Abstract
2-Chlorohexadecanal (2-ClHDA), a 16-carbon chain chlorinated fatty aldehyde that is produced by reactive chlorinating species attack of plasmalogens, is elevated in atherosclerotic plaques, infarcted myocardium, and activated leukocytes. We tested the hypothesis that 2-ClHDA and its metabolites, 2-chlorohexadecanoic acid (2-ClHA) and 2-chlorohexadecanol (2-ClHOH), induce COX-2 expression in human coronary artery endothelial cells (HCAEC). COX-2 protein expression increased in response to 2-ClHDA treatments at 8 and 20 h. 2-ClHA also increased COX-2 expression following an 8 h treatment. Quantitative PCR showed that 2-ClHDA treatment increased COX-2 mRNA over 8 h, while 2-ClHA treatment led to a modest increase by 1 h and those levels remained constant over 8 h. 2-ClHDA led to a significant increase in 6-keto-PGF1α release (a measure of PGI2 release) by HCAEC. These data suggest that 2-ClHDA and its metabolite 2-ClHA, which are produced during leukocyte activation, may alter vascular endothelial cell function by upregulation of COX-2 expression.
Keywords: Myeloperoxidase, reactive chlorinating species (RCS), fatty acid, fatty alcohol, plasmalogen, prostacyclin
Introduction
The inducible form of cyclooxygenase (COX-2) is highly expressed in atherosclerotic lesions and nearly absent in normal arterial tissue [1]. Both COX-1, which is constitutively active, and COX-2 catalyze the production of prostaglandin H2 (PGH2) from phospholipid-derived arachidonic acid [2,3]. PGH2 is subsequently converted by cell-specific isomerases into several structurally different eicosanoids. Activation of the coronary endothelium, leads to the production and release of PGI2 [4,5], which has vasodilatory and anti-atherogenic properties [6–8] that is partially mediated by upregulation of COX-2 [9].
Myeloperoxidase (MPO), highly expressed in atherosclerotic lesions [10], catalyzes the production of potent oxidants collectively termed reactive chlorinating species (RCS) [11]. The collateral damage caused by MPO-derived RCS to host tissue includes the formation of chlorinated tyrosine [12], cholesterol [12], and lipids [13–15]. Plasmalogens, a subclass of choline glycerophospholipids that contains a vinyl ether linkage between the sn-1 aliphatic chain and the glycerol backbone, are particularly abundant in the heart vasculature [16–18]. We have shown that RCS attack the vinyl ether bond of plasmenylcholine, resulting in the release of 2-ClHDA (2-chlorohexadecanal) [19], which accumulates in activated neutrophils [14], monocytes [20,21], ischemic/reperfused myocardium [22] and atherosclerotic aorta [21]. Thus far, 2-ClHDA has been shown to reduce eNOS (endothelial nitric oxide synthase) expression in endothelial cells [23] and elicit neutrophil chemoattraction [14].
2-ClHDA is oxidized and reduced to 2-chlorohexadecanoic acid (2-ClHA) and 2-chlorohexadecanol (2-ClHOH), respectively [24]. Little is known about the signaling properties of this unique class of chlorinated lipids resulting from MPO-mediated RCS attack of plasmalogens. In this study, we examined the effect of 2-ClHDA, 2-ClHA, and 2-ClHOH to induce COX-2 expression in HCAEC. The results suggest that 2-ClHDA and 2-ClHA, but not 2-ClHOH, induce COX-2 protein expression and PGI2 release by increasing COX-2 gene expression.
Experimental Procedures
Lipid Preparation
2-ClHDA was prepared by treating 1-O-hexadec-1’-enyl-glycero-3-phosphocholine (100 mg) with freshly prepared hypochlorous acid (final concentration 1.5 mM) in phosphate buffer (pH 4) for 5 min at 37 °C [24]. Reactions were terminated by lipid extraction and the chloroform layer was collected [25]. 2-ClHDA was purified by HPLC utilizing a Dynamax Si column (21.4 mm × 250 mm; 8 µm), and gradient elution over 3 h with hexane as the initial mobile phase and chloroform as the final mobile phase at a flow rate of 8 ml/min. Purity of synthetic 2-ClHDA was confirmed by GC-MS, and quantified by acid methanolysis and GC-FID (HP 5890A) using arachidic acid as an internal standard. 2-ClHA was synthesized and purified as previously described [24]. To synthesize 2-ClHOH, 2-ClHDA was resuspended in 2 ml radical free ethyl ether and 0.5 ml benzene and treated with Red Al™ reagent for 30 min at 37 °C [24]. 2-ClHOH was purified by TLC (petroleum ether/ethyl ether/acetic acid (70/30/1, v/v/v) (RF = 0.41).
Cell Culture
HCAEC (Cell Applications, San Diego, CA) were seeded onto 60-mm2 or 35-mm2 6-well polystyrene dishes and cultured in EGM-2-MV growth medium (Lonza, Walkersville, MD) at 37 °C in a 5 % CO2 and 95 % air atmosphere. Experiments were performed on confluent monolayers (~1×106 cells/60-mm2 dish) between passages 5 and 9. HCAEC growth medium was replaced with growth medium containing 2 % FBS for 4–6 h prior to experiments. Lipids were dried under a stream of nitrogen and reconstituted in ethanol to inject into culture medium at a final ethanol concentration of 0.1 %. Lipids were mixed with basal medium and added to each dish by pipette for a final concentration of 50 µM. TNF-α was mixed with basal medium containing 0.1% ethanol and added to dishes for a final concentration of 50 ng/mL. Control conditions included incubations with basal medium containing a final ethanol concentration of 0.1 %.
Western Blotting
Following each experimental condition, the medium was aspirated from the dishes and HCAEC monolayers were rinsed with PBS. Cells were scraped in SDS sample buffer, boiled, and protein was subjected to SDS-PAGE utilizing 10–12.5% polyacrylamide gels followed by transfer of proteins to polyvinylidene difluoride (PVDF)-plus membranes (GE Osmonics Inc., Trevose, PA) for Western blot analysis. Blots were probed with a monoclonal anti-COX-2 (1:1000) (Zymed, San Francisco, CA) and an HRP-conjugated secondary antibody (1:7000) (BioRad, Hercules, CA). Blots were stripped using 0.1 M glycine (pH 2.5) and, reprobed with a monoclonal anti-IκB (1:2500) (BD Transduction Laboratories, San Jose, CA). Blots were stripped again and probed with a polyclonal β-Actin (1:5000) (Abcam, Cambridge, MA) and probed with an HRP-conjugated goat-anti-rabbit (1:7000) (Sigma, St. Louis, MO). Immunoreactive bands were visualized by exposure to autoradiographic film following incubation with enhanced chemiluminescence reagent (GE Healthcare, Piscataway, NJ). Densitometric analyses of immunoreactive bands were performed using ImageQuant software.
RNA Extraction, Reverse Transcription, and Quantitative PCR
RNA was extracted and purified using an RNeasy Mini Kit (Qiagen, Valencia, CA) by following the manufacturer’s instructions. Less than half of the mRNA elute was incubated with 0.5 µg Oligo (dT)12–18 primer (Invitrogen, Carlsbad, CA) at 70 °C for 10 min to yield cDNA. RNA and reaction mixture (200 units M-MLV H- reverse transcriptase (Promega, Madison, WI), buffer, dNTPs (Invitrogen, Carlsbad, CA), and 0.1 M DTT) were incubated at 42 °C for 50 min and terminated at 70 °C for 15 min. Reaction mix was incubated with 1U RNase H (Invitrogen, Carlsbad, CA) at 37 °C for 20 min. Real-time PCR of the cDNA library was performed by mixing 1 µl cDNA, primers for COX-2 or GAPDH-1, dNTPs, TAQ polymerase, buffer, and SYBR Green fluorescence probe followed by quantitative RT-PCR (BioRad OpticonII) using the following parameters: 40 cycles of 95 °C for 1 min, 56 °C for 0.5 min, 72 °C for 0.5 min, 87 °C for 3 sec. A melt curve from 80–96 °C was performed. The COX-2 primer pairs (SuperArray, Frederick, MD) and the primer sequences (5’ to 3’) for GAPDH-1 are GCATCTTC-TTTTGCGTCGCC (forward), and GTCATTGATGGCAACAATATCC (reverse) were used. Data were analyzed using BioRad Opticon II software and COX-2 levels were normalized to GAPDH-1 levels for each sample. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of products on a 2 % agarose gel.
6-keto-PGF1α Quantification
Following experimental conditions, the medium from each 60-mm2 HCAEC monolayer dish was removed and centrifuged. The internal standard d4-6-keto-PGF1α at 10 pg was added to each sample followed by acidification with HCl to 2.5 pH for 30 min. C-18 SPE columns (Supelco, Bellefonte, PA) were washed with acetonitrile and equilibrated with water prior to addition of samples. Following sample loading, columns were sequentially washed with acidic water (2.5 pH) and water/ethanol (85:15 v/v) prior to elution of prostaglandins with ethyl acetate. Samples were dried under a stream of nitrogen and derivatized by treatment with O-methoxamine and PFB-Br followed by BSTFA as described previously [26]. Samples were reconstituted in ethyl acetate for GC-MS analysis. PGI2 was measured as its stable hydrolytic product 6-keto-PGF1α. Samples were analyzed by selected ion monitoring on a Hewlett Packard 6890 GC coupled to a Hewlett Packard 5973 MS as described previously [27].
Cell Death
Following experimental conditions, cell medium was removed from 60-mm2 dishes, centrifuged, and assayed for general cell death using a lactate dehydrogenase release assay (Sigma, St. Louis, MO) following the manufacturer’s instructions.
Data Analysis
Statistical analyses of data were performed by one-way ANOVA followed by a Dunnett post hoc test, as appropriate. P ≤ 0.05 was considered statistically significant.
Results
Effects of TNF-α, 2-ClHDA and 2-ClHA on COX-2 protein expression
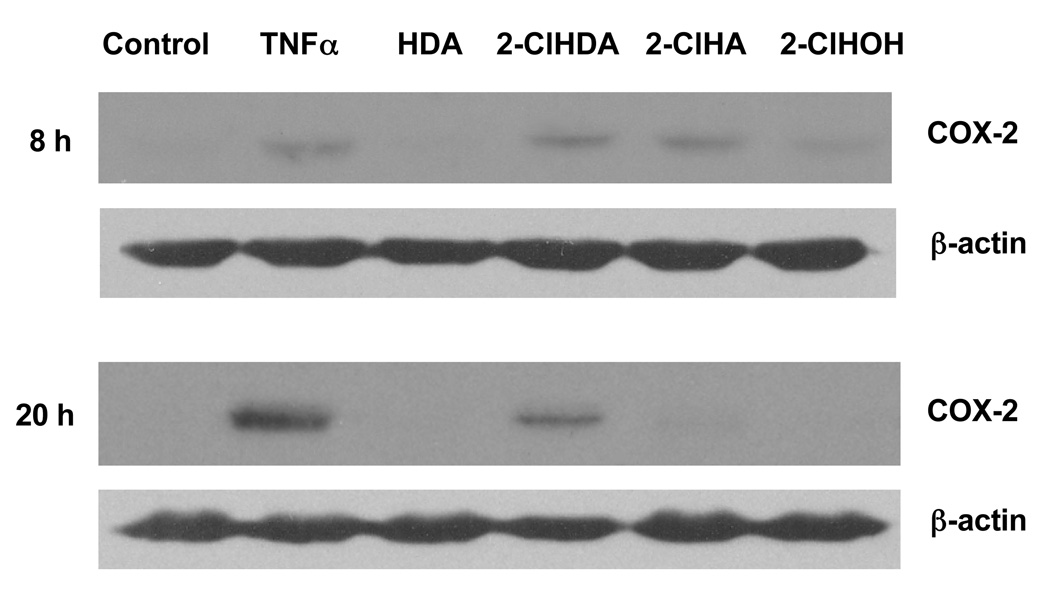
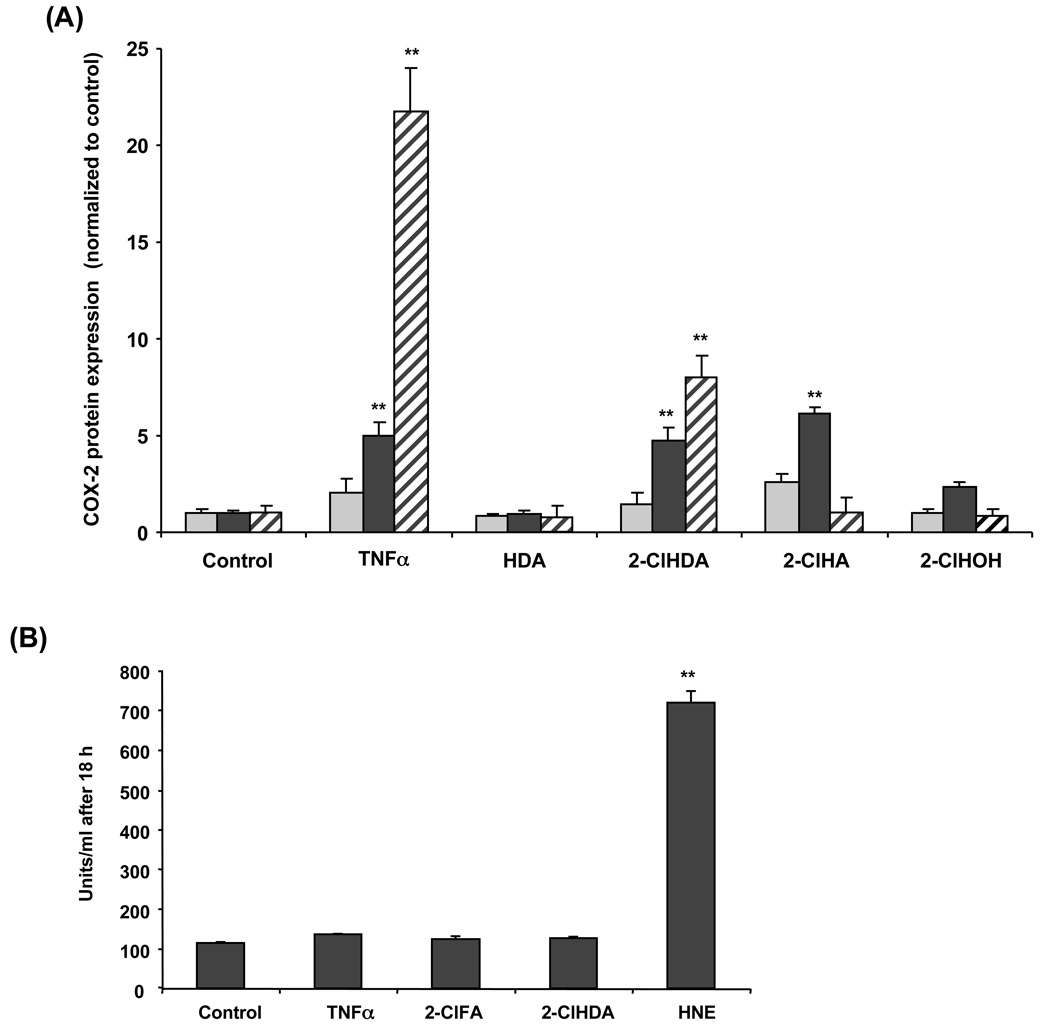
COX-2 expression in vascular endothelial cells is induced in response to several cytokines such as TNF-α and IL-1β [9,28,29] suggesting that COX-2 may be involved in the pathophysiology of inflammation [30]. Induction of COX-2 in the endothelium leads to the production and release of PGI2 [31], which has vasodilatory and anti-atherogenic properties. A time course analysis of TNF-α-treated HCAEC monolayers shows an increase in COX-2 protein expression by 8 h, reaching maximal expression at 20 h (Fig 1 and Fig 2A). In comparison to TNF-α at 20 h, 50 µM 2-ClHDA induced COX-2 expression to a lesser degree (Fig 1 and Fig 2A). 2-ClHA increased COX-2 expression within 8 h, and returned to baseline by 20 h. 2-ClHDA, however, induced COX-2 expression above control at both 8 and 20 h (Fig 1 and Fig 2A). Neither 2-ClHOH nor hexadecanal (HDA), the non-chlorinated aldehyde, induced COX-2 protein expression. Parallel experiments were performed showing that these chlorinated lipids did not cause significant cell death as determined by a lack of LDH release (Fig 2B). Normal LDH concentrations fall between 100–350 U/mL, while elevated LDH concentrations occur above 500 U/mL. Treatment of 100 µM 4-hydroxy-2-nonenal (HNE), known to induce cell death, was used as a positive control.
Figure 1. 2-chlorohexadecanal and 2-chlorohexadecanoic acid induce COX-2 protein expression in HCAEC.
Cell lysates were prepared from HCAEC treated with ethanol vehicle, 50 ng/mL TNF-α, or 50µM of hexadecanal (HDA), 2-chlorohexadecanal (2-ClHDA), 2-chlorohexadecanoic acid (2-ClHA), or 2-chlorohexadecanol (2-ClHOH) for 8 or 20 h as indicated. Samples were subjected to SDS-PAGE and western blotting for COX-2 expression.
Figure 2. Time course of 2-chlorohexadecanal and 2-chlorohexadecanoic acid elicited COX-2 protein expression in HCAEC.
Cell lysates were prepared from HCAEC treated with ethanol vehicle, 50 ng/mL TNF-α, or 50µM of hexadecanal (HDA), 2-chlorohexadecanal (2-ClHDA), 2-chlorohexadecanoic acid (2-ClHA), or 2-chlorohexadecanol (2-ClHOH) for 2 h (light grey bars,  ), 8 h (black bars,
), 8 h (black bars,  ), and 20 h (striped bars,
), and 20 h (striped bars,  ). Samples were subjected to SDS-PAGE and western blotting for COX-2 expression and analyzed by densitometry of immunoreactive bands (A). Following COX-2 analysis, blots were subsequently stripped and then subjected to western blotting for β-actin, which was used to normalize each experimental analysis of COX-2 expression. Cell medium was assayed for general cell death using a lactate dehydrogenase release assay (B). Values (mean ± SEM) are expressed as fold increase over control-treated cells for three independent determinations, (**) represents p<0.01.
). Samples were subjected to SDS-PAGE and western blotting for COX-2 expression and analyzed by densitometry of immunoreactive bands (A). Following COX-2 analysis, blots were subsequently stripped and then subjected to western blotting for β-actin, which was used to normalize each experimental analysis of COX-2 expression. Cell medium was assayed for general cell death using a lactate dehydrogenase release assay (B). Values (mean ± SEM) are expressed as fold increase over control-treated cells for three independent determinations, (**) represents p<0.01.
Effects of TNF-α, 2-ClHDA and 2-ClHA on COX-2 gene expression
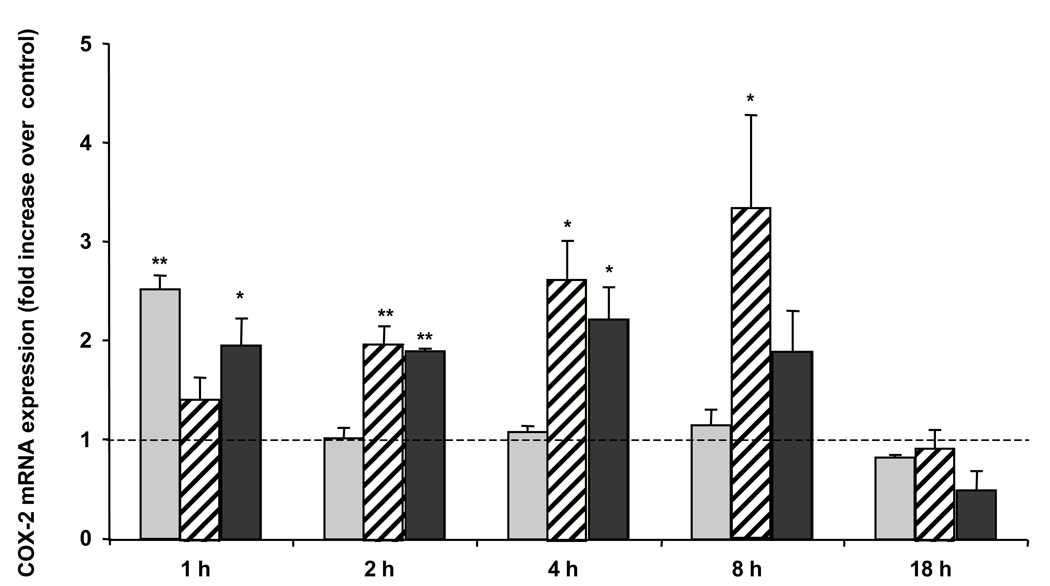
Quantitative PCR was performed to explore the effects of HCAEC monolayers treated with TNF-α, 2-ClHDA, 2-ClHA, or vehicle on COX-2 gene expression. Treatment with TNF-α led to a rapid 2.5-fold increase in mRNA expression by 1 h and levels returned to control levels by 2 h (Fig 3). COX-2 mRNA expression following 2-ClHDA treatment increased by ~ 3 fold by 8 h. HCAEC treatment with 2-ClHA induced a 2-fold increase in COX-2 mRNA by 2 h and remained elevated by 8 h. In all treatment conditions, COX-2 mRNA levels returned to near control levels by 18 h (Fig 3).
Figure 3. 2-Chlorohexadecanal and 2-chlorohexadecanoic acid induce COX-2 mRNA expression.
HCAEC were treated with either ethanol vehicle, 50 ng/mL TNF-α (light gray bars,  ), or 50 µM 2-chlorohexadecanal (2-ClHDA) (striped bars,
), or 50 µM 2-chlorohexadecanal (2-ClHDA) (striped bars,  ) or 50 µM 2-chlorohexadecanoic acid (2-ClHA) (black bars,
) or 50 µM 2-chlorohexadecanoic acid (2-ClHA) (black bars,  ) for the times indicated. Cells were scraped in lysis buffer, cDNA was prepared from purified mRNA, and samples were analyzed by quantitative PCR using primers for COX-2. Values (mean ± SEM) were normalized to GAPDH and expressed as fold increase over control-treated cells at each time point for three independent determinations. SEM of control values was less than 5% of the mean at all time points and there was no significant increase in control values over time, (* and **) represent p< 0.05 and p<0.01, respectively
) for the times indicated. Cells were scraped in lysis buffer, cDNA was prepared from purified mRNA, and samples were analyzed by quantitative PCR using primers for COX-2. Values (mean ± SEM) were normalized to GAPDH and expressed as fold increase over control-treated cells at each time point for three independent determinations. SEM of control values was less than 5% of the mean at all time points and there was no significant increase in control values over time, (* and **) represent p< 0.05 and p<0.01, respectively
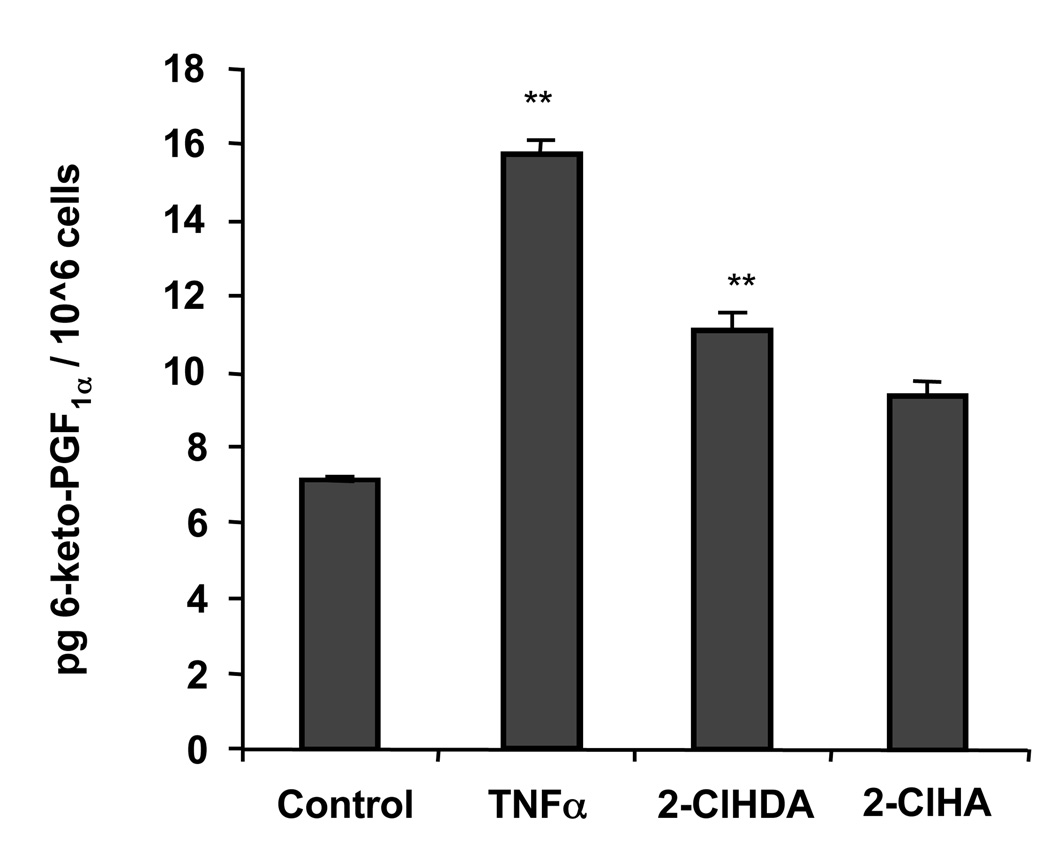
2-ClHDA and 2-ClHA elicit PGI2 release
The predominant COX-derived arachidonic acid product in coronary endothelial cells is PGI2 [4,5,7]. 6-keto-PGF1α, the stable hydrolytic product of PGI2, was measured in cultured medium following solid phase extraction, derivatization, and GC-MS analysis [26,27]. TNF-α treatment led to nearly a 2-fold increase in 6-keto-PGF1α, while 2-ClHDA, but not 2-ClHA at 20 h, led to lower but significant increases in 6-keto-PGF1α, (Fig 4), analogous to the pattern seen in protein expression (Fig 2A).
Figure 4. 2-Chlorohexadecanal and 2-chlorohexadecanoic acid lead to PGI2 release at 20 h.
HCAEC were treated with ethanol vehicle, 50 ng/mL TNFα, 50 µM 2-chlorohexadecanal (2-ClHDA), or 50 µM 2-chlorohexadecanoic acid (2-ClHA) for 20 h (n = 4 for each condition). Culture medium was removed and eicosanoids were purified by reverse SPE columns, converted to PFB esters, silylated using BSTFA, and analyzed by GC-MS, (**) represents p<0.01.
NF-κB signaling pathways by TNF-α, 2-ClHDA
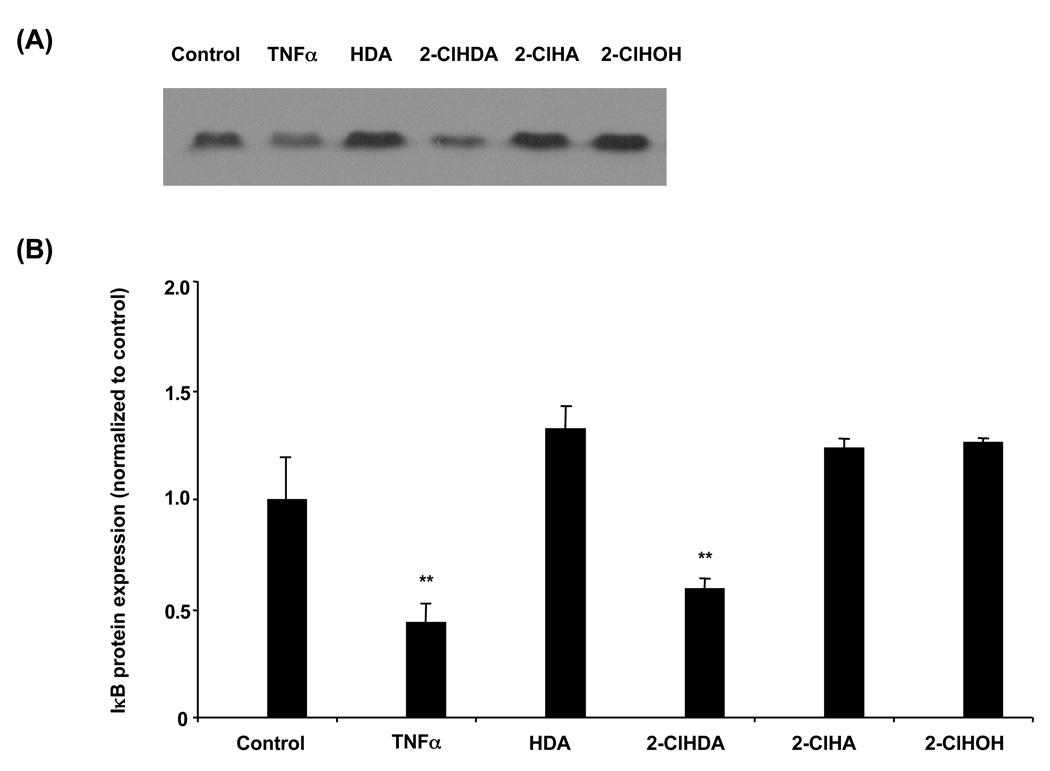
NF-κB is normally sequestered in the cytosol by IκB under non-inflammatory conditions. Upon stimulation of the NF-κB pathway, IκB is targeted for degradation and NF-κB is free to translocate to the nucleus where it binds to NF-κB DNA sites in the promoter of various genes. It has been shown in several cell types that treatment with TNF-α leads to NF-κB binding to the COX-2 promoter and reporter gene expression [31–33]. In HCAEC, TNF-α and 2-ClHDA led to significant IκB protein disappearance at 20 h, while 2-ClHA or other tested lipids did not to lead to a significant decrease in IκB protein levels (Fig 5A & 5B).
Figure 5. 2-Chlorohexadecanal decreases IκB protein expression.
Cell lysates were prepared from HCAEC treated with ethanol vehicle, 50 ng/mL TNF-α, or 50 µM hexadecanal (HDA), 2-chlorohexadecanal (2-ClHDA), 2-chlorohexadecanoic acid (2-ClHA), 2-chlorohexadecanol (2-ClHOH) for 20 h. Samples were subjected to SDS-PAGE and western blotting for IκB expression (A) and analyzed by densitometry of immunoreactive bands (B). Values (mean ± SEM) are expressed as fold increase over control treated cell at each time point, (**) is p < 0.01.
Discussion
Oxidative attack of plasmalogens by RCS leads to the production of reactive lipid aldehydes, such as 2-ClHDA [19]. Little is know about the possible signaling roles these naturally occurring halogenated lipids play. Marsche et al. (2004) discovered that HDL treated with hypochlorous acid, a component of RCS, led to a reduction in eNOS expression in endothelial cells and, upon further investigation, found that 2-ClHDA was responsible for the reduction in eNOS. An abundant non-chlorinated lipid aldehyde, HNE, has been shown to induce COX-2 expression in RAW264.7 macrophages [34,35] and in human osteoarthritic chondrocytes [35]. In the present study, we show that TNF-α, 2-ClHDA, and 2-ClHA, but not 2-ClHOH, induce an increase in COX-2 protein expression in human coronary artery endothelial cells. 2-ClHDA is produced by the production of RCS from phagocyte-derived myeloperoxidase [19]. 2-ClHDA concentrations range from 25–100 µM in human neutrophils [14]. The concentration of 2-ClHDA used in these studies is within the range that is physiologically produced from activated leukocytes. Although 2-ClHDA has been shown to have proatherogenic properties by decreasing eNOS [23] and increasing neutrophil chemotaxis [14], we show that 2-ClHDA increases COX-2 and PGI2 levels (as determined by increases in 6-keto-PGF1α levels). Interestingly comparisons of the time course of 2-ClHDA and 2-ClHA elicited COX-2 expression show that 2-ClHDA has a sustained impact on COX-2 expression. This may reflect disparate half-lives of these chlorinated lipids. In fact, 2-ClHDA is the precursor of 2-ClHA [24]. Thus, over time while 2-ClHDA levels decrease, the sustained COX-2 expression may be elicited by the product of 2-ClHDA metabolism, 2-ClHA.
The increase in COX-2 protein expression from TNF-α, 2-ClHDA, and 2-ClHA appears to occur by increased transcription of the COX-2 gene demonstrated by the results from quantitative PCR. The COX-2 gene contains 17 copies of the AUUUA motif in its 3’-UTR and can be regulated post-transcriptionally by elements that bind the AU-rich regions in its messenger RNA [36,37]. Previous studies have indicated that shear stress stabilizes COX-2 mRNA levels in HUVECs [37]. In this study, although we have shown that 2-ClHDA and 2-ClHA induce gene expression, we cannot rule out that 2-ClHDA and/or 2-ClHA also lead to a stabilization of COX-2 mRNA.
The human COX-2 promoter includes the NF-κB, cyclic AMP response element (CRE), activator protein–2 (AP2), signal transducers and activators of transcription (STAT3), SP1 and CCAAT/enhancer-binding protein (C/EBPb) [38–44]. The NF-κB pathway is considered to be a general inflammatory response pathway that can lead to gene transcription of various chemokines, adhesion molecules, and other pro-inflammatory molecules. In the present study we have shown that 2-chlorohexadecanal decreases IκB protein levels in HCAEC, suggesting that 2-ClHDA is signaling in part through the NF-κB pathway. Additionally it should be appreciated that COX-2 mRNA is elevated prior to IκB degradation in response to 2-chlorohexadecanal suggesting that additional pathways may play an important role in COX-2 expression in response to 2-chlorohexadecanal.
The release of 2-chloro fatty aldehydes from plasmalogens followed by its metabolism to 2-chloro fatty acids and 2-chloro fatty alcohols collectively suggest that a chlorinated lipidome is produced as a result of RCS attack of plasmalogens. It is now essential to determine the physiological role of these novel chlorinated lipids and their potential role in inflammation. The results herein demonstrate that 2-ClHA induces COX-2 expression and prostacyclin production in human coronary artery endothelial cells. Furthermore, the precursor of 2-ClHA, 2-ClHDA, also elicits COX-2 expression and prostacyclin production. It can be envisioned that the effect of these same chlorinated lipids on cells such as monocytes and neutrophils, which contain COX-2 associated isomerases that lead to pro-inflammatory eicosanoids, may have important roles in inflammation.
Acknowledgement
This research was supported by National Institutes of Health grants HL 74214 (DAF).
Abbreviations
- RCS
reactive chlorinating species
- 2-ClHDA
2-chlorohexadecanal
- 2-ClHA
2-chlorohexadecanoic acid
- 2-ClHOH
2-chlorohexadecanol
- HDA
hexadecanal
- COX-2
cyclooxygenase-2
- NF-κB
nuclear factor kappa B
- TNF-α
tumor necrosis factor-alpha
- HCAEC
human coronary artery endothelial cells
- MPO
myeloperoxidase
References
- 1.Schonbeck U, Sukhova GK, Graber P, Coulter S, Libby P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am J Pathol. 1999;155:1281–1291. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masferrer JL, Seibert K, Zweifel B, Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992;89:3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraemer SA, Meade EA, DeWitt DL. Prostaglandin endoperoxide synthase gene structure: identification of the transcriptional start site and 5'-flanking regulatory sequences. Arch Biochem Biophys. 1992;293:391–400. doi: 10.1016/0003-9861(92)90411-o. [DOI] [PubMed] [Google Scholar]
- 4.Whittaker N, Bunting S, Salmon J, Moncada S, Vane JR, Johnson RA, Morton DR, Kinner JH, Gorman RR, McGuire JC, Sun FF. The chemical structure of prostaglandin X (prostacyclin) Prostaglandins. 1976;12:915–928. doi: 10.1016/0090-6980(76)90126-x. [DOI] [PubMed] [Google Scholar]
- 5.Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 6.Dusting GJ, Moncada S, Vane JR. Prostacyclin (PGX) is the endogenous metabolite responsible for relaxation of coronary arteries induced by arachindonic acid. Prostaglandins. 1977;13:3–15. doi: 10.1016/0090-6980(77)90037-5. [DOI] [PubMed] [Google Scholar]
- 7.Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977;74:3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willems C, van Aken WG. Production of prostacyclin by vascular endothelial cells. Haemostasis. 1979;8:266–273. doi: 10.1159/000214317. [DOI] [PubMed] [Google Scholar]
- 9.Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993;268:9049–9054. [PubMed] [Google Scholar]
- 10.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. Journal of Clinical Investigation. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. Journal of Biological Chemistry. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 12.Hazen SL, Hsu FF, Duffin K, Heinecke JW. Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. Journal of Biological Chemistry. 1996;271:23080–23088. doi: 10.1074/jbc.271.38.23080. [DOI] [PubMed] [Google Scholar]
- 13.Winterbourn CC, van den Berg JJ, Roitman E, Kuypers FA. Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Archives of Biochemistry & Biophysics. 1992;296:547–555. doi: 10.1016/0003-9861(92)90609-z. [DOI] [PubMed] [Google Scholar]
- 14.Thukkani AK, Hsu FF, Crowley JR, Wysolmerski RB, Albert CJ, Ford DA. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. Journal of Biological Chemistry. 2002;277:3842–3849. doi: 10.1074/jbc.M109489200. [DOI] [PubMed] [Google Scholar]
- 15.Messner MC, Albert CJ, Hsu FF, Ford DA. Selective plasmenylcholine oxidation by hypochlorous acid: formation of lysophosphatidylcholine chlorohydrins. Chem Phys Lipids. 2006;144:34–44. doi: 10.1016/j.chemphyslip.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Gross RW. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984;23:158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- 17.Han X, Gross RW. Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Post JA, Verkleij AJ, Roelofsen B, Op de Kamp JA. Plasmalogen content and distribution in the sarcolemma of cultured neonatal rat myocytes. FEBS Letters. 1988;240:78–82. doi: 10.1016/0014-5793(88)80343-0. [DOI] [PubMed] [Google Scholar]
- 19.Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. Journal of Biological Chemistry. 2001;276:23733–23741. doi: 10.1074/jbc.M101447200. [DOI] [PubMed] [Google Scholar]
- 20.Thukkani AK, Albert CJ, Wildsmith KR, Messner MC, Martinson BD, Hsu FF, Ford DA. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. Journal of Biological Chemistry. 2003;278:36365–36372. doi: 10.1074/jbc.M305449200. [DOI] [PubMed] [Google Scholar]
- 21.Thukkani AK, McHowat J, Hsu FF, Brennan ML, Hazen SL, Ford DA. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003;108:3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. [DOI] [PubMed] [Google Scholar]
- 22.Thukkani AK, Martinson BD, Albert CJ, Vogler GA, Ford DA. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. American Journal of Physiology - Heart & Circulatory Physiology. 2005;288:H2955–H2964. doi: 10.1152/ajpheart.00834.2004. [DOI] [PubMed] [Google Scholar]
- 23.Marsche G, Heller R, Fauler G, Kovacevic A, Nuszkowski A, Graier W, Sattler W, Malle E. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arteriosclerosis, Thrombosis & Vascular Biology. 2004;24:2302–2306. doi: 10.1161/01.ATV.0000148703.43429.25. [DOI] [PubMed] [Google Scholar]
- 24.Wildsmith KR, Albert CJ, Anbukumar DS, Ford DA. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J. Biol. Chem. 2006;281:16849–16860. doi: 10.1074/jbc.M602505200. [DOI] [PubMed] [Google Scholar]
- 25.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Liu MC, Bleecker ER, Proud D, McLemore TL, Hubbard WC. Profiling of bisenoic prostaglandins and thromboxane B2 in bronchoalveolar fluid from the lower respiratory tract of human subjects by combined capillary gas chromatography-mass spectrometry. Prostaglandins. 1988;35:67–79. doi: 10.1016/0090-6980(88)90275-4. [DOI] [PubMed] [Google Scholar]
- 27.Schweer H, Kammer J, Seyberth HW. Simultaneous determination of prostanoids in plasma by gas chromatography-negative-ion chemical-ionization mass spectrometry. J Chromatogr. 1985;338:273–280. doi: 10.1016/0378-4347(85)80098-0. [DOI] [PubMed] [Google Scholar]
- 28.Eligini S, Stella Barbieri S, Cavalca V, Camera M, Brambilla M, De Franceschi M, Tremoli E, Colli S. Diversity and similarity in signaling events leading to rapid Cox-2 induction by tumor necrosis factor-alpha and phorbol ester in human endothelial cells. Cardiovasc Res. 2005;65:683–693. doi: 10.1016/j.cardiores.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Said FA, Werts C, Elalamy I, Couetil JP, Jacquemin C, Hatmi M. TNF-alpha, inefficient by itself, potentiates IL-1beta-induced PGHS-2 expression in human pulmonary microvascular endothelial cells: requirement of NF-kappaB and p38 MAPK pathways. Br J Pharmacol. 2002;136:1005–1014. doi: 10.1038/sj.bjp.0704811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu KK. Control of cyclooxygenase-2 transcriptional activation by pro-inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids. 2005;72:89–93. doi: 10.1016/j.plefa.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Syeda F, Grosjean J, Houliston RA, Keogh RJ, Carter TD, Paleolog E, Wheeler-Jones CP. Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-kappaB pathways. J Biol Chem. 2006;281:11792–11804. doi: 10.1074/jbc.M509292200. [DOI] [PubMed] [Google Scholar]
- 32.Chen CC. Signal transduction pathways of inflammatory gene expressions and therapeutic implications. Curr Pharm Des. 2006;12:3497–3508. doi: 10.2174/138161206778343028. [DOI] [PubMed] [Google Scholar]
- 33.Duque J, Diaz-Munoz MD, Fresno M, Iniguez MA. Up-regulation of cyclooxygenase-2 by interleukin-1beta in colon carcinoma cells. Cell Signal. 2006;18:1262–1269. doi: 10.1016/j.cellsig.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- 35.Vaillancourt F, Morquette B, Shi Q, Fahmi H, Lavigne P, Di Battista JA, Fernandes JC, Benderdour M. Differential regulation of cyclooxygenase-2 and inducible nitric oxide synthase by 4- hydroxynonenal in human osteoarthritic chondrocytes through ATF-2/CREB-1 transactivation and concomitant inhibition of NF-kappaB signaling cascade. J Cell Biochem. 2007;100:1217–1231. doi: 10.1002/jcb.21110. [DOI] [PubMed] [Google Scholar]
- 36.Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM. Post-transcriptional control of cyclooxygenase-2 gene expression The role of the 3'-untranslated region. J Biol Chem. 2000;275:11750–11757. doi: 10.1074/jbc.275.16.11750. [DOI] [PubMed] [Google Scholar]
- 37.Inoue H, Taba Y, Miwa Y, Yokota C, Miyagi M, Sasaguri T. Transcriptional and posttranscriptional regulation of cyclooxygenase-2 expression by fluid shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:1415–1420. doi: 10.1161/01.atv.0000028816.13582.13. [DOI] [PubMed] [Google Scholar]
- 38.Allport VC, Slater DM, Newton R, Bennett PR. NF-kappaB and AP-1 are required for cyclooxygenase 2 gene expression in amnion epithelial cell line (WISH) Mol Hum Reprod. 2000;6:561–565. doi: 10.1093/molehr/6.6.561. [DOI] [PubMed] [Google Scholar]
- 39.Billack B, Heck DE, Mariano TM, Gardner CR, Sur R, Laskin DL, Laskin JD. Induction of cyclooxygenase-2 by heat shock protein 60 in macrophages and endothelial cells. Am J Physiol Cell Physiol. 2002;283:C1267–C1277. doi: 10.1152/ajpcell.00609.2001. [DOI] [PubMed] [Google Scholar]
- 40.Iniguez MA, Martinez-Martinez S, Punzon C, Redondo JM, Fresno M. An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J Biol Chem. 2000;275:23627–23635. doi: 10.1074/jbc.M001381200. [DOI] [PubMed] [Google Scholar]
- 41.Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem. 1995;270:24965–24971. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- 42.Kirtikara K, Raghow R, Laulederkind SJ, Goorha S, Kanekura T, Ballou LR. Transcriptional regulation of cyclooxygenase-2 in the human microvascular endothelial cell line, HMEC-1: control by the combinatorial actions of AP2, NF-IL-6 and CRE elements. Mol Cell Biochem. 2000;203:41–51. doi: 10.1023/a:1007045600664. [DOI] [PubMed] [Google Scholar]
- 43.Kosaka T, Miyata A, Ihara H, Hara S, Sugimoto T, Takeda O, Takahashi E, Tanabe T. Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur J Biochem. 1994;221:889–897. doi: 10.1111/j.1432-1033.1994.tb18804.x. [DOI] [PubMed] [Google Scholar]
- 44.Xu Q, Ji YS, Schmedtje JF., Jr Sp1 increases expression of cyclooxygenase-2 in hypoxic vascular endothelium. Implications for the mechanisms of aortic aneurysm and heart failure. J Biol Chem. 2000;275:24583–24589. doi: 10.1074/jbc.M003894200. [DOI] [PubMed] [Google Scholar]