Abstract
We performed an updated quantitative meta-analysis of 162 neuroimaging studies of emotion using a novel multi-level kernel-based approach, focusing on locating brain regions consistently activated in emotional tasks and their functional organization into distributed functional groups, independent of semantically defined emotion category labels (e.g., “anger,” “fear”). Such brain-based analyses are critical if our ways of labeling emotions are to be evaluated and revised based on consistency with brain data. Consistent activations were limited to specific cortical sub-regions, including multiple functional areas within medial, orbital, and inferior lateral frontal cortices. Consistent with a wealth of animal literature, multiple subcortical activations were identified, including amygdala, ventral striatum, thalamus, hypothalamus, and periaqueductal gray. We used multivariate parcellation and clustering techniques to identify groups of co-activated brain regions across studies. These analyses identified six distributed functional groups, including medial and lateral frontal groups, two posterior cortical groups, and paralimbic and core limbic/brainstem groups. These functional groups provide information on potential organization of brain regions into large-scale networks. Specific follow-up analyses focused on amygdala, periaqueductal gray (PAG), and hypothalamic (Hy) activations, and identified frontal cortical areas co-activated with these core limbic structures. While multiple areas of frontal cortex co-activated with amygdala sub-regions, a specific region of dorsomedial prefrontal cortex (dmPFC, Brodmann’s Area 9/32) was the only area co-activated with both PAG and Hy. Subsequent mediation analyses were consistent with a pathway from dmPFC through PAG to Hy. These results suggest that medial frontal areas are more closely associated with core limbic activation than their lateral counterparts, and that dmPFC may play a particularly important role in the cognitive generation of emotional states.
Introduction
In recent years, the number of neuroimaging studies of emotion has risen dramatically, providing new information on how the human brain creates emotion. At the time of writing, upwards of 200 neuroimaging studies have investigated the brain correlates of emotional processing; the general approach is to induce an affective state and then identify associated patterns of reliable signal increases in the brain. Affective states are most often categorized into one of several “discrete emotions” that correspond to English words such as “anger,” “fear,” “happiness,” and “disgust” or according to broader affective dimensions, such as hedonic valence (positive/negative), arousal (high/low), or approach/withdrawal. These studies have been summarized by several meta-analyses, which have served to localize the most consistent findings across studies and their specificity for particular affective states (e.g., Murphy et al., 2003; Phan et al., 2002; Wager et al., 2003).
One important limiting factor for individual studies and meta-analyses alike is that the brain–psychological mappings that they investigate are only as reliable as the categories they employ. Whether, and under what conditions, affective states can be grouped meaningfully into discrete categories and/or dimensions is currently debated (Barrett, 2006a). Perhaps in part because of this limitation, meta-analyses have not yielded strong evidence that human-defined categories of emotion can be consistently discerned from neuroimaging studies, and there is some inconsistency on this point across meta-analyses (for a discussion, see Barrett and Wager, 2006).
In this paper, we take a fundamentally different approach: In an updated meta-analysis of 162 neuroimaging studies of emotion (1990–2005), we use multivariate analyses to identify consistent patterns of co-activation across studies – which we herein refer to as functional groups – without reference to their particular affective labels. While our analyses are still constrained by the nature of the individual studies that largely employ these affective or emotional labels, the functional groups that emerge from our analyses are not defined based on identification with particular psychological categories. Thus, we can identify the most consistently activated areas in studies of emotion and the large-scale functional groups to which they belong, even if the mappings between psychological categories and functional groups is not a simple one. This inductive (data-driven) analytic approach can identify meaningful units of analysis at both the level of brain region and brain network that are far less limited by the construct validity of psychological categories of emotion, compared to individual studies and traditional meta-analyses. Such brain-based analyses of multistudy neuroimaging datasets may ultimately help to provide a physiological basis against which to evaluate the quality of psychological taxonomies of emotion. In specific follow-up analyses, we focus on identifying frontal–subcortical pathways, particularly those involving the amygdala, periaqueductal gray (PAG) and hypothalamus (Hy), regions critical for emotion in animal models (see Table 1 for a list of abbreviations of all brain region names).
Table 1.
Abbreviations
| Localization prefixes/suffixes | |
| v | ventral |
| a | anterior |
| p | posterior |
| d | dorsal |
| r | rostra |
| s | superior |
| fr | frontal |
| sg | subgenual |
| pg | pregenual |
| lat/l | lateral |
| med/m | medial |
| BA | brodmann’s area |
| Basal telencephalon | |
| Amy | amygdala |
| HCMP | hippocampus |
| Hy | hypothalamus |
| BF | basal forebrain (cholinergic) |
| Basal ganglia | |
| Cau | caudate |
| Str | striatum (Cau/Put) |
| GP | globus pallidus |
| NAC | nucleus accumbens |
| Cerebellum | |
| CB | cerebellum |
| Brainstem | |
| Thalamus | |
| Thal | thalamus |
| MD | mediodorsal nucleus |
| CM | centromedian nucleus |
| Midbrain | |
| Midb | midbrain |
| PAG | periaqueductal gray |
| VTA | ventral tegmental area |
| SN | substia nigra |
| Cortical regions | |
| Temporal | |
| TP | temporal pole |
| TC | temporal cortex |
| STS | superior temporal sulcus |
| MTL | medial temporal lobe |
| Orbital/Insular | |
| Ins | insula |
| OFC | orbitofrontal cortex |
| frOP | frontal operculum |
| Medial wall | |
| ACC | anterior cingulate cortex |
| PFC | prefrontal cortex |
| preSMA | pre-supplementary motor area |
| PCC | posterior cingulate cortex |
| Lateral frontal | |
| IFG | inferior frontal gyrus |
| Occipital | |
| OCC | occipital cortex |
| V1 | primary visual cortex |
Note. Abbreviations for brain regions, organized by anatomical structure.
The need for data-driven, brain-based analyses
It has long been recognized that emotion is a complex organism-level response to a situation or stimulus. This makes defining emotion difficult, with the result that there is no single, agreed upon definition of an emotional response (e.g., Ekman and Davidson, 1994). Furthermore, many models of emotion are grounded in particular kinds of experiences (either one's own feelings or the experience of seeing someone else's behavior as emotional (for a review, see Barrett et al., 2007b). For example, the “basic emotion” view (e.g., Ekman, 1992; Izard, 1993; Panksepp, 1998) contends that there are several discrete, biologically bounded categories emotion, and these correspond to the folk categories of fear, anger, happiness, disgust, and fear. “Discrete emotion” approaches assume that instances of emotion also correspond to these categories but derive from a componential meaning analysis of the situation (for a review, see Ellsworth and Scherer, 2003). Some dimensional models of emotion tend to emphasize broad commonalities across emotional states, such as positive and negative affect (e.g., Cacioppo et al., 1999; Watson and Tellegen, 1985) or valence and arousal (Russell, 2003; Russell and Barrett, 1999). Neuroimaging studies of emotion, including meta-analyses, have largely focused on finding neural correlates of basic emotion categories or dimensions (e.g. “anger” or “positive affect”). Therefore, all brain maps and emerging functional groups are discussed and interpreted in light of these constructs and ultimately depend on them; the consistency and specificity of the brain–psychology mapping can only be as good as these constructs are.
Importantly, however, there is no guarantee that what intuitively seem like basic categories of emotion at the psychological level will actually turn out to be basic categories of emotion in the brain. In fact, there is substantial evidence to suggest they are not. Researchers have searched extensively for unique “signatures” for anger, sadness, fear, and so on, in patterns of peripheral physiological responses (patterns of heart rate, blood pressure, skin conductance, facial expression, etc.), but have largely failed to find consistent, co-occurring sets of physiological features that discriminate these categories (Barrett, 2006a). While positive and negative affect are easier to discriminate physiologically (Cacioppo et al., 2000), it is still unclear whether these boundaries are respected by the brain, in the sense that it is unlikely that there are dedicated brain circuits or neurotransmitter systems for only positive or only negative affect. For example, midbrain dopamine (DA) was once thought to be a marker for hedonic pleasure, but a growing literature suggests roles in goal-directed behavior and motivated learning rather than hedonic experience per se. for example, DA can be released in response to both positive (reward), negative (tail shock), or simply salient events (Horvitz, 2000); and dopaminergic enhancement increases drive to pursue and consume without apparently increasing subjective pleasure (Berridge, 2007). The amygdala has been considered by some groups as a marker for fear, but damage to the amygdala does not necessarily change the subjective experience of fear (Phelps and Anderson, 1997), and amygdala activity plays a prominent role in positive expectation (Paton et al., 2006), reward (Baxter and Murray, 2002; Everitt et al., 1999; Holland and Gallagher, 2004) and novelty (Wright et al., 2006). These are just a few examples, but the larger point is that while continuing to search for brain correlates of defined emotional categories is useful and important, a complementary strategy of data-driven analyses of emotion-related brain activity – temporarily ignoring the psychological labels given to these emotional states – might produce new insights into the structure of emotion in the brain. Therefore, in the present meta-analysis we opted for this complementary strategy. While we selected for inclusion in the metaanalysis those studies whose associated affect mapped onto what is typically considered emotion in psychological models of specific emotion categories or dimensions, our analyses are data-driven insofar as they ignore those labels and treat all reported peaks as equal.
A constructivist view: Emotion as combinations of basic brain-psychological processes
This strategy is consistent with a psychological constructionist approach to emotion, where the assumption is that the mental states called “anger,” “sadness,” and “fear” result from the interplay of more basic psychological processes that may not, themselves, be specific to emotion, but may combine in various ways (recipes, if you will) to produce varied emotional and affective states (e.g., Barrett, 2006b, 2007c; Russell, 2003). Just what these more basic psychologist processes are, however, are a matter for scientific discovery. Using a meta-analytic data-driven approach, we can inductively discover which processes exist by identifying functional groups that are active during the psychological states called “anger” or “perceiving anger.” Further, the data-driven approach may allow us to explore alternative ways of classifying emotions and eventually even develop a new typology, one based on the relative involvement of various psychological processes (and their corresponding functional groups), each reflected by activity in a distributed functional brain group. One group may be related to physiological regulation, another to action generation and inhibition, a third to meaning analysis (and consequently retrieval and processing of memories appropriate for the context), a fourth to perceptual processing relevant to the emotioneliciting situation, and so on. In such a typology, the category now called “fear” may be decomposed into distinctly different scientifically meaningful categories. In one kind of “fear,” a single stimulus signals potential threat, which produces alerting, orienting to the visual and auditory environments in a threat- and species-specific manner, brief heart-rate deceleration followed by increased blood flow to the limbs, and retrieval from memory of potential sources of threat in the situation. Another kind of “fear” may be elicited by complex (rather than simple) cues about long-term status and result in increased attention to the self and body state, withdrawal of blood from the limbs, and autobiographical memory retrieval. The differential involvement of several brain groups may differentiate these states; however, as of yet, no data-driven, inductive identification of affective functional brain groups exists to provide a basis for such a typology. Identifying relevant brain regions and functional groups is a goal of the present paper.
The present approach: methodological overview and neuroscientific questions
Using newly developed meta-analytic techniques, we first identified the brain regions most consistently activated in studies of emotion, following which we identified canonical patterns of co-occurring activations, or “functional groups.” Co-occurrence in this context means that across studies, contrasts (e.g., anger vs. neutral) that activate one particular brain region also tend to activate other regions in the group; and while these groups may be indicative of functional networks with anatomical connectivity, such connectivity cannot be assumed. These functional groups may be coherently activated because they form a neural circuit for a particular kind of emotion category (such as “aggression”), or because they implement a set of more basic processes such as action inhibition, memory retrieval, and other elements that participate in many emotions, although to different degrees in different emotional contexts. In either case, identifying coherent patterns of co-activation is an important step towards testing these alternatives.
Below, we describe the methodological framework and the particular hypotheses of interest in the meta-analysis. The analysis involved several steps. First, we analyzed the spatial density of reported peak coordinates from published studies using multilevel kernel density analysis (MKDA; (Wager et al., 2007a)), which identified brain voxels with more nearby reported peaks than would be expected by chance (see Methods). Then, these voxels were grouped according to their patterns of co-activation across studies and spatial contiguity at several spatial scales. First, singular value decomposition (SVD; a data reduction technique) was used to group voxels into contiguous nearby parcels that tended to be co-activated across studies. Then, parcels were subjected to nonmetric multidimensional scaling analysis (NMDS) and hierarchical clustering that grouped the parcels into co-activated regions (importantly, regions are not composed only of spatially contiguous voxels and may be spatially distributed). Then, the regions were subjected to a second iteration of the NMDS and clustering analysis, which assembled them into large-scale distributed functional groups, defined as such by their co-activation across studies. We characterized and visualized co-activation among regions and interpreted the patterns that emerged in terms of the functional groups to which they belong. Finally, we searched for frontal-amygdala interactions, and conducted mediation analyses to identify functional pathways connecting frontal cortex, brainstem, and hypothalamic regions that are involved in emotional processing.
Our neuroscientific hypotheses focus on several questions that have not been addressed in previous meta-analyses of emotion: in particular, on a) the consistency of activation in regions heavily implicated in animal studies of affective behavior, particularly the PAG and Hy; and b) the relationships between activation of frontal cortical regions and activation of Amygdala, PAG, and Hy. Specifically, we addressed the following questions:
Many human imaging studies of emotion activate the cortex. Which regions are most consistently activated, and can they be separated into distinct cortical functional groups?
Which cortical regions, if any, are associated with amygdala activation?
Do human imaging studies (like animal studies) reliably activate PAG and Hy, and which cortical regions, if any, are associated with PAG and Hy activation? (These regions are likely to be particularly important for cognitive appraisals with physiological consequences).
There is a pressing need to evaluate the consistency of activations in PAG and Hy and nearby regions in the basal forebrain and ventral striatum because they provide a crucial link between animal and human studies. Many animal studies focus on affective behavior and physiology, and PAG and Hy are principal regulators of affect-induced physiological responses. In such studies, stimulation of different longitudinal columns of PAG elicits distinct coordinated, organism-wide responses that are often labeled as “emotional” and involve coordinated autonomic responses that include changes in heart rate, blood pressure, peripheral blood flow, pupillary responses, piloerection, and other physiological effects (Bandler and Shipley, 1994; Behbehani, 1995; Carrive et al., 1989; Gregg and Siegel, 2001; Holstege and Georgiadis, 2004; Keay and Bandler, 2002; Kim et al., 1993; Lovick, 1992; Schenberg et al., 2001; Van der Horst and Holstege, 1998). These effects have earned PAG a central role in some conceptions of emotion (Panksepp, 1998). The Hy provides the primary brain control over the endocrine system through interactions with the pituitary. It plays a major role in the regulation of motivated behavior and homeostatic processes (Sewards and Sewards, 2003; Valenstein et al., 1970), regulates the release of cortisol and other hormones into the bloodstream with diverse effects (Tsigos and Chrousos, 2002), and interacts with the autonomic nervous system through large reciprocal connections with the PAG and other brainstem nuclei (Saper et al., 1976). Based on these and many other findings, the Hy plays a central role in brain responses to threat and stress and regulation of the body. Therefore, the studies that activate PAG and Hy are likely to be those that elicit the strongest physiological effects – with corresponding sequelae for physical health – and, perhaps, the strongest subjective emotional experiences.
Methods
Study selection
To collect as many published reports as possible we employed a three-pronged approach. First, we searched peer-reviewed journals (as indexed in large databases such as MEDLINE) for English-language manuscripts of neuroimaging studies of emotion induction or emotion experience published between January 1990 and December 2005. Second, we mined the articles found by the first method for other studies by searching through their reference sections. Third, we searched publications by known emotion researchers for additional reports. Published reports were included if they met the following criteria: (1) They involved unmedicated healthy adults; (2) They measured regional cerebral blood flow (e.g., O15H2O-PET) or blood oxygenation (e.g., BOLD-fMRI) across the entire brain (i.e., excluding studies that focused on limited regions of the brain); (3) They used the image subtraction methodology to determine activation foci; (4) They provided standard Talairach (Talairach and Tournoux, 1988) or Montreal Neurologic Institute (MNI) coordinates, allowing for comparison of findings across different studies and different laboratories. Published report were excluded if (1) induction method included fear conditioning, pain, or pain conditioning; (2) comparisons assessed learning, explicit memory, or evaluative priming; (3) the nature of the affective states were unclear such as romantic love, sexual desire, hunger, thirst, reward or emotional states labeled as more than one category; and (4) comparisons were not specific to emotion (e.g. self vs. other contrasts with emotional stimuli).
We included findings from a total of 162 neuroimaging studies (57 PET and 105 fMRI), yielding 437 contrasts (summarized in Table 2). For each study, foci of activation for these contrasts were included when reported as significant by the criteria designated in the individual studies. Relative decreases in activation in emotion-related tasks were not analyzed.1 Finally, for each paper, the type of analysis was coded as “fixed” or “random effects” by two different coders from a team of four trained raters. Raters were in complete agreement.
Table 2.
Studies
| First author | Year | Modality | N | Analysis | Contrasts | First author | Year | Modality | N | Analysis | Contrasts |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Schafer | 2005 | fMRI | 40 | Fixed | 8 | Nomura | 2004 | fMRI | 9 | Fixed | 4 |
| Grimm | 2005 | fMRI | 29 | Random | 4 | O’Doherty (b) | 2001 | fMRI | 9 | Random | 2 |
| Das | 2005 | fMRI | 28 | Random | 1 | Small | 2003 | fMRI | 9 | Random | 7 |
| Hutcherson | 2005 | fMRI | 28 | Random | 4 | Tabert | 2001 | fMRI | 9 | Random | 2 |
| Tessitore | 2005 | fMRI | 27 | Random | 1 | Dolan | 1996 | fMRI | 8 | Fixed | 1 |
| Cato | 2004 | fMRI | 26 | Fixed | 2 | Narumoto | 2000 | fMRI | 8 | Fixed | 4 |
| Habel | 2005 | fMRI | 26 | Random | 4 | Phillips | 2004 | fMRI | 8 | Random | 8 |
| Liddell | 2005 | fMRI | 25 | Random | 2 | Phillips (b) | 1998 | fMRI | 8 | Random | 2 |
| Fischer | 2004 | fMRI | 24 | Random | 1 | Whalen | 1998 | fMRI | 8 | Random | 2 |
| Williams_L | 2004 | fMRI | 22 | Fixed | 1 | Whalen | 2001 | fMRI | 8 | Fixed | 5 |
| KeslerWest | 2001 | fMRI | 21 | Fixed | 4 | Wright | 2004 | fMRI | 8 | Fixed | 3 |
| Pessoa | 2002 | fMRI | 21 | Fixed | 5 | Beauregard | 1998 | fMRI | 7 | Fixed | 1 |
| Eugene | 2003 | fMRI | 20 | Random | 4 | Moll | 2002 | fMRI | 7 | Fixed | 1 |
| Fitzgerald | 2005 | fMRI | 20 | Random | 9 | O’Doherty (a) | 2001 | fMRI | 7 | Random | 2 |
| Grobras | 2005 | fMRI | 20 | Random | 1 | Phan | 2004 | fMRI | 7 | Fixed | 1 |
| Kuchinke | 2005 | fMRI | 20 | Random | 4 | Phillips | 1997 | fMRI | 7 | Random | 6 |
| Lang | 1998 | fMRI | 20 | Random | 4 | Bystritsky | 2001 | fMRI | 6 | Random | 1 |
| Levesque | 2003 | fMRI | 20 | Random | 1 | Herpetz | 2001 | fMRI | 6 | Fixed | 1 |
| Schroeder | 2004 | fMRI | 20 | Random | 3 | Nitschke | 2004 | fMRI | 6 | Fixed | 1 |
| Stark | 2003 | fMRI | 19 | Random | 2 | Phillips (a) | 1998 | fMRI | 6 | Random | 8 |
| Aron | 2005 | fMRI | 17 | Random | 1 | Sprengelmeyer | 1998 | fMRI | 6 | Fixed | 3 |
| Crosson | 1999 | fMRI | 17 | Fixed | 1 | Teasdale | 1999 | fMRI | 6 | Fixed | 3 |
| Simpson | 2000 | fMRI | 17 | Random | 1 | Francis | 1999 | fMRI | 4 | Random | 3 |
| Anderson | 2003 | fMRI | 16 | Random | 2 | Lorberbaum | 1999 | fMRI | 4 | Fixed | 1 |
| Dolcos | 2004 | fMRI | 16 | Random | 2 | Damasio | 2000 | PET | 25* | Fixed | 6 |
| Hariri | 2000 | fMRI | 16 | Fixed | 4 | George | 1994 | PET | 21 | Fixed | 1 |
| Somerville | 2004 | fMRI | 16 | Random | 2 | Paradiso | 1999 | PET | 17 | Fixed | 2 |
| Fecteau | 2005 | fMRI | 15 | Random | 1 | Paradiso | 2003 | PET | 17 | Fixed | 4 |
| Gottfried | 2002 | fMRI | 15 | Random | 5 | Kimbrell | 1999 | PET | 16 | Fixed | 2 |
| Grandjean | 2005 | fMRI | 15 | Random | 1 | Pietrini | 2000 | PET | 15 | Random | 2 |
| Reinders | 2005 | fMRI | 15 | Random | 1 | Taylor | 2000 | PET | 14 | Fixed | 8 |
| Stark | 2005 | fMRI | 15 | Random | 6 | Blair | 1999 | PET | 13 | Fixed | 4 |
| Canli | 1998 | fMRI | 14 | Fixed | 2 | George | 1996 | PET | 13 | Fixed | 2 |
| Goel | 2001 | fMRI | 14 | Random | 1 | Kilts | 2003 | PET | 13 | Random | 4 |
| Gur | 2002 | fMRI | 14 | Random | 1 | Lane | 1998 | PET | 12 | Random | 2 |
| Wicker | 2003 | fMRI | 14 | Random | 7 | Lane (a) | 1997 | PET | 12 | Fixed | 3 |
| Fulbright | 1998 | fMRI | 13 | Fixed | 2 | Partiot | 1995 | PET | 12 | Fixed | 1 |
| Goldin | 2005 | fMRI | 13 | Random | 4 | Reiman | 1997 | PET | 12 | Fixed | 2 |
| Heinzel | 2005 | fMRI | 13 | Random | 1 | Royet | 2000 | PET | 12 | Fixed | 2 |
| Markowitch | 2003 | fMRI | 13 | Random | 4 | Royet | 2001 | PET | 12 | Fixed | 2 |
| Moll | 2005 | fMRI | 13 | Fixed | 1 | Zald | 1997 | PET | 12 | Fixed | 3 |
| Northoff | 2004 | fMRI | 13 | Random | 5 | Zatorre | 2000 | PET | 12 | Fixed | 1 |
| Shin | 2005 | fMRI | 13 | Fixed | 2 | Aalto | 2002 | PET | 11 | Random | 4 |
| Shirao | 2005 | fMRI | 13 | Random | 1 | Aalto | 2005 | PET | 11 | Random | 2 |
| Williams_L | 2005 | fMRI | 13 | Random | 6 | Frey | 2000 | PET | 11 | Random | 2 |
| Williams_M | 2005 | fMRI | 13 | Random | 1 | Gemar | 1996 | PET | 11 | Fixed | 2 |
| Dolan | 2001 | fMRI | 12 | Fixed | 3 | George | 1995 | PET | 11 | Fixed | 1 |
| Elliott | 2000 | fMRI | 12 | Random | 2 | Lane (c) | 1997 | PET | 11 | Fixed | 6 |
| Fitzgerald | 2004 | fMRI | 12 | Random | 1 | Baker | 1997 | PET | 10 | Fixed | 2 |
| Hariri | 2002 | fMRI | 12 | Random | 5 | Beauregard | 1997 | PET | 10 | Fixed | 1 |
| Iidaka | 2001 | fMRI | 12 | Random | 4 | Blood | 1999 | PET | 10 | Fixed | 2 |
| Killgore | 2004 | fMRI | 12 | Random | 7 | Blood | 2001 | PET | 10 | Random | 1 |
| Maratos | 2001 | fMRI | 12 | Random | 5 | Dolan | 2000 | PET | 10 | Fixed | 1 |
| Schienle | 2002 | fMRI | 12 | Random | 4 | George | 1996 | PET | 10 | Fixed | 6 |
| Schienle | 2006 | fMRI | 12 | Random | 4 | Lane (b) | 1997 | PET | 10 | Fixed | 1 |
| Strange | 2000 | fMRI | 12 | Random | 1 | Liberzon | 2000 | PET | 10 | Fixed | 3 |
| Vuilleumier | 2001 | fMRI | 12 | Random | 2 | Liberzon | 2003 | PET | 10 | Random | 5 |
| Wang | 2005 | fMRI | 12 | Random | 1 | Taylor | 2003 | PET | 10 | Fixed | 6 |
| Adams | 2003 | fMRI | 11 | Random | 1 | George | 1993 | PET | 9 | Fixed | 2 |
| DeAraujo | 2003 | fMRI | 11 | Random | 3 | Redoute | 2000 | PET | 9 | Fixed | 2 |
| Hariri | 2003 | fMRI | 11 | Random | 2 | Zald | 1998 | PET | 9 | Fixed | 3 |
| Rolls | 2003 | fMRI | 11 | Random | 4 | Dougherty | 1999 | PET | 8 | Fixed | 2 |
| Williams_L | 2001 | fMRI | 11 | Fixed | 1 | Liotti | 2000 | PET | 8 | Fixed | 2 |
| Beauregard | 2001 | fMRI | 10 | Random | 2 | Mayberg | 1999 | PET | 8 | Fixed | 1 |
| Breiter | 1996 | fMRI | 10 | Fixed | 2 | Ottowitz | 2004 | PET | 8 | Fixed | 1 |
| Buchanan | 2000 | fMRI | 10 | Random | 4 | Paradiso | 1997 | PET | 8 | Fixed | 5 |
| Canli | 2000 | fMRI | 10 | Random | 1 | Pourtois | 2005 | PET | 8 | Random | 3 |
| Gorno_tempini | 2001 | fMRI | 10 | Random | 2 | Rauch | 1999 | PET | 8 | Fixed | 2 |
| Hare | 2005 | fMRI | 10 | Random | 5 | Sergent | 1994 | PET | 8 | Fixed | 1 |
| Klein | 2003 | fMRI | 10 | Random | 1 | Shin | 2000 | PET | 8 | Fixed | 1 |
| Lee | 2004 | fMRI | 10 | Random | 2 | Taylor | 1998 | PET | 8 | Fixed | 2 |
| Maddock | 1997 | fMRI | 10 | Fixed | 1 | Kosslyn | 1996 | PET | 7 | Fixed | 3 |
| McCullough | 2005 | fMRI | 10 | Fixed | 2 | Nakamura | 1999 | PET | 7 | Fixed | 2 |
| Ruby | 2004 | fMRI | 10 | Random | 1 | Pardo | 1993 | PET | 7 | Fixed | 1 |
| Sato | 2004 | fMRI | 10 | Random | 1 | Fischer | 1996 | PET | 6 | Random | 1 |
| Wildgruber | 2005 | fMRI | 10 | Random | 1 | Imaizumi | 1997 | PET | 6 | Random | 1 |
| Wrase | 2003 | fMRI | 10 | Random | 4 | Isenberg | 1999 | PET | 6 | Fixed | 1 |
| Yamasaki | 2002 | fMRI | 10 | Fixed | 1 | Lane | 1999 | PET | 6 | Fixed | 3 |
| Critchley | 2000 | fMRI | 9 | Fixed | 2 | Morris | 1999 | PET | 6 | Fixed | 2 |
| Kringelbach | 2003 | fMRI | 9 | Random | 1 | Morris | 1996 | PET | 5 | Fixed | 3 |
| Lange | 2003 | fMRI | 9 | Random | 3 | Morris | 1998 | PET | 5 | Fixed | 4 |
Note. Table of studies used in the meta-analysis, with numbers of subjects (N) and numbers of contrast maps for each study. Analysis: Fixed refers to fixed-effects analysis, and random refers to random-effects analysis.
Damasio 2000 had differing numbers of subjects for each emotion, ranging from 16 for the lowest and 25 for the highest.
Data analysis: overview and rationale
The goal of this meta-analysis was to initially identify areas that were most consistently activated by emotional experience and emotion perception across the 437 contrasts in our database, and subsequently, to identify functional groups of areas that are co-activated across the brain in association with emotions. We address the first goal using Multi-level Kernel Density Analysis (MKDA), and the second goal using functional group analyses. We describe the overview and rationale for each method in turn, followed by details of each method.
Multilevel kernel density analysis: overview and rationale
As in previously published meta-analyses (Fox et al., 1998; Laird et al., 2005; Nielsen et al., 2005; Wager et al., 2003, 2004a), our method analyzes the distribution of peak coordinates from published studies across the brain. However, the present analytic strategy, which we refer to as Multi-level Kernel Density Analysis (MKDA), is unique, because it treats reported peaks as nested within contrasts, and therefore treats contrast maps (not peaks) as the unit of analysis (Wager et al., 2007a). This thereby creates two levels of analysis: within-contrast and between-contrasts. Inferences are made at the between-contrasts level, based on the number of contrast maps reporting peaks in the vicinity of each region of the brain.
The MKDA procedure has several important advantages over voxel-wise meta-analysis approaches used previously. First, other approaches have typically analyzed the peak locations from a set of studies, ignoring the nesting of peaks within contrasts. That is, they have treated reported peak coordinates as the unit of analysis, and used reported peaks as independent from one another, even when they are derived from the same contrast map. Such procedures thus treat each peak activation as a fixed effect and allow a single study to dominate the meta-analysis, especially if it reports multiple nearby peaks (as is the often the case, due to low spatial smoothness, reporting conventions, low thresholds, or choice of voxel sizes). For example, one of the papers included in this meta-analysis (Damasio et al., 2000) reported 90 peaks from 6 contrasts; thus, if many nearby peaks were reported in some brain regions, meta-analyses ignoring the study identifier could yield a significant meta-analytic result in those regions based on this study alone. The current MKDA approach, by contrast, takes account of the multilevel nature of the data; study contrast map is treated as a random effect, and no one contrast map can contribute disproportionately to the overall results, even if many nearby peaks are reported. A second advantage is that contrasts are weighted based on sample size and the quality of the statistical analysis used in the primary paper. These weights allow for larger and more rigorously performed studies to exert more influence on the meta-analytic results. Finally, a third advantage is that the meta-analytic summary statistic we use is straightforward to interpret: it is proportion of contrasts (P) activating within 10 mm of each voxel.
Another approach to would be to account for the strength of activation of each reported peak using the z-scores as weights. There are some advantages to this approach, but there are also significant disadvantages. One disadvantage is that z-scores from different studies are often not comparable because of the different analysis methods used (most importantly, fixed vs. random effects analyses). A second disadvantage is that because many statistical tests are performed in each study across thousands of voxels, and because results of small studies are inherently more variable, small studies can potentially capitalize on chance by identifying regions which happen to have low inter-subject variability in the small sample and thus report inflated z-scores. An analysis that weights only by z-score would give higher weights to these small and variable studies. A third disadvantage is that if peaks were weighted by z-scores, the meta-analytic summary statistic would no longer be transparently interpretable. Therefore, while z-score weighting would confer an important advantage in that information about the relative reliability of activation within studies would be preserved, weighting by z-scores would make information about the replicability of activation across studies (which are the unit of analysis here) more difficult to interpret.
Functional group analysis: overview and rationale
To identify functional brain regions and test whether co-activation patterns across contrasts show evidence for coherent functional groups across the whole brain, we subjected data derived from the MKDA analysis to multivariate analyses across the brain. This procedure is the meta-analytic equivalent of functional connectivity analyses in individual studies. It provides information on whether the same contrasts that showed increased activation near one voxel also showed increases near other voxels. Furthermore, this analysis allows the organization of consistently co-activated voxels into functionally similar subunits, which may be related as parts of a large-scale distributed functional group. Related procedures have been developed for use in meta-analysis by other groups (Nielsen and Hansen, 2006).
The goal of this analysis was to group voxels that were consistently activated across contrast maps from different studies, based on their functional properties (i.e. whether they are co-activated by the same set of contrast maps). Indeed, such activated voxels can be grouped at several spatial scales. We first describe the levels of analysis in brief and then provide more detail on the analysis methods.
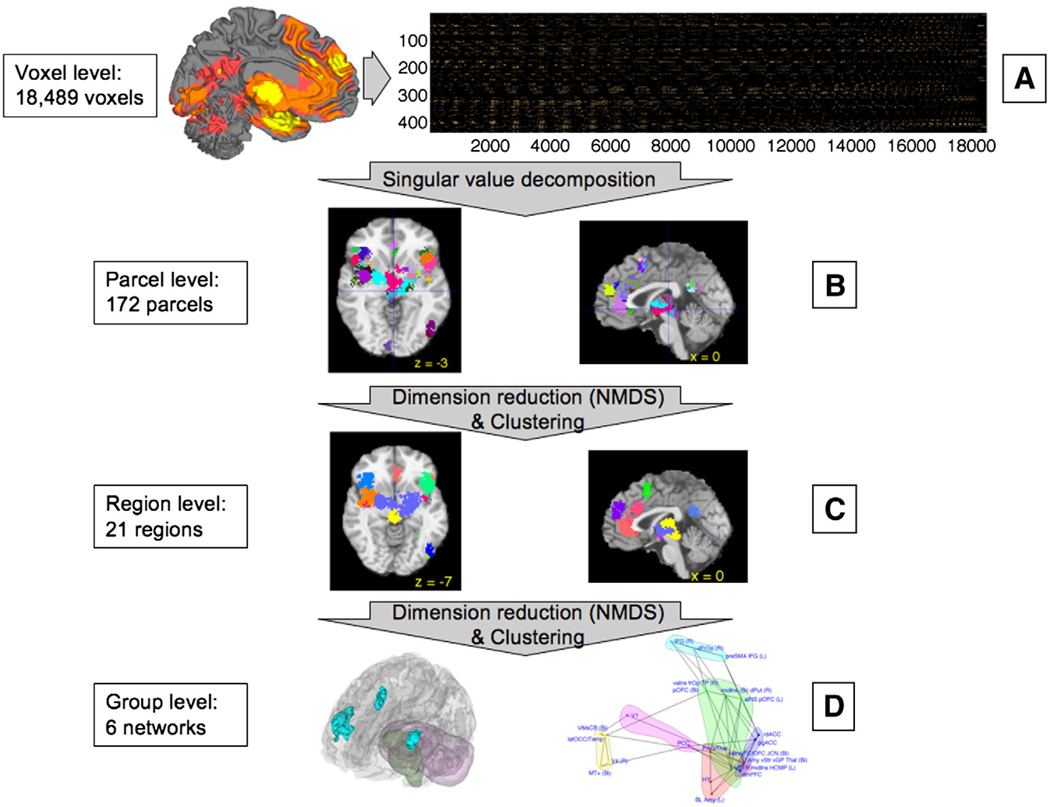
Voxel level: We identified the voxels that passed height-based or the most spatially-specific cluster-extent-based thresholds for significant consistency across studies in the MKDA analysis (discussed below). We refer to these as “suprathreshold” voxels, and these voxels were subjected to grouping based on co-activation across contrast maps in subsequent stages.
Parcel level: A parcel is a functional subregion of contiguous suprathreshold voxels that have similar patterns of activation across studies. Suprathreshold voxels were grouped into parcels using Singular Value Decomposition (SVD), as described below. 172 parcels were identified. As described in the results, we found that the parcels were typically close to the size of known functional subregions based on morphological analyses. For example, Area 24c', a specific part of rostral dorsal ACC, is a functional subregion (Vogt et al., 1992) with a corresponding identified parcel in the meta-analysis. Although parcellation is an area of current development (Flandin et al., 2002; Thirion et al., 2006), imaging studies do not typically discuss or formulate hypotheses at this level, as the spatial scale is still too small (although the potential exists with higher-resolution imaging and precise functional localizers).
Region level: Regions are functional units that encompass several parcels. We subjected the 172 parcels to Nonmetric Multidimensional Scaling (NMDS), a dimension-reduction technique, and then clustered parcels in the reduced-dimensional space into functional regions. As described below, 21 functional regions were identified, and the spatial extent of regions corresponded roughly to the size of larger functional subunits, such as pregenual anterior cingulate (pgACC), dorsal anterior cingulate (dACC), and Brodmann's Area 47. This level of description appears to be closest to the spatial specificity and replicability of functional imaging data in our database. While regions were not constrained to have contiguous voxels and some ‘regions’ are distributed across several brain areas, most regions encompassed contiguous areas of cortex, some spanning several contiguous parcels. Thus, it was necessary to perform an additional clustering analysis on the regions in order to identify large-scale functional groups.
Group level: Functional groups represent interactions among large-scale anatomical regions that are functionally related. In the neuroimaging literature, “networks” are often colloquially identified based on observation of similar patterns of activation across studies; to avoid confusion with time-series-derived networks, we adopt the term “groups” and we formalized the co-activation inference using clustering procedures. We repeated the NMDS and clustering procedure, this time using regions rather than parcels as the input data, and found evidence for significant clustering of regions into six large-scale functional groups. Examples of other distributed functional networks include a fronto-parietal attention network (Pinsk et al., 2004), a medial temporal–frontal–posterior cingulate memory network (Vincent et al., 2006), among others.
Data analysis: implementation
Identifying suprathreshold voxels: multilevel kernel density analysis (MKDA) implementation
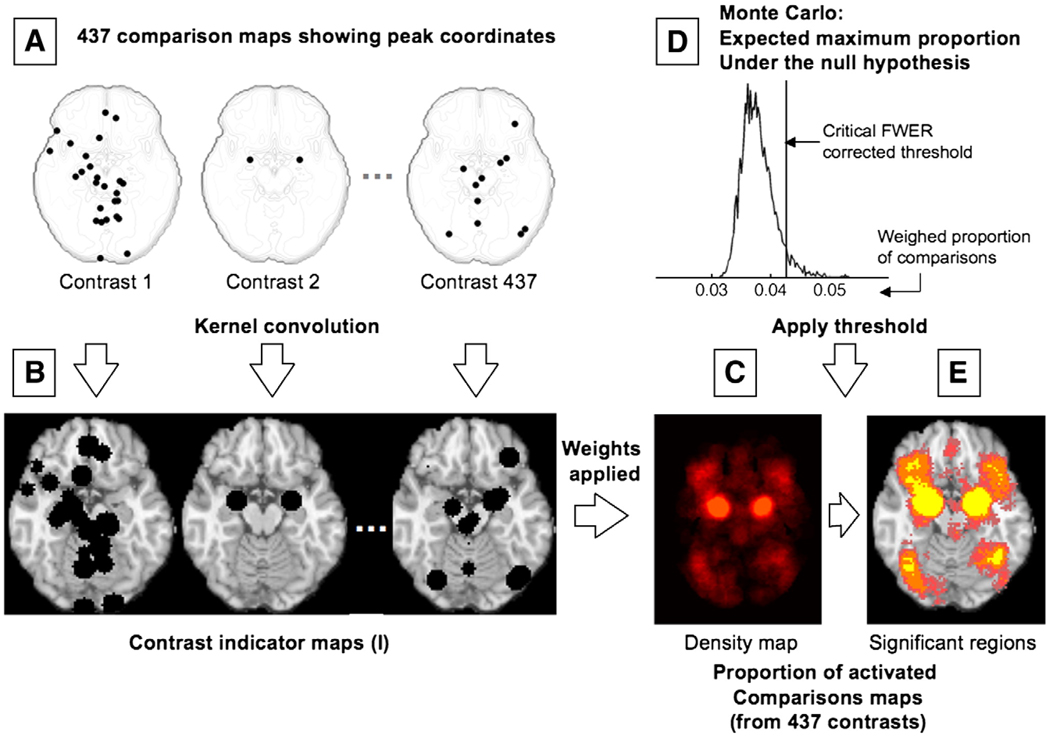
Each of the 162 studies included in our meta-analysis reported peak coordinates from one or more contrasts (statistical comparisons within a study) that map the difference between two conditions (e.g., the experience of sadness vs. neutral experience) — for a total of 437 included contrasts. For illustration, reported peak activations from three such contrasts are depicted in Fig. 1A.
Fig. 1.
Schematic representation of the procedures for multilevel kernel density analysis (MKDA). (A) Peak coordinates in three of the 437 comparison maps included in this meta-analysis. (B) Peak coordinates in each map were separately convolved with a 10 mm kernel, generating comparison indicator maps (CIMs) of values 0 or 1 (1 shown in black). (C) The weighted average of the CIMs (weights based on sample size and analysis type) is thresholded by the maximum proportion of activated comparison maps expected under the null hypothesis (shown in D) to produce significant results. (E) Significant results: yellow voxels are familywise error rate (FWER) corrected at p<.05. Other colored regions are FWER corrected for spatial extent at p<.05 with primary alpha levels of .001 (orange), and .01(pink).
In the vicinity of the axial brain slice shown in Fig. 1A (z=− 16± 10 mm), contrast 1 (left) reports 26 peaks, contrast 2 (middle) reports only 2 peaks, and contrast 437 (right) reports 11. Peaks from each contrast were transferred to a standard brain from the Montreal Neurologic Institute avg152T1.img, as distributed with SPM2 software (Wellcome Department of Imaging Neuroscience, London, UK) and were then convolved with a 10 mm spherical kernel to generate a map of “active” voxels that were within 10 mm of a reported peak for that contrast. The resulting Contrast Indicator Map (CIM, denoted as I in Eq. (1)) is thus limited to values of 0 (“this contrast did not activate near this voxel”) or 1 (“this contrast activated near this voxel”). Such indicator maps are depicted in Fig. 1B, in which such “active” voxels with a value of 1 are shown as black spheres. The procedure is described by Equation 1 below:
| (1) |
where vk is the [x, y, z] triplet in mm for voxel k's location in MNI space, and x is the [x, y, z] triplet for the nearest reported peak.
Once CIMs were constructed for every contrast, a density map across all contrasts was obtained showing the proportion of contrasts that activate near each voxel by taking a weighed average of the indicator maps. The weights for each study were the square root of the sample size (providing a measure closer to effect size and weighing large studies more heavily), multiplied by an adjustment weight (δ) for the type of analysis used for the population inference (fixed or random). Specifically, studies that used “random-effects” models appropriate for population inference were given δ=1, and those that used “fixed-effects” models, which generally produce much higher effect sizes for the same data, were given δ=0.75. Finally, the meta-analysis statistic at each voxel (P, or “density”) was the proportion (P) of contrasts that activated within 10 mm of that voxel, weighed by the quality of information provided by each study (as shown in Fig. 1C), according to the following Eq. (2):
| (2) |
where P is the weighted proportion of activated comparisons, c indexes comparison maps I, δ is the fixed-effect discounting factor, and N is the sample size that contributed to each comparison map.
Finally, to threshold the results, P was compared with a null-hypothesis density P0 established through Monte Carlo simulation. The null hypothesis was a uniform random distribution of peaks within each comparison in gray matter in the standard brain. For each CIM, we identified contiguous activation blobs of suprathreshold voxels. In each of 5000 Monte Carlo iterations, the locations of the activation blobs were selected at random within a graymatter mask (smoothed to include an 8 mm border, derived from segmentation of the avg152T1.img template using SPM2). Shape of the activation blobs was held constant (i.e., we conditioned on activation blob size and shape within each CIM). After each iteration, the maximum across-study density statistic (P) over the whole brain is saved. The critical Familywise Error Rate (FWER)-controlled threshold is the proportion that exceeds the whole-brain maximum in 95% of the Monte Carlo maps — controlling for the chance of seeing false positives anywhere in the brain at p<.05 corrected (this thresholding procedure is depicted in Fig. 1D).
Numerical stability analyses indicated that 5000 iterations were sufficient to achieve adequate stability of the Monte Carlo thresholding procedure: The maximum absolute deviation of the critical threshold from the final chosen threshold for the last 500 iterations was 4.29×10−5, or ±0.01 comparison maps. Additionally, after each Monte Carlo iteration, the largest cluster of contiguous voxels was saved, and a cluster extent threshold was set at the 95th percentile of these values across iterations, following the concept behind “cluster extent-based” multiple comparison correction implemented in SPM software (Friston et al., 1994).
Fig. 1E shows an example axial slice (z=−16 mm) of the results. In yellow-colored voxels the peak density was high enough that the null hypothesis chances of finding a single significant voxel anywhere in gray matter is p<.05, corrected for multiple comparisons as described above (FWER). Other colored areas show FWER-corrected regions based on cluster extent at two levels of spatial specificity: in orange-colored regions, a cluster of the size observed at p<.001 is unlikely to occur by chance (p<.05 FWER corrected), and in pink-colored regions, a cluster of the size observed at p<.01 is unlikely to occur by chance (p<.05 FWER corrected).
Functional group analysis: implementation
Parcellation
The goal of parcellation was to initially reduce the data from the voxel level to the parcel level and group voxels that were closely related (i.e., activated in largely the same contrasts). This step was necessary to make subsequent NMDS and clustering analyses computationally tractable. Input data was an indicator matrix of 437 comparison maps×18,489 suprathreshold voxels (defined as those voxels that survived the height threshold or the most stringent cluster threshold). Indicator values were either 0 (“this contrast did not activate within 10 mm of this voxel”) or 1 (“this contrast did activate within 10 mm of this voxel”), as shown in Fig. 2A. This 437×18,489 indicator matrix was subjected to SVD to allow reduction of the data to a smaller set of voxels whose activation profile across studies explains most of the variance in the larger set. Examination of the eigenvalues revealed an elbow at ∼50 components. Thus, we saved 50 components and assigned them index values of 1 through 50. Associations between each voxel and each component were assessed using Kendall's Tau-b, referred to here as τ (Gibbons, 1993; Gibbons et al., 2003), a nonparametric measure of association that does not require normally distributed data and thus, unlike Pearson’s r, is appropriate for indicator data. For each voxel, we computed τ with each component. Highest values of τ between a voxel and one of the components led to the assignment of the component 1–50 ID to that voxel. Consequently, sets of contiguous voxels that had the same ID value were defined as parcels–that is, parcels were made of voxels that correlated most highly with the same component, indicating co-activation. As our parcellation algorithm was a hard-clustering algorithm, each voxel was assigned to the component on which it loaded most highly, even if it was closely related to two components. While other methods such as the NMDS approach we used in subsequent steps can address issues of ambiguous groupings, this was not computationally feasible at the voxel level. Importantly, this procedure allowed us to reduce the voxel space enough to make the subsequent NMDS possible and to use the preferred measure of association, τ. At the conclusion of this stage, as illustrated in Fig. 2B, 172 parcels, ranging in size from 10 to 1060 voxels with a mean of 99.72 voxels (from 80 mm3 to 8480 mm3, with a mean of 797.76 mm3) were found across the brain. These are roughly comparable in size to known functional subregions in anatomical studies.
Fig. 2.
Schematic representation of the procedures for multivariate co-activation analysis. (A) Indicator matrix of comparison indicator map (CIM) values for suprathreshold voxels used as input — those that were found to be consistently and significantly activated in the previous MKDA analysis. Rows are contrasts, and columns are significant voxels. (B) Singular value decomposition was applied to these voxels, resulting in 172 parcels, or functional subregion of contiguous suprathreshold voxels that have similar patterns of activation across studies. (C) Parcels were subjected to dimension reduction (Nonmetric Multidimensional Scaling; NMDS) and clustering, resulting in 21 regions that were consistently co-activated across emotion studies. (D) The procedure described in (C) was repeated a second time, resulting in a best-estimate of 6 functional groups, shown in schematic view only (see Fig. 7 for high-resolution image of the functional groups). The groups describe functional relationships among large-scale anatomical regions.
Identifying regions
To further investigate whether parcels are part of larger regions or functional distributed groups (e.g. move from the parcel level to the region level), clustering could theoretically be performed on the 172 parcels identified by SVD. However, variability between parcels is likely to be accounted for by far fewer dimensions, rendering the to-be-clustered space sparse and consequently, noisy. Therefore, the goal of this portion of the analysis was to reduce the number of dimensions in the data, in order to improve the quality of clustering before we attempted to identify larger regions or distributed functional groups. Nonmetric Multidimensional Scaling (NMDS; (Kruskal, 1964; Shepard, 1962, 1980) is a dimension reduction technique similar to principal components analysis (PCA) and to independent components analysis (ICA), but that does not assume that the reduced space is metric (e.g. that distances in the reduced space are linearly related to distances in full space). Once SVD identified 172 clusters, we used NMDS to further reduce the data dimensionality. NMDS was then followed by a clustering procedure for assessing the quality and number of clusters in the component space created by the 2 These procedures are implemented in the function mdscale. NMDS. m in Matlab 7.3 (Mathworks, Natick, MA).
For this purpose, new CIMs were created for parcels, with a resulting 437 comparison maps×172 parcels CIMs that encoded whether each contrast reported an activation coordinate anywhere within each parcel. As previously described, indicator values were of either 0 (“this contrast did not activate somewhere in this parcel”) or 1 (“this contrast did activate somewhere in this parcel”). Based on these indicator values, associations were computed for each pair of parcels to create a matrix of τ values (172 parcels × 172 parcels). This matrix was then converted into a dissimilarity matrix (D) using the formula Dij=(1−τij)/2, where i and j index rows and columns of D (corresponding to a pair of parcels). Thus, complete agreement between two parcels, indicated by τ=1, yields D=0, (no dissimilarity) while complete disagreement, indicated by τ=−1, yields D=1 (maximum dissimilarity). NMDS decomposes D into a smaller set of latent components such that the distances among the parcels in the reduced component space (D̂) reproduce the original dissimilarity matrix as accurately as possible, with as few dimensions as possible. Shepard (Shepard, 1980) proposed that when the observed dissimilarities are plotted against the model-implied distances, a non-linear relationship indicates that the metric model is inadequate. As illustrated in Fig. 3A the Shepard plot for this dimension reduction process indicates that a nonmetric MDS captures the dissimilarities better than would a linear decomposition. Therefore, to avoid the constraint that D and D̂ be related linearly, which implies that the underlying distances are metric, we used NMDS, which requires only that D and D̂ be monotonically related.
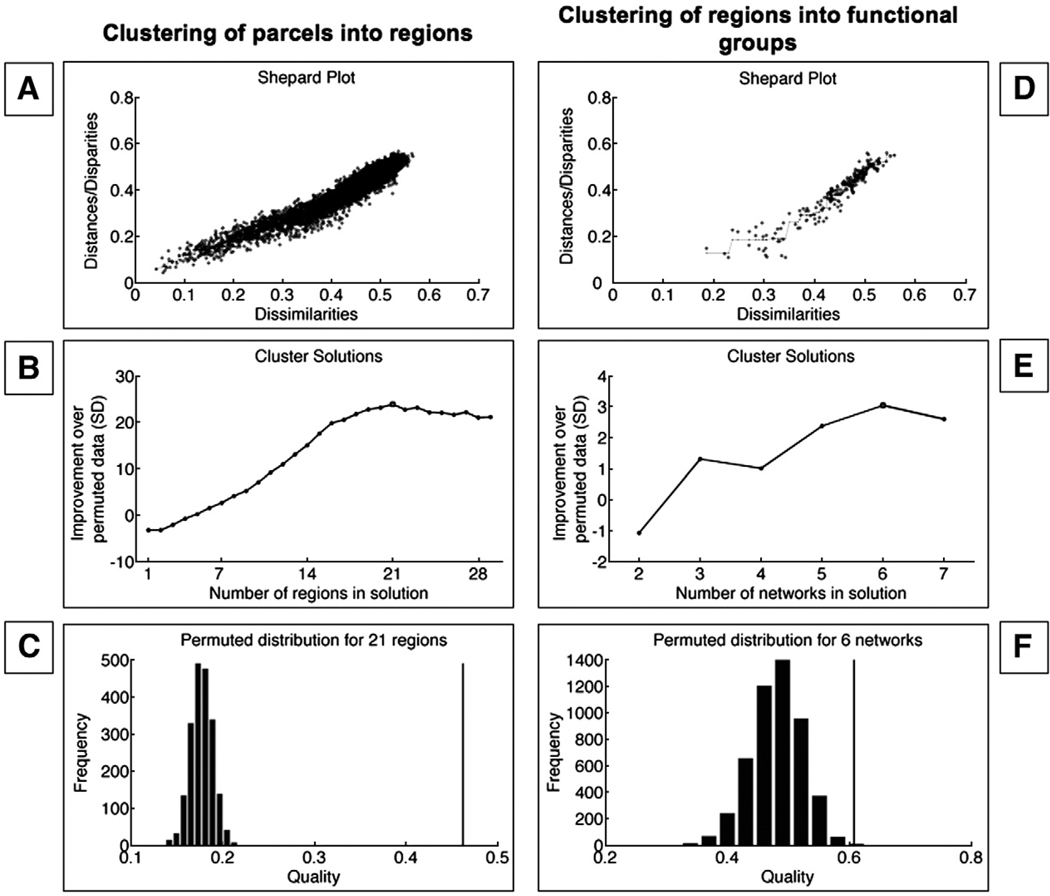
Fig. 3.
Plots showing results of the NMDS and clustering process. (A) Plot of observed dissimilarities between the parcels (in the process of clustering parcels into regions), against the model-implied distance. A non-linear relationship indicates that the metric model is inadequate. The nonlinear relationship suggests that NMDS is a more appropriate procedure than linear methods in this case. (B) Z-scores of clustering quality relative to the clustering quality of permuted data (y-axis) plotted as a function of number of clusters in the solution (x-axis). A 21-cluster solution was associated with the greatest improvement over permuted data, and was therefore chosen as the best estimate. (C) Cluster quality for observed data (vertical line) compared with a histogram of clustering quality for permuted data for the 21-cluster solution. Figs. 3D–F show similar plots for the second iteration of the algorithm, clustering regions into large-scale groups. (D) NMDS is indicated by the non-linear relationship. (E) A six-cluster solution was associated with the highest improvement over permuted data, and was therefore chosen. (F) The quality of the selected six-cluster solution (compared with permuted solutions) is indicated by the vertical line.
We use the standard error metric of stress in the decomposition (Kruskal, 1964), minimized on the best-fitting monotonic function of D according to the following formula:
where f(Dij) indicates predicted distance values between i and j according to the best-fitting monotonically increasing function. In this analysis, 20 dimension solutions were tested, and a 16-dimensional space accurately reproduced the data (stress values <.05), and captured most of the variance.
Once 16 dimensions of variance were selected using NMDS, component scores were recorded for each parcel in a matrix (172 parcels × 16 dimensions). Clustering algorithms are particularly well suited to finding sets of regions because they are designed to identify classes of nearby objects (e.g. brain regions) in a multi-dimensional space (rather than on a single component). Here, we used hierarchical clustering to identify sets of regions (implemented in clusterdata.m in Matlab 7.3). The NMDS component scores were used as estimated coordinates in the 16-dimensional space, and regions were grouped based on distances in this space. We then used a permutation test to choose the optimal number of clusters (i.e., regions). For each possible solution between 2 and 30 clusters, we first computed a measure of clustering quality. In a high quality solution, similarity will be high among members of the group, and low among members of different groups. Clustering quality is defined in:
where D denotes distance, Di0 is the distance from parcel i to the center of its own set, Dinn is the distance to the nearest neighboring set, and k indexes over parcels (Struyf et al., 1996). Once clustering quality q for a solution was established, we then permuted the columns of the component scores, re-applied the clustering algorithm, and calculated cluster quality q based on the permuted data. This permutation procedure disrupts relationships between parcels by exchanging their locations in each dimension with those of other parcels, while preserving the marginal distribution of scores in each dimension. This process was repeated 5000 times to assess average cluster quality for solutions of that size, and ultimately, to develop a null-hypothesis distribution of q for each of 2–30 possible cluster solutions.
Fig. 3B shows the number of regions in the possible solutions on the x-axis, plotted against the improvement over permuted solutions on the y-axis; a 21-region solution gives the maximum improvement over the permuted data. The permuted-data distribution of quality is shown in Fig. 3C for the 21-region solution. Quality for the observed-data solution is shown by the vertical black line. Because the 21 clusters of parcels found by this method were predominantly contiguous in space (a minority was distributed, often homologous), we refer to them as regions rather than groups, as illustrated in Fig. 2C.
Identifying groups
To investigate whether co-activation patterns across contrasts culminated in coherent functional groups across the whole brain (and to move from the region level to the group level), we computed new indicator maps for each of these 21 regions, culminating in a 437 comparison maps×21 regions indicator matrix. The indicator maps encoded whether each contrast reported an activation coordinate anywhere within each region. We then repeated the dimension reduction and clustering steps as described above. These NMDS procedures (Shepard plot in Fig. 3D) yielded 16 dimensions, and the subsequent clustering resulted in six functional groups, as illustrated in Fig. 2D and Fig. 3E–F.
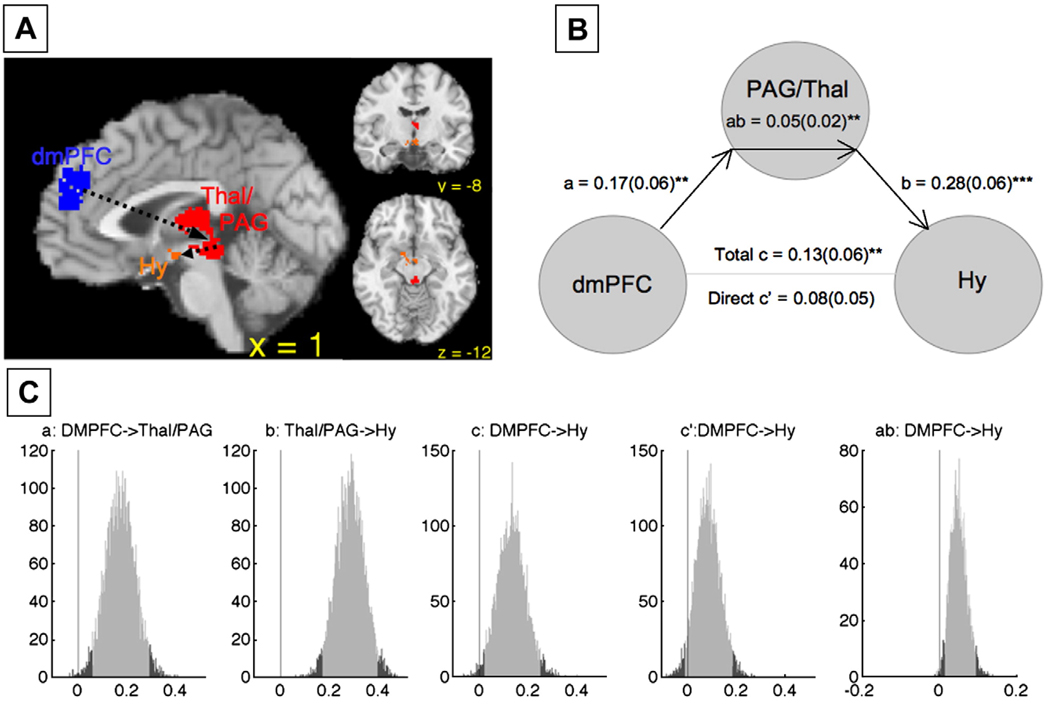
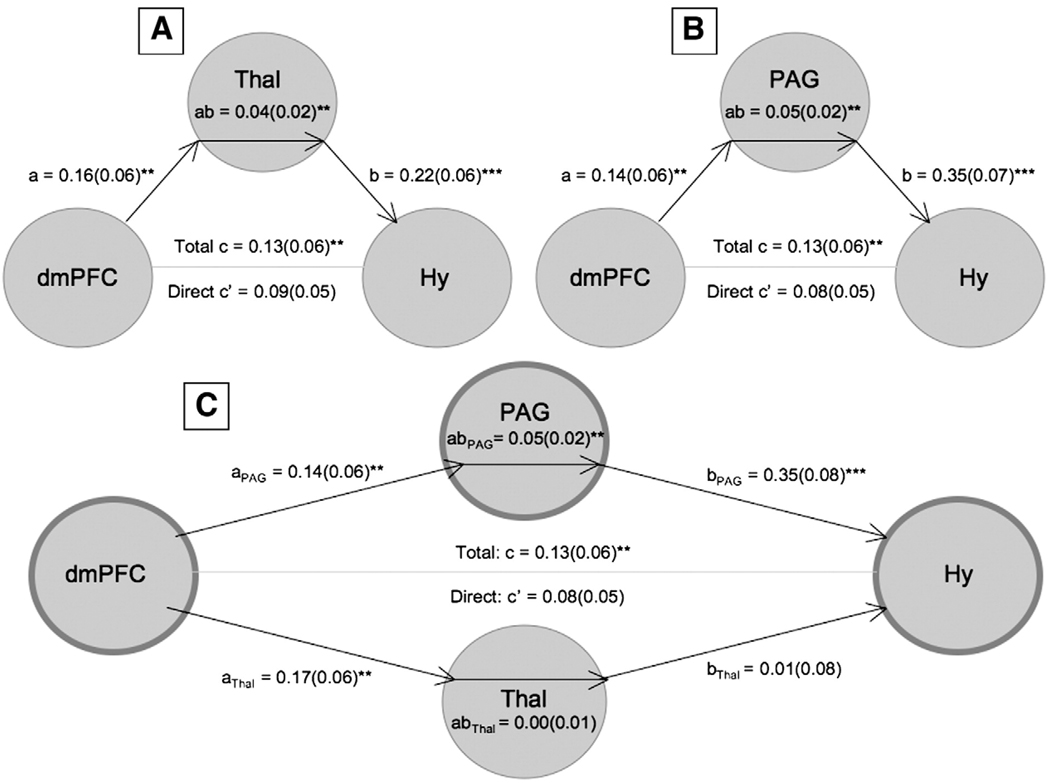
Mediation analysis
In the right panel of Fig. 2D, regions that comprise each of the six functional groups are plotted in the space of the first two of the 16 dimensions found in the second NMDS analysis (on the x- and y-axes, respectively). Points that are closer together tend to have positive co-activation, and connected lines represent significant Tau-b (τ) association values between pairs of two regions (FDR corrected). Importantly, the relationships depicted by these lines are only those that were not completely mediated by any other relationship between regions. To prune the association map in this way, we performed a mediation analysis on a region-by-region basis for each significant association.
We examined whether the relation (τ) between CIM values for any two regions (denoted as X and Y) can be explained by virtue of the values from a third region (denoted as M), using the standard logic of mediation (see Baron and Kenny, 1986). If M is a true mediator, then the relationship between CIM values for X and M must be significant, the relationship between the values for Y and M must be significant, and importantly, the relationship between values for X and Y must become insignificant when M is controlled. In a case where parameters a and b are path coefficients for the relationships between X and M and M and Y, respectively, a product a*b that is significantly different from zero indicates that M mediates the relationship between X and Y. Importantly, because this product a*b is not normally distributed, and because our measure of association is τ (as opposed to a Pearson's r), Sobel's test (Sobel, 1982) would not be appropriate here, and bias-corrected, accelerated bootstrapping (Efron and Tibshirani, 1993) was used to assess significance of mediation (Shrout and Bolger, 2002). To this end, rows in each of the indicator maps for X, Y, and M representing activations in specific contrasts were re-sampled with replacement, creating new bootstrapped-sample indicator maps of 437 activations for each of the three regions. This procedure was repeated 10,000 times; the mediation analysis (comparison of the a*b product against zero) was performed at each iteration, and the coefficients a, b, c, c', and the product a*b were saved at each iteration, to create null hypothesis distributions with 95% confidence intervals, that allowed the identification of significant τ relationships that are mediated by activation of an intervening region.
Visualization and localization
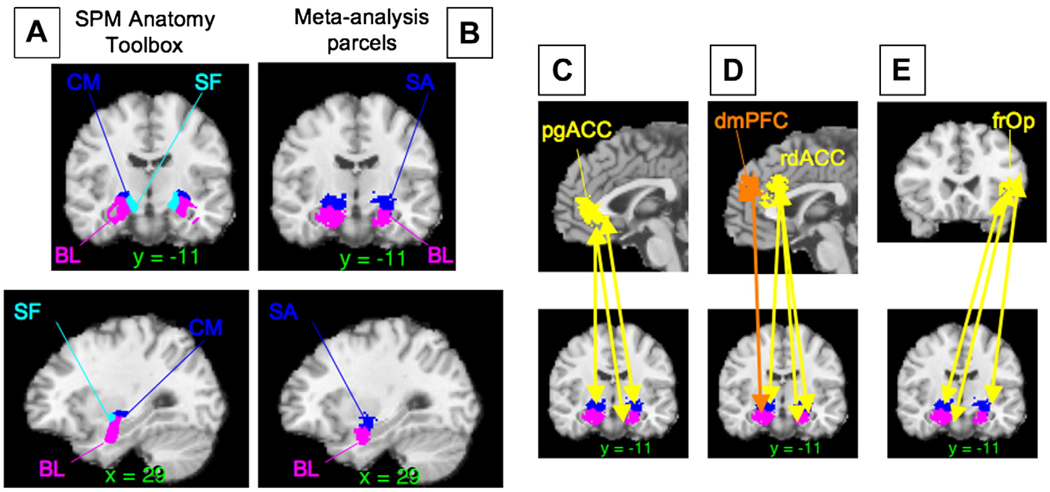
The three-dimensional renderings and figures with brain slices presented in the results section were reconstructed from a canonical MRI image (colin27.img, the single-subject template in SPM2; http://www.fil.ion.ucl.ac.uk/spm/software/spm2/). This brain was coregistered with the international standard Montreal Neurologic Institute (MNI) brain template (avg152T1.img) which is itself based on the average of 152 brains registered roughly to landmarks from the atlas of Talairach and Tourneaux (Talairach and Tournoux, 1988). To localize highly replicable regions, we overlaid significant voxels on the MNI average template and determined their locations using the atlases by Duvernoy (Duvernoy, 1995; Duvernoy and Bourgouin, 1999; Haines, 2000; Martin, 1996), work by Ongur and colleagues (Ongur et al., 2003), and Caret software (Van Essen et al., 2001); these methods provided more accurate results than automated labeling systems. We use the single-subject brain only for visualization in figures because its anatomical detail makes brain landmarks more identifiable to readers. We do not report Brodmann areas because their boundaries cannot be identified with sufficient accuracy on this template brain, unless they are in regions shown in Ongur et al., 2003, who provide labels registered to the MNI brain. While variation across labs and software packages in nominally similar warping to “Talairach space” produces inconsistencies among reported coordinates (Brett et al., 2002), the MNI template brain is the most popular template for electronic registration, so using it minimizes localization errors in the meta-analysis results.
Results and discussion
Identification of suprathreshold voxels
The results of the MKDA analysis were reported earlier in an abbreviated form (Wager et al., 2008) and are discussed and interpreted below in fuller detail in the context of the functional group analysis. Panels A–C in Fig. 4 show the (unweighted) activation peaks from all the 437 CIMs plotted on the orbital, lateral, and medial surfaces on the brain (respectively). As can be seen, activations across studies are distributed throughout the cortex; to achieve significance in the MKDA analysis, any single voxel has to be consistently activated by over 4% of the contrasts in our metaanalysis (e.g. ∼ 18 contrasts or more activating in the same location, depending on the study weights). Panels D–F in Fig. 4 show regions that were consistently activated across neuroimaging studies as determined by our analysis. Yellow regions represent peak activation foci, FWER corrected at p<.05. Other colored regions are FWER corrected for spatial extent at p<.05 with primary alpha levels of .001 (orange), and .01 (pink). Slices corresponding to key regions are shown in Fig. 5D,E. Stereotactic coordinates for the peak activation foci and the weighted percentage of activating CIMs in each contiguous corrected region (yellow in Fig. 4 and Fig. 5) are listed in Table 3. While many of these appear low in terms of absolute percentage of CIMs, there are sufficient numbers of reported activations in the same location to inspire confidence in their reliability.
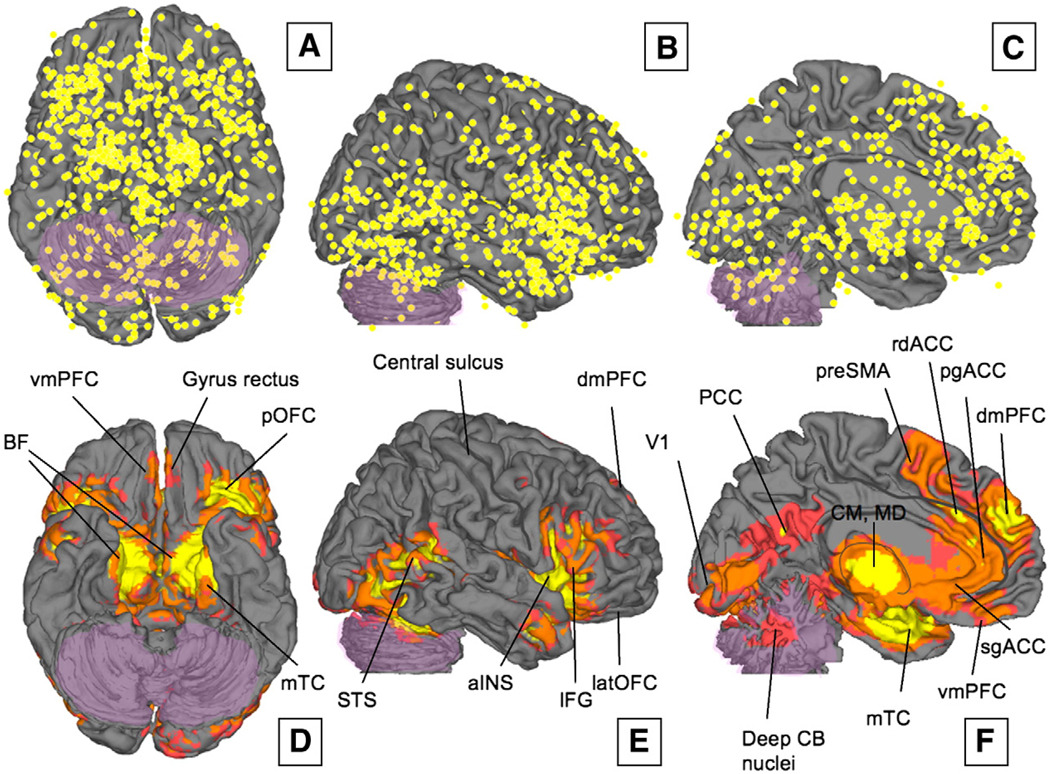
Fig. 4.
(A–C) Un-weighted peak activations from all 437 contrasts in our meta-analysis are plotted on the lateral, orbital, and medial surfaces on the brain, respectively. Activations across studies are distributed throughout the cortex, though clusters of consistent results are concentrated in some areas. (D×F) Regions that were consistently activated across neuroimaging studies as determined by multi-level kernel density analysis. To achieve significance in our analysis, any single voxel had to be activated by at least ∼4% of the contrasts in our meta-analysis (e.g. 18 contrasts or more, depending on the study weights). Yellow voxels are family-wise error rate (FWER) corrected at p<.05. Other colored regions are Family-wise Error Rate corrected for spatial extent at p<.05 with primary alpha levels of .001 (orange), and .01(pink). See Table 1 for abbreviations of brain region names.
Fig. 5.
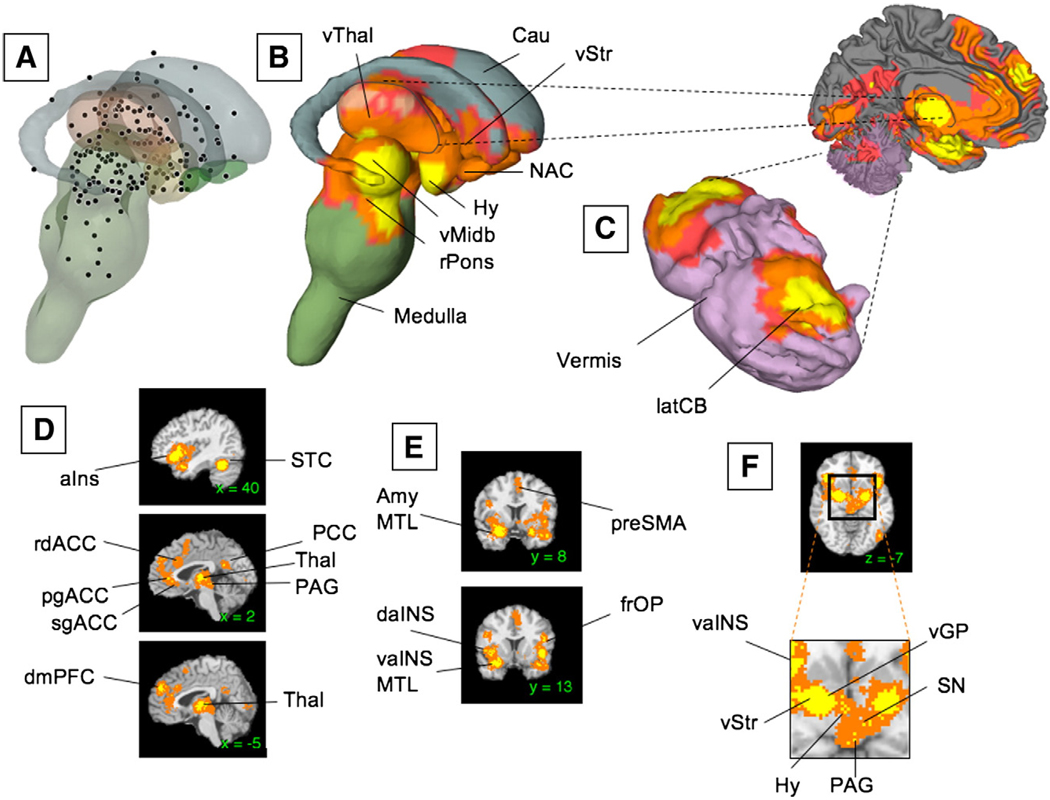
(A) Unweighted peak activations from all the 437 contrasts in our meta-analysis are plotted on the subcortical surface. (B) Regions that were consistently activated across neuroimaging studies as determined by the meta-analysis are plotted on the subcortical surface. (C) Significant activations in the Cerebellum (CB). (D–F) Cortical as well as subcortical regions of activation are shown in sagittal, coronal, and axial slices. Yellow voxels are family-wise error rate (FWER) corrected at p<.05. Other colored regions are FWER corrected for spatial extent at p<.05 with primary alpha levels of .001 (orange), and .01(pink). See Table 1 for abbreviations.
Table 3.
Peak Activation Foci
| Name | Lat | X | Y | Z | Vol | %Act |
|---|---|---|---|---|---|---|
| Orbital/Insular | ||||||
| vaIns (Ag),TP | L | −28 | 6 | −22 | 2344 | 25 |
| aIns/frOP | L | −40 | 24 | −6 | 4248 | 25 |
| aIns | L | −34 | 12 | −10 | 1160 | 19 |
| vaIns (Ag)/TP | L | −40 | 10 | −20 | 2040 | 18 |
| aIns | R | 42 | 24 | −8 | 1712 | 18 |
| vIns | R | 40 | 4 | −14 | 1040 | 17 |
| aIns | R | 44 | 16 | −2 | 2032 | 17 |
| aIns | L | −32 | 12 | 0 | 1224 | 15 |
| midIns | L | −40 | 0 | −2 | 1752 | 15 |
| OFC (47/12m,l),vaIns | R | 36 | 26 | −16 | 2160 | 14 |
| OFC (47/12l),IFG | L | −44 | 26 | −16 | 1304 | 14 |
| midIns (dors.) | R | 42 | 4 | 10 | 736 | 12 |
| frOP | L | −46 | 16 | 0 | 552 | 12 |
| midIns | R | 44 | −4 | 0 | 1576 | 9 |
| midIns | R | 40 | 6 | 0 | 48 | 7 |
| OFC (47/12m,13l) | L | −28 | 34 | −18 | 8 | 4 |
| Basal telencephalon | ||||||
| Amy/BF | L | −20 | −6 | −18 | 6800 | 30 |
| vStr/BF | L | −30 | 2 | −12 | 1832 | 26 |
| Amy/BF | R | 20 | −4 | −20 | 5744 | 25 |
| vStr/vGP | R | 26 | 0 | −10 | 1704 | 24 |
| vGP/vIns | L | −30 | −8 | −10 | 1984 | 24 |
| HCMP/vGP | R | 22 | −12 | −12 | 1944 | 21 |
| vGP/Hy/STN | L | −10 | −6 | −6 | 1104 | 19 |
| vGP | R | 14 | 6 | −8 | 1208 | 17 |
| Put | R | 26 | 2 | 0 | 1936 | 15 |
| HCMP | L | −18 | −14 | −24 | 584 | 15 |
| Amy (BL) | R | 30 | −2 | −28 | 576 | 15 |
| vStr/HCMP | R | 32 | −10 | −8 | 624 | 14 |
| Put | L | −28 | 0 | 0 | 1008 | 14 |
| vGP | L | −22 | 8 | −12 | 432 | 13 |
| Hy | L | −10 | 0 | −14 | 512 | 13 |
| Hy | R | −8 | −10 | −12 | 864 | 13 |
| Uncus | L | −12 | 0 | −26 | 440 | 13 |
| Uncus | R | 12 | −2 | −26 | 480 | 12 |
| HCMP | L | −30 | −12 | −24 | 408 | 12 |
| TP (med.) | R | 38 | 12 | −24 | 280 | 11 |
| TP (lat.) | R | 50 | 8 | −26 | 176 | 6 |
| para-HCMP | L | −28 | −24 | −18 | 200 | 5 |
| Brainstem | ||||||
| STN | R | 16 | −8 | 0 | 768 | 15 |
| Thal (DM,CM) | 0 | −16 | 4 | 1376 | 14 | |
| STN | R | 12 | −18 | −6 | 280 | 14 |
| dPAG/SC | – | 2 | −30 | −6 | 24 | 24 |
| Posterior temporal/occipital cortices | ||||||
| iTC/sCB | R | 40 | −54 | −22 | 2016 | 15 |
| TC/OCC | R | 44 | −60 | −14 | 792 | 12 |
| STG | L | −48 | −8 | −2 | 1096 | 10 |
| iTC/sCB | L | −38 | −64 | −18 | 936 | 10 |
| STS (post.) | R | 54 | −46 | 10 | 720 | 10 |
| V1 (BA 17) | L | −6 | −88 | 0 | 1120 | 9 |
| TC/OCC | R | 52 | −58 | 8 | 280 | 9 |
| OCC (lat.) | R | 48 | −70 | −2 | 128 | 9 |
| PCC | R | 6 | −60 | 18 | 176 | 6 |
| iOCC | L | −32 | −78 | −18 | 16 | 6 |
| PCC | – | 2 | 52 | 24 | 32 | 6 |
| OCC (lat.) | L | −48 | −70 | 10 | 48 | 5 |
| iTC | L | −36 | −50 | −20 | 8 | 5 |
| Medial/lateral frontal cortex | ||||||
| IFG/frOP | R | 48 | 22 | 12 | 1744 | 19 |
| MPFC (24,10m/r) | – | 2 | 32 | −4 | 1672 | 11 |
| MPFC (BA 9) | −4 | 52 | 30 | 648 | 11 | |
| IFG | L | −50 | 24 | 6 | 400 | 10 |
| pre-SMA | − | 0 | 10 | 60 | 1280 | 8 |
| pre-SMA | – | 4 | 12 | 48 | 1208 | 8 |
| dACC | – | 0 | 24 | 32 | 64 | 8 |
| pgACC | – | 2 | 40 | 2 | 168 | 8 |
| IFS | L | −46 | 10 | 24 | 48 | 6 |
| IFS | L | −48 | 22 | 18 | 24 | 5 |
| MPFC (BA 9/32) | – | 0 | 52 | 20 | 24 | 5 |
| rdACC | – | −4 | 34 | 18 | 8 | 4 |
Note. Stereotactic coordinates for the most consistent peak activation foci across all studies, with laterality (Right or Left), XYZ coordinates, number of voxels in each cluster (Vol) and the weighted percentage of Contrast Indicator Maps (CIMs) that activated in each cluster (%Act). See Table 1 for abbreviations of brain regions.
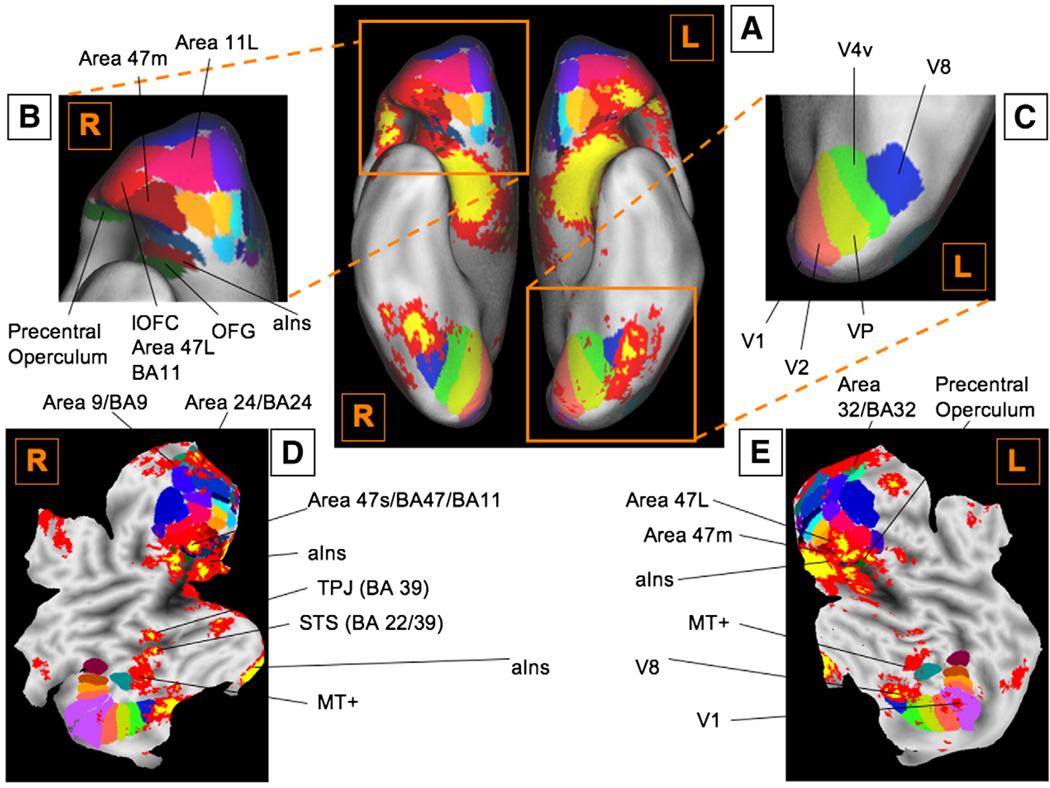
First, and importantly, significant activations were found in a subset of frontal cortical regions. While individual studies reported activations spanning the lateral surface of the cortex (Fig. 4B and Table 3), only a few activations were found to be consistent across all studies (Fig. 4E). Significant activations in inferior frontal gyrus (IFG) bilaterally extend from the pars opercularis (Broca's area, BA 44) through pars triangularis (BA 45) and pars orbitalis on the inferior frontal convexity (BA 47) and into the frontal operculum (frOP), and are contiguous with activations in posterior OFC and the anterior insula (aIns; see Fig. 4D and E, and Fig. 6 for details).
Fig. 6.
Detailed maps of orbitofrontal and visuotopic subregions. (A) Orbitofrontal and visuotopic subregions as well as significant activations are overlaid on the orbital surface. Orbitofrontal regions are as described by Ongur, Ferry, and Price (2003) as implemented in Caret software (v5.5; http://brainmap.wustl.edu/caret/). (B) An enlarged view of the orbitofrontal cortex. Consistent activations are centered Area 47 m/L, ventral anterior insula, and frontal operculum bilaterally. (C) An enlarged ventral view of visuotopic regions, as defined in Caret (D. C. Van Essen, 2004). Activations are centered in V1, ventral V2, V8, and MT+, with extension into V4v and VP. (D) A flat map of the cortical surface of the right hemisphere, overlaid by both orbitofrontal and visuotopic regions, and significant activations. (E) A flat map of the cortical surface of the left hemisphere, overlaid by both orbitofrontal and visuotopic regions, and significant activations. See Table 1 for abbreviations.
On the medial surface (Fig. 4F), activations were found in pre-SMA, dmPFC (BA 9 extending back to BA 32), and the cingulate cortex. Cingulate activations were largely limited to the rostral half of the ACC, corresponding to both the “affective” and “cognitive” zones (Bush et al., 2000; Etkin et al., 2006). Strikingly, these cingulate activations were clustered into three distinct foci, corresponding to rdACC (BA 24a/b, the so called “cognitive” zone in (Bush et al., 2000), pgACC (BA24), and sgACC (corresponding to the so-called “affective” zone; See Fig. 4F).
Activations were found in temporal, occipital, and parietal association cortices. Consistent activations were also observed in a right-lateralized area within posterior STS, and the medial and lateral anterior temporal cortex near the TPJ (Fig. 4E and Fig. 6D). Regions of medial temporal cortex bordering on vaIns and parahippocampal cortex were consistently activated as well. Occipital activations were surprisingly relatively well localized to visual areas along the ventral stream from V1 to V8, and in MT+ (see Fig. 6A and E for details). Significant activations were also found in a region in posterior cingulate (PCC). These are discussed in the context of the group analyses, below.
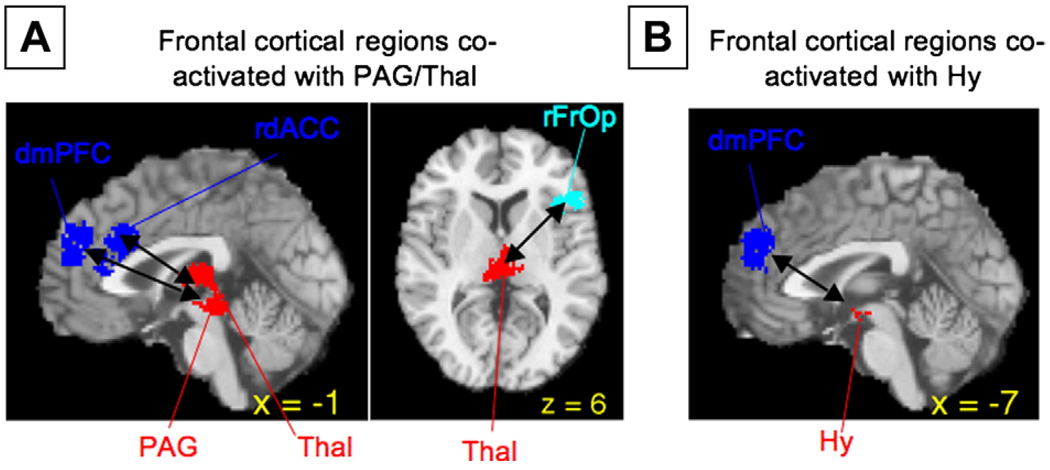
Fig. 5A shows the unweighted activations from all the 437 CIMs plotted on the subcortical surface. Fig. 5B show regions that were consistently activated across neuroimaging studies plotted on the same surface. Large regions of activation were observed in or around the amygdala (both dorsal and ventral; Fig. 5E), extending into the ventral Striatum (vStr; Fig. 5F), pallidum, nucleus accumbens, hippocampus (HCMP), and the basal forebrain (possibly encompassing sites of cholinergic nuclei). Notably, significant activations in the HCMP were found only in those areas in anterior HCMP that were contiguous to activations in the amygdala. In the brainstem, consistent activations were found in specific, mostly dorsal nuclei, which correspond to findings in animal studies. As shown in the slice in Fig. 5F, PAG and SN were consistently activated, as was the rostral pons (Fig. 5B), although lower brainstem centers in the pons and medulla were not consistently activated. Consistently activated diencephalic regions included the hypothalamus. Finally, while in many studies cerebellar activations are not reported (and therefore activations might be under-represented in this analysis), consistent activations were found in both lateral and deep nuclei of the cerebellum (CB; Fig. 5C). Taken together, these results suggest that – like animal studies – human neuroimaging studies do reliably activate subcortical regions such as the Hy or PAG.
Functional groups associated with emotion and affect
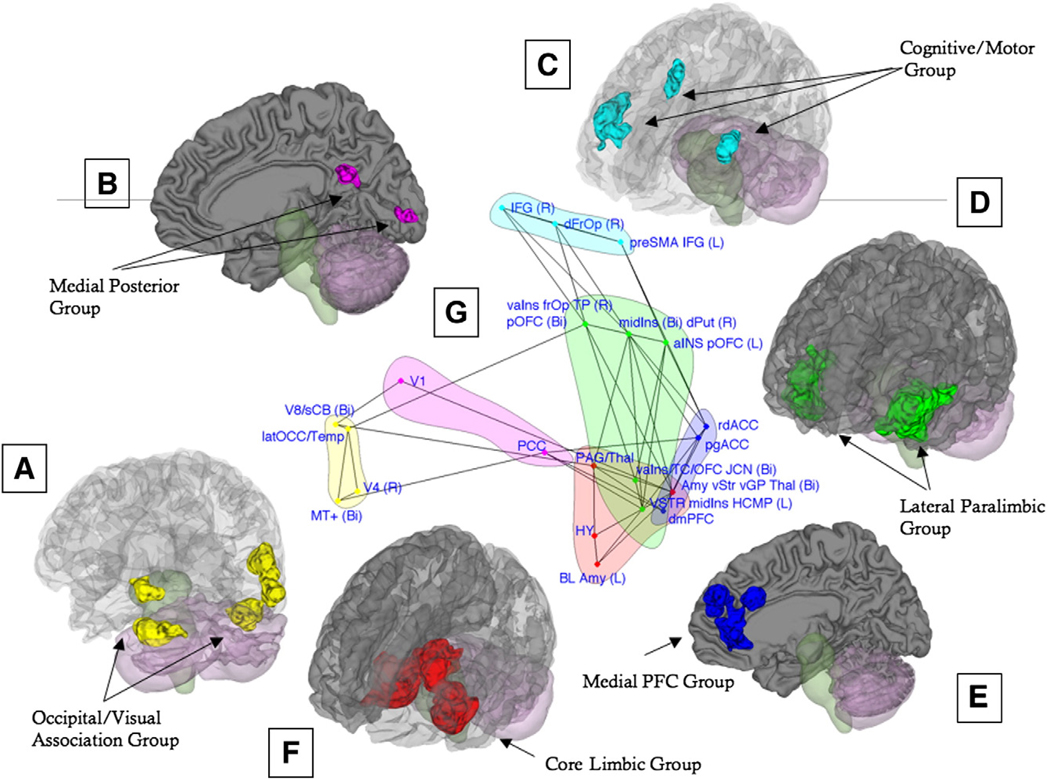
We identified six groups of regions that were consistently co-activated across studies of emotion and affect – each group is discussed below followed by a discussion of the connections between them. The functional groups are depicted in 3D rendering on the single-subject brain in Fig. 7A–F; Clockwise from the bottom left, the Lateral Occipital/Visual Association group (Fig. 7A, in yellow) includes cortical regions in the right and left lateral occipital gyrus and right occipital/temporal cortex, and CB (contiguous with inferior temporal cortex). Closely related is the Medial Posterior group (Panel B, in magenta), which includes V1 (Primary Visual cortex) and PCC. The Cognitive/Motor group (Panel C, in light blue) includes the right frOP, bilateral IFG, and the preSMA/left middle frontal gyrus. The Cognitive/Motor group connects along multiple association routes to the Lateral Paralimbic group (Panel D, in green), which includes the vStr, vpIns, daIns, vaIns/posterior orbital gyrus, and temporal pole. We also identified a Medial PFC group (Panel E, in dark blue), which includes rostral–dorsal and pregenual subsection of the anterior cingulate cortex as well as dmPFC. Both the Lateral Paralimbic group and the Medial PFC group importantly connects to the Core Limbic group (F, in red), which includes the amygdala/left HCMP, thalamus extending into PAG, additional areas of ventral striatum, and lateral Hy. This functional group includes areas typically considered to be critical for affective behavior in animals (Bandler and Shipley, 1994; Berridge, 1999; LeDoux, 2000; Panksepp, 1998) and therefore provides a crucial link between animal and human studies. Panel G shows the co-activation patterns between these functional groups (for details, see the next section and the figure legend). Table 4 lists the regions found in the MKDA analysis as well as their group affiliation.
Fig. 7.
(A–F) The six functional groups revealed by our multivariate analysis are depicted in 3D rendering on the single-subject brain. Regions in each group are rendered in a unique color. (G) To visualize the relationships among the regions in each group, both regions and co-activation lines are displayed on a “flattened” map of the connectivity space along the first two dimensions determined by NMDS (see Methods). Colors correspond to those in panels A–F and identify each network. Points closer together on the graph tend to have stronger positive co-activation, and connected lines represent significant Tau-b (τ) association values between pairs of regions. The connectivity map has been “pruned” such that the relationships depicted are direct, meaning that they were not completely mediated by any other single intervening region. Direct relationships were assessed by mediation analyses considering each possible mediating region in turn, with 1000 bootstrap samples per analysis. See Table 1 for abbreviations.
Table 4.
Regions and their Functional Group Affiliation
| Name | X | Y | Z | Vol | %Act | #Act | Functional Groups |
|---|---|---|---|---|---|---|---|
| V8/sCB (Bi) | −11 | −62 | −20 | 1130 | 17 | 116 | Occipital/Visual Association |
| MT+ (Bi) | −28 | −69 | 9 | 325 | 19 | 68 | Occipital/Visual Association |
| V4 (R) | 44 | −79 | −4 | 20 | 26 | 18 | Occipital/Visual Association |
| latOCC/Temp (R) | 49 | −56 | −2 | 1234 | 16 | 111 | Occipital/Visual Association |
| PCC | 1 | −54 | 25 | 200 | 19 | 35 | Media Posterior |
| V1 | −7 | −89 | 0 | 125 | 16 | 36 | Media Posterior |
| dmPFC | −2 | 51 | 29 | 496 | 35 | 67 | Media PFC |
| pgACC | 1 | 39 | 2 | 573 | 29 | 67 | Media PFC |
| rdACC | 0 | 29 | 25 | 629 | 4 | 88 | Media PFC |
| vaIns/frOP/TP (R) pOFC (Bi) | 38 | 19 | −11 | 1543 | 36 | 159 | Lateral Paralimbic |
| aINS pOFC (Bi) | −40 | 23 | −6 | 1563 | 30 | 132 | Lateral Paralimbic |
| vaIns/TC/OFC JCN (Bi) | −7 | 12 | −23 | 639 | 14 | 109 | Lateral Paralimbic |
| midIns (Bi) dPut (R) | 21 | 0 | 2 | 668 | 8 | 123 | Lateral Paralimbic |
| VSTR midIns HCMP (L) | −36 | 0 | −8 | 920 | 19 | 155 | Lateral Paralimbic |
| BL Amy (L) | −23 | −3 | −21 | 1281 | 53 | 153 | Core Limbic |
| Hy | −7 | −5 | −13 | 68 | 15 | 69 | Core Limbic |
| PAG/Thal | −1 | −23 | 2 | 662 | 36 | 80 | Core Limbic |
| Amy vStr vGP Thal (Bi) | 11 | −6 | −10 | 3544 | 27 | 232 | Core Limbic |
| frOP (R) | 46 | 24 | 6 | 350 | 24 | 82 | Cognitive/Motor Network |
| IFG (R) | 49 | 20 | 19 | 554 | 19 | 70 | Cognitive/Motor Network |
| preSMA, IFG (L) | −23 | 13 | 37 | 629 | 8 | 79 | Cognitive/Motor Network |
Note. Stereotactic coordinates for the regions found in the NMDS analysis, with laterality (Right or Left), XYZ coordinates, number of voxels in each cluster (Vol) and the weighted percentage as well as number of Contrast Indicator Maps that activated in each cluster (% Act and # Act, respectively). The regions are orders See Table 1 for abbreviations of brain regions.
Lateral Occipital/Visual Association group and Medial Posterior group
Regions in these functional groups (Fig. 7A–B) are closely connected structurally, as well as functionally, and are likely to play a joint role in visual processing and attention to emotional stimuli; we therefore discuss their functional roles together.
The first important observation here is that consistent activations in visual areas were found. 75% of the studies included in this meta-analysis used visual stimuli to elicit emotions, and we examined whether visual activations were limited largely to these studies. Of the 28 studies (producing 37 contrasts) that activated in the V1 region described in our analysis, 26 used visual stimuli; only 2 studies activating in this area used other methods (olfactory stimuli, e.g. Royet et al., 2001; Zatorre et al., 2000). All of the visually induced emotion studies compared emotional conditions with comparable neutral control conditions. Thus, the localization of increased visual activation to V1, V4, and V8 (as seen in Fig. 6) suggests that both early and late visual processing (primarily in the ventral stream) is enhanced in emotion when compared to neutral control conditions.
One possible explanation for this finding is that emotionally evocative stimuli are more visually complex or systematically differ from neutral stimuli in their perceptual characteristics. This alternative cannot be definitively ruled out, although most emotion studies compare affective and neutral stimuli matched fairly well on perceptual characteristics (Taylor et al., 2000). A second possibility, supported by the neuroanatomical evidence, is that projections from limbic circuitry enhance activation in the ventral stream when viewing emotional content (for a discussion, see Duncan and Barrett, 2007). According to this alternative, the affective salience of a stimulus directly influences visual activity. This interpretation is supported by several lines of evidence: a) recent electrophysiology work shows reward-timing prediction by neurons in V1 (Shuler and Bear, 2006); b) recent ERP work shows that the C1 potential, associated with primary visual cortex, can be influenced by prior conditioning with affective International Affective Picture System (IAPS) images (Stolarova et al., 2006); c) anatomical studies show direct projections from amygdala to V1 (Freese and Amaral, 2005); and d) a neuroimaging study showed that amygdala-damaged patients show reduced visual cortical responses to emotional faces (Vuilleumier et al., 2004). Consistent with this hypothesis, we found that V1 was directly associated and co-activated with the region encompassing vStr, GP, and the right amygdala, as can be seen in Fig. 7G. Either by this means, or because of top–down, goal related influences (e.g., due to differential eye fixation patterns when viewing emotional stimuli; van Reekum et al., 2007), evocative stimuli may elicit different patterns of fixation during viewing and consequently different visual activation.
Activations in TPJ and STS are often implicated in Theory of Mind (Saxe and Kanwisher, 2003) and in intentional action, respectively (Saxe et al., 2004). The inclusion of these areas in a posterior–cortical functional group consisting of occipital regions may reflect the frequent use of “social” visual stimuli to evoke emotions (e.g. faces or IAPS images featuring people, used in over 50% of the studies in the meta-analysis, and in the vast majority of those activating regions in this functional group, as illustrated above). However, this area is more generally considered multimodal association cortex (Kandel et al., 2000; Macaluso and Driver, 2001), and it may play a more general role in high-level visual processing. In studies of attention, similar activations result from salient changes in visual information or attention (Corbetta and Shulman, 2002); a common theme may be making inferences and processing the conceptual relevance of visual elements.
Although the precise function of PCC in emotion remains unclear, several roles have been proposed. It may play a role in memory-guided representation of context important for conceptual processing in emotion (Maddock, 1999; Mantani et al., 2005; Minoshima et al., 1997) or in allocating spatial attention (Mesulam et al., 2001; Small et al., 2003). Also, along with activations in vmPFC and dmPFC, activation of PCC might be a part of the resting state or “default” network, which appears to mediate associative, internally-generated thought processes (Greicius et al., 2003; Gusnard and Raichle, 2001), suggesting a role for self-directed attention in emotional events. Consistently, activation in PCC has shown linear increases with self-relevance (Moran et al., 2006), and recent TMS work has shown that PCC is essential for episodic memory retrieval of self-representations (but not for other-representations; Lou et al., 2004). However, an alternative interpretation given the resting state activity pattern attributed to the PCC is that activations in contrasts comparing emotional conditions with control conditions may in fact reflect lower levels of deactivations in the emotion condition compared to the control condition.
Given several potential major roles for PCC (discussed above), the co-activation results support the visual attention hypothesis, although further research is needed to disambiguate the affective functions of the PCC. This need is underscored by the apparent importance of PCC in this functional group analysis; as seen in Fig. 7G, the PCC serves as a “relay-station” to other functional groups, as it directly connects to the dmPFC and pgACC in the Medial PFC group (which is consistent with the default network), and to the vStr, midIns, and HCMP in the Lateral Paralimbic group.
The consistent cerebellar activation we observed might be related to increased demands on motor planning during affective and emotional states, but there is accumulating evidence for a more direct cerebellar role in emotion-related processing more generally. Electrical stimulation of deep cerebellar nuclei in humans has been shown to induce activity in mesolimbic affect-related areas (Heath et al., 1978) and, in some cases, to elicit states of profound rage (Heath et al., 1974). Conversely, CB damage often leads to emotion dysregulation, characterized by fluctuations between flattened affect and inappropriate social behaviors (Schmahmann and Sherman, 1998) reminiscent of social and emotional deficits with OFC damage. The CB is connected with specific prefrontal regions in topographically mapped reciprocal circuits (Middleton and Strick, 1994, 2000) and with “limbic” regions, including the Hy (Haines and Dietrichs, 1984), OFC, dmPFC, portions of IFG, and inferior frontal convexity (BA 46/12) (Middleton and Strick, 2001). Cerebellar efferents to these areas pass largely through DM in the thalamus, which we also find is consistently activated in human emotion. One hypothesis is that the CB might contribute to the processing of situational context (Schmahmann and Sherman, 1998) as part of a complex pattern-recognition system (Albus, 1971). However, it is also possible that the inclusion of the superior cerebellum in this functional group is an artifact due to inconsistent spatial normalization across studies, as warping procedures typically behave poorly in this region (Diedrichsen, 2006). Thus, the connectivity with occipital regions may be driven by peaks that actually lie within the inferior temporal–occipital cortex. Nevertheless, this does not preclude the notion that the superior lateral neocerebellum may be consistently activated in studies of emotion and affect.
Cognitive/Motor group
Like the two functional groups discussed above, activation of this functional group (Fig. 7C, in light blue) is most likely not specific to emotion, consistent with the idea that emotions are mental events constructed from a number of component cognitive, affective, perceptual, and motor processes (Barrett, 2006b; Barrett et al., 2007b). The group consists of pre-SMA, bilateral IFG, and frOP. The pre-SMA is thought to underlie representation of intentional action (Lau et al., 2004), or in related conceptions, energization of cognitive systems to orient and respond based on internal cues (Stuss and Alexander, 2007). Both the IFG and the frOP have been previously reported not only in many studies of emotion, but also in neuroimaging studies of response inhibition and selection, task switching, and working memory (Aron et al., 2003, 2004b; Badre et al., 2005; Gabrieli et al., 1998; Martin and Chao, 2001; Poldrack et al., 1999; Wager et al., 2005; Wagner et al., 2001). The right IFG and right frOP in particular are hypothesized to be parts of a ventral frontoparietal network of attention to sensory stimuli of potential behavioral significance (Corbetta and Shulman, 2002) and have been specifically implicated in manipulation of information in working memory (Wager and Smith, 2003) and in response inhibition across domains (Aron et al., 2004a; Nee et al., 2007). A general role for BA 44/45 and the operculum might be context-based selection among competing stimulus–response mappings or sets (Thompson-Schill et al., 1997), with left specialization for semantic and right specialization for late-stage response selection (Nee et al., 2007).
Though the precise information-processing roles of the regions in this functional group may be dissociable, meta-analyses have shown that activation of all regions—pre-SMA, IFG, and frOP—is a consistent feature of cognitive control operations (Nee et al., 2007; Wager et al., 2004a; Wager and Smith, 2003). Prior studies of functional connectivity have also shown that bilateral vlPFC/ frOP and preSMA form a functional network during cognitive control tasks (Wager et al., 2005). Given this evidence for functional integration in cognitive tasks and the functional roles discussed above—namely, the role of IFG/frOP in attention for action (Corbetta and Shulman, 2002), right IFG in response inhibition (Nee et al., 2007), and preSMA in selection for action—this functional group is likely to play an important role in context-appropriate selection of actions and attention for action.
In emotion-related phenomena, these frontal–lateral region may be important for the information selection that is integral to analysis of meaning of emotional input, such as that associated with appraisal or conceptual categorization and labeling of affect, (Lieberman et al., 2007) and in shaping subsequent behavior and physiological responses via projections to subcortical areas such as the PAG (An et al., 1998). In support of this notion, emotion- and pain-related activity in IFG and the operculum is modified by manipulations of the context in which affective stimuli are presented, such as when pain expectations are changed (Benedetti et al., 2005; Kong et al., 2006; Wager et al., 2004b). Activity in these areas is also modified by voluntary regulation of emotional responses, such as when participants are asked to use cognitive strategies to reduce or increase negative emotion, (Johnstone et al., 2007; Ochsner et al., 2002, 2004b). Further, in emotion regulation, the IFG was found to be inversely correlated with amygdala via mPFC, a relationship that is disrupted in depression (Johnstone et al., 2007).
Lateral Paralimbic group
This functional group is likely to play an important role in motivation. Both the OFC (including posterior orbital gyrus) and the vStr are thought to contribute to valuation of stimuli in general and rewards in particular. Specifically, vStr, is sensitive to both reward and punishment (Delgado et al., 2000) and has been implicated in reward prediction (O'Doherty et al., 2004). Similarly, OFC is sensititve to both rewards and punishment (Kringelbach and Rolls, 2004; O'Doherty et al., 2001), and is capable of reward discrimination, valuation, and prediction in both animals and humans (Gottfried et al., 2003; Schultz et al., 2000). Sub regions of the OFC activated in this meta-analysis (Fig. 6) as well as the insula are thought to belong to the Orbital Sensory network proposed by Ongur & Price and are known to receive olfactory, visceral, and gustatory inputs (Ongur and Price, 2000). Importantly, these OFC sub-regions are neuroanatomically interconnected with the amygdala and vStr (Ongur and Price, 2000), connections that are also evident in the group analysis (Fig. 7G). Consistently, one possible role for the OFC is to facilitate associative flexibility that is coded in such subcortical structures (for discussion, see Barrett et al., 2007c).
In a recent meta-analysis of insula activation across studies of pain, emotion, attention shifting, and working memory (Wager and Barrett, 2004). vaIns was associated relatively specifically with emotion, particularly emotion elicited by autobiographical memory retrieval, which is likely to elicit relatively strong experienced emotion. By contrast, daIns was activated primarily by pain and cognitive response selection tasks that require motivated selection for action. Based on these findings, the authors proposed a continuum from vIns, which is most closely connected to brainstem and other core limbic regions and is functionally related to emotional and affective sensory experience, through dIns and frOP, which are more heavily interconnected with lateral frontal cognitive and motor systems. The present results provide some support for this view: the daIns region appears to form a bridge between the pre-SMA and the vaIns (Fig. 7G). The ventral insular regions, but not the dorsal ones, connect directly to the “core limbic” regions of ventral Thal, Hy, PAG, and vStr.
Lastly, activity in right daIns has been associated with interoception, or representation of bodily responses (Craig, 2002; Critchley et al., 2004), which contribute to both broad and specific affective states. Therefore, it is likely that this functional group has a role in evaluating “bottom–up” internal as well as external affective signals and integrating them into motivational states with associated goals. Indeed, the close links between this group and both the Cognitive/Motor group and the Medial PFC group further emphasizes its integrative role with cognitive inputs processed in medial and lateral prefrontal cortex.
Interestingly, the HCMP was part of early definitions of the limbic system (Maclean, 1949; Papez, 1937) and was a focus of early human brain stimulation work on emotion (Sem-Jacobsen, 1968), but its role in emotion has not been emphasized as much recently because of a lack of obvious emotional deficits in animals with hippocampal lesions (but cf. Gray and McNaughton, 2000; Maren et al., 1997). Activation in HCMP suggests that memory retrieval might play a role in emotion. Further, the concurrent activation of PCC, MTL, and mPFC might represent elements of a memory retrieval circuit that has been previously identified (Vincent et al., 2006) that could interact with the frontal information selection and appraisal circuit of IFG/dmPFC.
Medial PFC group
This functional group, which encompasses the ACC subregions that were activated in this meta-analysis as well as dmPFC, is likely to play a role in both the generation and regulation of emotion. The regions in this functional group have been identified as a part of a medial visceromotor network (Ongur and Price, 2000) and each region shows evidence for context-based generation and modulation of emotion. While the regions cluster into the same functional group in our analyses, there is evidence that their roles are related but may also be dissociable. In humans, activity in pgACC has classically been associated with resolution of conflict in emotional Stroop tasks (Bush et al., 2000; Etkin et al., 2006), but also correlated with heart rate during cognitively generated stress (Wager et al., 2006) and motivated selection and/or maintenance of task goals (Summerfield et al., 2006; Wager et al., 2005). Further, pgACC shows increases in activity and opioid release with placebo treatment in pain (Petrovic et al., 2002; Wager et al., 2004a,b; Zubieta et al., 2005). In addition, the rdACC has been directly implicated in the cognitive modulation of pain affect (Faymonville et al., 2000; Rainville et al., 1997; Wager et al., 2004b).
Interestingly, a recent meta-analysis of anxiety disorders (Etkin and Wager, 2007) showed rdACC to be hyperactive in patients with specific phobias (compared to controls), and in fear conditioning in nonclinical populations; the rdACC and mOFC/sgACC were also observed to be consistently hypoactive in PTSD patients compared to controls, suggesting a direct link between altered function in this region and dysregulation of affect. Importantly, the pgACC peak found in our analysis extends into this area of sgACC, which has been functionally implicated in depression in several ways. Namely, the sgACC has been found to be hypoactivate in depression Drevets et al., 2002, 1997, and to be modulated by treatment with selective serotonin reuptake inhibitors (SSRIs; Drevets et al., 2002; Mayberg et al., 2000) as well as by deep brain stimulation in treatment-resistant depression (Mayberg et al., 2005). Metabolic decreases in this area were related to clinical improvement in these studies, and may be mediated by connectivity to OFC, pgACC, hypothalamus, NAcc, and the amygdala/HCMP (Johansen-Berg et al., 2008). In addition, regions of vmPFC (just anterior to sgACC) have been shown to inversely correlate with amygdala during successful emotion regulation. Additional findings that this inverse pattern predicts a more adaptive diurnal rhythm of circulating free cortisol in older adults and is absent in depressed individuals, further implicates medial frontal regions in affective functioning (Johnstone et al., 2007; Urry et al., 2006).
The functional contributions of mPFC have yet to be precisely determined, but recent research and theorizing suggests that these brain areas jointly contribute to making mental state attributions (for reviews, see Adolphs, 2001; Blakemore et al., 2004; Lane and McRae, 2004) such as when a person makes judgments about the psychological states of another person, or monitors, introspects, or makes inferences about his or her own moment-to-moment feelings (also see Ochsner et al., 2004b). Further, it has also been implicated in anticipation of pain (Porro et al., 2002), with a potential role in generating second-order appraisals leading to secondary pain affect (Price, 2000). Further, it has been suggested that the dorsal portions of mPFC are uniquely associated with the metacognitive ability to monitor, re-represent, or re-describe affective inputs, as occurs in cognitive generation of emotion and in emotion regulation (Ochsner and Gross, 2005; Ochsner et al., 2004a).
One surprising feature of these results is that while the Medial PFC group is directly connected to both the Core Limbic group and the Lateral Paralimbic group, we did not observe direct functional connectivity between the Cognitive/Motor group and the Medial PFC group; rather, dorsal and posterior insular regions (in the Paralimbic group; Fig. 7G) served as a bridge between the two groups. This suggests that the Medial PFC group is more related to core affective responses than to cognitive–motor activity. Consistent with these findings, areas of the medial wall including pgACC, are known to have direct projections to the Hy and lower brainstem autonomic effectors (Saper, 1995), have been related to visceromotor control (Vogt et al., 1992), and to extinction of conditioned fear responses (Milad and Quirk, 2002) in animal studies. Further, different subregions of mPFC appear to play different and perhaps opposing roles in the generation and regulation of hypothalamic–pituitary–adrenal “stress” responses (Sullivan and Gratton, 2002). Taken together, it seems likely that this Medial PFC group interfaces between cognitive context and core affect, and as some subregions may be more associated with cognitive representation and context-generated emotion (dmPFC), others may be more closely related to control over affective physiology (pgACC).
Core Limbic group
This functional group represents the closest parallel to findings in the animal literature. The PAG is thought to participate in regulation of autonomic responses related to the psychophysiology of emotion, and it is likely that in humans, like in animals, it serves as an integrative emotional center, receiving direct cortical inputs (e.g. mPFC, OFC, Ins), and projecting to the lower brainstem neuclei as well as to the hypothalamus. Further, it is known that dopaminergic neurons in SN fire in response to rewarding (Schultz et al., 1997) and to simply salient events (Horvitz, 2000), and recent work has shown that phasic firing of these neurons specifically indicates error in reward prediction in animals (Schultz and Dickinson, 2000) as well as humans (Montague et al., 2004). Similar responses were recorded in humans to cognitive feedback even in absence of reward (Aron et al., 2004b). Lastly, SN projects to vStr, which is itself implicated in reward prediction (O'Doherty et al., 2004). It is likely, therefore, that the SN and vStr play a role in appetitive learning and in response to salient events in the environment, including emotion-related stimuli and possibly emotional experience.
Significant activations were also found throughout the dorsal Thal, with maximal consistency in the central medial zone, around the “limbic” mediodorsal and centromedian nuclei (Fig. 4F). The subnuclei of the thalamus are heavily connected in reciprocal circuits with much of the forebrain and provide avenues of communication among cortical regions (see Edelman and Tononi, 2001, for review). As described above, consistent activations in the Hy in studies of emotion (69 contrasts, from 52 studies) is likely to reflect its role in regulation of stress responses, motivated behavior, and homeostatic processes, as well as its interaction with the autonomic nervous system and control of bodily states via the endocrine system.
While the amygdala is consistently activated in neuroimaging studies of emotion, its role in emotion generation or experience is not clear, as findings in individual studies are somewhat inconsistent. One possibility is that the amygdala may set the stage for, but not be intrinsic to the implementation of, emotion experience (Barrett et al., 2007a). Some of the discrepancy in findings may also stem from the highly differentiated structure of the amygdala; it is organized into three sub-complexes, the basolateral, cortico-medial, and central, which are further comprised of over 20 distinct nuclei, thought to perform distinct operations (Swanson and Petrovich, 1998). Indeed, many roles have been proposed for the amygdala; some argue that the amygdala is critical to fear-related processing (LeDoux, 2000), although two recent meta-analyses (Murphy et al., 2003; Phan et al., 2002) reported that only 40%–60% of the studies involving the experience or perception of fear showed increased amygdala activation (Barrett and Wager, 2006). Moreover, neither is amygdala activity specific to the category “fear.” We previously reported that the most fear-related region in the amygdala (the superior amygdala) is just as responsive to disgust-related stimuli (Wager et al., 2008). In individual studies as well, amygdala activation is found not only for fearful faces, but also for happy and angry faces, compared to neutral faces (Breiter et al., 1996; Whalen et al., 2001, respectively). At the level of the single neuron, single cells in the primate's amygdala respond to stimuli that acquire both positive and negative value during conditioning (Paton et al., 2006). Consistently, it has been suggested that amygdala activity has a general role in evaluation of affective significance (Phelps and Anderson, 1997), as well as other roles in mediating emotional memory (LaBar and Cabeza, 2006; Mather, 2007), and as related to a general dispositional style (Davidson and Irwin, 1999). Importantly, however, the human amygdala also responds to novelty (Wright et al., 2006) and habituates quickly once the stimulus acquires a clear and certain predictive value (Fischer et al., 2003), which is consistent with an alternate view that the amygdala tags stimuli of unknown or uncertain predictive value to increase attention, so that more can be learned about their value on the next encounter or trial (Barrett et al., 2007a).
Connections Among Functional Groups
Once we identified the six functional groups most associated with emotion, we examined patterns of co-activation among these groups across studies, testing for co-activation between areas within groups. To visualize the relationships among the regions in each functional group, Fig. 7G displays both regions and co-activation lines on a “flattened” map of the connectivity space along the first two dimensions determined by NMDS (as described in the methods section), and with matching colors to the 3D groups. Points that are closer together on this map tend to have positive co-activation, and connected lines represent significant Tau-b (τ) association values between pairs of two regions (FDR corrected). Importantly, this map has been “pruned” such that the relationships depicted by these lines represent only those functional relationships between regions that were not completely mediated by any other region.
As can be seen from the “pruned” flattened map, there appears to be a strong relationship between the Medial PFC group and the Core Limbic group. Medial PFC group regions such as dmPFC and pgACC are directly coactivated with PAG/Thal and vStr/Amygdala, respectively, both in the Core Limbic group. In addition, these two groups appear to be indirectly connected along multiple routes via the Lateral Paralimbic group (via regions such as the vpIns).
Frontal areas in the Cognitive/Motor group are also directly associated with areas in the Lateral Paralimbic group; in turn, these areas (e.g. vaIns) are directly related to areas in the Core Limbic group. Therefore, areas that are part of the Cognitive/Motor group such as frOP may be co-activated with brainstem areas such as PAG in the Core Limbic group, through coactivations in the Lateral Paralimbic group or through concurrent coactivations in both the Lateral Paralimbic and the Medial PFC group. This pattern of connectivity could indicate that while appraisals generated in the Medial PFC group directly influence activity in limbic regions, they also converge in insular regions with other core limbic inputs to influence more general motivational states, which could influence attention and selection of action in the Cognitive/Motor group.
Importantly, in further analyses of these associations (described in the next section), we chose to focus on frontal–posterior co-activations, as these are thought to be critical components of emotion-inducing appraisal in humans; we therefore tested frontalamygdala, frontal-PAG and frontal-Hy links. Positive findings would suggest the presence of anatomically specific cortical–limbic and cortical–brainstem pathways that may be tested in future individual neuroimaging studies.
Frontal interaction with amygdala
Recently, neuroimaging studies have highlighted fronto-amygdala interactions, especially because medial PFC regions are thought to be involved in regulation of amygdala responses to negative events. Several studies have reported negative correlations between amygdala and activity in both vmPFC (Johnstone et al., 2007; Urry et al., 2006) and lateral PFC (Lieberman et al., 2007; Ochsner et al., 2002). Both animal and human neuroimaging studies show mPFC is critical for fear extinction and modulating learned fear responses via amygdala (Gottfried and Dolan, 2004; Milad and Quirk, 2002; Phelps et al., 2004). In PTSD, hypo-frontality is functionally associated with hyperactivity in amygdala (Etkin and Wager, 2007). However, other studies have shown mPFC sub-regions relate positively to stress and negative emotion—e.g. in animals studies, stimulation of prelimbic cortex causes Hy increases and HPA axis activation and cortisol release (Sullivan and Gratton, 2002). Therefore, it is of interest to know which frontal regions are most strongly co-activated with amygdala, whether different frontal regions co-activate with distinct amygdala sub-regions, whether these are the same frontal regions that co-activated with PAG and Hy, and whether any regions show negative co-activation (which would imply that activation of PFC region results in reduced probability of a study activating amygdala).
To that end, we selected parcels that corresponded to the basolateral complex and the superior amygdala (corresponding to centro-medial complex, the superficial amygdala, and the central nucleus) bilaterally using the SPM Anatomy Toolbox (V15) as a guide (Eickhoff et al., 2006, 2005). Fig. 8A shows the regions depicted in the toolbox; Fig. 8B shows the parcels that were found in this meta-analysis. Once we identified four distinct parcels—namely, Right BasoLateral (RBL), Left BasoLateral (LBL), Right Superior Amygdala (RSA), and Left Superior Amygdala (LSA)—we looked for any individual frontal regions that were co-activated with each of the amygdala sub-regions, by re-computing Tau-b (τ) associations for each frontal–amygdala pair of regions. Results were FDR corrected, and are shown in Fig. 8C–E.
Fig. 8.
(A) Amygdala sub-regions specified in the SPM Anatomical Toolbox. The Basolateral Amygdala (BL) is depicted in magenta; the Centro-Medial complex (CM) is depicted in blue; the Superficial Amygdala (SF) is depicted in cyan. (B) Amygdala sub-regions identified in this meta-analysis. The Basolateral Amygdala is depicted in magenta (BL); the Superior Amygdala (SA) encompasses both the Superficial and CentroMedial complex, and is depicted in cyan. (C–E) Co-activation of frontal and amygdala sub-regions. Areas that were co-activated with three or more amygdala regions are depicted yellow; dmPFC, the only region co-activated with only one amygdala region, is depicted in orange.
Several frontal regions were significantly co-activated with amygdala sub-regions, especially along the medial wall, and ventral areas showed more frequent associations with amygdala than dorsal. The most strongly connected regions (in the sense that they correlated significantly with multiple amygdala sub-regions) were pgACC (Fig. 8C), rdACC (Fig. 8D), and right frOP (Fig. 8E), which are consistent with the literature discussed above. More specifically, dmPFC was co-activated only with one amygdala sub-region, LSA3, the right frOP was co-activated with LBL, RSA, and LSA (but not the RBL)4, the rdACC was co-activated with RBL, RSA, and LSA (but not LBL).5 Finally, the pgACC was co-activated with RBL, RSA, LSA (but not LBL).6 These results illustrate that each of the frontal regions has a unique pattern of co-activation with amygdala sub-regions in this analysis, and that amygdala sub-regions have unique patterns of co-activation with frontal regions. Indeed, the only amygdala sub-region that showed strong association with all frontal regions in this analysis is the LSA; this is consistent with previous meta-analyses of emotion that found amygdala activation to be left-lateralized and superior (Wager et al., 2003).
However, while the regions identified were consistent with our predictions, we did not find any inverse co-activation between any frontal regions and amygdala. This may be attributed to the absence of emotion regulation studies from this meta-analysis. It is possible that such an inverse functional relationship is expressed when emotional experience is influenced by context or processes of valuation, rather than valence per se (Wager et al., 2003). Lastly, as we show below, mPFC regions are also functionally associated with PAG/Hy activity, although there is evidence for a different pattern within mFPC.
Frontal interaction with PAG and Hy
In animal models of emotion, the PAG and Hy are considered both central and critical. While they have been shown to mediate physiological effects of emotion and stress through the HPA axis and autonomic nervous system, whether they broadly mediate the instantiation of emotional states is unknown. For example, Mobbs et al. (2007) recently reported PAG activation in conditions of proximal threat, along with correlations between PAG activity and the experience of dread, suggesting parallelism between human and animal responses to threat. Consistent with these findings we find the cluster encompassing PAG and Thal and a region centered on Hy activated consistently in a range of emotion conditions, including those in experimental imaging studies by cognitive factors (memory for emotional events, cognitively mediated threat, etc.). Therefore, although PAG and Hy may serve similar functional roles in animals and humans, cortical generators of PAG and Hy responses are likely to be different in humans and animals. This potential difference underscores the need to identify those cortical correlates of PAG and HY in humans, especially in PFC.
Functional co-activation with PAG and Hy
The group analyses discussed above shows that areas in medial PFC are functionally positioned in close proximity to subcortical brainstem limbic structures, such as PAG and Hy, while lateral PFC regions in the Cognitive/Motor group were only associated with these subcortical structures through areas in the Lateral Paralimbic group. However, regions such as lateral PFC figure prominently in some theories of cognitive generation and control of emotion (Ochsner and Gross, 2005; Wager, 2005). Therefore, we first looked for any individual frontal regions that were co-activated with PAG and Hy. Results are shown in Fig. 9A. Right frOP, rdACC, and dmPFC are co-activated with the Core Limbic group region encompassing the Thal and PAG.7 Importantly, this is a subset of the regions that were co-activated with amygdala. However, only two of these three regions were also connected with multiple amygdala structures (namely, rdACC and frOP). Conversely, and as shown in Fig. 9B, dmPFC is the only frontal region co-activated with Hy in our analysis.8
Fig. 9.
Co-activation of frontal and subcortical regions. (A) Frontal cortical regions that are co-activated with PAG. Color of activated areas indicate the network to which they belong — dark blue for the mPFC network (dmPFC, rdACC), light blue for the Cognitive/Motor network (rFrOP), and red for the Core Limbic network (PAG/Thal, the region tested for co-activity with frontal regions). (B) Frontal cortical regions that are co-activated with Hy (shown in red). Colors are as in (A). See Table 1 for abbreviations.
Testing mediations in the DMPFC-PAG-Hy pathway
Because Hy and PAG/Thal were found to be co-activated in the functional groups analysis (both are part of the Core Limbic group), and because dmPFC was found to be the only frontal area co-activated with both regions in subsequent analysis, we focus on this relationship in the mediation analyses. We do not report mediation analyses involving amygdala here, because frontal connections with amygdala sub-regions were more numerous and less specific, making path modeling less informative and less reliable. Table 5A–C summarize the two-way patterns of co-activation among the dmPFC, PAG, and Hy. Table 5D and E summarize the three-way contingencies of dmPFC and Hy when PAG/Thal region is active and inactive, respectively. As can be seen from the tables, dmPFC and Hy are more likely to be active when PAG/Thal is active than when it is inactive.
Table 5.
Co-activations
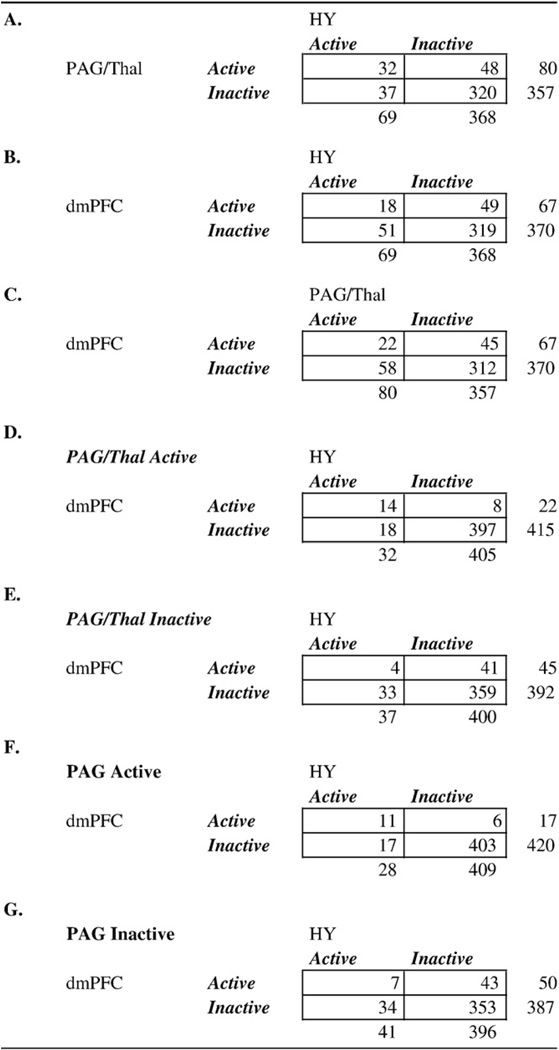 |
Note. (A) Two-way contingency tables showing co-activation of Hy and PAG/Thal. Active refers to the number of CIMs that are activated in the region. Inactive refers to the number of CIMs that did not activate within the region. (B) Two-way contingency tables showing co-activation of Hy and dmPFC. (C) Two-way contingency tables showing co-activation of PAG/Thal and dmPFC. (D–E) Three-way contingency tables showing coactivation of Hy and dmPFC with PAG/Thal Active or Inactive. (F–G) Three-way contingency tables showing co-activation of Hy and dmPFC with PAG Active or Inactive.
To further our understanding of this relationship, we examined the neuroanatomical projections between these regions. Although the human–monkey homology is often not entirely clear, it is known that monkey PAG and Hy have massive bidirectional projections (Beitz, 1982; Cameron et al., 1995). Similarly, it had been suggested that the region of dmPFC homologous to the one we observed (superior dmPFC/BA9; e.g. Ongur and Price, 2000) is connected via a unidirectional projection to PAG (An et al., 1998; Mantyh, 1983), and that the same area of dmPFC projects heavily to Hy as well (Ongur et al., 1998; Ongur and Price, 2000). Therefore, based on this and the activation and co-activation evidence presented above, we reasoned that dmPFC is part of an appraisal system involved in the cognitive generation of emotion. Consequently, we predicted a functional pathway in co-activation across studies from dmPFC through PAG to Hy, and tested this hypothesis using path analysis, first using the PAG/Thal region that was identified in the NMDS analysis, and then testing PAG and Thal parcels separately.
The functional regions in question are shown in Fig. 10A in which the arrows illustrate the potential directionality of a top–down projection from dmPFC through PAG/Thal to Hy. The critical test of this model is of the indirect pathway between dmPFC and Hy, mediated by PAG (e.g. whether a pathway through PAG explains a significant amount of the co-activation between dmPFC and Hy). Importantly, this mediation is not a test of directional (i.e., causal) association; we believe that making directional inferences with confidence in this case requires manipulation of variables (Rubin, 1986). However, the results of such analyses may serve to inform directional hypotheses, especially when coupled with examination of directionality of anatomical projections. Further, such results may serve to both inform and inspire future empirical work that could directly test the related hypotheses.
Fig. 10.
Mediation model and analyses for the association between dmPFC, Hy, and PAG/Thal. (A) Visualization of the locations of regions tested in the model and their connectivity in the model tested. (B) PAG/Thal is a complete mediator of dmPFC–Hy co-activation. Path coefficients are shown next to arrows indicating each link in the analysis, with standard errors in parentheses. a refers to the path from dmPFC to PAG/Thal. b refers to the direct link between PAG/Thal and Hy. c refers to the total association between dmPFC and Hy, without the mediator (PAG/Thal). *p<.05; **, p<.01; ***, p<.001, two-tailed. (C) Bootstrap distributions for this mediation analysis, based on 10,000 bootstrap samples. The histogram shows the bootstrapped sampling distribution, with the tails (p<.05) shaded in dark gray. The vertical line indicates the null hypothesis path coefficient value (zero). A significant result occurs when the null hypothesis value falls in the tail of the bootstrapped sampling distribution. See Table 1 for abbreviations.
As shown in Fig. 10B, dmPFC and Hy are positively co-activated (total effect c=0.13, SE=0.06, p<.01). However, in the mediation bootstrap test with the PAG/Thal entered into the model, the relationship was no longer significant (direct effect c' = 0.08, SE=0.05, ns). In addition, the test showed that PAG/Thal was co-activated with Hy controlling for dmPFC (b=0.28, SE=0.06, p<.001) and significantly mediated the dmPFC–Hy relationship (ab=0.05, SE=0.02, p<.01). Together, the results suggest that the PAG/Thal region was a complete mediator of dmPFC and Hy co-activation. Fig. 10C shows the distributions of the variables as created during the bootstrapping procedure.
Additionally, as the PAG/Thal region identified in the NMDS analysis encompassed two anatomical regions, we performed a set of mediation analyses on each parcel separately, to investigate whether each one independently mediates the relationship between dmPFC and Hy. The Mediation results are presented in Fig. 11. As can be seen in Fig. 11A, Thal alone was a complete mediator of the dmPFC–Hy association (ab=0.04, SE=0.02, p<.01; additional statistics are shown in Fig. 11A). As can be seen in Fig. 10B, PAG alone was also a complete mediator of the dmPFC–Hy association (ab=0.05, SE=0.02, p<.01; additional statistics are shown in Fig. 11B). However, when both PAG and Thal were entered into the model as simultaneous mediators (Fig. 11C), PAG was found to fully mediate the dmPFC–Hy relationship (abPAG= 0.05, SE=0.02, p<.01; c' = 0.08, SE=0.05, ns). Conversely, Thal was no longer a significant mediator (abThal=0.00, SE=0.01, ns) and the Thal–Hy association was no longer significant (bThal=0.01, SE=0.08, ns). This result implies that the co-activation of dmPFC and Hy is mediated by activation in PAG. Three-way contingency tables showing frequency as a function of activation status in the three regions are shown in Table 5F–G. In summary, dmPFC was the only frontal region co-activated with both PAG and Hy. Further, PAG appears to be a mediator of dmPFC–Hy co-activation, as suggested by anatomical evidence, which in turn implies a functional pathway from specific portions of the medial frontal cortex (dmPFC) to brainstem and hypothalamic regions thought to be critical for effects of emotion on the body, and possibly for emotional experience as well. This functional pathway implies a role for dmPFC in cognitive generation of emotion, and may be tested in future studies of brain–physiology interactions related to emotion.
Fig. 11.
Thal and PAG as separate mediators of the dmPFC–Hy co-activation. Abbreviations for path coefficients are as in Fig. 9. (A) Thal is a complete mediator of the dmPFC–Hy co-activation. (B) PAG is a complete mediator of the dmPFC–Hy co-activation. (C) When both PAG and Thal are entered into the mediation model as two separate mediators, only PAG is a complete mediator of the dmPFC–Hy co-activation. See Table 1 for abbreviations.
Limitations
An important limitation of our approach, shared by the meta-analytic methods most frequently applied to neuroimaging data, is that it relies on peak coordinates reported by individual studies. Importantly, the use of peak coordinates involves the assumption that peak coordinates are representative of the activation maps from which they came, which may not always be the case; indeed, activation “blobs” may not be captured in size or shape by mere peak-reporting. Further, peak coordinates may be subject to quite varied data-collection procedures, analyses, and standards of reporting, and are rarely accompanied by effect sizes. Here, we attempted to reduce sensitivity of the results to such differences and improve on previous methods by using the multi-level KDA analysis, which treats contrasts as the unit of analysis. Thus we improve on previous methods and do not treat study identity as a fixed effect and do not assume that there are no inter-studies differences in number and location of peaks, smoothness, false-positive rates, and statistical power. One future direction might be to move beyond peaks to shape and size of activation “blobs” by using the whole-brain statistical maps from studies included in the meta-analysis, or unthresholded maps with significant clusters marked with contours (if those were made available by study authors or in databases). Such maps would better capture the nature of the activations found in each study and lead to more accurate meta-analytic results. Another statistical limitation that may benefit from refinement in future work is that contrast maps are treated as independent, which is not strictly the case. Future advances could be made in modeling this non-independence.
In addition, inherent limitations in signal acquisition and analysis in the studies we analyzed may preclude finding many areas that are important in emotion. Anterior orbitofrontal regions, for example, were not consistently identified in our meta-analysis. This could be because of signal dropout in this region in fMRI studies in particular. The same limitations may apply to other regions. In addition, the variation of signal-to-noise ratio (SNR) across the brain, across subjects, and across studies may spatially bias the results in favor of areas with higher SNR. Such areas will be over-represented in this meta-analysis while other areas with low SNR may be under-represented.
Conclusions
Taken as a whole, these results suggest that human neuroimaging studies of emotion activate a wide range of areas across the brain including cortical regions such as dmPFC, ACC, OFC, IFG, Ins, OCC. In addition, like animal studies, these studies reliably activate subcortical areas such as Thal, vStr Amy, PAG and Hy. PAG and Hy are of particular interest because they are often neglected in discussions of human neuroimaging studies. The functional roles of PAG and Hy in emotional responding remain to be elucidated.
Further, while many cortical and subcortical regions activate during emotional states, these regions can be separated into distinct functional groups that appear to subserve different functions in emotion. A frontal Cognitive/Motor group was functionally associated with a Paralimbic group (consisting of insular, striatal, and OFC regions), which in turn was closely associated with a Core Limbic group (PAG, Hy, vStr, amygdala, and Thal). A medial PFC group was closely associated with the Core Limbic group and appeared to provide a more direct cortical connection to the core limbic regions. This finding may be surprising given emphasis on lateral PFC in emotion, but it is broadly consistent with anatomical and physiological patterns in animal studies. Two posterior visual/ associative functional groups (including V1, lat. Occ, PCC, STS, TPJ, and superior CB) were largely activated by studies involving visual stimulation, and were functionally connected to dmPFC and the Paralimbic group through the PCC. None of these activation patterns is likely to be specific to emotion per se, though this supposition needs more careful testing with respect to PAG, Hy, and dmPFC, which do not appear to be consistently activated in studies that do not involve affect. The generality of these functional groups suggests that a constructivist approach appears most sensible at this point: Emotions such as anger, sadness, and fear, and even broad categories such as positive and negative affect, are likely to be generated via the interplay of more basic processes in perceptual, attentional, and mnemonic systems that are not unique to emotion. Whether there are gross brain regions unique to affective states requires further testing in studies that build on and refine the results presented here.
The broad patterns of connectivity captured in the functional groups were further investigated by co-activation and mediation analyses on specific regions. Four frontal areas were found to co-activate with any of four amygdala sub-regions (dmPFC, right frOp, pgACC, rdACC), three of which were co-activated with more than one amygdala sub-region. There were no negative co-activations between amygdala and frontal regions. Notably, of the frontal areas associated amygdala, dmPFC was co-activated with only one amygdala sub-region (LSA). Conversely, dmPFC was the only frontal region to co-activate with both PAG and Hy. Mediation analyses of these co-activations were consistent with a model based on patterns of anatomical connectivity in which dmPFC influences Hy through the PAG. Coupled with evidence that dmPFC activity is often found when people evaluate the significance of situational context for the self or others, these results suggest a uniquely important role for dmPFC in the perception and experience of emotion.
Acknowledgments
We would like to thank Matthew Davidson, Seth Duncan, Jennifer Mize, Heepeel Chang, and Sara Steele for assistance with the preparation of the database and other materials, and Martin Lindquist, Niall Bolger, Tom Nichols, Sam Gershman, and Bryan Denny for helpful discussion. We would also like to thank the authors of SPM at the Functional Imaging Laboratory and Caret at the Van Essen Lab for making their software freely available. This research and the preparation of this manuscript were supported in part by National Science Foundation grant (SES631637) and National Institute of Mental Health grant (R01MH076136) to Tor D. Wager, by National Institutes of Health Director's Pioneer Award (DP1OD003312), a National Institute of Mental Health's Independent Scientist Research Award (K02 MH001981), a National Institute of Aging grant (AG030311) and National Science Foundation grants (BCS 0721260; BCS 0527440) to Lisa Feldman Barrett, and by the National Science Foundation Graduate Research Fellowships to Hedy Kober and Kristen Lindquist. Author contributions: Concept: TDW, LFB, HK; Software specification: TDW, LFB; Software implementation: TDW; Database preparation: LFB, HK, JJ, EBM, KL; Analysis: HK, TDW, LFB; Writing: HK, TDW, LFB.
Appendix A. Papers included in the meta-analysis
- Aalto S, Naatanen P, Wallius E, Metsahonkala L, Stenman H, Niem PM, et al. Neuroanatomical substrata of amusement and sadness: a PET activation study using film stimuli. Neuroreport. 2002;13(1):67–73. doi: 10.1097/00001756-200201210-00018. [DOI] [PubMed] [Google Scholar]
- Aalto S, Wallius E, Naatanen P, Hiltunen J, Metsahonkala L, Sipila H, et al. Regression analysis utilizing subjective evaluation of emotional experience in PET studies on emotions. Brain Research Protocols. 2005;15(3):142–154. doi: 10.1016/j.brainresprot.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Adams RB, Jr, Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300(5625):1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94(1):327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Baker SC, Frith CD, Dolan RJ. The interaction between mood and cognitive function studied with PET. Psychological Medicine. 1997;27(3):565–578. doi: 10.1017/s0033291797004856. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Chertkow H, Bub D, Murtha S, Dixon R, Evans A. The Neural Substrate for Concrete, Abstract, and Emotional Word Lexica: A Positron Emission Tomography Study. Journal of Cognitive Neuroscience. 1997;9(4):441–461. doi: 10.1162/jocn.1997.9.4.441. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, et al. The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. Neuroreport. 1998;9(14):3253–3258. doi: 10.1097/00001756-199810050-00022. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Sciences. 2001;98(20):11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nature Neuroscience. 1999;2(4):382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lutz K, Mirzazade S, Specht K, Shah NJ, Zilles K, et al. Recognition of emotional prosody and verbal components of spoken language: an fMRI study. Cognitive Brain Research. 2000;9(3):227–238. doi: 10.1016/s0926-6410(99)00060-9. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Pontillo D, Powers M, Sabb FW, Craske MG, Bookheimer SY. Functional MRI changes during panic anticipation and imagery exposure. Neuroreport. 2001;12(18):3953–3957. doi: 10.1097/00001756-200112210-00020. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport. 1998;9(14):3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. The Journal of Neuroscience. 2000;20(19):RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, et al. Processing words with emotional connotation: an FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. Journal of Cognitive Neuroscience. 2004;16(2):167–177. doi: 10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9(2):93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Radonovich K, Sadek JR, Gokcay D, Bauer RM, Fischler IS, et al. Left-hemisphere processing of emotional connotation during word generation. Neuroreport. 1999;10(12):2449–2455. doi: 10.1097/00001756-199908200-00003. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, et al. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26(1):141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. European Journal of Neuroscience. 2003;18(7):2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher P, Morris J, Kapur N, Deakin JF, Frith CD. Neural activation during covert processing of positive emotional facial expressions. Neuroimage. 1996;4(3):194–200. doi: 10.1006/nimg.1996.0070. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, Fletcher P. Dissociable temporal lobe activations during emotional episodic memory retrieval. Neuroimage. 2000;11(3):203–209. doi: 10.1006/nimg.2000.0538. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Morris JS, de Gelder B. Crossmodal binding of fear in voice and face. Proceedings of the National Academy of Sciences. 2001;98(17):10006–10010. doi: 10.1073/pnas.171288598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23(1):64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Lasko M, et al. Anger in healthy men: a PET study using script-driven imagery. Biological Psychiatry. 1999;46(4):466–472. doi: 10.1016/s0006-3223(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11(8):1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Eugene F, Levesque J, Mensour B, Leroux JM, Beaudoin G, Bourgouin P, et al. The impact of individual differences on the neural circuitry underlying sadness. Neuroimage. 2003;19(2):354–364. doi: 10.1016/s1053-8119(03)00121-6. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Armony JL, Joanette Y, Belin P. Sensitivity to voice in human prefrontal cortex. Journal of Neurophysiology. 2005;94(3):2251–2254. doi: 10.1152/jn.00329.2005. [DOI] [PubMed] [Google Scholar]
- Fischer H, Fransson P, Wright CI, Backman L. Enhanced occipital and anterior cingulate activation in men but not in women during exposure to angry and fearful male faces. Cognitive, Affective, and Behavioral Neuroscience. 2004;4(3):326–334. doi: 10.3758/cabn.4.3.326. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wik G, Fredrikson M. Functional neuroanatomy of robbery re-experience: affective memories studied with PET. Neuroreport. 1996;7(13):2081–2086. doi: 10.1097/00001756-199609020-00005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2005;30(4):1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Posse S, Moore GJ, Tancer ME, Nathan PJ, Phan KL. Neural correlates of internally-generated disgust via autobiographical recall: a functional magnetic resonance imaging investigation. Neuroscience Letters. 2004;370(2–3):91–96. doi: 10.1016/j.neulet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O’Doherty J, Browning A, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10(3):453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Frey S, Kostopoulos P, Petrides M. Orbitofrontal involvement in the processing of unpleasant auditory information. European Journal of Neuroscience. 2000;12(10):3709–3712. doi: 10.1046/j.1460-9568.2000.00227.x. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Skudlarski P, Lacadie CM, Warrenburg S, Bowers AA, Gore JC, et al. Functional MR imaging of regional brain responses to pleasant and unpleasant odors. American Journal of Neuroradiology. 1998;19(9):1721–1726. [PMC free article] [PubMed] [Google Scholar]
- Gemar MC, Kapur S, Segal ZV, Brown GM, Houle S. Effects of self-generated sad mood on regional cerebral activity: a PET study in normal subjects. Depression. 1996;4(2):81–88. doi: 10.1002/(SICI)1522-7162(1996)4:2<81::AID-DEPR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Gill DS, Haxby JV, Ungerleider LG, Herscovitch P, et al. Brain regions involved in recognizing facial emotion or identity: an oxygen-15 PET study. Journal of neuropsychiatry and clinical neurosciences. 1993;5(4):384–394. doi: 10.1176/jnp.5.4.384. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Herscovitch P, Post RM. Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biological Psychiatry. 1996;40(9):859–871. doi: 10.1016/0006-3223(95)00572-2. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. American Journal of Psychiatry. 1995;152(3):341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, et al. Regional brain activity when selecting a response despite interference: an H2 15 O PET study of the Stroop and an emotional Stroop. Human Brain Mapping. 1994;1:194–209. doi: 10.1002/hbm.460010305. [DOI] [PubMed] [Google Scholar]
- George MS, Parekh PI, Rosinsky N, Ketter TA, Kimbrell TA, Heilman KM, et al. Understanding emotional prosody activates right hemisphere regions. Archives of Neurology. 1996;53(7):665–670. doi: 10.1001/archneur.1996.00550070103017. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. The functional anatomy of humor: segregating cognitive and affective components. Nature Neuroscience. 2001;4(3):237–238. doi: 10.1038/85076. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Hutcherson CA, Ochsner KN, Glover GH, Gabrieli JD, Gross JJ. The neural bases of amusement and sadness: a comparison of block contrast and subject-specific emotion intensity regression approaches. Neuroimage. 2005;27(1):26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, et al. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14(2):465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. The Journal of Neuroscience. 2002;22(24):10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean D, Sander D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, et al. The voices of wrath: brain responses to angry prosody in meaningless speech. Nature Neuroscience. 2005;8(2):145–146. doi: 10.1038/nn1392. [DOI] [PubMed] [Google Scholar]
- Grimm S, Schmidt CF, Bermpohl F, Heinzel A, Dahlem Y, Wyss M, et al. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex—an fMRI study. Neuroimage. 2006;30(1):325–340. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Grosbas M, Paus T. Brain networks involved in viewing angry hands or faces. Cerebral Cortex. 2006;16(8):1087–1096. doi: 10.1093/cercor/bhj050. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16(3 Pt 1):651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Habel U, Klein M, Kellermann T, Shah NJ, Schneider F. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage. 2005;26(1):206–214. doi: 10.1016/j.neuroimage.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57(6):624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heinzel A, Bermpohl F, Niese R, Pfennig A, Pascual-Leone A, Schlaug G, et al. How do we modulate our emotions? Parametric fMRI reveals cortical midline structures as regions specifically involved in the processing of emotional valences. Cognitive Brain Research. 2005;25(1):348–358. doi: 10.1016/j.cogbrainres.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry. 2001;50(4):292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JD, Barrett LF, Gross JJ. Attention and emotion: does rating emotion alter neural responses to amusing and sad films? Neuroimage. 2005;27(3):656–668. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, et al. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. Journal of Cognitive Neuroscience. 2001;13(8):1035–1047. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- Imaizumi S, Mori K, Kiritani S, Kawashima R, Sugiura M, Fukuda H, et al. Vocal identification of speaker and emotion activates different brain regions. Neuroreport. 1997;8(12):2809–2812. doi: 10.1097/00001756-199708180-00031. [DOI] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, et al. Linguistic threat activates the human amygdala. Proceedings of the National Academy of Sciences. 1999;96(18):10456–10459. doi: 10.1073/pnas.96.18.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler-West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, et al. Neural substrates of facial emotion processing using fMRI. Cognitive Brain Research. 2001;11(2):213–226. doi: 10.1016/s0926-6410(00)00073-2. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21(4):1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Egan G, Gideon DA, Ely TD, Hoffman JM. Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. Neuroimage. 2003;18(1):156–168. doi: 10.1006/nimg.2002.1323. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, George MS, Parekh PI, Ketter TA, Podell DM, Danielson AL, et al. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biological Psychiatry. 1999;46(4):454–465. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- Klein S, Smolka MN, Wrase J, Gruesser SM, Mann K, Braus DF, et al. The influence of gender and emotional valence of visual cues on FMRI activation in humans. Pharmacopsychiatry. 2003;36 Suppl 3:S191–S194. doi: 10.1055/s-2003-45129. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Shin LM, Thompson WL, McNally RJ, Rauch SL, Pitman RK, et al. Neural effects of visualizing and perceiving aversive stimuli: a PET investigation. Neuroreport. 1996;7(10):1569–1576. doi: 10.1097/00001756-199607080-00007. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo ML, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: an fMRI study. Neuroimage. 2005;28(4):1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37(9):989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8(18):3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. American Journal of Psychiatry. 1997;154(7):926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness: evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10(4):525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35(11):1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35(2):199–210. [PubMed] [Google Scholar]
- Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SC, et al. Task instructions modulate neural responses to fearful facial expressions. Biological Psychiatry. 2003;53(3):226–232. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- Lee GP, Meador KJ, Loring DW, Allison JD, Brown WS, Paul LK, et al. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cognitive and Behavioral Neurology. 2004;17(1):9–17. doi: 10.1097/00146965-200403000-00002. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53(6):502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28(4):726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshima S. Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology. 2000;23(5):508–516. doi: 10.1016/S0893-133X(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, et al. A direct brainstem—amygdala—cortical 'alarm' system for subliminal signals of fear. Neuroimage. 2005;24(1):235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic—cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biological Psychiatry. 2000;48(1):30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, et al. Feasibility of using fMRI to study mothers responding to infant cries. Depression and Anxiety. 1999;10(3):99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH. Activation of left posterior cingulate gyrus by the auditory presentation of threat-related words: an fMRI study. Psychiatry Research: Neuroimaging. 1997;75(1):1–14. doi: 10.1016/s0925-4927(97)00018-8. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RN, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39(9):910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Vandekerckhove MM, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex. 2003;39(4–5):643–665. doi: 10.1016/s0010-9452(08)70858-x. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McCullough S, Emmorey K, Sereno M. Neural organization for recognition of grammatical and emotional facial expressions in deaf ASL signers and hearing nonsigners. Cognitive Brain Research. 2005;22(2):193–203. doi: 10.1016/j.cogbrainres.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, Bramati IE, Mourao-Miranda J, Andreiuolo PA, et al. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. The Journal of Neuroscience. 2002;22(7):2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Moll FT, Ignacio FA, Bramati IE, Caparelli-Daquer EM, et al. The moral affiliations of disgust: a functional MRI study. Cognitive and Behavioral Neurology. 2005;18(1):68–78. doi: 10.1097/01.wnn.0000152236.46475.a7. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Morris JS, Scott SK, Dolan RJ. Saying it with feeling: neural responses to emotional vocalizations. Neuropsychologia. 1999;37(10):1155–1163. doi: 10.1016/s0028-3932(99)00015-9. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Ito K, Sugiura M, Kato T, Nakamura A, et al. Activation of the right inferior frontal cortex during assessment of facial emotion. Journal of Neurophysiology. 1999;82(3):1610–1614. doi: 10.1152/jn.1999.82.3.1610. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Yamada H, Iidaka T, Sadato N, Fukui K, Itoh H, et al. Brain regions involved in verbal or non-verbal aspects of facial emotion recognition. Neuroreport. 2000;11(11):2571–2576. doi: 10.1097/00001756-200008030-00044. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21(2):583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, et al. Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fMRI study. Neuroimage. 2004;21(1):352–363. doi: 10.1016/j.neuroimage.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Bermpohl F, Niese R, Pfennig A, Pascual-Leone A, et al. Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Human Brain Mapping. 2004;21(3):202–212. doi: 10.1002/hbm.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Sirota A, Niaura R, Rauch SL, Brown WA. Neural and endocrine correlates of sadness in women: implications for neural network regulation of HPA activity. Journal of neuropsychiatry and clinical neurosciences. 2004;16(4):446–455. doi: 10.1176/jnp.16.4.446. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Johnson DL, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, et al. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. American Journal of Psychiatry. 1999;156(10):1618–1629. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Robinson RG, Andreasen NC, Downhill JE, Davidson RJ, Kirchner PT, et al. Emotional activation of limbic circuitry in elderly normal subjects in a PET study. American Journal of Psychiatry. 1997;154(3):384–389. doi: 10.1176/ajp.154.3.384. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Robinson RG, Boles Ponto LL, Watkins GL, Hichwa RD. Regional cerebral blood flow changes during visually induced subjective sadness in healthy elderly persons. Journal of neuropsychiatry and clinical neurosciences. 2003;15(1):35–44. doi: 10.1176/jnp.15.1.35. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Raichle ME. Neural correlates of self-induced dysphoria. American Journal of Psychiatry. 1993;150(5):713–719. doi: 10.1176/ajp.150.5.713. [DOI] [PubMed] [Google Scholar]
- Partiot A, Grafman J, Sadato N, Wachs J, Hallett M. Brain activation during the generation of non-emotional and emotional plans. Neuroreport. 1995;6(10):1397–1400. doi: 10.1097/00001756-199507100-00009. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Gao K, Moore GJ, Tancer ME, Posse S. Real-time fMRI of cortico-limbic brain activity during emotional processing. Neuroreport. 2004;15(3):527–532. doi: 10.1097/00001756-200403010-00029. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, Williams SC, et al. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Research: Neuroimaging. 1998;83(3):127–138. doi: 10.1016/s0925-4927(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, et al. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21(4):1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1998;265(1408):1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. American Journal of Psychiatry. 2000;157(11):1772–1781. doi: 10.1176/appi.ajp.157.11.1772. [DOI] [PubMed] [Google Scholar]
- Pourtois G, de Gelder B, Bol A, Crommelinck M. Perception of facial expressions and voices and of their combination in the human brain. Cortex. 2005;41(1):49–59. doi: 10.1016/s0010-9452(08)70177-1. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Dougherty DD, Alpert NM, Orr SP, Lasko M, et al. Neural activation during sexual and competitive arousal in healthy men. Psychiatry Research: Neuroimaging. 1999;91(1):1–10. doi: 10.1016/s0925-4927(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Redoute J, Stoleru S, Gregoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Human Brain Mapping. 2000;11(3):162–177. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, et al. Neuroanatomical correlates of externally and internally generated human emotion. American Journal of Psychiatry. 1997;154(7):918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Reinders AA, den Boer JA, Buchel C. The robustness of perception. European Journal of Neuroscience. 2005;22(2):524–530. doi: 10.1111/j.1460-9568.2005.04212.x. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, de Araujo IE. Different representations of pleasant and unpleasant odours in the human brain. European Journal of Neuroscience. 2003;18(3):695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Royet JP, Hudry J, Zald DH, Godinot D, Gregoire MC, Lavenne F, et al. Functional neuroanatomy of different olfactory judgments. Neuroimage. 2001;13(3):506–519. doi: 10.1006/nimg.2000.0704. [DOI] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. Journal of Neuroscience. 2000;20(20):7752–7759. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16(6):988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Sato W, Yoshikawa S, Kochiyama T, Matsumura M. The amygdala processes the emotional significance of facial expressions: an fMRI investigation using the interaction between expression and face direction. Neuroimage. 2004;22(2):1006–1013. doi: 10.1016/j.neuroimage.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Schafer A, Schienle A, Vaitl D. Stimulus type and design influence hemodynamic responses towards visual disgust and fear elicitors. International Journal of Psychophysiology. 2005;57(1):53–59. doi: 10.1016/j.ijpsycho.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Walter B, Stark R, Vaitl D. fMRI responses to pictures of mutilation and contamination. Neuroscience Letters. 2006;393(2–3):174–178. doi: 10.1016/j.neulet.2005.09.072. [DOI] [PubMed] [Google Scholar]
- Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, et al. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. 2002;13(16):2023–2026. doi: 10.1097/00001756-200211150-00006. [DOI] [PubMed] [Google Scholar]
- Schroeder U, Hennenlotter A, Erhard P, Haslinger B, Stahl R, Lange KW, et al. Functional neuroanatomy of perceiving surprised faces. Human Brain Mapping. 2004;23(4):181–187. doi: 10.1002/hbm.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B, Zuck E. Segregated processing of facial identity and emotion in the human brain: a PET study. Visual Cognition. 1994;1:349–369. [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biological Psychiatry. 2000;48(1):43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shirao N, Okamoto Y, Okada G, Ueda K, Yamawaki S. Gender differences in brain activity toward unpleasant linguistic stimuli concerning interpersonal relationships: an fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2005;255(5):327–333. doi: 10.1007/s00406-005-0566-x. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Ongur D, Akbudak E, Conturo TE, Ollinger JM, Snyder AZ, et al. The emotional modulation of cognitive processing: an fMRI study. Journal of Cognitive Neuroscience. 2000;12 Suppl 2:157–170. doi: 10.1162/089892900564019. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39(4):701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biological Psychiatry. 2004;55(9):897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1998;265(1409):1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Schienle A, Sarlo M, Palomba D, Walter B, Vaitl D. Influences of disgust sensitivity on hemodynamic responses towards a disgust-inducing film clip. International Journal of Psychophysiology. 2005;57(1):61–67. doi: 10.1016/j.ijpsycho.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Stark R, Schienle A, Walter B, Kirsch P, Sammer G, Ott U, et al. Hemodynamic responses to fear and disgust-inducing pictures: an fMRI study. International Journal of Psychophysiology. 2003;50(3):225–234. doi: 10.1016/s0167-8760(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Strange BA, Henson RN, Friston KJ, Dolan RJ. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. Neuroimage. 2000;12(4):425–433. doi: 10.1006/nimg.2000.0637. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Borod JC, Tang CY, Lange G, Wei TC, Johnson R, et al. Differential amygdala activation during emotional decision and recognition memory tasks using unpleasant words: an fMRI study. Neuropsychologia. 2001;39(6):556–573. doi: 10.1016/s0028-3932(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Fig LM, Decker LR, Minoshima S, Koeppe RA. The effect of emotional content on visual recognition memory: a PET activation study. Neuroimage. 1998;8(2):188–197. doi: 10.1006/nimg.1998.0356. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38(10):1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18(3):650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SC, et al. Functional MRI study of the cognitive generation of affect. American Journal of Psychiatry. 1999;156(2):209–215. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, et al. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Research: Neuroimaging. 2005;139(1):9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30(3):829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wang L, McCarthy G, Song AW, Labar KS. Amygdala activation to sad pictures during high-field 4 tesla functional magnetic resonance imaging. Emotion. 2005;5(1):12–22. doi: 10.1037/1528-3542.5.1.12. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Riecker A, Hertrich I, Erb M, Grodd W, Ethofer T, et al. Identification of emotional intonation evaluated by fMRI. Neuroimage. 2005;24(4):1233–1241. doi: 10.1016/j.neuroimage.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Das P, Boucsein W, Sokolov EN, Brammer MJ, et al. The dynamics of cortico-amygdala and autonomic activity over the experimental time course of fear perception. Cognitive Brain Research. 2004;21(1):114–123. doi: 10.1016/j.cogbrainres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell B, Olivieri G, Peduto A, Brammer MJ, et al. BOLD, sweat and fears: fMRI and skin conductance distinguish facial fear signals. Neuroreport. 2005;16(1):49–52. doi: 10.1097/00001756-200501190-00012. [DOI] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14(5):1070–1079. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Williams MA, McGlone F, Abbott DF, Mattingley JB. Differential amygdala responses to happy and fearful facial expressions depend on selective attention. Neuroimage. 2005;24(2):417–425. doi: 10.1016/j.neuroimage.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Hermann D, Flor H, Mann K, et al. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neuroscience Letters. 2003;348(1):41–45. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]
- Wright P, He G, Shapira NA, Goodman WK, Liu Y. Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport. 2004;15(15):2347–2351. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences. 2002;99(17):11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998;121(Pt 6):1143–1154. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proceedings of the National Academy of Sciences. 1997;94(8):4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M, Rouby C. Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport. 2000;11(12):2711–2716. doi: 10.1097/00001756-200008210-00021. [DOI] [PubMed] [Google Scholar]
Footnotes
Available online on ScienceDirect (http://www.sciencedirect.com)
We considered activations only and ignored deactivations, as those are inconsistently reported and therefore do not allow for meaningful generalization. Also, the neural mechanisms underlying reported deactivations remain undetermined and their interpretations remain inconclusive or unclear (Gusnard and Raichle, 2001; Hutchinson et al., 1999).
While this was not computationally tractable with the full set of 18,489 voxels, it was with the reduced set of 172 parcels; the 172-parcel analysis took 16 h on a 2007 Intel Power Macintosh with 8 GB of RAM.
When dmPFC was active, 34% of contrasts (N=67) activated LSA. When dmPFC was inactive, 23% of contrasts (N=370) activated LSA.
When frOP was active, 35% of contrasts (N=82) activated LBL, 35% activated LSA, and 33% activated RSA. When frOP was inactive, 27% of contrasts (N=355) activated LBL, 22% activated LSA, and 24% activated RSA.
When rdACC was active, 31% of contrasts (N=88) activated RBL, 32% activated LSA, and 35% activated RSA. When rdACC was inactive, 23% of contrasts (N=349) activated RBL, 23% activated LSA, and 30% activated RSA.
When pgACC was active, 36% of contrasts (N=67) activated RBL, 33% activated LSA, and 33% activated RSA. When pgACC was inactive, 23% of contrasts (N=370) activated RBL, 23% activated LSA, and 25% activated RSA.
When frOP was active, 29% of contrasts (N=82) activated PAG/Thal. When frOP was inactive, 16% of contrasts (N=355) activated PAG/Thal. When rdACC was active, 27%of contrasts(N=88) activated PAG/Thal. When rdACC was inactive, 16% of contrasts (N=349) activated PAG/Thal. When dmPFCwas active, 33% of contrasts (N=67) activated PAG/Thal. When dmPFC was inactive, 16% of contrasts (N=370) activated PAG/Thal.
When dmPFC was active, 27% of contrasts activated Hy. When dmPFC was inactive, 14% of contrasts activated Hy.
References
- Adolphs R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Albus JS. A theory of cerebellar function. Math. Biosci. 1971;10:25–61. [Google Scholar]
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in Macaque monkeys. J. Comp. Neurol. 1998;401(4):455–479. [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore T, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004a;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron AR, Shohamy D, Clark J, Myers C, Gluck MA, Poldrack RA. Human midbrain sensitivity to cognitive feedback and uncertainty during classification learning. J. Neurophysiol. 2004b;92(2):1144–1152. doi: 10.1152/jn.01209.2003. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrievel and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17(9):379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Are emotions natural kinds. Perspect. Psychol. Sci. 2006a;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Solving the emotion paradox: categorization and the experience of emotion. Personal. Soc. Psychol. Rev. 2006b;10(1):20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Wager TD. The structure of emotion: evidence from neuroimaging studies. Curr. Dir. Psychol. Sci. 2006;15(2):79–83. [Google Scholar]
- Barrett LF, Lindquist KA, Bliss-Moreau E, Duncan S, Gendron M, Mize J, et al. Of mice and men: natural kinds of emotions in the mammalian brain? A response to Panksepp and Izard. Perspect. Psychol. Sci. 2007a;2(3):297–312. doi: 10.1111/j.1745-6916.2007.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annu. Rev. Psychol. 2007b;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Ochsner KN, Gross JJ. On the automaticity of emotion. In: Bargh J, editor. Social psychology and the unconscious: The automaticity of higher mental processes. New York: Psychology Press; 2007c. [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat. Rev., Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog. Neurobiol. 1995;46(6):575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7(1):133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J. Neurosci. 2005;25(45):10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Well-being: Foundations of hedonic psychology. New York: Russell Sage Foundation; 1999. Pleasure, pain, desire, and dread: hidden core processes of emotion; pp. 527–559. [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Winston J, Frith U. Social cognitive neuroscience: where are we heading? Trends Cogn. Sci. 2004;8:216–222. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat. Rev., Neurosci. 2002;3(3):243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components: form follows function. J. Pers. Soc. Psychol. 1999;76(5):839–855. [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, Ito TA. The psychophysiology of emotion. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotion. 2nd ed. New York: Guilford; 2000. pp. 173–191. [Google Scholar]
- Cameron AA, Khan IA, Westlund KN. The efferent projections of the periaqueductal gray in the rat: APhaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J. Comp. Neurol. 1995;351(4):568–584. doi: 10.1002/cne.903510407. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R, Dampney RA. Somatic and autonomic integration in the midbrain of the unanesthetized decerebrate cat: a distinctive pattern evoked by excitation of neurones in the subtentorial portion of the midbrain periaqueductal grey. Brain Res. 1989;483(2):251–258. doi: 10.1016/0006-8993(89)90169-8. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev., Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev., Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Oehman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33(1):127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur. Neuropsychopharmacol. 2002;12(6):527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Duncan S, Barrett LF. The role of the amygdala in visual awareness. Trends Cogn. Sci. 2007;11(5):190–192. doi: 10.1016/j.tics.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain Stem and Cerebellum: Surface, Structure, Vascularization, and Three-dimensional Sectional Anatomy with MRI. Springer-Verlag Wien. 1995 [Google Scholar]
- Duvernoy HM, Bourgouin P. The Human Brain: Surface, Three-dimensional Sectional Anatomy with MRI, and Blood Supply. Springer; 1999. [Google Scholar]
- Edelman GM, Tononi G. A Universe of Consciousness: how matter becomes imagination. Basic Books. 2001 [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage. 2006;32(2):570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Ekman P. An argument for basic emotions. Cogn. Emot. 1992;6:169–200. [Google Scholar]
- Ekman P, Davidson RJ. The nature of emotion: fundamental questions. Oxford University Press; 1994. [Google Scholar]
- Ellsworth PC, Scherer KR. Appraisal processes in emotion. Handbook of affective sciences. 2003:572–595. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a metaanalysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward: the role of amygdala-ventral striatal subsystems. Ann. N. Y. Acad. Sci. 1999;877(1):412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Faymonville ME, Laureys S, Degueldre C, Del Fiore G, Luxen A, Franck G, et al. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology. 2000;92(5):1257–1267. doi: 10.1097/00000542-200005000-00013. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res. Bull. 2003;59(5):387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Flandin G, Kherif F, Pennec X, Riviere D, Ayache N, Poline JB, et al. Parcellation of brain images with anatomical and functional constraints for fMRI data analysis. Biomedical Imaging. 2002:907–910. 2002. Proceedings. 2002 IEEE International Symposium on. [Google Scholar]
- Fox PT, Parsons LM, Lancaster JL. Beyond the single study: function/location metanalysis in cognitive neuroimaging. Curr. Opin. Neurobiol. 1998;8(2):178–187. doi: 10.1016/s0959-4388(98)80138-4. [DOI] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V 1 in the macaque monkey. J. Comp. Neurol. 2005;486(4):295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc. Natl. Acad. Sci. 1998;95(3):906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons JD. Nonparametric Measures of Association. Sage Publications Inc; 1993. [Google Scholar]
- Gibbons JD, Chakraborti S, Gibbons JGD. Nonparametric Statistical Inference. Marcel Dekker. 2003 [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat. Neurosci. 2004;7(10):1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding Predictive Reward Value in Human Amygdala and Orbitofrontal Cortex. American Association for the Advancement of Science. 2003 doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. Fundamentals of the septo-hippocampal system. 2nd ed. Oxford, UK: Oxford University Press; 2000. [Google Scholar]
- Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25(1):91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: fnuctionl imaging and the resting human brain. Nat. Rev., Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haines DE. Philadelphia: Lippincott Williams & Wilkins; 2000. Neuroanatomy: An Atlas of Structures, Sections, and Systems. [Google Scholar]
- Haines DE, Dietrichs E. An HRP study of hypothalamo-cerebellar and cerebello-hypothalamic connections in squirrel monkey Saimiri sciureus. J. Comput. Neurol. 1984;229(4):559–575. doi: 10.1002/cne.902290409. [DOI] [PubMed] [Google Scholar]
- Heath RG, Cox AW, Lustick LS. Brain activity during emotional states. Am. J. Psychiatr. 1974;131(8):858–862. doi: 10.1176/ajp.131.8.858. [DOI] [PubMed] [Google Scholar]
- Heath RG, Dempesy CW, Fontana CJ, Myers WA. Cerebellar stimulation: effects on septal region, hippocampus, and amygdala of cats and rats. Biol. Psychiatry. 1978;13(5):501–529. [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr. Opin. Neurobiol. 2004;14(2):148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Holstege G, Georgiadis JR. The emotional brain: neural correlates of cat sexual behavior and human male ejaculation. Prog. Brain Res. 2004;143:39–45. doi: 10.1016/S0079-6123(03)43004-5. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Schiffer W, Joseffer S, Liu A, Schlosser R, Dikshit S, et al. Task-specific deactivation patterns in functional magnetic resonance imaging. Magn. Reson. Imaging. 1999;17(10):1427–1436. doi: 10.1016/s0730-725x(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Izard CE. Four systems for emotion activation: cognitive and noncognitive processes. Psychol. Rev. 1993;100(1):68–90. doi: 10.1037/0033-295x.100.1.68. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex. 2008;18(6):1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 2000 [Google Scholar]
- Keay KA, Bandler R. Distinct central representations of inescapable and escapable pain: observations and speculation. Exp. Physiol. 2002;87(2):275–279. doi: 10.1113/eph8702355. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short-and long-term contextual fear. Behav. Neurosci. 1993;107(6):1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J. Neurosci. 2006;26(2):381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kruskal JB. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika. 1964;29(1):1–27. [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat. Rev., Neurosci. 2006;7(4):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 2005;25(1):155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, McRae K. Neural substrates of conscious emotional experience: a cognitive-neuroscientific perspective. In: Beauregard M, editor. Consciousness, emotional self-regulation and the brain. Amsterdamn: John Benjamins; 2004. pp. 87–122. [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. American Association for the Advancement of Science. 2004;vol 303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom S, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol. Sci. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, et al. Parietal cortex and representation of the mental self. Proc. Natl. Acad. Sci. 2004;101(17):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA. Inhibitory modulation of the cardiovascular defence response by the ventrolateral periaqueductal grey matter in rats. Exp. Brain Res. 1992;89(1):133–139. doi: 10.1007/BF00229010. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Driver J. Spatial attention and crossmodal interactions between vision and touch. Neuropsychologia. 2001;39(12):1304–1316. doi: 10.1016/s0028-3932(01)00119-1. [DOI] [PubMed] [Google Scholar]
- Maclean PD. Psychosomatic disease and the "visceral brain" recent developments bearing on the papez theory of emotion. Psychosom. Med. 1949;11(6):338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22(7):310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Mantani T, Okamoto Y, Shirao N, Okada G, Yamawaki S. Reduced activation of posterior cingulate cortex during imagery in subjects with high degrees of alexithymia: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:982–990. doi: 10.1016/j.biopsych.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Connections of midbrain periaqueductal gray in the monkey. I. Ascending efferent projections. J. Neurophysiol. 1983;49(3):567–581. doi: 10.1152/jn.1983.49.3.567. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 1997;88(2):261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: Structure and processes. Curr. Opin. Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Martin JH. 2nd ed. Stamford, CT: Appleton & Lange; 1996. Neuroanatomy: Text and Atlas. [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspect. Psychol. Sci. 2007;2(1):33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol. Psychiatry. 2000;48(8):830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Nobre AC, Kim YH, Parrish TB, Gitelman DR. Heterogeneity of cingulate contributions to spatial attention. NeuroImage. 2001;13(6 Part 1):1065–1072. doi: 10.1006/nimg.2001.0768. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 2001;21(2):700. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Brain Res. Rev. 2000;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann. Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J. Cogn. Neurosci. 2006;18(9):1586. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3(3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn. Affect. Behav. Neurosci. 2007;7(7):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nielsen FA, Hansen LK. A functional meta-analytic atlas with non-negative partial least squares; Paper presented at the Annual Meeting of the Organization for Human Brain Mapping..2006. [Google Scholar]
- Nielsen FA, Copenhagen D, Lyngby D. Mass meta-analysis in Talairach space. Adv. Neural Inf. Process. Syst. 2005;17:985–992. [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable Roles of Ventral and Dorsal Striatum in Instrumental Conditioning. American Association for the Advancement of Science. 2004 doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004a;16(10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, et al. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage. 2004b;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in Macaque monkeys. J. Comp. Neurol. 1998;401(4):480–505. [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Oxford University Press; 1998. Affective Neuroscience: The Foundations of Human and Animal Emotions. [Google Scholar]
- Papez JW. A Proposed Mechanism of Emotion: American Medical Association. 1937 [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439(7078):865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and Opioid Analgesia—c Imaging a Shared Neuronal Network. 2002;vol. 295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Anderson AK. Emotional memory: what does the amygdala do. Curr. Biol. 1997;7(5) doi: 10.1016/s0960-9822(06)00146-1. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Doniger GM, Kastner S. Push-pull mechanism of selective attention in human extrastriate cortex. J. Neurophys. 2004;92(1):622–629. doi: 10.1152/jn.00974.2003. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuro-Image. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, et al. Does anticipation of pain affect cortical nociceptive systems? J. Neurosci. 2002;22(8):3206. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288(5472):1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Royet JP, Hudry J, Zald DH, Godinot D, Gregoire MC, Lavenne F, et al. Functional neuroanatomy of different olfactory judgments. NeuroImage. 2001;13(3):506–519. doi: 10.1006/nimg.2000.0704. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Which ifs have causal answers. J. Am. Stat. Assoc. 1986;81:961–962. [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychol. Rev. 2003;110(1):145–172. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J. Pers. Soc. Psychol. 1999;76(5):805–819. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central autonomic system. The Rat Nervous System. 1995:107–135. [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117(2):305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42(11):1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Schenberg LC, Bittencourt AS, Sudre EC, Vargas LC. Modeling panic attacks. Neurosci. Biobehav. Rev. 2001;25(7–8):647–659. doi: 10.1016/s0149-7634(01)00060-4. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 2000;23(1):473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb. Cortex. 2000;10(3):272–283. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sem-Jacobsen CW. CC Thomas; 1968. Depth-electrographic Stimulation of the Human Brain and Behavior: From Fourteen Years of Studies and Treatment of Parkinson’s Disease and Mental Disorders with Implanted Electrodes. [Google Scholar]
- Sewards TV, Sewards MA. Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Res. Bull. 2003;61(1):25–49. doi: 10.1016/s0361-9230(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Shepard RN. The analysis of proximities: multidimensional scaling with an unknown distance function. I. Psychometrika. 1962;27(2):125–140. [Google Scholar]
- Shepard RN. Multidimensional scaling, tree-fitting, and clustering. Science. 1980;210(4468):390. doi: 10.1126/science.210.4468.390. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311(5767):1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuro-Image. 2003;18(3):633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol. Method. 1982;13:290–312. [Google Scholar]
- Stolarova M, Keil A, Moratti S. Modulation of the C1 visual event-related component by conditioned stimuli: evidence for sensory plasticity in early affective perception. Cereb. Cortex. 2006;16(6):876–887. doi: 10.1093/cercor/bhj031. [DOI] [PubMed] [Google Scholar]
- Struyf A, Hubert M, Rousseeuw P. Clustering in an object-oriented environment. J. Stat. Softw. 1996;1(4):1–30. [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos. Trans. R. Soc. B: Biol. Sci. 2007;362(1481):901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27(1–2):99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J. Predictive codes for forthcoming perception in the frontal cortex. Science. 2006;314(5803):1311. doi: 10.1126/science.1132028. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. New York, NY: Thieme Medical Publishers; 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System — an Approach to Cerebral Imaging. [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38(10):1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Thirion B, Flandin G, Pinel P, Roche A, Ciuciu P, Poline JB. Dealing with the shortcomings of spatial normalization: multi-subject parcellation of fMRI datasets. Hum. Brain Mapp. 2006;27(8):678–693. doi: 10.1002/hbm.20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge. A reevaluation. National Acad Sciences. 1997 doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Reexamination of the role of the hypothalamus in motivation. Psychol. Rev. 1970;77(1):16–31. doi: 10.1037/h0028581. [DOI] [PubMed] [Google Scholar]
- Van der Horst VG, Holstege G. Sensory and motor components of reproductive behavior: pathways and plasticity. Behav. Brain Res. 1998;92(2):157–167. doi: 10.1016/s0166-4328(97)00188-5. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. Organization of visual areas in macaque and human cerebral cortex. In: Chalupa L, Werner JS, editors. The Visual Neurosciences. Cambridge, MA: MIT Press; 2004. pp. 507–521. [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J. Am. Med. Inform. Assoc. 2001;8(5):443. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. NeuroImage. 2007;36(3):1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J. Neurophysiol. 2006;96(6):3517. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb. Cortex. 1992;2(6):435. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat. Neurosci. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wager TD. The neural bases of placebo effects in pain. Curr. Dir. Psychol. Sci. 2005;14(4):175–179. [Google Scholar]
- Wager TD, Barrett LF. From affect to control: Functional specialization of the insula in motivation and regulation. Published online at PsychExtra. 2004 [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19(3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wager TD, Reading S, Jonides J. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage. 2004a;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004b;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Smith EE, Nichols TE. Towards a taxonomy of attention-shifting: individual differences in fMRI during multiple shift types. Cogn. Affect. Behav. Neurosci. 2005;5(2):127–143. doi: 10.3758/cabn.5.2.127. [DOI] [PubMed] [Google Scholar]
- Wager TD, Waugh C, Lindquist MA, Fredrickson B, Noll DC, Taylot SF. The role of ventromedial prefrontal cortex in anxiety and emotional resilience. Paper presented at the Human Brain MApping. 2006 [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: Current and future directions. Soc. Cogn. Affect. Neurosci. 2007a;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human muopioid activity during pain. Proc. Natl. Acad. Sci. 2007b;104(26):11056. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, et al. The Neuroimaging of Emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotion. 3rd Edition. New York: Guilford; 2008. pp. 249–271. [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral Prefrontal cortex. NeuroImage. 2001;14(6):1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychol. Bull. 1985;98(2):219–235. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiol. Aging. 2006;27(2):361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M, Rouby C. Neural mechanisms involved in odor pleasantness and intensity judgments. NeuroReport. 2000;11(12):2711–2716. doi: 10.1097/00001756-200008210-00021. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J. Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]