Summary
There is broad consensus that olfactory signalling in vertebrates and the nematode C. elegans uses canonical G protein-coupled receptor transduction pathways. In contrast, mechanisms of insect olfactory signal transduction remain deeply controversial. Genetic disruption of G proteins and chemosensory ion channels in mice and worms leads to profound impairment in olfaction, while similar mutations in the fly show more subtle phenotypes. The literature contains contradictory claims that insect olfaction uses cAMP, cGMP, or IP3 as second messengers; that insect odorant receptors couple to Gαs or Gαq pathways; and that insect odorant receptors are GPCRs or odor-gated ion channels. Here we consider all the evidence and offer a consensus model for a non-canonical mechanism of olfactory signal transduction in insects.
Introduction
“What sense is it that informs this great butterfly of the whereabouts of his mate, and leads him wandering through the night? What organ does this sense affect? One suspects the antennæ; in the male butterfly they actually seem to be sounding, interrogating empty space with their long feathery plumes. Are these splendid plumes merely items of finery, or do they really play a part in the perception of the effluvia which guide the lover?”
--Social Life in the Insect World by J.H. Fabre [1]
Insects show robust and extremely sensitive behaviors that are elicited by chemical cues in a species-specific manner [2]. In the 1870s the French biologist Jean-Henri Fabre described the phenomenon that the female peacock moth releases invisible odor signals (termed “pheromones” 50 years later [3]) to attract the male [1]. Surprisingly, in spite of a long and prolific history of research into insect olfaction (reviewed in [2,4]), the molecular mechanisms of insect olfactory signal transduction remain unclear.
In all animals, odor cues are detected by membrane receptors that signal the identity and quantity of chemicals in the environment by inducing electrical activity in primary olfactory sensory neurons (OSNs). Classic work in vertebrates indicated that odors stimulate adenylate cyclase activity [5,6]. This led to the subsequent identification of an olfactory-specific adenylate cyclase (ACIII) and Gαs protein (Gαolf) [7] and later the discovery of a large family of genes encoding seven transmembrane domain G protein-coupled odorant receptors (ORs) [8]. Genetic deletion of signaling components in the mouse severely disrupts olfactory function. Similarly clear results in the nematode C. elegans (reviewed in [4]) affirmed the universally accepted view that all animals smell through G protein-coupled receptors (GPCRs) that activate canonical signaling pathways.
These evolutionary considerations have guided studies of insect olfactory signal transduction for several decades, leading workers in the field to assume that GPCRs and the signal transduction cascades activated by them will also operate in insects. However, the primary data to support these assumptions are surprisingly contradictory (Table 1). This article reviews the history of investigation into the problem and proposes a consensus model for a non-canonical mechanism of olfactory signalling in insects.
Table 1.
Signaling systems implicated in insect olfactory transduction
| Pathway/Components | Effect/evidence | Reference |
|---|---|---|
| Gαq: Ca+2/IP3/DAG/PLC | ||
| Gαq | Expressed in olfactory neurons | [15-17-20] |
| dgαq mutant/knockdown | Decreased response | [19,28] |
| PKC activators | Activate AC1 channel | [13] |
| DAG kinase (rdgA) mutant | Decreased response | [19] |
| PI-TP | Expressed in olfactory neurons | [23] |
| PI-TP (rdgB) mutant | Decreased response | [23] |
| IP3 kinase1 | Expressed in olfactory neurons | [26] |
| IP3 kinase1overexpression | Altered response | [26] |
| IP3 | Odor-evoked increase | [10,11] |
| PLC | Expressed in olfactory neurons | [24] |
| PLC (norpA) mutant | Decreased response | [24] |
| PLCβ (plc21C) mutant | Decreased response | [19] |
| Gαs: Cyclic nucleotides | ||
| Gαs | Expressed in olfactory neurons | [17] |
| Gαs mutant | Decreased response | [25,27] |
| AC and PDE | Expressed in olfactory neurons | [25] |
| AC mutant (rut) | Altered response | [25,27] |
| PDE mutant (dnc) | Altered response | [25,27] |
| cAMP | Odor-evoked increase | [10,52] |
| cAMP | No odor-evoked increase | [10,12,51] |
| cGMP | Odor-evoked increase | [12] |
| cGMP | Activates AC1 channel | [13] |
| cAMP/cGMP | Activates Or83b | [52] |
| cAMP/cGMP | Does not activate Or83b | [51] |
| CNG channel | Expressed in olfactory neurons | [21] |
| CNG K+ (eag) mutant | Decreased response | [22] |
| Or83b co-receptor | ||
| Function requires Or83b | Imaging/electrophysiology | [34,40,41,51] |
| Or83b enhances function | Imaging/electrophysiology | [37,42,43] |
| Function without Or83b | Imaging/electrophysiology | [47–50] |
| Function with G-protein | Imaging/electrophysiology | [48,49,52] |
| Function without G-protein | Imaging/electrophysiology | [37,42,43,50,51] |
Abbreviations: PKC, protein kinase C; DAG, diacylglycerol; PI-TP, phosphatidylinositol transfer protein; IP3, inositol 1,4,5-trisphosphate; PLC, phospholipase C; CNG, cyclic nucleotide-gated; AC, adenylate cyclase; PDE, phosphodiesterase; cAMP, 3′-5′-cyclic adenosine monophosphate; cGMP, 3′-5′-cyclic guanosine monophosphate.
Pheromone-evoked physiological responses in insect olfactory neurons
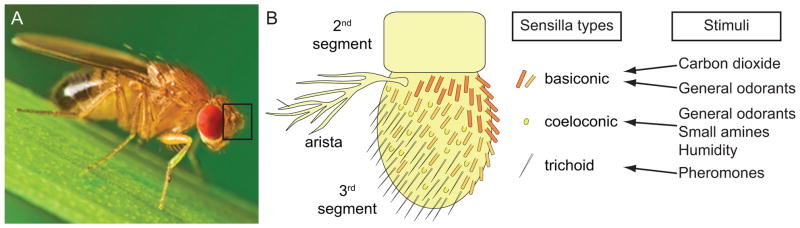
Insects are equipped with two pairs of head appendages, the antennae and maxillary palps, which are decorated with thousands of olfactory hairs called sensilla that in Drosophila each house between one and four OSNs (Figure 1) [2]. In other insects, a sensillum may house as many as 30 OSNs. Different classes of sensilla respond to different odor types (Figure 1B). Chemical cues pass through the pores in the sensillum wall, interact with ORs present on the membranes of sensory dendrites emanating from the OSN, and change the frequency of action potentials in these neurons. OSNs exhibit characteristic levels of spontaneous activity that depend on the specific odorant receptor expressed in the OSN and odors can either increase or decrease spiking frequency [9].
Figure 1. Insect olfactory sensilla.
(A) Adult male vinegar fly, Drosophila melanogaster, on a blade of grass. Black box indicates the position of the chemosensory antennae. Adapted from a royalty-free photo (© Studiotouch #8408777, Fotolia.com).
(B) Cartoon of one antenna, with the segments labeled and the position of three different types of chemosensory hairs on the third segment indicated, along with the classes of stimuli that activate neurons in these sensilla. Adapted from [34], published by the Public Library of Science, which uses the Creative Commons Attribution License.
Modern studies into how odor cues activate insect OSNs began with Dietrich Schneider and colleagues, who used electrophysiology to record the electrical activity of the pheromone-tuned OSNs in the antenna of the male silkmoth [2]. Later biochemical work by Breer and colleagues indicated that pheromones induce rapid production of inositol 1,4,5-trisphosphate (IP3) [10,11] but found no evidence for production of 3′-5′-cyclic adenosine monophosphate (cAMP) [10]. IP3 production required the activity of a pertussis-toxin sensitive G protein signalling pathway [11]. Ziegelberger et al. confirmed that cAMP was not produced, but detected pheromone-induced production of 3′-5′-cyclic guanosine monophosphate (cGMP) on a slower time-scale more consistent with a role in modulating OSN sensitivity [12]. By patch clamping of the moth OSN dendritic membrane, Zufall and Hatt found a pheromone-gated nonselective cation channel (AC1) that could also be activated by protein kinase C (PKC) activators and cGMP but not cAMP or IP3 [13]. They proposed a model of dual activation in which pheromones activate AC1 to produce a rapid response via PKC and a more sustained response via cGMP [13]. Stengl found multiple pheromone-evoked currents in moth neurons operating at different time scales, the first a very rapid calcium current that declines in 100 msec that could not be blocked by PKC inhibitors, a second IP3-stimulated cation current that declines in less than 3 sec, and a third inward current that was sustained over several seconds [14]. The molecular identity of the moth AC1 and IP3-activated channels is still unknown.
Evidence for G-protein signaling in insect olfactory transduction?
These biochemical and electrophysiological studies implicating second messengers in insect olfactory signal transduction prompted a search for olfactory-enriched signaling proteins. G-protein subunits of Gαs, Gαq, and Gαo subtypes were found in OSNs in diverse insects (Table 1) [15–19]. Gαs and Gαq were found to be enriched in sensory dendrites, implicating them in transduction mechanisms, but Gαo was localized only to the olfactory axon bundle, making it less likely that Gαo signaling is directly involved in transduction [17,20]. In addition to G proteins, olfactory cyclic nucleotide- and IP3-gated ion channels were described [14,21,22].
Starting in the 1990s, genetic analysis in Drosophila made it possible to test the functional relevance of these various signaling pathways in insect olfaction. Carlson and co-workers investigated the Gαq pathway and found reduced responses in flies mutant for PLC and associated phosphoinositide signaling components, but the effects were subtle and limited to subtypes of OSNs [23,24]. Alcorta and co-workers found evidence for both IP3 and cAMP in insect olfactory behavior, but again the phenotypes were quite subtle [25–27]. Flies in which Gαq was knocked down [19,28] or deleted [19] showed reduced electrophysiological and behavioral sensitivity to odors, and these Gαq defects synergized with mutations in DAG kinase and PLC [19]. Recent in vivo work from Kain et al. [19] represents the strongest evidence available that G protein signaling coupled to phosphoinositides is required for maximal sensitivity of the insect odor response but not for the odor response itself. Kain and colleagues examined Drosophila Gαq (dgq) null OSNs generated either by genetic mosaic techniques or RNA interference and found a shift to lower sensitivity in the absence of dgq. This phenotype was enhanced when OSNs also lacked PLCβ21C or a diacylglycerol kinase encoded by the rdgA gene. The authors conclude that a phospholipid intermediate triggered by Gαq is crucial to optimal sensitivity of insect OSNs. We will revisit the question of G protein signaling in the concluding remarks of this review.
Unconventional topology and heteromeric assembly of insect odorant receptors
Understanding the molecular basis of odor responses in insects required the identification of insect ORs. After many years of failed GPCR homology-based searches for insect ORs, a combination of difference cloning [29] and genomic analysis [29–31] yielded a family of divergent seven transmembrane domain proteins. Subsequent functional analysis in flies confirmed that these membrane proteins indeed confer odor-specific responses in the antenna [9,32,33]. Carlson and colleagues made the important observation that an individual OR governed not only ligand-specificity, but also levels of spontaneous activity, activation kinetics, and whether a cell was inhibited or activated by a given odor [9]. Based on these results, Hallem et al. hypothesized that the insect OR is poised between an inactive state that is insensitive to G proteins and an active state that can lead to the activation of a G protein-mediated signal transduction cascade [9]. Inhibitory odorants would lock the receptor into an inactive state and excitatory odorants would engage a G protein signaling pathway that increases the frequency of action potentials in the OSN.
Although insect ORs were widely assumed to be GPCRs, in vitro and in vivo structural analysis revealed that the membrane topology of insect ORs is inverted compared to conventional GPCRs [34–37]. Further, insect ORs have no amino acid homology to ORs in vertebrates or C. elegans or to any other class of GPCR. Accordingly, conventional binding sites for G proteins are not obviously present in the insect ORs. This implied that the insect olfactory system may utilize atypical molecular mechanisms, distinct from vertebrates and nematodes.
Aside from differences in OR protein sequence, insect ORs differ from vertebrates in the expression of multiple ORs per cell—a ligand binding OR and a second member of the gene family that is called Or83b in Drosophila and called either OR2 or OR7 in other insects. OR83b and its orthologues in other insects is highly conserved across insect species [38–40] and is necessary for trafficking of ORs to dendritic membrane in vivo [34,41]. Throughout this review we will use the term OR83b to refer generically to the insect OR co-receptor. Biochemical studies showed that OR and OR83b proteins physically interact [34,42], leading to the suggestion that OR83b functions as a co-receptor for the ORs, although OR83b does not itself respond to odors. Thus in contrast to the GPCR-type OR mediating odor responses in vertebrates, the insect OR appears to be a heteromultimeric receptor complex.
A diversity of receptor types and ligands in insects
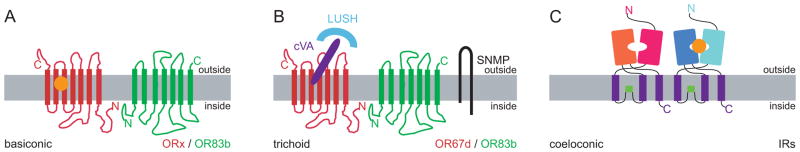
Insect respond to a very wide array of chemical substances and recent advances in Drosophila have begun to explain how this is achieved [4]. The specific OR/OR83b subunit composition governs whether the neuron will respond to general odorants or pheromones (Figure 2A–B) [9,33,43]. Pheromone receptors are a subset of the OR gene superfamily that, along with OR83b, require a CD36 homologue called SNMP for function [44,45] (Figure 2B). How these proteins interact to modulate pheromone sensitivity is an active and exciting area of investigation. Finally, a completely new receptor family—the Ionotropic Receptors (IRs), divergent, insect-specific members of the ionotropic glutamate receptor family—was recently proposed to explain the odor sensitivity of OSNs housed in coeloconic sensilla [46] (Figure 2C). These recent advances in receptor cloning were important in allowing the field to understand the molecular basis of odor recognition in a given OSN, but shed little light on how these receptors actually couple odor recognition with OSN activation.
Figure 2. Diverse chemosensory receptors mediating olfaction in insects.
(A) Basiconic sensilla are tuned to carbon dioxide detection using Gr21a/Gr63a (not shown) and general odorant detection using OR/OR83b complexes. The general odorant is indicated by the orange dot and interacts with the OR subunit in the complex.
(B) Pheromones are detected with distinct OR/OR83b complexes that act in concert with a CD36 homologue called SNMP [44,45]. For the detection of cis-vaccenyl acetate (cVA), a soluble odorant binding protein called LUSH is required [55]. cVA is indicated by the purple ellipse and interacts with the OR67d subunit in the complex.
(C) A newly described family of chemosensory receptors is encoded by variant ionotropic receptors (IRs) [46]. IRs are expressed in coeloconic sensilla that detect general odorants, small amines, and humidity. Ligands (indicated by the orange dot) are presumed to be bound by extracellular domains of these receptors, but the nature of the IR receptor complex and what subunit(s) bind ligands remain to be determined.
Insect odor transduction mechanisms probed through heterologous expression
To clarify how insect ORs are activated, multiple groups have turned to heterologous expression of these receptors in various cell types. Heterologous expression confers the benefits that ORs can be studied in isolation and subjected to pharmacological analysis, but conclusions must always be tempered because the receptors are not in their native environment in the insect OSN. Different groups chose different cells types—mammalian tissue culture cells or frog (Xenopus laevis) oocytes—and expressed ORs alone or with OR83b and with or without exogenous G proteins [37,42,43,47–52].
Initially, several groups reported that insect ORs could function in vitro in the absence of the OR83b co-receptor but with high odor concentrations and the addition of exogenous G proteins (promiscuous Gα15 or insect Gαq) [47–50]. Upon addition of the appropriate odor, inward cation currents and an influx of extracellular calcium ions were detected [47–50]. Given the absolute requirement for OR83b for OR function in vivo [34,40,41], the basis for how ORs function in vitro without OR83b is not currently understood. Some of these same groups later showed that co-transfection of ORs with OR83b significantly enhances the proportion of responding cells and also increases both odor sensitivity and the magnitude of the evoked response, even in the absence of exogenous Gαq [37,42,43]. Touhara and colleagues [43] were the first to argue not only that OR/OR83b responses can occur in the absence of exogenous Gαq but that the responses are biophysically different in the absence of G proteins.
The most recent work in this field has directly questioned whether insect ORs function as GPCRs (Figure 3), and has yielded complex answers. Three groups, led by Touhara and Vosshall [51], Newcomb [37], and Hansson [52], expressed ligand-specific ORs from various insects along with the corresponding OR83b co-receptor in mammalian or insect tissue culture cells or frog eggs.
Figure 3. Models of insect olfactory receptor signal transduction.
(A) Canonical G protein signaling in the mammalian olfactory system (ACIII, adenylate cyclase III; CNGC, cyclic nucleotide-gated channel).
(B) Sato et al. [51] propose that insect ORs form ligand-gated nonselective cation channels activated rapidly by odors in the absence of G-protein signaling.
(C) Wicher et al. [52] propose that the variable ORx subunit is a G protein-coupled receptor and that OR83b is a cyclic nucleotide-gated ion channel. Odor activation of ORx triggers two pathways, a fast short ionotropic pathway and a slow prolonged metabotropic pathway. The metabotropic pathway involves Gs coupling of ORx, leading to the production of intracellular cAMP, which activates OR83b.
(D) Integrative model of insect olfactory signal transduction proposed in this review article. See text for details. Abbreviations: CaM, calmodulin; PKC, protein kinase C; PKA, protein kinase A; PKG, protein kinase G; PLC, phospholipase C; AC, adenylate cyclase; GC, guanylate cyclase.
Sato et al. expressed multiple different ORs along with Drosophila OR83b or its orthologue from moth, and mosquito in various heterologous cell types [51] and characterized a very fast ionotropic response that persisted in the presence of PLC inhibitors and GDPβS, a general inhibitor of G protein signaling. This led these authors to conclude that odor-evoked currents mediated by insect ORs are independent of known G protein signaling pathways. While Sato and coworkers found no evidence for odor-stimulated cAMP production, they did note that particular OR complexes showed odor-independent cyclic nucleotide sensitivity. Further they showed that the specific subunit composition governs the biophysical properties of the OR/OR83b receptor, strongly suggesting that these proteins function as a complex to form an odor-gated ion channel whose initial activation does not depend on G protein signaling (Figure 3B).
Working with Drosophila OR43b/OR83b, Smart et al. found that inhibitors of the Gq pathway and the general G protein inhibitor GDPβS did not block odor-evoked calcium increases, but changed the inactivation kinetics [37]. This led the authors to conclude that while G protein signaling is not required to activate the receptors, it may be involved in post-activation modulation.
In part agreeing with these results, Wicher et al. [52] found that Drosophila OR22a/OR83b could be activated by odors to produce a very rapid ionotropic response that did not require G proteins. However, they also noted and studied in depth a considerably slower metabotropic response that depended on Gαs but did not involve Gαq pathways. Activation of the metabotropic pathway was shown to produce intracellular cAMP. OR83b was shown to be gated directly by cAMP or cGMP. Odor-evoked currents persisted in the presence of a general inhibitor of G protein signaling, GDPβS, but cells were less sensitive to odors. Thus this group concluded that ligand-binding ORs couple to Gαs to produce cyclic nucleotides, which in turn activate OR83b, which they hypothesize has the properties of a cyclic-nucleotide gated non-selective cation channel. This model posits that the OR functions like a GPCR and that OR83b functions as an ion channel (Figure 3C), although direct interaction of insect ORs with G proteins remains to be shown.
Such dual activation is conceptually similar to the dual activation properties of moth OSNs found in early work by Zufall, Hatt, Stengl, and coworkers [13,14], although the details of the relevant second messenger differ across all of these studies. Dual activation may work to extend the range of sensitivity to odors, with the ionotropic pathway operating at higher odor concentrations in a G protein-independent manner and the metabotropic pathway being important to amplify signal strength at low odor concentrations. This would be consistent with the in vivo results from Kain et al., which showed that eliminating Gαq signaling pathways reduced the sensitivity of OSNs but did not eliminate responses to odors [19]. Although the time course of metabotropic activation found by Wicher et al. in heterologous cells was quite slow, it is conceivable that signaling is more rapid in native OSNs. For instance, it is possible that ORs and effector/modulator proteins can be physically linked in a signaling complex that affords more rapid activation than that achievable in heterologous cells. Strong evidence for such a signaling complex held together by scaffolding proteins has previously been shown for the Drosophila photoreceptor signaling system (see [53] for a review).
Prospects for a consensus model of insect olfactory signal transduction
How are we to reconcile all of these disparate findings? Is there a single unifying mechanism of insect olfactory signal transduction, or are there multiple pathways that depend on the specific OR and cell type being examined? In these concluding remarks, we summarize what we believe to be the points of consensus in the field and attempt to address the outstanding issues. The various possibilities for a consensus signaling model are schematized in Figure 3D.
While the proposal that insect ORs adopt a membrane topology opposite to GPCRs [34] was initially greeted with skepticism, mounting structural [35–37] and functional [37,51,52] data now strongly suggest that insect ORs are a novel class of membrane receptor unrelated to GPCRs. There seems to be broad agreement that while the initial response to odors can occur in the absence of G protein signaling [19,37,51,52], second messenger-mediated responses on a slower time scale may make these receptors more sensitive to odors [19,37,52].
Since insect ORs have no homology to GPCRs, how would these proteins be integrated into a signaling framework that includes modulation by G proteins? A straightforward model to reconcile all of these data would posit that OR/OR83b forms a non-selective cation channel that fluxes cations including calcium upon odor binding by the ligand-specific OR in the subunit. This initial, rapid ionotropic response would be a property of the OR/OR83b protein alone (Figure 3B–C). The influx of calcium would trigger a slower metabotropic response to produce post-activation modulation of the OR/OR83b complex and increase sensitivity by increasing the open probability of the receptor (Figure 3C–D). Since it seems unlikely to us that the OR/OR83b complex directly interacts with G proteins, we are left with the problem of how G proteins are activated secondary to OR/OR83b activation. One possibility is that as yet undescribed membrane receptors (brown circles in Figure 3D) are co-stimulated by OR/OR83b activation, thus triggering conventional G protein signaling. Another possibility is that a solely intracellular signaling network acts to stimulate G proteins directly. There are at present no available data to support either model and more work is needed to clarify exactly how and where G proteins act in insect olfactory signaling. Further, the conflicting evidence for an involvement of Gαq vs Gαs signaling pathways must be resolved. One possibility is that different OR/OR83b complexes couple to different signaling pathways. Alternatively, some of the observations in heterologous cells may not reflect the coupling properties of insect ORs in vivo.
Although many details are lacking, a blended ionotropic/metabotropic model would be consistent with the early observations made by Zufall, Hatt, and Stengl [13,14] of a pheromone-gated ion channel that has a rapid primary response not modulated by second messengers, and secondary responses that are sensitive to pharmacological perturbation of second messenger pathways. Based on subsequent work, it is conceivable that the AC1 current is a property of a pheromone-sensitive OR/OR83b complex in the moth.
While neither the ORs nor OR83b have any obvious homology to cyclic-nucleotide binding domains, they could conceivably have a novel nucleotide activation domain. Perhaps more likely, cyclic nucleotides could act indirectly by activating kinases that phosphorylate the OR/OR83b complex (Figure 3D). The intracellular domain of OR83b is enriched in multiple consensus phosphorylation sites by calcium- and cyclic-nucleotide activated kinases (Pellegrino and Vosshall, unpublished observation), making post-translational modification plausible for this receptor.
Such a model of blended ionotropic/metabotropic modulation is typical for ion channels as a class of signaling proteins. Nearly all ion channels are subject to extensive post-translational modification by phosphorylation or lipid, nucleotide, or calcium-calmodulin binding to alter open probability and inactivation kinetics. For example, the TRPV1 capsaicin-sensitive ion channel is directly gated by chemicals and heat, but can be potentiated by GPCR-mediated second messenger pathways that either phosphorylate the ion channel or produce phosphoinositides that directly modulate TRPV1 (for review see [54]). Thus our consensus model is only surprising in light of the old and, as we argue, incorrect assumption that insect ORs are GPCRs.
Why might insects have evolved a non-canonical mechanism to translate odor binding to OSN activation? One obvious cost of an ionotropic mechanism is the loss of signal amplification provided by GPCRs. In contrast, an obvious benefit is the speed of signaling permitted by direct activation independent of second messenger pathways. Our consensus model would accommodate both modes of signaling, perhaps acting at different ranges of odor concentration.
Much remains to be done to provide truly convincing data for this integrated model of olfactory signal transduction. The evidence that the OR/OR83b complex forms an odor-gated ion channel is still quite preliminary. If these are ion channels, the ion-conducting pore must be identified, be it formed at the OR/OR83b interface (Figure 3B) or solely within OR83b (Figure 3C). Structural information on the stoichiometry of the OR complex as well as a high-resolution x-ray crystal structure will go a long way toward solving the ongoing debate on the mechanism of action of this class of receptors. If OR and OR83b are subject to post-translational modification subsequent to the initial ionotropic activation, these sites of modification must be identified by a comprehensive structure-function analysis. Finally, all of these hypotheses must be validated in an in vivo preparation with careful electrophysiology, pharmacology, and genetics. Exciting days lie ahead in this field.
Acknowledgments
Work in the authors’ laboratory is supported by the National Institutes of Health (DC006711, DC008600), the Howard Hughes Medical Institute, and funded in part by a grant to R. Axel and L.B.V. from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative. The authors thank Kazushige Touhara, Dieter Wicher, and Bill Hansson for constructive comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Fabre JH. Social Life In The Insect World. Harmondsworth, UK: Penguin; 1911. [Google Scholar]
- 2.Schneider D. Insect olfaction: deciphering system for chemical messages. Science. 1969;163:1031–1037. doi: 10.1126/science.163.3871.1031. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt TD. Fifty years of pheromones. Nature. 2009;457:262–263. doi: 10.1038/457262a. [DOI] [PubMed] [Google Scholar]
- 4.Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- 5.Pace U, Hanski E, Salomon Y, Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985;316:255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- 6.Sklar PB, Anholt RR, Snyder SH. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986;261:15538–15543. [PubMed] [Google Scholar]
- 7.Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244:790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- 8.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 9.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Breer H, Boekhoff I, Tareilus E. Rapid kinetics of second messenger formation in olfactory transduction. Nature. 1990;345:65–68. doi: 10.1038/345065a0. [DOI] [PubMed] [Google Scholar]
- 11.Boekhoff I, Raming K, Breer H. Pheromone-induced stimulation of inositol trisphosphate formation in insect antennae is mediated by G-proteins. J Comp Physiol [B] 1990;160:99. [Google Scholar]
- 12.Ziegelberger G, van den Berg MJ, Kaissling KE, Klumpp S, Schultz JE. Cyclic GMP levels and guanylate cyclase activity in pheromone-sensitive antennae of the silkmoths Antheraea polyphemus and Bombyx mori. J Neurosci. 1990;10:1217–1225. doi: 10.1523/JNEUROSCI.10-04-01217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zufall F, Hatt H. Dual activation of a sex pheromone-dependent ion channel from insect olfactory dendrites by protein kinase C activators and cyclic GMP. Proc Natl Acad Sci U S A. 1991;88:8520–8524. doi: 10.1073/pnas.88.19.8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stengl M. Inositol-trisphosphate-dependent calcium currents precede cation currents in insect olfactory receptor neurons in vitro. J Comp Physiol [A] 1994;174:187–194. doi: 10.1007/BF00193785. [DOI] [PubMed] [Google Scholar]
- 15.Talluri S, Bhatt A, Smith DP. Identification of a Drosophila G protein alpha subunit (dGq alpha-3) expressed in chemosensory cells and central neurons. Proc Natl Acad Sci U S A. 1995;92:11475–11479. doi: 10.1073/pnas.92.25.11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laue M, Maida R, Redkozubov A. G-protein activation, identification and immunolocalization in pheromone-sensitive sensilla trichodea of moths. Cell Tissue Res. 1997;288:149–158. doi: 10.1007/s004410050802. [DOI] [PubMed] [Google Scholar]
- 17.Miura N, Atsumi S, Tabunoki H, Sato R. Expression and localization of three G protein alpha subunits, G(o), G(q), and G(s), in adult antennae of the silkmoth (Bombyx mori) J Comp Neurol. 2005;485:143–152. doi: 10.1002/cne.20488. [DOI] [PubMed] [Google Scholar]
- 18.Jacquin-Joly E, Francois MC, Burnet M, Lucas P, Bourrat F, Maida R. Expression pattern in the antennae of a newly isolated lepidopteran Gq protein alpha subunit cDNA. Eur J Biochem. 2002;269:2133–2142. doi: 10.1046/j.1432-1033.2002.02863.x. [DOI] [PubMed] [Google Scholar]
- 19 ••.Kain P, Chakraborty TS, Sundaram S, Siddiqi O, Rodrigues V, Hasan G. Reduced odor responses from antennal neurons of Gqα, phospholipase Cβ, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J Neurosci. 2008;28:4745–4755. doi: 10.1523/JNEUROSCI.5306-07.2008. The authors performed in vivo electrophysiological measurements in mutants of Drosophila dGqα and the phospholipase Cβ to provide evidence that olfactory transduction in Drosophila is modulated by Gαq protein and a phospholipid second messenger cascade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutzler M, Lu T, Zwiebel LJ. Gα encoding gene family of the malaria vector mosquito Anopheles gambiae: expression analysis and immunolocalization of AGαq and AGαo in female antennae. J Comp Neurol. 2006;499:533–545. doi: 10.1002/cne.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumann A, Frings S, Godde M, Seifert R, Kaupp UB. Primary structure and functional expression of a Drosophila cyclic nucleotide-gated channel present in eyes and antennae. EMBO J. 1994;13:5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubin AE, Liles MM, Harris GL. The K+ channel gene ether a go-go is required for the transduction of a subset of odorants in adult Drosophila melanogaster. J Neurosci. 1998;18:5603–5613. doi: 10.1523/JNEUROSCI.18-15-05603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riesgo-Escovar JR, Woodard C, Carlson JR. Olfactory physiology in the Drosophila maxillary palp requires the visual system gene rdgB. J Comp Physiol [A] 1994;175:687–693. doi: 10.1007/BF00191841. [DOI] [PubMed] [Google Scholar]
- 24.Riesgo-Escovar J, Raha D, Carlson JR. Requirement for a phospholipase C in odor response: overlap between olfaction and vision in Drosophila. Proc Natl Acad Sci U S A. 1995;92:2864–2868. doi: 10.1073/pnas.92.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin F, Charro MJ, Alcorta E. Mutations affecting the cAMP transduction pathway modify olfaction in Drosophila. J Comp Physiol [A] 2001;187:359–370. doi: 10.1007/s003590100208. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Diaz C, Martin F, Alcorta E. The Inositol 1,4,5-triphosphate kinase1 gene affects olfactory reception in Drosophila melanogaster. Behav Genet. 2006;36:309–321. doi: 10.1007/s10519-005-9031-x. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Diaz C, Martin F, Alcorta E. The cAMP transduction cascade mediates olfactory reception in Drosophila melanogaster. Behav Genet. 2004;34:395–406. doi: 10.1023/B:BEGE.0000023645.02710.fe. [DOI] [PubMed] [Google Scholar]
- 28.Kalidas S, Smith DP. Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron. 2002;33:177–184. doi: 10.1016/s0896-6273(02)00560-3. [DOI] [PubMed] [Google Scholar]
- 29.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 30.Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 31.Gao Q, Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. doi: 10.1006/geno.1999.5894. [DOI] [PubMed] [Google Scholar]
- 32.Stortkuhl KF, Kettler R. Functional analysis of an olfactory receptor in Drosophila melanogaster. Proc Natl Acad Sci USA. 2001;98:9381–9385. doi: 10.1073/pnas.151105698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 34 ••.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. This paper was the first to question the dogma that insect ORs are GPCRs. Experiments herein define insect ORs and OR83b as a novel transmembrane family with a distinct topology from conventional GPCRs. Moreover, OR83b is shown to be essential and sufficient to maintain the OR/OR83b complex on the membrane of the olfactory neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35 •.Wistrand M, Kall L, Sonnhammer EL. A general model of G protein-coupled receptor sequences and its application to detect remote homologs. Protein Sci. 2006;15:509–521. doi: 10.1110/ps.051745906. This paper describes a novel structural search algorithm to identify GPCRs and concludes based on bioinformatic evidence that insect ORs are distinct from GPCRs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36 •.Lundin C, Käll L, Kreher KA, Kapp K, Sonnhammer EL, Carlson JR, von Heijne G, Nilsson I. Membrane topology of the Drosophila OR83b odorant receptor. FEBS Lett. 2007;581:5601–5604. doi: 10.1016/j.febslet.2007.11.007. This paper uses careful glycosylation site mapping to confirm the membrane topology of OR83b proposed by Benton et al. [34], most importantly confirming the inferred extracellular disposition of the C terminus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37 •.Smart R, Kiely A, Beale M, Vargas E, Carraher C, Kralicek AV, Christie DL, Chen C, Newcomb RD, Warr CG. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Mol Biol. 2008;38:770–780. doi: 10.1016/j.ibmb.2008.05.002. In this paper, the authors used pharmacological inhibitors of several G protein-mediated pathways to investigate the signaling mechanism of insect OR, and found that odorant-evoked responses were independent of conventional G protein-coupled second messenger pathways. They also carry out careful analysis of the topology of OR22a, confirming for a single OR the conclusions reached for the OR83b co-receptor. [DOI] [PubMed] [Google Scholar]
- 38.Krieger J, Klink O, Mohl C, Raming K, Breer H. A candidate olfactory receptor subtype highly conserved across different insect orders. J Comp Physiol A. 2003;189:519–526. doi: 10.1007/s00359-003-0427-x. [DOI] [PubMed] [Google Scholar]
- 39.Pitts RJ, Fox AN, Zwiebel LJ. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci U S A. 2004;101:5058–5063. doi: 10.1073/pnas.0308146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones WD, Nguyen TA, Kloss B, Lee KJ, Vosshall LB. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr Biol. 2005;15:R119–R121. doi: 10.1016/j.cub.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Neuhaus EM, Gisselmann G, Zhang W, Dooley R, Stortkuhl K, Hatt H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat Neurosci. 2005;8:15–17. doi: 10.1038/nn1371. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307:1638–1642. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- 44 •.Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450:289–293. doi: 10.1038/nature06328. This paper shows the first functional evidence that Drosophila CD36 homologue, SNMP, is required for the detection of cVA, male-specific fatty acid-derived pheromone. Functional expression of a moth pheromone receptor in Drosophila also requires SNMP. This result provides new insight into the molecular basis of pheromone detection in insects. [DOI] [PubMed] [Google Scholar]
- 45 •.Jin X, Ha TS, Smith DP. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci USA. 2008;105:10996–11001. doi: 10.1073/pnas.0803309105. Using a forward genetic screen for cVA-insensitive mutants, the authors demonstrate that SNMP is required for the detection of cVA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46 ••.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. The authors identified a novel class of candidate chemosensory receptors, iGluR-related genes (IRs) in Drosophila using a bioinformatic screen for olfactory-enriched genes. Detailed functional and expression analysis of this new family provides evidence that the IRs function as odorant receptors in coeloconic sensilla. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wetzel CH, Behrendt H-J, Gisselmann G, Störtkuhl KF, Hovemann B, Hatt H. Functional expression and characterization of a Drosophila odorant receptor in a heterologous cell system. Proc Natl Acad Sci U S A. 2001;98:9377–9380. doi: 10.1073/pnas.151103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakurai T, Nakagawa T, Mitsuno H, Mori H, Endo Y, Tanoue S, Yasukochi Y, Touhara K, Nishioka T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc Natl Acad Sci U S A. 2004;101:16653–16658. doi: 10.1073/pnas.0407596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krieger J, Grosse-Wilde E, Gohl T, Breer H. Candidate pheromone receptors of the silkmoth Bombyx mori. Eur J Neurosci. 2005;21:2167–2176. doi: 10.1111/j.1460-9568.2005.04058.x. [DOI] [PubMed] [Google Scholar]
- 50.Kiely A, Authier A, Kralicek AV, Warr CG, Newcomb RD. Functional analysis of a Drosophila melanogaster olfactory receptor expressed in Sf9 cells. J Neurosci Methods. 2007;159:189–194. doi: 10.1016/j.jneumeth.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 51 ••.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. Based on electrophysiological and biochemical analysis on the insect ORs expressed in various heterologous expression systems, the authors reveal that the insect OR/OR83b complex itself produces odorant-evoked cation channel activity, independent of G-protein signaling. [DOI] [PubMed] [Google Scholar]
- 52 ••.Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. By electrophysiological recordings, the authors show that G-protein mediated cAMP is generated in response to odorants. Moreover, they also reveal that OR83b alone functions as a cyclic nucleotide gated channel. [DOI] [PubMed] [Google Scholar]
- 53.Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Front Cell Neurosci. 2009;3:1–17. doi: 10.3389/neuro.03.002.2009. Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 55 ••.Laughlin JD, Ha TS, Jones DN, Smith DP. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133:1255–1265. doi: 10.1016/j.cell.2008.04.046. Structural analysis of LUSH protein shows that a dominant negative LUSH mutant can activate pheromone-sensitive neurons in the absence of cVA by mimicking the conformational change of LUSH normally induced by pheromone. This result suggests that odorant- and pheromone-binding proteins seem to be more than simple carriers, and might play an important role in odor transduction in insects. [DOI] [PMC free article] [PubMed] [Google Scholar]