Abstract
Objectives
To study the influence of genetics on the development of hip osteoarthritis as determined by structural change on plain radiographs.
Design
Sibling study.
Setting
Nottinghamshire, England.
Participants
392 index participants with hip osteoarthritis of sufficient severity to warrant total hip replacement, 604 siblings of the index participants, and 1718 participants who had undergone intravenous urography.
Main outcome measure
Odds ratios for hip osteoarthritis in siblings.
Results
The age adjusted odds ratios in siblings were 4.9 (95% confidence interval, 3.9 to 6.4) for probable hip osteoarthritis and 6.4 (4.5 to 9.1) for definite hip osteoarthritis. These values were not significantly altered by adjusting for other risk factors.
Conclusion
Siblings have a high risk of hip osteoarthritis as shown by structural changes on plain radiographs. One explanation is that hip osteoarthritis is under strong genetic influence.
Introduction
Osteoarthritis of the hip is a major cause of pain and disability in the community, resulting in high medical and social costs.1 For any individual, development of hip osteoarthritis is likely to be multifactorial and depends on individual constitutional and “genetic” susceptibility and local mechanical risk factors. The most studied risk factors associated with hip osteoarthritis are obesity, heavy lifting at work (particularly in farm labourers), and developmental dysplasia, although few cases can be directly attributable to such risk factors in the United Kingdom.2–7
Evidence for a genetic predisposition to osteoarthritis is strongest in the subset of patients with generalised nodal osteoarthritis. This was initially reported half a century ago, although robust epidemiological evidence has only recently been forthcoming.8 In a classic twin study, the heritability was estimated as 39% for osteoarthritis of the knee and 65% for osteoarthritis of the hand.9 Further evidence of familial aggregation of hand and knee osteoarthritis has come from cohort studies of volunteers in Framingham and Baltimore.10,11 Neither study has, however, reported data for hip osteoarthritis, partly because of its lower prevalence compared with hand and knee osteoarthritis. Although there have been two previous attempts to determine whether hip osteoarthritis clusters in families, these studies have been either small or based on questionnaire responses only, with inadequate control groups.12,13 We aimed to determine whether there is an important genetic influence on the development of hip osteoarthritis and, if so, whether this relation is independent of other risk factors such as body mass index and nodal status.
Participants and methods
Approval for our study was obtained from the research ethics committees of Nottingham City Hospital and north Nottinghamshire. We compared the prevalence of hip osteoarthritis, as determined by structural changes on plain radiographs, in two groups of participants. These groups differed only in their level of exposure to a genetic risk of hip osteoarthritis. The groups comprised: (a) index participants with primary hip osteoarthritis of sufficient severity to warrant a total hip replacement, a surrogate measure of severe symptomatic and structural disease3,14; (b) siblings of these index participants, who have a high exposure to genetic risk of hip osteoarthritis; and (c) participants who had undergone intravenous urography (urography participants), assumed to represent the average genetic susceptibility of the general population. There is no theoretical reason why these participants should be at any increased or decreased risk of having hip osteoarthritis.
Case ascertainment
Index participants
We identified index participants as patients who had undergone a total hip replacement (primary or revision) at two major orthopaedic centres in Nottinghamshire (Nottingham City and Harlow Wood Hospital) between 1990 and 1996 or who were awaiting this procedure. We only included patients if they had a diagnostic code of primary hip osteoarthritis, and we excluded patients aged over 90 or those who had had surgery at under 40. Index participants were sent a questionnaire designed to examine risk factors for hip osteoarthritis, including height, weight, job title, and self reported finger nodes using a validated diagram (“nodal” was defined as one Heberden's node on at least one finger of each hand).15
Respondents were asked to provide the names and addresses of all living siblings, irrespective of loss of regular contact or whether the sibling had known joint problems. A reminder was sent to non-responders after 4-6 weeks.
Siblings
We identified siblings from the above responses. For practical reasons we elected to study only Nottinghamshire residents. An identical questionnaire was mailed to the siblings, including an invitation to undergo pelvic radiography. We excluded siblings aged under 40 because the low prevalence of change identified by radiography below this age did not justify exposure to radiation.
We emphasised that the invitation was regardless of hip pain or arthritis, and we requested that the questionnaires were returned even if radiography was not wanted. A reminder was sent to non-responders after 4-6 weeks.
Urography participants
We obtained the radiographs of patients aged 40-85 years who had undergone intravenous urography between 1994 and 1996. We also included in the study a “control” urogram that visualised the pelvis, if available. To increase study power, we aimed to recruit about three times as many urography participants of the same sex and similar age (within two years) than siblings. Those alive in 1998 were sent a questionnaire inquiring about risk factors for hip osteoarthritis.
Radiographic assessment
To enable a direct comparison of radiographs, the siblings underwent a modified pelvic radiography with the same radiographic technique used for a “control” urogram (tube to film distance 100 cm, beam centred to allow visualisation of the L1 vertebra to the lesser trochanter). All radiographs were read blind by a single observer. Individual radiographic features of osteoarthritis (narrowing, osteophyte, sclerosis, and cysts) were graded 0-3 according to a standard atlas.16 An overall qualitative grade of 0-5 was assigned according to a recommended method.17 Minimum hip joint space at the site of maximum narrowing was measured by a metered dial calliper (RS Components, Switzerland) to within 0.1 mm. Three standard definitions of hip osteoarthritis were utilised according to the minimum hip joint space: 2.5 mm or less (probable hip osteoarthritis), 1.5 mm or less (definite hip osteoarthritis), and an overall qualitative grade of 3-5, about equivalent to Kellgren and Lawrence grades 3-4.17 All definitions were assumed to have been satisfied if a total hip replacement was present. Reproducibility was assessed in a subset of 40 radiographs, read blind about one month apart.
Statistics
We quantified the levels of agreement with the kappa statistic18; reproducibility of continuous variables was assessed by using Bland and Altman's method.19 We performed unmatched analyses by selecting the “worst hip” per participant—that is, the one showing the greatest severity of the feature under study. We calculated odds ratios by unconditional logistic regression with SPSS.
Results
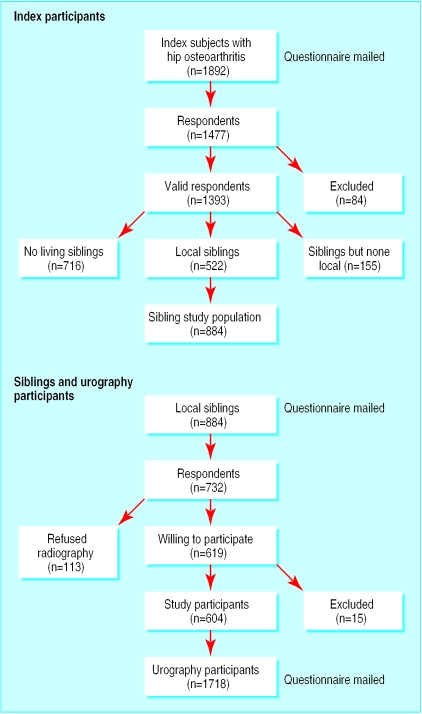
Questionnaires were sent to 1892 index participants, comprising 1134 women and 758 men. Overall, 1477 replies were received, a response rate of 78% (fig 1). Forty five questionnaires were returned uncompleted, and 39 subjects were excluded who indicated alternative diagnoses, the most common being rheumatoid arthritis and hip fracture (11 participants each). Of the 1393 valid respondents, 716 (51%) had no living siblings within the United Kingdom and 155 (11%) had siblings but none of whom resided locally. The remaining 522 index participants (37%) had at least one sibling resident in Nottinghamshire.
Figure 1.
Recruitment of participants
Questionnaires were sent to 884 siblings from these 522 families. Overall, 735 replied, a response rate of 83% (fig 1). Of these respondents, 619 (84%) agreed to undergo pelvic radiography or had a pre-existing recent radiograph. Four respondents were excluded because they were under 40, and 11 were excluded because review of the radiographs of their index hip participants showed alternative diagnoses: rheumatoid arthritis (three participants), Paget's disease (one), and hip fracture (one). The remaining 604 sibling participants (354 women, 250 men) comprised 70% of all eligible local siblings and were derived from 392 families (table 1).
Table 1.
Age of participants by sex
| Index participants
|
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All
|
With siblings*
|
Siblings
|
Controls
|
||||||||
| Women (n=833) | Men (n=560) | Women (n=241) | Men (n=151) | Women (n=354) | Men (n=250) | Women (n=981) | Men (n=737) | ||||
| Age: | |||||||||||
| Mean (95% CI) | 73 (72.4 to 73.6) |
71 (70.3 to 71.7) |
70.7 (69.6 to 71.8) |
68.7 (67.5 to 69.9) |
65.5 (64.5 to 66.5) |
65.2 (64 to 66.4) |
65.2 (64.6 to 65.8) |
65.1 (64.4 to 65.8) |
|||
| Range | 42-90 | 46-90 | 43-87 | 51-86 | 42-89 | 41-88 | 43-90 | 43-86 | |||
Who subsequently underwent pelvic radiography.
Overall, 1718 “control” urograms were analysed, comprising 981 women and 737 men (table 1). A further 21 were not included because the relevant urograms were missing or did not visualise the pelvis. In total, 985 replies were received from the 1442 participants still alive in 1998, a response rate of 68%.
Reproducibility
Reproducibility of the measurement of the width of the joint space was good (mean difference 0.02 mm, 95% limits of agreement 0.5 (SD) mm). Kappa values for narrowing, femoral head osteophyte, cysts, and overall grade were good (greater than 0.7), with lower values for femoral neck and acetabular osteophyte and sclerosis (see table A on website).
Prevalence of hip osteoarthritis
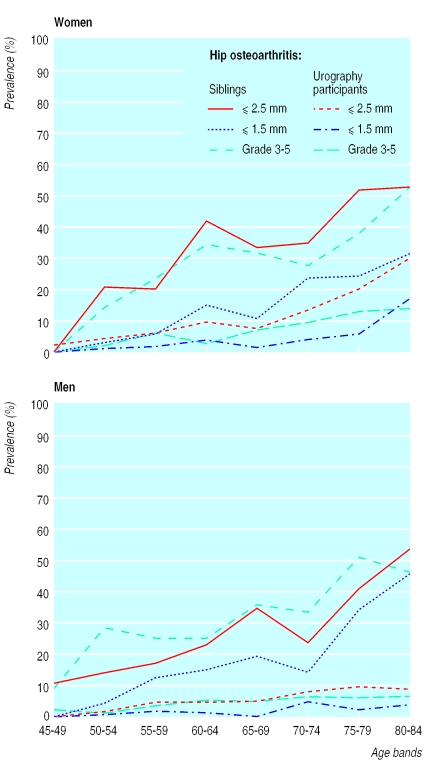
The overall prevalence of hip osteoarthritis in urography participants varied between 3.8%-11% in women and 2.1%-5.9% in men, according to disease definition (table 2). Prevalence increased with age, with a noticeable increase above 75 years found in women only (fig 2). Among siblings, prevalence was about threefold to eightfold higher (table 2). For individual radiographic features there was an increased frequency of all grades of change (1-3) in siblings.
Table 2.
Prevalence of radiographic change in siblings and controls. Values are numbers (percentages)
| Women
|
Men
|
||||
|---|---|---|---|---|---|
| Siblings (n=354) | Controls (n=981) | Siblings (n=250) | Controls (n=737) | ||
| Joint space narrowing (minimum hip joint space): | |||||
| >2.5 mm | 235 (66.4) | 874 (89.1) | 181 (72.4) | 693 (94.0) | |
| ⩽2.5 mm | 119 (33.6) | 107 (10.9) | 69 (27.6) | 44 (6.0) | |
| ⩽1.5 mm | 52 (14.7) | 40 (4.1) | 46 (18.4) | 15 (2.0) | |
| Bilateral ⩽2.5 mm | 47 (13.3) | 34 (3.5) | 29 (11.6) | 8 (1.1) | |
| Osteophyte grade*: | |||||
| 0 | 207 (64.5) | 924 (89.3) | 146 (64.9) | 652 (86.7) | |
| 1 | 56 (17.4) | 67 (6.5) | 31 (13.8) | 52 (6.9) | |
| 2 | 48 (15.0) | 39 (3.8) | 31 (13.8) | 41 (5.5) | |
| 3 | 10 (3.1) | 5 (0.5) | 17 (7.6) | 7 (0.9) | |
| Narrowing grade*: | |||||
| 0 | 162 (50.5) | 840 (81.2) | 118 (52.4) | 636 (84.6) | |
| 1 | 117 (36.4) | 153 (14.8) | 77 (34.2) | 94 (12.5) | |
| 2 | 29 (9.0) | 32 (3.1) | 16 (7.1) | 14 (1.9) | |
| 3 | 13 (4.0) | 10 (1.0) | 14 (6.2) | 8 (1.1) | |
| Overall grade: | |||||
| 0 | 131 (37.0) | 738 (75.2) | 104 (41.6) | 563 (76.4) | |
| 1 | 33 (9.3) | 54 (5.5) | 16 (6.4) | 59 (8.0) | |
| 2 | 86 (24.3) | 120 (12.2) | 47 (18.8) | 78 (10.6) | |
| 3 | 55 (15.5) | 39 (4.0) | 36 (14.4) | 21 (2.8) | |
| 4 | 14 (4.0) | 14 (1.4) | 18 (7.2) | 8 (1.1) | |
| 5 | 2 (0.6) | 3 (0.3) | 4 (1.6) | 2 (0.3) | |
| 3-5† | 103 (29.1) | 69 (7.0) | 83 (33.2) | 37 (5.3) | |
| Total hip replacement | 32 (9.0) | 13 (1.3) | 25 (10) | 6 (0.8) | |
Participants with bilateral total hip replacement excluded owing to inability to categorise osteophyte or narrowing.
Including total hip replacement.
Figure 2.
Prevalence of hip osteoarthritis in siblings and urography participants
In male siblings, the odds ratios of hip osteoarthritis were 6.4 for a minimum hip joint space of 2.5 mm or less and 11.8 for one of 1.5 mm or less; the odds were lower in females (4.5 and 4.2 respectively) and did not vary significantly according to the severity of the changes on radiography (table 3).
Table 3.
Odds ratios (95% confidence intervals) of osteoarthritis of hip in siblings. Values are adjusted for age
| Definition | Women | Men | Both sexes |
|---|---|---|---|
| Minimum hip joint space: | |||
| ⩽2.5 mm | 4.45 (3.26 to 6.07) | 6.41 (4.19 to 9.78) | 5.03 (3.93 to 6.46) |
| ⩽1.5 mm | 4.21 (2.76 to 6.56) | 11.85 (6.39 to 21.9) | 6.21 (4.36 to 8.84) |
| Bilateral ⩽2.5 mm | 4.30 (2.71 to 6.84) | 12.5 (5.60 to 27.9) | 5.86 (3.95 to 8.68) |
| Grade 3-5 | 5.72 (4.06 to 8.07) | 9.85 (6.41 to 15.1) | 7.12 (6.45 to 9.31) |
| Total hip replacement | 7.73 (4.0 to 14.9) | 14.4 (5.77 to 35.8) | 9.76 (5.74 to 16.6) |
Selecting for analysis of only one sibling per family (using random number tables) did not alter these results. If the most severe response bias is assumed—that is, that none of the 265 non-participants had osteoarthritis, although the odds are reduced at 2.8 for probable and 4 for definite osteoarthritis, they remain highly significant.
Potential confounding factors—No significant differences were found in mean body mass index or nodal status (28% of women and 8% of men) between siblings and urography participants, and adjusting for these potential confounders did not alter the odds ratios.
Discussion
Siblings of patients who have undergone a hip replacement are at a substantially increased risk of osteoarthritis of the hip. This increased risk exists across all age groups and is present regardless of which aspects of structural change are used for disease definition. These results robustly support the hypothesis that the development of hip osteoarthritis is under strong genetic influence.
What is already known on this topic
Osteoarthritis of the hip is a major cause of pain and disability in the community
The risk of developing hip osteoarthritis is likely to be multifactorial, depending on individual genetic susceptibility and local mechanical risk factors
Several studies have provided evidence for familial aggregation of osteoarthritis of the hand and knee
What this study adds
Siblings of patients who have undergone a hip replacement for osteoarthritis are at a substantially increased risk of hip osteoarthritis
This finding supports the hypothesis that the development of hip osteoarthritis is under strong genetic influence
Clearly, familial aggregation of disease might also be explained by non-genetic factors, owing to siblings sharing environmental as well as genetic influences. For example, the effects of minor abnormalities of structural development or skeletal growth patterns reflecting the environment in utero might have influenced these results. This effect could have been inflated by including clusters of related individuals—that is, more than one sibling per family. Attempting to restrict these effects by analysing only one random sibling per family, however, did not alter our results. Moreover, rigorous attempts were made at several stages to exclude atypical families with developmental dysplasia; index participants were excluded if they had undergone premature surgery (more likely to be dysplasia), they had a diagnostic code other than primary hip osteoarthritis, or their questionnaire responses suggested alternative diagnoses. We are therefore confident that the participants are representative of families in the community in which one sibling has hip osteoarthritis.
It is possible that index participants may have preferentially provided details of siblings if they were known to have hip pain or arthritis. We attempted to limit this potential bias by emphasising a wish to know about all living siblings, regardless of hip pain. Only 70% of the eligible siblings participated, and it is likely that non-participants had a much lower prevalence of hip osteoarthritis. Even assuming the most severe possible bias, however, siblings remain at a significantly increased risk. For practical reasons we only studied siblings who were resident in Nottinghamshire. Although this could have introduced bias (owing to local siblings possibly sharing similar environmental or occupational influences), the main advantage of this approach was that siblings and urography participants were derived from the same geographical population.
Utilising “control” urograms is a well recognised methodology and avoids the need to radiograph a de novo population, thus limiting both exposure to radiation and costs.17,20 The main indications are to identify calculi and assess structure in the presence of urine abnormalities or suspected malignancy. None of these conditions are known to have any positive or negative association with hip osteoarthritis. In view of these important indications, it is unlikely that hip osteoarthritis or pain would reduce the eligibility for this examination. Thus, the urography participants can be regarded as representative of the background population in terms of risk factors for hip osteoarthritis. Moreover, the prevalence of osteoarthritis in the urography participants broadly agrees with other studies.21–25
Our reported genetic influence is greater than in other studies. This is largely explained by methodological differences in study power and design. Lindberg reported a relative risk of 1.86 among siblings of patients who had undergone hip replacement, but utilised only an unspecified number of pre-existing pelvic radiographs rather than attempting to radiograph all siblings.12 A UK study reported a relative risk for hip replacement of 1.7813; this was determined from questionnaire responses only, and spouses (who share environmental risk factors and might themselves be biased towards surgery) were the urography participant group. These differences might explain the lower risks reported.
Supplementary Material
Acknowledgments
We thank the participants and their families, the orthopaedic surgeons who allowed us to identify their patients, Professor P Croft and Dr J Fairbairn for radiological advice, and Mr B Palmer for statistical advice.
Footnotes
Funding: Arthritis Research Campaign project grant L0510.
Competing interests: None declared.
The reproducibility of ordinal variables appears on the BMJ's website
References
- 1.Gabriel SE, Crowson CS, O'Fallon WM. Costs of osteoarthritis: estimates from a geographically defined population. J Rheumatol. 1995;22(suppl 43):23–25. [PubMed] [Google Scholar]
- 2.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national health and nutrition examination survey (HANES 1). Evidence for an association with overweight, race and physical demands of work. Am J Epidemiol. 1988;128:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 3.Cooper C, Inkskip H, Croft PR, Campbell L, Smith G, McLaren M, et al. Individual risk factors for hip osteoarthritis: obesity, hip injury and physical activity. Am J Epidemiol. 1998;147:516–522. doi: 10.1093/oxfordjournals.aje.a009482. [DOI] [PubMed] [Google Scholar]
- 4.Felson D. Does excess weight cause osteoarthritis and, if so, why? Ann Rheum Dis. 1996;55:668–669. doi: 10.1136/ard.55.9.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thelin A. Hip joint arthrosis: an occupational disorder amongst farmers. Am J Ind Med. 1990;18:339–343. doi: 10.1002/ajim.4700180316. [DOI] [PubMed] [Google Scholar]
- 6.Croft P, Coggon D, Cruddas M, Cooper C. Osteoarthritis of the hip: an occupational disease in farmers. BMJ. 1992;304:1269–1272. doi: 10.1136/bmj.304.6837.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien T, Moran R, McGoldrick F. The aetiology of degenerative disease of the hip. Ir J Med Sci. 1989;158:63–66. doi: 10.1007/BF02942144. [DOI] [PubMed] [Google Scholar]
- 8.Stecher RM. Heberden's nodes. Hereditary in hypertrophic arthritis of the finger joints. Am J Med Sci. 1941;201:801–809. [Google Scholar]
- 9.Spector TD, Cicuttini FM, Baker J, Loughlin J, Hart DJ. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312:940–944. doi: 10.1136/bmj.312.7036.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felson DT, Couropmitree N, Chaisson C, Hannan MT, Zhang Y, McAlindon TE, et al. Evidence for a Mendelian gene in a segregation analysis of generalised osteoarthritis. Arthritis Rheum. 1998;41:1064–1071. doi: 10.1002/1529-0131(199806)41:6<1064::AID-ART13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch R, Lethbridge-Çejku M, Hanson R, Scott WW, Reichle R, Plato CC, et al. Familial aggregation of osteoarthritis. Data from the Baltimore longitudinal study on aging. Arthritis Rheum. 1998;41:1227–1232. doi: 10.1002/1529-0131(199807)41:7<1227::AID-ART13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg H. Prevalence of primary coxarthrosis in siblings of patients with primary coxarthrosis. Clin Orthop. 1986;203:273–275. [PubMed] [Google Scholar]
- 13.Chitnavis J, Sinsheimer J, Clipsham K, Loughlin J, Sykes B, Burge P, et al. Genetic influences in end-stage osteoarthritis. J Bone Joint Surg (Br) 1997;79-B(4):660–664. doi: 10.1302/0301-620x.79b4.7437. [DOI] [PubMed] [Google Scholar]
- 14.Fox K, Hochberg MC, Resnik C, Kenzora J, Hebel R, Zimmerman S, et al. Severity of radiographic findings in hip osteoarthritis associated with total hip arthroplasty. J Rheumatol. 1996;23:693–697. [PubMed] [Google Scholar]
- 15.O'Reilly S, Doherty S, Johnson S, Muir K, Doherty M. Screening for hand osteoarthritis using a postal survey. Osteoarthritis Cartilage. 1999;7:461–465. doi: 10.1053/joca.1999.0240. [DOI] [PubMed] [Google Scholar]
- 16.Altman RD, Hochberg MC, Murphy WA, Wolfe F. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(suppl A):3–70. [PubMed] [Google Scholar]
- 17.Croft P, Cooper C, Wickham C, Coggon D. Defining osteoarthritis of the hip for epidemiologic studies. Am J Epidemiol. 1990;132:514–522. doi: 10.1093/oxfordjournals.aje.a115687. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 19.Bland J, Altman DG. Statistical methods for assessing agreement between two methods of clinical assessment. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- 20.Roach KE, Persky V, Miles T, Budiman-Mak E. Biomechanical aspects of occupation and osteoarthritis of the hip: a case-control study. J Rheumatol. 1994;21:2334–2340. [PubMed] [Google Scholar]
- 21.Tepper S, Hochberg MC. Factors associated with hip osteoarthritis: data from the first national health and nutrition examination survey (NHANES-I) Am J Epidemiol. 1993;137:1081–1088. doi: 10.1093/oxfordjournals.aje.a116611. [DOI] [PubMed] [Google Scholar]
- 22.Danielsson L. Incidence and prognosis of coxarthrosis. Acta Orthop Scand Suppl. 1964;66:1–114. doi: 10.3109/ort.1964.35.suppl-66.01. [DOI] [PubMed] [Google Scholar]
- 23.Jorring K. Osteoarthritis of the hip. Acta Orthop Scand. 1980;51:523–530. doi: 10.3109/17453678008990835. [DOI] [PubMed] [Google Scholar]
- 24.Danielsson L, Lindberg H, Nilsson B. Prevalence of coxarthrosis. Clin Orthop. 1984;191:110–115. [PubMed] [Google Scholar]
- 25.Smith R, Egger P, Coggon D, Cawley MID, Cooper C. Osteoarthritis of the hip joint and acetabular dysplasia in women. Ann Rheum Dis. 1995;54:179–181. doi: 10.1136/ard.54.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.