Introduction
Secondhand smoke exposure is an important trigger of childhood asthma.1,2,3 Motivating parents to reduce their child's exposure to smoke is an important step in achieving asthma control.4 Understanding parents' beliefs about the extent and health effects of smoke exposure, and their readiness to change behaviors to reduce such exposure are important for designing smoke exposure reduction interventions.
Readiness to cease personal smoking has been described 5,6,7,8; however, there has been relatively little research describing parental readiness to make changes to reduce smoke exposure in their children. Readiness to change one's own smoking, having a nonsmoking partner, and having a child in the home have been associated with greater in-home smoking restrictions.9,10,11 Research on parents of children with asthma is more limited. One study12 of parents of wheezing children presenting to a pediatric emergency department found that most parents who smoked wanted to quit and knew that secondhand smoke could contribute to asthma. The aims of this analysis were as follows: (1) to examine associations between sources of tobacco smoke exposure and biomarkers of smoke exposure in smoke-exposed children with asthma; (2) to describe parental perceptions about the extent and effects of tobacco smoke exposure, and their relationship to biomarkers of their child's smoke exposure; and (3) to assess parental readiness to make changes to reduce or eliminate secondhand tobacco smoke exposure in their child.
Materials and Methods
Study methods were reviewed and approved by the Institutional Review Boards of the Kaiser Permanente Northern California Region and the Palo Alto Medical Foundation Research Institute.
Sample/Population
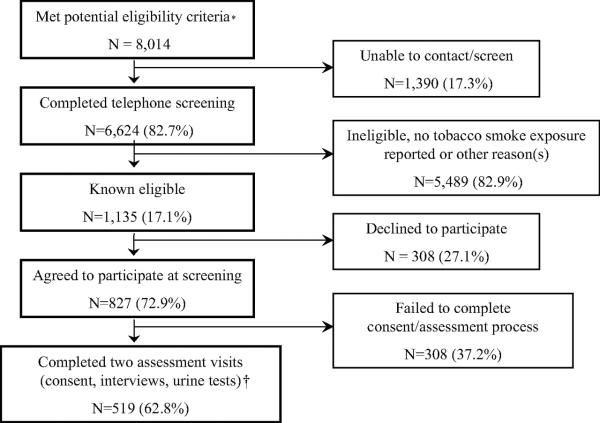
For potential recruitment into a randomized controlled clinical trial of a secondhand smoke exposure reduction intervention, children 3 to 12 years old with medication use and/or a physician diagnosis suggesting persistent asthma were identified from health-plan computerized databases: (1) four or more β-agonist dispensing events in the prior year; (2) four or more antiinflammatory asthma medication dispensing events in the prior year; and (3) a physician diagnosis of either mild persistent, moderate persistent, or severe persistent asthma. Eligibility criteria and the recruitment flowcharts are presented in Figure 1. With the child's primary care physician's approval, the parents were sent a letter; if they did not decline further contact, they were telephoned by research staff. The parents/primary caregiver were identified through the child's electronic medical record and confirmed during the initial telephone contact. If the parent reported current exposure to tobacco smoke at home or in any other place that their child regularly spent time, the parent was invited to bring the child to a study visit. The parent or legal guardian who provided informed consent and came to the study visits was considered the primary caregiver.
Figure 1.
Recruitment process results.
*From administrative data: age 3 to 12 years, Kaiser Permamente member for ≥ 1 year, one or more asthma care visits in prior year, and met one of the following three criteria suggesting persistent asthma: physician diagnosis code of persistent asthma (based on 1997 National Asthma Education and Prevention Program guidelines4), or pharmacy records documenting four or more β-agonist dispensing events in prior year or four or more antiinflammatory asthma controller medication dispensing events in prior year, and physician-approved recruitment of the family.
†The analysis sample (N = 519) represents 45.7% of the known eligible children.
After the caregiver completed an interviewer-administered questionnaire, urine was collected from the child; if the child was ≥ 5 years old, he or she underwent spirometry with low-dose bronchodilator challenge. At a second assessment visit at least 1 week later, an additional questionnaire was completed, another urine sample was obtained, and spirometry was repeated. The parent received $30 after the second visit; the child received an inexpensive toy at each visit.
Data Collection
Primary Caregiver Questionnaire
Sources of tobacco smoke exposure were determined from questions about the primary caregiver's own smoking, smoking by others who lived in the home, smoking at other places the child visited regularly, and smoke exposure in day-care or child-care settings. Parental stage of change with respect to behaviors that would reduce secondhand smoke exposure was determined from the parents' answers to questions patterned on those typically used to classify stage of change within the framework of the transtheoretical model of health behavior change.13
This model describes five stages: precontemplation, contemplation, preparation, action, and maintenance.5 In precontemplation, the person is not thinking about making the specific change in question. In the contemplation stage, the person is intending to make the change within the next 6 months but there is no commitment to action. In preparation, the person is intending to make the change within the coming month. In the action stage, the behavior change has been made within the prior 6 months. Finally, maintenance, although not relevant for the analyses presented herein, is reached when the health behavior change has been sustained for > 6 months. The questionnaire examined four areas of behavior change: (1) personal smoking cessation; (2) making the primary home smoke free; (3) making other places where the child spends time smoke free; and (4) keeping the child out of smoke-exposed locations not in the home. Parents were also asked about their perception of the child's smoke exposure and its effects on asthma.
Biomarkers of Tobacco Smoke Exposure
Cotinine is the major proximate metabolite of nicotine.14,15 Urine cotinine levels are directly correlated with tobacco exposure over the preceding 3 to 4 days.14,16,17 After collection, urine specimens were frozen and transported to the Palo Alto Medical Foundation Research Institute Immunology and Infectious Disease Research Laboratory.
Cotinine Assay
Cotinine levels are typically about six times greater in urine than in serum or saliva.16 To detect the lower urine cotinine concentrations associated with secondhand smoke exposure, we used the OraSure Cotinine Saliva Micro-Plate EIA assay (OraSure Technologies, Inc.; Bethlehem, PA), which is sensitive in the range of 5 to 100 ng/mL that is typically associated with secondhand smoke exposure. This assay was validated on urine samples of volunteers and by using both the saliva kit calibrators and urine kit calibrators across a range of concentrations. All calibrators and each urine sample were subjected to triplicate assay. If two or more of the values were within 5% of each other, the average of these values was used. If not, the sample was assayed again.
Creatinine Assay
Urine creatinine was assessed using the QuantiChrom Creatinine Assay Kit (BioAssay Systems; Hayward, CA). Urine specimens were assayed in duplicate. If the urine creatinine values were within 5% of each other, the average was used. If not the sample was assayed again.
Cotinine to Creatinine Ratio
The cotinine to creatinine ratio (CCR)18 was used to correct for individual variation in urine concentration and was calculated as follows: (100 × cotinine in nanograms per milliliter/creatinine in milligrams per deciliter). We used the mean of two CCR values in our analyses.
Spirometry
Spirometry was performed using a Brass Fleisch-type pneumotach connected to a computer (KoKo Spirometer; Pulmonary Data Systems; Louisville, KY) conforming to 1994 American Thoracic Society standards.19 Postbronchodilator testing was performed at least 15 min after two puffs (0.18 mg) of albuterol via metered-dose inhaler with an AeroChamber VHC (Monaghan Medical Corporation; Plattsburgh, NY). Standing height was measured using a stadiometer. Lung function predicted values were determined according to Wang et al20 for children 5 to 8 years old, and Hankinson et al21 for children ≥ 9 years old.
All maneuvers were reviewed by a pediatric pulmonologist (H.J.F.) to ensure consistency with American Thoracic Society standards.19 FEV1 was accepted but other parameters rejected for maneuvers with > 1 s maximal effort but incomplete exhalation.
Statistical Methods
Differences in group means were tested using analysis of variance and the Duncan test. Differences in proportions were tested using the X2 test and, for paired data, a McNemar test. Statistical significance was accepted as p < 0.05.
Results
Telephone eligibility screening was completed for 82.7% of the children with asthma identified. Of those, 17.1% met the initial eligibility criteria: tobacco smoke exposure, English speaking, and planning to remain a health plan member for the next 12 months. Five hundred nineteen child/primary caregiver dyads provided informed consent and completed both assessments (Fig 1).
Urine cotinine concentrations ranged from 0 to 69.5 ng/mL (mean, 17.7 ng/mL; SD, 14.9 ng.mL); creatinine values ranged from 6.0 to 282.0 mg/dL (mean, 95.6 mg/dL; SD, 44.8 mg/dL). CCRs ranged from 0 to 128.5 ng/mg (mean, 20.1 ng/mg; SD, 19.9 ng/mg).
Child and Caregiver Characteristics
Consistent with other studies3 of childhood asthma, more than half of the children were male (Table 1). The sample included similar numbers of children (approximately 52) at each year of age. Most primary caregivers were mothers/foster mothers or grandmothers. The sample was socioeconomically and ethnically diverse (Table 1). More than three fourths of the children met criteria for not well or very poorly controlled asthma (Table 2).
Table 1.
Demographic Characteristics of the Study Sample (n = 519)
| Characteristics | No. (%) |
|---|---|
| Age, yr | |
| 3 to 5 | 153 (29.5) |
| 6 to 7 | 100 (19.3) |
| 8 to 12 | 266 (51.2) |
| Gender | |
| Male | 306 (59.0) |
| Female | 213 (41.0) |
| Primary caregiver* | |
| Mother | 431 (84.5) |
| Grandmother | 25 (4.9) |
| Father | 43 (8.4) |
| Other | 11 (2.2) |
| Primary caregiver smoking status† | |
| Yes | 191 (37.2) |
| No | 323 (62.8) |
| Parent education‡ | |
| High school or less | 140 (27.9) |
| Some college | 277 (55.3) |
| 4-yr college graduate or higher | 84 (16.8) |
| Family income§ | |
| ≤ $20,000 | 74 (15.7) |
| $20,001 to $40,000 | 116 (24.7) |
| $40,001 to $60,000 | 118 (25.1) |
| $60,001 to $80,000 | 83 (17.7) |
| > $80,000 | 79 (16.8) |
| Ethnicity‖ | |
| African American | 142 (27.4) |
| White | 130 (25.1) |
| Asian/Pacific islander | 60 (11.6) |
| Hispanic/Mexican/Latino | 53 (10.2) |
| Multiethnic ancestry | 128 (24.7) |
| Other ancestry | 5 (1.0) |
| Sites of exposure¶ | |
| Primary caregiver's home | 439 (84.6) |
| Grandparent's | 49 (9.4) |
| Day-care setting# | 88 (17.0) |
| Other child care setting** | 8 (1.5) |
| History of asthma hospitalization†† | 109 (21.6) |
| Increase in FEV1 ≥ 12% after two puffs of albuterol‡‡ | 42 (15.4) |
| Increase in forced expiratory flow, midexpiratory phase ≥ 20% after two puffs of albuterol§§ | 100 (49.5) |
Data are missing for nine participants.
Data are missing for five participants.
Data are missing for 18 participants.
Data are missing for 49 participants.
Data are missing for one participant.
Percentages add to > 100% because of multiple sites of exposure; percentages are based on 519 participants.
Day-care center includes day-care center, home day care, or relative/sitter child care. Other child-care setting includes Head Start program, preschool, elementary school, or middle school.
Data are missing for 15 participants.
Valid prebronchodilator and postbronchodilator FEV1 data were available from only 273 participants ≥ 5 yr old.
Valid prebronchodilator and postbronchodilator data were available from only 202 participants ≥ 5 yr old.
Table 2.
Proportions of Participants Having Various Characteristics Indicative of Level of Asthma Control (n = 519)
| Asthma Control Criteria | No. (%) |
|---|---|
| Not well or very poorly controlled asthma: met one or more of the criteria below* | 401 (77.3) |
| Asthma symptom-free days ≤ 10 within prior 2 wk† | 200 (39.1) |
| Caregiver's sleep interrupted two or more times in past 4 wk due to child's asthma | 188 (36.2) |
| Used rescue medication > 2 d in prior wk | 149 (28.7) |
| Received two or more bursts of oral corticosteroids in the last yr‡ | 100 (20.4) |
| Normal activity in past 2 wk was somewhat or extremely limited§ | 232 (44.9) |
| FEV1 < 80% of predicted‖ | 65 (23.8) |
| FEV1/FVC < 80%‖ | 77 (28.0) |
Asthma control criteria are based on Figures 4-3a and 4-3b in the National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health, 2007; publication No. 08-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed December 15, 2007.
Data are missing for seven participants.
Data are missing for 29 participants.
Data are missing for two participants.
Valid prebronchodilator data were available only on 273 participants ≥ 5 yr of age.
Sites of Exposure
The child's primary home was the most often cited site of exposure (84.6%), followed by a day-care or child-care setting (18.5%), and a grandparent's house (9.4%) [Table 1]. Sixty-four percent of the children had one site of exposure, and 34.3% were exposed at two or more sites (Table 3). There was no statistically significant difference in the CCR between children with only one site of exposure and those reporting two or more sites (p = 0.19).
Table 3.
Mean CCR in Relation to Number and Type of Sites of Secondhand Tobacco Smoke Exposure
| CCR, ng/mg | ||||||
|---|---|---|---|---|---|---|
| Variables | No. (%) | Mean | SD M | Minimum | Maximum | p Value* |
| Sites, No.† | ||||||
| 1 | 332 (64.0) | 19.2 | 18.8 | 0.0 | 109.9 | 0.19 |
| 2 | 152 (29.3) | 22.7 | 22.9 | 0.0 | 128.5 | |
| ≥ 3 | 26 (5.0) | 20.9 | 16.2 | 0.0 | 58.4 | |
| Type of site/source‡ | ||||||
| Both primary caregiver and day-care setting§ | 22 (4.3) | 39.6A | 27.5 | 1.5 | 102.4 | < 0.0001‖ |
| Primary caregiver only | 162 (31.8) | 26.3B | 22.2 | 0.0 | 128.5 | |
| Day-care setting only | 68 (13.4) | 22.2B | 21.3 | 0.0 | 105.0 | |
| Neither day-care setting nor primary caregiver | 257 (50.5) | 14.0C | 14.4 | 0.0 | 83.6 | |
| Total sample | 519 | 20.1 | 19.9 | 0.0 | 128.5 | |
Analysis of variance test of overall differences in mean CCR values.
Nine children were reported to have no sites of smoke exposure.
For 10 children data were missing on caregiver smoking status or else a urine sample was not obtainable or was lost/compromised during transfer to the testing laboratory.
Day care refers to day care provided in someone's home, with a relative/sitter, or at a day-care center.
A is different from B, C is different from both A and B; p < 0.05, Duncan test.
Smoke exposure in day care was an important contributor to overall smoke exposure (Table 3). Children who were not smoke exposed by either their primary caregiver or day-care provider had the lowest mean CCR (mean, 14.0; SD, 14.4). Mean CCR was greater if either the primary caregiver or day-care provider smoked (mean, 26.3; SD, 22.2; and mean, 22.2; SD, 21.3; respectively), and greater still if both were smokers (mean, 39.6; SD, 27.5) [p < 0.05]. Of the 451 children in day care, 90 children (20.0%) reported smoke exposure at day care. Of these, 80 children (91.1%) were exposed in day care provided in someone's home, with a relative, or with a sitter.
Parental Perceptions
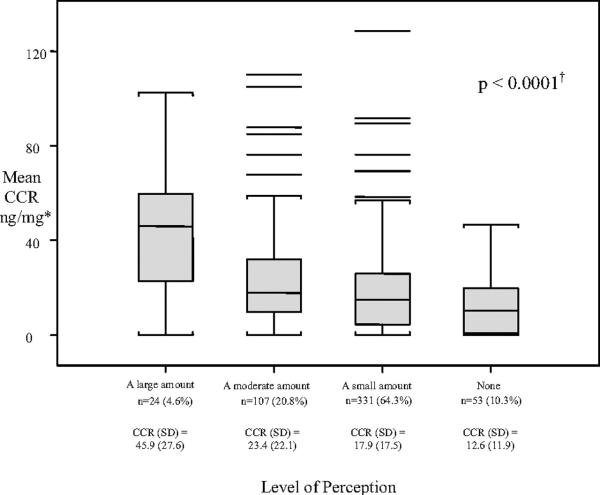
Parental perception of a child's level of smoke exposure was significantly associated with the child's CCR (p < 0.0001, Fig 2), but the relationship was weak (r2 = 0.11). When parents reported a large amount of exposure, the children's mean CCR generally confirmed the report. A report of small, moderate, or no exposure, however, was relatively uninformative about the child's actual exposure. Most parents believed that smoke exposure had only a small or no negative effect on their child's asthma (Table 4). Neither parental perception of the negative effect of smoke exposure nor the severity of their child's asthma was associated with the child's CCR.
Figure 2.
Mean sCCR associated with parent's perception of their child's exposure to tobacco smoke.
*Assessed by the question, “On a typical day, how much tobacco smoke do you think (child's name) is actually exposed to considering all locations?” Data are missing for four subjects.
†Overall difference in means is significant at p < 0.0001. Specifically, large amount is greater than moderate amount, small amount, or none; moderate amount is greater than none at p < 0.05 (Duncan test).
Table 4.
Mean CCR Associated with Parental Perceptions of the Child's Exposure to Secondhand Tobacco Smoke (n = 519)
| Parental Perception | No. (%) | Mean CCR, ng/mg (SD) | Value* |
|---|---|---|---|
| How much effect do you think exposure to tobacco smoke has on (child's name) asthma?† | |||
| No negative effect | 50 (10.9) | 17.2 (17.8) | 0.30 |
| A small negative effect | 217 (47.4) | 21.2 (19.6) | |
| A moderate negative effect | 109 (23.8) | 23.2 (24.0) | |
| A large negative effect | 82 (17.9) | 19.0 (19.3) | |
| How severe do you feel (child's name) asthma is? | |||
| Mild | 200 (38.5) | 18.8 (19.7) | 0.59 |
| Moderate | 236 (45.5) | 20.8 (20.5) | |
| Severe | 63 (12.1) | 20.4 (19.0) | |
| Do not know | 20 (3.9) | 24.0 (18.6) |
Analysis of variance test of overall differences in mean CCR values among parental perception variables.
Data are missing for 61 participants.
Stages of Change
Personal smoking cessation was relevant for 36.7% of parents, making the child's primary home smoke free for 84.2%, making areas not in the home smoke free for 46.6%, and keeping the child out of smoke-exposed locations for 57.8% (Table 5). Most primary caregivers were willing to consider relevant changes including personal smoking cessation (61.3%), making areas out of the home smoke free (66.9%), and keeping their child out of smoke-exposed locations (72.7%). CCRs did not differ by primary caregiver stage of change for any of these exposure reduction actions. Regardless of smoking status, when more than one exposure reduction behavior was relevant, caregivers were more likely to be in precontemplation about making the child's primary home smoke free than about keeping the child out of smoke-exposed locations (56.8% vs 25.0%, respectively; p < 0.0001), keeping areas out of the home smoke free (55.3% vs 36.0%, respectively; p = 0.0002), or their own smoking cessation (48.4% vs 39.0%, p = 0.036).
Table 5.
Mean CCRs Stratified by Primary Caregiver Stage of Change for Specific Behaviors Needed in Order To Reduce Child's Exposure to Secondhand Smoke
| Behavior and Stage of Change | No. (%) | Mean CCR (SD), ng/mg | Value* |
|---|---|---|---|
| Primary caregiver smoking cessation | |||
| Precontemplation | 74 (38.7) | 28.2 (25.8) | 0.54 |
| Contemplation | 57 (29.8) | 25.2 (17.0) | |
| Preparation | 51 (26.7) | 30.3 (25.6) | |
| Action | 9 (4.7) | 20.7 (16.2) | |
| Total† | 191 | ||
| Making child's primary home smoke free | |||
| Precontemplation | 222 (50.8) | 19.8 (19.1) | 0.13 |
| Contemplation | 50 (11.4) | 27.1 (21.9) | |
| Preparation | 161 (36.8) | 20.3 (20.4) | |
| Action | 4 (0.9) | 17.6 (16.9) | |
| Total† | 437 | ||
| Making child's area out of home smoke free | |||
| Precontemplation | 80 (33.1) | 20.6 (22.2) | 0.39 |
| Contemplation | 11 (4.6) | 25.1 (25.4) | |
| Preparation | 103 (42.6) | 22.4 (22.0) | |
| Action | 48 (19.8) | 16.2 (20.5) | |
| Total† | 242 | ||
| Keeping child out of smoke-exposed locations out of the home | |||
| Precontemplation | 82 (27.3) | 19.7 (19.9) | 0.16 |
| Contemplation | 8 (2.7) | 24.3 (17.5) | |
| Preparation | 92 (30.7) | 24.4 (23.3) | |
| Action | 118 (39.3) | 17.7 (22.0) | |
| Total† | 300 |
Analysis of variance test of overall differences in mean CCR values among stage of change groups; does not include those for whom a given source of exposure was not relevant (eg, those whose primary caregiver did not smoke).
Total equals the number of persons for whom a given exposure reduction action is relevant. There were four caregivers who were not staged for making the child's primary home smoke free, one who was not staged for making the child's area out of the home smoke free, and three who were not staged for keeping the child out of smoke-exposed locations outside the home.
Discussion
The smoking status of the primary caregiver and the day-care provider had an additive effect on children's secondhand smoke exposure. The mean CCR was lowest if neither the primary caregiver nor the day-care provider smoked, greater if either smoked, and greatest if both smoked. Day-care exposure sites mostly included home-based day care. These findings are consistent with previous research that found higher CCRs among children exposed by their primary caregiver,22,23 and that smoke exposure in a child care setting is an independent contributor to an infant's CCR.24 Our results extend these findings to toddlers and school-age children with asthma, and highlight the importance of inquiring about tobacco smoke exposure in child-care settings as part of the assessment of secondhand smoke exposure of a child with asthma.
The observation of CCR values of zero in some children (n = 78) was not unexpected. Parental perceptions about exposure may be inaccurate. Some children were intermittently exposed at another parent's home. Others may have been temporarily away from the primary exposure source prior to that sample collection. Parental knowledge that urine testing was to occur may have led to behavior changes for some individuals.
Parental perceptions about their child's secondhand smoke exposure were only weakly associated (r2 = 0.11) with the child's CCR. This finding is consistent with a study from Spain that found parental perception about smokiness of the home had only a modest association with CCR in 3- to 6-year-old children (r2 = 0.32)25.
The 2006 US Surgeon General's report states that the “scientific evidence indicates that there is no risk-free level of exposure to secondhand smoke.”26 Our results suggest that parents of smoke-exposed children with asthma often underestimate the harm to their child. Most of the primary caregivers who smoked were contemplating cessation; fewer were ready to do so within the next month or had recently quit. To eliminate their child's exposure, close to one third of caregivers would have to modify smoking behaviors at two or more sites. Among those where more than one behavior change was relevant, making the primary home smoke free appeared to be more difficult to contemplate or initiate than other changes, including personal smoking cessation. This suggests that primary caregivers consider it more challenging to change the smoking behavior of other adults in the home than to change their own behaviors.
Limitations
Persons lacking health insurance were not represented in our sample. We also excluded parent-child dyads who were not willing to consent to participation in the subsequent randomized clinical trial. Our study was conducted in Northern California, where tobacco smoking in and around public facilities is not allowed or is highly restricted. Exposure may be significantly greater in communities where there are fewer such restrictions. Assessment of stage of change was based on self-report. Finally, intention to change a behavior in the near future does not guarantee that the behavior change will be initiated or accomplished.
Conclusions
Smoking by the child's primary caregiver and day-care provider are important sources of tobacco smoke exposure for children with asthma. Parental assessment of the level of their child's smoke exposure cannot be relied on as a complete assessment of that exposure. Although the harm of tobacco smoke exposure was frequently underestimated, most parents were receptive to taking action to reduce their child's exposure. Making the primary home smoke free, when there were smokers other than the child's primary caregiver, appeared to be the most challenging change to ask of the primary caregiver.
Our findings on the contribution of passive smoke exposure from day-care providers have important implications for public policy, parent education, and physician counseling. Further research is needed to determine effective interventions to reduce tobacco smoke exposure for children with asthma.
Acknowledgments
The authors gratefully acknowledge the contributions of the patients, physicians, and staff of the participating Kaiser Permanente Medical Offices. Arndt Herz, MD; Myngoc Nguyen, MD; Laura Prager, MD; Peg Strub, MD; Madelyn Weiss, MD; Clifford Yee, MD; and Kim Trood, RN facilitated study implementation. Linda Bertorello, RRT; Lisa Caine, RCP; and Veronica Luna coordinated study implementation, recruited subjects, and conducted assessments. Paulina Ayres, Karen Kriete, Andrea Norcia, Debbie Schide, and Jodi Thirtyacre recruited and assessed subjects. Kathy Stamm, BSMT, and Teri Slifer at the Palo Alto Medical Foundation Immunology and Infectious Disease Research Laboratory analyzed the urine specimens for cotinine and creatinine concentration. Ai Lin Tsai assisted with data extraction. The helpful comments of Peg Strub, MD, during preparation of this manuscript are gratefully acknowledged. This support and the support of the Project Officers; Robert Smith, PhD; and Virginia Taggart, PhD are gratefully acknowledged.
This research was supported by grant NIH R01 HL70012 (Dr. Wilson, principal investigator) and conducted at Kaiser Permanente Northern California Medical Centers and the Palo Alto Medical Foundation Research Institute.
Glossary
Abbreviation
- CCR
cotinine to creatinine ratio
Footnotes
OraSure Technologies, Inc. generously underwrote a substantial portion of the costs of the kits used for the cotinine analyses. Monaghan Medical Corporation generously donated AeroChamber Plus VHC devices for use in the study.
No authors have any personal or financial support or author involvement with organizations with financial interest in the subject matter nor any actual or potential conflicts of interest.
References
- 1.Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993;328:1665–1669. doi: 10.1056/NEJM199306103282303. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409–415. doi: 10.1378/chest.122.2.409. [DOI] [PubMed] [Google Scholar]
- 3.Farber HJ, Wattigney W, Berenson G. Trends in asthma prevalence: the Bogalusa Heart Study. Ann Allergy Asthma Immunol. 1997;78:265–269. doi: 10.1016/S1081-1206(10)63179-1. [DOI] [PubMed] [Google Scholar]
- 4.National Asthma Education and Prevention Program . Expert panel report 2: guidelines for the diagnosis and management of asthma. National Institutes of Health, National Heart, Lung, and Blood Institute; Bethesda, MD: 1997. publication No. 97-4051. [Google Scholar]
- 5.Velicer WF, Prochaska JO. An expert system intervention for smoking cessations. Patient Educ Couns. 1999;36:119–129. doi: 10.1016/s0738-3991(98)00129-3. [DOI] [PubMed] [Google Scholar]
- 6.Velicer WF, Fava JL, Prochaska JO, et al. Distribution of smokers by stage in three representative samples. Prev Med. 1995;24:401–411. doi: 10.1006/pmed.1995.1065. [DOI] [PubMed] [Google Scholar]
- 7.Wewers ME, Stillman FA, Hartman AM, et al. Distribution of daily smokers by stage of change: current Population Survey results. Prev Med. 2003;36:710–720. doi: 10.1016/s0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 8.Fava JL, Velicer WF, Prochaska JO. Applying the transtheoretical model to a representative sample of smokers. Addict Behav. 1995;20:189–203. doi: 10.1016/0306-4603(94)00062-x. [DOI] [PubMed] [Google Scholar]
- 9.Okah FA, Okuyemi KS, McCarter KS, et al. Predicting adoption of home smoking restriction by inner-city black smokers. Arch Pediatr Adolesc Med. 2003;157:1202–1205. doi: 10.1001/archpedi.157.12.1202. [DOI] [PubMed] [Google Scholar]
- 10.Okah FA, Choi WS, Okuyemi KS, et al. Effect of children on home smoking restriction by inner city smokers. 2002. Pediatrics. 109:244–249. doi: 10.1542/peds.109.2.244. [DOI] [PubMed] [Google Scholar]
- 11.Gilpin EA, White MM, Farkas AJ, et al. Home smoking restrictions: which smokers have them and how they are associated with smoking behavior. Nicotine Tob Res. 1999;1:153–162. doi: 10.1080/14622299050011261. [DOI] [PubMed] [Google Scholar]
- 12.Mahabee-Gittens M. Smoking in parents of children with asthma and bronchiolitis in a pediatric emergency department. Pediatr Emerg Care. 2002;18:4–7. doi: 10.1097/00006565-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 14.Leong JW, Dore ND, Shelley K, et al. The elimination half-life of urinary cotinine in children of tobacco-smoking mothers. Pulm Pharmacol Ther. 1998;11:287–290. doi: 10.1006/pupt.1998.0153. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis MJ, Russell MA, Benowitz NL, et al. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78:696–698. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107(suppl 2):349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherer G, Meger-Kossien I, Riedel K, et al. Assessment of the exposure of children to environmental tobacco smoke (ETS) by different methods. Hum Exp Toxicol. 1999;18:297–301. doi: 10.1191/096032799678840075. [DOI] [PubMed] [Google Scholar]
- 18.Fried PA, Perkins SL, Watkinson B, et al. Association between creatinine-adjusted and unadjusted urine cotinine values in children and mother's report of exposure to environmental tobacco smoke. Clin Biochem. 1995;28:415–420. doi: 10.1016/0009-9120(94)00092-a. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Dockery DW, Wypij D, et al. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Irvine L, Crombie IK, Clark RA, et al. What determines levels of passive smoking in children with asthma? Thorax. 1997;52:766–769. doi: 10.1136/thx.52.9.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook DG, Whincup PH, Jarvis MJ, et al. Passive exposure to tobacco smoke in children aged 5-7 years: individual, family, and community factors. BMJ. 1994;308:384–389. doi: 10.1136/bmj.308.6925.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ownby DR, Johnson CC, Perterson EL. Passive cigarette smoke exposure of infants: importance of nonparental sources. Arch Pediatr Adolesc Med. 2000;154:1237–1241. doi: 10.1001/archpedi.154.12.1237. [DOI] [PubMed] [Google Scholar]
- 25.Jurado D, Munoz C, Luna JD, et al. Environmental tobacco smoke exposure in children: parental perception of smokiness at home and other factors associated with urinary cotinine in preschool children. J Expo Anal Environ Epidemiol. 2004;14:330–336. doi: 10.1038/sj.jea.7500329. [DOI] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services . The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Centers for Disease Control and Prevention, Office of Smoking and Health; Atlanta, GA: 2006. [PubMed] [Google Scholar]