Abstract
Prenatal exposure to environmental contaminants, such as Benzo(a)pyrene [B(a)P] has been shown to impair brain development. The overarching hypothesis of our work is that glutamate receptor subunit expression is crucial for cortical evoked responses and that prenatal B(a)P exposure modulates the temporal developmental expression of glutamatergic receptor subunits in the somatosensory cortex. To characterize prenatal B(a)P exposure on the development of cortical function, pregnant Long Evans rats were exposed to low-level B(a)P (300μg/kg BW) by oral gavage on gestational days 14 to 17. At this exposure dose, there was no significant effect of B(a)P on 1) the number of pups born per litter, 2) the pre-weaning growth curves and 3) initial and final brain to body weight ratios. Control and B(a)P-exposed offspring were profiled for B(a)P metabolites in plasma and whole brain during the pre-weaning period. No detectable levels of metabolites were found in the control offspring. However, a time-dependent decrease in total metabolite concentration was observed in B(a)P-exposed offspring. On PND100-120, cerebrocortical mRNA expression was determined for the glutamatergic NMDA receptor subunit (NR2B) in control and B(a)P-exposed offspring. Neural activity was also recorded from neurons in primary somatic sensory (barrel) cortex. Semiquantitative PCR from B(a)P-exposed offspring revealed a significant 50% reduction in NR2B mRNA expression in B(a)P-exposed offspring relative to controls. Recordings from B(a)P-exposed offspring revealed that N-methyl-D-aspartate (NMDA) receptor -dependent neuronal activity in barrel cortex evoked by whisker stimulation was also significantly reduced (70%) as compared to controls. Analysis showed that the greatest deficit in cortical neuronal responses occurred in the shorter latency epochs from 5-20ms post-stimulus. The results suggest that in utero exposure to benzo(a)pyrene results in diminished mRNA expression of the NMDA NR2B receptor subunit to result in late life deficits in cortical neuronal activity in the offspring. The findings from this study lead to a strong prediction that in utero exposure to benzo(a)pyrene at a time when synapses are first formed and adjusted in strength by activity in the sensory pathways will produce a strong negative effect on brain function in offspring progeny.
Keywords: polycyclic aromatic hydrocarbon-(PAH), benzo(a)pyrene-B(a)P, World Trade Center (WTC), small for gestational age (SGA), intrauterine growth restriction (IUGR), Bailey Scales of Infant Development (BSID-II), susceptibility-exposure paradigm, somatosensory cortex-S1 cortex, cortical neuronal activity and behavior, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA), environmental aryl hydrocarbon receptor agonists-eAhR agonist, developmental neurotoxicity
INTRODUCTION
Benzo(a)pyrene is the prototypical polycyclic aromatic hydrocarbon (PAH). It is produced by the incomplete combustion of organic substances in processes, such as automobile exhaust, trash incineration, coal and charcoal grilling, as well as in wood and paper processing (Ramesh et al., 2004; Dahlgren et al., 2003; ASTDR, 1995). Human exposure to B(a)P occurs through the ingestion of contaminated food and water (Ramesh et al., 2004; Phillips, 1999) or the inhalation of particulates in the ambient air, such as after the collapse of the World Trade Center on September 11, 2001(ATSDR, 1995; Perera et al., 2005). Evidence from human epidemiological studies has shown that unintended prenatal exposure of the fetus to B(a)P adversely affects fetal development, resulting in low birth weight and reduced head circumference, as well as neurobehavioral deficits in the offspring. These deficits have been quantified in human populations as low scores on selective types of cognitive and neuromotor functioning (Hack et al., 1991; Perera et al., 2003; Landrigan et al., 2004). Studies of B(a)P exposed populations provide evidence that environmental contaminants (of the polycyclic aromatic hydrocarbon class) at environmentally relevant levels adversely affect cognitive development during childhood (Perera et al., 2006).
Studies have also documented the neurotoxic effects resulting from exposure to polycyclic aromatic hydrocarbons on learning and memory mechanisms in rodent models utilizing different exposure regimens. Several studies have reported robust neurotoxic effects as evidenced by deficits in learning and memory mechanisms (assessed by Y-maze, fixed-ratio operant conditioning, 2-lever reversal conditioning and morris water maze performance) subsequent to prenatal or sub-acute benzo(a)pyrene exposure (Grova et al., 2007; Wormley et al., 2004). In a report from Konstandi et al. (1997), evidence was presented that subsequent to repeated treatment of adult rats with a 3-methylcholanthrene (a member of the polyclyclic aromatic hydrocarbon family) resulted in a significant reduction in learning ability in a two-way active avoidance procedure.
Recently, we hypothesized that the mechanism for deficits in cortical neuronal activity following prenatal B(a)P exposure in offspring involved the downregulation of early ionotrophic glutamatergic receptor subunit expression during the critical period of postnatal synaptogenesis (Brown et. al., 2007). The rat primary somatosensory (S1) cortex was selected as a model to test our mechanistic hypothesis because of the unique organization of the rat whisker to cortex pathway, and the already demonstrated effect of other toxins (Hood et a., 2007), such as alcohol and lead on this brain region (Rema et al., 1998; Rema and Ebner, 1999; Wilson et al., 2000; Benuskova et al., 2001). Rat whiskers are arranged in horizontal rows and vertical arcs on both sides of the face. Each whisker projects via 2 relays to a cellular aggregate called a barrel in layer IV of the S1 Cortex (see Figure 1; adapted from Schubert and Staiger, 2002; Woolsey and Van der Loos, 1970). This arrangement of the whisker to barrel pathway makes the S1 cortex in rats an ideal system to quantify alterations in stimulus-response relationships. Additionally, because the rat cortex is in a relatively undeveloped state at birth, it serves as an excellent model to study map development within the context of characterizing the influence of toxicants during its development on its final form. Several studies have shown that the major features of the somatotopic map are formed within the first week of life (for review, see Killackey et al., 1990). The thalamocortical afferents cluster into barrels over the first 4 or 5 d (Erzurumlu and Jhaveri, 1990) and the cortical cells in layer IV form into the characteristic cell sparse hollows and cell-dense barrel walls during the same period (Rice et al., 1985).
Figure 1. Main elements in the neural pathway from the axons innervating the whisker follicles on a rat’s face (lower left) to the primary somatic sensory cortex (upper right).
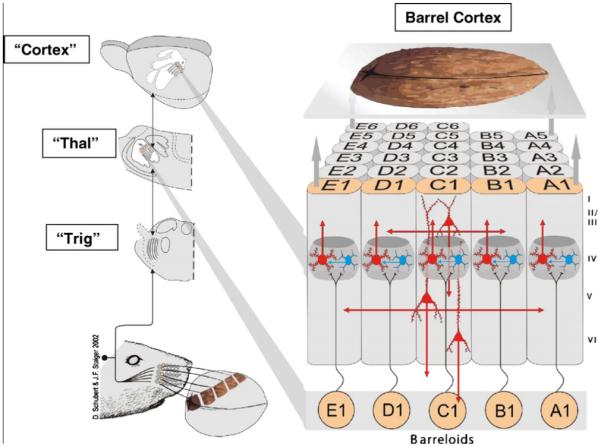
The diagram illustrates the rat palpating a walnut with its whiskers and conceptualizes the walnut being perceived through activity transmitted in the sensory pathway to cortex. The pathway contains a first synapse in the brainstem trigeminal nuclei (“Trig”), a second synapse in the contralateral thalamus (“Thal”) and a third relay from thalamus to primary sensory (SI) cortex (called barrel cortex). There is a separate channel for each whisker on the rat’s face up to and including primary sensory cortex as illustrated on the right, each labeled by a row (from A dorsal to E ventral) and a number starting from posterior and counting anteriorly. The roman numerals to the right of barrel cortex identify the six cortical layers. The results in the present paper were generated by analyzing neurons in the barrel cortex using single cell electrophysiology and biochemical techniques. [Permission was originally obtained from Brain Res. Adapted from D. Schubert and J. F. Staiger, (2002); Woolsey and van der Loos (1970)].
A recent study from our group characterized the impact of prenatal exposure to B(a)P on modulation of glutamate receptor subunit expression that is critical for the maintenance of synaptic plasticity mechanisms during cortical development. The results of this study revealed significant down-regulation of developmental ionotropic glutamate receptor subunit expression, namely the N-methyl-D-aspartate receptor (NMDAR) and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptor (AMPAR). This previous study also demonstrated (using whole cell patch clamp analysis of primary cortical neuronal cultures from B(a)P[exposed offspring) that significant voltage-dependent decreases in the amplitude of inward currents occur at negative potentials in B(a)P-treated cortical neuronal membranes (Brown et al., 2007). Overall, the results from our previous study suggested that plasticity and behavioral deficits produced as a result of prenatal B(a)P exposure are at least, in part, a result of down-regulation of early developmental glutamatergic receptor subunit expression and function at a time when excitatory synapses are being formed for the first time in the developing central nervous system.
The present study extends the findings from the previous study in that the objective of the present study is to determine whether in utero B(a)P exposure interferes with cortical neuronal responses reflected by deficits in cortical driven activity as a result reductions in mRNA expression of glutamatergic receptor subunit expression in the offspring. The vast majority of excitatory and inhibitory synapses are formed during the first 3 postnatal weeks (Blue and Parnevalas, J. Neurocytol., 198x). Glutamate receptor subunits (such as NR2B) are expressed in increasing numbers during this period of synaptogenesis and each subunit has a different developmental profile (Nakayama, et al., 2005). The early and late components of cortical neuronal responses to whisker stimulation in mature rat barrel cortex show differing dependence on iontotropic glutamate receptors: the early response, associated with thalamocortical activation of barrel neurons, is heavily dependent on AMPA receptor activation, while the late response (including surround whisker responses) is NMDA dependent (Armstrong-James and Callahan, 1993). In an earlier study, Fox (1998) reported that pulling out vibrissae was sufficient to alter the connectivity of the barrel cortex but insufficient, in altering the larger features of cortical somatotopic organization. The observed changes were remarkable but did not totally abolish the cortical neuronal activity in the deprived pathways. A conclusion from this study was that pulling out vibrissae reduces the amount of activity in the pathways with which they are associated. The major conclusion was when the follicle is left intact following vibrissae removal-along with the attendant nerve endings; activity in the primary thalamocortical afferents, whether spontaneous or caused by mechanical stimulation is likely to propagate through to the cortex. Here we report that in utero exposure to B(a)P results in significant reductions in the early and late response components of cortical neuronal activity. This B(a)P-induced robust reduction in cortical neuronal activity corresponds with a significant downregulation of NMDA-NR2B subunit expression in offspring rats.
MATERIALS AND METHODS
Power analysis was used to evaluate the proper number of offspring animals required from the standpoint of number of litters as the statistical unit. From this analysis, three cohorts of randomized timed-pregnant dams between two experimental groups were utilized: 1) a control group (vehicle exposed), and 2) a 300μg/kg body weight (BW) B(a)P exposed group. Thirty offspring animals were utilized consisting of fifteen control and fifteen 300μg/kg body weight (BW) B(a)P-exposed offspring from eleven total litters (5 control and 6 B(a)P-exposed) were used to collect the electrophysiological data. Power analysis indicated that three repetitions of each cohort were required and it was estimated that the variance between measures from litters would be 10% of the mean response. Power analysis established that three successful experiments in each cohort would be required to detect 20% change in any of the experimental end-points with 80% power and a type-I error rate of 5%. This estimate held true as we were able to detect differences +/- the variance in the effect of B(a)P-exposed offspring relative to offspring controls.
Animal Husbandry
All experiments were approved by the Institutional Animal Care and Use Committees (IACUC) of Vanderbilt University and Meharry Medical College and were performed according to Guidelines for Animal Experimentation as set forth by the NIH and both institutions. Timed-pregnant Long-Evans Hooded dams were obtained from Harlan Sprague Dawley (St. Louis, M0) on gestational day (GD) 11. Upon arrival, animals were housed individually in clear plastic cages with laboratory grade (heat-treated) pine shavings as bedding. Animals were quarantined for 2 days in the AAALAC accredited Meharry Medical College animal care facility and were maintained in a controlled environment with a temperature at 21 ± 2°C and relative humidity of 50 ± 10% with a 12/12 hr light/dark cycle. Dams were fed commercial food (Rat Chow 5012: Purina Mills, St. Louis, MO). Water and food was available ad libitum. Each dam was randomly assigned to either a control or experimental group and on GD14-17, timed-pregnant dams were exposed (using our susceptibility-exposure paradigm; Brown et al., 2007) by oral gavage in a total volume of 0.875ml to 1) peanut oil alone or to 2) 300μg/kg B(a)P in peanut oil. Offspring control and B(a)P-exposed animals were maintained in standard plastic cages until PND100-120 at which time they were anesthetized and their cortical neuronal activity assessed using the previously described procedures described in Hood et al., (2006).
B(a)P Disposition Analysis
Plasma and whole brain tissue from control and B(a)P-exposed offspring was processed for bioavailable levels of B(a)P metabolites by liquid-liquid extraction and reverse phase high performance liquid chromatography as previously outlined in Brown et al., (2007).
Anesthesia and Surgery
For electrophysiological recordings, male animals between PND90-120 were used. These animals were anesthetized with urethane (1.5 gm/kg ip; 30% solution in water). Core body temperature was maintained at 37° C with a heating pad electronically controlled by feedback from a rectal thermometer (Harvard Apparatus). Because anesthetic levels profoundly affect response characteristics (Armstrong-James and George, 1988; Friedberg et al., 1999), they were maintained by supplemental urethane injections. Anesthesia was maintained at surgical levels (Friedberg, et al., 1999), and when spontaneous burst rates became more frequent than 3-4/s repeat doses (10% of the original dose) were administered to maintain the depth of anesthesia. This condition assured that rats were maintained under full anesthesia with no signs of movement or stress. Respiration was regular (within 80 -110 breaths/ min) and there were no overt whisking movements, spontaneous limb movement or whisker tremor. An opening was made in the skull from 4 to 7 mm lateral to the midline and from 0 to 4 mm posterior to Bregma to expose the barrel field cortex. Small openings were made in the dura using the tip of a 30 gauge needle to facilitate electrode penetration.
Electrophysiology
Action potentials were recorded in the barrel field using carbon fiber microelectrodes (Armstrong-James and Millar, 1979). A three-dimensional stereotaxic apparatus (Narashigi Corp) was used to control the x-y position and angle of the penetrations, while the z-axis depth from the cortical surface was measured by an electronic microdrive with an accuracy of ~ 10 μm (David Kopf Instruments). The “best whisker” (BW) response for each penetration was determined by stimulation of each whisker manually. The whisker that evoked the largest magnitude of response was selected for recording in each electrode penetration. Only one stimulus intensity was tested, and evoked responses did not achieve control levels at the stimulus intensity that was employed. Recordings were made at a depths of 300 (layer II-III), 600 (layer IV) and 900 (layer V) microns below the pial surface (Li and Ebner, 2005). Whiskers were stimulated precisely by deflecting individual whiskers caudally with a computer-controlled piezoelectric bimorph stimulator positioned with a micromanipulator just rostral to the whisker so that the whisker was stimulated in an anterior to posterior direction. Each block of trials consisted of 50 stimuli (3 ms duration) delivered at 1 Hz. A 50v stimulus deflected the piezoelement approximately 600 microns (see Figure 2 in Hood et al., 2006). For all neurons analyzed, one block of stimulus trials was presented to the BW.
Figure 2. Representative Traces of S1 Barrel Field Cortex Electrode Localization in Control and B(a)P-exposed Offspring.
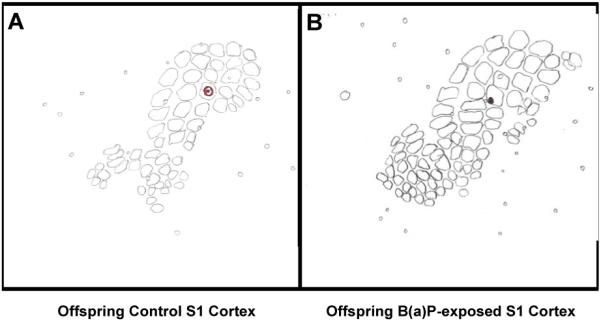
Maps for control and B(a)P-exposed animals were constructed from cytochrome oxidase stained tangential sections through layer IV of barrel field cortex. Both electrode tracks are in the C3 barrel column, so their principal whisker was the C3 whisker. Panel A; Verification example of electrode localization from control offspring. Panel B; Verification example of electrode localization from in utero B(a)P-exposed offspring.
Electrophysiology Statistical Analysis
The magnitude of cortical neuronal response or the response evoked from the best whisker stimulation was the mean ± S.E. of 50 trials delivered at 1 Hz at each recording location, for each sorted unit. For all forms of post stimulus time histograms (PSTH) analysis, the counts in each bin were adjusted for spontaneous activity by subtracting the number of spikes per 1 ms bin generated 50 ms before the stimulus from the number in each bin post-stimulus. Multiunit post-stimulus time histograms (PSTHs) at 1 ms bin resolution were generated on-line using a CED 1401 plus processor and software controlled by a PC computer. All action potential data was stored on a hard disk for off-line analysis. Individual neurons from continuously recorded multiple units were sorted with an Offline Sorter (Plexon Inc.) in the template matching mode to separate waveforms. PSTHs of responses within a 40 millisecond epoch were generated for the sorted units with NeuroExplorer (Nex Technologies). Data from all three recording depths 300μ, 600μ and 900μ corresponding to cortical layers II-III, IV and V was collapsed across control and B(a)P-exposed offspring. For determining the significance of cortical neuronal responses, non-parametric statistical analysis of the data was performed using the Wilcoxon matched-pair sign rank test and Mann-Whitney U-tests. Latency histograms were constructed from the same data as the PSTH to test the possibility that prenatal B(a)P exposure changed the timing of cortical activation. Latency histograms (LHs) were constructed in a manner similar to PSTHs except that only the first spike occurring >3 ms post-stimulus (i.e., only one spike/stimulus) was used to construct the histogram. The modal latency was identified for each whisker in a cell’s receptive field as the bin in the latency histogram with the greatest number of spikes. Neurons failing to generate a definitive latency grouping were listed as “no fixed latency”.
Histology
After an initial characterization of this type, it was imperative that the recording site be verified in each control and B(a)P-exposed offspring animal at the conclusion of a recording session. Therefore at the end of every recording session a DC current was passed (2μA for 5 s) through the electrode tip to produce an easily identifiable lesion roughly 50 μm in diameter to mark the recording site. These lesions were made at three depths along the penetration. A representative offspring control (left) and B(a)P-exposed (right) flattened section of S1 cortex is presented as Figure 2. The electrode location is circled for ease of identification. Electrode tracks were considered to be within a certain barrel only if the recording site could be localized within the boundaries of the cytochrome oxidase (CO) stained barrel column (Wong-Riley and Welt, 1980). The electrodes were considered to be within a certain barrel only if the recording site could be localized within the boundaries of the CO stained barrel column. Flattened cortices were generated as follows: At the end of the experiment the animal was perfused with 0.1 M phosphate buffer followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were removed from the skull and the neocortex was removed from the underlying structures and post fixed overnight. The preparation was then allowed to sink in 30% sucrose then flattened between slides.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Cerebrocortical tissue stored in liquid nitrogen from control (n=5) and B(a)P-exposed (n=5) offspring littermates of animals utilized in electrophysiology studies was used for semiquantitative PCR analysis. The cDNA synthesis and RT-PCR amplification were produced in one step using Promega’s Access RT-PCR System. The sequences of designed primers was:
NR2B Forward: 5′-AGA AAC CTG TCC TTC AGC GA-3′
NR2B Reverse: 5′-GTC AAC CAC CTC TGA CCG TT-3′
The size of the amplified product is 534 bp.
The final concentration of reagents in the PCR reaction system were as follows: 1X RT-PCR buffer, 1mM MgSO4, 0.2mM of each deoxynucleosidetriphsphate (dNTP), 1μM of each primer, 0.1u/μl Reverse Transcriptase, 0.1u/μl DNA Polymerase, and 20ng of total RNA was used as template in each 50μl reaction volume. For quantitative analysis of gene expression, Ambion (Austin, TX) QuantumRNA™ 18sRNA Internal Standards Kit was used to amplify 18sRNA together with the specific target gene in one tube. The optimal ratio of 18sRNA primer: competimer for each gene was determined prior to RT-PCR so that two bands can be visualized together. RT-PCR cycling conditions were as follows: 48°C 45 minutes for reverse transcription, and then 94°C, 2 minutes for RT inactivation; 94°C 30 minutes→60°C 1 minute→68°C 2 minutes for PCR, there were 35 cycles. All PCR reactions were linear at the PCR cycling conditions given above. The PCR products were electrophoresed on 1.2% agarose gel with 0.5μg/ml ethidium bromide. The specificity of each band was confirmed by the size of the amplified products, corresponding, for example, to 534bp for NR2B, and 324bp for 18sRNA. The density of each band was measured by an AlphaImager™ 2000 Digital Imaging System (Alpha Innotech Corp; San Leandro, CA., using the protocol described in Hood et al., 2000). The levels of target gene mRNA expression are shown as the relative ratio between the intensity of the target-specific band and that of the 18sRNA band.
Statistical Analysis of NMDA NR2B mRNA Expression
Values are given as means ± S.E.M. in figures. A two-way analysis of variance (ANOVA) was used to determine differences between control and B(a)P exposed groups in NMDA NR2B mRNA gene expression levels relative to 18sRNA. 18S was used as an internal standard for the RT-PCR analysis because this gene has been demonstrated to not be modulated as a result of B(a)P exposure and thus, not affect the expression of mRNA. The 18S comprises up to 80% of a total RNA sample, thus when the concentration of a total RNA sample is determined from spectrophotometric readings, the sample is essentially already being normalized to the amount of rRNA it contains. rRNAs are also transcribed by a distinct polymerase from mRNAs, which may result in a different pattern of regulation of expression. 18S rRNAs have been found to be uniform in all rodent tissues tested including liver, brain, thymus, heart, lung, spleen, testes, ovary, kidney and embryo. Therefore, the choice of a housekeeping gene in toxicology studies is based on empirical determinations as to the effect of the toxicant on the system under study.
Due to the expected within and between litter variation, NR2B mRNA expression levels were tested for statistical differences from tissues from different litters kept in liquid nitrogen. All pairwise multiple comparisons were then performed using the Student-Newman-Keuls method. Three separate determinations were performed and the criterion for statistical significance was p < 0.05 in all cases.
RESULTS
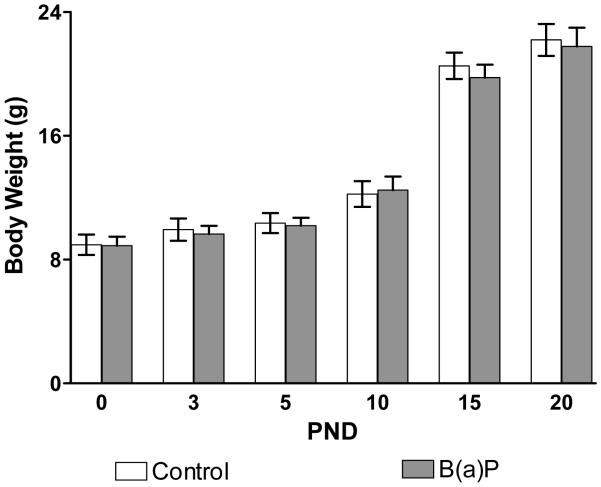
There we no significant differences found in the number of pups born per litter between control and B(a)P-exposed (300μg/kg BW) dams as shown in Table 1 consistent with a recent previous report from our group (Brown et al., 2007). During the prenatal exposure period as well as during the subsequent pre-weaning period, there were no identifiable B(a)P-related effects on conventional/reproductive indices of toxicity. No convulsions, tremors, or abnormal movements were noted in any of the dams or offspring in control or B(a)P-exposed litters. There were no significant differences in the liver to body weight ratios of the B(a)P-exposed offspring as compared to control offspring over the course of this study. Treatment-related differences in the brain to body weight ratios were observed only on PND15 and PND30 between control and B(a)P-exposed offspring. In the present study, the ratios are slightly higher on PND15 and PND30 where ratios from B(a)P-exposed offspring were greater than controls (data not shown). It is interesting to note that postnatal modulatory effects on PND15 and PND30 quantified as robust reductions in temporal developmental expression of glutamatergic receptor subunits has been previously reported subsequent to in utero exposure to environmental aryl hydrocarbon receptor agonists (Hood et al., 2006, Brown et al, 2007). The body weight growth curves for control and in utero B(a)P-exposed offspring are shown as Figure 3. These data reveal no significant differences in the pre-weaning growth curves between control and in utero B(a)P-exposed offspring.
Table 1.
| Group | Dams | Dose | Sites/dam | Pups Born/litter |
|---|---|---|---|---|
| Control | 5 | ---- | 10.2 ± 2.6 | 9.3 ± 2.1 |
| B(a)P (GD 14-17) |
6 | 300μg/kg BW | 11.2 ± 0.6 | 10.8 ± 0.8 |
Values represent the mean ± SEM, α=0.05.
Figure 3. Body weight gain for Control and B(a)P exposed offspring.
Shown are the gross body weights representing 3 cohorts over the period from PND0 through PND70. White bars represent control offspring, grey bars represent in utero B(a)P-exposed offspring. (Values are mean ± SEM with p<0.05) The data represent litter means of body weight from a representative subset [n=3 of control litters; n=3 for B(a)P litters] of total litters [n=5 for control litters; n=6 for B(a)P litters].
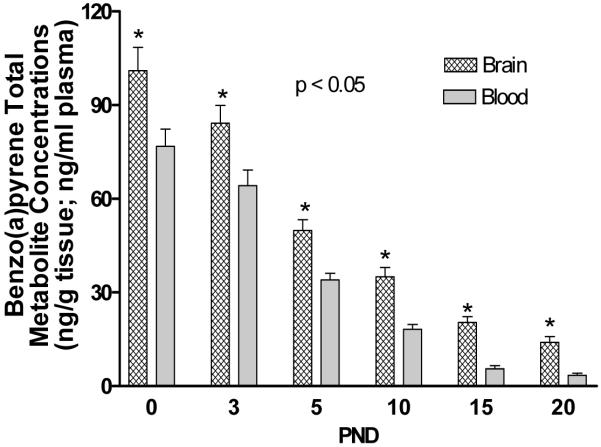
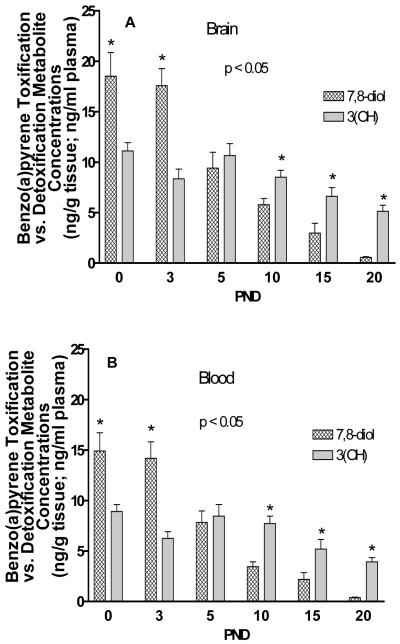
Control and in utero B(a)P-exposed offspring blood and whole brain tissue was also submitted for total B(a)P metabolite analysis. No detectable levels of metabolites were found in the control offspring. However, a time-dependent decrease in total B(a)P metabolite concentration was observed in plasma and whole brain tissue and is shown in Figure 4. Additionally, the total B(a)P metabolite load in brain and blood, the concentrations of B(a)P metabolites, such as B(a)P 7,8-diol 9,10-epoxide and 3(OH) B(a)P indicate active B(a)P toxification and detoxification pathways respectively and are shown in Figure 5. The concentration of B(a)P 7,8-diol metabolites were shown to be elevated during the pre-weaning period at PND5 but decreased by PND10. The 3-hydroxy metabolite showed elevated concentrations on PND10 and PND20. The differences between these two metabolite groups at each of the pre-weaning (PND) time points tested were statistically significant (p< 0.005).
Figure 4.
Time-course distribution of bioavailable B(a)P total metabolites in brain tissues and plasma. Timed-pregnant dams received 300 μg B(a)P/kg BW via gavage on GD14-17. Offspring pups were sacrificed on PND 0, 3, 5, 10, 15, and 20 and metabolite levels were determined as outlined in the methods and materials section. The detection limit (evaluated by a minimum signal to noise ratio of 3) of B(a)P metabolites by HPLC was approximately 300femtograms/total sample on column. Values represent mean + S.E.M. Owing to the limited volume of blood that could be obtained at PND0 & PND5, whole blood was used in lieu of plasma, whereas plasma was used for the remaining time points for metabolite analysis. Values are mean ± SEM with *p<0.05 for n=3 litters for control and n=3 litters for B(a)P-exposed cerebrocortical brain tissue for metabolite concentrations in brain tissues as compared to plasma. The asterisks denote statistical significance.
Figure 5.
Time-course distribution of B(a)P metabolites representative of toxification (BaP 7,8-diol) and detoxification (3-OH B(a)P) processes in A) brain tissues and B) plasma. Timed-pregnant dams received 300 μg B(a)P/kg BW via gavage on GD14-17. Offspring pups were sacrificed on PND 0, 3, 5, 10, 15, and 20 and metabolite levels were determined as outlined in the methods and materials section. Values represent mean + S.E.M. Owing to the limited volume of blood that could be obtained at PND0 & PND5, whole blood was used in lieu of plasma, whereas plasma was used for the remaining time points for metabolite analysis. Values are mean ± SEM with *p<0.05 for n=3 litters for control and n=3 litters for B(a)P-exposed cerebrocortical brain tissue for metabolite concentrations in brain tissues as compared to plasma. The asterisks denote statistical significance.
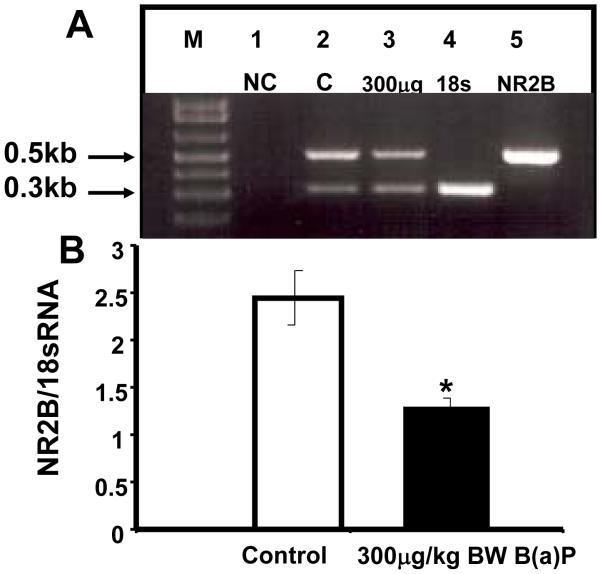
Previous data from our group (Wormley et al., 2004; Hood et al., 2006; Brown et al., 2007) has suggested that glutamate receptor subunit expression is modulated subsequent to in utero B(a)P exposure. We also maintain that in utero exposure has the potential to reduce cortical neuronal activity via alterations in glutamatergic receptor subunit expression thereby affecting NMDA and AMPA receptor activity. Therefore, the expression level of the glutamate receptor subunits NMDA-NR2B was evaluated on PND90-120, in parallel with electrophysiology studies which assayed B(a)P effects on cortical neuronal activity. The cerebrocortical mRNA expression for NR2B normalized to 18sRNA expression in Control and B(a)P-exposed offspring is shown as Figure 6. The results for control offspring are consistent with previous reports (Grova et al., Nagahashi et al, Brown et al., 2007). The RT-PCR results from 300μg/kg BW B(a)P-exposed offspring demonstrate a significant 50% reduction in NR2B mRNA expression in B(a)P-exposed offspring relative to control offspring.
Figure 6. Control and B(a)P-exposed offspring cerebrocortical NR2B mRNA expression on PND100.
Panel A shows a representative (n=3) agarose gel electrophoresis result from semi-quantitative RT-PCR analysis. The upper band is the NR2B (534 bp) subunit and the lower band is the internal control, 18sRNA (324 bp). M represents the DNA marker. (lane 1) negative control; offspring control cerebrocortical template cDNA minus NR2B primers; (lane 2) positive control; offspring control cerebrocortical template cDNA with NR2B + 18sRNA primers; (lane 3) 300 μg/kg BW B(a)P-exposed offspring cerebrocortical template cDNA with NR2B + 18sRNA primers; (lane 4) control cerebrocortical template cDNA + 18sRNA primers; (Lane 5) control cerebrocortical template cDNA + NR2B primers; Panel B displays a histogram of the densitometric quantitation of the relative expression of NR2B to internal 18sRNA control quantified from the ratios in lane 2 (white bar-control) and lane 3 (black bar300 μg/kg BW B(a)P-exposed -) from panel A.. *p < 0.05 relative to control in the PND100 samples.
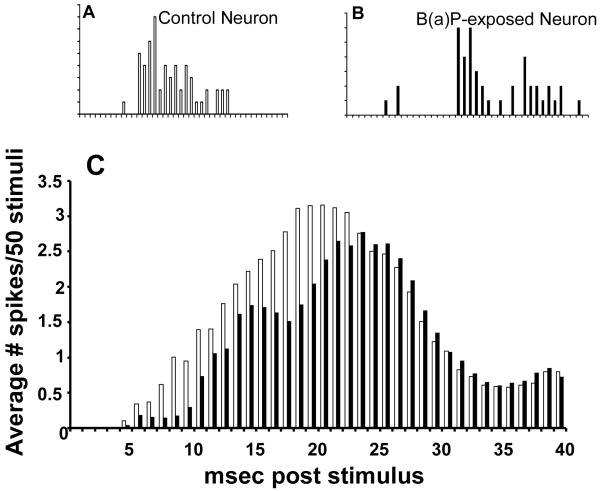
Lastly and importantly, in utero B(a)P-exposed offspring exhibited robust deficits in stimulus-evoked cortical neuronal activity in the barrel field cortex. B(a)P-induced suppression of evoked cortical neuronal activity was greatest in the earlier latency components of the response after stimulation of the best whisker (Figure 7;1-20 ms post stimulus). These earlier latency components are specifically dependent on glutamatergic receptor subunit expression. The 11-20ms epoch is totally dependent on NMDA receptor expression and is approximately 50% reduced in this epoch consistent with the approximate 50% reduction in NMDA-NR2B subunit expression as quantified by semiquantitative PCR analysis. The overall reduction in evoked cortical neuronal activity in the in utero B(a)P-exposed offspring can be easily visualized after subdividing the data into 10ms epoch responses recorded from the many neurons/animals shown in Figure 7. The consistency of the reduction in short latency response is inherent to the population PSTH (Figure 8), where it is also evident that the delay in onset of the cortical neuronal responses is reflected by reduction in number of spikes in the shorter as well as the longer latency intervals. The response of typical single neurons, one from a control offspring animal and one from an in utero B(a)P-exposed offspring animal is shown as panel A and B, respectively as insets in Figure 8.
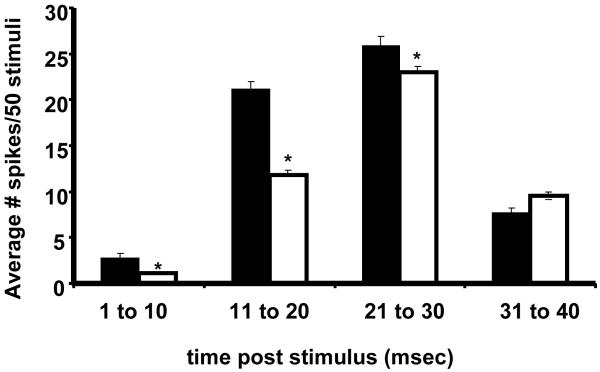
Figure 7. Bar graph of the mean ± SE Stimulus Evoked Activity from Control and B(a)P-exposed (300μg/kg BW) Offspring.
The mean number of spikes (spikes evoked/50 stimuli: y axis) occurring at 10ms epochs (x axis) for the duration of 40ms trial. Black bars represent peanut oil control offspring and white bars represent B(a)P-exposed offspring. Data is derived from a suprathreshold intensity (50 V stimulus applied to piezoelectric element) with whiskers deflected approximately 600 μm from the face. Response magnitudes (+SEM) were assessed for statistical differences within each group (* p<0.05, ANOVA). The epoch analysis shows that prenatal B(a)P exposure significantly impacts the shorter latency components.
Figure 8. Post-Stimulus Time Histograms Illustrate the Deficit in Short Latency Responses to Whisker Stimulation by S1 Cortical Neurons.
Panel A and Panel B; Responses to a single round of 50 whisker stimulations from Control and B(a)P-exposed neurons, respectively. Panel C; Comparison of average responses from all Control versus all B(a)P-exposed neurons recorded.
DISCUSSION
The central finding of the present study is that prenatal B(a)P exposure produces reproducible alterations in the form of diminished cortical neuronal activity to sensory input in the offspring. Results from the present study allow for the consolidation of interpretations from the previous two studies from our group. Specifically, that normal function of the somatic sensory cortex is impaired for at least 4 months after birth as a result of in utero B(a)P-exposure. The suppression of evoked cortical neuronal activity does, in fact, correlate with a reduction in NMDA-NR2B receptor subunit expression. The physiological deficit is characterized by robust reductions in sensory stimulus evoked cortical neuronal activity. The persistent reduction in the initial shorter latency epoch (3-20msec) from in utero B(a)P-exposed offspring is similar to what was previously observed in TCDD-exposed offspring (Hood et al., 2006). In normal and control animals, this shorter latency response is almost completely dependent on glutamatergic receptors (Armstrong-James et al., 1993) localized in thalamocortical synapses of cortical layer IV. The responses with latencies between 11-20msec depend largely on NMDA type glutamate receptors subunit expression (Armstrong-James et al., 1993; Rema et al., 1998). Thus, the suppression of these early latency responses in B(a)P-exposed offspring predicts a perturbation of NMDA receptor subunit function, and possibly of other types of glutamate receptors. Collectively, these results establish that in utero exposure to low doses of B(a)P during the period of cortical neuron formation and migration result in a strong negative effect on glutamate receptor subunit expression.
The fact that in utero exposure to B(a)P results in deficits in cortical neuronal activity in adult offspring poses an interesting question as to whether sufficient amounts of the maternal B(a)P dose is bioavailable in offspring so as to cause the observed neurotoxicity. Our findings on the disposition of B(a)P/metabolites in blood and whole brain tissues from offspring suggest that in utero exposure to B(a)P results in accumulation of metabolites, which persist in offspring tissues up to PND20. Findings from the present study corroborate our earlier findings (Wu et al., 2003; Brown et al., 2007) demonstrating transplacental translocation of metabolites from dam to fetus during gestation and subsequent persistence in tissues throughout the preweaning period. Lactational transfer of B(a)P from mother to the newborn has been reported for rats (Yoshiko et al., 2004), ruminants (Lapole et al., 2007) and humans (Zanieri et al., 2007). Thus, the developing rat pups will not only have a constant infusion of B(a)P in utero (via placental transfer; Sanyal and Li, 2007) but also during the neonatal period (lactational transfer; cited above). In addition to transplacental transfer of maternal metabolites, fetal metabolic conversion of B(a)P transferred through the placenta (Kihlström, 1986) may also contribute to the global B(a)P metabolite pool in the offspring pups. The bioavailable reactive metabolites of B(a)P such as the B(a)P 7,8-diol 9,10-epoxide in neuronal tissues may also potentiate the reported deficits in cortical neuronal activity.
The formation and accumulation of the 7, 8-diol during this early-critical period of synaptogenesis is interesting in that this diol can be converted further into B(a)P dihydrodiol epoxide (BPDE). The covalent interaction of BPDE with nucleophilic centers in cellular macromolecules, such as DNA and protein, is a critical event in the initiation of toxicity. The sub-acute exposure of pregnant dams to B(a)P may have contributed to an increased production of BPDE from 7,8-diol and an elevated uptake of BPDE by neurons. The lipophilicity of BPDE has been reported to allow partitioning across membranes to reach all cellular compartments (Reed and Jones, 1996). The modification of cellular macromolecules or alteration of cellular signaling events by this B(a)P derivative may be a critical determinant in the resulting neurotoxic response. The predominance of 3-hydroxy metabolites at PND15 and PND20 indicate that the mechanism of detoxification may be more prominent at later stages of development. As toxification overrides the detoxification processes during the critical period of synaptogenesis, the preferential disposition of B(a)P 7,8-diol to brain tissues and its bioavailability provide sufficient evidence to implicate this metabolite as a potential causative agent of the observed neurotoxicity.
The pharmacokinetic properties of B(a)P favor a greater residence time for this toxicant in target tissues. Studies conducted in F-344 rats from our group (Ramesh et al., 2001) and those of others in Sprague-Dawley rats (Moir et al., 1998) have documented that the half-life of B(a)P subsequent to a single exposure is 10 hours. Hence, there may be greater tissue accumulation of B(a)P metabolites due to the sub-acute dosing regimen (since dosing would occur again before all the compound has cleared), and that lactational transfer may be occurring since the parent compound and metabolites are found in the pups well after dosing has ended. We did not determine the half-life in either the dams or offspring and the repeated dosing regimen and lactational transfer make it difficult to do so or to speculate about this.
Prenatal B(a)P exposure could produce many of its long lasting effects by adding to cortical-based sensory deprivation caused by B(a)P depression of glutamate receptor subunits during the early postnatal period. When these results from low input activity are compared with similar effects of prenatal B(a)P exposure, they raise the possibility that B(a)P produces a “central” deprivation by reducing cortical activity below the levels needed for normal experience-dependent maturation of synaptic function. It remains a possibility that the delay in response onset could be due to slow conduction and “sluggish” synapses in the S1 circuit pathway. Furthermore, both the magnitude of response and latency could be affected via trigeminal or thalamic relay neurons in the S1 pathway. The effect of prenatal B(a)P exposure on glutamate receptor subunit expression in the offspring cortex is consistent with the deficits observed in the hippocampus. Prenatal effects of B(a)P have been shown by Wormley et al, (2004) to inhibit the induction of Long Term Potentiation (LTP). LTP is defined as a persistent, activity-dependent increase in the strength of synaptic transmission induced by high frequency stimulation of excitatory inputs to hippocampal cells. The potentiated effect persists for hours to weeks and requires postsynaptic depolarization sufficient to recruit NMDA receptor activation and subsequent Ca++ influx into the cell through receptor-activated voltage-dependent channels (Collinridge, 1987). Using patch-clamp electrophysiology we have shown a voltage-dependent decrease in inward current of rat cortical neurons exposed to 25nM B(a)P. Recently, in a blind study we have measured a similar decrease in cortical neuronal activity from ex vivo neuronal cultures from mice that were exposed in utero to 150μg/kg BW B(a)P (in preparation). The current-voltage relationship and the average reversal potential (Erev = 1.6 mV) are in agreement with findings by Li et al., (2004). The values for E rev found in our studies and that of Li et al., are close to the equilibrium potential for a non-specific cation channel, such as NMDA. Studies are currently underway to characterize the nature of B(a)P-induced decreases in cortical neuronal activity.
Several laboratories have shown that toxicant exposure, in general, has differential effects on specialized subunits of glutamate receptors. (Grova et al., 2007; Hood et al., 2006; Chen et al., 2004; Wormley et al., 2004; Guilarte and McGlothan, 1998 & Nihei and Guilarte, 1999). For the first time, we establish that in utero exposure to benzo(a)pyrene degrades the function of the somatic sensory cortex in a distinctive and quantifiable manner. The physiological deficits reported herein are supported by additional data demonstrating reductions in glutamatergic receptor subunit NMDA NR2B mRNA levels. These new data corroborate earlier observations (Hood et al., 2006; Brown et al., 2007) and demonstrate that prenatal B(a)P induced effects in offspring occur at a time when excitatory synapses are being formed for the first time in the somatosensory cortex, consistent with diminished expression of NMDA receptor subunits.
ACKNOWLEDGEMENTS
This work was supported by NIH grants S11ES014156-02 (DBH, MA and AR) and U54NS041071-0002 (DBH) and R03CA130112-01 (AR). Also critical to the conduct of the studies described in this article are private foundation grants from the Simons Foundation and Nancy Lurie Marks Foundation. Institutional grants G12RRO3032 and S06GM08037 to MMC, and pre-doctoral support for Monique M McCallister from the Meharry Medical College-Vanderbilt University Alliance Diversity Neuroscience Training Grant, T32MH065782 are also acknowledged. We also thank the Meharry-Vanderbilt ARCH Consortium internal advisory board for critical review of the revised manuscript prior to submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Armstrong-James M, George MJ. Influence of anesthesia on spontaneous activity and receptive field size of single units in rat Sm1 neocortex. Exp Neurol. 1988;99:369–87. doi: 10.1016/0014-4886(88)90155-0. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Miller JM. Carbon fiber microelectrodes. Journal of Neuroscience Methods. 1979;1:279–87. doi: 10.1016/0165-0270(79)90039-6. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Welker E, Callahan CA. The contribution of NMDA and non-NMDA receptors to fast and slow transmission of sensory information in the rat S-I barrel cortex. J Neurosci. 1993;13:2149–60. doi: 10.1523/JNEUROSCI.13-05-02149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile of Pentachlorophenol (update) ATSDR; Atlanta, GA: 1995. [Google Scholar]
- Benuskova L, Velayudhan R, Armstong-James M, Ebner FF. Theory for normal and impaired experience-dependent response level in neocortex of adult rats. Proc Nat Acad Sci. 2001;98:2797–802. doi: 10.1073/pnas.051346398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat. I. Qualitative analysis. J Neurocytol. 1983;12:599–616. doi: 10.1007/BF01181526. [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat. II. Quantitative analysis. J Neurocytol. 1983;12:697–712. doi: 10.1007/BF01181531. [DOI] [PubMed] [Google Scholar]
- Brown LA, Khousbouei H, Goodwin SJ, Irvin-Wilson C, Ramesh A, Sheng L, et al. Downregulation of Early Ionotrophic Glutamate Receptor Subunit Developmental Expression as a Mechanism for Observed Response level Deficits Following Gestational Exposure to Benzo(a)pyrene. NeuroToxicology. 2007;28(5):965–78. doi: 10.1016/j.neuro.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Lee YF, Chan MH, Lo PS. The role of N-methyl-D-aspartate receptors in neurobehavioral changes induced by toluene exposure during synaptogenesis. Ann NY Acad Sci. 2004;1025:552–5. doi: 10.1196/annals.1316.067. [DOI] [PubMed] [Google Scholar]
- Collingridge G. Synaptic response level. The role of NMDA receptors in learning and memory. Nature. 1987;330:604–5. doi: 10.1038/330604a0. [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Warshaw R, Horsak RD, Parker FM, Takhar H. Exposure assessments of residents living near a wood treatment plant. Environ Res. 2003a;92:99–109. doi: 10.1016/s0013-9351(02)00064-6. [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Warshaw R, Thornton J, Anderson-Mahoney CP, Takhar H. Health effects on nearby residents of a wood treatment plant. Environ Res. 2003b;92:92–8. doi: 10.1016/s0013-9351(02)00065-8. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Res Dev Brain Res. 1990;56:229–34. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12(5):1826–38. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by depth of anesthesia. J Neurophysiol. 1999;81:2243–52. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mundy W, Crofton KM. Spatial learning and long-term potentiation in the dentate gyrus of the hippocampus in animals developmentally exposed to Aroclor 1254. Toxicol Sci. 2000;57:102–11. doi: 10.1093/toxsci/57.1.102. [DOI] [PubMed] [Google Scholar]
- Grova N, Valley A, Turner JD, Morel A, Muller CP, Schroeder H. Modulation of behavior and NMDA-R1 gene mRNA expression in adult female mice after sub-acute administration of benzo(a)pyrene. NeuroToxicology. 2007;28:630–6. doi: 10.1016/j.neuro.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL. Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res. 1998;790:98–107. doi: 10.1016/s0006-8993(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Hack M, Breslau N, Weissman B, Aram D, Klein N, Borawski E. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. N Engl J Med. 1991;325:231–7. doi: 10.1056/NEJM199107253250403. [DOI] [PubMed] [Google Scholar]
- Hood DB, Nayyar T, Greenwood M, Ramesh A, Inyang F. Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo(a)pyrene following maternal inhalation. Inhalation Toxicology. 2000;12:511–35. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- Hood DB, Woods L, Brown L, Johnson S, Ebner FF. Gestational 2,3,7,8,-tetrachlorobenzo-p-doxin exposure effects on sensory cortex function. NeuroToxicology. 2006;27:1032–42. doi: 10.1016/j.neuro.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Kashani AH, Qiu Z, Jurata L, Lee SK, Pfaff S, Goebbels S, et al. Calcium activation of the LMO4 transcription complex and its role in the patterning of thalamocortical connections. J Neurosci. 2006:8398–408. doi: 10.1523/JNEUROSCI.0618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and response level in rat primary auditory cortex. Proc Nat Acad Sci. 2007;104:7646–51. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström I. Placental transfer of benzo(a)pyrene and its hydrophilic metabolites in the guinea pig. Acta Pharmacol Toxicol. 1986;58:272–6. doi: 10.1111/j.1600-0773.1986.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–7. doi: 10.1016/0166-2236(95)93937-s. Review. [DOI] [PubMed] [Google Scholar]
- Konstandi M, Pappas P, Johnson E, Lecklin A, Marselos M. Suppression of the acquisition of conditioned avoidance behavior in the rat by 3-methylcholanthrene. Pharmacol Biochem Behav. 1997;56:637–41. doi: 10.1016/s0091-3057(96)00407-8. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Lioy PJ, Thurston G, Berkowitz G, Chen LC, Chillrud SN, et al. NIEHS World Trade Center Working Group Health and environmental consequences of the world trade center disaster. Environ Health Perspect. 2004;112:731–9. doi: 10.1289/ehp.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapole D, Rychen G, Grova N, Monteau F, Le Bizec B, Feidt C. Milk and urine excretion of polycyclic aromatic hydrocarbons and their hydroxylated metabolites after a single oral administration in ruminants. J Dairy Sci. 2007;90:2624–9. doi: 10.3168/jds.2006-806. [DOI] [PubMed] [Google Scholar]
- Li L, Ebner FF. Cortical modulation of spatial and angular tuning maps in the rat thalamus. J Neurosci. 2007;27:167–79. doi: 10.1523/JNEUROSCI.4165-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rema V, Ebner FF. Chronic suppression of activity in barrel field cortex downregulates sensory responses in contralateral barrel field cortex. J Neurophysiol. 2005;94(5):3342–56. doi: 10.1152/jn.00357.2005. [DOI] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004 Jun;109:443–52. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Moir D, Viau A, Chu I, Withey J, McMullen E. Pharmacokinetics of benzo[a]pyrene in the rat. J Toxicol Environ Health A. 1998;53:507–30. doi: 10.1080/009841098159114. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Tamaki M, Ozaki Y, Arasaki K. Origins of short latency somatosensory evoked potentials to median nerve stimulation. Electroencephalogr Clin Neurophysiol. 1983;56:74–85. doi: 10.1016/0013-4694(83)90008-1. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kiyosue K, Taguchi T. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J Neurosci. 2005;25:4040–51. doi: 10.1523/JNEUROSCI.4115-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei M, Guilarte TR. NMDAR-2A subunit protein expression is reduced in the hippocampus of rats exposed to Pb2+ during development. Mol Brain Res. 1999;66:42–9. doi: 10.1016/s0169-328x(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–5. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of Prenatal Exposure to Airborne Polycyclic Aromatic Hydrocarbons on Neurodevelopment in the First 3 Years of Life among Inner-City Children. Environ Health Perspect. 2006;114:1287–92. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Rauh V, Lester K, Tsai WY, Tu H, et al. Relationships among Polycyclic Aromatic Hydrocarbon-DNA Adducts, Proximity to the World Trade Center, and Effects on Fetal Growth. Environ Health Perspec. 2005;113:1062–7. doi: 10.1289/ehp.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Smith AB, Burse VW, Steele GK, Needham LL, Hannon WH. Half-life of polychlorinated biphenyls in occupationally exposed workers. Arch Environ Health. 1989;44:351–4. doi: 10.1080/00039896.1989.9935905. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Walker S, Hood DB, Guillen M, Schneider K, Weiland E. Bioavailability and Risk Assessment of Orally Ingested Polycyclic Aromatic Hydrocarbons. International Journal of Toxicology. 2004;23:301–33. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Inyang F, Hood DB, Archibong AE, Knuckle ME, Nyanda AM. Metabolism, bioavailability, and toxicokinetics of benzo(a)pyrene in F-344 rats following oral administration. Exp toxicol pathol. 2001;53:275–90. doi: 10.1078/0940-2993-00192. [DOI] [PubMed] [Google Scholar]
- Reed GA, Jones BC. Enhancement of benzo(a)pyrene diol epoxide mutagenicity by sulfite in a mammalian test system. Carcinogenesis. 17:1063–1068. doi: 10.1093/carcin/17.5.1063. [DOI] [PubMed] [Google Scholar]
- Rema V, Armstrong-James M, Ebner FF. Experience-dependent plasticity of adult rat S1 cortex requires local NMDA receptor activation. J Neurosci. 1998;18:10196–206. doi: 10.1523/JNEUROSCI.18-23-10196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rema V, Ebner FF. Effect of enriched environment rearing on impairments in cortical excitability and plasticity after prenatal alcohol exposure. J Neurosci. 1999;19:10993–1006. doi: 10.1523/JNEUROSCI.19-24-10993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice FL, Gomez C, Barstow C, Burnet A, Sands P. A comparative analysis of the development of the primary somatosensory cortex: interspecies similarities during barrel and laminar development. J Comp Neurol. 1985;236:477–95. doi: 10.1002/cne.902360405. [DOI] [PubMed] [Google Scholar]
- Sanyal MK, Li YL. Deleterious effects of polynuclear aromatic hydrocabon on blook vascular system of the rat fetus. Birth Defects Res B Dev Reprod Toxicol. 2007;80:367–73. doi: 10.1002/bdrb.20122. [DOI] [PubMed] [Google Scholar]
- Schubert D, Staiger JF, Cho N, Kotter R, Zilles K, Luhmann HJ. Layer-specific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. J Neurosci. 2001;21:3580–92. doi: 10.1523/JNEUROSCI.21-10-03580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Seo BW, Strupp BJ, Seegal RF, Schantz SL. Effects of perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicol Teratol. 2003;25:459–71. doi: 10.1016/s0892-0362(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Johnston MV, Goldstein GW, Blue ME. Neonatal lead exposure impairs development of rodent barrel filed cortex. Proc Natl Acad Sci USA. 2000;9:5540–5. doi: 10.1073/pnas.97.10.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci U S A. 1980;77:2333–7. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (S-I) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitetural units. Brain Res. 1970;17:205–32. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Wormley D, Chirwa S, Harris E, Nayyar T, Wu J, Hood DB. Inhaled Benzo(a)pyrene Impairs Long Term potentiation in Rat Dentate Gyrus: Reduced Capacity for Long-term Potentiation in the F1 generation. Cell and Mol Biol. 2004b;50:715–21. [PubMed] [Google Scholar]
- Wormley D, Ramesh A, Hood DB. Environmental contaminant-mixture effects on cns development, plasticity and behavior. Toxicol Appl Pharm. 2004a;197:49–65. doi: 10.1016/j.taap.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Wu J, Ramesh A, Nayyar T, Hood DB. Assessment of metabolites and AhR and CYP1A1 mRNA expression subsequent to prenatal exposure to inhaled benzo(a)pyrene. Int J Dev Neurosci. 2003;21:333–46. doi: 10.1016/s0736-5748(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Yoshiko T, Nobue W, Masanobu O, Akira T, Ryoichi K, KazuichI H. Transfer of polycyclic aromatic hydrocarbons to fetus and breast milk of rats exposed to diesel exhaust. Journal of Health Sciences. 2004;50:497–502. [Google Scholar]
- Zanieri L, Galvan P, Checchini L, Cincinelli A, Lepri L, Donzelli GP, et al. Polycyclic aromatic hydrocarbons (PAHs) in human milk from Italian women: influence of cigarette smoking and residential area. Chemosphere. 2007;67:1265–74. doi: 10.1016/j.chemosphere.2006.12.011. [DOI] [PubMed] [Google Scholar]