed for CCI alone. The common sciatic nerves at the level of the CCI with or without NT-3 infusion were processed to detect either Nav1.8 or Nav1.9 protein. Consistent with previous reports (Devor et al., 1989; England et al., 1994, 1996; Amir et al., 1999), both Nav1.8 and Nav1.9 protein levels were more highly localized to the constriction sites 7 days following CCI (CCI; Figure 7 bottom). Infusion of NT-3 attenuated this redistribution as evidenced by a reduction in relative levels of Nav1.8 and Nav1.9 protein at the constriction sites of the CCI + NT-3 treated nerves (Figure 7 bottom). This bolsters our proposal that although CCI can effect a redistribution of sodium channels to the injured axon tips, the extremely low transcript levels following NT-3 treatment result in protein levels that are not sufficient to sustain this response. Alternatively, exogenous NT-3 may also dampen the signals that effect this translocation.
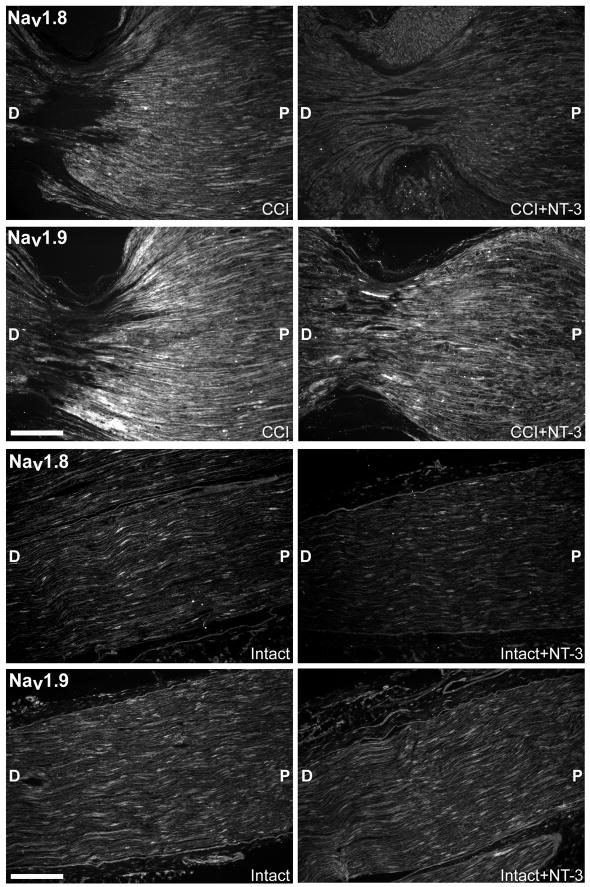
Figure 7. NT-3 infusion results in decreased expression of Nav1.8 and Nav1.9 protein in the common sciatic nerve following CCI.
Fluorescence photomicrographs demonstrate the levels of Nav1.8 and Nav1.9 protein in 10 μm sections of the common sciatic nerve either ipsilateral to CCI with (CCI + NT-3) or without (CCI) immediate intrathecal infusion of NT-3 (Top), or contralateral to CCI with (Intact + NT-3) or without (Intact) immediate intrathecal infusion of NT-3 (Bottom) as indicated. P, proximal nerve; D, distal nerve. Scale bars, 60 μm. Note: There is increased Nav1.8 and Nav1.9 protein localization proximal to the first constriction site in the CCI only nerves. Intrathecal infusion of NT-3 at the time of injury (CCI + NT-3) results in noticeably lower levels of Nav1.8 and Nav1.9 protein localization near the constriction sites.
While channel localization was elevated at the ligature sites following CCI, relative levels of expression in the uninjured contralateral sciatic nerve (Intact) for both of these sodium channels is only low (Nav1.8 ) to moderate (Nav1.9; Figure 7 top). In accordance with our findings for the DRG, infusion of NT-3 effected a subtle decrease on the relative levels of Nav1.8 protein expression observed in the intact nerves (Intact + NT-3) (Figure 7 top). However, infusion of NT-3 did not result in any perceptible changes in the levels of Nav1.9 protein expression in the uninjured nerve (Intact + NT-3) (Figure 7 top).
Expression of the common neurotrophin receptor p75 is altered in response to CCI and NT-3 infusion
Our previous studies have demonstrated that DRG neurons co-express two isoforms of trkA, one that selectively binds and is activated by NGF and one with a 6 amino acid insert in the extracellular domain that binds and is activated by both NGF and NT-3 (Karchewski et al., 1999; Barker et al., 1993; Clary and Reichardt 1994). In other cell models, the common neurotrophin receptor, p75, inhibits NT-3 signaling through trkA (Mischel et al., 2001). As the ability of NT-3 to attenuate expression of Nav1.8 and Nav1.9 is always most pronounced ipsilateral to CCI we examined whether expression of p75 might be altered in response to CCI and NT-3 in a manner consistent with that which might confer an enhanced ability of NT-3 to signal via trkA.
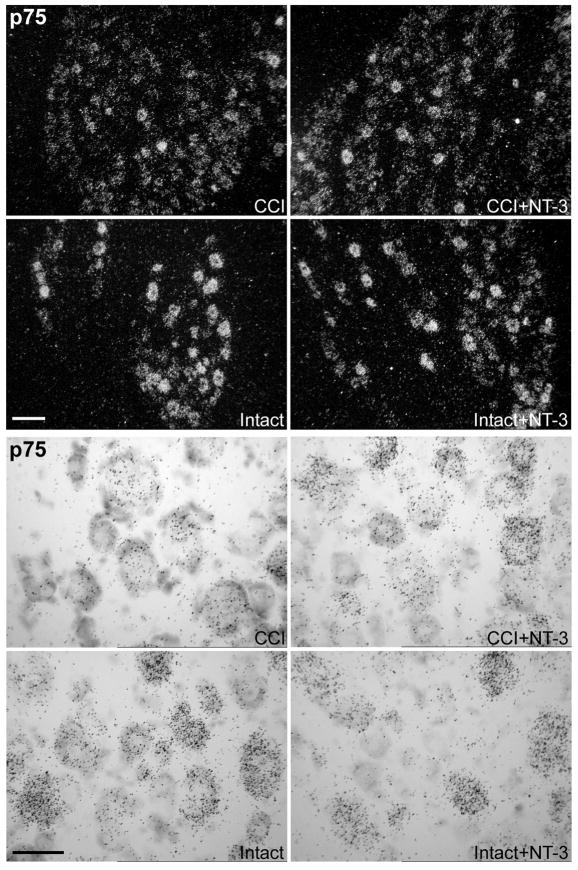
Qualitative examination of p75 mRNA expression in DRG subjected to one week unilateral CCI with or without NT-3 infusion begun at the time of injury, revealed that in intact DRG neurons p75 is heterogenously expressed at moderate to high levels across all size ranges of neurons (Figure 8), consistent with that previously reported (Verge et al., 1992, Karchewski et al., 1999). CCI results in a dramatic reduction in neuronal p75 expression (Figure 8). NT-3 infusion did not discernibly alter p75 expression in small and medium size neurons but did result in increased neuronal expression of p75 mRNA within a subpopulation of primarily large size neurons ipsilateral to CCI (Figure 8). This is consistent with our previously reported data showing that NT-3 can reverse the downregulation in p75 expression that occurs in spinal nerve injured neurons, but only in those large size neurons expressing trkC (Verge et al., 1996).
Figure 8. Message levels for the neurotrophin receptor p75 are modulated in response to CCI and NT-3 treatment.
Darkfield photomicrographs and brightfield photomicrographs of 6 μm thick adult rat L5 DRG sections processed for in situ hybridization to detect p75 transcripts contralateral (Intact) or ipsilateral to 7d CCI (CCI) and after a 7d unilateral CCI plus intrathecal infusion of 600 ng/μl/hr NT-3 (Intact+NT-3; CCI+NT-3) as indicated. Scale bar = 100μm. Note: Levels of p75 mRNA are markedly decreased following CCI. Treatment with NT-3 increases neuronal levels of p75 mRNA in DRG ipsilateral to CCI (CCI + NT-3), but only in a subpopulation of primarily larger sized DRG neurons. Scale Bar = 60 μm.
Discussion
Attenuation of Nav1.8 and Nav1.9 expression correlates with the regulation of thermal hyperalgesia by NT-3 following CCI
We have previously reported that administration of exogenous NT-3 at the time of CCI prevents the development of thermal hyperalgesia and attenuates expression of one of the molecules and associated signalling pathways complicit in this behavior (Wilson-Gerwing et al., 2005). In concert with these findings, we now show that exogenous NT-3 at the time of injury significantly decreases levels of expression of two TTX-R sodium channels, Nav1.8 and Nav1.9, and thus, presumably mitigates the hyperexcitable state associated with this pathology.
In support of a role for Nav1.8 in the development of thermal hyperalgesia, it has been shown that if Nav1.8 is blocked by antisense oligonucleotides, the development of thermal hyperalgesia is reduced in both the spinal nerve ligation and CCI models (Porreca et al., 1999; Lai et al., 2002; Joshi et al., 2006). However, if antisense oligonucleotides to Nav1.9 or genetic ablation of Nav1.9 are employed, there appears to be no effect on thermal or mechanical hypersensitivity in the neuropathic rat (Porreca et al., 1999; Priest et al., 2005; Amaya et al., 2006). Interestingly, it appears that Nav1.9 does play a role in hypersensitivity produced by the application of inflammatory mediators to the peripheral terminals of the nociceptors (Amaya et al., 2006). It has been proposed that Nav1.9 plays a crucial role in setting the resting membrane potential of a neuron and that an increased density of this channel, such as is seen with the accumulation of voltage gated sodium channels at the tips of the injured neurons (Devor et al., 1989; England et al., 1994, 1996), may hyperpolarize the neuron (Herzog et al., 2001). It thus appears that the decreased expression of Nav1.8 by exogenous NT-3 likely plays an important role in preventing the development of thermal hyperalgesia, while the decreased expression of Nav1.9 may prevent hyperexcitablity and/or repetitive firing of the neuron by increasing the resting membrane potential of these neurons. The ability of NT-3 to modulate sodium channel expression is not limited to Nav1.8 and Nav1.9. In preliminary studies, we have obtained similar findings for Nav1.3 (TDW-G and VMKV, unpublished findings), a TTX-sensitive sodium channel whose expression is elevated in response to nerve injury (Lindia et al., 2005). The effect of NT-3 is also not just a global nonselective affect that inhibits translation, as we have recently found somatostatin expression not to be altered by NT-3 treatment (TDW-G and VMKV, unpublished findings), and also observed expression of other markers to be upregulated in injured neurons expressing trkC, including expression of trkC (Verge et al., 1996; Jongsma Wallin et al., 2001; Karchewski et al., 2002)
Potential mechanisms of modulation of sodium channel expression by NT-3
Inflammatory mediators (including NGF) are capable of increasing the expression voltage gated sodium channels (Dib-Hajj et al., 1998; Fjell et al., 1999; Gould et al., 2000; Fang et al., 2005; Amaya et al., 2006). It has also been postulated that the NGF regulation of Nav1.8 is limited by availability of its cognate receptor trkA (Fang et al., 2005). It therefore seems plausible that the ability of NT-3 to downregulate expression of trkA (Wilson-Gerwing and Verge, 2006) and to act in an antagonistic fashion to the pro-inflammatory effects of NGF (Wilson-Gerwing et al., 2005) may also underlie its ability to attenuate expression of the TTX-R sodium channel Nav1.8 and possibly Nav1.9. NT-3 can mitigate many aspects of trkA-associated nociceptive phenotype in vivo, including effecting a notable reduction in expression of trkA, NGF high-affinity binding sites, BDNF, substance P, and PACAP in the nociceptive subpopulation (Jongsma Wallin et al., 2001; Karchewski et al., 2002; Gratto and Verge, 2003). Up to half of trkA neurons express a low level of the cognate NT-3 receptor, trkC, while all express the common neurotrophin receptor p75 (Karchewski et al., 1999). It is unlikely that NT-3 signaling by trkC greatly influences the anti-nociceptive responses observed in intact neurons, because the downregulation of BDNF, substance P, and PACAP occurs predominantly in the subpopulation of trkA neurons that lack trkC and express the neuropeptide substance P (Jongsma Wallin et al., 2001; Karchewski et al., 2002; Gratto and Verge, 2003). The ability of NT-3 to differentially regulate phenotype in trkC versus trkA neurons may be linked to its differential influence on activated MAPK signaling in these two populations. In preliminary work, we find that NT-3 treated neurons expressing only trkA have a significantly reduced level of activated/nuclear localized ERK1/2 signaling relative to control, while those expressing only trkC have elevated levels of activated/nuclear localized ERK1/2, with no net effect observed for neurons expressing both trkA and trkC (Wilson-Gerwing and Verge, 2005).
Whether NT-3 mediates it affect on the nociceptive subpopulation by signaling directly through trkA is currently being investigated. While NT3 is best known for its ability to signal through its cognate receptor trkC, it is also able to interact with trkA (Lamballe et al., 1991; Ip et al., 1993). Signaling via the trkA family of receptors may also be modulated by select expression of isoforms of full length trkA receptors. The splice variants of the trkA receptor encode two receptors with or without a 6 amino acid (a.a.) insert in the extracellular binding domain. This insert region does not appear to affect the receptor’s binding specificity or functional response to NGF, but can confer enhanced responsiveness of the receptor to NT-3 (Barker et al., 1993; Clary et al., 1994). We have shown virtually all trkA sensory neurons to co-express both isoforms, thus providing an anatomical substrate for this interaction (Karchewski et al., 1999). In addition, because in other cell types p75 has been shown to inhibit NT-3 signaling via trkA (Mischel et al., 2001), our observed downregulation of neuronal p75 expression following CCI may allow for enhanced signaling via trkA by NT-3. Even though NT-3 infusion resulted in some neurons expressing higher levels of p75, this effect was limited to large size neurons that are likely to be trkC-positive (Verge et al., 1996), while expression in small and medium size neurons consistent with the nociceptive subpopulation remained depressed. An enhanced ability of NT-3-to signal via trkA when p75 levels are decreased may account for the more robust response observed ipsilateral to CCI in this and past studies (Wilson-Gerwing et al., 2005; Wilson-Gerwing and Verge 2006).
Finally, there is some evidence that NT-3 may be able to act through the glial derived neurotrophic factor (GDNF) receptor c-Ret (Kobayashi and Masuoka, 2000) and thus may exert its effects on Nav1.8 and Nav1.9 in this manner. Similar to NGF, GDNF has been shown to upregulate Nav1.8 and Nav1.9 following sciatic nerve transection (Cummins et al., 2000) and to reduce ectopic neuronal discharges (spontaneous pain) after nerve injury (Boucher et al., 2000) – a property of neuropathic pain attributed to Nav1.9.
Clinical relevance of reduced Nav1.8 and Nav1.9 expression
The use of sodium channel blockers to treat both neuropathic and inflammatory pain in a clinical setting is well known to result in analgesia (Galer, 1995; McQuay et al., 1995; Clayton et al., 1997; Evans et al., 1997; Trezise and Xie, 1997). These include such treatments as topical creams (for example, lidocaine), anticonvulsants, and tricyclic antidepressants (reviewed in Rogers et al., 2006).
It therefore becomes important to ask: Does the decreased expression of Nav1.8 and Nav1.9 also result in a diminished channel activity? It has been demonstrated that a significant downregulation of these two channels also resulted in the significant reduction of the TTX-R sodium current (Dib-Hajj et al., 1999). In addition, phosphorylation of Nav1.8 by p38 MAPK, a signalling pathway implicated in the regulation of TRPV1 (Wilson Gerwing et al., 2005), increases current density within DRG neurons (Hudmon et al., 2008). We have previously reported that exogenous NT-3 greatly dampens p38 MAPK signalling in DRG neurons subjected to CCI (Wilson Gerwing et al., 2005). This, in addition to its ability to attenuate sodium channel expression could have a significant impact on overall channel activity and contributions to the neuropathic pain state.
In conclusion, investigation into the ability of exogenous NT-3 to modulate expression of two TTX-R sodium channels, Nav1.8 and Nav1.9 that are implicated in the generation of neuropathic pain, reveals that NT-3 can effectively antagonize yet another pro-inflammatory aspect of the neuropathic pain state and in doing so presumably alter the electrophysiological properties of these neurons.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research to VMKV (Grant #MOP74747). TDW-G is supported by a scholarship from the University of Saskatchewan, Saskatchewan Health Research Foundation – RPP, and the Canadian Institutes of Health Research. We thank Regeneron Pharmaceuticals (Tarrytown, NY) for the generous supply of NT-3 used in this study.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–12860. doi: 10.1523/JNEUROSCI.4015-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN. GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol. 2003;548:373–382. doi: 10.1113/jphysiol.2003.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PA, Lomen-Hoerth C, Gensch EM, Meakin SO, Glass DJ, Shooter EM. Tissue-specific alternative splicing generates two isoforms of the trk A receptor. J Biol Chem. 1993;268:15150–15157. [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Brain Res Mol Brain Res. 1996;43:117–131. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108:237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- Clary DO, Reichardt LF. An alternatively spliced form of the nerve growth factor receptor TrkA confers an enhanced response to neurotrophin 3. Proc Natl Acad Sci U S A. 1994;91:11133–7. doi: 10.1073/pnas.91.23.11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton NM, Collins E, Sargent R, Brown T, Nobbs M, Bountra C. The anti-hypersensitivity activity of the sodium channel blocker 4030W92 in models of acute and chronic inflammatory pain and neuropathic pain in the rat. American Pain Society. 1997;16:163. [Google Scholar]
- Cummins TR, Black JA, Dib-Hajj SD, Waxman SG. Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J Neurosci. 2000;20:8754–8761. doi: 10.1523/JNEUROSCI.20-23-08754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci. 1999;19:RC43. doi: 10.1523/JNEUROSCI.19-24-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagerlind A, Friberg K, Bean AJ, Hokfelt T. Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry. 1992;98:39–49. doi: 10.1007/BF00716936. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Ji RR, Abdi S, Tate S, Woolf CJ. The pattern of expression of the voltage-gated sodium channels Na(v)1.8 and Na(v)1.9 does not change in uninjured primary sensory neurons in experimental neuropathic pain models. Pain. 2002;96:269–277. doi: 10.1016/S0304-3959(01)00456-0. [DOI] [PubMed] [Google Scholar]
- Devor M, Keller CH, Deerinck TJ, Levinson SR, Ellisman MH. Na+ channel accumulation on axolemma of afferent endings in nerve end neuromas in Apteronotus. Neurosci Lett. 1989;102:149–154. doi: 10.1016/0304-3940(89)90070-0. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Fjell J, Cummins TR, Zheng Z, Fried K, LaMotte R, Black JA, Waxman SG. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain. 1999;83:591–600. doi: 10.1016/S0304-3959(99)00169-4. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci U S A. 1998;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol. 2003;550:739–752. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England JD, Gamboni F, Ferguson MA, Levinson SR. Sodium channels accumulate at the tips of injured axons. Muscle Nerve. 1994;17:593–598. doi: 10.1002/mus.880170605. [DOI] [PubMed] [Google Scholar]
- England JD, Happel LT, Kline DG, Gamboni F, Thouron CL, Liu ZP, Levinson SR. Sodium channel accumulation in humans with painful neuromas. Neurology. 1996;47:272–276. doi: 10.1212/wnl.47.1.272. [DOI] [PubMed] [Google Scholar]
- Evans KS, Scott CM, Bountra C. The effect of the novel sodium channel blocker 4030W92 on a carrageenin induced cutaneous hypersensitivity in the anaesthetised rat. American Pain Society. 1997;16:186. [Google Scholar]
- Fang X, Djouhri L, Black JA, Dib-Hajj SD, Waxman SG, Lawson SN. The presence and role of the tetrodotoxin-resistant sodium channel Na(v)1.9 (NaN) in nociceptive primary afferent neurons. J Neurosci. 2002;22:7425–7433. doi: 10.1523/JNEUROSCI.22-17-07425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Okuse K, Waxman SG, Lawson SN. trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci. 2005;25:4868–4878. doi: 10.1523/JNEUROSCI.0249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell J, Cummins TR, Davis BM, Albers KM, Fried K, Waxman SG, Black JA. Sodium channel expression in NGF-overexpressing transgenic mice. J Neurosci Res. 1999;57:39–47. doi: 10.1002/(SICI)1097-4547(19990701)57:1<39::AID-JNR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Galer BS. Neuropathic pain of peripheral origin: advances in pharmacologic treatment. Neurology. 1995;45:S17–25. doi: 10.1212/wnl.45.12_suppl_9.s17. discussion S35–16. [DOI] [PubMed] [Google Scholar]
- Gold MS, Weinreich D, Kim CS, Wang R, Treanor J, Porreca F, Lai J. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, 3rd, England JD, Soignier RD, Nolan P, Minor LD, Liu ZP, Levinson SR, Paul D. Ibuprofen blocks changes in Na v 1.7 and 1.8 sodium channels associated with complete Freund’s adjuvant-induced inflammation in rat. J Pain. 2004;5:270–280. doi: 10.1016/j.jpain.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Gould HJ, 3rd, Gould TN, England JD, Paul D, Liu ZP, Levinson SR. A possible role for nerve growth factor in the augmentation of sodium channels in models of chronic pain. Brain Res. 2000;854:19–29. doi: 10.1016/s0006-8993(99)02216-7. [DOI] [PubMed] [Google Scholar]
- Gratto KA, Verge VM. Neurotrophin-3 down-regulates trkA mRNA, NGF high-affinity binding sites, and associated phenotype in adult DRG neurons. Eur J Neurosci. 2003;18:1535–1548. doi: 10.1046/j.1460-9568.2003.02881.x. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Cummins TR, Waxman SG. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J Neurophysiol. 2001;86:1351–1364. doi: 10.1152/jn.2001.86.3.1351. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Lin JH, Stanisz A, Grundy D, Aerssens J, Peeters PJ, Moechars D, Coulie B, Stead RH. Dissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null mice. J Physiol. 2006;576:257–267. doi: 10.1113/jphysiol.2006.113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Wiley JW. Altered expression and function of sodium channels in large DRG neurons and myelinated A-fibers in early diabetic neuropathy in the rat. Biochem Biophys Res Commun. 2006;339:652–660. doi: 10.1016/j.bbrc.2005.11.057. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci. 2008;28:3190–201. doi: 10.1523/JNEUROSCI.4403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, Stitt TN, tapley P, Klein R, Glass DJ, Fandi J, Greene LA, Barbacid M, Yancopoulos GD. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- Jongsma Wallin H, Danielsen N, Johnston JM, Gratto KA, Karchewski LA, Verge VM. Exogenous NT-3 and NGF differentially modulate PACAP expression in adult sensory neurons, suggesting distinct roles in injury and inflammation. Eur J Neurosci. 2001;14:267–282. doi: 10.1046/j.0953-816x.2001.01641.x. [DOI] [PubMed] [Google Scholar]
- Joshi SK, Mikusa JP, Hernandez G, Baker S, Shieh CC, Neelands T, Zhang XF, Niforatos W, Kage K, Han P, Krafte D, Faltynek C, Sullivan JP, Jarvis MF, Honore P. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. Pain. 2006;123:75–82. doi: 10.1016/j.pain.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- Karchewski LA, Kim FA, Johnston J, McKnight RM, Verge VMK. Anatomical evidence supporting the potential for modulation by multiple neurotrophins in the majority of adult lumbar sensory neurons. J Comp Neurol. 1999;413(2):327–41. doi: 10.1002/(sici)1096-9861(19991018)413:2<327::aid-cne11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Karchewski LA, Gratto KA, Wetmore C, Verge VM. Dynamic patterns of BDNF expression in injured sensory neurons: differential modulation by NGF and NT-3. Eur J Neurosci. 2002;16:1449–1462. doi: 10.1046/j.1460-9568.2002.02205.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Matsuoka I. Enhancement of sympathetic neuron survival by synergistic action of NT3 and GDNF. Neuroreport. 2000;11:2541–2545. doi: 10.1097/00001756-200008030-00039. [DOI] [PubMed] [Google Scholar]
- Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95:143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Lamballe F, Klein R, Barbacid M. TrkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991;66:967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Lindia JA, Kohler MG, Martin WJ, Abbadie C. Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain. 2005;117:145–53. doi: 10.1016/j.pain.2005.05.027. [DOI] [PubMed] [Google Scholar]
- McQuay H, Carroll D, Jadad AR, Wiffen P, Moore A. Anticonvulsant drugs for management of pain: a systematic review. Bmj. 1995;311:1047–1052. doi: 10.1136/bmj.311.7012.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel PS, Smith SG, Vining ER, Valetta JS, Mobley WC, Reichardt LF. The extracellular domain of p75NTR is necessary to inhibit neurotrophin-3 signaling through TrkA. J Biol Chem. 2001;276:11294–11301. doi: 10.1074/jbc.M005132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Lai J, Bian D, Wegert S, Ossipov MH, Eglen RM, Kassotakis L, Novakovic S, Rabert DK, Sangameswaran L, Hunter JC. A comparison of the potential role of the tetrodotoxin-insensitive sodium channels, PN3/SNS and NaN/SNS2, in rat models of chronic pain. Proc Natl Acad Sci U S A. 1999;96:7640–7644. doi: 10.1073/pnas.96.14.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest BT, Murphy BA, Lindia JA, Diaz C, Abbadie C, Ritter AM, Liberator P, Iyer LM, Kash SF, Kohler MG, Kaczorowski GJ, MacIntyre DE, Martin WJ. Contribution of the tetrodotoxin-resistant voltage-gated sodium channel NaV1.9 to sensory transmission and nociceptive behavior. Proc Natl Acad Sci U S A. 2005;102:9382–9387. doi: 10.1073/pnas.0501549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–7. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Hormuzdiar WN, Waxman SG. alpha-SNS produces the slow TTX-resistant sodium current in large cutaneous afferent DRG neurons. J Neurophysiol. 2000;84:710–718. doi: 10.1152/jn.2000.84.2.710. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Verge VM, Riopelle RJ. Quantitative radioautography for NGF receptors. In: Rush AM, editor. Nerve Growth Factors. John Wiley & Sons; London: 1989. pp. 315–326. [Google Scholar]
- Rogers M, Tang L, Madge DJ, Stevens EB. The role of sodium channels in neuropathic pain. Semin Cell Dev Biol. 2006 doi: 10.1016/j.semcdb.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Rush AM, Brau ME, Elliott AA, Elliott JR. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia. J Physiol. 1998;511 (Pt 3):771–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Waxman SG. PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res. 2004;1023:264–271. doi: 10.1016/j.brainres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport. 1998;9:967–972. doi: 10.1097/00001756-199804200-00003. [DOI] [PubMed] [Google Scholar]
- Trezise DJ, Xie X. Sodium channel blocking properties of the novel antihypersensitivity agent, 4030W92, in rat sensory neurons in vitro. American Pain Society. 1997;16:170. [Google Scholar]
- Verge VM, Gratto KA, Karchewski LA, Richardson PM. Neurotrophins and nerve injury in the adult. Philos Trans R Soc Lond B Biol Sci. 1996;351:423–430. doi: 10.1098/rstb.1996.0038. [DOI] [PubMed] [Google Scholar]
- Verge VM, Richardson PM, Benoit R, Riopelle RJ. Histochemical characterization of sensory neurons with high-affinity receptors for nerve growth factor. J Neurocytol. 1989;18:583–591. doi: 10.1007/BF01187079. [DOI] [PubMed] [Google Scholar]
- Verge VM, Richardson PM, Wiesenfeld-Hallin Z, Hokfelt T. Differential influence of nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons. J Neurosci. 1995;15:2081–2096. doi: 10.1523/JNEUROSCI.15-03-02081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge VM, Xu Z, Xu XJ, Wiesenfeld-Hallin Z, Hokfelt T. Marked increase in nitric oxide synthase mRNA in rat dorsal root ganglia after peripheral axotomy: in situ hybridization and functional studies. Proc Natl Acad Sci U S A. 1992;89:11617–11621. doi: 10.1073/pnas.89.23.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Gerwing TD, Verge VMK. Neurotrophin-3 (NT-3) differentially modulates levels of activated extracellular signal-related kinases (ERKs) in intact adult rat dorsal root ganglion (DRG) Soc for Neurosci Abstracts 860.14 2005 [Google Scholar]
- Wilson-Gerwing TD, Dmyterko MV, Zochodne DW, Johnston JM, Verge VM. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J Neurosci. 2005;25:758–767. doi: 10.1523/JNEUROSCI.3909-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Gerwing TD, Verge VM. Neurotrophin-3 attenuates galanin expression in the chronic constriction injury model of neuropathic pain. Neuroscience. 2006;141:2075–2085. doi: 10.1016/j.neuroscience.2006.05.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.