Abstract
Adaptive immune responses are critical for the control and clearance of influenza A virus (IAV) infection. However, in recent years, it has become increasingly apparent that innate immune cells, including natural killer cells, alveolar macrophages (aMφ), and dendritic cells (DC) are essential following IAV infection in the direct control of viral replication or in the induction and regulation of virus-specific adaptive immune responses. This review will discuss the role of these innate immune cells following IAV infection, with a particular focus on DC and their ability to induce and regulate the adaptive IAV-specific immune response.
Keywords: NK cells, macrophages, dendritic cells
Introduction
Influenza A virus (IAV) infections represent a significant public health threat, particularly in the case of children, the elderly, and those with underlying diseases, all of whom are at a significantly increased risk for disease complications and death following IAV infection [1,2,3]. Seasonal outbreaks alone cause an estimated 200,000 hospitalizations and over 30,000 deaths annually in the United States [4]. In addition to typical seasonal infections, IAV can also undergo substantial changes (through recombination/ antigenic shift) that leave us with little to no protective immunity and increase influenza’s mortality rate even among healthy young adults [5,6,7]. In the last century alone, we have observed three major pandemics: the 1918 Spanish flu, 1957 Asian flu, and the 1968 Hong Kong flu; with the 1918 pandemic being the most significant, causing an estimated 30–50 million deaths worldwide [5, 8]. Furthermore, the recent appearance of IAV strains with pandemic potential, such as H1N1 “swine flu” and H5N1 avian influenza, have highlighted the importance of research into IAV infections and the innate and adaptive immune responses that control and eliminate infection. This review will focus upon the important role innate immunity has in both initial viral control and in inducing and regulating the adaptive immune response to IAV.
NATURAL KILLER CELLS
Natural killer (NK) cells are innate lymphocytes that are best known for their potent cytotoxic activities and robust production of inflammatory cytokines, including IFNγ, TNFα, and MIP-1α [9,10,11]. NK cells recognize and help control a wide range of pathogens, including viruses, bacteria, and intracellular parasites [12,13,14]; however, a role for NK cells in the control of the virus following IAV infection remains relatively understudied. An early report examining the role of NK cells following IAV infection demonstrated that NK cell depletion using antiserum to asialo-GM1 prior to IAV infection renders mice and hamsters more susceptible to IAV-induced morbidity and mortality [15]. More recently, it has been shown that NK cells utilize the natural cytotoxicity receptors (NCR) NKp46 (NCR-1 in the mouse) and NKp44 to recognize IAV hemagglutinins on virally infected target cells [16, 17]. An elegant study by Gazit et al. subsequently demonstrated that mice specifically lacking NCR-1 exhibit increased morbidity and mortality following IAV infection, suggesting an essential role for NKp46 expression on NK cells during immunity to IAV infection [18]. A similar observation of enhanced disease results from NK cell depletion in both C57BL/6 and BALB/c strains. In this model, protection is restored by adoptive transfer of donor NK cells that reconstitute the lungs to <20% of the endogenous pulmonary NK cell content, suggesting that the pulmonary NK cell response to intranasal IAV infection is quite potent (J. McGill, K. Legge, and J. Heusel, unpublished results). Curiously, the majority of pulmonary NK cells display a surface phenotype of cytolytically mature NK cells, whereas NK cells in lymph nodes, including those draining the IAV-infected lung, display the phenotype of cytokine-producing NK cells [19]. This suggests the potential for functionally distinct NK cell responses at these sites during acute IAV infection. Although the above studies suggest an important role for NK cells following IAV infection, it currently remains unclear whether this increased susceptibility results from the lack of direct NK cell cytotoxicity toward infected cells or through the lack of NK cell crosstalk with other immune cell types, a mechanism that has been proposed to enhance both DC [20] and adaptive T-cell responses [21] following IAV infection. More recently, it has been reported that human NK cells respond to IAV-infected DC through multiple cytokine pathways; NK cell cytotoxicity (in vitro) is dependent upon IFN-α, whereas IFNγ production is IL-12 dependent and requires both NKp46 and NKG2D-mediated NK cell activation [22].
ALVEOLAR MACROPHAGES
The lung contains two primary phagocyte populations, DC and alveolar macrophages (aMφ), with the latter being the predominant APC population in the airways during steady-state conditions. Classically, aMφ are thought to have a regulatory phenotype in the lungs, existing in a relatively quiescent state during homeostasis [23]. These resting aMφ produce only low levels of inflammatory cytokines and are less phagocytic than their counterparts in other tissues [23]. Importantly, aMφ have also been shown to suppress the induction of innate and adaptive immunity [24,25,26,27]. In vivo depletion of aMφ by clodronate-liposome administration leads to excessive inflammation and immunity to otherwise harmless inhaled antigens [27], while in vitro incubation of antigen-presenting DC in the presence of aMφ can suppress T-cell activation via a mechanism that involves nitric oxide (NO), IL-10, transforming growth factor-β (TGF-β), and prostaglandins [24]. More recently, it has become evident that one of the primary mechanisms of aMφ-mediated suppression is inhibition of DC maturation through the production of NO [24, 25].
Despite this role as a regulatory cell during steady-state conditions, the inhibitory phenotype of aMφ can be overcome in the face of IAV infection in order to mount potent antiviral immune responses. Following activation, aMφ convert into highly phagocytic cells that produce robust amounts of inflammatory cytokines, including IL-6 and TNFα [28]. In addition to aMφ activation, IAV infection induces significant recruitment of inflammatory monocytes via CCR2 [29], which differentiate into monocyte-derived DC (moDC) and inflammatory “exudate” macrophages [30,31,32,33,34]. While initially inflammatory cells during the course of infection, emerging evidence suggests that these exudate macrophages will eventually develop the suppressor phenotype of lung-resident aMφ, helping to restore preinfection homeostasis in the lungs over the course of a few days [35, 36]. Although aMφ were originally described as the cell type responsible for pulmonary monocyte recruitment during IAV infection, a recent study by Herold et al. suggests that instead the majority of the recruitment results from alveolar epithelial cells that produce high levels of CCL2 (MCP-1), a ligand for CCR2, following infection [34].
In addition to their role in immunity to more “seasonal” strains of IAV, infection with highly pathogenic IAV are also known to induce significant recruitment of aMφ to the lungs [37,38,39]. Although this excessive inflammation was initially thought to contribute to the pathogenicity of the virus, a recent report by Tumpey et al. demonstrates an essential role for these macrophages in regulating antiviral immunity in mice [39]. Depletion of aMφ prior to, but not 3 or 5 days following, IAV infection results in uncontrolled viral replication and a significant increase in IAV-associated mortality [39]. These results suggest that macrophages play a key role in the early control of virus infection prior to the induction of adaptive responses. In accordance with this idea, Kim et al. have demonstrated a similar essential role for aMφ following IAV infection of swine [40]. In this model, although no mortality was observed, swine depleted of aMφ prior to IAV infection exhibited increased respiratory stress, increased induction of IL-10, and a significant reduction in pulmonary TNFα levels. Further, aMφ depletion resulted in reduced antibody titers and reduced numbers of virus-specific CD8 T cells in the lungs at later time points post infection [40]. It has been suggested that aMφ phagocytosis is the key mechanism through which these cells regulate IAV infection, as clearance of apoptotic host cells is essential to limit virus spread. Therein, not surprisingly, inhibition of aMφ and neutrophil phagocytosis in the lungs following IAV infection results in increased viral titers and IAV-associated mortality [41]. Together, the results above suggest an important role for macrophages in the innate immune response in the lungs following IAV infection.
Despite the apparently beneficial role that these cells play in controlling early viral replication, several contrasting reports have demonstrated a more deleterious role for aMφ following IAV infection. Excessive inflammation in the lung is detrimental following a variety of respiratory challenges [42, 43]. The majority of immunopathology associated with IAV infection has been attributed to NOS2 and TNFα, as IAV infection of mice deficient in either exhibit decreased mortality [44,45,46], and antioxidant treatment (which inhibits NOS2) of IAV-infected mice results in improved lung function and accelerated disease resolution [47]. Importantly, both cytokines appear to contribute to pulmonary damage and destruction, but they have little effect in controlling viral replication [48, 49].
Until recently, the phenotype of the cells that produced the majority of NOS2 and TNFα in the lungs following IAV infection was unknown. However, recent work has demonstrated that IAV infection of CCR2-deficient mice results in decreased monocyte/macrophage recruitment to the lungs, as well as reduced lung pathology and mortality compared with wild-type controls [29, 34, 50]. Similarly, a dependence on CCL2 for recruitment of macrophages was observed in mice constitutively overexpressing the CCR2 ligand, CCL2. IAV-infection of these mice significantly increased monocyte/macrophage recruitment to the lungs, immunopathology, and mortality [30]. Finally, Lin et al. have demonstrated that the moDC and exudate macrophages recruited to the lungs during IAV infections via CCR2 are responsible for the majority of NOS2 and TNFα production in the lungs following infection. Together, these studies suggest that macrophages may be a predominant cell population contributing to IAV-associated immunopathology [30].
In addition to their role in promoting cytokine-associated immunopathology, Herold et al. have shown a direct role for macrophages in promoting increased lung epithelial apoptosis following IAV infection. Exudate macrophages express increased levels of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in the lungs following IAV infection [50], and the abrogation of TRAIL signaling by exudate macrophages results in a significant reduction in epithelial cell apoptosis in the lungs, decreased immunopathology, and decreased IAV-associated mortality. Together, these results suggest that macrophage-derived TRAIL may play an important role in promoting epithelial cell apoptosis and lung immunopathology [50].
The above results accentuate the importance of a balanced immune response, particularly in a delicate tissue such as the lungs. Although several groups have demonstrated an essential role for macrophage-derived innate immunity following IAV infection, excessive inflammatory cytokine production, and immune cell recruitment, resulting from activated macrophages can result in increased immunopathology and increased susceptibility to virus-associated mortality.
DC
DC play a key role in bridging innate and adaptive immune responses following IAV infection. In the naïve steady state, DC are located throughout the respiratory tract, including the airway epithelium, lung parenchyma, and the alveolar spaces of the lung [51,52,53,54,55,56,57,58,59], where they constantly survey for invading pathogens or foreign material. Lung-resident DC are a heterogeneous population with respect to surface phenotype and function; however, the predominant DC in the naïve lungs are airway and alveolar DC (aDC), characterized as being CD11c+MHC II+CD11bnegCD4negCD8neg, and interstitial DC (iDC) characterized as being CD11c+MHC II+CD11bhiCD4negCD8neg [52, 53, 60,61,62]. Following a pulmonary insult or infection, there is a significant influx of CD11c+MHC II+ DC into the lungs. This increase is due to both an increase in aDC and iDC numbers, as well as the recruitment of additional subsets such as inflammatory monocyte-derived DC, plasmacytoid DC (pDC), and CD8α+ DC [30, 61,62,63,64,65,66,67,68].
DC ACTIVATION FOLLOWING IAV INFECTION
Induction of adaptive IAV-specific CD8 T-cell responses requires the presentation of peptide antigens by MHC molecules on the surface of mature APCs within the draining LN [69,70,71,72]. Therefore, early studies were focused on identifying the primary cell population involved in initiating virus-specific T-cell immunity following IAV infection. In vitro exposure of DC to IAV was shown to be sufficient to stimulate a primary virus-specific CD8 T-cell response from LN-purified T cells [73,74,75,76]. Further, IAV-infected DC were particularly potent inducers of IAV-specific immunity relative to other APC subsets and could trigger significant CD8 T-cell responses without the addition of exogenous cytokines [73, 75]. Together, these results suggested that DC are likely to be the primary population responsible for the induction of virus-specific CD8 T-cell responses.
Prior to their interactions with naïve T cells in the LN, however, DC within the lungs must acquire IAV-antigen and mature. Although the primary route of DC antigen acquisition in vivo remains unclear, it is likely that DC acquire antigen via two distinct mechanisms: through direct infection with IAV [73, 75,76,77] or through phagocytosis of either dead or dying epithelial cells [69, 71, 78,79,80,81,82,83]. Early in vitro studies demonstrated that DC are susceptible to direct infection by IAV and that IAV infection induces robust maturation and confers DC with the ability to induce potent virus-specific T-cell responses [73, 75, 76]. Interestingly, a fraction of respiratory DC (rDC) examined directly ex vivo from draining LN contain intact influenza NP antigen [84], as well as the nonstructural influenza protein NS1 [85], and early studies have suggested the presence of plaqueable virus in DC isolated from the LN [77], together indicating that a direct infection of DC by IAV may occur in vivo. In support of this idea, recent studies in our laboratory using RT-PCR analysis to examine IAV NS2 mRNA expression in rDC purified from the draining LN suggest that ∼5-10% of DC migrating from the lungs to the LN may be directly infected with virus (R. VanOosten and K. Legge, unpublished results). Interestingly, we have detected only a minor correlation between the initial viral inoculum and the frequency of NS2 mRNA+ DC among migrating rDC in vivo. Instead, it appears that at increasing inoculum doses of IAV, the migrating rDC that are infected carry higher IAV mRNA loads, suggesting an increased MOI per DC at high or lethal doses of infection relative to low or sublethal doses of infection (R. VanOosten and K. Legge, unpublished results).
Studies have demonstrated that the degree of IAV infection of DC can significantly alter their virus-induced cytokine profile and ability to prime IAV-specific CD8 T-cell responses [84, 86,87,88,89]. Interestingly, while in vitro infections with low MOI results in enhanced CD8 T-cell responses following in vitro stimulation, DC infections with high MOI resulted in truncated CD8 T-cell responses, increased IL-12p40 production [89] and increased production of the anti-inflammatory cytokines IL-10 and TGF-β [88]. Given that a lethal dose inoculum of mice with IAV is known to induce increased IL-12 p40 production and a truncated virus-specific CD8 T-cell response relative to a sublethal dose infection [85], these results suggest a potentially important role for direct infection of DC and MOI in the regulation of adaptive immune responses.
In addition to the route of direct infection, DC can also acquire antigen through phagocytosis of dead or dying epithelial cells [69, 71, 78,79,80,81,82,83]. Infection with IAV induces apoptosis of the respiratory epithelium [79, 80, 90]. Encounter of naïve T cells with influenza antigen-bearing immature DC does not drive T-cell activation but rather induces tolerance to viral antigens [91, 92]. However, coupled with IAV-induced inflammation, DC uptake and present apoptosis-derived IAV antigens, mature and initiate an effective adaptive immune response [69, 71, 81, 82]. Albert et al. demonstrated that human DC were able to acquire influenza antigen from apoptotic bodies and induce potent CD8 T-cell responses in vitro [78, 93]. Importantly, neither model of antigen acquisition is mutually exclusive, and it is likely that both direct infection and uptake of apoptotic bodies from dying, virally infected epithelial cells contribute to the ability of rDC to acquire and present viral antigens on their surface.
Until recently, cellular recognition of IAV was thought to be mediated by two families of pattern recognition receptors (PRRs): toll-like receptors (TLRs) 3, 7/8, and 9, which recognize double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), and CpG DNA motifs, respectively [94]; and RIG-I-like helicases (RLHs), which consist primarily of retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene product (MDA-5), which recognize cytoplasmic uncapped 5′-triphosphate RNAs and cytoplasmic dsRNA, respectively [95]. More recently, several groups have demonstrated an important role for the inflammasome, in particular, the components caspase-1 and cryopyrin, in the cellular recognition of IAV and the initiation and regulation of both innate and adaptive antiviral immune responses [96,97,98], suggesting that cells can utilize a variety of redundant pathways to aid in recognition and protection from IAV. Classical DC, which include rDC, are thought to primarily utilize the RLH family of PRRs in the recognition of IAV [99, 100], while pDC are thought to utilize TLR-3, -7, -9 and PKR [100, 101].
Following infection by or encounter with IAV, DC initiate production of proinflammatory cytokines and chemokines that can include IL-6, IL-12, TNF-α, IL-8, IP-10, RANTES, MIP-1β, and most importantly, type 1 interferons (IFNα and IFNβ) [70]. Type I IFNs possess potent antiviral properties and are critical to the control of IAV infection. IFN stimulation induces transcription of several IFN-responsive genes, many of which can protect and interfere with the establishment of IAV infection in uninfected cells [102, 103]. Further, IFN stimulation can regulate innate and adaptive immune responses through its ability to enhance rDC maturation and antigen cross-priming by CD8α+ DC, as well as promote the survival and development of effector functions by activated CD8 T cells [104,105,106,107,108,109,110,111,112,113,114,115].
Given the importance of type I IFN in regulating antiviral immunity, it is not surprising that IAV has evolved to produce a potent IFN antagonist, the NS1 protein. NS1 has been shown to inhibit type I IFN production by infected cells, including classical DC [116,117,118,119,120], as well as to inhibit rDC maturation in vitro, resulting in a poor ability to stimulate virus-specific CD8 T cell responses [116, 117]. Interestingly, this inability to mature in the face of the IAV NS1 protein results not from inhibition of secreted IFN, but instead to an inhibition of the intracellular IFN induction pathway [117]. The ability of NS1 to inhibit DC maturation is timing dependent as pretreatment of human DC with type I IFN allows the DC to respond more efficiently to IAV infection and overcome NS1-mediated inhibition [121]. These results suggest that in vivo, only those DC directly infected by influenza virus early in the infection prior to IFN release, would be at risk for NS1-mediated inhibition as noninfected DC, which uptake viral antigen (i.e., NS1lo to negative cells) and DC infected with virus after type I IFN release, would be less susceptible to this inhibition. In contrast to rDC, pDC are not susceptible to NS1-mediated IFN inhibition and produce robust levels of type I IFN following IAV infection in vitro [121]. However, interestingly, pretreatment with type I IFN prior to IAV exposure can also enhance pDC-mediated cytokine secretion. Together, these results suggest that, in addition to its previously demonstrated role in the inhibition of viral replication, type I IFN can also function to promote enhanced DC activation, and subsequently, more robust adaptive immune responses.
DC MIGRATION FROM THE LUNGS TO THE LN
Migration of rDC from the lungs to the LN is a key step in the initiation of adaptive immune responses following IAV infection. Most studies examining DC trafficking have used intranasal or intratracheal installation of fluorescent dyes [65, 122], fluorescent dye-tagged latex particles or beads [123], or fluorescently coupled macromolecules [54, 124, 125] to track cell migration out of the lungs to the draining LN. Under steady-state conditions, DC migrate continuously, but at low levels, from the lungs to the draining LN, and arrive therein in a semimature state [65, 126]. This continual migration is thought to be important in promoting tolerance to innocuous antigens [53, 58, 59, 127, 128].
Following IAV infection or treatment with TLR agonists, we and others have demonstrated that DC trafficking from the lungs to the LN is rapidly enhanced, inducing a significant influx of mature, lung-derived DC into the draining LN [65, 122, 125]. Using i.n. CFSE administration, we demonstrated that this rapid augmentation of DC migration into the LN is transient, however, peaking within 18 h post infection and returning to baseline levels by 48 h post infection [65, 129]. Contrasting reports have demonstrated a slightly longer period of enhanced DC migration from the lungs to the LN, sometimes as long as 5-7 days post infection [122, 125], suggesting that the period of enhanced migration may be dependent upon the strain of IAV and the dose of infection.
While the influx of lung-derived CD11c+MHC II+ DC into the LN following IAV infection is well described, the phenotype of the antigen-bearing migratory cells remains controversial. Wikstrom et al. demonstrated that, following instillation with FITC-conjugated OVA plus adjuvant, the primary DC subset ferrying antigen from the lungs to the LN expressed low levels of CD11b and CD8α, suggesting the DC were derived from an interstitial DC population [130]. In contrast, Vermaelen et al. suggested that an airway-derived population was more prevalent in the draining LN following instillation by FITC-OVA [54], a result confirmed by Belz et al. [122, 131] and GeurtsvanKessel et al. [132], who independently demonstrated that the primary cells trafficking to the LN following IAV infection are the CD8αnegCD11bneg airway and alveolar DC subsets. More recently, using the FITC-OVA model of DC tracking, Kim and Braciale have demonstrated that two major lung-derived subsets accumulate in the LN following IAV infection: a CD11bhiCD103neg interstitial population and a CD11b+/negCD103+ airway-derived population, the latter of which stained positive for intact IAV proteins [125]. Interestingly, while both DC subsets were shown to express CD8α in the LN, it is likely that this expression was derived via extraction of CD8 from CD8 T cells during antigen-presenting interactions, as this expression was not detected on DC from RAG−/− mice [125]. Confirming Kim’s data in a model of respiratory syncytial virus (RSV) infection and CFSE installation, Lukens et al. also recently observed the migration of both a CD11bhiCD103neg and a CD11bnegCD103+ DC subset from the lungs into the LN [133].
Recently, the factors that regulate DC migration from the lungs to the LN under steady-state or inflammatory conditions have begun to be elucidated. Similar to DC migration from the skin to draining LN, DC utilize CCR7 and its ligands CCL19 and CCL21 to migrate from the lungs to the LN during steady-state conditions [123, 134] and following IAV infection [135]. Consistent with this idea, CCR7−/− mice almost completely lack DC trafficking from the lungs to the LN during IAV infections [135], resulting in undetectable IAV-specific CD8 T-cell responses and a significant reduction in IAV-specific CD4 T-cell responses [135].
In addition to chemokine signals, lipid metabolites, including sphigosine-1-phosphate (S1P) and prostaglandins, are thought to play important roles in regulating DC migration during both steady-state and inflammatory conditions. S1P is known to play a role in a variety of immune cell functions, including migration and cytokine release [136, 137], and intratracheal administration of the sphingosine analog (R)-2-amino-4-(4-heptyloxyphenyl)-2-methylbutanol (AAL-R) following IAV infection inhibits the development of virus-specific CD8 T cell-responses in the lungs, not through a direct effect on the T-cell response itself, but rather through the inhibition of DC trafficking from the lungs to the LN [138, 139]. Additionally, PGD2 has been shown to inhibit the migration of rDC from the lungs to the LN [124], and recent studies within our laboratory have shown that blockade of the interaction of PGD2 with its receptor, DP1, reverses the inhibition of DC migration from the lungs to the LN that occurs starting around 18 h post IAV infection [65] (A. Katewa and K. Legge, unpublished results).
DC AND THE INDUCTION OF ADAPTIVE IMMUNE RESPONSES
Although rDC migration from the lungs to the LN is key for the initiation of adaptive immune responses, as blockade [65, 122] or depletion of rDC [132] prior to IAV infection inhibits activation of naïve IAV-specific CD8 T cells, rDC alone are not the only DC population responsible for activating virus-specific T-cell responses. Belz et al. recently examined the ability of migratory rDC, CD8α+ LN resident, CD8α− LN resident or pDC purified from the lung draining LN of IAV -infected mice to activate naïve, virus-specific CD8 T cells in vitro. These experiments revealed that both rDC that had migrated from the lungs to the LN and CD8α+ LN-resident DC (LNDC) were able to activate naïve virus-specific CD8 T cells [122, 131, 140, 141]. It is likely that the CD8α+ LNDC, which are known to cross-present antigens, acquired viral antigens from the migrating rDC populations, thus allowing them to initiate IAV-specific CD8 T cell responses in the LN [140, 141]. Several subsequent publications examining herpes simplex virus-1 [122, 141,142,143], vaccinia virus [131], lymphocytic choriomeningitis virus, and Listeria monocytogenes [144] have supported the hypothesis that migrating DC subsets transfer antigen to CD8α+ LNDC upon arrival in the draining LN, suggesting that this may be a more global mechanism of promoting robust primary effector T-cell responses.
Interestingly, this transfer of antigen may have a role outside of simply increasing the number of DC presenting viral antigen in the LN, as Belz et al. have recently shown a division of labor between migratory rDC and CD8α+ LNDC in activating naïve and memory virus-specific CD8 T cells following secondary virus challenge [145]. Following IAV infection, both naïve and memory CD8 T cells responded to antigen-bearing CD8α+ DC within the LN, but only naïve CD8 T cells responded to lung-derived migratory rDC. This diminished capacity of pulmonary DC to promote memory CD8 T-cell expansion during subsequent challenge infections may be dependent upon expression of CD70, as LNDC promoted memory CD8 T-cell expansion through CD70/CD27 interactions, while rDC stimulation of naive T cells was CD70 independent [145]. Importantly, these results suggest a unique mechanism for continually promoting naïve CD8 T-cell expansion, even in the face of preexisting memory. Unlike CD8 T-cell activation, naïve IAV-specific CD4 T cells appear to be instead limited to activation via migratory DC populations [146]. Importantly, while the ability to present antigen to IAV-specific T cells is limited to only a few distinct subsets of DC, it appears that IAV antigens are transferred from migratory rDC to several DC subsets within the LN [132]. Therefore, in the future, it will be important to more clearly elucidate the mechanisms and implications of this division of labor between DC subsets in priming/expansion of adaptive immune cells during primary, secondary, tertiary, etc. IAV challenges and following vaccination and boosting responses against IAV.
The kinetics of antigen presentation following IAV infection continues to remain a controversial subject. Antigen presentation and early activation of IAV-specific CD8 T cells in the lung-draining LN initiates at low levels as early as 1-2 days post infection [122, 129, 147], consistent with the data suggesting that the influx of rDC from the lungs peaks at 18 h post infection [65, 129]. However, the peak of antigen presentation within the LN is thought to occur at day 3 post infection and can be sustained out to at least 9 days post infection [122], lending support to the evidence that migratory rDC transfer antigen to LN-resident CD8α+ DC and suggesting that the predominant antigen-presenting population at these later time points is, in fact, the CD8α+ LNDC subset [122, 131, 140, 141]. Until recently, IAV was thought to cause an acute infection in immunologically competent hosts, and several groups, using a variety of approaches, including direct analysis for plaqueable virus, PCR for IAV genome or probing for MHC-IAV peptide complexes, have shown that IAV antigen is undetectable in the host by 15-18 days post infection [77, 148,149,150,151,152,153]. However, recent data using the adoptive transfer of IAV-specific T cells have suggested that, while undetectable by PCR, low levels of IAV-antigen may persist in the host for extended periods of time post infection and, more importantly, that this persistent antigen pool is sufficient to generate/maintain virus-specific T-cell responses for several months after infection [150, 152, 154, 155]. In contrast, using a similar approach, Mintern et al. have convincingly demonstrated that IAV-peptide-MHC complexes do not persist at later times (i.e., days 30+) following IAV infections [153]. In the coming years, it will be important to resolve the differences in these findings, as knowledge of the length of antigen presentation following IAV infections is essential to our understanding of the initiation and maintenance of effector and memory adaptive IAV-specific immunity.
In addition to the influx of rDC from the lungs to the LN following IAV infection, there is also recruitment of inflammatory DC subsets from the lymphatics and blood. Amongst these populations are CCR2+ monocyte-derived inflammatory DC (moDC) [156] and pDC [132]. Although the accumulation of CCR2+ moDC in the LN following both IAV infection and other challenges has been described [157,158,159,160], their role remains unclear. Recently, however, Nakano et al. have suggested that recruitment of blood-derived moDC is essential to promote the development of robust Th1 responses within the LN following IAV infection. Although CD8α+ DC are known to induce robust Th1 responses, the authors demonstrate that moDC induce even more robust Th1 responses and that, while their absence from the LN following IAV infection does not limit the magnitude of the virus-specific T-cell response itself, it leads to a significant decrease in IL-12 levels and T cell-derived IFNγ production therein [156].
In addition to the involvement of conventional DC in IAV immunity, several groups have described recruitment of pDC from the blood into the LN following IAV challenge [122, 132]. However, despite the ability of pDC to prime robust IAV-specific CD8 T cells in vitro [161,162,163,164], it appears that, while pDC can acquire IAV antigen, they do not present this antigen to CD8 T cells in vivo or directly ex vivo [122, 125, 131, 132]. Instead pDC may play an important role in promoting antibody production, as pDC depletion prior to IAV infection results in significantly reduced levels of neutralizing antibody [132].
Recent data from our laboratory suggest a more deleterious role for pDC in the LN during lethal, but not sublethal, IAV infections. Our findings demonstrate a novel regulation of FasL on LNDC during lethal vs. sublethal infections [85]. During steady-state conditions, LNDC express FasL. However, following a sublethal IAV infection, rDC and LNDC down-regulate expression of the IL-12p40 homodimer, and therein, subsequently, down-regulate expression of FasL. In contrast, LNDC and rDC from lethal dose IAV infections express high levels of IL-12p40, leading to sustained expression of FasL within the LN. Following activation, CD8 T cells up-regulate Fas, rendering them susceptible to FasL-mediated apoptosis. While CD8 T cells accumulate in the LN of sublethally IAV-infected mice because of the absence of LNDC FasL, CD8 T cells are eliminated via LNDC-induced FasL-mediated apoptosis in the LN of lethally infected mice. This early T-cell elimination within the LN results in significantly reduced CD8 T-cell responses in the lungs, and, subsequently increased mortality. Importantly, blockade or absence of functional FasL during lethal IAV infection is sufficient to rescue CD8 T-cell immunity and prevent IAV-associated mortality [85]. Subsequent work in our laboratory has determined that among the LN-resident DC subsets, the pDC population is the cell type responsible for the elimination of virus-specific CD8 T cells in the LN following lethal IAV infection (R. Langlois and K. Legge, unpublished results).
Together, the results detailed above suggest a key role for both migratory lung-derived DC and LN-resident DC in the induction and regulation of virus-specific CD8 T-cell responses following IAV infection.
LOCAL ROLE FOR PULMONARY DC IN IAV IMMUNITY
Following IAV infection or encounter with a foreign antigen, we and others have demonstrated a massive recruitment of CD11c+MHC II+ conventional DC and pDC into the lungs for at least 6 days post infection [63, 65,66,67,68, 132, 165]. These recruited DC subsets do not subsequently migrate to the draining LN [65, 122], suggesting that they do not participate in the initiation of adaptive immune responses. However, there is accumulating evidence suggesting that these DC may play a role in shaping the ensuing adaptive immune response in the lungs.
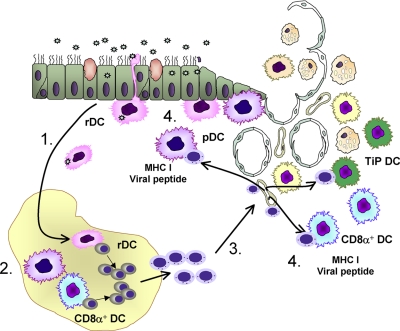
We recently described a novel role for lung-recruited pulmonary DC subsets, particularly pDC and CD8α+ DC in promoting increased virus-specific CD8 T cells in the lungs (Fig. 1) [66]. Depletion of aDC at 48 h post IAV infection (i.e., following the conclusion of DC trafficking from the lungs to the LN) resulted in increased mortality, increased pulmonary viral titers and reduced virus-specific CD8 T cells in the lungs. Further, aDC depletion resulted in significantly reduced recruitment of pDC and CD8α+ DC to the lungs. Importantly, reconstitution of the lungs with purified pDC and CD8α+ was able to rescue the IAV-specific CD8 T-cell response. This reconstitution of the virus-specific CD8 T-cell response required direct cell-cell interactions between DC and T cells in the lungs and required MHC I and viral antigens on the reconstituted DC [66]. At that time, it was unclear what additional mechanisms were contributing to the enhanced accumulation of virus-specific CD8 T cells in the lungs following pulmonary DC reconstitution. However, we have subsequently demonstrated that pulmonary DC provide key survival signals to virus-specific CD8 T cells in the lungs, as, in their absence, the T cells undergo significantly increased levels of apoptosis (J. McGill and K. Legge, unpublished results).
Figure 1.
The role of DC in the initiation and regulation of adaptive CD8 T-cell responses following IAV infection. 1. rDC lining the airways and alveolar spaces of the lungs acquire influenza antigen through direct infection or uptake of apoptotic bodies from infected epithelial cells. This causes rDC to mature and migrate to the lung draining LN. 2. Once in the LN, rDC pass on antigen to LN-resident CD8a+ DC. LN-resident CD8a+ DC and rDC interact with naïve antigen-specific CD8 T cells in the LN and initiate a program of activation, proliferation, and differentiation into cytotoxic effector cells. 3. Activated effector CD8 T cells migrate from the LN to the lungs. 4. CD8 T cells undergo a second direct interaction with MHC I expressing, viral antigen-presenting pulmonary pDC, CD8a+ DC or TiP DC in order to accumulate to sufficient numbers to mediate viral clearance and protection.
In accordance with our findings, Aldridge et al. recently demonstrated that TNF-/inducible nitric oxide synthase (iNOS)- producing DC (tipDC) that accumulate in the lungs following IAV infection interact directly with effector CD8 T cells in the lungs to promote increased CD8 T cell accumulation therein [166]. In this model, CCR2−/− mice, which fail to recruit tipDC to the lungs following infection, have a significantly reduced virus-specific CD8 T cell response compared with wild-type controls. However, reconstitution of CCR2−/− lungs with purified tipDC promoted increased CD8 T-cell accumulation in a manner that required IAV antigen [166].
In further agreement, using a model of RSV infection, Smit et al. have recently demonstrated a requirement for pDC in the lungs in promoting virus-specific CD8 T-cell responses therein [167]. In their model, Flt3L-induced expansion of pDC in the lungs resulted in increased virus-specific CD8 T cell responses in the lungs, while a specific depletion of pDC resulted in a significant reduction in the magnitude of the T-cell response [167], together confirming an important role for lung-recruited pDC in shaping the magnitude of the RSV-specific CD8 T-cell response. Overall, the above studies indicate an emerging role for pulmonary DC in regulating the magnitude and character of the adaptive CD8 T-cell response in the lungs following infection by both IAV and other respiratory pathogens.
CONCLUDING REMARKS
In conclusion, although adaptive immunity is essential for the clearance of IAV infection, innate immune cells also play essential roles in the control of IAV both through their ability to control early viral replication and through their ability to promote and regulate adaptive T- and B-cell responses. Importantly, however, like excessive adaptive immunity, an overly robust innate immune response can also lead to enhanced disease and mortality following infection, accentuating the importance of balance during both innate and adaptive immune responses during IAV infection.
Acknowledgments
This work was supported by National Institutes of Health AI071085 and AI076989 to K. L. L. We thank Ryan Langlois and Steve Varga for critical reading of this manuscript.
Footnotes
Abbreviations: aDC=alveolar DC, aMφ=alveolar macrophage(s), DC=dendritic cell(s), IAV=influenza A virus; iDC= interstitial DC, LN=lymph node, moDC,=monocyte-derived DC, NCR=natural cytotoxicity receptors, NK=natural killer, NO=nitric oxide, RLHs=RIG-I-like helicases, S1P,=sphigosine-1-phosphate
References
- Barker W H, Mullooly J P. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980;112:798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- Barker W H, Mullooly J P. Pneumonia and influenza deaths during epidemics: implications for prevention. Arch Intern Med. 1982;142:85–89. [PubMed] [Google Scholar]
- Singleton J A, Wortley P, Lu P J. Influenza vaccination of persons with cardiovascular disease in the United States. Tex Heart Inst J. 2004;31:22–27. [PMC free article] [PubMed] [Google Scholar]
- Thompson W W, Comanor L, Shay D K. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194:S82–S91. doi: 10.1086/507558. [DOI] [PubMed] [Google Scholar]
- Horimoto T, Kawaoka Y. Pandemic threat posed by avian influenza A viruses. Clin Microbiol Rev. 2001;14:129–149. doi: 10.1128/CMR.14.1.129-149.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Murphy B R, Fauci A S. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M J, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. [PubMed] [Google Scholar]
- Tay C H, Szomolanyi-Tsuda E, Welsh R M. Control of infections by NK cells. Curr Top Microbiol Immunol. 1998;230:193–220. doi: 10.1007/978-3-642-46859-9_12. [DOI] [PubMed] [Google Scholar]
- Yokoyama W M, Kim S, French A R. The dynamic life of natural killer cells. Ann Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- Vankayalapati R, Wizel B, Weis S E, Safi H, Lakey D L, Mandelboim O, Samten B, Porgador A, Barnes P F. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168:3451–3457. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- Warfield K L, Perkins J G, Swenson D L, Deal E M, Bosio C M, Aman M J, Yokoyama W M, Young H A, Bavari S. Role of natural killer cells in innate protection against lethal ebola virus infection. J Exp Med. 2004;200:169–179. doi: 10.1084/jem.20032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136:1435–1441. [PubMed] [Google Scholar]
- Mandelboim O, Lieberman N, Lev M, Paul L, Arnon T I, Bushkin Y, Davis D M, Strominger J L, Yowdel J W, Pargador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- Arnon T I, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Gazit R, Gruda R, Elboim M, Arnon T I, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Smyth M J. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchiere G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- He X S, Draghi M, Mahmood K, Holmes T H, Kemble G W, Dekker C L, Arvin A M, Parham P, Greenberg H B. T cell-dependent production of IFN-γ by NK cells in response to influenza A virus. J Clin Invest. 2004;114:1812–1819. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghi M, Pashine A, Sanjanwala B, Gendzekhadze K, Cantoni C, Cosman D, Moretta A, Valiante N M, Parham P. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178:2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- Holt P G. Inhibitory activity of unstimulated alveolar macrophages on T-lymphocyte blastogenic response. Am Rev Respir Dis. 1978;118:791–793. doi: 10.1164/arrd.1978.118.4.791. [DOI] [PubMed] [Google Scholar]
- Holt P G, Oliver J, Bilyk N, McMenamin C, McMenamin P G, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilyk N, Holt P G. Cytokine modulation of the immunosuppressive phenotype of pulmonary alveolar macrophage populations. Immunology. 1995;86:231–237. [PMC free article] [PubMed] [Google Scholar]
- Strickland D H, Thepen T, Kees U R, Kraal G, Holt P G. Regulation of T-cell function in lung tissue by pulmonary alveolar macrophages. Immunology. 1993;80:266–272. [PMC free article] [PubMed] [Google Scholar]
- Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Quay J, Soukup J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J Immunol. 1991;147:4307–4312. [PubMed] [Google Scholar]
- Dawson T C, Beck M A, Kuziel W A, Henderson F, Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156:1951–1959. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K L, Suzuki Y, Nakano H, Ramsburg E, Gunn M D. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- Perrone L A, Plowden J K, Garcia-Sastre A, Katz J M, Tumpey T M. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing M D, Shea A L, Inglis C A, Dias P B, Sarawar S R. CXCR2 is required for neutrophil recruitment to the lung during influenza virus infection, but is not essential for viral clearance. Viral Immunol. 2007;20:369–378. doi: 10.1089/vim.2006.0101. [DOI] [PubMed] [Google Scholar]
- Wareing M D, Lyon A, Inglis C, Giannoni F, Charo I, Sarawar S R. Chemokine regulation of the inflammatory response to a low-dose influenza infection in CCR2−/− mice. J Leukoc Biol. 2007;81:793–801. doi: 10.1189/jlb.0506299. [DOI] [PubMed] [Google Scholar]
- Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel W A, Mack M, Srivastava M, Seeger W, Maus U A, Lohmeyer J. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol. 2006;177:1817–1824. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- Bilyk N, Holt P G. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993;177:1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taut K, Winter C, Briles D E, Paton J C, Christman J W, Maus R, Baumann R, Welte T, Maus U A. Macrophage turnover kinetics in the lungs of mice infected with Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2008;38:105–113. doi: 10.1165/rcmb.2007-0132OC. [DOI] [PubMed] [Google Scholar]
- Baskin C R, Bielefeldt-Ohmann H, Tumpey T M, Sabourin P J, Long J P, Garcia-Sastre A, Tolnay A E, Albrecht R, Pyles J A, Olson P H, Aicher L D, Rosenzweig E R, Murali-Krishna K, Clark E A, Kotur M S, Fornek J L, Proll S, Palermo R E, Sabourin C L, Katze M G. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc Natl Acad Sci USA. 2009;106:3455–3460. doi: 10.1073/pnas.0813234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones S M, Shinya K, Kash J C, Copps J, Ebihara H, Hatta Y, Kim J H, Halfmann P, Hatta M, Feldmann F, Alimonti J B, Fernando L, Li Y, Katze M G, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Tumpey T M, Garcia-Sastre A, Taubenberger J K, Palese P, Swayne D E, Pantin-Jackwood M J, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz J M, Basler C F. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H M, Lee Y W, Lee K J, Kim H S, Cho S W, van Rooijen N, Guan Y, Seo S H. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J Virol. 2008;82:4265–4274. doi: 10.1128/JVI.02602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- Xu L, Yoon H, Zhao M Q, Liu J, Ramana C V, Enelow R I. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-α by antiviral CD8+ T cells. J Immunol. 2004;173:721–725. doi: 10.4049/jimmunol.173.2.721. [DOI] [PubMed] [Google Scholar]
- Hussell T, Pennycook A, Openshaw P J. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Peper R L, Van Campen H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb Pathog. 1995;19:175–183. doi: 10.1006/mpat.1995.0056. [DOI] [PubMed] [Google Scholar]
- Karupiah G, Chen J H, Nathan C F, Mahalingam S, MacMicking J D. Identification of nitric oxide synthase 2 as an innate resistance locus against ectromelia virus infection. J Virol. 1998;72:7703–7706. doi: 10.1128/jvi.72.9.7703-7706.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasekera J P, Vinuesa C G, Karupiah G, King N J. Enhanced antiviral antibody secretion and attenuated immunopathology during influenza virus infection in nitric oxide synthase-2-deficient mice. J Gen Virol. 2006;87:3361–3371. doi: 10.1099/vir.0.82131-0. [DOI] [PubMed] [Google Scholar]
- Snelgrove R J, Edwards L, Rae A J, Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur J Immunol. 2006;36:1364–1373. doi: 10.1002/eji.200635977. [DOI] [PubMed] [Google Scholar]
- Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–308. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis I, Matalon S. Reactive species in viral pneumonitis: lessons from animal models. News Physiol Sci. 2001;16:185–190. doi: 10.1152/physiologyonline.2001.16.4.185. [DOI] [PubMed] [Google Scholar]
- Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, Mack M, Kuziel W A, Corazza N, Brunner T, Seeger W, Lohmeyer J. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P G, Nelson D J, McWilliam A S. Population dynamics and functions of respiratory tract dendritic cells in the rat. Adv Exp Med Biol. 1995;378:177–181. doi: 10.1007/978-1-4615-1971-3_39. [DOI] [PubMed] [Google Scholar]
- Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A. 2004;61:170–177. doi: 10.1002/cyto.a.20064. [DOI] [PubMed] [Google Scholar]
- Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med. 2005;172:530–551. doi: 10.1164/rccm.200410-1384SO. [DOI] [PubMed] [Google Scholar]
- Vermaelen K Y, Carro-Muino I, Lambrecht B N, Pauwels R A. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas J A, Strickland D H, Holt P G, Stumbles P A. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005;175:1609–1618. doi: 10.4049/jimmunol.175.3.1609. [DOI] [PubMed] [Google Scholar]
- Pollard A M, Lipscomb M F. Characterization of murine lung dendritic cells: similarities to Langerhans cells and thymic dendritic cells. J Exp Med. 1990;172:159–167. doi: 10.1084/jem.172.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P G, Schon-Hegrad M A, Oliver J. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat. Regulation of antigen presentation activity by endogenous macrophage populations. J Exp Med. 1988;167:262–274. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumbles P A, Upham J W, Holt P G. Airway dendritic cells: co-ordinators of immunological homeostasis and immunity in the respiratory tract. APMIS. 2003;111:741–755. doi: 10.1034/j.1600-0463.2003.11107806.x. [DOI] [PubMed] [Google Scholar]
- Lambrecht B N, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- Shortman K, Liu Y J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- McWilliam A S, Napoli S, Marsh A M, Pemper F L, Nelson D J, Pimm C L, Stumbles P A, Wells T N, Holt P G. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–2432. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam A S, Nelson D, Thomas J A, Holt P G. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byersdorfer CA, Chaplin DD. Visualization of early APC/T cell interactions in the mouse lung following intranasal challenge. J Immunol. 2001;167:6756–6764. doi: 10.4049/jimmunol.167.12.6756. [DOI] [PubMed] [Google Scholar]
- De Heer H J, Hammad H, Soullie T, Hijdra D, Vos N, Willart M A, Hoogsteden H C, Lambrecht B N. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge K L, Braciale T J. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- McGill J, van Rooijen N, Legge K L. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Pinto C E, Kradin R L. The antigen-presenting activities of Ia+ dendritic cells shift dynamically from lung to lymph node after an airway challenge with soluble antigen. J Exp Med. 1995;181:1275–1283. doi: 10.1084/jem.181.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Suzuki S, Shirai A, Suzuki M, Nakazawa M, Nagashima Y, Okubo T. Dendritic cells are associated with augmentation of antigen sensitization by influenza A virus infection in mice. Eur J Immunol. 2000;30:316–326. doi: 10.1002/1521-4141(200001)30:1<316::AID-IMMU316>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- Norbury C C, Malide D, Gibbs J S, Bennink J R, Yewdell J W. Visualizing priming of virus-specific CD8 T cells by infected dendritic cells in vivo. Nat Immunol. 2002;3:265–271. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Bender A, Gonzalez N, Bui L K, Garrett M C, Steinman R M. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Invest. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N, Bender A, Gonzalez N, Bui L K, Garrett M C, Steinman R M. Stimulation of human anti-viral CD8+ cytolytic T lymphocytes by dendritic cells. Adv Exp Med Biol. 1995;378:375–379. doi: 10.1007/978-1-4615-1971-3_84. [DOI] [PubMed] [Google Scholar]
- Macatonia S E, Taylor P M, Knight S C, Askonas B A. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989;169:1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonacs R, Humborg C, Tam J P, Steinman R M. Mechanisms of mouse spleen dendritic cell function in the generation of influenza-specific, cytolytic T lymphocytes. J Exp Med. 1992;176:519–529. doi: 10.1084/jem.176.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Easton A, Eichelberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza infected mice. J Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Brydon E W, Smith J, Sweet C. Influenza A virus-induced apoptosis in brochiolar epithelial cells limits pro-inflammatory cytokine release. J Gen Virol. 2003;84:2389–2400. doi: 10.1099/vir.0.18913-0. [DOI] [PubMed] [Google Scholar]
- Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimua Y. In vivo induction of apoptosis by influenza virus. J Gen Virol. 1995;76:2869–2873. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- Sauter B, Albert M, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- Wolff A, Technau A, Ihling C, Technau-Ihling K, Erber R, Bosch F X, Brandner G. Evidence that wild-type p53 in neuroblastoma cells is in a conformation refractory to integration into the transcriptional complex. Oncogene. 2001;20:1307–1317. doi: 10.1038/sj.onc.1204251. [DOI] [PubMed] [Google Scholar]
- Hao X, Kim T S, Braciale T J. Differential response of respiratory dendritic cell subsets to influenza virus infection. J Virol. 2008;82:4908–4919. doi: 10.1128/JVI.02367-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge K L, Braciale T J. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Oh S, Belz G T, Eichelberger M C. Viral neuraminidase treatment of dendritic cells enhances antigen-specific CD8(+) T cell proliferation, but does not account for the CD4(+) T cell independence of the CD8(+) T cell response during influenza virus infection. Virology. 2001;286:403–411. doi: 10.1006/viro.2001.0992. [DOI] [PubMed] [Google Scholar]
- Oh S, Eichelberger M C. Polarization of allogeneic T-cell responses by influenza virus-infected dendritic cells. J Virol. 2000;74:7738–7744. doi: 10.1128/jvi.74.17.7738-7744.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, McCaffery J M, Eichelberger M C. Dose-dependent changes in influenza virus-infected dendritic cells result in increased allogeneic T-cell proliferation at low, but not high, doses of virus. J Virol. 2000;74:5460–5469. doi: 10.1128/jvi.74.12.5460-5469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Eichelberger M C. Influenza virus neuraminidase alters allogeneic T cell proliferation. Virology. 1999;264:427–435. doi: 10.1006/viro.1999.0019. [DOI] [PubMed] [Google Scholar]
- Technau-Ihling K, Ihling C, Kromeier J, Brandner G. Influenza A virus infection of mice induces nuclear accumulation of the tumorsuppressor protein p53 in the lung. Arch Virol. 2001;146:1655–1666. doi: 10.1007/s007050170054. [DOI] [PubMed] [Google Scholar]
- Dhodapkar M V, Steinman R M. Antigen-bearing immature dendritic cells induce peptide-specific CD8+ regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- Dhodapkar M V, Steinman R M, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M L, Pearce S F, Francisco L M, Sauter B, Roy P, Silverstein R L, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Lee M S, Kim Y J. Pattern-recognition receptor signaling initiated from extracellular, membrane, and cytoplasmic space. Mol Cells. 2007;23:1–10. [PubMed] [Google Scholar]
- Ichinohe T, Lee H K, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P G, Dash P, Aldridge J R, Jr, Ellebedy A H, Reynolds C, Funk A J, Martin W J, Lamkanfi M, Webby R J, Boyd K L, Doherty P C, Kanneganti T D. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen I C, Scull M A, Moore C B, Holl E K, McElvania-TeKippe E, Taxman D J, Guthrie E H, Pickles R J, Ting J P. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C B, Moltedo B, Alexopoulou L, Bonifaz L, Flavell R A, Moran T M. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J Immunol. 2004;173:6882–6889. doi: 10.4049/jimmunol.173.11.6882. [DOI] [PubMed] [Google Scholar]
- Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu Y J. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- Der S D, Zhou A, Williams B R, Silverman R H. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D C, Gopalkrishnan R V, Wu Q, Jankowsky E, Pyle A M, Fisher P B. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G E, Gaszewska-Masterlzar A, Moskophidis D. The role of α/β and γ interferons in development of immunity to influenza A virus in mice. J Virol. 2000;74:3996–4003. doi: 10.1128/jvi.74.9.3996-4003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger J M, Lins D C, Mescher M F. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J Immunol. 2005;175:4392–4399. doi: 10.4049/jimmunol.175.7.4392. [DOI] [PubMed] [Google Scholar]
- Curtsinger J M, Schmidt C S, Mondino A, Lins D C, Kedl R M, Jenkins M K, Mescher M F. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- Durbin J E, Fernandez-Sesma A, Lee C K, Rao T D, Frey A B, Moran T M, Vukmanovic S, García-Sastre A, Levy DE. Type I IFN modulates innate and specific antiviral immunity. J Immunol. 2000;164:4220–4228. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- Horisberger M A. Interferons, Mx genes, and resistance to influenza virus. Am J Respir Crit Care Med. 1995;152:S67–S71. doi: 10.1164/ajrccm/152.4_Pt_2.S67. [DOI] [PubMed] [Google Scholar]
- Horisberger M A, Hochkeppel H K. An interferon-induced mouse protein involved in the mechanism of resistance to influenza viruses. Its purification to homogeneity and characterization by polyclonal antibodies. J Biol Chem. 1985;260:1730–1733. [PubMed] [Google Scholar]
- Kolumam G A, Thomas S, Thompson L J, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough D F. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough D F. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- Yamada Y K, Meager A, Yamada A, Ennis F A. Human interferon α and γ production by lymphocytes during the generation of influenza virus-specific cytotoxic T lymphocytes. J Gen Virol. 1986;67:2325–2334. doi: 10.1099/0022-1317-67-11-2325. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy D E, Durbin J E, Palese P, Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sesma A, Marukian S, Ebersole B J, Kaminski D, Park M S, Yuen T, Sealfon S C, Garcia-Sastre A, Moran T M. Influenza virus evades innate and adaptive immunity via the NS1 protein. J Virol. 2006;80:6295–6304. doi: 10.1128/JVI.02381-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochs G, Garcia-Sastre A, Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. J Virol. 2007;81:7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li M, Zheng H, Muster T, Palese P, Beg A A, Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NF-κB and induction of α/β interferon. J Virol. 2000;74:11566–11573. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C B, Garcia-Sastre A, Williams B R, Moran T M. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J Infect Dis. 2003;187:1126–1136. doi: 10.1086/368381. [DOI] [PubMed] [Google Scholar]
- Phipps-Yonas H, Seto J, Sealfon S C, Moran T M, Fernandez-Sesma A. Interferon-β pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog. 2008;4:e1000193. doi: 10.1371/journal.ppat.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz G T, Smith C M, Kleinert L, Reading P, Brooks A, Shortman K, Carbone F R, Heath W R. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci USA. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph G J. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- Hammad H, de Heer H J, Soullie T, Hoogsteden H C, Trottein F, Lambrecht B N. Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J Immunol. 2003;171:3936–3940. doi: 10.4049/jimmunol.171.8.3936. [DOI] [PubMed] [Google Scholar]
- Kim T S, Braciale T J. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS ONE. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B N, De Veerman M, Coyle A J, Butierrez-Ramos J C, Thielemans K, Pauwels R A. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P G, Stumbles P A. Regulation of immunologic homeostasis in peripheral tissues by dendritic cells: the respiratory tract as a paradigm. J Allergy Clin Immunol. 2000;105:421–429. doi: 10.1067/mai.2000.105010. [DOI] [PubMed] [Google Scholar]
- Holt P G. Pulmonary dendritic cells in local immunity to inert and pathogenic antigens in the respiratory tract. Proc Am Thorac Soc. 2005;2:116–120. doi: 10.1513/pats.200502-017AW. [DOI] [PubMed] [Google Scholar]
- Yoon H, Legge K L, Sung S S, Braciale T J. Sequential activation of CD8+ T cells in the draining lymph nodes in response to pulmonary virus infection. J Immunol. 2007;179:391–399. doi: 10.4049/jimmunol.179.1.391. [DOI] [PubMed] [Google Scholar]
- Wikstrom M E, Batanero E, Smith M, Thomas J A, von Garnier C, Holt P G, Stumbles P A. Influence of mucosal adjuvants on antigen passage and CD4+ T cell activation during the primary response to airborne allergen. J Immunol. 2006;177:913–924. doi: 10.4049/jimmunol.177.2.913. [DOI] [PubMed] [Google Scholar]
- Belz G T, Smith C M, Eichner D, Shortman K, Karupiah G, Carbone F R, Heath W R. Cutting edge: conventional CD8 α+ dendritic cells are generally involved in priming CTL immunity to viruses. J immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel C H, Willart M A, van Rijt L S, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen B E, Hoogsteden H C, Osterhaus A D, Rimmelzwaan G F, Lambrecht B N. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens M V, Kruijsen D, Coenjaerts F E, Kimpen J L, van Bleek G M. Respiratory syncytial virus induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung draining lymph node. J Virol. 2009;83:7235–7243. doi: 10.1128/JVI.00452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Lambrecht B N, Pochard P, Gosset P, Marquillies P, Tonnel A B, Pestel J. Monocyte-derived dendritic cells induce a house dust mite-specific Th2 allergic inflammation in the lung of humanized SCID mice: involvement of CCR7. J Immunol. 2002;169:1524–1534. doi: 10.4049/jimmunol.169.3.1524. [DOI] [PubMed] [Google Scholar]
- Heer A K, Harris N L, Kopf M, Marsland B J. CD4+ and CD8+ T cells exhibit differential requirements for CCR7-mediated antigen transport during influenza infection. J Immunol. 2008;181:6984–6994. doi: 10.4049/jimmunol.181.10.6984. [DOI] [PubMed] [Google Scholar]
- Graler M H, Goetzl E J. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim Biophys Acta. 2002;1582:168–174. doi: 10.1016/s1388-1981(02)00152-x. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Walsh K B, Edelmann K H, McGavern D, Hatta Y, Kawaoka Y, Rosen H, Oldstone M B. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci USA. 2009;106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Edelmann K H, Walsh K B, Guerrero M, Hatta Y, Kawaoka Y, Roberts E, Oldstone M B, Rosen H. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol Pharmacol. 2008;74:896–903. doi: 10.1124/mol.108.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F R, Belz G T, Heath W R. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004;25:655–658. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Allan R S, Waithman J, Bedoui S, Jones C M, Villadangos J A, Zhan Y, Lew A M, Shortman K, Heath W R, Carbone F R. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Allan R S, Smith C M, Belz G T, van Lint A L, Wakim L M, Heath W R, Carbone F R. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- Smith C M, Belz G T, Wilson N S, Villadangos J A, Shortman K, Carbone F R, Heath W R. Cutting edge: conventional CD8 α+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J Immunol. 2003;170:4437–4440. doi: 10.4049/jimmunol.170.9.4437. [DOI] [PubMed] [Google Scholar]
- Belz G T, Shortman K, Bevan M J, Heath W R. CD8α+ dendritic cells selectively present MHC Class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz G T, Bedoui S, Kupresanin F, Carbone F R, Heath W R. Minimal activation of memory CD8(+) T cell by tissue-derived dendritic cells favors the stimulation of naive CD8(+) T cells. Nat Immunol. 2007;8:1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- Mount A M, Smith C M, Kupresanin F, Stoermer K, Heath W R, Belz G T. Multiple dendritic cell populations activate CD4+ T cells after viral stimulation. PLoS ONE. 2008;3:e1691. doi: 10.1371/journal.pone.0001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingulli E, Funatake C, Jacovetty E L, Zanetti M. Cutting edge: antigen presentation to CD8 T cells after influenza A virus infection. J Immunol. 2009;182:29–33. doi: 10.4049/jimmunol.182.1.29. [DOI] [PubMed] [Google Scholar]
- Eichelberger M C, Wang M L, Allan W, Webster R G, Doherty P C. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J Gen Virol. 1991;72:1695–1698. doi: 10.1099/0022-1317-72-7-1695. [DOI] [PubMed] [Google Scholar]
- Flynn K J, Riberdy J M, Christensen J P, Altman J D, Doherty P C. In vivo proliferation of naive and memory influenza-specific CD8(+) T cells. Proc Natl Acad Sci USA. 1999;96:8597–8602. doi: 10.1073/pnas.96.15.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelley-Gibbs D M, Dibble J P, Brown D M, Strutt T M, McKinstry K K, Swain S L. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178:7563–7570. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- Lawrence C W, Braciale T J. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- Zammit D J, Turner D L, Klonowski K D, Lefrancois L, Cauley L S. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintern J D, Bedoui S, Davey G M, Moffat J M, Doherty P C, Turner S J. Transience of MHC Class I-restricted antigen presentation after influenza A virus infection. Proc Natl Acad Sci USA. 2009;106:6724–6729. doi: 10.1073/pnas.0901128106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelley-Gibbs D M, Brown D M, Dibble J P, Haynes L, Eaton S M, Swain S L. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna K M, Aguila C C, Redman J M, Suarez-Ramirez J E, Lefrancois L, Cauley L S. In situ imaging reveals different responses by naive and memory CD8 T cells to late antigen presentation by lymph node DC after influenza virus infection. Eur J Immunol. 2008;38:3304–3315. doi: 10.1002/eji.200838602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Lin K L, Yanagita M, Charbonneau C, Cook D N, Kakiuchi T, Gunn M D. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Naik S H. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- Qu C, Edwards E W, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque N S, Peters W, van Rooijen N, Sanchez-Torres C, Bromberg J, Charo I F, Jung S, Lira S A, Randolph G J. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells-virus experts of innate immunity. Semin Immunol. 2005;17:253–261. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- Fonteneau J F, Gilliet M, Larsson M, Dasilva I, Munz C, Liu Y J, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- Schlecht G, Garcia S, Escriou N, Freitas A A, Leclerc C, Dadaglio G. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood. 2004;104:1808–1815. doi: 10.1182/blood-2004-02-0426. [DOI] [PubMed] [Google Scholar]
- De Heer H J, Hammad H, Kool M, Lambrecht B N. Dendritic cell subsets and immune regulation in the lung. Semin Immunol. 2005;17:295–303. doi: 10.1016/j.smim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Aldridge J R, Jr, Moseley C E, Boltz D A, Negovetich N J, Reynolds C, Franks J, Brown S A, Doherty P C, Webster R G, Thomas P G. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci USA. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J J, Lindell D M, Boon L, Kool M, Lambrecht B N, Lukacs N W. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS ONE. 2008;3:e1720. doi: 10.1371/journal.pone.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]