Abstract
Context
Five-year survival rates for early-stage colorectal, breast and prostate cancer currently exceed 90% and are increasing. Cancer survivors are at greater risk for second malignancies, other co-morbidities, and accelerated functional decline. Lifestyle interventions may provide benefit, but it is unknown whether long-term cancer survivors can modify their lifestyle behaviors sufficiently to improve functional status.
Objective
To determine whether a telephone counseling and mailed material-based diet-exercise intervention is effective in reorienting functional decline in older, overweight cancer survivors.
Design
Randomized controlled trial in which survivors were randomly assigned to intervention (Intervention, n=319) or delayed-intervention control arms (Control, n=322).
Setting
Home-based from Canada, United Kingdom and 21 United States
Participants
641 overweight (body mass index [BMI] ≥ 25), long-term (≥ 5 years) survivors (ages 65–91) of colorectal, breast and prostate cancer recruited July 2005-May 2007.
Intervention
12-month home-based tailored program of telephone counseling and mailed materials promoting exercise, improved diet quality, and modest weight loss. Control group wait-listed for 12 months.
Main Outcome Measures
Change in self-reported physical function (SF-36 physical function subscale: 0–100, high score indicates better function) from baseline to 12 months was the primary endpoint. Secondary outcomes included changes in basic and advanced lower extremity function (0–100), physical activity, BMI, and overall health quality-of-life.
Results
From an average baseline score of 75.7 to 12-month follow-up, SF-36 function scores declined less rapidly in Intervention [−2.15(95% CI-0.36,−3.93)] versus Control [−4.84(−3.04,−6.63)] arms (p=0.03). Likewise, changes in basic lower extremity function were +0.34(−0.84,1.52) versus −1.89(−0.70,−3.09) from an average baseline score of 78.2, p=0.005. Physical activity, dietary behaviors and overall quality of life increased significantly in Intervention versus Control arms, and weight loss also was greater, −2.06(−1.69, −2.43) versus −0.92 (−0.51,−1.33) kg, respectively (p<0.0001).
Conclusion
Among older long-term colorectal, breast, and prostate cancer survivors, a diet and exercise intervention reduced the rate of self-reported functional decline compared to no intervention.
INTRODUCTION
In 2008, the Centers for Medicare and Medicaid Services declared mobility maintenance and functional independence among at-risk elders as the sole priority in aging research.1 Older cancer survivors represent an important target because cancer and its treatment are associated with accelerated functional decline.2 Furthermore, cancer survivors face increased risk for second malignancies, and other chronic diseases such as cardiovascular disease, osteoporosis and diabetes, all of which are associated with increased functional limitations.3–5
The practice of healthy lifestyle behaviors may reduce risk for disease and functional decline. However, many older cancer survivors report poor lifestyle behaviors, and few meet recommended health promotion guidelines. While most older cancer survivors are non-smokers, their dietary and physical activity behaviors are sub-optimal.6 Though survivors report high interest in exercise- and diet-related interventions, this is most pronounced among younger survivors with a relatively recent cancer diagnosis.7 We previously showed positive trends in reversing functional decline with a home-based physical activity and dietary intervention among 182 older adults with newly-diagnosed cancers.7 It is unknown whether long-term cancer survivors, whose cancer diagnoses are in the distant past, would partake and benefit from a similar intervention.
RENEW (Reach out to ENhancE Wellness) is a randomized controlled trial that tested a home-based diet and exercise intervention on reorienting the functional trajectory of older, long-term survivors of breast, prostate and colorectal cancer. Overweight/obese survivors were targeted for this study given the increase in obesity among older adults,8 and the likelihood that obesity would further compromise functional status. Change in physical function over 12-months in intervention versus control arms served as the primary endpoint. Secondary endpoints included measures of physical activity, body mass index (BMI), and overall health-related quality-of-life.
METHODS
Study Design
Methods of the RENEW trial have been published elsewhere.9 In brief, this randomized controlled trial compared a 12-month diet and exercise intervention delivered via telephone counseling and tailored mailed materials versus a delayed-intervention control arm. The research protocol was approved by the Duke University institutional review board and North Carolina Central Cancer Registry (NCCCR), and written consent was obtained from all study participants.
Study Participants
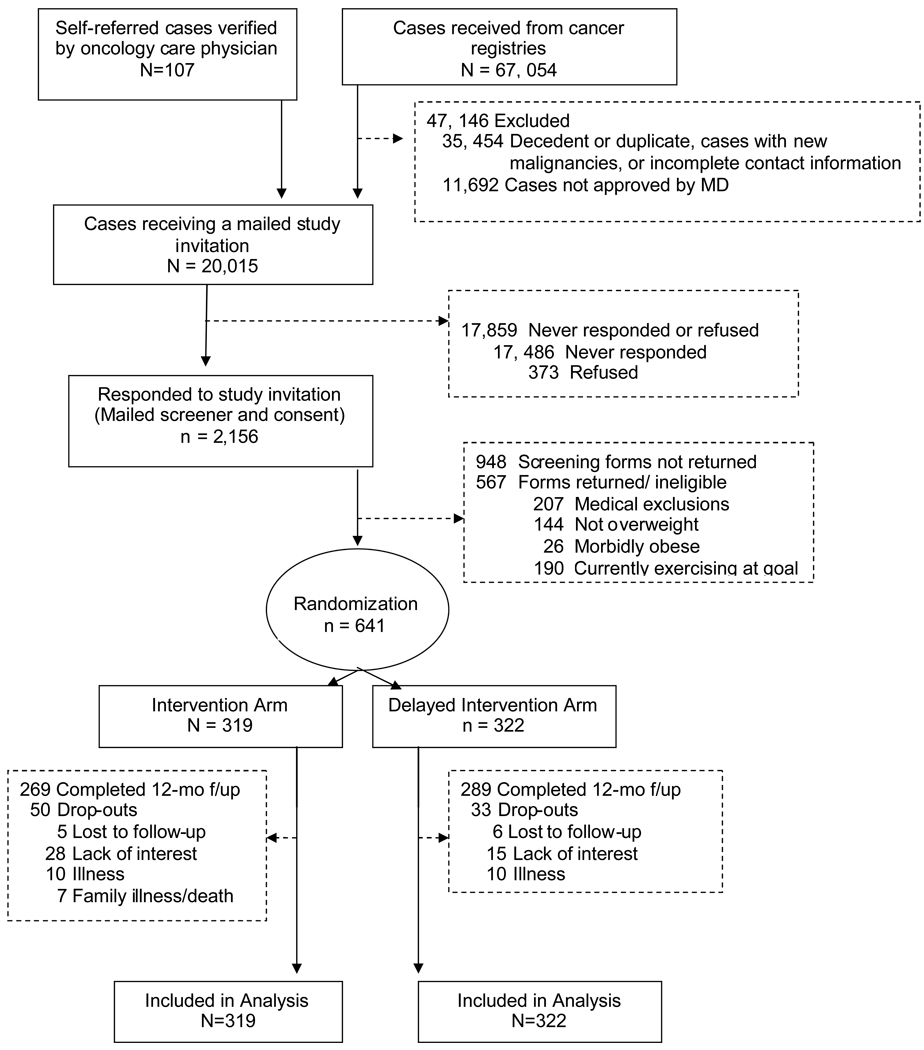
Figure 1 depicts the multi-tiered screening and enrollment process. The trial relied on self-referred participants and those ascertained from the NCCCR through which we identified 67,161 breast, prostate and colorectal cancer cases diagnosed at least five years ago and currently aged 65 years and older with no evidence of progressive disease or second cancers. After comparing case lists to state mortality tapes and conducting further follow-up, duplicates were found and those deceased or with missing contact information were excluded for potential contact. We also excluded cases whose physicians denied us permission to contact their patients. Study letters of invitation were mailed to 20,015 individuals. Of these, 2,156 expressed interest in participation and received a consent form and brief questionnaire to screen for eligibility. Responses were received from 1,208 individuals of whom 567 individuals were deemed ineligible based on the following: 1) non-community dwelling; 2) BMI <25 or >40; 3) severe hearing or speaking impairment; 4) non-English speaking/writing; 5) contraindications to unsupervised exercise (angina, myocardial infarction within 6-months, congestive heart failure, chronic obstructive pulmonary disease, plans to have a hip or knee replacement, walker or wheelchair-use, recent stroke with hemiparesis); or 6) greater than 150 minutes of moderate-to-vigorous exercise per week (already meeting recommended physical activity guidelines). A total of 641 individuals were deemed eligible and block randomized (by a statistician with no participant contact) on race, cancer-type and gender with even distribution into an intervention (n=319) or delayed-intervention control arm (n=322). Participants were recruited from July 1, 2005 to May 17, 2007.
Figure 1.
Consort flow diagram
RENEW Intervention
The RENEW intervention consisted of a personally-tailored workbook and series of quarterly newsletters, along with a program of telephone counseling and automated prompts (i.e., 15 sessions and eight prompts over the 12-month period).9 Costs for materials (including development), telephone counseling, and postage were $1000 per person. The intervention was theoretically-based using Social Cognitive Theory and the Transtheoretical Model.10
Workbook and materials
Upon assignment to the RENEW intervention, participants received a personalized workbook. The introductory pages featured colorful bar graphs comparing participants’ current lifestyle behaviors and weight status to recommended levels: 1) 15 minutes of strength training exercise every other day; 30 minutes of endurance exercise each day; consumption of at least seven servings (for women) or nine servings (for men) of fruits and vegetables per day;11 restriction of saturated fat to less than 10% of energy intake; and a 10% weight loss goal during the 12-month study period. Workbook chapters provided standardized content on exercise and a healthy caloric-restricted diet. Participants also received a pedometer, exercise bands (three levels of resistance), an exercise poster depicting six lower extremity strength exercises, Portion Doctor® tableware to guide food portioning (Portion Health Products, St. Augustine Beach, FL), and personalized record logs to self-monitor daily exercise and dietary intake (including a fat gram booklet to assist with self-monitoring).
Telephone counseling
Each participant was assigned a health counselor (“personal trainer”), for the 12-month period. Counseling sessions were conducted weekly during the first three weeks, every other week for one month, and then monthly. Each telephone session was 15–30 minutes in duration and served to enhance social support and self-efficacy. During each telephone call the counselor worked with the participant to monitor progress, provide reinforcement, explore strategies in overcoming barriers, field questions, direct participants to appropriate resources and establish future goals.
Telephone prompts
Automated telephone messages by the study principal investigator (WDW) provided additional, intermittent reinforcement.
Tailored progress reports
Every 12-weeks, participants received a tailored two-page progress report-newsletter with a motivational greeting, a graph depicting behavioral change in the target behaviors related to the RENEW goals and a sign-off message that was tailored to stage-of-readiness.
Delayed intervention control
Like many lifestyle interventions,12 we employed a delayed-intervention, wait-list control.
Outcomes
The primary outcome, change in functional status between baseline and 12 months, was assessed using the physical function subscale of the Medical Outcomes Study Short Form-36 (SF-36) questionnaire as an indicator of overall physical function.13 The physical function subscale assesses the impact of health on the performance of activities ranging from basic self-care to vigorous physical activity, and has been widely used with good construct validity, and sensitivity to change.14 Given that lower extremity function is central to the maintenance of independence,15, 16 we also assessed function using the Basic and Advanced Lower Extremity Function subscales of the Late Life Function and Disability Index.17, 18 In addition, the entire SF-36 was administered at each wave of data collection, thus providing health-related quality-of-life outcomes on general health, pain, vitality, social functioning, physical and emotional roles, and mental health. Raw data from all of these scales are normalized and range from 0–100 with higher scores indicating better function.
Secondary outcomes related to target behaviors of physical activity, diet and weight loss. Physical activity was assessed using the Community Health Activities Model Program for Seniors (CHAMPS) questionnaire.19 CHAMPS was developed for use in older adults, has been tested with home-based interventions and is sensitive to change.20 For this study, we scored strength and endurance items separately as indicators of our physical activity target behaviors. Dietary intake data were averaged from two unannounced 24-hour recalls at baseline and 12-months using the interactive Nutrition Data System for Research (NDSR) software. (Version 2006, Nutrition Coordinating Center, Minneapolis, MN). Self-reported height and weight were collected for estimation of BMI and weight loss.
Other items collected on the surveys included a count of the six most prevalent medical conditions from our previous research (arthritis, hypertension, heart problems, circulatory problems, osteoporosis, and cataracts), a checklist of 22 symptoms, social support, income, smoking status, and cancer treatment. All surveys were conducted at baseline and 12-months by interviewers at the Diet Assessment Center of Pennsylvania State University who had no knowledge of study design or outcomes. Additional short quarterly surveys were conducted for self-reported physical function.
All study participants were provided with a telephone number to report any significant changes in health status. Changes in health also were assessed during each counseling call and during each of the outcome assessments and surveys. When appropriate, medical clearance was obtained for continuation or cessation of the intervention. Health events were classified by the investigative team who were blinded to arm assignment, and who classified events as “serious” versus “non-serious”, and either “non-attributable,” “possibly attributable” or “attributable” to the intervention.
Statistical Analyses
The sample size was set at 544 to give the t-test (two-tailed alpha of 0.05) at least 80% power to detect an arm difference of 3.9 in change in the physical function subscale from baseline to 1-year. A standard deviation of 16.2 was assumed. Data from our intervention development study informed these calculations.21 Under the assumption that at most 15% of the participants would drop out by 1-year, the sample size was inflated to 640. While physical function was the primary outcome, arm differences in 17 secondary outcomes were also tested. The alpha level for these 17 tests was controlled at an overall two-sided 0.05 using Holm’s procedure.22 Finally, we examined whether the arms differed on the percentage of participants who met study goals for our behavioral outcomes. These analyses, using logistic regression models, were considered exploratory and p-values are presented without reference to an alpha level.
Covariate-adjusted arm effects for 17 of the 18 continuous outcomes were tested with the general linear model, after verifying the approximate normality of the residuals. Duration of endurance exercise had a skewed distribution and the arm effect on this outcome was tested with the proportional odds logistic regression model23 by transforming number of minutes into a 10-level ordinal variable. The following covariates were included in all models regardless of their p-values: baseline value of the outcome, age, race, number of co-morbidities, number of symptoms, education, cancer-type, BMI, and physical function (SF-36 subscale). These covariates were selected apriori based on their clinical significance. Drop-outs were included in all analyses by imputing change across time to be zero. We also conducted sensitivity analyses by testing the arm effect in only the non-dropouts. Arm means are presented with standard errors (se); differences between arm means are presented with 95% confidence intervals. Covariate-adjusted and unadjusted p-values for the arm effect are presented. All analyses were performed using SAS Version 9.1, Cary, NC.
RESULTS
Study participants largely resided in 21 U.S. states, though a small number of participants were from Canada and the United Kingdom. Characteristics of the study sample are reported in Table 1. Despite our efforts to attract an ethnically diverse sample, like most lifestyle intervention studies, our sample was largely Caucasian, and a majority reported some college education.24 Furthermore, participants differed from non-respondents on age (younger), gender (more females), cancer-type (fewer colorectal cancer cases), and time elapsed since diagnosis (more proximal).
Table 1.
Baseline participant characteristics
| Characteristics | Intervention (n=319) | Control (n=322) |
|---|---|---|
| Age, mean (SD), years | 73.0 (5.0) | 73.1 (5.1) |
| Race, (n) % white | (284) 89.0 | (285) 88.5 |
| Gender, (n) % male | (147) 46.1 | (145) 45.0 |
| Education, (n) % any college | (201) 63.0 | (194) 60.3 |
| Co-morbidity, mean number (SD) | 2.0 (1.3) | 2.0 (1.2) |
| Current Smokers, (n) % | (17) 5.3 | (20) 6.2 |
| Cancer type, (n) % | ||
| Breast | (143) 44.8 | (146) 45.3 |
| Prostate | (131) 41.1 | (130) 40.4 |
| Colorectal | (45) 14.1 | (46) 14.3 |
| Years since diagnosis, mean (SD) | 8.5 (2.7) | 8.7 (2.7) |
| BMI, mean (SD) | 29.1 (3.3) | 29.2 (3.6) |
| SF-36 Physical Function, mean (SD) | 75.9 (18.7) | 75.6 (19.1) |
The three most prevalent medical conditions reported were arthritis, hypertension and circulatory problems, and the most frequently cited symptoms were shortness of breath with exertion, muscle cramps, and sleep problems. The mean baseline physical function score of 75.7 is comparable to the median score for males and females ages 65 and over.14 The mean baseline score of 78 on the basic lower extremity scale indicated that study participants had relatively few difficulties in performing basic tasks, i.e., going up-and-down a flight of stairs, stepping-up and-down from a curb, or using a step stool. But the mean score of 53 on the advanced lower extremity scale indicated more difficulties in the performance of advanced tasks such as walking a mile, going-up and down stairs without a handrail, and running a short distance, such as to catch a bus.
Of 641 individuals randomized, 558 (87%) completed the 12-month follow-up. Attrition was within the projected rate (15%) used for power calculations and reasons for withdrawal are listed in Figure 1. Differences between dropouts and individuals completing the 12-month intervention were examined by age, race, education, number of co-morbidities and symptoms, BMI, cancer-type, physical function, and study arm. Higher rates of attrition were observed among those in the Intervention arm (p=0.04) or with a higher BMI [29.9(0.40) versus 29.1(0.14), p=0.05].
Change in physical function
For the SF-36 physical function subscale, the Control arm experienced a −4.84(0.09) point change in physical function, more than double that of the Intervention arm [−2.15(0.09)], (Table 2). There was a statistically significant difference between arms in basic lower extremity function; i.e., function changed negligibly in the Intervention arm [+0.34(0.6) points], whereas the Control arm showed a decrease [−1.89(0.6) points]. Advanced lower extremity function followed a similar pattern but did not achieve statistical significance. Sensitivity analyses were performed by testing the arm effect in only the non-dropouts. P-values from these sensitivity analyses were similar to those from analyses using imputed data.
Table 2.
Arm means and differences between arm means for all outcomesa
| Outcomes | Intervention (n=319) |
Control (n=322) |
Mean arm difference (95% CI) |
P-value of arm effect: covariate- adjusted (unadjusted) |
Holm’s Procedureb Alpha Level |
||
|---|---|---|---|---|---|---|---|
| Baseline Mean (se) |
Change Mean (se) |
Baseline Mean (se) |
Change Mean (se) |
||||
| Primary Outcome | |||||||
| SF-Physical Function | 75.9 (1.1) | −2.15 (0.9) | 75.6 (1.1) | −4.84 (0.9) | 2.69 | 0.03 (0.03) | |
| Range 10–100 | (0.17, 5.21) | ||||||
| Secondary Outcomes | |||||||
| LLF- Basic Lower Extremity | 78.4 (0.8) | +0.34 (0.6) | 78.1 (0.9) | −1.89 (0.6) | 2.24 (0.56, 3.91) | 0.005 (0.005) | 0.0050 |
| Range 45.6–100 | |||||||
| LLF-Advanced Lower Extremity | 52.8 (0.8) | −0.37 (0.5) | 52.9 (0.8) | −2.30 (0.6) | 1.92 (0.45, 3.39) | 0.02 (0.01) | 0.0063 |
| Range 0–100 | |||||||
| Behavioral Targets | |||||||
| Duration of Strength Exercise (minutes/wk) | 7.0 (2.1) | +18.7 (2.4) | 11.5 (2.5) | +0.5 (2.7) | 18.2 (11.2, 25.2) | <0.0001 (<0.0001) | 0.0029 |
| Range 0–600c | |||||||
| Duration of Endurance Exercise (minutes/wk) | 24.6 (2.1) | +36.3 (4.9) | 28.7 (2.3) | +23.4 (5.6) | 12.9 (1.89, 27.6) | 0.004 (0.003) | 0.0042 |
| Range 0–149 | |||||||
| Strength Exercise Frequency (sessions/wk) | 0.5 (0.1) | +1.4 (0.2) | 0.5 (0.1) | +0.2 (0.1) | 1.12 (0.70, 1.54) | <0.0001 (<0.0001) | 0.0031 |
| Range 0–7 | |||||||
| Endurance Exercise Frequency (sessions/wk) | 1.6 (0.1) | +1.6 (0.2) | 1.8 (0.2) | +0.5 (0.2) | 1.05 (0.39, 1.72) | 0.005 | 0.0045 |
| Range 0–15 | |||||||
| Fruits & Vegetables (daily servings) | 3.72 (0.1) | +1.24 (0.14) | 3.54 (0.1) | +0.13 (0.11) | 1.11 (0.76, 1.47) | <0.0001 (<0.0001) | 0.0033 |
| Range 0–15.80 | |||||||
| Saturated Fat Intake (g/day) | 19.6 (0.5) | −3.06 (0.51) | 19.32 (0.5) | −1.07 (0.49) | −1.99 (−0.58, −3.40) | 0.002 (<0.0001) | 0.0038 |
| Range 2 −57 | |||||||
| Weight (kg) | 85.7 (0.7) | −2.06 (0.19) | 84.7 (0.7) | −0.92 (0.2) | −1.14 | <0.0001 | 0.0036 |
| Range 59.1 – 125.5 | (−0.59, −1.69) | ||||||
| Body mass index | 29.1 (0.2) | −0.69 (0.07) | 29.2 (0.2) | −.031 (0.08) | −0.38 | <0.0001 | 0.0038 |
| Range 25.0 – 47.0 | (−0.19, −0.57) | ||||||
| Health Quality of Life | |||||||
| SF-General Health | 71.8 (0.9) | +0.77 (0.72) | 72.6 (0.9) | −1.94 (0.80) | 2.71 | 0.03 (0.02) | 0.0071 |
| Range 15 −100 | (0.58, 4.84) | ||||||
| SF-Pain | 72.2 (1.2) | −0.78 (1.07) | 72.6 (1.2) | −3.19 (1.22) | 2.40 | 0.16 (0.13) | 0.0167 |
| Range 10–100 | (−0.79, 5.59) | ||||||
| SF-Vitality | 61.9 (0.9) | −0.47 (0.89) | 61.5 (1.0) | −2.42 (0.98) | 1.95 | 0.10 (0.09) | 0.0125 |
| Range 0 – 100 | (−0.64, 4.55) | ||||||
| SF-Social Functioning | 90.2 (1.0) | −1.29 (1.05) | 90.8 (0.9) | −5.05 (1.22) | 3.75 | 0.03 (0.02) | 0.0083 |
| Range 12.5 – 100 | (0.58, 6.92) | ||||||
| SF- Mental Health | 85.6 (0.7) | +0.50 (0.53) | 86.3 (0.7) | −2.04 (0.74) | 2.54 | 0.01 (0.08) | 0.0056 |
| Range 32 – 100 | (0.75, 4.33) | ||||||
| SF- Role Physical | 75.7 (1.9.) | −2.43 (2.02) | 78.6 (1.9) | −4.68 (2.14) | 2.25 | 0.32 (0.30) | 0.0250 |
| Range 0 – 100 | (−3.54, 8.05) | ||||||
| SF-Role Emotional | 92.1 (1.2) | −0.73 (1.32) | 92.0 (1.2) | −0.62 (1.38) | −0.11 | 0.93 (0.98) | 0.0500 |
| Range 0 – 100 | (−3.86, 3.64) | ||||||
Abbreviations: se, standard error; CI, confidence interval
Since the observed means and covariate-adjusted means were almost identical, only the observed means are shown. Both covariate-adjusted and unadjusted p-values for the test of the arm effects are given. Missing year-1 outcomes were imputed to baseline value.
Holm’s procedure first ranks the 17 p-values from lowest to highest. The first (lowest) p-value has to be less than 0.0029 (0.05/17) to be statistically significant and to permit continuation to the other t-tests. The Holm’s procedure continues sequentially in this fashion using alpha levels of 0.0.0031 (0.05/16), 0.0033 (0.05/15), … , and 0.05 (0.05/1) for the remaining 16 tests, respectively.
600 minutes of light strength training is an outlier (next highest value is 210). Because we were unable to validate the accuracy of this data point it was included in the analysis. Inclusion or exclusion of this value made no difference on the estimates of the p-value.
Change in targeted behaviors
There were significant differences between the Intervention and Control arms for all targeted behaviors except endurance exercise frequency indicating successful uptake of the intervention (Table 2). Duration of strength training exercise minutes increased in the Intervention [+18.7(2.4)] and remained stable among Controls [0.5(2.7)]. Likewise, duration of endurance exercise minutes increased in the Intervention arm [36.3(4.9)] and was stable in among Controls [23.4(5.6)]. Mean intake of fruits and vegetables increased by 1.24(0.14) daily servings in the Intervention arm and by 0.1(0.1) among Controls. Mean consumption of saturated fat decreased by 3.06 grams per day in the Intervention arm and by only 1.07(0.5) among Controls. Furthermore, participants in the Intervention arm reported a mean kilogram weight loss of 2.06(0.2), which was more than twice that reported by Controls 0.92(0.2).
In exploratory analyses, we compared the arms on percentage of participants meeting study goals at 12 months. At baseline, only 8% of the sample was doing strength training at the recommended number of minutes. At 12-months, the percentage of participants performing recommended strength training was 28%(0.03) within the Intervention arm versus 11%(0.02) among Controls, p <0.001). Our screening criteria excluded individuals performing more than 150 minutes per week of endurance exercise at baseline, but at the 12-month follow-up 15%(0.02) of participants in the Intervention arm versus 11%(0.02) in the Control arm were meeting weekly national guidelines for endurance exercise (p=0.07). At baseline 6% of participants met fruit and vegetable guidelines but at 12 months, 16%(0.02) of Intervention versus 4%(0.01) of Controls met guidelines, (p<0.001). At baseline, 36% of participants consumed fewer than 10% of total calories from saturated fat; at 12-months the percentage of participants meeting this guideline was 49%(0.03) in the Intervention arm and 38%(0.03) among Controls, p=0.001.
Change in health quality-of-life
Overall health related quality-of-life decreased in every subscale in the Control arm throughout the 12-month period. In the Intervention arm decreases in subscale scores were of lower magnitude and sustained for overall and mental health, see Table 2.
Adverse Events
Changes in health status were identified at each telephone contact (survey or counseling) or by self-report. A total of 201 events were reported, reviewed and classified, with the majority coded as non-serious. Because any cardiac, musculoskeletal, or digestive concerns were considered “possibly attributable” to the diet-exercise intervention, 106 of the 201 events were classified as such. Of these, 32 involved hospitalization and were thus considered serious. Only five events were considered directly attributable to the study: (1) increased blood pressure with exercise (physician clearance obtained for continued participation); (2) hip pain with exercise; (3) pulled hamstring while walking; (4) fall during hiking; and (5) calf pain and stiffness using exercise bands. Upon analysis of these events, there were no differences between the Intervention and Control arms in the total number of events, nor events in any subcategory.
COMMENT
The major finding of this trial is that older, overweight long-term cancer survivors successfully engaged in a behavioral lifestyle intervention that resulted in an amelioration of functional decline. To our knowledge, this is the first report of a long-term (i.e. 12-month) intervention directed at maintaining function in long-term cancer survivors. The majority of physical activity or dietary interventions for cancer patients have targeted younger patients, those undergoing treatment, or those with recent diagnoses.25 This intervention was ambitious in that older, long-term cancer survivors were asked to change both diet and exercise behaviors. Even with modest change, they experienced clinically meaningful improvements in both physical function and other health related quality-of-life domains. The functional benefits were clinically meaningful in that global physical function, as assessed by the SF-36 Physical Function subscale, declined by 4.8 points in as little as 12-months in the Control arm, but only by half as much in the Intervention arm. A decline of 6.5 points over a 4-year period is associated with a 10% higher mortality risk within a subsequent three year window.26 whereas, a decline of two points is considered too small to be clinically detectable.26 To our knowledge no other telephone counseling studies with functional outcomes directed at older adults have advocated changes in both physical activity and diet simultaneously. These findings contrast to those of Kolt et al, who found no significant differences in SF-36 physical function subscale scores among older adults in a primary care setting using a telephone counseling intervention of similar duration; however it is unknown whether differences in study findings are due to differences in sample size and characteristics or that our intervention also promoted dietary change and weight loss.27
Further evidence of functional benefit is indicated by the measures of lower extremity functioning which were targeted for strength training by the intervention. Although the changes observed within the two study arms were modest, participants in the Intervention arm maintained their lower extremity function whereas Controls experienced a decline. A parallel pattern of similar magnitude was observed in these two measures in a group of stroke patients following a 12-week progressive resistance training program in comparison to control.28 Although the samples in these two studies differ, it is important to note that our home-based strength training intervention provided only moderate-to-low doses, and yet was still able to preserve lower extremity functioning. Indeed, the intervention in total not only enhanced physical functioning, but overall health and quality-of-life. Previous cross-sectional reports have found that quality-of-life is significantly higher among cancer survivors who meet public health physical activity guidelines, and results from this study provide cause-and-effect evidence which supports this association.29
Maintenance of function is particularly important for older adults. Our results of our study are timely since in 2008, the Centers for Medicare and Medicaid Services declared research aimed at maintaining mobility and function in at-risk elders as the only aging priority.1 A comparison of older cancer survivors with age-matched individuals with no cancer history found significantly more functional limitations among those having had cancer.30 The 12-month decline in physical function experienced by the Control arm in this study would be comparable to the added burden of ischemic heart disease.31 Attenuation or reversal of functional decline in this population is therefore clinically relevant. Furthermore, the type of intervention, home-based with no requirement for travel or clinic visits, is likely to appeal to many older adults with potential to reach even those in rural settings.
As noted, improved physical function was dependent upon our ability to successfully modify unhealthy behaviors. This may be particularly relevant since adherence to physical activity and dietary guidelines tend to be low in cancer survivors. Data from the RAND-36 Health Status Inventory indicate that 39–47% of cancer survivors meet physical activity recommendations, 15–19% meet recommendations for fruit and vegetable consumption, and only 5% meet recommendations for all behaviors.6 Data from the National Health Interview Survey suggest that adherence to physical activity is even lower, about 25%, for cancer survivors who are older.30 Our results are remarkable in that our Intervention participants made improvements in all of the targeted behaviors. Yet, careful examination of the data indicates that there is still much to be done. For example, while minutes of endurance physical activity increased significantly in the Intervention arm compared to Controls, the mean number of minutes of endurance physical activity at 12-months within the Intervention arm was still low (70 minutes/week) and only 15% of the sample achieved the recommended level of 150 minutes or more endurance exercise per week. Newly released physical activity guidelines acknowledge the challenges posed by meeting the recommended amounts of physical activity among individuals with chronic conditions and advise adults with chronic conditions to be as physically active as their condition allows.32
Our design had multiple features worthy of mention. First, we targeted overweight cancer survivors because overweight/obesity place individuals at higher risk for functional decline. Data from a recently completed trial of physical activity and dietary fat intake indicate that overweight/obese individuals were more likely to successfully change behavior than individuals of normal weight, which perhaps contributed to the success of our intervention.33 Second, our pilot study provided foundational experience and in designing this trial we added a lower extremity strengthening component to enhance physical function, given the known association between lower extremity function and maintenance of independence.15 Of note, newly published physical activity guidelines for older adults now include recommendations for strength training.34 We believe successful implementation of strength training in this study played an important role in the observed reorientation of functional trajectory.
This study however has some limitations to consider which may affect generalizability. First, all of the outcomes are based on self-report, and it is possible that some of the behavioral outcomes are subject to over- or under- estimation of desired behaviors. On the other hand, the fact that there were no clinical or research visits in this trial enhanced our ability to deliver the intervention anywhere in the United States and other English speaking countries. Second, our demographics indicate that this intervention was most likely delivered to highly motivated individuals. Recruitment for this study was extremely difficult and has been detailed previously.9 It should be noted however, that long-term cancer survivors present a particular challenge to recruitment for the following reasons: 1) their follow-up with oncologists (who usually serve as points of entry to oncology-based trials) is frequently discontinued, 2) their contact information may no longer be accurate; 3) they may have died; and 4) the teachable moment that often accompanies a cancer diagnosis wanes over time and diminishes interest in participating in clinical trials.35 Despite these challenges, we were able to overcome obstacles in reaching this population which heretofore has been understudied in survivorship research.36
In conclusion, this study provides a major contribution to the literature pertaining to cancer survivorship, particularly since it provides data on a long overlooked, yet important faction, i.e. older long-term cancer survivors. Long-term survivors of colorectal, breast and prostate cancer participating in a diet and exercise intervention reduced the rate of self-reported physical function decline in comparison to a group receiving no intervention. Future efforts should be directed toward health promotion programs among older cancer survivors, not only in those who are well beyond their diagnosis, but also in those who are more newly diagnosed and perhaps more motivated to participate in clinical trials targeting lifestyle change. Studies should also address whether overweight older adults with other conditions might benefit from similar interventions, especially given the current paradoxical controversy over weight loss as beneficial or detrimental in overweight older adults.37, 38 Future studies should not only assess the impact on health and well-being, but also should address cost related outcomes, especially given that the economic burden associated with functional decline and loss of independence is exceedingly high.39
Additional Acknowledgements
Funding/Support: RENEW is supported by the National Institutes of Health through the following grants CA106919, P30AG028716 and a grant from the Veterans Affairs Research & Development E3386R.
Role of Sponsor: Sponsors did not participate in the design or conduct of the study, collection, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript.
Additional Acknowledgements:
The authors dedicate this manuscript in memory of our esteemed and beloved colleague, Dr. Elizabeth C. Clipp. We wish to thank Carl Pieper Dr.PH, ,a compensated Duke affiliate, for his guidance in areas of grant preparation, analysis, and design. We are grateful for the contributions of David Farrell, MPH Jetze Beers, B.A.and staff of People Designs, Inc who helped craft the RENEW intervention materials. People Designs, Inc. was compensated for their work We thank Phil Page, PT at Thera-Band Academy for donating exercise bands via a company grant program. The authors also wish to thank the following current or former Duke affiliates compensated for their efforts in the execution of this project: Valeda Stull, B.A., Teresa Baker, Emily Hill, M.S, Jennifer Laheta, MEd, Pam Eberle Wiley, MS, RD and Nanyamka Williams, B.S. We thank Linda Phelps, B.A. for her compensated contributions from Pennsylvania State University. We also are grateful to our participating institutions and the many oncology care providers.
Footnotes
Trial Registration clinical trials.gov identifier: NCT00303875
Author Contributions: Dr. Morey had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Morey, Snyder, Sloane, Cohen, Demark-Wahnefried.
Acquisition of data: Snyder, Sloane, Miller, Mitchell.
Analysis and interpretation of data: Morey, Snyder, Sloane, Peterson, Demark-Wahnefried
Drafting of the manuscript: Morey.
Critical revision of the manuscript for important intellectual content: Snyder, Sloane, Cohen, Peterson, Hartman, Miller, Mitchell, Demark-Wahnefried.
Statistical analysis: Morey, Snyder, Sloane, Peterson, Demark-Wahnefried.
Obtained funding: Morey, Snyder, Sloane, Cohen, Demark-Wahnefried.
Study supervision: Snyder, Demark-Wahnefried
Financial Disclosures: None reported.
Conflicts of interest: No such conflicts were reported by any of the authors.
REFERENCES
- 1.Centers for Medicare & Medicaid Services. Medicare Evidentiary Priorities 2008. 2008 August 15; http://www.cms.hhs.gov/coverageGenInfo/07_EvidentiaryPriorities.asp.
- 2.Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97(3):674–681. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- 3.Deimling GT, Sterns S, Bowman KF, et al. Functioning and activity participation restrictions among older adult, long-term cancer survivors. Cancer Invest. 2007;25(2):106–116. doi: 10.1080/07357900701224813. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt M, Greenfield S, Stovall EL, editors. Committee on Cancer Survivorship. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2008. Feb 26, http://www.nap.edu/openbook.php?isbn=0309095956. [Google Scholar]
- 5.Rowland J, Mariotto A, Aziz N, Tesauro G, Feuer E. Cancer Survivorship United States, 1971–2001. MMWR. 2004;53(24):526–529. [PubMed] [Google Scholar]
- 6.Blanchard CM, Courneya KS, Stein K, et al. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 7.Demark-Wahnfried W, Clipp EC, Morey MC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: Outcomes from Project LEAD. J Clin Oncol. 2006;24:3465–3473. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Older Americans Update 2006: Key indicators of well-being. Washington, D.C.: U.S. Government Printing Office; 2006. May, Federal Interagency Forum on Aging-Related Statistics. [Google Scholar]
- 9.Snyder DC, Morey MC, Sloane R, et al. Reach out to enhance wellness in older cancer survivors (RENEW): Design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer. Psycho Oncol. 2008 doi: 10.1002/pon.1491. Published online in Wiley InterScience. DOI: 10.1002/pon.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus BH, King TK, Clark MM, Pinto BM, Bock BC. Theories and techniques for promoting physical activity behaviors. Sports Med. 1996;22(5):321–331. doi: 10.2165/00007256-199622050-00005. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans 2005. 2008 October 30; http://www.health.gov/dietaryguidelines/dga2005/document/.
- 12.Madsen S, Mizra M, Holm S, Hilsted K, Kampmann K, Riis P. Attitudes towards clinical research among participants and nonparticipants. J Intern Med. 2002;251(2):156–168. doi: 10.1046/j.1365-2796.2002.00949.x. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36) Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 14.Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36® Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric Incorporated; 2000. [Google Scholar]
- 15.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol: MS. 2000;55A(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. NEJM. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haley SM, Jette AM, Coster WJ et al. Late life function and disability instrument: II. development and evaluation of the function component. J Gerontol: MS. 2002;57A(4):M217–M222. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- 18.Jette AM, Haley SM, Coster WJ et al. Late life function and disability instrument: I. development and evaluation of the disability component. J Gerontol: MS. 2002;57A(4):M209–M216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 19.Stewart AL, Mills KM, King AC, Haskell WL, Gillis DE, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AL, Verboncoeur CJ, McLellan BY et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol: MS. 2001;56A(8):M465–M470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demark-Wahnefried W, Morey MC, Clipp EC, et al. Leading the Way in Exercise and Diet (Project LEAD): intervening to improve function among older breast and prostate cancer survivors. Control Clin Trials. 2003;24(2):206–223. doi: 10.1016/s0197-2456(02)00266-0. [DOI] [PubMed] [Google Scholar]
- 22.Holm S. A simple sequentially reactive multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 23.McCullagh P. Regression models for ordinal data (with discussion) J R Stat Soc B. 1980;42:109–142. [Google Scholar]
- 24.Stull VB, Snyder DC, Demark-Wahnefried W, Stull VB, Snyder DC, Demark-Wahnefried W. Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. J Nutr. 2007;137(1 Suppl):243S, 248S. doi: 10.1093/jn/137.1.243S. [DOI] [PubMed] [Google Scholar]
- 25.Courneya KS, Friedenreich CM, Courneya KS, Friedenreich CM. Physical activity and cancer control. Semin Oncol Nurs. 2007;23(4):242–252. doi: 10.1016/j.soncn.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Bayliss MS, Rogers WH, Kosinski M, Tarlov AR. Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems. JAMA. 1996;276(13):1039–1047. [PubMed] [Google Scholar]
- 27.Kolt GS, Schofield GM, Kerse N, et al. Effect of telephone counseling on physical activity for low-active older people in primary care: a randomized, controlled trial. J Am Geriatr Soc. 2007;55(7):986–992. doi: 10.1111/j.1532-5415.2007.01203.x. [DOI] [PubMed] [Google Scholar]
- 28.Ouellette MM, LeBrasseur NK, Bean JF, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004;35(6):1404–1409. doi: 10.1161/01.STR.0000127785.73065.34. [DOI] [PubMed] [Google Scholar]
- 29.Peddle CJ, Au HJ, Courneya KS, Peddle CJ, Au H-J, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Dis Colon Rectum. 2008;51(8):1242–1248. doi: 10.1007/s10350-008-9324-2. [DOI] [PubMed] [Google Scholar]
- 30.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. J Clin Oncol. 2005;23(34):8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 31.Alonso J, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res. 2004;13(2):283–298. doi: 10.1023/b:qure.0000018472.46236.05. [DOI] [PubMed] [Google Scholar]
- 32.Dept of Health and Human Services. 2008 Physical Activity Guidelines for Americans. 2008 November 28; http://www.health.gov/paguidelines/
- 33.Vandelanotte C, Reeves MM, Brug J, De Bourdeaudhuij I. A randomized trial of sequential and simultaneous multiple behavior change interventions for physical activity and fat intake. Prev Med. 2008;46(3):232–237. doi: 10.1016/j.ypmed.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Nelson ME, Rejeski WJ, Blair SN et al. Physical activity and public health in older adults: recommendation from the American College of Sport Medicine and the American Heart Association. Circulation. 2007;116(9):1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 35.Demark-Wahnefried W, Aziz NM, Rowland JH, et al. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer.[see comment] J Clin Oncol. 2005 Aug 20;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courneya KS, Karvinen KH, Courneya KS, Karvinen KH. Exercise, aging, and cancer. Appl Physiol Nutr Metab. 2007;32(6):1001–1007. doi: 10.1139/H07-074. [DOI] [PubMed] [Google Scholar]
- 37.Bales CW, Buhr G, Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc. 2008;9(5):302–312. doi: 10.1016/j.jamda.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Newman AB, Yanez D, Harris T, et al. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49(10):1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 39.Yabroff KR, Lawrence WF, Clauser S, et al. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96(17):1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]