Abstract
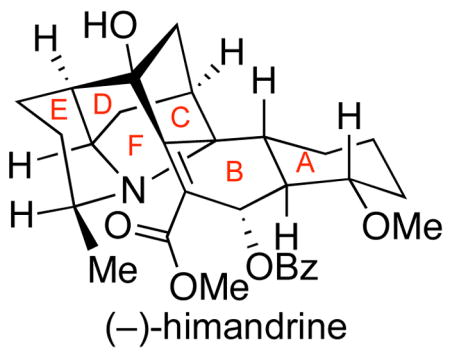
We describe the first total synthesis of (−)-himandrine, a member of the class II galbulimima alkaloids. Noteworthy features of this chemistry include a diastereoselective Diels-Alder reaction in the rapid synthesis of the tricycle ABC-ring system in enantiomerically enriched form, the use of a formal [3+3] annulation strategy to secure the CDE-ring system with complete diastereoselection, and successful implementation of our biogenetically inspired oxidative spirocyclization of an advanced intermediate. The successful and direct late-stage formation of the F-ring in the hexacyclic core of himandrine drew on the power of biogenetic considerations and fully utilized the inherent chemistry of a plausible biosynthetic intermediate.
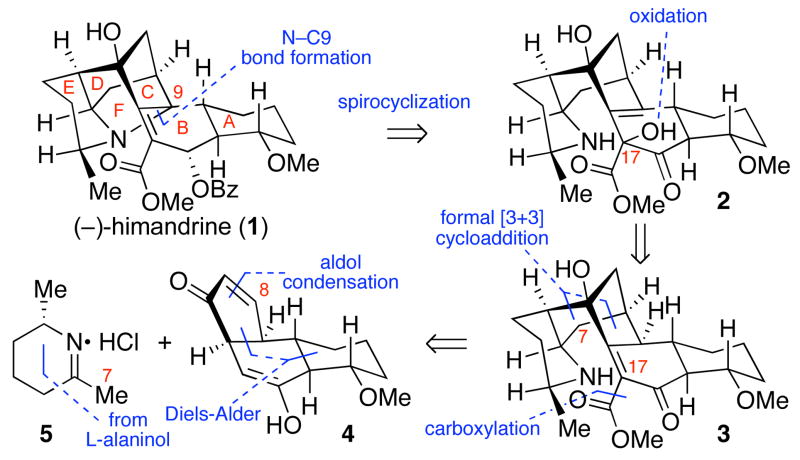
The galbulimima alkaloid (−)-himandrine (1) is a topologically fascinating compound isolated from the bark of Galbulimima belgraveana, a tree indigenous to Papua New Guinea and northern Australia.1 The promise that natural and synthetic derivatives of galbulimima alkaloids have shown for treatment of human ailments2 has resulted in substantial attention in both academia and industry. We previously reported the first enantioselective total synthesis of (−)-galbulimima alkaloid 13 (class III)3 and revised the absolute stereochemical assignment of the class II and III derivatives.4 While there have been several outstanding syntheses of class I5 and III6 galbulimima alkaloids, no total synthesis of the class II7 galbulimima alkaloids possessing the unique N-C9 spirofused polycyclic framework has been reported. Herein we describe our total synthesis of class II alkaloid 1 guided by our previously disclosed hypothesis for its biogenesis,3b featuring a final stage oxidative spirocyclization to secure the BCF ring juncture.
Inspired by Mander, Ritchie and Taylor’s 1967 proposal relating all galbulimima alkaloids to a single polyacetate precursor,1d and consistent with our specific hypothesis for the biogenesis of class II and III galbulimima alkaloids,3b we identified aminoketoester 3 (Scheme 1) as a plausible point of divergence en route to more complex alkaloids. Our retrosynthetic analysis of (−)-1 follows this hypothesis,3b in which the N-C9 bond is introduced by a late stage oxidative spirocyclization of the pentacyclic aminoketoester 3. We envisioned that oxidation of 3, potentially facilitated by dienol formation, would afford allylic alcohol 2, which is poised for the critical condensative spirocyclization. We expected that application of our annulation methodology8 to enone 4 and iminium chloride 5 would convergently assemble a pentacycle primed for our proposed biomimetic oxidative spirocyclization.
Scheme 1.
Retrosynthetic analysis of (−)-himandrine (1).
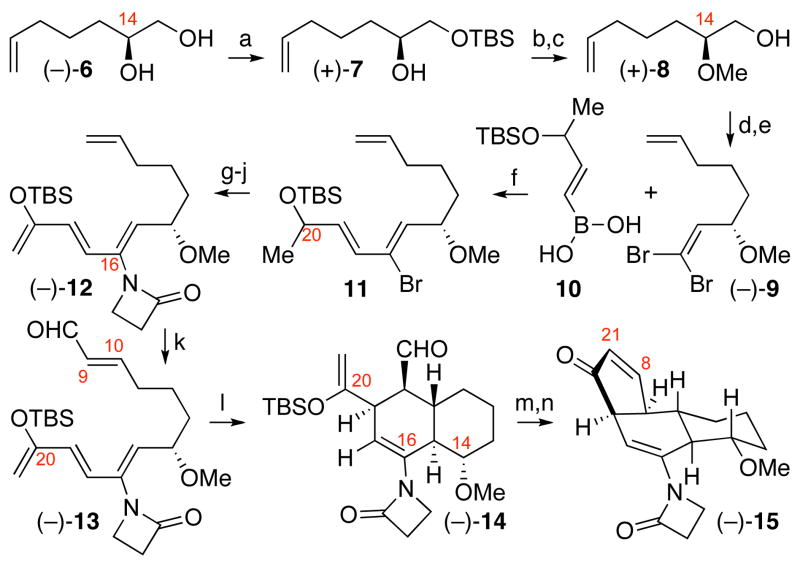
Our enantioselective synthesis of a tricyclic enone 4 mimic is illustrated in Scheme 2. The C14-stereochemistry, introduced through the use of MacMillan’s D-proline–catalyzed α-oxidation9,10 of hept-6-enal, afforded the enantiomerically enriched diol (−)-6 (>98.5% ee).11 The C14-methyl ether was then secured by etherification12 of alcohol (+)-7 to provide the methoxyalcohol (+)-8, setting the stage for the substrate-directed synthesis of the trans-decalin AB-ring system. Oxidation13 of alcohol (+)-8 followed by conversion of the corresponding aldehyde to dibromoolefin provided (−)-9. A highly efficient Suzuki cross-coupling reaction3,14 involving boronic acid 1010 and dibromide (−)-9 afforded the cis-vinyl bromide 11 in 97% yield. A copper-promoted coupling15 of vinyl bromide 11 with 2-azetidinone followed by conversion of the C20-silyl ether to the corresponding C20-silyl enol ether gave the desired tetraene (−)-12. The 2-azetidinone grouping at C16 was strategically introduced to serve a dual role in facilitating the planned Diels–Alder reaction by providing a 2-N-acylaminodiene with greater preference for s-cis C16-C17 conformation, and masking the C16-carbonyl for subsequent transformations. A Ru-catalyzed olefin cross-metathesis reaction16 with acrolein enabled selective functionalization of the acid sensitive tetraene (−)-12 to the corresponding unsaturated aldehyde (−)-13 in 85% yield. Heating a solution of tetraenal (−)-13 in acetonitrile at 95 °C afforded the desired trans-decalin aldehyde (−)-14 as the major endo Diels-Alder product (75%, dr = 5:1) as supported by nOe studies.10 Treatment of aldehyde (−)-14 with titanium tetrachloride provided the corresponding Mukaiyama aldol17 product, which upon exposure to Martin sulfurane18 afforded the oxygen and acid sensitive enone (−)-15 in 57% yield over two steps (Scheme 2).
Scheme 2.
Enantioselective synthesis of tricyclic enone (−)-15.a
aConditions: (a) TBSCl, imidazole, DMAP, DMF, 0 ºC, 94%. (b) 4Å-MS, Proton Sponge®, Me3O•BF4, CH2Cl2, 23 ºC, 93%. (c) HCl, MeOH, 23 ºC, 98%. (d) DMSO, iPr2NEt, SO3•pyr, CH2Cl2, 23 ºC. (e) CBr4, PPh3, CH2Cl2, 0 ºC, 65% (2-steps). (f) Pd(PPh3)4, Tl2CO3, THF, H2O, 23 °C, 97%. (g) 2-azetidinone, CuI, K2CO3, (MeNHCH2)2, PhMe, 120 °C, 85%. (h) TBAF, THF, 0→23 ºC. (i) DMSO, iPr2NEt, SO3•pyr, CH2Cl2, 23 ºC, 80% (2-steps). (j) TBSOTf, Et3N, CH2Cl2, −78 °C, 82%. (k) acrolein, 4,5-dihydroIMesCl2Ru=CH(2-iPrO)Ph (10 mol%), CH2Cl2, 23 °C, 85%. (l) BHT, N,N-diethylaniline, MeCN, 95 °C, 75%, 5:1 dr. (m) TiCl4, CH2Cl2, −78 °C. (n) Martin sulfurane, PhH, 23 ºC, 57% (2-steps).
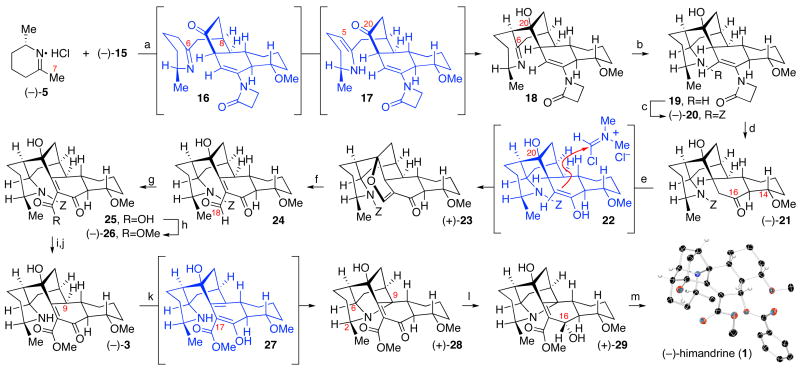
Lithiation of the readily available iminium chloride (−)-53 followed by copper-promoted conjugate addition8 to the enantiomerically enriched enone (−)-15 afforded the highly air sensitive pentacyclic iminoalcohol 18 (Scheme 3). Importantly, blocking of the si-face of the enone by the trans-decalin AB-ring system imposed exquisite stereochemical control during the C7-C8 bond formation. Rapid tautomerization of the transiently formed iminoketone 16 enabled nucleophilic addition of C5 to the C20-ketone of 17, consistent with our biosynthetic hypothesis for the D-ring formation.3 Addition of sodium borohydride to crude imino alcohol 18 resulted in completely diastereoselective C6-imine reduction, at which point benzyloxycarbonylation of the resulting aminoalcohol 19 provided the desired product (−)-20 (Scheme 3, Z = benzyloxycarbonyl) in 50% yield over three steps. Importantly, the formal cycloaddition between iminium chloride (−)-5 and enone (−)-15 and subsequent C6-reduction of imine 18 secured four stereogenic centers and expediently assembled the pentacyclic substructure of (−)-himandrine (1) as a single diastereomer.
Scheme 3.
Enantioselective total synthesis of (−)-himandrine (1).a
aConditions: (a) nBuLi, THF, (−)-5, −78→ 0 °C; CuBr•SMe2; (−)-15, −78→ −10 °C. (b) NaBH4, EtOH, 0 ºC. (c) ClCO2Bn, iPr2NEt, K2CO3, THF, H2O, 50% (3-steps). (d) TsOH•H2O, PhH, 23 °C, 81%. (e) POCl3, DMF, CH2Cl2, 0→23 °C, 71%. (f) DDQ, SiO2, MeCN, H2O, 23 °C. (g) NaClO2, NaH2PO4•H2O, 2-methyl-2-butene, tBuOH, H2O, 23 ºC. (h) CH2N2, THF, 0 ºC, 61% (3-steps). (i) TMS-I, 2,6-di-tBu-4-Me-Pyr, CH2Cl2, 0 ºC, 66%. (j) Et3N•(HF)3, THF, 23 ºC, 90%. (k) NCS, MeCN, 23 ºC, 45 min, 89%. (l) NaBH4, EtOH, 0 ºC, 90%. (m) BzCl, pyridine, 23 ºC, 7 d, 87%.
Mild hydrolysis of N-vinyl-carbamate (−)-20 with p-toluene sulfonic acid monohydrate in benzene afforded the key intermediate ketone (−)-21 in 75% yield. The mild reaction conditions used in hydrolysis of the C16 N-vinyl lactam circumvent the competing acid catalyzed β-elimination of the C14-methyl ether in ketone (−)-21.19 At this juncture, we required a mild method for introduction of the C17-methoxycarbonyl group in order to access the aminoketoester 3. After exploring a variety of strategies, we found that treatment of ketone (−)-21 with Vilsmeier’s reagent provided the vinyl ether (+)-23 in 71% yield, likely occurring through nucleophilic addition of enol 22 (Scheme 3) followed by trapping with the C20-alcohol. Exposure of vinyl ether (+)-23 to 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)20 furnished the corresponding unsaturated β-ketoaldehyde 24. Immediate oxidation of the acid sensitive C18-aldehyde 24 to the corresponding carboxylic acid 25 followed by treatment with diazomethane provided the desired β-ketoester (−)-26 in 61% yield over three steps (Scheme 3). Sequential treatment of carbamate (−)-26 with trimethylsilyliodide (TMS-I)3,21 and triethylamine trihydrofluoride provided pentacyclic aminoketoester (−)-3, our proposed common biosynthetic intermediate to the class II and class III galbulimima alkaloids.
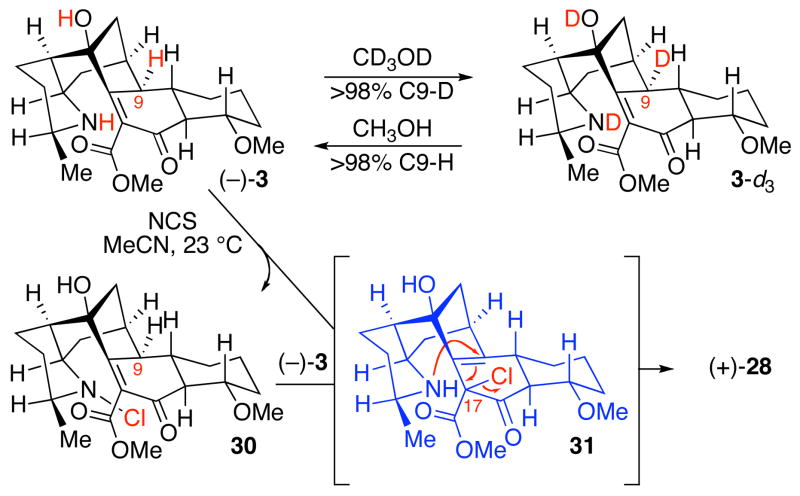
With the key intermediate in hand, we were able to evaluate the feasibility of our postulated biomimetic late stage N-C9 bond formation. We expected that β-ketoester 3 might undergo rapid tautomerization to the electron rich dienol 27, enabling facile C17 oxidation as a prelude to intramolecular allylic displacement by the amine to give the N-C9 spirocycle. Deuterium labeling studies were employed in monitoring H/D exchange at C9 (Scheme 4). Compellingly, simple dilution of aminoketoester (−)-3 in methanol-d4 resulted in immediate and quantitative deuterium incorporation at C9 in the form of aminoketoester 3-d3 (Scheme 4).22 Dissolution of aminoketoester 3-d3 in methanol returned aminoketoester (−)-3, indicating the exchange of C9-methine occurs with retention of C9-stereochemistry.23 Cumulatively, our results are consistent with the amino group being intimately involved in facilitating C9-deprotonation.24,25 The preservation of the C9-stereochemistry is consistent with intramolecular protium/deuterium delivery by the corresponding ammonium ion from the more sterically hindered face of the C19-C9 tetrasubstituted alkene of 27.
Scheme 4.
Key observations relevant to N-C9 bond formation.
Guided by our biosynthetic hypothesis and with evidence for rapid dienol formation from aminoketoester (−)-3, we wished to capitalize on the inherent chemistry of this plausible biosynthetic intermediate. In the event, treatment of aminoketoester (−)-3 with N-chlorosuccinimide (NCS) in acetonitrile at 23 °C over 45 min afforded the desired spirofused hexacyclic enone (+)-28 in 89% yield. The structure of (+)-28 was supported through detailed 2D NMR analysis.26 Treatment of ketone (+)-28 with sodium borohydride in ethanol effected completely diastereoselective C16-reduction to the desired diol (+)-29 in 90% yield. Benzoylation of the C16-hydroxyl group of diol (+)-29 proceeded to give the first synthetic sample of (−)-himandrine (1) in 87% yield ([α]22 D = −21 (c, 0.12 CHCl3); Lit.1a ([α] = −38 (c 1.22, CHCl3).27 All spectroscopic data for synthetic (−)-1 matched those reported for the natural compound.10 The structure of our synthetic (−)-1 was unequivocally confirmed by X-ray crystallographic analysis.
Intrigued by the high efficiency in the conversion of aminoketoester (−)-3 to hexacyclic enone (+)-28, we sought to gain further mechanistic insight into this transformation. Close monitoring of the reaction mixture indicated that exposure of aminoketoester (−)-3 to NCS in acetonitrile resulted in concomitant formation of hexacycle (+)-28 and the light–sensitive N-chloro pentacycle 30 (Scheme 4) that would disappear by the end of the reaction. Use of benzene as solvent for this transformation reduced the overall rate of hexacycle (+)-28 formation and allowed for the isolation of N-chloro pentacycle 30.28 Importantly, dissolution of N-chloro pentacycle 30 in acetonitrile for 12 h did not result in formation of any hexacycle (+)-28 and completely returned 30.29,30 Additionally, when the deuterium incorporation studies described above were conducted with 30, there was no evidence for formation of the corresponding dienol, suggesting the nitrogen of 30 is not basic enough to enable deprotonation at C9. Interestingly, exposure of aminoketoester (−)-3 to samples of N-chloro pentacycle 30 in acetonitrile resulted in formation of hexacycle (+)-28 and (−)-3.31 This result is consistent with an intermolecular N to C halogen transfer from N-chloro pentacycle 30 to aminoketoester (−)-3 (likely via 27). A plausible mechanism for conversion of aminoketoester (−)-3 to spirofused hexacycle (+)-28 is halogenation of the dienol 27 to give α-chloroester 31,32 followed by intramolecular allylic displacement by the amine. Significantly, this mechanism is consistent with our proposed biomimetic hypothesis for the advanced stage oxidative spirocyclization of aminoketoester (−)-3.3b
We have described the first total synthesis of (−)-himandrine (1), a member of the class II galbulimima alkaloids. Noteworthy features of this chemistry include the diastereoselective Diels-Alder reaction for rapid synthesis of the trans-decalin containing tricycle (−)-15 in enantiomerically enriched form, the formal [3+3] annulation strategy to secure the CDE-ring system with complete diastereoselection, and successful implementation of our biogenetically inspired oxidative spirocyclization in converting 30 to (+)-28. The successful and direct conversion of (−)-3 to (+)-28 drew on the power of biogenetic considerations and fully utilized the inherent chemistry of this plausible biosynthetic intermediate.
Supplementary Material
Acknowledgments
M.M. is an Alfred P. Sloan Research Fellow, a Beckman Young Investigator, and a Camille Dreyfus Teacher-Scholar. M.T. acknowledges BMS and Novartis graduate fellowships. We thank Justin Kim and Dr. Peter Müller for X-ray crystallographic analysis of (−)-1. We thank Professor Robert G. Griffin and Dr. Tony Bielecki for use of a high field instrument at the MIT-Harvard Center for Magnetic Resonance (EB002026). We acknowledge financial support by NIH-NIGMS (GM074825).
Footnotes
Supporting Information Available: Experimental procedures, spectroscopic data, copies of 1H and 13C NMR spectra, and X-ray structure of (−)-1. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Binns SV, Dustan PJ, Guise GB, Holder GM, Hollis AF, McCredie RS, Pinhey JT, Prager RH, Rasmussen M, Ritchie E, Taylor WC. Aust J Chem. 1965;18:569. [Google Scholar]; (b) Guise GB, Mander LN, Prager RH, Rasmussen M, Ritchie E, Taylor WC. Aust J Chem. 1967;20:1029. [Google Scholar]; (c) Mander LN, Prager RH, Rasmussen M, Ritchie E, Taylor WC. Aust J Chem. 1967;20:1473. [Google Scholar]; (d) Mander LN, Prager RH, Rasmussen M, Ritchie E, Taylor WC. Aust J Chem. 1967;20:1705. [Google Scholar]
- 2.For SCH-530348, a galbulimima alkaloid derived antiplatelet agent, in Phase-III clinical trials for acute coronary syndrome, see Chackalamannil S, Wang Y, Greenlee WJ, Hu Z, Xia Y, Ahn HS, Boykow G, Hsieh Y, Palamanda J, Agans-Fantuzzi J, Kurowski S, Graziano M, Chintala M. J Med Chem. 2008;51:3061. doi: 10.1021/jm800180e.Malaska MJ, Fauq AH, Kozikowski AP, Aagaard PJ, McKinney M. Bioorg Med Chem Lett. 1995;5:61.
- 3.Movassaghi M, Hunt DK, Tjandra M. J Am Chem Soc. 2006;128:8126. doi: 10.1021/ja0626180.(b) Please see SI of ref 3a (page S4, Scheme S2) for a detailed unified biosynthetic hypothesis for class II and III galbulimima alkaloids.
- 4.(a) The direct stereochemical and structural relationship between (−)-galbulimima alkaloid 13 (class III) and (−)-himandrine (1, class II) was established through instructive chemical degradation studies in the context of the isolation work (ref. 1c). (b) For revision of the stereochemical assignment based on new X-ray analysis, see Willis AC, O’Connor PD, Taylor WC, Mander LN. Aust J Chem. 2006;59:629.
- 5.For prior syntheses of class I galbulimima alkaloids, see Hart DJ, Wu WL, Kozikowski AP. J Am Chem Soc. 1995;117:9369.Chackalamannil S, Davies RJ, Aserom T, Doller D, Leone D. J Am Chem Soc. 1996;118:9812.Tchabanenko K, Adlington RM, Cowley AR, Baldwin JE. Org Lett. 2005;7:585. doi: 10.1021/ol047676+.
- 6.For prior syntheses of class III galbulimima alkaloids, see Mander LN, McLachlan MM. J Am Chem Soc. 2003;125:2400. doi: 10.1021/ja029725o.Shah U, Chackalamannil S, Ganguly AK, Chelliah M, Kolotuchin S, Bulevich A, McPhail A. J Am Chem Soc. 2006;128:12654. doi: 10.1021/ja065198n.Evans DA, Adams DJ. J Am Chem Soc. 2007;129:1048. doi: 10.1021/ja0684996.
- 7.For an intriguing synthesis of the skeleton of 1, see O’Connor PD, Mander LN, McLachlan MMW. Org Lett. 2004;6:703. doi: 10.1021/ol036308n.
- 8.Movassaghi M, Chen B. Angew Chem Int, Engl Ed. 2007;46:565. doi: 10.1002/anie.200603302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown SP, Brochu MP, Sinz CJ, MacMillan DWC. J Am Chem Soc. 2003;125:10808. doi: 10.1021/ja037096s. [DOI] [PubMed] [Google Scholar]
- 10.See Supporting Information for details.
- 11.Diol (−)-6 is prepared in 3-steps from commercially available 7-octene-1,2-diol on greater than 8-g scale.
- 12.Meerwein H. Methoden Org Chem (Houben-Weyl) 1965;6:325. [Google Scholar]
- 13.Parikh JP, Doering WE. J Am Chem Soc. 1967;89:5505. [Google Scholar]
- 14.Miyaura N, Yamada K, Suginome H, Suzuki A. J Am Chem Soc. 1985;107:972.(b) For rate enhancement with TlOH, see Uenishi J-i, Beau J-M, Armstrong RW, Kishi Y. J Am Chem Soc. 1987;109:4756.Roush WR, Moriarty KJ, Brown BB. Tetrahedron Lett. 1990;31:6509.Evans DA, Starr JT. J Am Chem Soc. 2003;125:13531. doi: 10.1021/ja037643+.
- 15.Jiang L, Job GE, Klapars A, Buchwald SL. Org Lett. 2003;5:3667. doi: 10.1021/ol035355c. [DOI] [PubMed] [Google Scholar]
- 16.(a) Chatterjee AK, Morgan JP, Scholl M, Grubbs RH. J Am Chem Soc. 2000;122:3783. [Google Scholar]; (b) Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J Am Chem Soc. 2000;122:8168. [Google Scholar]
- 17.Mukaiyama T, Marasaka K, Banno K. Chem Lett. 1973:1011. [Google Scholar]
- 18.Martin JC, Arhart RJ. J Am Chem Soc. 1971;93:4327. [Google Scholar]
- 19.The more forcing reaction conditions needed for hydrolysis of an N-vinyl oxazolidinone (ref. 3) derivative of 20 led to competing β-elimination of the C14-methyl ether.
- 20.(a) Liu HJ, Tran DDP. Tetrahedron Lett. 1999;40:3827. [Google Scholar]; (b) Walker D, Hiebert JD. Chem Rev. 1967;67:153. doi: 10.1021/cr60246a002. [DOI] [PubMed] [Google Scholar]
- 21.Jung ME, Lyster MA. J Chem Soc, Chem Comm. 1978:315. [Google Scholar]
- 22.The 1H NMR spectrum of 3-d3 was the same as the starting aminoketoester (−)-3 with the exception of the C9-methine spin systems.
- 23.Similar results were obtained using a C20 O-trimethylsilylated derivative of (−)-3.
- 24.Attempts at intermolecular C9 deprotonation were unsuccessful; the proximity of the amine to C9-methine seems to facilitate tautomerization.
- 25.Use of derivatives of aminoketoester 3 not possessing a basic amine (i.e., N-Cbz, N-Cl) resulted in no C9-deuterium incorporation over 24 h.
- 26.Key HMBC correlations between C9/C2-H and C9/C6-H confirmed the N-C9 bond connectivity.
- 27.The C14-methyl ether and the C17-methoxycarbonyl substituents greatly shield the C16 alcohol leading to slow benzoylation.
- 28.The reaction had to be stopped within 10 min otherwise significantly more hexacycle (+)-28 would be generated.
- 29.Addition of succinimide does not lead to conversion of 30 to (+)-28.
- 30.While the sensitivity of 30 precluded its derivatization, use of its more stable C20-O-trimethylsilyl derivative under basic, acidic, or photochemical conditions predominantly led to elimination and decomposition.
- 31.Complete mass balance was observed and the amount of hexacycle (+)-28 formed was exactly proportional to amount of 30 used.
- 32.While C9 halogenation cannot be ruled out, C17 halogenation is consistent with the lack of product formation using the C20-Otrimethylsilyl derivative of 30 with significantly blocked access to C17.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.