Abstract
Type I interferons (IFN) and dendritic cells (DC) share an overlapping history, with rapidly accumulating evidence for vital roles for both production of type 1 IFN by DC and the interaction of this IFN both with DC and components of the innate and adaptive immune responses. Within the innate immune response, the plasmacytoid DC (pDC) are the “professional” IFN producing cells, expressing specialized toll-like receptors (TLR7 and -9) and high constitutive expression of IRF-7 that allow them to respond to viruses with rapid and extremely robust IFN production; following activation and production of IFN, the pDC subsequently mature into antigen presenting cells that help to shape the adaptive immune response. However, like most cells in the body, the myeloid or conventional DC (mDC or cDC) also produce type I IFNs, albeit typically at a lower level than that observed with pDC, and this IFN is also important in innate and adaptive immunity induced by these classic antigen presenting cells. These two major DC subsets and their IFN products interact both with each other as well as with NK cells, monocytes, T helper cells, T cytotoxic cells, T regulatory cells and B cells to orchestrate the early immune response. This review will discuss some of the converging history of DC and IFN as well as mechanisms for IFN induction in DC and the effects of this IFN on the developing immune response.
Keywords: dendritic cells, plasmacytoid dendritic cells, type I interferon, IFN
1. Introduction
This year marks the 50th anniversary of the first report by Isaacs and Lindenmann on an anti-viral substance they termed “interferon” [1]. Interferon was described in this ground-breaking manuscript as a supernatant factor from influenza virus-infected chick chorioallontoic membrane cell cultures that could “interfere” with the replication of virus in a previously uninfected culture. This initial description of interferon followed two decades of research by various groups into the phenomenon of viral interference, whereby one virus is able to block the replication of another virus when both are used to infect the same culture. The novelty in this landmark 1957 paper was the demonstration that the phenomenon of viral interference was independent of the transfer of virions, and therefore was not directly mediated by viruses; rather, the viral interference was dependent on a soluble protein that itself had no direct effect on viruses, but, instead, directly acted on cells.
Although interferon was quickly hailed as an important anti-viral agent with obvious clinical potential, its recognition as a key player in the immune response came only much later. The reason for this delay is that the field of cellular immunology was in its infancy in the 1950’s and 1960’s when the antiviral effects of interferon were first described. During this period, the role of the thymus and the bursa were just being elucidated, followed by the definition of T cells and B cells as distinct subsets of lymphocytes. Over a period of many years, it became clear that interferon was rapidly produced in large quantities in vivo in response to viral infection and that stimulation of human peripheral blood mononuclear cells (PBMC) with enveloped viruses in vitro resulted in the release of large quantities of IFN-α from a rare cell type distinct from the T cells, B cells, monocytes and NK cells [2–5].
The nature of these primary cells in human peripheral blood that produce large quantities of interferon remained elusive until the rapidly developing field of dendritic cell (DC) biology intersected with the interferon field. Early evidence pointed to a DC as being the main producer of IFN-α in response to stimulation with viruses [6–8], but it wasn’t until there was recognition of different subsets and differentiation states of DC that the precise nature of the professional interferon producing cells as immature plasmacytoid dendritic cells (pDC) could be defined. However, while the major producer of IFN-α is the pDC, myeloid dendritic cells also can produce IFN, albeit at lower levels, in response to some viruses and there is clear evidence for communication between these two dendritic cell subsets. In this review, the role of DC subsets in interferon biology will be discussed – with DC acting both as producers of and responders to interferon. In addition, how the DC and the interferon contribute to the development of innate and adaptive immunity will be discussed.
2. Interferon – the first cytokine
Interferon was by far the earliest described member of the class of protein molecules now known as cytokines and quintessentially fits the definition for a cytokine given in any first course in immunology – it is a soluble product released from stimulated cells that serves to communicate between cells of the immune system. IFN is both pleiotropic and redundant; the pleiotropic functions of IFN range from induction of antiviral activity to pro-and anti-apoptotic activity to growth inhibition to immunomodulation of a panoply of immune cell responses. Although often referred to simply as “interferon”, this term in fact refers to a large family of genes comprising at least three different major subtypes. The type I interferon family, the members of which signal through the common IFNα/β receptor, include the closely-related members of the IFN-α family, which in humans are encoded by a family of 13 contiguous genes as well as several pseudogenes, the single gene for IFNB, which shares about 50% homology with the IFNAs, as well as genes for IFNκ and IFNω [9]. The single type II interferon, IFN-γ, is genetically unrelated to the type I IFNs and signals through its own receptor, but shares some overlapping functions with the type I IFNs. A third family of IFNs has recently been described, the IFN-λs, which are also known as IL-28A and B (IFN-λ2 and IFN-λ3) and IL-29 (IFN-λ1) and which signal through the IFN-λ receptor, consisting of the IL10R2 chain shared with the IL-10 and IL-22 receptors and a unique IFN-λ receptor chain [10, 11]. The redundancy in IFNs exists both within the class of type I interferons that all signal through the same receptor, as well as in the overlapping functions with some of the activities of type II (IFN-γ) and type III (IFN-λ) interferons as well as with some functions shared with cytokines outside of the IFN family.
3. Production of IFN in response to virus and virus-infected cells
Early literature on the nature of interferon producing cells in the blood was strongly influenced by a study by Saksela et al. [12]. These investigators serendipitously chose Sendai virus, a murine paramyxovirus for their studies of IFN production in PBMC cultures. From their studies, they concluded that monocytes were the primary IFN-producing cells in human periphal blood. Indeed, by intracellular flow, monocytes can clearly be seen to produce IFN-α in response to Sendai virus (Fitzgerald-Bocarsly, unpublished); this response, while vigorous, differs from the PBMC response to HSV in that each monocyte produces on the order of 10–100-fold less IFN than the NIPC, and unlike the response to HSV, requires the virus to be viable for IFN induction [13]. In the 1970’s, the Trinchieri laboratory demonstrated that type I IFN was produced upon co-culture of PBMC with virus [14]. This IFN was also found to be produced by co-culture of herpes simplex virus-infected fibroblasts with human PBMC during the course of natural killer cell lysis of the virus-infected fibroblasts [15]. One of the first clues that hinted towards a specialized population of peripheral blood cells that could respond to virus stimulation came from my observation while still a post-doc in the laboratory of Dr. Carlos Lopez that NK cell-mediated lysis of HSV-infected fibroblasts was depleted by treatment of PBMC with anti-HLA-DR plus complement, whereas lysis of the prototypical NK target, K562, was independent of HLA-DR expression [16]. Subsequently, it was found that the cells in PBMC that produced IFN-α in response to virus-infected cells were HLA-DR+ cells that lack cell surface markers typical of T cells, B cells, monocytes and NK cells [4, 5]. The cells were called “natural interferon producing cells” by the Alm group [17] to indicate that they were part of the early, or natural immune response, now known as innate immunity.
By using limiting dilution assays and IFN-α specific ELISpot assays, the NIPC responding to HSV or HSV-infected cells were found to be low frequency cells (~1-2:1000 PBMC) [3], and a similar low frequency IFN producing cell response was observed in response to a wide range of enveloped viruses [18]. Moreover, it was found that virus did not have to be viable in order to induce IFN in the NIPC, with UV-inactivated virus [15, 19] or even formalin-fixed HSV-infected fibroblasts [20] able to induce IFN. Calculations based on the total amount of IFN produced in HSV-stimulated cultures vs. the frequency of NIPC in the cultures (as determined by ELISpot) led to the conclusion that each functional NIPC could produce between 1–2 IU of IFN-α, which corresponds to 3–10 pg/cell [3, 13]. Subsequently, it was determined that the reason for the sensitivity of NK-mediated lysis of virus-infected fibroblasts to depletion of HLA-DR+ cells was not that the NK cells themselves expressed HLA-DR, but, rather, that the NK lysis of some virus-infected targets required the presence of both CD16+ NK cells as well as a lineage negative, HLA-DR+ accessory population that was, by itself, not lytic [21–23]. This HLA-DR+ population co-enriched with the population of cells that responded vigorously with the production of IFN-α in response to virus stimulation [24].
4. Plasmacytoid and myeloid dendritic cells: the professional and not so professional interferon producing cells
4.1. Plasmacytoid DC as IFN-producing cells
The precise identity of the small put potent population of NIPC in the peripheral blood remained elusive for a number of years, but evidence suggested that these cells belonged to the relatively newly-described class of cells known as dendritic cells (DC) [25, 26]. Like DC, the NIPC were found to enrich in light-density fractions of Percoll or Metrizamide gradients [5, 6], and the major IFN-α activity could be recovered in cell fractions enriched for DC by cell sorting [7, 8]. The sorted cells were found to exhibit distinct, abundant endoplasmic reticulum, somewhat reminiscent of plasma cells. However, although we and the Rinaldo group concluded that the NIPC were within the DC populations, it was clear that the NIPC could be distinguished from cultured, enriched conventional DC as the latter cells failed to produce IFN-α [27]. The most comprehensive phenotypic descriptions of the NIPC indicated that the NIPC (functionally defined as IFN-α+ cells within peripheral blood stimulated with HSV) as HLA-DR+, CD4+, CD45RA+ cells that were negative for a variety of lineage- and activation-associated markers (CD11c, CD11b, CD14, CD13, CD33, CD16, CD80 and CD86) [28].
The next breakthrough in DC biology was the recognition that peripheral blood contains distinct subsets of DC that differed in cell surface phenotype. O’Doherty et al. reported the presence of two distinct populations of blood DC that differed on the basis of CD11c expression [29] and Olweus et al. further defined the subsets of peripheral blood dendritic cells, which expressed differing levels of expression of the IL-3R alpha chain, CD123 [30]. These CD11c-, CD123+ cells were found to be the same as those being described by other investigators as either “plasmacytoid monocytes”, “plasmacytoid T cells” or “plasmacytoid dendritic cells” [31, 32].
In retrospect, it was realized that the cells now known as pDC had first been described in 1958 by pathologists as cells with a plasma cell-like morphology located in what was subsequently found to be T cell zones of human lymphoid tissues [33], including tonsil and lymph nodes and were found in large numbers in lymph nodes in certain pathological conditions such as lymphadenitis, Castleman’s disease, and Hodgkin’s lymphoma (reviewed in [34]). Thus, like the history of IFN itself, the history of the pDC spans half a century, and similar to IFN, the significance of these cells in the host immune response was not appreciated for decades after their initial discovery. Subsequently, Y-J Liu and colleagues described the plasmacytoid nature of the DC population isolated from the T cell-rich areas near high endothelial venules of tonsils and demonstrated that these CD4+, CD3+, CD11c- cells could differentiate into mature DCs when cultured with IL-3 plus CD40L [32] and they called these cells “DC2” because cells matured in this way induced Th2 immune responses. The identification of the DC2 (now known as the plasmacytoid dendritic cells or pDC) as being the same as the NIPC came in 1999 when the sorted DC2/pDC fractions from peripheral blood were found to produce 100–1000-fold more IFN-α in response to UV-inactivated HSV than the CD11c+ conventional DC [19], a finding that was soon confirmed by other groups [35]. The pDC have subsequently been found to respond with vigorous IFN-α production in response to a variety of viral stimuli and to synthetic TLR agonists CpG [36–38] and members of the imiquimod family [39].
The description of human pDC as being the NIPC was followed by descriptions of pDC/NIPC in mice [40, 41]. While similar in many respects, murine pDC differ from human pDC with respect to phenotype and some functions; these differences, some of which are highlighted below, have been previously reviewed by Barchet et al. [42].
4.2 Interferon production by myeloid dendritic cells
While it is well-established that pDC are the most potent IFN producing cells, it is clear that a plethora of cell types are capable of producing IFN in response to viral infection, with the magnitude of the response dependent upon both the cell type and the infecting virus. Among these other non-pDC IFN producing cells are myeloid DC. The majority of studies of IFN-α production by human myeloid DC have focused on MDDC because of the ease with which MDDC can be derived in large numbers from monocytes. We evaluated the production of type 1 IFN by MDDC in response to virus stimulation; while MDDC were relatively weak producers of type 1 IFN in response to HSV, there was a significant expression of IFN in Sendai virus stimulated MDDC [43]; this type 1 IFN response was weaker than the response of either pDC or monocytes to Sendai virus, but like the responses of both of these other cell types, the MDDC expressed a number of different IFNA genes. Coccia et al. reported the expression of multiple subtypes of IFN-α genes as well as IFN-β produced by MDDC in response to influenza virus. For each of the subtypes, however, the magnitude of the response of purified pDC was much greater than that for MDDC. Likewise, they observed that purified pDC produced 100-fold more IFN-α and IFN-β protein in response to Flu than did the MDDC [44]. In addition, both MDDC and pDC were found in that study to express IFN-λ mRNA in response to Flu. Interestingly, unlike mature pDC, which dramatically down-regulate their ability to produce IFN-α (reviewed in [45]), the matured MDDC retained the ability to express IFN-α,–β and -λ message in response to Flu or Sendai virus infection.
MDDC have been shown to be susceptible to productive infection with HSV [46], and this infection leads to expression of activation markers on the MDDC. HSV-stimulated MDDC cultures are able to produce only very low, but biologically significant, levels of IFN-α. In one study by Katz and colleagues, actual infection of the MDDC by HSV was not required in that UV-inactivated HSV was found to induce similar levels of IFN in the DC as did viable virus [46]. In contrast to this finding, however, Melchjorsen et al. recently demonstrated that human macrophages failed to produce IFNs α, β and λ in response to UV-inactivated HSV, and that MDDC failed to produce IFN-λ in response to UV-inactivated HSV (the production of IFN-α and –β were not discussed in this context) [47]. MDDC have also been reported to produce IFN-α/β in response to infection with Mycobacterium tuberculosis [48], indicating a potentially broad range of pathogens that are recognized by these innate immune effectors.
5. Relationship between plasmacytoid and myeloid dendritic cells
While it is clear that both pDC and mDC (also known as conventional or cDC) derive from a common hematopoietic precursor, the exact relationship between these two lineages is controversial. Early reports of the cells now known as pDC described these cells as either plasmacytoid monocytes or plasmacytoid T cells [31, 35]. mDC can be derived from myeloid precursors, and the most commonly studied form of MDC are the monocyte-derived dendritic cells (MDDC), which are obtained by culturing CD14+ monocytes with GMCSF and IL-4. Murine pDC and cDC can be derived in a Flt3L-dependent manner from the common lymphoid or myeloid progenitors within the bone marrow [49–54]. Moreover, administration of Flt3L in vivo mobilizes both myeloid and plasmacytoid DC as well as NK cells and other hematopoietic cell types [49, 50, 53]. A myeloid derivation of pDC was suggested by the expression of GMCSF receptors on the cells [30]. Alternatively, studies have suggested a lymphoid origin of pDC because of their expression of certain lymphoid-related gene products including the pre-T cell receptor α (pTα), V-preB, Spi-B, Notch-1 and a rearranged immunoglobulin heavy chain (IgH) D-J [55]. Additionally, Spits et al. demonstrated that ectopic expression of Id3 and Id2 inhibited development of CD34+ progenitor cells into T cells, B cell and pDC, again suggesting a common lymphoid precursor for these cell types [56]. Interestingly, Shigematsu et al., however, demonstrated that not only could the pDC be derived from either myeloid or lymphoid precursors, the human pTα as well as the IgH and RAG transcripts could be found both the myeloid and lymphoid-derived pDC [54]. Together, these data support the conclusion that so-called myeloid and plasmacytoid DC can each be derived from myeloid and lymphoid lineages, underscoring the plasticity of both DC lineages as well as myeloid and lymphoid lineages. In that respect, the adoption of the term “conventional” DC or cDC as opposed to “myeloid DC” to distinguish these cells from pDC is probably a good idea, even though it carries with it the implicit implication that pDC are somehow “unconventional”.
This developmental plasticity of the DC subsets echoes and fits in well with the changing dogma in which the classical notion of common myeloid and lymphoid progenitors is giving way to the idea of a common lymphoid/myeloid progenitor that gives rise to progenitors for both T cells and myeloid cells, as well as progenitors that give rise to B cells and myeloid cells (reviewed by [57]). In this model, myeloid potential is retained in the thymus such that thymic progenitors can produce both T cells and DC; this is supported by the observation that pDC in the thymus express the pTα and other T-lineage products [58]. Likewise, according to H. Kawamoto, a common B cell/myeloid progenitor could give rise to either B cells or DC subsets; indeed, B cells have much in common with DC in terms of phagocytosis, antigen processing and antigen presentation, as well as expression of rearranged IgH genes and other B cell transcripts [57].
Mouse studies have recently highlighted the importance of transcription factors that regulate the development of DC subsets. For example, mice doubly knocked-out for IRF-4 and IRF-8 lacked all splenic DC subsets whereas reintroduction of these transcription factors restored both cDC and pDC development. A role for both IRF-8 and, to a lesser degree, IRF-4 in the development of pDC was found using single and double knock-out mice for these transcription factors [59], whereas lack of only IRF-4 resulted in decreased CD4+ DC while lack of IRF-8 resulted in a lack of CD8+ DC. IRF-8 but not IRF-4 transduced into the double knock-out DCs allowed these cells to produce IFN-a and IL-12 in response to the TLR9 agonist, CpG, a potent stimulator of pDC. Human peripheral blood pDC also express both IRF-4 and IRF-8 [43], but whether either is required for the production of IFN-α by these cells is not known.
Ikaros is a zinc finger protein that serves mainly as a repressor and is important for the development of several lineages. It was recently demonstrated that mice with low levels of Ikaros expression lack peripheral blood pDC, but not other DC subsets, and fail to produce IFN-α after stimulation with TLR7 and -9 agonists or after murine cytomegalovirus infection. The bone marrow of these mice, however, contained precursors of pDC that failed to undergo terminal differentiation in response to Flt-3L; these bone marrow pDC had upregulation of genes that are normally poorly expressed in pDC from wild type animals, suggesting that the function of Ikaros is to silence an array of genes to allow pDC differentiation [60].
Along with clear evidence for plasticity of developmental origin of cDC and pDC, recent studies have demonstrated that the DC subsets maintain plasticity even after differentiation. In this regard, the Oldstone group observed that bone marrow-derived murine pDC can differentiate into myeloid (conventional) DC upon infection with lymphocytic choriomeningitis virus (LCMV) [61]. The DC transformation was dependent upon infection of the pDC precursors and involved IFN-α, but was independent of cell division and was limited to pDC in the bone marrow but not in the periphery, suggesting that the plasticity is limited to the less mature pDC. In what is perhaps a related observation, Reis e Sousa and colleagues demonstrated that cDC from mice infected with DC-tropic LCMV make high levels of type I IFN and that non-pDC secrete high levels of type I IFN when double-stranded RNA is introduced into the cytoplasm to mimic viral infection [62]. O’Garra and Trinchieri in a recent commentary have asked, tongue-in-cheek, whether DC are “afraid of commitment” and have suggested that the reversible commitment of DC subsets and the reprogramming of pDC to differentiation into cDC may serve to efficiently sustain the adaptive immune response [63].
Part of the evidence for conversion of pDC to cDC lineage was the acquisition of the ability to recognize different microbial structures through TLR4 [61]. However, recognizing antigen through TLR4 may not be an unusual property of pDC; although pDC were previously reported not to express TLR4 [64], our group demonstrated that human peripheral blood pDC express basal levels of TLR4 and respond to LPS stimulation with upregulation of TLR4 message and protein as well as up-regulate the expression of the transcription factor, IRF-7 [65]. LPS, however, did not lead to translocation of IRF-7 to the nucleus of the pDC, but upon subsequent stimulation with HSV-1, the LPS-stimulated PDC showed increased levels of IFN-α expression with enhanced kinetics. In addition, murine pDC have also been shown to be activated by TLR4 ligands [66].
Although functional plasticity of DC subsets appears to exist both at the developmental and post-developmental stages, one must be cautious against over-interpretation of results from studies using non-highly purified populations of cells. For example, while it is established that murine pDC produce IL-12 in response to viruses or TLR7 or -9 stimuli [67], whether human pDC also do so has been controversial. Y-J Liu and colleagues obtained highly purified pDC and found that the purified pDC produced hundreds-fold more IFN-α than mDC and the mDC produced 13-fold more IL-12 p70 than the purified pDC in response to TLR and CD40 ligands [68]. The pDC were found to express transcription factors necessary for type I IFN transcription, but not for IL-12 transcription. These authors attributed previous reports of significant levels of IL-12 production by human pDC [35, 69] to contaminating mDCs.
6. Mechanisms for induction of IFN in plasmacytoid vs. myeloid dendritic cells
As described above, pDC are, by far, the professionals of the interferon world. They are equipped with abundant rough endoplasmic reticulum that is reminiscent of antibody-secreting plasma cells (hence the term “plasmacytoid”) [19, 70]. The pDC can produce massive amounts of type I IFN in response to stimulation with a wide range of DNA and RNA viruses that signal through TLR9 and TLR7, respectively [71, 72]. Evidence obtained by clonal analysis of material from virus-stimulated pDC indicates that these cells express a wide range of IFN-α subtypes as well as IFNs –β, κ, ω, and λ [43, 68] that appears to be somewhat broader than the subtypes represented in the more modest type I IFN responses in monocytes or MDDC [73]. Although pDC upregulate a number of different genes, including those for several other cytokines including TNF-α and IL-6 [8, 74], chemokines such as CXCL10 and CCL4 [74–76], and human beta defensin 1 [77] in response to viral stimulus, the type I IFN and type III (IFN-λ) genes were recently reported to represent ~60% of the novel transcripts of the stimulated human pDC, thus making these cells true IFN factories [68]. The signaling pathways involved in type I IFN production by human pDC are summarized in Figure 1 and described below:
Figure 1. Type I IFN production pathways in human pDC and cDC/mDC.
pDC utilize a variety of cell-surface receptors to endocytose viruses into endosomes where the viral nucleic acid is uncoated. There, the RNA or DNA interacts with TLR7 and -9, respectively, to induce type I IFN gene expression independent of viral replication and in a MyD88-dependent manner. pDC express high constitutive levels of the transcription factor, IRF-7 that is required for the induction of IFN-α. cDC also utilize a TLR-dependent pathway for the sensing of viral RNA – TLR3, which recognizes dsRNA within the endosomes. This IFN response, however, requires autocrine IFN feedback to upregulate IRF-7 expression to get the full range of IFN subtype expression. In addition to the TLR-dendent IFN induction, both types of DC have MyD88 independent mechanisms for recognition of cytoplasmic viruses following infection of the cells with virus, including the RIG-I system for recognition of cytoplasmic dsRNA and an as yet undefined cytoplasmic sensor for DNA.
6.1 Intracellular pathways in induction of IFN in DC
According to the classic pathway of type I IFN induction, virus stimulation leads to the phosphorylation of IRF-3, its translocation to the nucleus and subsequent upregulation of a subset of early type I IFN genes. These IFNs are translated, then secreted, and signal through the IFN-α/β receptor and, through ISGF3 (comprised of STAT1, STAT2 and IRF-9), upregulate IRF-7 expression which is needed for the expression of the full range and magnitude of the IFN-α genes. In contrast to this model, which was originally worked out in fibroblasts [78], pDC (but not blood cDC and MDDC or any other cell type present in peripheral blood) express high constitutive levels of IRF-7 that rapidly translocates to the nucleus upon viral stimulation [43, 68, 79–81]. In mice as well as humans, this IFN induction pathway in pDC proceeds even in the absence of feedback signaling [79, 82]. Indeed, IRF-7 has been called the “master regulator” of type I IFN gene expression in both Myd88-independent and -dependent IFN-α responses, and pDC are wholly dependent upon this for IFN responses to TLR9 agonists [83]. pDC also express high levels of IRF-4, -5, and -8 [43, 68]. IRF-4 and IRF-8 are involved in the development of pDC, and in the mouse IRF-8 appears to be required in the differentiated pDC for IFN-α expression [59]; whether the latter is true in human pDC is currently being investigated in our laboratory in collaboration with Dr. Keiko Ozato. The role of the IRF-4 in pDC is unknown. Both pDC and cDC have are known to express IRF-5 [43, 44, 68, 84]. While the major role of IRF-5 in pDC may be in the cellular maturation pathway, our collaborator, Dr. Betsy Barnes, has implicated IRF-5 in the induction of type I IFN genes as well [85].
A distinct difference between human peripheral blood CD11c+ DC (cDC) and pDC is the high levels of expression of TLR7 and TLR9 in the endosome by the latter but not the former [86]. TLR9 is the receptor for both CpG sequences and double-stranded DNA from herpes simplex virus and cytomegalovirus [72, 87–89], while the ligands for TLR7 include the imadazoquinolones and single stranded RNA such as found in viruses like HIV-1. [39, 90–93]. MyD88, which is required for signaling through TLR7 and TLR9, forms a complex with IRF-7 that is required for the signaling for type I IFN production in the pDC [94]. This association, which requires that the TLR and ligand remain for an extended time in the endosome as opposed to being transferred to the lysosome for induction of IFN, also requires IRAK-4 [95] and the ubiquitin ligase action of TRAF6 for the activation of IRF-7 [96], [97]. A recent study demonstrated that the kinase that interacts with and phosphorylates the IRF-7 is IRAK-1; IRAK-1 deficient mice were found to be severely compromised in their ability to activate IRF-7 and induce type 1 IFN [98].
Classically, it is virus replication in cells that leads to the induction of type I IFN. Interestingly, while MDDC productively replicate HSV-1, pDC are not infected by this virus. Indeed, both live and UV-inactivated HSV-1 as well as HSV-infected cells are able to induce IFN-α production in pDC [3, 15, 19]. Although pDC can be productively infected with HIV-1 [99], UV- or AT-2-inactivated HIV-1 as well as live virus can all induce IFN-α in pDC. Diebold et al. [91] have shown that cDC taken from mice with a DC-tropic strain of LCMV make high levels of type I IFN on subsequent culture; likewise they reported that non-pDC secrete high levels of IFN in response to dsRNA introduced into the cytoplasm, mimicking virus infection. This latter induction was TLR3 (sensor for dsRNA) independent and partially PKR dependent. Although pDC production of IFN is believed to be important in host defense, mice lacking either TLR9 or MyD88 (and hence NIPC function) are able to adequately control HSV replication in vivo [89], suggesting that there is functional redundancy in protective anti-viral measures. These redundant mechanisms may be comprised of IFN induction in other DC or non-DC populations or may represent alternate pathways within pDC for IFN-α induction. In support of the latter, Hornung et al. [100] demonstrated that pDC produce IFN-α in response to a single stranded RNA virus, and similar results have been obtained for TLR9-independent IFN-α induction by HSV in both pDC and non-plasmacytoid DC [87].
Although cDC (including blood and tissue mDC as well as monocyte-derived DC) typically lack the ability to produce high levels of type I IFN, they do have the machinery to produce IFN upon viral infection. As in the case for the TLR9-independent responses to HSV described above, these IFN responses typically require viral replication and are largely due to intracellular recognition of viral nucleic acid by cytoplasmic receptors. A common replicative intermediate of many viruses is dsRNA. dsRNA is well-known for its ability to activate PKR, but, more recently, the role for the retinoic acid-inducible gene-I (RIG-I) encoded helicases and melanoma differentiation-associated gene 5 (MDA-5) have been strongly implicated as the prevalent cytoplasmic receptors for dsRNA leading to IFN-α production [101, 102]. A role for an as yet unidentified, TLR9-independent intracellular receptor for DNA has also been reported recently, thus expanding the potential repertoire for recognition of intracellular pathogens and viruses [103]. cDC have an additional mechanism for the induction of type I IFN in response to dsRNA viruses that goes via the dsRNA sensor, TLR3 in the endosomes. TLR3 signaling utilizes the adaptor molecule TRIF rather than the IPS-1/MAV5 used in the RIG-I/MDA-5 pathway [104]. The early IFN produced in these pathways then acts in an autocrine fashion to upregulate IRF-7, which can then lead to the virus-dependent IFN-α production (reviewed in [105]).
While, as noted above, many viruses do not require viral replication for induction of IFN-α in pDC, other viruses such as VSV require viral gene expression. In recent studies, Isakawa et al. have reported that cytoplasmic viral RNA can be transferred to the endosomes of pDC via autophagy, thus explaining how cytoplasmic virus enters into TLR7-containing compartments [106].
The redundancy in the DC subtypes that respond to viral infection with IFN production, the range of IFN subtypes produced and the different mechanisms of IFN induction have obvious beneficial implications for the host; since viruses expend considerable genetic energy in evasion of the IFN response, the redundancies in the host response serve to provide the host with overlapping protection. As reviewed in Haller et al., there are a plethora of anti-IFN strategies employed by viruses, ranging across the entire spectrum of the IFN response, such as the sequestration of dsRNA by the NS1 protein of flu, paramyxovirus inhibition of MDA5, P protein of Rabies virus and Ebola virus inhibition of IRF-3, to viral IRF mimics that exert dominant-negative effects (reviewed in Haller [105]). Interestingly, Kaposi’s sarcoma virus (HHV-8) encodes and inhibitor of IRF-7, thus directly targeting the type I IFN pathway [107]. Moreover, poxviruses express soluble IFN-binding proteins that compete with cellular receptors for IFN; these so-called “viroceptors” neutralize the activity of IFNs and viroceptors specific for IFN-α and IFN-λ have been isolated [105].
6.2. Dendritic cell-surface receptors involved in induction of IFN-α
Although much has been learned about the intracellular mechanisms of induction of IFN-α within dendritic cells, interactions of extracellular viruses with the surface of DC is less well understood. In pDC, a requirement for viral envelope glycosylation in IFN-α induction, presumably by interaction of the glycoproteins with cell surface receptors, has been demonstrated. Examples of this include the requirement for gD, a glycoprotein of HSV-1 required for viral entry for induction of IFN-α in pDC [108, 109] and the mutation of a single amino acid in the M protein of porcine transmissible gastroenteritis virus that renders the virus non-interferogenic [110, 111], and the dependence on gp120 of HIV for IFN induction by pDC [7, 112]. Proposed candidate receptors for these viral glycoproteins are C-type lectin receptors. BDCA-2 is a C-type lectin receptor on pDC that has been shown to be endocytic [113, 114]; cross-linking of this receptor leads to negative regulation of the ability of pDC to produce IFN [114]. We have reported the expression of low levels of the mannose receptor (CD206) on pDC (which is also expressed but at much higher levels on MDDC) [115]. Antibody blockade of this receptor was found to inhibit production of IFN-α in response to HSV and HIV [115], but did not affect monocyte IFN-α production. However, we have recently reported that pDC are exquisitely sensitive to down-regulation of IFN-α production in response to viruses following cross-linking of a variety of different cell-surface receptors including BDCA-2, BDCA-4, MHC Class I, MHC Class II, CD123 and CD4 [80]. Thus, care must be taken to not overinterpret antibody blocking studies in pDC as being necessarily indicative of blocking of uptake receptors. Likewise, since BDCA-4 cross-linking by magnetic immunobeads downregulates IFN-α production [80], positive selection of BDCA-4 expressing pDC in this manner should be avoided. Cao et al. [116] have recently described the expression of the ILT7 receptor on human pDC and demonstrated that it associates with the signal adapter protein FcεR1γ to form a receptor complex. They further reported that cross-linking of this receptor on the pDC inhibits CpG and Flu- induced type I IFN and cytokine expression by these cells. While interesting, these results are subject to the same caveat described above.
While non-enveloped viruses such as polio virus are generally not interferogenic in pDC, when complexed with antibody, can induce IFN-α [117]. The virus-immune complexes are taken up via FcγRII, which is expressed on pDC [118]. This receptor is also been shown to mediate uptake of immune complexes containing DNA in lupus [119–121], which then stimulates IFN production through TLR9. A role for scavenger receptors (CD204), which bind and internalize a wide range of negatively charged macromolecules, have been implicated in the uptake of material by both human and macaque MDDC [122, 123], and scavenger receptor A knock-out mice display an increased susceptibility to HSV-1 infection [124].
A number of other C-type lectin receptors are also expressed on subsets of DC and are reported to be involved in uptake of viruses. Some of these include DC-SIGN [125], which is expressed on MDDC but not on the majority of circulating mDC or pDC [126], DEC205 and dectin. The exact role of these in uptake of pathogens by DC is under intense investigation, but studies have indicated that DC-SIGN binds and internalizes HIV-1, and the DC subsequently serve as a Trojan horse to transmit virus to uninfected cells [127, 128].
7. Effects of IFN produced by DC on innate and adaptive immunity
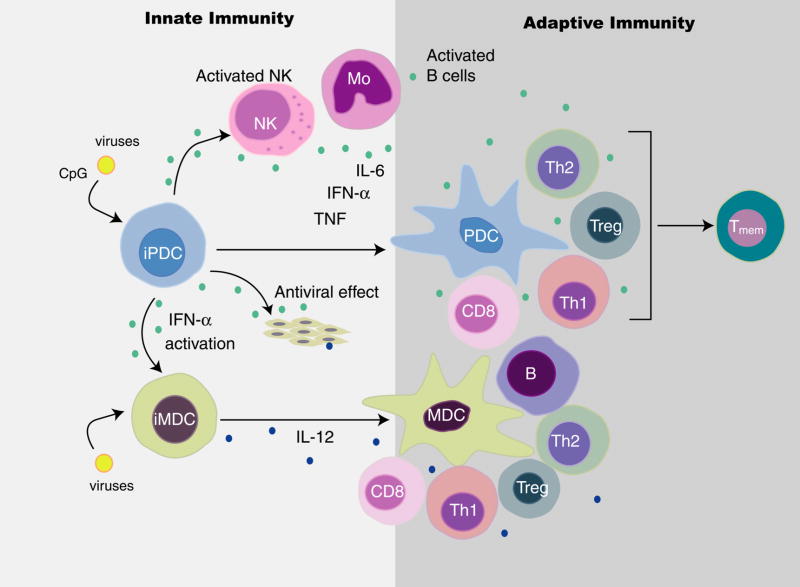
The functional role of IFN produced by DC is a topic of intensive investigation. These studies have focused both on the effects of exogenous IFN on DC and other components of the immune response as well as in vivo and in vitro studies of the scope of immune responses in the presence or absence of DC and their derived IFN. Some of these interactions are summarized in Figure 2 and are described below:
Figure 2. DC-derived IFN induces DC subset interaction and influences innate and adaptive immune responses.
Type I IFN produced in response to virus by pDC (and in some cases cDC) leads to antiviral activity, recruitment and upregulation of NK activity, activation of monocytes as well as activation and maturation of cDC. Depending on the nature of the stimulus received, the mDC and pDC have the capacity to activate B cells for the production of antibody and activate Th1, Th2, Treg and CD8+ T cell activity and T memory, with IFN-α playing a central role in these activities.
7.1 Effects of type I IFN on DC
Type I IFNs are known to have a number of effects on DC maturation and function. For example, IFN-α (as well as inducers of IFN-α such as TLR9 agonists CpGA or HSV, or TLR7 agonists), along with IL-3 act as survival factors, and to some extent, as maturation factors for pDC [86, 129–131]. In addition, IFN-α was also shown to enhance the maturation of CD11c+ human blood mDC; mDC matured in this way led to the induction of IL-10 producing T-reg cells [130] and contribute to the dsRNA or virus-induced maturation of murine DC [132]. MDDC generated by culture of human monocytes with IFN-α plus GM-CSF (rather than the classic IFN-γ plus GM-CSF) expressed higher levels of MHC class I molecules and acquired TLR7, which could then secrete IFN-α in response to TLR7 agonists and activated CD8+ cells [133]. In a model of generation of human mDC, cultures from unstimulated DC were found to contain low levels of IFN-α that enhanced maturation and activated the DC [134]. In this same study, IFN-α was found to induce the migration of human split skin-derived DC, suggesting a role for IFN-α in mobilization of Langerhans cells [134]. Asselin-Paturel et al., in a murine model, demonstrated that both pDC and cDC respond to TLR4, -7 and -9 ligands, with IFN-α important in response to all three ligands in pDC, whereas cDC required IFN-α for TLR9, and to a lesser degree, TLR7 responses [66].
Cross-talk between pDC and mDC via type I IFN has been implicated in some pathological situations. In individuals with lupus, for example, there is a chronic activation of IFN-α production by pDC leading to the maturation of mDC that induce a strong autoimmune Th1 response (reviewed in [119]).
One role of pDC-produced IFN-α is to upregulate cytokines and chemokines in pDC in an autocrine or paracrine manner. Virus-stimulated pDC produce CXCL10 (IP-10) as well as other chemokines [74–76]; in our study, we demonstrated that IFN-α of PBMC can lead to direct upregulation of CXCL10 in pDC. However, neutralization of IFN-α in HSV-stimulated pDC only partially reduced the expression of CXCL10, suggesting that both IFN and direct virus stimulation of the pDC contribute to the CXCL10 expression [74]. In the situation where cDC are infected by HSV, there is maturation of both infected and uninfected DC, with type I IFN priming the maturation and secretion of cytokines by the uninfected pDC [46]. Likewise, IFN-α efficiently primes for the production of both IFN-α and IFN-λ by pDC (Yin et al, in preparation).
7.2 Effects of DC-derived type I IFN on natural killer cells
As described above, lysis of many virus-infected targets by NK cells requires the contribution of DC as effector cells which function in both an IFN-dependent and independent manner [21–23, 135]. More recently, both cDC and mDC have been shown to contribute to the virus-induced activation of NK cells through IFN-α-dependent and -independent mechanisms [88, 136, 137]. In an in vivo murine cytomegalovirus infection model, pDC were induced to activate NK cells [88] and in the case of human pDC, not only do they produce IFN-α that can activate NK cells, they also produce chemokines that selectively attract NK cells and activated T cells [74]. This recruitment of NK cells and activated T cells has also been reported for MDDC in that M. tuberculosis-infected human MDDC produced IFN and chemokines that both chemoattracted and activated NK cells [138].
7.3 Effects of DC-derived type I IFN on T cell responses
As professional antigen presenting cells (APC), DC are able to activate a variety of CD4+ T cells, and, with their unique ability to efficiently cross-present antigens, are also efficient at induction of cytotoxic CD8+ T cells. By most accounts, cDC (of which MDDC are the best studied) are more efficient APC than non-activated pDC due to their higher rate of endocytosis of antigen and higher expression of MHC Class II [32]. However, pDC do effectively internalize antigen, and internalization of viruses into endosomes followed by endosomal acidification is required for IFN-α production [18, 80]. Once activated, the pDC upregulate both their expression of co-stimulatory molecules as well as MHC Class II and are able to activate both CD4+ and CD8+ cells [32, 139–141].
For pDC, IFN-α production is central to their activation of Th1 cells, and, indeed, IFN-α itself is a Th1-biasing cytokine, able to induce the production of IFN-γ by Th cells [142]. In support of this link between IFN-α production by pDC and Th1 cells, virus-stimulated pDC mature into DC that could stimulate naïve T cells to produce IFN-γ and IL-10 [131, 143]. In contrast, pDC activated in the presence of IL-3 and CD40L were found to skew the T cell responses towards Th2 responses [32, 144].
Vital roles for IFN-α in priming or cross-priming of CD8+ T cells by virus-stimulated DC have been described. DC generated in the presence of IFN-α plus GM-CSF (“IFN-DC”) were found to be superior to CD40L-matured MDDC generated with IL-4 plus GM-CSF for cross-priming responses to hepatitis C virus or inactivated HIV-1 [145]. pDC stimulated by CpGA (which induces pDC production of IFN-α) or CpGB (which induces pDC maturation, but little IFN-α) were able to induce CD8 memory cells, whereas CpGB was more efficient than CpGA in inducing priming of naïve CD8+ cells [146], thus suggesting both IFN-dependent and –independent pathways of CD8 T cell activation by pDC. Such activation of CD8+ cells is not always advantageous; for example, it has recently been demonstrated that pDC derived IFN-α can stimulate cytotoxic T cell activity in atherosclerotic plaque through the induction of TRAIL [141]. Likewise, pDC derived IFN-α has been implicated in killing of T cells in HIV-1 infection, also in a TRAIL-dependent manner [147].
In addition to its positive roles in Th and CTL responses, DC-derived IFN-a can also contribute to the development of the CD4+IL-10+ subset of Treg cells [148]. Moreover, continuous activation of pDC by HIV-1 and their production of IFN-α impedes early T-cell lymphopoiesis [149].
7.4 Effects of DC-derived type I IFN on antibody responses
In addition to their effects on NK and T cell responses, type I IFN and dendritic cells can affect humoral immunity as well. Le Bon et al. [150] demonstrated that type I IFN potently enhanced the antibody response to soluble antigen, allowing for class-switching and development of immunological memory in a DC-dependent manner. Furthermore, type I IFN was found to be essential for the adjuvant activity of complete Freund’s adjuvant in this study. pDC have also been reported to induce the secretion of influenza-specific and polyclonal antibodies in human culture; moreover, pDC triggered with virus induced B cells to differentiate into plasma cells, with pDC-derived IFN generating the non-Ig –secreting plasmablasts and pDC-derived IL-6 inducing their differentiation into Ig-secreting plasma cells [151, 152].
8. Conclusions
After fifty years of study, type I IFNs have claimed a central position in host defense not only as important mediators of innate anti-viral immunity, but also as links between the innate and adaptive immune systems. These proteins have become true blockbusters in the pharmaceutical industry even without a full understanding of their mechanisms of action. With the acceleration of immunological research and the recognition of distinct roles for pDC and mDC in the immune response, there has been an explosion in our understanding of the interplay between IFN and these DC subsets. It can be anticipated that in the next fifty years, and certainly sooner, even more effective manipulation of the IFN system as it relates to DC wiill be possible and will contribute to improved therapeutics for cancer, infectious disease and autoimmunity.
Abbreviations
- PBMC
peripheral blood mononuclear cells
- NK
natural killer cell
- pDC
plasmacytoid dendritic cell
- mDC/cDC
myeloid or conventional dendritic cell
- IFN
interferon
- NIPC
natural IFN producing cell
- TLR
toll-like receptor
- IRF
interferon response factor
- MDDC
monocyte-derived dendritic cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isaacs A, Lindenmann J. Virus interference. 1. The interferon. Proc Royal Society Biology. 1957;147:258. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 2.Ronnblom L, Ramstedt U, Alm GV. Properties of human natural interferon-producing cells stimulated by tumor cell lines. European Journal of Immunology. 1983;13:471–476. doi: 10.1002/eji.1830130608. [DOI] [PubMed] [Google Scholar]
- 3.Cederblad B, Alm G. Infrequent but efficient interferon-α-producing human mononuclear leukocytes induced by herpes simplex virus in vitro studies by immunoplaque and limiting dilution assays. J Interferon Res. 1990;10:65–73. doi: 10.1089/jir.1990.10.65. [DOI] [PubMed] [Google Scholar]
- 4.Perussia B, Fanning V, Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell-Growth Regul. 1985;4:120–137. [PubMed] [Google Scholar]
- 5.Fitzgerald-Bocarsly P, Feldman M, Mendelsohn M, Curl S, Lopez C. Human mononuclear cells which produce interferon-alpha during NK (HSV-FS) assays are HLA-DR positive cells distinct from cytolytic natural killer effectors. J Leukocyte Biol. 1988;43:323–334. doi: 10.1002/jlb.43.4.323. [DOI] [PubMed] [Google Scholar]
- 6.Feldman M, Fitzgerald-Bocarsly P. Sequential enrichment and immunocytochemical visualization of human interferon-α producing cells. J Interferon Res. 1990;10:435–446. doi: 10.1089/jir.1990.10.435. [DOI] [PubMed] [Google Scholar]
- 7.Ferbas JJ, Toso JF, Logar AJ, Navratil JS, Rinaldo CR. CD4+ blood dendritic cells are potent producers of IFN-α in response to in vitro HIV-1 infection. J Immunol. 1994;152:4649–4662. [PubMed] [Google Scholar]
- 8.Ghanekar S, Zheng L, Logar A, Navratil J, Borowski L, Gupta P, Rinaldo C. Cytokine expression by human peripheral blood dendritic cells stimulated in vitro with HIV-1 and herpes simplex. J Immunol. 1996;157:4028–4036. [PubMed] [Google Scholar]
- 9.Uze G, Lutfalla G, Mogensen KE. Alpha and beta interferons and their receptor and their friends and relations. J Interferon Cytokine Res. 1995;15:3–26. doi: 10.1089/jir.1995.15.3. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol. 2004;76:314–321. doi: 10.1189/jlb.0204117. [DOI] [PubMed] [Google Scholar]
- 11.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nature immunology. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 12.Saksela E, Virtanen I, Hovi T, Secher DS, Cantell K. Monocyte is the main producer of human leukocyte alpha interferons following Sendai virus induction. Prog Med Virol. 1984;30:78–86. [PubMed] [Google Scholar]
- 13.Feldman SB, Milone MC, Kloser P, Fitzgerald-Bocarsly P. Functional deficiencies in two distinct IFN-α producing cell populations in PBMC from human immunodeficiency virus seropositive patients. J Leuk Biol. 1995;57:214–220. doi: 10.1002/jlb.57.2.214. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G, Santoli D, Dee RR, Knowles BB. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. J Exp Med. 1977;147:1299. doi: 10.1084/jem.147.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald PA, von Wussow P, Lopez C. Role of interferon in natural kill of HSV-1 infected fibroblasts. J Immunol. 1982;129:819–824. [PubMed] [Google Scholar]
- 16.Fitzgerald PA, Evans R, Kirkpatrick D, Lopez C. Heterogeneity of human NK cells: Comparison of effectors that lyse HSV-1-infected fibroblasts and K562 erthroleukemia targets. J Immunol. 1983;130:1663–1667. [PubMed] [Google Scholar]
- 17.Ronnblom L, Ramstedt U, Alm GV. Properties of human natural interferon-producng cells stimulated by tumor cell lines. Eur J Immunol. 1983;13:471–476. doi: 10.1002/eji.1830130608. [DOI] [PubMed] [Google Scholar]
- 18.Feldman SB, Ferraro M, Zheng HM, Patel N, Gould-Fogerite S, Fitzgerald-Bocarsly P. Viral induction of low frequency interferon-alpha producing cells. Virology. 1994;204:1–7. doi: 10.1006/viro.1994.1504. [DOI] [PubMed] [Google Scholar]
- 19.Siegal F, Kadowaki N, Shodell M, Fitzgerald-Bocarsly P, Shah K, Ho S, Antonenko A, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 20.Sandberg K, Matsson P, Alm GV. A distinct population of non-phagocytic and CD4+ null lymphocytes produce interferon-α after stimulation by Herpes simplex virus infected cells. J Immunol. 1990;145:1015–1020. [PubMed] [Google Scholar]
- 21.Bandyopadhyay S, Perussia B, Trinchieri G, Miller DS, Starr S. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. J Exp Med. 1986;164:180–195. doi: 10.1084/jem.164.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh SH, Trinchieri G, Bandyopadhyay S, Starr SE. Natural killer cell-mediated lysis of herpes simplex virus-infected fibroblasts: Inability to detect soluble factors that contribute to lysis. Cell Immunol. 1990;127:221–229. doi: 10.1016/0008-8749(90)90127-d. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald-Bocarsly P, Feldman M, Curl S, Schnell J, Denny T. Positively selected Leu-11a (CD16+) cells require the presence of accessory cells or factors for the lysis of HSV-infected fibroblasts but not HSV-infected Raji. J Immunol. 1989;143:1318–1326. [PubMed] [Google Scholar]
- 24.Feldman M, Howell D, Fitzgerald-Bocarsly P. Interferon dependent and independent participation of accessory cells in natural killer cell mediated lysis of HSV-1 infected fibroblasts. J Leuk Biol. 1992;52:473–482. doi: 10.1002/jlb.52.5.473. [DOI] [PubMed] [Google Scholar]
- 25.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice III. functional properties in vivo. J Exp Med. 1974;139:1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinman RM, Kaplan G, Witmer MD, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs. V. Purification of spleen dendritic cells, new surface markers and maintenance in vitro. J Exp Med. 1979;149:1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chehimi J, Starr SE, Kawashima H, Miller DS, Trinchieri G, Perussia B, Bandyopadhyay S. Dendritic cells and IFN-a producing cells are two functionally distinct non-B, non-monocytic HLA-DR+ cell subsets in human peripheral blood. Immunol. 1989;68:486–490. [PMC free article] [PubMed] [Google Scholar]
- 28.Svensson H, Cederblad B, Lindahl M, Alm G. Stimulation of natural interferon-α/β-producing cells by Staphyococcus aureus. J Interferon and Cytokine Research. 1996;16:7–16. doi: 10.1089/jir.1996.16.7. [DOI] [PubMed] [Google Scholar]
- 29.O’Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 30.Olweus J, BitMansour A, Warnke R, Thompson P, Carballido J, Picker L, Lund-Johansen F. Dendritic cell ontogeny: A human dendritic cell lineage of myeloid origin. Proc Natl Acad Sci, USA. 1997;94:12551–12556. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Facchetti F, Wold-Peeters C, Mason D, Pulford K, Van den Oord J, Desmet V. Plasmacytoid T cells. Immunohistochemical evidence for their monocyte/macrophage origin. Am J Path. 1988;133:15–21. [PMC free article] [PubMed] [Google Scholar]
- 32.Grouard G, Rissoan M, Filguiera L, Durand I, Banchereau J, Liu J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin-3 and CD40 ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennert K, Remmele W. Karyometrische untersuchungen an lymphknotenzell des menschen I: Mitt germinoblasten, lymphoblasten und lymphozyten. Acta Haematol. 1958;19:99–113. doi: 10.1159/000205419. [DOI] [PubMed] [Google Scholar]
- 34.Galibert L, Maliszewski C, Vandenabeele S. Plasmacytoid monocytes/T cells: a dendritic cell lineage? Seminars in immunology. 2001;13:283–289. doi: 10.1006/smim.2001.0324. [DOI] [PubMed] [Google Scholar]
- 35.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 36.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c- type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. Journal of Immunology. 2001;166:2291–2295. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 37.Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J of Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 38.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cellular Immunology. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 40.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nature immunology. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 41.Nakano H, Yanagita M, Gunn MD. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. The Journal of experimental medicine. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Seminars in immunology. 2005;17:253–261. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Izaguirre A, Barnes BJ, Amrute S, Yeow WS, Megjugorac N, Dai J, Feng D, Chung E, Pitha PM, Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 44.Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 45.Fitzgerald-Bocarsly P. Natural interferon producing cells: the plasmacytoid dendritic cells. Biotechniques. 2002;33:S16–S29. [PubMed] [Google Scholar]
- 46.Pollara G, Jones M, Handley ME, Rajpopat M, Kwan A, Coffin RS, Foster G, Chain B, Katz DR. Herpes simplex virus type-1-induced activation of myeloid dendritic cells: the roles of virus cell interaction and paracrine type I IFN secretion. J Immunol. 2004;173:4108–4119. doi: 10.4049/jimmunol.173.6.4108. [DOI] [PubMed] [Google Scholar]
- 47.Melchjorsen J, Siren J, Julkunen I, Paludan SR, Matikainen S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J Gen Virol. 2006;87:1099–1108. doi: 10.1099/vir.0.81541-0. [DOI] [PubMed] [Google Scholar]
- 48.Remoli ME, Giacomini E, Lutfalla G, Dondi E, Orefici G, Battistini A, Uze G, Pellegrini S, Coccia EM. Selective expression of type I IFN genes in human dendritic cells infected with Mycobacterium tuberculosis. J Immunol. 2002;169:366–374. doi: 10.4049/jimmunol.169.1.366. [DOI] [PubMed] [Google Scholar]
- 49.Karsunky H, Merad M, Mende I, Manz MG, Engleman EG, Weissman IL. Developmental origin of interferon-alpha-producing dendritic cells from hematopoietic precursors. Exp Hematol. 2005;33:173–181. doi: 10.1016/j.exphem.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. The Journal of experimental medicine. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blom B, Ho S, Antonenko S, Liu YJ. Generation of interferon alpha-producing predendritic cell (Pre-DC)2 from human CD34(+) hematopoietic stem cells. The Journal of experimental medicine. 2000;192:1785–1796. doi: 10.1084/jem.192.12.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blom B, Ligthart SJ, Schotte R, Spits H. Developmental origin of pre-DC2. Hum Immunol. 2002;63:1072–1080. doi: 10.1016/s0198-8859(02)00745-0. [DOI] [PubMed] [Google Scholar]
- 53.D’Amico A, Wu L. The Early Progenitors of Mouse Dendritic Cells and Plasmacytoid Predendritic Cells Are within the Bone Marrow Hemopoietic Precursors Expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shigematsu H, Reizis B, Iwasaki H, Mizuno S, Hu D, Traver D, Leder P, Sakaguchi N, Akashi K. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 2004;21:43–53. doi: 10.1016/j.immuni.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Corcoran L, Ferrero I, Vremec D, Lucas K, Waithman J, O’Keeffe M, Wu L, Wilson A, Shortman K. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J Immunol. 2003;170:4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- 56.Spits H, Couwenberg F, Bakker AQ, Weijer K, Uittenbogaart CH. Id2 and Id3 inhibit development of CD34(+) stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. The Journal of experimental medicine. 2000;192:1775–1784. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawamoto H. A close developmental relationship between the lymphoid and myeloid lineages. Trends Immunol. 2006;27:169–175. doi: 10.1016/j.it.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Bendriss-Vermare N, Barthelemy C, Durand I, Bruand C, Dezutter-Dambuyant C, Moulian N, Berrih-Aknin S, Caux C, Trinchieri G, Briere F. Human thymus contains IFN-{{alpha}}-producing CD11c-, myeloid CD11c+, and mature interdigitating dendritic cells. J Clin Invest. 2001;107:835–844. doi: 10.1172/JCI11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O’Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 60.Allman D, Dalod M, Asselin-Paturel C, Delale T, Robbins SH, Trinchieri G, Biron CA, Kastner P, Chan S. Ikaros is required for plasmacytoid dendritic cell differentiation. Blood. 2006;108:4025–4034. doi: 10.1182/blood-2006-03-007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nature immunology. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, Al-Shamkhani A, Flavell R, Borrow P, Reis e Sousa C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 63.O’Garra A, Trinchieri G. Are dendritic cells afraid of commitment? Nature immunology. 2004;5:1206–1208. doi: 10.1038/ni1204-1206. [DOI] [PubMed] [Google Scholar]
- 64.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dai J, Megjugorac NJ, Amrute SB, Fitzgerald-Bocarsly P. Regulation of IFN Regulatory Factor-7 and IFN-{alpha} Production by Enveloped Virus and Lipopolysaccharide in Human Plasmacytoid Dendritic Cells. J Immunol. 2004;173:1535–1548. doi: 10.4049/jimmunol.173.3.1535. [DOI] [PubMed] [Google Scholar]
- 66.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O’Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. The Journal of experimental medicine. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- 68.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 69.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 70.Ferbas J, Rinaldo C. Purified dendritic cells are potent producers of IFN-α. J Leuk Biol Suppl. 1992;3:24. [Google Scholar]
- 71.Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol. 2005;26:221–229. doi: 10.1007/s00281-004-0180-4. [DOI] [PubMed] [Google Scholar]
- 72.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. The Journal of experimental medicine. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Izaguirre A, Barnes B, Amrute S, Yeow YZ, Megjugorac N, Dai J, Feng D, Chung E, Pitha P, Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte derived dendritic cells. J Leuk Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 74.Megjugorac N, Young HA, Amrute S, Olshalsky S, Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J Leukoc Biol. 2004;75:504–514. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- 75.Penna G, Vulcano M, Roncari A, Facchetti F, Sozzani S, Adorini L. Cutting Edge: Differential Chemokine Production by Myeloid and Plasmacytoid Dendritic Cells. J Immunol. 2002:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- 76.Penna G, Vulcano M, Sozzani S, Adorini L. Differential Migration Behavior and Chemokine Production by Myeloid and Plasmacytoid Dendritic Cells. Human Immunology. 2002;63:1164–1171. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 77.Ryan LK, Diamond G, Amrute S, Feng Z, Weinberg A, Fitzgerald-Bocarsly P. Detection of HBD1 peptide in peripheral blood mononuclear cell subpopulations by intracellular flow cytometry. Peptides. 2003;24:1785–1794. doi: 10.1016/j.peptides.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 78.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 79.Dai J, Megjugorac NJ, Amrute SB, Fitzgerald-Bocarsly P. Regulation of IFN regulatory factor-7 and IFN-alpha production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J Immunol. 2004;173:1535–1548. doi: 10.4049/jimmunol.173.3.1535. [DOI] [PubMed] [Google Scholar]
- 80.Fanning SL, George TC, Feng D, Feldman SB, Megjugorac NJ, Izaguirre AG, Fitzgerald-Bocarsly P. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-alpha production. J Immunol. 2006;177:5829–5839. doi: 10.4049/jimmunol.177.9.5829. [DOI] [PubMed] [Google Scholar]
- 81.George TC, Fanning SL, Fitzgeral-Bocarsly P, Medeiros RB, Highfill S, Shimizu Y, Hall BE, Frost K, Basiji D, Ortyn WE, Morrissey PJ, Lynch DH. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J Immunol Methods. 2006;311:117–129. doi: 10.1016/j.jim.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 82.Barchet W, Cella M, Odermatt B, Asselin-Paturel C, Colonna M, Kalinke U. Virus-induced interferon a production by a dendritic cell subset in the absence of feedback signaling in vivo. J Exp Med. 2002;195:507–516. doi: 10.1084/jem.20011666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 84.Mancl ME, Hu G, Sangster-Guity N, Olshalsky SL, Hoops K, Fitzgerald-Bocarsly P, Pitha PM, Pinder K, Barnes BJ. Two discrete promoters regulate the alternatively spliced human interferon regulatory factor-5 isoforms. Multiple isoforms with distinct cell type-specific expression, localization, regulation, and function. J Biol Chem. 2005;280:21078–21090. doi: 10.1074/jbc.M500543200. [DOI] [PubMed] [Google Scholar]
- 85.Barnes BJ, Field AE, Pitha-Rowe PM. Virus-induced heterodimer formation between IRF-5 and IRF-7 modulates assembly of the IFNA enhanceosome in vivo and transcriptional activity of IFNA genes. J Biol Chem. 2003;278:16630–16641. doi: 10.1074/jbc.M212609200. [DOI] [PubMed] [Google Scholar]
- 86.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. The Journal of experimental medicine. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hochrein H, Schlatter B, O’Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 90.Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A, Liu YJ, Lifson JD, Littman DR, Bhardwaj N. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 92.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 95.Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Marodi L, Davidson D, Speert D, Roifman C, Garty BZ, Ozinsky A, Barrat FJ, Coffman RL, Miller RL, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann F, Jouanguy E, Casanova JL. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, Takeuchi O, Akira S. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nature immunology. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 97.Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC, Taniguchi T. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci U S A. 2004;101:15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, Takeuchi O, Akira S. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. The Journal of experimental medicine. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fong L, Mengozzi M, Abbey NW, Herndier BG, Engleman EG. Productive Infection of Plasmacytoid Dendritic Cells with Human Immunodeficiency Virus Type 1 Is Triggered by CD40 Ligation. J Virol. 2002;76:11033–11041. doi: 10.1128/JVI.76.21.11033-11041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- 101.Rothenfusser S, Goutagny N, DiPerna G, Gong M, Monks BG, Schoenemeyer A, Yamamoto M, Akira S, Fitzgerald KA. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 102.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 103.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 104.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 105.Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 107.Wang J, Zhang J, Zhang L, Harrington W, Jr, West JT, Wood C. Modulation of human herpesvirus 8/Kaposi’s sarcoma-associated herpesvirus replication and transcription activator transactivation by interferon regulatory factor 7. J Virol. 2005;79:2420–2431. doi: 10.1128/JVI.79.4.2420-2431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ankel H, Westra DF, Welling-Wester S, Lebon P. Induction of interferon-α by glycoprotein D of herpes simplex virus: a possible role of chemokine receptors. Virology. 1998;251:317–326. doi: 10.1006/viro.1998.9432. [DOI] [PubMed] [Google Scholar]
- 109.Lebon P. Inhibition of herpes simplex virus type-1 induced interferon synthesis by monoclonal antibodies against viral glycoprotein D and by lysosomotropic drugs. J Gen Virol. 1985;66:2781–2786. doi: 10.1099/0022-1317-66-12-2781. [DOI] [PubMed] [Google Scholar]
- 110.Charley B, Levenant L, Delmas B. Glycosylation is required for coronavirus TGEV to induce an efficient production of IFN-α by blood mononuclear cells. Scand J Immunol. 1991;33:435–440. doi: 10.1111/j.1365-3083.1991.tb01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laude H, Gelfi J, Lavenant L, Charley B. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastoenteritis virus. J Virol. 1992;66:743–749. doi: 10.1128/jvi.66.2.743-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ankel H, Capobianchi MR, Castilletti C, Dianzani F. Interferon induction by HIV glycoprotein 120: role of the V3 loop. Virology. 1994;205:34–43. doi: 10.1006/viro.1994.1617. [DOI] [PubMed] [Google Scholar]
- 113.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 114.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a Novel Plasmacytoid Dendritic Cell-specific Type II C-type Lectin, Mediates Antigen Capture and Is a Potent Inhibitor of Interferon {alpha}/{beta} Induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Milone MC, Fitzgerald-Bocarsly P. The mannose receptor mediates induction of IFN-alpha in peripheral blood dendritic cells by enveloped RNA and DNA viruses. J Immunol. 1998;161:2391–2399. [PubMed] [Google Scholar]
- 116.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. The Journal of experimental medicine. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palmer P, Charley B, Rombaut B, Daeron M, Lebon P. Antibody-dependent induction of type 1 interferons by poliovirus in mononuclear blood cells reuires the type II Fcγ receptor (CD32) Virol. 2000;278:86–94. doi: 10.1006/viro.2000.0627. [DOI] [PubMed] [Google Scholar]
- 118.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 119.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006;18:676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 120.Palucka AK, Banchereau J, Blanco P, Pascual V. The interplay of dendritic cell subsets in systemic lupus erythematosus. Immunol Cell Biol. 2002;80:484–488. doi: 10.1046/j.1440-1711.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 121.Ronnblom L, Alm G. A pivitol role for the natural interferon α-proudcing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J Exp Med. 2001;194:F59–F63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harshyne LA, Zimmer MI, Watkins S, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunoi. 2003;170:2302–2309. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 123.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 124.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Kodama T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 125.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 126.Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, Coleman N, Lee B. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 127.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 128.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16:135–144. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 129.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. The Journal of experimental medicine. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 131.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. The Journal of experimental medicine. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, Seya T, Taniguchi T. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci U S A. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, Gaugler B. IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J Immunol. 2003;171:3385–3393. doi: 10.4049/jimmunol.171.7.3385. [DOI] [PubMed] [Google Scholar]
- 134.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 135.Bandyopadhyay S, Oh SH, Chehimi J, Ziegner U, Miller DS, Hoxie JA, Starr SE. Natural killer cell-mediated lysis of target cells infected with cytomegalovirus or human immunodeficiency virus. In: Ades EW, Lopez C, editors. 5th International NK Workshop; Hilton Head, South Carolina: Karger; 1988. pp. 114–119. [Google Scholar]
- 136.Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nature immunology. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 137.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 138.Lande R, Giacomini E, Grassi T, Remoli ME, Iona E, Miettinen M, Julkunen I, Coccia EM. IFN-alpha beta released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: selective recruitment of NK and activated T cells. J Immunol. 2003;170:1174–1182. doi: 10.4049/jimmunol.170.3.1174. [DOI] [PubMed] [Google Scholar]
- 139.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nature immunology. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 140.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. [see comments] Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 141.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–2489. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 142.Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon α increases the frequency of interferon γ-producing human CD4+ T cell. J Exp Med. 1993;178:1655–1663. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. [see comments] Nature Immunology. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 144.Rissoan M-C, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu Y-J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 145.Lapenta C, Santini SM, Spada M, Donati S, Urbani F, Accapezzato D, Franceschini D, Andreotti M, Barnaba V, Belardelli F. IFN-alpha-conditioned dendritic cells are highly efficient in inducing cross-priming CD8(+) T cells against exogenous viral antigens. Eur J Immunol. 2006;36:2046–2060. doi: 10.1002/eji.200535579. [DOI] [PubMed] [Google Scholar]
- 146.Rothenfusser S, Hornung V, Ayyoub M, Britsch S, Towarowski A, Krug A, Sarris A, Lubenow N, Speiser D, Endres S, Hartmann G. CpG-A and CpG-B oligonucleotides differentially enhance human peptide-specific primary and memory CD8+ T-cell responses in vitro. Blood. 2004;103:2162–2169. doi: 10.1182/blood-2003-04-1091. [DOI] [PubMed] [Google Scholar]
- 147.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: How good interferon turns bad. Clin Immunol. 2006 doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dikopoulos N, Bertoletti A, Kroger A, Hauser H, Schirmbeck R, Reimann J. Type I IFN negatively regulates CD8+ T cell responses through IL-10-producing CD4+ T regulatory 1 cells. J Immunol. 2005;174:99–109. doi: 10.4049/jimmunol.174.1.99. [DOI] [PubMed] [Google Scholar]
- 149.Schmidlin H, Dontje W, Groot F, Ligthart SJ, Colantonio AD, Oud ME, Schilder-Tol EJ, Spaargaren M, Spits H, Uittenbogaart CH, Blom B. Stimulated plasmacytoid dendritic cells impair human T-cell development. Blood. 2006;108:3792–3800. doi: 10.1182/blood-2006-02-004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 151.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 152.Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, Venditti M, Capone I, Seif I, De Maeyer E, Tough D, Donatelli I, Belardelli F. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol. 2002;169:375–383. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]