Abstract
In many parts of the vertebrate nervous system, synaptic connections are remodeled during early postnatal life. Neural activity plays an important role in regulating one such rearrangement, synapse elimination, in the developing neuromuscular system, but there is little direct evidence on roles of pre- or postsynaptic activity in regulating synapse elimination in the developing brain. To address this issue, we expressed a chloride channel-yellow fluorescent protein fusion in cerebellar Purkinje cells (PCs) of transgenic mice to decrease their excitability. We then assessed elimination of supernumerary climbing fiber inputs to PCs. Individual PCs are innervated by multiple climbing fibers at birth; all but one are eliminated during the first three postnatal weeks in wild-type mice, but multiple innervation persists for at least three months in the transgenic mice. The normal redistribution of climbing fiber synapses from PC somata to proximal dendrites was also blunted in transgenics. These results show that normal electrical activity of the postsynaptic cell is required for it to attain a mature innervation pattern.
Keywords: chloride channel, climbing fiber, Purkinje cell
Throughout the vertebrate nervous system, neurons receive more inputs early in development than they maintain in adulthood. During a circumscribed period in early postnatal life, supernumerary inputs are removed by a process called synapse elimination, and the remaining inputs then become stronger. This process has been analyzed in greatest detail at a peripheral synapse, the skeletal neuromuscular junction, at which all motor axons but one are eliminated, leaving each muscle fiber with a single input. There, the process has been shown to be activity dependent and competitive, with the relative activities of the inputs playing a decisive role in the outcome (1–4).
Neural activity also regulates synaptic rearrangements in the developing central nervous system (5–8). In the cerebellar cortex, one of the best-studied systems in this respect, each Purkinje cell (PC) is innervated by multiple climbing fibers at birth. Between postnatal day (P) 5 and P15–20, supernumerary climbing fibers are withdrawn, leaving each PC innervated by just a single climbing fiber (7–10). In parallel, climbing fiber terminations are redistributed on the PC, with a loss of perisomatic synapses and an increase in number of synapses on proximal dendrites (10, 11). Early studies showed that supernumerary climbing fiber inputs were retained in mutants with developmental defects in cerebellar maturation (12–15), and subsequent analysis demonstrated a critical role for the other main input to PCs, the parallel fibers, in the elimination of redundant inputs (9, 16–25). Conversely, harmaline administration to inferior olivary neurons, from which climbing fibers arise, leads to synchronous activation and to the persistence of multiple innervation (26).
Limitations of these studies are that most of them involved perturbation of a particular neurotransmitter or signaling system (16, 18–25), and many of them involved global rather than cell type specific perturbations (12–15). Questions therefore remain as to whether electrical (action potential) activity per se is required for elimination of supernumerary climbing fiber inputs and, if so, whether presynaptic or postsynaptic activity (or both) is required. Here, we have devised a transgenic strategy to address these issues. We expressed a chloride channel in PCs postnatally, to selectively decrease the excitability of these cells during the period of synapse elimination. We then assessed the effects of this perturbation on the firing properties and the innervation of these cells. We show that decreasing the excitability of PCs prevents elimination of supernumerary climbing fiber inputs and decreases their redistribution from somata to proximal dendrites. The results demonstrate that firing properties of target neurons regulate refinement of synapses during the development in the CNS.
Results
Use of a Recombinant Chloride Channel to Decrease Neuronal Excitability.
A promising means of reducing excitability is to increase membrane permeability to K+ or Cl−, thereby making it more difficult for excitatory potentials to bring the neuron to threshold. In preliminary studies, we used biolistic transfection to express recombinant K+ and Cl− channel subunits, along with the green fluorescent protein (EGFP), in isolated rat superior cervical ganglion (SCG) neurons. Transfected cells were identified by EGFP fluorescence and targeted for recording under voltage- and current-clamp conditions. As demonstrated previously, the properties of cells expressing EGFP alone are indistinguishable from those of wild-type cells (27). Some of the channel constructs tested, such as Kir1.1 (KCNJ1), which generates weakly inwardly rectifying K+ channels, had no measurable effects on the resting or active membrane properties of SCG neurons, whereas expression of the Kir2.1 (KCNJ2) subunit, which generates strongly inwardly rectifying K+ channels (28), markedly hyperpolarized cells and rendered them inexcitable: Action potentials could not be generated in Kir2.1-expressing SCG neurons regardless of the amplitudes of the current injections.
In contrast, expression of the skeletal muscle chloride channel subunit ClC-1 (CLCN1) (29) reduced the excitability of SCG neurons without rendering them inexcitable. Inward and outward Cl− currents were readily recorded from ClC-1-expressing sympathetic neurons, and from ClC-1-expressing HEK-293 cells (Fig. S1). Expression of ClC-1 resulted in marked hyperpolarization of resting membrane potential (Vm) and decreased the input resistance (Rin) and the amplitude of afterhyperpolarizations in sympathetic neurons (Fig. S2 A–C). In addition, the mean amplitude of action potentials (APA) was significantly higher in cells expressing CLC-1 than in controls (Fig. S2C). Most important, significantly larger currents were required to evoke action potentials in cells expressing ClC-1, compared with cells expressing EGFP alone (Fig. S2D). Interestingly, however, action potential durations did not differ between EGFP− and ClC-1 + EGFP-expressing cells (Fig. S2D), demonstrating that expression of ClC-1 reduces, but does not block, sympathetic cell excitability. Consistent with this conclusion, larger currents were required to evoke repetitive firing (Fig. S2 E and F) and maximal firing rates were reduced (Fig. S2G) in ClC-1-expressing SCG neurons compared with controls.
Based on these results, we devised a strategy to selectively express ClC-1 in defined neuronal populations. First, we generated a ClC-1-yellow fluorescent protein (YFP) fusion protein, which we term CLY. The biophysical and functional properties of this fusion protein in heterologous cells were indistinguishable from those of wild-type ClC-1. We then generated transgenic mice in which the CLY coding sequences were separated from neuron-specific regulatory elements of the Thy1 gene by sequences that terminated transcription and translation (STOP sequences). These sequences were flanked by two loxP sites, making expression of CLY dependent on excision of the STOP sequences by Cre recombinase. In initial studies, several lines of these mice, called Thy1-SCLY, were mated to mice that expressed Cre ubiquitously (Beta-actin-Cre) (30). In one line, double transgenic offspring (Beta-actin-Cre;Thy1-SCLY) were nearly immobile and died perinatally, presumably reflecting decreased excitability of motor neurons. Histological examination of the brains and spinal cords of these animals revealed no detectable abnormalities.
Expression of Chloride Channels in Purkinje Cells.
To selectively inactivate PCs, we used mice in which regulatory elements from the L7/PcP2 gene drive expression of Cre recombinase (31). Previous studies have shown PC-specific expression of Cre in this line (31). Doubly transgenic mice (L7-Cre;Thy1-SCLY, called L7-SCLY hereafter) were identified by PCR (Fig. S3) and assayed for expression of the CLY fusion protein by immunostaining with antibodies to GFP. CLY was readily detectable in PCs but not in any other cells in the cerebellum or elsewhere in the brain (Fig. 1A). In contrast, CLY was broadly expressed in brain and spinal cord of Beta-actin-Cre;Thy1-SCLY mice, and no expression of CLY was detectable in Thy1-SCLY mice that had not been mated to a Cre-expressing line. Within PCs, the CLY fusion protein was present throughout the soma and dendrites, and extended into proximal axons (Fig. 1A).
Fig. 1.
Expression of chloride channel-YFP fusion protein in Purkinje cells. (A) Sections of cerebellum from transgenic mice show that the ClC-YFP fusion protein, stained with anti-GFP, is present in somata (a1), dendrites (a2), and proximal axons (a3). (B) Sections from transgenic mice at different ages stained with the Purkinje cell specific marker anti-calbindin (red) and anti-GFP (green) to detect ClC-YFP fusion protein. (Scale bars: 50 μm.) (C) Percentage of PCs expressing the fusion protein. Mean ± SEM is plotted as function of the age.
To determine the time course of CLY expression, we examined parasagittal sections of the cerebellar vermis of L7-SCLY mice at ages ranging from P2 to P30. Sections were double labeled with anti-calbindin, to detect all PCs. We detected no CLY-positive PCs at P2, but the fraction of positive cells increased rapidly to approximately 60% by P5–9 (Fig. 1 B and C). Levels of CLY per cell increased over the subsequent weeks (Fig. 1B), and expression persisted until at least 1 year of age. Because climbing fibers are normally eliminated between P5 and P21 (10), CLY is present in a majority of PCs during the period of synaptic rearrangement. Conversely, lack of CLY expression before this period implies that the transgene is unlikely to significantly affect early events in PC maturation.
We compared L7-SCLY and control cerebella to determine whether expression of CLY affected cellular structure. We detected no difference between transgenic and control animals at P30–40 in any of the following parameters: Cerebellar size, cerebellar thickness, cerebellar foliation, PC size, or level of calbindin staining in PCs. PCs in the L7-SCLY transgenics showed no evidence of axonal or dendritic degeneration.
Electrophysiological Analysis of Transgenic Purkinje Cells.
To analyze spiking activity of PCs, we recorded extracellularly from single cells in vivo (12 cells from 8 P11–16 control mice, 20 cells from 7 P30 controls, 21 cells from 14 P30 transgenics, and 8 cells from 6 P90 transgenics). Representative records are shown in Fig. 2 A–D. Two qualitatively different types of activity are evident in these records. One is the simple spike, which corresponds to a single PC action potential. The other is an all-or-nothing complex spike, consisting of several components of diminishing amplitude (see also Fig. S4). Previous studies have demonstrated that complex and simple spikes are generated in response to climbing fiber and parallel fiber activity, respectively (32). Each complex spike represents the response to a single, suprathreshold climbing fiber input and each simple spike represents the response to multiple, subthreshold parallel fiber inputs (32). Importantly, different climbing fiber inputs to a single PC generate complex spikes with distinct waveforms (10), so the number of inputs to a PC can be estimated from the fine structure of the complex spike, determined as shown in Fig. S4. In wild-type mice, multiple waveforms were evident in recordings from 75% of PCs at P11–16 but in no PCs at P30 (Fig. 2 A, B, E, and F), consistent with elimination of supernumerary climbing fiber inputs during the third postnatal week (10). In contrast, we detected multiple complex spike waveforms in 90% of PCs in L7-SCLY mice at P30 (Fig. 2 C, E, and F). To determine whether the elimination of supernumerary innervation was blocked or only transiently delayed in L7-SCLY mice, we also recorded from PCs at P90, and found that >75% showed multiple complex spike waveforms (Fig. 2 D–F). Thus, multiple innervation of PCs by climbing fibers persists in mature L7-SCLY transgenics. In addition, the complex spike rate from transgenics at P30 and P90 was significantly higher than in wild-type P30 PCs and was comparable to that of wild-type P11-P16 PCs (Fig. 2G and Table S1).
Fig. 2.
Multiple climbing fiber responses and increased complex spike rate in Purkinje cells of L7-SCLY mice. (A–D) Extracellular single-unit recordings are shown. (Left) Traces show the occurrence of complex spikes (CS). (Right) Expanded traces show complex spike waveforms marked by different symbols in the Left. Simple spikes (SS; arrows) are shown for comparison. (E) Percentage of Purkinje cells showing significantly distinct complex spike waveforms in indicated groups. Multiple climbing fiber responses persisted in transgenic (Tg) mice. (F) Average number of different complex spike waveforms detected across PCs within each neuronal group. (G) Average complex spike rate (Hz) of the different PC groups. Complex spike rate in PCs recorded from P30 and P90 transgenics was higher than in wild-type P30 PC.
We also analyzed simple spikes to ask whether signaling from parallel fibers to PCs differed between control and transgenic mice. In young wild-type mice, PCs display a tonic or unimodal pattern of simple spikes, whereas by one month of age, the pattern is bimodal, with periods of high-frequency simple spikes intermingled with periods of relative inactivity (Fig. 3 A and B) (33, 34). Compared with mature PCs, immature PCs displayed a decreased probability of showing silent pauses and an increased duration of activity periods (Fig. 3 E and F and Table S1). Notably, PCs from one month-old L7-SCLY mice displayed a simple spike firing pattern similar to that of PCs in P15 controls and quiescent periods were rare in these cells (Fig. 3C). Such a unimodal firing pattern was maintained in transgenic PCs recorded at P90 (Fig. 3 D and E). On average, however, simple spike rates were similar in mature control and transgenic PCs (Fig. 3G and Table S1).
Fig. 3.
Absence of bimodal firing pattern in Purkinje cells of L7-SCLY mice. (A–D) Longer intervals from records shown in Fig. 2 are used to illustrate simple spike (SS) firing patterns. Frequency distribution histograms from single unit data (bin size, 2 Hz) are shown (Right). WT PCs recorded at P30 (B) displayed long trains of simple spikes interspersed with quiescent periods. WT P15 (A), transgenic (Tg) P30 (C), and Tg P90 (D) PCs displayed long tonic periods of simple spike activity with absent or sporadic short quiescent periods. (E) Average occurrence probability of quiescent periods for each neuronal group. (F) Average SS burst duration for each neuronal group. (G) Average SS rate for each neuronal group.
Decreased PC Excitability Blocks Withdrawal of Climbing Fiber Inputs.
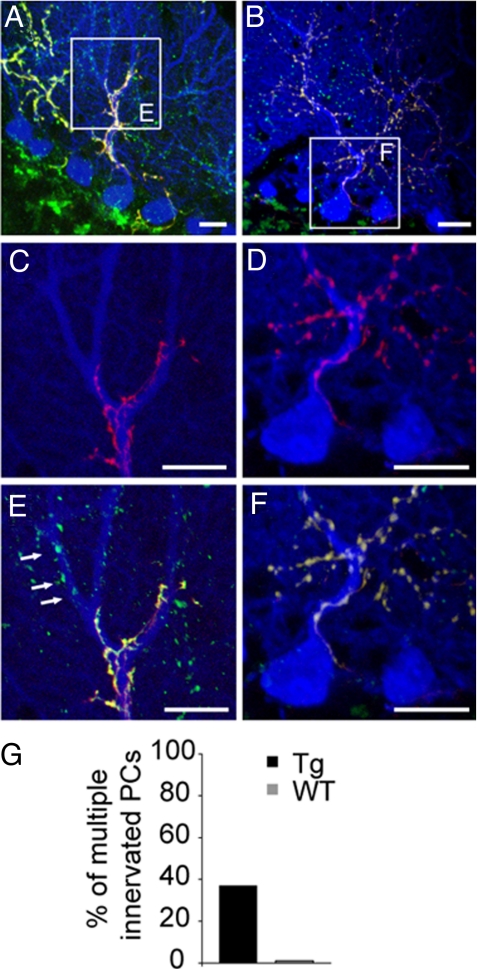
To determine the anatomical basis for the supernumerary inputs recorded electrophysiologically, we labeled a subset of climbing fibers by injection of biotinylated dextran (BDA) into the inferior olivary nucleus, which contains the somata of the neurons from which climbing fibers arise. Injections were made at P20-P30, shortly after climbing fiber elimination is complete in wild-type mice, and cerebella were examined 7–10 days later. PCs were stained with anti-calbindin or anti-GFP, all climbing fiber synapses were marked with antibodies to VGluT2, and anterogradely transported BDA was revealed with streptavidin conjugated Alexa 594. As shown in Fig. 4A, C, and E, some individual PC cells were contacted by both BDA-positive and BDA-negative climbing fibers, demonstrating that they were innervated by at least two climbing fibers. Approximately 40% of transgenic PCs (13/30 from five mice), but no wild-type PCs (0/30 from four mice), analyzed in this way were innervated by multiple climbing fibers (Fig. 4 B, D, F, and G). It is important to note that this method underestimates the extent of polyneuronal innervation, because PCs innervated by two BDA-negative or two BDA-positive climbing fibers would not be scored as multiply innervated. If half of the climbing fibers were filled with BDA and if BDA-positive and -negative climbing fibers were randomly intermingled, the real incidence of double innervated PCs would be twice that scored in these experiments. Thus, the value of approximately 40% multiple innervation determined morphologically is not inconsistent with the value of 90% determined electrophysiologically (Figs. 2 E and 4G).
Fig. 4.
Polyneuronal innervation of Purkinje cells in transgenic mice. (A) Triple fluorescent labeling for BDA (red), VGluT2 (green), and chloride channel-YFP expressing PC (blue) of cerebellar slices from P40 transgenic mice demonstrating the multiple climbing fiber innervation of Purkinje cells. A Inset is enlarged in C and E. (B) Wild-type PC innervated by only one double-labeled climbing fiber. B Inset is enlarged in D and F. (G) Percentage of multiple innervated PCs assayed by confocal microscopy. (Scale bars: 20 μm.)
Further analysis showed that climbing fiber morphology was heterogeneous in L7-SCLY mice. Some fibers were highly ramified and apparently normal, whereas others were reduced to a thin branch with few varicosities. Still others retained immature features, extending many branches in the first half of the molecular layer but only one or two branches upward (Fig. S5). Thus, histological and physiological maturation of climbing fibers is disrupted in L7-SCLY mice.
Number and Position of Climbing Fiber Varicosities on Transgenic Purkinje Cells.
Supernumerary climbing fibers persisting on a transgenic PC might retain as many synapses as single climbing fibers in wild-type mice. Alternatively, the total number of climbing fiber synapses on each PC in transgenics might be similar to that in controls, in which case each transgenic climbing fiber would make fewer synapses than a single control climbing fiber. To distinguish these possibilities, we used VGluT2 staining to count the total density of climbing fiber varicosities on PC somata and proximal dendrites (Fig. 5 A–C). The density of climbing fiber varicosities was approximately 50% higher in transgenic PCs, than in controls (Fig. 5D). This result is consistent with the increase in climbing fiber numbers and suggests that multiple climbing fibers form a full complement of synapses on transgenic PCs.
Fig. 5.
Increase of climbing and parallel fibers termination densities. (A–C) Confocal images of VGluT2-labeled climbing fiber terminations (green) on PCs stained for Calbindin in control mice (A) or GFP (B) in transgenic mice (both red). Small portions of the molecular layer were acquired at high magnification (C) to estimate the number of VgluT2 varicosities per μm of dendrite length (D). An increase of VGluT2 varicosities was found in transgenic mice (P < 0.001 by t test; number of measured dendrites in parentheses). (E–G) Confocal images of VGluT1-labeled parallel fibers terminations (green) on PCs stained for Calbindin (E) or GFP (F) as in A–C. Small portions of the molecular layer were acquired at high magnification (G) to estimate the density of VGluT1 varicosities on selected peridendritic areas (H). An increase of VGluT1 varicosities was found in transgenic mice (P < 0.001 by t test; number of measured areas in parentheses). (Scale bars: 50 μm in A, B, E, and F; 10 μm in C; 5 μm in G.)
We also asked whether the position of climbing fiber terminations on PCs was affected by expression of CLY. During the early postnatal period when supernumerary climbing fibers are lost from PCs, the synapses of the persisting climbing fibers are redistributed from the soma to proximal dendrites (10, 11). To ask whether climbing fiber redistribution occurred in L7-SCLY animals, we analyzed the distribution of VGluT2-positive climbing fiber synapses on calbindin- or GFP-stained PCs (Fig. 6 A and B). At P7–8, somatic climbing fiber synapses were present on nearly all transgenic and control PCs (Fig. 6C). Somatic synapses were subsequently lost from both control and transgenic PCs, but significantly more transgenic than control PCs retained somatic climbing fiber synapses at P15–18 and P30–60. Consistent with this decreased or delayed progression of synapses from soma to dendrites, the extent of VGluT2-positive synapses along PC dendrites was significantly less in transgenics than in controls (Fig. 6D).
Fig. 6.
Persistence of perisomatic climbing fiber synapses in transgenic PCs. (A and B) Confocal images of P30 wild-type and transgenic mice showing PCs in red (stained with anti-Calbindin and anti-GFP, respectively) and climbing fiber terminations labeled with anti-VGluT2 (green). Climbing fiber terminations are absent from somata of P30 control PCs but present on many PC somata in transgenic mice (B). (Scale bars: 25 μm.) (C) The percentage of PCs somatic profiles bearing climbing fiber synapses. Differences between groups: P < 0.02 at P15–18, P < 0.01 in P30–60 by t test. (D). The percentage of the distance from the apex of the Purkinje cell layer to the base of the molecular layer occupied by climbing fiber terminals. Extension is lower in transgenic mice than in controls (P < 0.001 by t test; number of mice in parentheses).
Finally, we asked whether the increased climbing fiber synapse density or the increased territory they occupied occurred at the expense of the other main class of excitatory synapses on PCs, those made by parallel fibers. We could distinguish these main synaptic types because parallel fiber terminals are VGluT1 positive whereas, as noted above, climbing fiber varicosities are VGluT2-positive. The density of VGluT1-positive parallel fiber synapses was 15% higher in transgenic than control mice (Fig. 5 E–H). Thus, increased innervation by climbing fibers does not endanger innervation by parallel fibers. Instead, the small but significant increase of VGluT1 raises the possibility that elimination of supernumerary parallel fibers may also be prevented in L7-SCLY PCs. However, because each PC is innervated by thousands of parallel fibers in wild-type mice, it is not feasible to test this idea directly.
Motor Coordination Is Impaired in Transgenic Mice.
To assess the functional consequence of PC inactivation and retained supernumerary innervation by climbing fibers, we assayed L7-SCLY mice with rotarod and footprint tests of motor coordination. Transgenic mice were less able to stay on a rotating rod (rotarod) than controls (Fig. S6A). Significant differences were observed at each of four speeds tested. Performance improved significantly and to a similar extent on successive trials in both groups (Fig. S6B), suggesting that the defect observed in transgenics reflects impaired motor coordination rather than impaired skill learning. We also assessed gait by measuring the distance between footprints (Fig. S6C). A slight shortening of stride length was found in transgenics (Fig. S6D), while other parameters, such as base width and distance between feet were similar between groups.
Discussion
To assess the role of neural activity in synapse elimination, we selectively expressed a chloride channel, CLC1, in cerebellar PCs. The transgene was expressed only postnatally, and accordingly had no detectable effect on the early development of these cells. However, polyneuronal innervation of PCs by climbing fibers, normally eliminated during the first three postnatal weeks, persisted in transgenic PCs, as assessed by electrophysiological and morphological criteria. Expression of CLC1 also decreased translocation of climbing fiber synapses from PC soma to proximal dendrites, and led to deficits in motor behavior consistent with cerebellar malfunction. These results provide strong genetic evidence that synapse elimination in the CNS requires appropriate amounts and/or patterns of electrical activity in the postsynaptic cell.
Many investigators have pointed out the parallels between elimination of supernumerary climbing fiber inputs to PCs and elimination of supernumerary motor axonal inputs to muscle fibers. In view of strong evidence that synapse elimination at the neuromuscular junction is activity-dependent, it has been attractive to suppose that the same is true for climbing fibers. Consistent with this idea, previous studies have indicated that the presence of functional parallel fiber synapses onto PCs is necessary for the postnatal elimination of supernumerary climbing fiber inputs. For example, climbing fiber elimination is delayed or prevented in spontaneously occurring mutant strains, including weaver, staggerer, and reeler, in which granule cells, from which parallel fibers arise, fail to form or degenerate (12–15, 35). Climbing fiber elimination is also impaired after local X-irradation of granule cell progenitors in newborn rats, thereby eliminating granule cells (36). Later studies revealed impairment of climbing fiber elimination after more precise lesions in mutant mice, such as mutations of the orphan glutamate receptor ∂2 subunit (GluR∂2) or the metabotropic glutamate receptor (mGluR1), both of which are highly expressed at parallel fiber-PC synapses, or of components of the mGluR1 signal transduction pathway, such as G alpha q, phospholipase C beta 4, and PKC gamma (16–25). Finally, pharmacological block of NMDA receptor activity at mossy fiber-granule cell synapses, during a relatively short critical period (P15–16), also blocks climbing fiber elimination (37, 38).
Although these results are consistent with the idea that climbing fiber elimination requires appropriate electrical activity in PCs, they are open to alternative interpretations. Elimination of parallel fiber inputs (12–15, 35, 36), for example, may remove sources of trophic support or developmental signals, and activity. Likewise, synaptic transmission at parallel fiber-PC synapses is mediated in large part by AMPA-type glutamate receptors, so inactivation of metabotropic receptors and downstream signal transduction cascades may affect PC metabolism in ways that are not strictly attributable to effects on electrical activity. Indeed, activation of mGluR1 produces a complex postsynaptic response consisting of a calcium-release signal from intracellular stores and a slow excitatory postsynaptic potential, and long-term depression at parallel fiber-PC synapses is deficient in mGluR1−/− mice (39). The recent finding that inactivation of the neurotrophin receptor, TrkB, slows elimination of climbing fibers (40, 41), might reflect altered electrical activity of PCs because inhibitory inputs develop abnormally, but this perturbation could alternatively upset trophic balance in an activity-independent fashion. Perhaps more direct are studies in which harmaline was administered to the inferior olivary nucleus, which contains the cell bodies from which climbing fiber originates. This treatment disrupted the normal pattern of activity in climbing fibers, increasing synchrony among climbing fibers, and led to a persistence of polyneuronal innervation of PCs (26). These results support the idea that the pattern of PC activity regulates competition among climbing fibers vying for domination of a PC, a conclusions similar to that supported by genetic, histological and physiological studies of the neuromuscular junction (1–4). Nonetheless, the effects of harmaline are complicated, and it is not clear whether the observed defects in synapse elimination result from changes in the amount/pattern of climbing fiber activity, or from other alterations in climbing fiber structure and/or function.
In light of these uncertainties, we aimed to perform a direct test of the role of neural activity, and more specifically of postsynaptic activity, in the elimination of climbing fibers. The use of L7-Cre;Thy1-SCLCY transgenic mice had several advantages for addressing this issue. First, perturbation was limited to PCs; their inputs and other cells in the cerebellum were not directly affected. Second, the effect was directly and specifically exerted on PC excitability, without direct interference with membrane receptors or intracellular pathways. Third, SCLY expression was undetectable until after P2, so early events in PC development or climbing fiber innervation were not perturbed. The links between altered activity and synapse elimination remain to be elucidated; they may include effects on retrograde trophic signals that impinge directly on climbing fibers or act indirectly through other cellular elements.
In addition to blocking synapse elimination, expression of SCLCY delayed the migration of climbing fiber synapses from soma to proximal dendrites (10, 11, 42). The molecular mechanisms underlying the translocations are unknown, but available data suggests that it is neither a cause nor a consequence of synapse elimination. Indeed, after some perturbations the location of climbing fibers inputs is abnormal but synapse elimination occurs on schedule (43, 44). Conversely, in TrkB mutants, synapse elimination is delayed but synaptic translocation occurs on schedule (41). These results suggest that these steps in maturations are regulated independently. We therefore conclude that postsynaptic activity exerts separate effects on the number of climbing fiber inputs to PCs and on their subcellular locations.
Materials and Methods
Animals.
All animal procedures were carried out in accordance with the policy of Washington University and the Italian Ministry of Health on the use of animals in research. Mice were kept on a 14/10-h day/night cycle with food and water ad libitum.
Trangenic mice with conditional over-expression of a muscle chloride channel-YFP fusion protein (Thy1-SCLY) were generated in our laboratory. The ClC-1 cDNA (45) was a generous gift from Dr. Klaus Steinmeyer (University of Hamburg, Germany). The ClC1-YFP fusion was made by inserting the ClC-1 cDNA into the HindIII/BamHI sites of pEYFP-N1. A LoxP-STOP-LoxP fragment (4) was introduced into the HindIII site of ClC-1-YFP to generate SCLY. SCLY (LoxP-STOP-LoxP-ClC1-YFP) was then cloned into a mouse Thy1.2 vector (46, 47). Transgenic mice were generated by standard methods. These mice were mated to transgenics that expressed Cre recombinase ubiquitously (ß-actin-Cre; 30) or selectively in Purkinje cells (L7-Cre; 31). Mice were genotyped by PCR (see Fig. S3B).
Electrophysiology.
Whole cell recordings were obtained from transfected HEK-293 cells and SCG neurons at room temperature (22–25 °C). Detailed protocols for transfection and electrophysiological analysis are presented in SI Text.
For in vivo extracellular single-unit recordings from cerebellar cortex, mice were anesthetized by i.p. injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) then placed in a stereotaxic apparatus. A craniotomy was performed above the cerebellum to expose a small region of the cerebellar vermis or hemispheres. The exposed area was covered with agar (4% in physiological solution). Heart rate and body temperature were continuously monitored. Extracellular recordings of Purkinje cell single-unit activity were obtained using glass microelectrodes. Protocols for recording, sorting, and analyzing simple and complex spikes are detailed in SI Text. On completion of the electrophysiological protocol, a small amount of methyl blue dye was deposited at the recording site by passing 1-mA current through the recording electrode for 5–8 min. The brain was then removed, fixed, and sectioned to verify electrode placement.
Histology.
Mice were transcardially perfused with 4% paraformaldehyde (PFA) in phosphate buffer solution (PBS), pH 7.2. Cerebella were postfixed, cryoprotected, and sectioned parasagittally at 30 μm in a cryostat. Sections were then immunostained and examined by epifluorescence or confocal microscopy. Detailed methods for immunofluorescence, confocal analysis and anterograde tracing are provided in SI Text.
Supplementary Material
Acknowledgments.
We thank M. Meyer (Max Planck Institut, Martinsried, Germany) for L7-Cre mice, G. Martin (University of California, San Francisco) for ß-actin-Cre mice, K. Steinmeyer (University of Hamburg, Germany) for reagents, Prof. A. Cangiano for comments, and the Genome Modification Core at Washington University. This work was supported by University of Verona, Fondazione Cariverona, and by grants from the National Institutes of Health to J.M.N and J.R.S.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907298106/DCSupplemental.
References
- 1.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 2.Buffelli M, Busetto G, Bidoia C, Favero M, Cangiano A. Activity-dependent synaptic competition at mammalian neuromuscular junctions. News Physiol Sci. 2004;19:85–91. doi: 10.1152/nips.01464.2003. [DOI] [PubMed] [Google Scholar]
- 3.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 4.Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 5.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 6.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009 May 27; doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Bosman LW, Konnerth A. Activity-dependent plasticity of developing climbing fiber-Purkinje cell synapses. Neuroscience. 2009;162:612–623. doi: 10.1016/j.neuroscience.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72:295–339. doi: 10.1016/j.pneurobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- 11.Crepel F. Regression of functional synapses in the immature mammalian cerebellum. Trends Neurosci. 1982;5:266–269. [Google Scholar]
- 12.Crepel F, Delhaye-Bouchaud N, Guastavino JM, Sampaio I. Multiple innervation of cerebellar Purkinje cells by climbing fibres in staggerer mutant mouse. Nature. 1980;283:483–484. doi: 10.1038/283483a0. [DOI] [PubMed] [Google Scholar]
- 13.Crepel F, Mariani J. Multiple innervation of Purkinje cells by climbing fibers in the cerebellum of the Weaver Mutant Mouse. J Neurobiol. 1976;7:579–582. doi: 10.1002/neu.480070610. [DOI] [PubMed] [Google Scholar]
- 14.Mariani J, Changeux JP. Multiple innervation of Purkinje cells by climbing fibers in the cerebellum of the adult staggerer mutant mouse. J Neurobiol. 1980;11:41–50. doi: 10.1002/neu.480110106. [DOI] [PubMed] [Google Scholar]
- 15.Mariani J, Crepel F, Mikoshiba K, Changeux JP, Sotelo C. Anatomical, physiological and biochemical studies of the cerebellum from Reeler mutant mouse. Philos Trans R Soc London Ser B. 1977;281:1–28. doi: 10.1098/rstb.1977.0121. [DOI] [PubMed] [Google Scholar]
- 16.Kano M, et al. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto K, Kano M. Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neurosci Res. 2005;53:221–228. doi: 10.1016/j.neures.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto K, et al. Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci. 2001;21:9701–9712. doi: 10.1523/JNEUROSCI.21-24-09701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto K, et al. Climbing fiber synapse elimination during postnatal cerebellar development requires signal transduction involving G alpha q and phospholipase C beta 4. Prog Brain Res. 2000;124:31–48. doi: 10.1016/S0079-6123(00)24006-5. [DOI] [PubMed] [Google Scholar]
- 20.Kano M, et al. Impaired synapse elimination during cerebellar development in PKC gamma mutant mice. Cell. 1995;83:1223–1231. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 21.Ichise T, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- 22.Offermanns S, et al. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Galphaq. Proc Natl Acad Sci USA. 1997;94:14089–14094. doi: 10.1073/pnas.94.25.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashiwabuchi N, et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 24.Kurihara H, et al. Impaired parallel fiber->Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor delta2 subunit. J Neurosci. 1997;17:9613–9623. doi: 10.1523/JNEUROSCI.17-24-09613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalouette A, Lohof A, Sotelo C, Guenet J, Mariani J. Neurobiological effects of a null mutation depend on genetic context: Comparison between two hotfoot alleles of the delta-2 ionotropic glutamate receptor. Neuroscience. 2001;105:443–455. doi: 10.1016/s0306-4522(01)00193-2. [DOI] [PubMed] [Google Scholar]
- 26.Andjus PR, Zhu L, Cesa R, Carulli D, Strata P. A change in the pattern of activity affects the developmental regression of the Purkinje cell polyinnervation by climbing fibers in the rat cerebellum. Neuroscience. 2003;121:563–572. doi: 10.1016/s0306-4522(03)00556-6. [DOI] [PubMed] [Google Scholar]
- 27.Malin SA, Nerbonne JM. Elimination of the fast transient in superior cervical ganglion neurons with expression of KV4.2W362F: Molecular dissection of IA. J Neurosci. 2000;20:5191–5199. doi: 10.1523/JNEUROSCI.20-14-05191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johns DC, Marx R, Mains RE, O'Rourke B, Marbán E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valverde MA. ClC channels: Leaving the dark ages on the verge of a new millennium. Curr Opin Cell Biol. 1999;11:509–516. doi: 10.1016/s0955-0674(99)80074-x. [DOI] [PubMed] [Google Scholar]
- 30.Lewandoski M, Martin GR. Cre-mediated chromosome loss in mice. Nat Genet. 1997;17:223–225. doi: 10.1038/ng1097-223. [DOI] [PubMed] [Google Scholar]
- 31.Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98. [PubMed] [Google Scholar]
- 32.Ebner TJ, Bloedel JR. Temporal patterning in simple spike discharge of Purkinje cells and its relationship to climbing fiber activity. J Neurophysiol. 1981;45:933–947. doi: 10.1152/jn.1981.45.5.933. [DOI] [PubMed] [Google Scholar]
- 33.McKay BE, Turner RW. Physiological and morphological development of the rat cerebellar Purkinje cell. J Physiol. 2005;567:829–850. doi: 10.1113/jphysiol.2005.089383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci. 2002;22:10603–10612. doi: 10.1523/JNEUROSCI.22-24-10603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinmayr M, et al. Staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice. Proc Natl Acad Sci USA. 1998;95:3960–3965. doi: 10.1073/pnas.95.7.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugihara I, Bailly Y, Mariani J. Olivocerebellar climbing fibers in the granuloprival cerebellum: Morphological study of individual axonal projections in the X-irradiated rat. J Neurosci. 2000;20:3745–3760. doi: 10.1523/JNEUROSCI.20-10-03745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabacchi S, Bailly Y, Delhaye-Bouchaud N, Mariani J. Involvement of the N-methyl D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science. 1992;256:1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- 38.Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartmann J, Konnerth A. Mechanisms of metabotropic glutamate receptor-mediated synaptic signaling in cerebellar Purkinje cells. Acta Physiol (Oxf) 2009;195:79–90. [Google Scholar]
- 40.Bosman LW, et al. Requirement of TrkB for synapse elimination in developing cerebellar Purkinje cells. Brain Cell Biol. 2006;35:87–101. doi: 10.1007/s11068-006-9002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson EM, Craig ET, Yeh HH. TrkB is necessary for pruning at the climbing fibre-Purkinje cell synapse in the developing murine cerebellum. J Physiol. 2007;582:629–646. doi: 10.1113/jphysiol.2007.133561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scelfo B, Strata P, Knöpfel T. Sodium imaging of climbing fiber innervation fields in developing mouse Purkinje cells. J Neurophysiol. 2003;89:2555–2563. doi: 10.1152/jn.00884.2002. [DOI] [PubMed] [Google Scholar]
- 43.Mariani J. Elimination of synapses during the development of the central nervous system. Prog Brain Res. 1983;58:383–392. doi: 10.1016/S0079-6123(08)60041-2. [DOI] [PubMed] [Google Scholar]
- 44.Sotelo C. Cerebellar synaptogenesis: What we can learn from mutant mice. J Exp Biol. 1990;153:225–249. doi: 10.1242/jeb.153.1.225. [DOI] [PubMed] [Google Scholar]
- 45.Koch MC, et al. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- 46.Caroni P. Overexpression of growth associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997;71:3–9. doi: 10.1016/s0165-0270(96)00121-5. [DOI] [PubMed] [Google Scholar]
- 47.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.