Abstract
Mercury (Hg) is an extremely toxic pollutant, and its biogeochemical cycle has been perturbed by anthropogenic emissions during recent centuries. In the atmosphere, gaseous elemental mercury (GEM; Hg°) is the predominant form of mercury (up to 95%). Here we report the evolution of atmospheric levels of GEM in mid- to high-northern latitudes inferred from the interstitial air of firn (perennial snowpack) at Summit, Greenland. GEM concentrations increased rapidly after World War II from ≈1.5 ng m−3 reaching a maximum of ≈3 ng m−3 around 1970 and decreased until stabilizing at ≈1.7 ng m−3 around 1995. This reconstruction reproduces real-time measurements available from the Arctic since 1995 and exhibits the same general trend observed in Europe since 1990. Anthropogenic emissions caused a two-fold rise in boreal atmospheric GEM concentrations before the 1970s, which likely contributed to higher deposition of mercury in both industrialized and remotes areas. Once deposited, this toxin becomes available for methylation and, subsequently, the contamination of ecosystems. Implementation of air pollution regulations, however, enabled a large-scale decline in atmospheric mercury levels during the 1980s. The results shown here suggest that potential increases in emissions in the coming decades could have a similar large-scale impact on atmospheric Hg levels.
Keywords: atmosphere, Greenland, past century, pollution
Mercury (Hg) is a worldwide pollutant globally dispersed in the environment. Lake and ocean sediment profiles, peat bogs, and ice—in both remote and industrialized regions—suggest that natural background levels of mercury have increased during the past 150 years (1). Based on these observations, global-scale Hg cycling models show that anthropogenic emissions have substantially perturbed the global biogeochemical mercury cycle since preindustrial times (2). Recently, efforts have been made to reduce Hg emissions from industry and power plants in both North America and Europe. Direct anthropogenic sources such as coal combustion, however, still release large amounts of inorganic mercury into the atmosphere, either as gaseous elemental mercury (GEM; Hg°) or as divalent gaseous mercury species (Hg2+) (3).
GEM, with an atmospheric lifetime in the range of 5–24 months, can be transported across long distances to remote locations before conversion to divalent Hg species by atmospheric oxidation processes (4). Divalent Hg species are subject to rapid wet and dry deposition in terrestrial ecosystems. Some of the Hg2+ deposited in aquatic systems and sediments is transformed into organic methylmercury (5). Although inorganic Hg species have severe health effects on humans, methylmercury is thought to be the highly toxic species involved in bioaccumulation in aquatic food chains. In thousands of lakes in North America, Europe, and Asia, methylmercury contamination of fish negatively impacts the health of humans and wildlife, particularly affecting populations whose traditional diet is based on seafood (e.g., in the Arctic) (5).
Many factors affect fish mercury levels (5), and the response of fish methylmercury concentrations to changes in mercury deposition has been difficult to establish. Harris et al. (6) recently reported, however, a whole ecosystem study demonstrating a rapid and direct change in mercury levels in fish resulting from a change in atmospheric mercury. Thus, recent evolution of the atmospheric mercury burden, strongly influenced by anthropogenic emissions, could have played a key role in contamination of many ecosystems. This linkage is of prime importance because many organizations (e.g., United Nations Environmental Program) and nations (e.g., the United States, Europe, and Canada) are presently debating the implementation and extension of mercury emission regulations and implementation of a global network to monitor mercury pollution.
To better assess the global influence of anthropogenic mercury emissions, new methodological approaches are needed. In particular, historical evolution of atmospheric GEM concentrations during the last few decades remains poorly characterized, even though GEM represents as much as 95% of the atmospheric mercury burden (4). The first direct measurements of total gaseous mercury (TGM, including GEM and divalent gaseous mercury species) were reported in the late 1970s during ship cruises across the Atlantic Ocean (7), but real-time atmospheric continuous monitoring at various European and Arctic locations was only initiated in the early 1990s (8). Lake sediment (9, 10), peat bog (11, 12), and ice and snow (13, 14) records have been powerful tools to reconstruct past evolution of atmospheric deposition of Hg2+ species at specific locations, although their interpretation is still debated (15, 16). These archives, however, do not inform directly about GEM, the major atmospheric Hg species. In this paper, we present results from a natural archive that provides a unique history of gaseous elemental mercury concentrations at middle and high northern latitudes. Specifically, we examined polar firn air as an archive for investigating the impact of anthropogenic emissions on the mercury cycle.
Results and Discussion
The potential of polar ice sheets to serve as an archive for reconstruction of past atmospheric compositions has been well established (17, 18). From the top surface to ≈60 to 120 m depth is the firn, an openly porous and permeable consolidated snow through which air can diffuse. Consequently, changes in the composition of the atmosphere propagate downward through the firn, such that the mean age of the air increases monotonically with depth. The firn air at Summit Station (72.6°N, 38.5°W) in central Greenland allowed us to reconstruct the decadal-average atmospheric history of GEM levels during the last ≈70 years. Using established methods (18, 19), we sampled firn air from one borehole at 14 depth intervals from 15 to 79.5 m and analyzed for GEM concentrations with a 2537A Tekran analyzer (see Materials and Methods). Evolution of GEM levels with depth in the firn air is reported in Fig. 1.
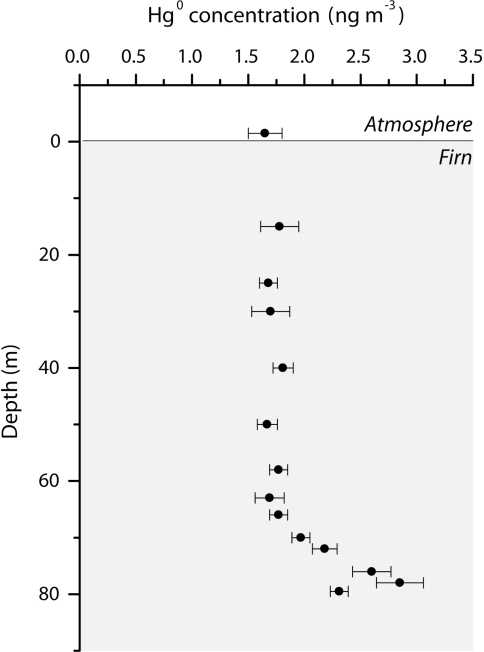
Fig. 1.
Gaseous elemental mercury (GEM; Hg°) concentrations measured in firn air at Summit Station from 15 to 79.5 m depth. The atmospheric level reported is a mean concentration from three weeks of measurements during both summer 2005 and spring 2006 (21). Above 69 m depth in the firn is the diffusion zone, where gas can diffuse rapidly. From 69 to 80 m is the lock-in zone, where impermeable winter layers prevent vertical diffusion of air, but persistent open porosity allows air pumping. The bars represent one standard deviation confidence interval.
Due to physical effects and, in some instances, chemical effects, the composition of firn air at any depth does not correspond exactly to the atmospheric composition at some time in the past (20). Thus, evaluation of all physical and chemical processes that may affect GEM between the shallow snowpack and the deep firn is required to correctly infer an atmospheric history from firn air concentrations (reported in Fig. 1). We recently investigated this transfer function for GEM under present conditions and proposed a specific conceptual model of the firn at Summit (21). Three primary zones were categorized: the chemical zone, the diffusion zone, and the lock-in zone. In the near surface snow air, we observed during late spring and summer variability of GEM concentrations on a daily timescale induced by chemical processes, as well as a seasonal shift in these chemical mechanisms. Such processes defined the chemical zone, which extends from the surface to ≈2.5 m depth. Daily variations of GEM concentrations are too rapid to influence the firn record, however, and the influence of seasonal variations does not extend below the first 15 m of the firn. The diffusion zone extends from the bottom of the chemical zone to the top of the lock-in zone and is comprised of an openly porous and permeable matrix in which air composition is solely determined by physical processes. Within the lock-in zone, the impermeable winter layer prevents vertical diffusion of air, but persistent open porosity in the summer layer allows extraction of samples. Hence, air in the lock-in zone cannot equilibrate with the overlying diffusive column. The transition between the diffusion zone and the lock-in zone is located at ≈69 m depth during this study. Above 69 m depth, the diffusion zone allows a smooth record of atmospheric changes. Below 69 m depth, the air ages at approximately the same rate as the surrounding ice. At still greater depths, when the overburden is sufficient, all pores become closed, and air can no longer be extracted. This defined the transition from firn to ice also referred to as close-off and is located at ≈79.5 m depth at Summit Station (20).
To infer the atmospheric record of GEM from firn air concentrations presented in Fig. 1, we determined a site specific, diffusivity-depth relationship. No changes in the physical structure of the firn required consideration: surface temperature and accumulation are the main parameters determining firn structure, and their limited evolution at Summit during the last century (22, 23) did not induce significant changes in either density or porosity of the firn. We next applied a one-dimensional firn diffusion model (24) to reconstruct GEM concentrations at all depths in the firn air from different atmospheric histories. A Monte Carlo approach enabled us to optimize agreement between these modeled concentration-depth profiles and measured GEM levels in the firn (see Materials and Methods). Atmospheric GEM concentrations in the boundary layer at Summit (a 1-σ envelope) during the last 66 years are shown in Fig. 2A. GEM concentrations start to clearly increase in the 1940s and reach a peak at ≈3 ng m−3 around 1970, subsequently decreasing and stabilizing around 1995 (Fig. 2A).
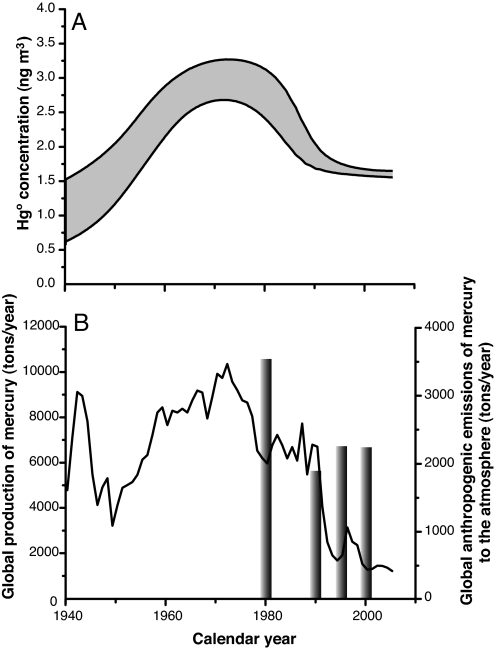
Fig. 2.
Impact of human emissions on the atmospheric mercury reservoir during recent decades. (A) Envelope of modeled concentrations for atmospheric GEM inferred from Summit firn air, central Greenland, during the last 66 years (gray area). (B) Global anthropogenic emissions of mercury to the atmosphere in 1980, 1990, 1995, and 2000 (ref. 3 and references therein) (gray bars) and worldwide production of mercury (black line) (49). Global anthropogenic emissions of mercury presented here are based on emission inventories and include industrial emissions as well as coal combustion. Worldwide production refers to the mercury extracted from geological reservoirs for industrial uses (including mining). Only a fraction of the mercury produced is transferred to the atmosphere by industrial processes.
Poorly characterized mechanisms driving both production and destruction of GEM in the interstitial air of snowpacks depend on environmental conditions (irradiation, temperature, and presence of water layer around snow grains) and the chemical composition of snow (e.g., presence of halogens or divalent mercury species as a consequence of surface deposition) (25). Greenland surface air temperature trends, including at the Summit site, have not shown persistent warming since 1930 in contrast to global average surface temperature (23). Liquid water in snowpacks could enhance GEM emission (e.g., during snowmelt) (26), but occurrences of surface snow melting at Summit are extremely improbable. Similarly, important changes in solar irradiation at Summit during the last century are unlikely. Direct radiative forcing induced by natural variability of solar activity has been extremely low during the last 200 years (27), and there has been no evidence for a large-scale destruction of stratospheric ozone in the Northern hemisphere (28). Arctic ozone levels exhibit high natural seasonal and interannual variability, however, driven primarily by atmospheric dynamics; and between 1979 and 2000, the trend in mean annual total column ozone over the Arctic was about −3% per decade (7% accumulated loss) (29). Photo-reduction of divalent mercury species in snow is enhanced by UV irradiation (30, 31). Although the amount of UV radiation reaching the snow surface at Summit is notably influenced by stratospheric ozone levels, the UV measurement time series available in the Arctic is not yet long enough to allow trends to be detected. A recent laboratory study showed that a 10% increase in UV radiation would have a negligible effect on GEM production in the interstitial air of alpine snow (31). How snow composition impacts mercury chemistry is still under challenge (32). Higher levels of sea salts favor reduction processes and thus GEM destruction in the snow interstitial air (30), but no long-term trends have been reported for sea salt impurities deposited on the Greenland ice sheet during the last century (33). Recent evolution of surface snow composition at Summit has been dominated by human-driven pollutants, but Hg2+ concentrations measured in a firn core dated from 1949 to 1989 exhibited low values and limited variation (range: 0.05–2.0 pg g−1; mean: 0.43 pg g−1) (13).
GEM does not adsorb on ice surfaces, as demonstrated by both field and laboratory measurements (34, 35), and we showed that present chemical processes in the shallow firn do not perturb the long-term record of past atmospheric GEM (21). We cannot eliminate the possibility that slow evolution of environmental conditions (e.g., surface temperature and irradiation) and snow composition (e.g., sea salts levels) induced slight evolution in chemical processes involving GEM in the shallow firn during recent decades. However, such processes are unlikely during winter and occur during a limited period of the year (typically from late spring to early fall, 21), and we argue that their past evolution will have a negligible impact on the deep firn atmospheric record. Thus, we conclude the firn air is a reliable archive to investigate historical changes of GEM atmospheric concentrations during the last ≈70 years.
Atmospheric trends inferred from polar archives inform about boundary layer levels, and the lack of information about free tropospheric background could be a concern for many gaseous species. Conversely, specific oxidation processes can lead to depletion of GEM in the upper troposphere and lower stratosphere (36), and GEM concentrations in the boundary layer could be relevant information when measured far from large pollution sources (e.g., at Summit). Active atmospheric chemistry has been reported for polar coastal areas: the so-called Atmospheric Mercury Depletion Events (AMDEs), occurring simultaneously with the postsolar sunrise destruction of ozone, can lead to complete depletions of atmospheric GEM during springtime (32). No atmospheric ozone depletion events were observed at Summit (37); and, consequently, AMDEs are unlikely to occur in central Greenland. No strong variations in atmospheric GEM concentrations are thus expected throughout the year at Summit. Furthermore, firn air in the lock-in zone has the useful property that it averages surface gas concentrations for a decade or so, due to diffusion in the firn.
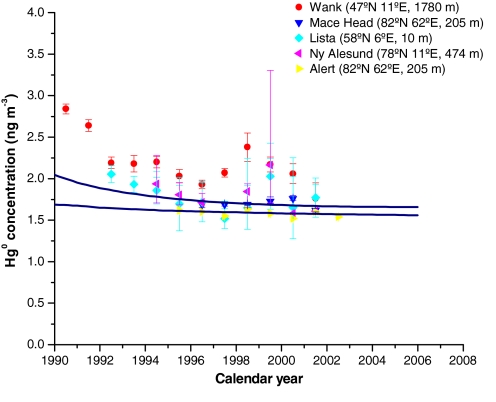
GEM atmospheric monitoring available from the Arctic at Alert (82°N, 62°E, 205 m, Canada) (38) and Ny-Ålesund (78°N, 11°E, 474 m, Spitzbergen) (8) as well as the GEM history reconstructed from the firn air at Summit exhibit stable GEM concentrations of about 1.7 ng m−3 during the last 10 years (Fig. 3). In Europe, atmospheric GEM monitoring started at the summit of the Wank mountain (47°N, 11°E, 1780 m, Germany), at Lista (58°N, 6°E, 10 m, Norway), and at Mace Head (53°N, 9°W, 10 m, Ireland) in 1990 (39), 1993 (40), and 1996 (41), respectively. The Wank station is located in a central and continental area and is more strongly influenced by regional sources than the Lista and Mace Head stations. From 1990 to 1996, a decrease in atmospheric GEM concentrations was observed at both the Wank and Lista stations, with higher levels at Wank (Fig. 3). This trend has been related to the 45% decrease in European anthropogenic emissions reported between 1990 and 1995 (42). Atmospheric GEM history reconstructed from the Summit record reproduces this decrease, but to a lesser extent. It is likely that the influence of European anthropogenic sources is diluted at northern latitudes, but the signal is still evident. Since 1996, Mace Head and Lista records exhibit stable GEM atmospheric levels around 1.7 ng m−3, which are well reconstructed using firn air from Summit.
Fig. 3.
Envelope of GEM atmospheric concentrations inferred from Summit firn air (blue curves), and atmospheric monitoring of GEM available for the Northern Hemisphere at different European and Arctic sites since 1990. Dots and errors bars represent the median and 95% confidence intervals. Data are reproduced from Slemr et al. (8) and Steffen et al. (38).
From 1980 to 1990, atmospheric GEM concentrations in the Northern Hemisphere were only investigated at one station, Rorvik, near Gothenburg (53°N, 11°E, 10 m, Sweden), which is likely to have been strongly affected by regional Hg sources (i.e., Eastern Europe before the breakup of the USSR). The Rorvik record exhibits maximum GEM concentrations in the late 1980s but is likely only representative of a local pattern as pointed out by a panel of international experts convened in Madison, Wisconsin as part of the 8th International Conference on Mercury as a Global Pollutant (August 2006) (1). Thus, the Rorvik record is not directly comparable to our Greenland firn air reconstruction. Before 1980, the only atmospheric GEM data were obtained during short ship cruises (i.e., a few weeks) across the Atlantic Ocean. These measurements exhibit strong latitudinal gradients with peak values around 40°N and mean values of ≈2 ng m−3 in the northern hemisphere (7, 43–45). While atmospheric GEM concentrations reconstructed from Summit firn air are higher than mean hemispheric values, they reproduce GEM levels observed shipboard between 40° and 50°N (see Fig. S1).
Reconstructions of the worldwide trend of GEM concentrations have been attempted using all direct measurements of atmospheric GEM available since 1977. Slemr et al. (8) suggested that GEM concentrations in the northern atmosphere had been increasing since 1977 to reach a maximum in the late 1980s. More recently, Lindberg et al. (1) concluded that a null hypothesis (i.e., little change in atmospheric GEM since 1977) could be as likely as the history proposed by Slemr et al. (8). Both reconstructions strongly relied on mean hemispheric GEM levels inferred from oceanographic expedition measurements. These ship-based records, however, are strongly influenced by short timescale variations of both natural and anthropogenic GEM sources and do not allow quantitative modeling of latitudinal distribution of GEM concentrations, even though they appear to exhibit a north-south structure.
Atmospheric GEM concentrations inferred from Greenland firn air reproduce the scarce GEM measurements reported from 1977 to 1980 across the Atlantic Ocean at mid-northern latitudes (7) (see Fig. S1 and SI Text for more details), confirming a spatially heterogeneous evolution of the Northern Hemisphere atmospheric mercury burden during recent decades. We conclude, therefore, that evolution of GEM concentrations reconstructed above Greenland cannot be generalized to the whole Northern Hemisphere, but is instead likely to be representative of middle and high northern latitudes. Most anthropogenic sources have been located at temperate northern latitudes, and global atmospheric circulation transfers to Greenland air masses from North America, Europe, and, to a lesser extent, Asia (46). Thus, firn air collected at Summit becomes a powerful archive to infer the impact of human emissions on the atmospheric reservoir during recent decades.
Globally, approximately half a million tons of metallic Hg have been produced from cinnabar and other ores for industrial applications (mainly nonferrous metal smelting, manufacturing, and chlor-alkali plants) since 1925 (47). Only a fraction of the mercury extracted from geological stocks has been emitted directly to the atmosphere by industrial processes [≈30% in recent decades (48)]. In both the United States and Europe, however, most sediment and peat record reconstructions show higher deposition of atmospheric divalent mercury during the 1970s (10, 11, 14), simultaneous with the peak in worldwide production of mercury of ≈107 kg year−1 (Fig. 2B) (49). Worldwide mercury production is, therefore, an indirect indicator of anthropogenic Hg emissions to the atmosphere. Such an indicator is extremely useful when direct estimates of anthropogenic mercury emissions by source inventories are not available (i.e., before 1980).
Atmospheric GEM concentrations, reconstructed from Greenland firn air and the worldwide production of mercury, peak at roughly the same time during the 1970s (Fig. 2). Later, from 1980 to 2000, the atmospheric trend of GEM concentrations and global estimates of anthropogenic emissions of mercury to the atmosphere (mainly emissions from coal combustion) exhibit a similar trend: a large decrease during the 1980s and then stabilization between 1990 and 2000 (3, 50, 51). Hence, atmospheric GEM concentrations inferred from Greenland firn air and global anthropogenic Hg emissions have exhibited consistently similar trends during the most recent decades (Fig. 2), suggesting that the atmospheric reservoir of mercury at mid- and high-northern latitudes has been driven mainly by anthropogenic emissions during the last decades. Consequently, rapid industrial development in the Northern Hemisphere may have caused the significant two-fold rise in boreal GEM atmospheric levels reported after World War II. The decline observed from the 1970s to the early 1990s is likely a response to emission controls implemented through the United States Clean Air Act of 1970 and the 1977 amendments. During the same period, air pollution regulations in Europe gradually eliminated uncontrolled Hg emission sources that were common in urban areas.
The atmospheric lifetime of mercury is long enough to allow transport of GEM from emission areas, mostly mid-northern latitudes, to remote locations. In the Arctic, the record of atmospheric deposition of Hg2+ species in lake sediments exhibits a similar global trend as atmospheric GEM concentrations inferred from Greenland firn: lake sediments show an increase in mercury deposition which parallels increasing industrialization (see ref. 15 for a critical review). However, deposition patterns differ among Arctic lakes, particularly within the last few decades where either increases or decreases in Hg accumulation rates have been reported in Alaska (52) as well as in Greenland (11). Cycling within individual lake and transport from surrounding catchment soils may amplify or diminish the atmospheric deposition signal. Discrepancy between sediment archives also could be related to recent climate warming that may have affected the limnology of High Arctic lakes (16). However, both sediment records of atmospheric deposition of Hg2+ species at high northern latitudes and atmospheric GEM concentrations inferred from Greenland firn air support the conclusion that transfer of anthropogenic inorganic mercury through the atmosphere to terrestrial and marine reservoirs occurs on a large scale. The connection between deposition of inorganic mercury, enhanced during the 1970s, and contamination of ecosystems, due to methylation processes is more complex. Mercury levels in lakes respond rapidly (within years) to changes in mercury deposition directly to their surfaces, but much more slowly (in decades) to changing inputs to their watersheds (6). Although decreases in fish mercury have been reported recently for lakes exposed to reduced mercury deposition (53), other aquatic ecosystems may continue to bio-accumulate Hg due to higher anthropogenic emissions in the 1970s.
Conclusion
Mercury emissions have decreased recently in Europe and North America, but these declines have been offset by increases in Asia, one of the fastest growing regions in the world (3). Asia has become the largest contributor of anthropogenic atmospheric Hg, responsible for more than half of global emissions, and a significant increase in emissions from this region is expected in the next few decades due to rapid economic and industrial development (54). Notably, anticipated growth in coal combustion, steel production, gold mining, and disposal of Hg-containing devices may considerably escalate Hg emissions in Asia. As was observed during the 1970s, we expect an increase in GEM concentrations at mid-northern latitudes as a result. This trend is likely to increase in the future, impacting high-northern latitude areas. Long-range transportation of mercury from Asia is already reported in the United States (55). Recently, more than 140 nations decided to begin negotiations for a mercury-limiting treaty in the framework of the United Nations Environment Program (UNEP). Implementation of such global regulations may be an effective strategy to avoid contamination of populated and remote areas up to the Arctic in the future.
Materials and Methods
Firn Air Sampling.
From May 25 to May 31, 2006, we investigated GEM in the firn air at a remote location 10 km away from Summit Station, central Greenland (72.6°N, 38.5°W, 3200 m elevation). We sampled firn air using established methods (18, 19, 56) from one borehole at depths of 15, 25, 30, 40, 50, 58, 63, 66, 70, 72, 74, 76, 78, and 79.5 m. A 4-m long bladder was lowered into the borehole after drilling to the sampling depth and pressurized with air from the bottom of the hole, effectively sealing the hole. Two Dekabon (polyethylene/aluminum composite) lines were used to pump firn air from a space left immediately below the bladder. These lines drew air from two separate openings above and below a horizontal stainless steel baffle that was nearly as wide as the borehole. Air was pumped from the upper opening at 20 L min−1 and directed to waste after measuring CO2 concentration (in situ measurements using an NDIR analyzer). When CO2 levels stabilized, indicating effective removal of contamination by younger and/or ambient air, sampling from the lower opening was initiated. Firn air was first collected in flasks for additional measurements (e.g., CH4 and CFCs) before GEM sampling actually started. Pumping from the upper opening continued during sampling (at 20 L min−1) to remove any air leaking from within or around the bladder. This technique also served to keep sampled air out of contact with the bladder itself.
GEM Analysis.
A gas phase mercury analyzer 2537A (Tekran Inc.) was used for determination of GEM concentrations in firn air. The prefiltered air stream (0.2-μm Teflon particle filter) was collected on two gold cartridges. GEM was thermally desorbed and detected by cold vapor atomic fluorescence spectrometry at 253.7 nm. Use of dual gold cartridges allowed alternate sampling and desorption, resulting in continuous measurement of GEM on a predefined time base. Set-up, accuracy, and precision of this instrument have been evaluated previously during field comparisons at an urban/industrial site (57) and a remote marine background location (58). The Tekran analyzer was operated with a five-minute sampling frequency, and the air was sampled at a flow rate of 1 L min−1. The analyzer was calibrated every 25 h with an internal automatic permeation source. The detection limit for GEM in this operation mode is about 0.15 ng m−3. Since the internal pump of the 2537A Tekran analyzer was not strong enough to pump firn air, particularly from deeper layers, a PTFE pump (MZ-2C; Vacuubrand, Inc.) was connected to the firn sampling line, upstream to the Tekran analyzer. This pump delivered firn air to the inlet of the 2537A at the desired flow rate of 1 L min−1. We measured blanks of the PTFE pump before and after sampling at all depths to quantify any contamination introduced by this additional pump.
At each depth, we sampled firn air for 40 min, resulting in approximately eight GEM measurements. We checked blanks of the Dekabon sampling line both at the beginning and end of the fieldwork; they were 0.08 ± 0.13 ng m−3 (n = 8, before sampling, on May 25, 2006) and 0.01 ± 0.06 ng m−3 (n = 18, after sampling, on June 1, 2006).
Diffusion Modeling.
We used a one-dimension gas diffusion model in Eulerian coordinates developed by Rommelaere et al. (24) to infer the atmospheric record of GEM from firn air concentrations (Fig. 1). Processes taken into account included (i) air mixing by pressure and temperature gradients down to a few meters below the surface (i.e., the so-called convection zone); (ii) molecular diffusion in the open pore space and gravitational fractionation (entrainment toward the deeper firn depends on concentration gradients, diffusivities, and molar mass); and (iii) a downward air flux in the open porosity zone due to bubble closure removing air from the open pores. Note that this removed air has to be replaced by air coming from the upper parts of the firn, thus creating a downward flux. We set temperature and accumulation rate to their present-day values [respectively, 241°K (20) and 224 kg m−2 year−1 (22)] and assumed these rates were constant throughout the model run. We used firn structure parameters (density and closed porosity) from the EUROCORE drilling (20) (see Fig. S2).
GEM diffusivity in firn air depends on both GEM diffusivity in air and firn tortuosity. Tortuosity of a porous medium represents the complexity of the pathway and is commonly calculated as the ratio of the mean path length to the minimum possible (straight line) path length. We used an inverse method (24, 59) to compute tortuosity at all depths in the firn from the CO2 atmospheric trend and from CO2 concentrations measured in the firn at Summit. In other words, we obtained a site specific, tortuosity-depth relationship by adjusting diffusivity until the model reproduced the observed CO2 firn-air profile when driven by the independently derived atmospheric CO2 history (see Fig. S3). We sampled air for CO2 analysis at the same depths as GEM.
We validated parameterization of the model diffusivity using CH4 and three halocarbon species (CFC11, CFC113, and CCl4) for which atmospheric histories have been estimated from emission scenarios and real-time measurements (60–62). Concentration-depth profiles were determined for these four species using the adjusted diffusivity-depth profile in the diffusion model, and good agreement with concentrations-depth profiles actually measured in the Summit firn was obtained (see Figs. S4 and S5).
Monte Carlo Modeling.
Little information is available about past atmospheric levels of GEM, but real-time measurements reported since 1977 (8) and industrial production figures (49) suggest that GEM concentrations could have peaked within the last 50 years. Thus, we chose a mathematical parameterization of atmospheric GEM history from 1940 to 2006 (firn air model input)—which allowed for the possibility of a constant level, monotonic increase or decrease, or peak concentration of GEM concentration during this timeframe, depending on the choice of parameter values. We tested a wide range of parameter values with the forward firn air model previously described (Monte Carlo approach), representing widely varying scenarios for atmospheric evolution of GEM concentrations. We then modeled a profile of GEM concentrations in the firn from each atmospheric history tested. Agreement between a modeled firn profile and the experimental firn profile (Fig. 1) was estimated using the χ2 parameter (see SI Text). According to Tarantola theory (63), we were able to associate a probability density with each atmospheric scenario tested. We finally assessed probability distributions for GEM concentrations with a 1-year time resolution, and we calculated means and standard deviations from all distributions (i.e., every year from 1940 to 2006) (see SI Text). The envelope of atmospheric GEM concentrations presented in this study corresponds to the mean concentrations plus or minus one standard deviation.
Supplementary Material
Acknowledgments.
We thank our Summit collaborators for their assistance during the field campaigns; the summer Summit crew, VECO Polar Resources, and the Air National Guard for providing logistical support during the field experiments; the Danish Polar Board and Greenlandic Home Rule Government for permission to work in Greenland, and the technical staff of Laboratoire de Glaciologie et Géophysique de l'Environnement for help in preparing our field campaigns. We are grateful to P. Martinerie, J. McConnell, F. Parennin, and three anonymous reviewers for their helpful comments; T. Berg, F. Slemr, and A. Steffen for providing data; and R. Kreidberg for providing help in editing the manuscript. This research was funded by the U.S. National Science Foundation Office of Polar Programs project NSF-OPP 0520445, the French Atmospheric Chemistry Program “Exchanges Neige Polaire,” the French Ministry of Research (Action Concertée Incitative Jeunes Chercheurs 3012), the Laboratoire de Glaciologie et Géophysique de l'Environnement, and the Institut Universitaire de France (to C.F. and C.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905117106/DCSupplemental.
References
- 1.Lindberg S, et al. A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. Ambio. 2007;36:19–32. doi: 10.1579/0044-7447(2007)36[19:asopau]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Mason RP, Sheu G-R. Role of the ocean in the global mercury cycle. Global Biogeochem Cycles. 2002;16:1093. [Google Scholar]
- 3.Pacyna EG, Pacyna JM, Steenhuisen F, Wilson S. Global anthropogenic mercury emission inventory for 2000. Atmos Environ. 2006;40:4048–4063. [Google Scholar]
- 4.Schroeder WH, Munthe J. Atmospheric mercury - An overview. Atmos Environ. 1998;32:809–822. [Google Scholar]
- 5.Munthe J, et al. Recovery of mercury-contaminated fisheries. Ambio. 2007;36:33–44. doi: 10.1579/0044-7447(2007)36[33:romf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Harris RC, et al. Whole-ecosystem study shows rapid fish-mercury response to changes in mercury deposition. Proc Natl Acad Sci USA. 2007;104:16586–16591. doi: 10.1073/pnas.0704186104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slemr F, Schuster G, Seiler W. Distribution, speciation, and budget of atmospheric mercury. J Atmos Chem. 1985;3:407–434. [Google Scholar]
- 8.Slemr F, et al. Worldwide trend of atmospheric mercury since 1977. Geophys Res Lett. 2003;30:1516. [Google Scholar]
- 9.Engstrom DR, Swain EB. Recent declines in atmospheric mercury deposition in the upper midwest. Environ Sci Technol. 1997;31:960–967. [Google Scholar]
- 10.Pirrone N, et al. Historical atmospheric mercury emissions and depositions in North America compared to mercury accumulations in sedimentary records. Atmos Environ. 1998;32:929–940. [Google Scholar]
- 11.Bindler R. Estimating the natural background atmospheric deposition rate of mercury utilizing ombrotrophic bogs in southern Sweden. Environ Sci Technol. 2003;37:40–46. doi: 10.1021/es020065x. [DOI] [PubMed] [Google Scholar]
- 12.Roos-Barraclough F, Shotyk W. Millennial-scale records of atmospheric mercury deposition obtained from ombrotrophic and minerotrophic peatlands in the swiss Jura mountains. Environ Sci Technol. 2003;37:235–244. doi: 10.1021/es0201496. [DOI] [PubMed] [Google Scholar]
- 13.Boutron CF, Vandal GM, Fitzgerald WF, Ferrari CP. A forty-year record of mercury in central Greenland snow. Geophys Res Lett. 1998;25:3315–3318. [Google Scholar]
- 14.Schuster PF, et al. Atmospheric mercury deposition during the last 270 years: A glacial ice core record of natural and anthropogenic sources. Environ Sci Technol. 2002;36:2303–2310. doi: 10.1021/es0157503. [DOI] [PubMed] [Google Scholar]
- 15.Biester H, Bindler R, Martinez-Cortizas A, Engstrom DR. Modeling the past atmospheric deposition of mercury using natural archives. Environ Sci Technol. 2007;41:4851–4860. doi: 10.1021/es0704232. [DOI] [PubMed] [Google Scholar]
- 16.Outridge PM, Sanei H, Stern GA, Hamilton PB, Goodarzi F. Evidence for control of mercury accumulation rates in Canadian High Arctic lake sediments by variations of aquatic primary productivity. Environ Sci Technol. 2007;41:5259–5265. doi: 10.1021/es070408x. [DOI] [PubMed] [Google Scholar]
- 17.Siegenthaler U, et al. Stable carbon cycle–climate relationship during the late pleistocene. Science. 2005;310:1313–1317. doi: 10.1126/science.1120130. [DOI] [PubMed] [Google Scholar]
- 18.Battle M, et al. Atmospheric gas concentrations over the past century measured in air from firn at the South Pole. Nature. 1996;383:231–235. [Google Scholar]
- 19.Schwander J, Stauffer B, Sigg A. Air mixing in firn and the age of the air at pore close-off. Ann Glaciol. 1988;10:141–145. [Google Scholar]
- 20.Schwander J, et al. The age of the air in the firn and the ice at Summit, Greenland. J Geophys Res. 1993;98:2831–2838. [Google Scholar]
- 21.Faïn X, et al. Mercury chemistry in the snowpack at Summit Station, Central Greenland, and implications for the study of the transfer function. Atmos Chem Phys. 2008;8:3441–3457. [Google Scholar]
- 22.Banta JR, McConnell JC. Annual accumulation over recent centuries at four sites in central Greenland. J Geophys Res. 2007;112:D10114. [Google Scholar]
- 23.Chylek P, Box JE, Lesins G. Global warming and the Greenland ice sheet. Climatic Change. 2004;63:201–221. [Google Scholar]
- 24.Rommelaere V, Arnaud L, Barnola J-M. Reconstructing recent atmospheric trace gas concentrations from polar firn and bubbly ice data by inverse methods. J Geophys Res. 1997;102 30 069–030 083. [Google Scholar]
- 25.Lalonde JD, Poulain AJ, Amyot M. The role of mercury redox reactions in snow on snow-to-air mercury transfer. Environ Sci Technol. 2002;36:174–178. doi: 10.1021/es010786g. [DOI] [PubMed] [Google Scholar]
- 26.Dommergue A, et al. The fate of mercury species in a sub-arctic snowpack during the snowmelt. Geophys Res Lett. 2003;30:1621. [Google Scholar]
- 27.IPCC. Climate change 2007 synthesis report. Fourth Assessment Report of the Intergovernmental Panel on Climate Change. 2007 [Google Scholar]
- 28.Solomon S. Stratospheric ozone depletion: A review of concepts and history. Rev Geophys. 1999;37:275–316. [Google Scholar]
- 29.Weatherhead EC, Andersen SB. The search for signs of recovery of the ozone layer. Nature. 2006;441:39–45. doi: 10.1038/nature04746. [DOI] [PubMed] [Google Scholar]
- 30.Lalonde JD, Amyot M, Doyon M-R, Auclair J-C. Photo-induced Hg(II) reduction in snow from the remote and temperate Experimental Lake Area (Ontario, Canada) J Geophys Res. 2003;108:4200. [Google Scholar]
- 31.Faïn X, et al. Diurnal production of gaseous mercury in the alpine snowpack before snowmelt. J Geophys Res. 2007;112:D21311. [Google Scholar]
- 32.Steffen A, et al. A synthesis of atmospheric mercury depletion event chemistry in the atmosphere and snow. Atmos Chem Phys. 2008;8:1445–1482. [Google Scholar]
- 33.Banta JR, McConnell JR, Edwards R, Engelbrecht JP. Delineation of carbonate dust, aluminous dust, and sea salt deposition in a Greenland glaciochemical array using positive matrix factorization. Geochem Geophys Geosyst. 2008 Jul 17; doi: 10.1029/2007GC001908. [DOI] [Google Scholar]
- 34.Bartels-Rausch T, Huthwelker T, Jori M, Gaggler HW, Ammann M. Interaction of gaseous elemental mercury with snow surfaces: Laboratory investigation. Environ Res Lett. 2008;3 045009. [Google Scholar]
- 35.Ferrari CP, Dommergue A, Boutron CF. Profiles of mercury in the snow pack at Station Nord, Greenland shortly after polar sunrise. Geophys Res Lett. 2004;31:L03401. [Google Scholar]
- 36.Talbot R, Mao H, Scheuer E, Dibb J, Avery M. Total depletion of Hg° in the upper troposphere-lower stratosphere. Geophys Res Lett. 2007:34. [Google Scholar]
- 37.Helmig D, et al. A review of surface ozone in the polar regions. Atmos Environ. 2007;41:5138–5161. [Google Scholar]
- 38.Steffen A, Schroeder B, MacDonald RW, Poissant L, Konoplav A. Mercury in the Arctic atmosphere: An analysis of eight years of measurements of GEM at Alert (Canada) and a comparison with observations at Amderma (Russia) and Kuujjuarapik (Canada) Sci Total Environ. 2005;342:185–198. doi: 10.1016/j.scitotenv.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 39.Slemr F, Scheel HE. Trends in atmospheric mercury concentrations at the summit of the Wank mountain, southern Germany. Atmos Environ. 1998;32:845–853. [Google Scholar]
- 40.Foltescu VL, et al. Airborne concentrations and deposition fluxes of major and trace species at marine stations in southern Scandinavia. Atmos Environ. 1996;30:3857–3872. [Google Scholar]
- 41.Ebinghaus R, et al. Long-term measurements of atmospheric mercury at Mace Head, Irish west coast, between 1995 and 2001. Atmos Environ. 2002;36:5267–5276. [Google Scholar]
- 42.Pacyna EG, Pacyna JM, Pirrone N. European emissions of atmospheric mercury from anthropogenic sources in 1995. Atmos Environ. 2001;35:2987–2996. [Google Scholar]
- 43.Slemr F, Junkermann W, Schmidt RWH, Sladovic R. Indication of change in global and regional trends of atmospheric mercury concentrations. Geophys Res Lett. 1995;22:2143–2146. [Google Scholar]
- 44.Slemr F, Langer E. Increase in global atmospheric concentrations of mercury inferred from measurements over the Atlantic Ocean. Nature. 1992;355:434–437. [Google Scholar]
- 45.Temme C, Slemr F, Ebinghaus R, Einax JW. Distribution of mercury over the Atlantic Ocean in 1996 and 1999–2001. Atmos Environ. 2003;37:1889–1897. [Google Scholar]
- 46.Khal JD, et al. Air mass trajectories to Summit, Greenland: A 44-year climatology and some episodic events. J Geophys Res. 1997;102:26861–26875. [Google Scholar]
- 47.Hylander L, Meili M. 500 years of mercury production: Global annual inventory by region until 2000 and associated emissions. Sci Total Environ. 2003;304:13–27. doi: 10.1016/S0048-9697(02)00553-3. [DOI] [PubMed] [Google Scholar]
- 48.Swain EB, et al. Socioeconomic consequences of mercury use and pollution. Ambio. 2007;36:45–61. doi: 10.1579/0044-7447(2007)36[45:scomua]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.USGS. US Geological Survey, Mercury Statistics. 2006. http://minerals.usgs.gov/ds/2005/2140/mercury.pdf.
- 50.Pacyna EG, Pacyna JM. Global emission of mercury from anthropogenic sources in 1995. Water Air Soil Poll. 2002;137:149–165. [Google Scholar]
- 51.Pacyna J, Pacyna EG. A report for the Arctic Monitoring and Assessment Programme (AMAP) 1996. Global emissions of mercury to the atmosphere and emissions from anthropogenic sources. [Google Scholar]
- 52.Fitzgerald WF, et al. Modern and historic atmospheric mercury fluxes in northern Alaska: Global sources and arctic depletion. Environ Sci Technol. 2005;39:557–568. doi: 10.1021/es049128x. [DOI] [PubMed] [Google Scholar]
- 53.Hrabik TR, Watras CJ. Recent declines in mercury concentration in a freshwater fishery: isolating the effects of de-acidification and decreased atmospheric mercury deposition in Little Rock Lake. Sci Total Environ. 2002;297:229–237. doi: 10.1016/s0048-9697(02)00138-9. [DOI] [PubMed] [Google Scholar]
- 54.Streets DG, Zhang Q, Wu Y. Projections of global mercury emissions in 2050. Environ Sci Technol. 2009;43:2983–2988. doi: 10.1021/es802474j. [DOI] [PubMed] [Google Scholar]
- 55.Jaffe D, et al. Export of atmospheric mercury from Asia. Atmos Environ. 2005;39:3029–3038. [Google Scholar]
- 56.Butler JH, et al. A record of atmospheric halocarbons during the twentieth century from polar firn air. Nature. 1999;399:749–755. [Google Scholar]
- 57.Schroeder WH, et al. International field intercomparison of atmospheric mercury measurement methods. Water Air Soil Poll. 1995;80:611–620. [Google Scholar]
- 58.Ebinghaus R, et al. International field intercomparison measurements of atmospheric mercury species at Mace Head, Ireland. Atmos Environ. 1999;33:3063–3073. [Google Scholar]
- 59.Fabre A, Barnola J-M, Arnaud L, Chappellaz J. Determination of gas diffusivity in polar firn: Comparison between experimental measurements and inverse modeling. Geophys Res Lett. 2000;27:557–560. [Google Scholar]
- 60.Etheridge DM, Steele LP, Francey RJ, Langenfelds RL. Atmospheric methane between 1000 AD and present: Evidence of anthropogenic emissions and climatic variability. J Geophys Res. 1998;103:15979–15993. [Google Scholar]
- 61.WDCGG. 2006. http://gaw.kishou.go.jp/wdcgg/
- 62.Martinerie P, et al. Long-lived halocarbon trends and budgets from atmospheric chemistry modelling constrained with measurements in polar firn. Atmos Chem Phys. 2009;9:3911–3934. [Google Scholar]
- 63.Tarantola A. Inverse Problem Theory: Method for Data Fitting and Model Parameter Estimation. New York: Elsevier; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.