Summary
Body condition affects the timing and magnitude of life history transitions. Therefore, identifying proximate mechanisms involved in assessing condition is critical to understanding how these mechanisms affect the expression of life history plasticity. Nutrient storage is an important body condition parameter, likely playing roles in both attaining minimum body-condition thresholds for life history transitions and expression of life history traits.
We manipulated protein availability for females of the flesh fly Sarcophaga crassipalpis to determine whether reproductive timing and output would remain plastic or become fixed. Liver was provided for 0, 2, 4, or 6 days of adult pre-reproductive development. Significantly, liver was removed after the feeding threshold had been attained and females had committed to producing a clutch.
We also identified the major storage proteins and monitored their abundances, because protein stores may serve as an index of body condition and therefore may play an important role in life history transitions and plasticity.
Flesh flies showed clear post-threshold plasticity in reproductive timing. Females fed protein for 2 days took ~30% longer to provision their clutch than those fed for 4 or 6 days. Observations of oogenesis showed the 2-day group expressed a different developmental program including slower egg provisioning.
Protein availability also affected reproductive output. Females fed protein for 2 days produced ~20% fewer eggs than females fed 4 or 6 days. Six-day treated females provisioned larger eggs than 4-day treated females, followed by 2-day treated females with the smallest eggs.
Two storage proteins were identified, LSP-1 and LSP-2. LSP-2 accumulation differed across feeding treatments. The 2- and 4-day treatment groups accumulated LSP-2 stores but depleted them during provisioning of the first clutch, whereas the 6-day group accumulated the greatest quantity of LSP-2 and had substantial LSP-2 stores remaining at the end of the clutch. This pattern of accumulation and depletion suggests that LSP-2 could play roles in both provisioning the current clutch and future clutches, making it a good candidate molecule for affecting reproductive timing and allotment. LSP-1 was not associated with post-threshold plasticity; it was carried over from larval feeding into adulthood and depleted uniformly across all feeding groups.
Keywords: reproductive timing, reproductive threshold, hexameric storage protein, phenotypic plasticity
Introduction
Life history transitions, such as metamorphosis, sexual maturation, and reproduction, are critical events in an organism’s lifecycle. Across organisms it is apparent that both the timing of life history transitions and the size at transition (e.g., body size at metamorphosis or clutch size at reproduction) can be highly plastic, offering individuals the opportunity to tune their lifecycles to environmental conditions (reviewed by Stearns & Koella 1986, Boutin 1990, Wheeler 1996, Nylin & Gotthard 1998).
Much work on life history plasticity in both metamorphosis and reproductive timing has been influenced by the amphibian metamorphosis model of Wilbur and Collins (1973). This model postulated that individuals must grow until they exceed a size threshold to be competent to undergo metamorphosis and that life history timing after reaching the threshold will show adaptive plasticity in response to environmental quality (Denver, Boorse, & Glennemeier 2002, Juliano et al. 2004). Studies on amphibian metamorphosis have found evidence for post-threshold plasticity in timing and size, supporting the Wilbur and Collins (1973) model; in contrast, studies on insect metamorphosis and reproduction typically have found no evidence for post-threshold plasticity, failing to support the model (e.g., Travis 1984, Alford & Harris 1988, Gebhardt & Stearns 1988, Hensley 1993, Leips & Travis 1994, Bradshaw & Johnson 1995, Twombly 1996, Moehrlin & Juliano 1998, Flanagin, Haase, & Juliano 2000, and Hentschel & Emlet 2000, Juliano et al. 2004, but see Shafiei, Moczek, & Nijhout 2001). In insect life history transitions, after reaching some developmental threshold, life history timing typically becomes fixed (i.e., canalized) first, while growth and reproductive parameters remain plastic longer (Hatle, Borst, & Juliano 2003).
Implicit in studies of plasticity and canalization in life history transitions is that organisms assess their internal body condition and that some measure of condition conveys when the animal has accumulated enough resources to make a life history transition, often referred to as a threshold. Therefore, a critical question is which aspects of physiological status (e.g., body size, protein storage) are involved in assessing condition, and how does this condition affect the expression of post-threshold plasticity? While organisms are likely simultaneously assessing multiple parameters of condition, nutrient storage has emerged as a key player in attaining reproductive thresholds, fecundity, and offspring success across a wide range of animals from mammals and birds to insects (Wheeler 1996, Meijer & Drent 1999, Hatle et al. 2003a, Schneider 2004, Schoech, Bowman, & Reynolds 2004, Ebling 2005). Unfortunately, few studies directly address the role of nutrient storage in post-threshold patterns of plasticity and canalization.
In this study, we use a diet-switching approach to test the hypothesis that post-threshold reproductive timing and allotment is flexible in our model for reproduction, the flesh fly Sarcophaga crassipalpis Macquart. We assess the putative role of protein storage in reproduction, specifically focusing on associations with patterns of plasticity and canalization.
EXPERIMENTAL TREATMENTS AND PREDICTIONS
Females of the flesh fly S. crassipalpis provision eggs in large clutches and can provision multiple clutches (Denlinger 1981). Sarcophaga crasssipalpis females are anautogenous, so they must feed on protein as an adult to reproduce, but like many other flesh-eating flies, a single high-protein meal of meat is sufficient to surpass their reproductive threshold (Yin, Qin, & Stoffolano 1999, Browne 2001, Wall, Wearmouth, & Smith 2002). While S. crassipalpis females can reach their reproductive threshold with a single large meal, if given the option they will feed for many days after reaching the minimum threshold for reproduction, allowing them to produce a larger clutch (Hahn, unpublished). We manipulated resource availability by allowing flies to feed on protein ad libitum for 0, 2, 4, or 6 days starting 4–8 hours after adult eclosion. The group of flies not fed protein served as a control that were expected to not mature eggs, while all treatment groups that received protein were well above the minimum threshold for reproduction. Previous pilot experiments showed that flies exposed to ad libitum protein for 6 days did not differ in reproductive output from flies fed ad libitum until the day of ovulation (Hahn, unpublished). Therefore, our treatments included a range of protein availability from below the threshold for reproduction to functionally ad libitum protein availability.
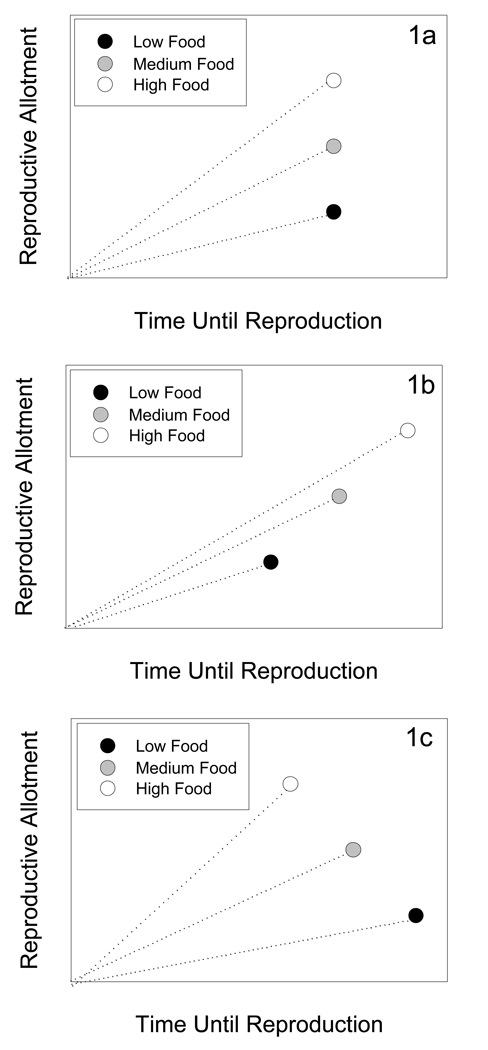
We expect that diet level will be positively correlated with reproductive output and protein storage regardless of timing. If reproductive timing shows plasticity, three outcomes are possible. First, the null hypothesis, representing post-threshold canalization, is that diet level will not affect reproductive timing and the maturation of eggs should be synchronous across treatments receiving protein (Fig. 1a). Second, individuals that received protein for only a short period (e.g., 2 days) could accelerate reproduction compared to individuals that received protein for a longer period (e.g., 6 days)(Fig. 1b). In this case, we expect that individuals would be expressing a different developmental program identifiable by accelerating the stages of egg maturation. This scenario would be consistent with the Wilbur and Collins (1973) model in which individuals under richer nutritional regimes would adaptively delay the timing of egg development to acquire more resources, whereas individuals receiving poor resources would speed up egg development to transition (e.g., Shafiei et al. 2001; Morey & Reznick 2000). Third, individuals that received protein for only a short period (e.g., 2 days) could delay reproduction compared to individuals that received protein for a longer period (e.g., 6 days)(Fig. 1c). This scenario is consistent with the idea that individuals may delay reproduction with the possibility that they could find more food to augment their first clutch, but after a certain point the costs of delaying reproduction outweigh the benefits of laying a small clutch (Day & Rowe 2002). Alternatively, individuals fed less could take longer to mature eggs due to the extra time necessary to mobilize storage to fully provision a clutch. These internal nutrients could be mobilized out of standing body stores from the larval stage or by reabsorbing some oocytes to provision others (Wheeler 1996). These scenarios for delayed reproduction in low fed individuals are not mutually exclusive; both represent modifications of the egg development program and both can be adaptive if they increase lifetime reproductive output. In this case, observing a decrease in the speed of egg maturation would be indicative of physiological plasticity.
Fig. 1.
Hypothetical relationships between reproductive timing and allotment under post-threshold conditions of low, medium, and high food availability under three different scenarios: (a) post-threshold reproductive timing is canalized, (b) post-threshold timing is plastic wherein individuals in better environments delay reproduction to accumulate more resources and individuals in poor environments accelerate reproduction as in Wilbur and Collins (1973), (c) post-threshold timing is plastic wherein individuals in better environments accelerate reproduction and individuals in poor environments delay reproduction, perhaps to accumulate more resources or to mobilize internal reserves.
Insect hexameric storage proteins act as a reservoir for amino acids and have been shown to contribute to reproduction in several insect species (Pan & Telfer, 1996; Wheeler & Buck 1996, Seo et al., 1998; Burmester 1999, Hatle et al. 2001, 2003a, Hahn & Wheeler 2003). Therefore, we characterized and quantified the hexameric storage proteins present in adult female flesh flies through time for all of our feeding treatments to determine whether the accumulation and disappearance of storage proteins are associated with reproductive traits. If storage hexamerins act as an important source of amino acids for reproduction, we expect that adults will accumulate hexamerin reserves during early feeding and deplete them during egg provisioning. Further, peak storage protein accumulation and timing will be correlated with reproductive timing and measures of reproductive output, specifically fecundity and egg size, across treatment groups.
Methods and Materials
ANIMAL REARING AND EXPERIMENTAL PROTOCOL
Studies were performed on a laboratory population of the flesh fly Sarcophaga crassipalpis reared at the University of Florida. Larvae were fed homogenized beef liver at a density of 80 individuals per 40 g of liver in a 25°C room with a 16L:8D light cycle. Liver was placed in an aluminum foil packet that rested on a bed of dry sawdust in a plastic shoebox (24 × 8 × 6 cm). After feeding, larvae crawled out of the aluminum foil packets and pupariated in the sawdust where they were held at 25°C until adult eclosion. On the day of eclosion individuals were placed in aluminum screened cages (30 × 30 × 30 cm) and incubated at 22.5°C with a 16L:8D light cycle for the duration of the experiment.
Newly-eclosed flies were sexed and sorted by hand into eight cages each containing 250 males and 250 females. All cages received water and sugar ad libitum throughout the experiment. Of the eight cages, two were never provided liver, two were provided liver for two days starting the day of eclosion, two were provided liver for four days, and two were provided liver for six days starting the day of eclosion.
Eight females were sampled from each cage daily until all individuals sampled contained fully-matured eggs. Females were promptly frozen at −20°C until the time of dissection. Frozen individuals were dissected in cold Ringer’s saline on ice and the ovaries and any eggs in the uterus were removed. The progression of oogenesis was quantified by removing the ovaries and opening them to observe the oocytes. The development of oocytes was staged using a modification of the scale of Adams & Reinecke (1979) wherein previtellogenic folicles were staged 1–2, vitellogenic follicles were staged from 3–7 progressively based on yolk content and nurse cell morphology, and fully-matured, chorionated eggs that had moved from the ovaries into the uterus were staged 8. Fully-matured stage 8 eggs were removed from the uterus and counted as a measure of fecundity. The length of four stage 8 eggs from each female was measured to the nearest 0.1 mm using a microscope-mounted ocular micrometer and the mean value for each female was used in further analyses. Female carcasses were promptly homogenized in 900 µL of phosphate buffered saline (pH 7.2) containing the Complete Protease Inhibitor Cocktail (Roche, Mannheim, Germany) and frozen at −20°C for later storage protein analysis.
BIOCHEMICAL ANALYSIS
The total soluble protein content of each homogenized female carcass was estimated using a Lowry-type assay, the DC Protein Assay Kit (Bio-Rad, Hercules, CA). A sample aliquot of known protein content was mixed with 10 µl of sample buffer. Native-PAGE was performed according to Laemmli (1970) with SDS and beta-mercaptoethanol omitted. Proteins were separated on a 8 × 5 cm 4–20% native-PAGE gradient gels composed of 29:1 acrylamide:bis-acrylamide topped by a 4% stacking gel and run at 100 v for 30 min followed by 4 h at 200 v. Gels were stained with 0.1% Coomassie Brilliant Blue R 250 dissolved in a 5:4:1 solution containing methanol, water, and acetic acid. Gels were destained in the same solution without Coomassie.
Putative S. crassipalpis storage proteins were first identified by their migration through gels compared to the literature. Bands of the appropriate migration position were excised from the gels and analyzed at the University of Florida Proteomics Core Facility. Proteins were extracted from gel slices and digested into fragments by trypsin. Capillary rpHPLC separation of protein digests was performed on a 15 cm × 75 µm i.d. PepMap C18 column (LC Packings, San Francisco, CA) in combination with an Ultimate Capillary HPLC System (LC Packings, San Francisco, CA) operated at a flow rate of 200 nL/min. Inline mass spectrometric analysis of the column eluate was accomplished by a hybrid quadrupole time-of-flight instrument (QSTAR, Applied Biosystems, Foster City, CA) equipped with a nanoelectrospray source. Fragment ion data generated by Information Dependent Acquisition (IDA) via the QSTAR-MS were searched against the NCBI nr sequence database using the Mascot (Matrix Science, Boston, MA) database search engine. Detailed analysis of protein identification data was carried out using Scaffold (Proteome Software), which is an implementation of the Peptide and Protein Prophet algorithms (Keller et al. 2002) along with protein identification by X!Tandem. Positively identified proteins were selected using the following parameters: 95% minimum peptide confidence, minimum number of peptides 4, and 95% minimum protein confidence, in addition to validation by manual interpretation of the tandem MS data.
The storage protein content of each sample was quantified by densitometry of digital images of native-PAGE gels ran as above using the program Image J (NIH, Bethesda, MD). Standard curves were generated using known quantities of bovine serum albumin that ranged from 0.02 to 4.0 mg. Internal standards of 0.05 and 1.0 mg bovine serum albumin were included on each gel to correct for gel to gel variation.
STATISTICAL ANALYSIS
Differences in the timing of egg maturation and reproductive output were assessed using One-Way ANOVAs. Both egg maturation rate during the vitellogenic period, starting on day 3 of adulthood through to completion, and depletion of the arylphorin/LSP-1 storage protein were reasonably approximated by linear functions through time among the treatment groups and relationships between the treatment groups were assessed using General Linear Models with time, treatment, and a time by treatment interaction as independent variables. The accumulation of the LSP-2 storage protein was distinctly hump-shaped and we modeled the patterns of LSP-2 accumulation in the three treatment groups fed protein using the equation LSP-2 Concentration = LSP-2 Maximum Accumulation * Exp (-(Time in Days − Maximum Time in Days)2 / 2(Half-Maximum Peak Width)2). Comparisons among treatment groups in LSP-2 accumulation were made by directly comparing the 95% confidence interval estimates for the maximum LSP-2 accumulation and the timing of maximum accumulation. Initial analyses included replicate cages within each treatment as a blocking factor. However no cage effects were detected; therefore in the interest of using the simplest effective models, cage was not included in final analyses. All analyses were performed using the JMP statistical package 5.1.2 (SAS Incorporated, 2004).
Results
REPRODUCTIVE TIMING AND REPRODUCTIVE OUTPUT
Individuals that did not receive liver (0-Day Treatment) began egg provisioning, but halted egg development early with oocytes containing very little yolk. By the end of the observation period these individuals began resorbing their partially-provisioned oocytes (Fig. 2). This group was excluded from further analyses. All other treatment groups were able to produce a substantial clutch of fully-matured eggs, validating that they had surpassed their intake threshold for reproduction. The treatment receiving 2 days of liver took longer to mature eggs than the 4-and 6-day treatments (ANOVA F2,29=97.63, p<0.001, Fig. 3a&3b). Beginning on day three of adulthood, when vitellogenesis was initiated in all fed treatments, the progression of oogenesis occurred linearly through time until eggs were matured. The rate of oogenesis was noticeably slower in the 2-day treatment, while there was no difference in the progression of oogenesis through time between the 4- and 6-day treatments (Table 1a, Fig. 2).
Fig. 2.
Progression of oogenesis through time across feeding treatments. Symbols represent the means and bars the standard errors. Flies fed no liver never complete development of eggs.
Fig. 3.
Relationships between reproductive timing and two different measures of reproductive output across the protein-fed treatments: (a) fecundity and time, (b) egg size and time. Bars represent 95% confidence intervals for each parameter.
Table 1.
Multivariable General Linear Model results for the effects of feeding treatment and timing on the progression of oogenesis (1a) and whole-body arylphorin/LSP-1 content (1b).
| 1a. Timing of Egg Development | |||
|---|---|---|---|
| df | F | p | |
| Whole Model | 5 | 152.29 | <0.001 |
| Treatment- Days of Liver | 3 | 283.69 | <0.001 |
| Age – Days Since Eclosion | 8 | 49.24 | <0.001 |
| Interaction- Treatment × Age | 24 | 30.38 | <0.001 |
| Error | 119 | ||
| Total | 154 | ||
| 1b. Arylphorin/LSP-1 Content | |||
| df | F | p | |
| Whole Model | 7 | 47.89 | <0.001 |
| Treatment – Days of Liver | 3 | 2.12 | 0.1 |
| Age − Days Since Eclosion | 1 | 157.52 | <0.001 |
| Interaction − Treatment × Age | 3 | 11.26 | <0.001 |
| Error | 164 | ||
| Total | 171 | ||
Feeding treatment also had a large effect on reproductive output. Individuals in the 2-day treatment matured fewer eggs than the treatments receiving the 4-and 6-days of food, which did not differ from each other (ANOVA F2,29=11.44, P<0.001, Fig. 3a). Mature eggs were smallest in the 2-day treatment, intermediate in the 4-day treatment, and largest in the 6-day treatment (ANOVA F2,29=21.72, P<0.001, Fig. 3b). Individuals fed liver for 2-days delayed egg maturation and produced fewer and smaller eggs than individuals in the 4-and 6-day treatments (Figs 3a & 3b), which is consistent with post-threshold plasticity in both timing and reproductive output as proposed in figure 1c.
STORAGE PROTEIN IDENTIFICATION AND QUANTIFICATION
Three bands in native-PAGE were identified as putative storage proteins based on their mobility and abundance (Fig. 4). Mass spectrometric analysis of tryptic fragments of the putative storage protein bands revealed multiple abundant fragments within each band having significant homology to known storage proteins from the blow fly Calliphora vicina(see Fig. 1 in Supplementary Material, Naumann & Scheller 1991, Burmester et al. 1998), as well as the fruit fly Drosophila melanogaster and the housefly Musca domestica(not shown, Smith et al. 1981, Capurro et al. 2000). Under these electrophoretic conditions, the S. crassipalpis arylphorin/LSP-1 homologue separates out into two different bands, one band runs higher and the second band runs just above the single LSP-2 band. This LSP-2 band appears almost as a doublet under the lower arylphorin/LSP-1 band, and only appears after four days of adulthood in the protein-fed treatments (Fig. 4).
Fig. 4.
Expanded views of the region of 4–20% native-PAGE gels showing the abundance of both storage hexamerins through the first seven days after eclosion in females from the high-fed treatment which were provided liver for six days after eclosion (top) and females from the treatment not provided any protein (bottom). Dashed-grey arrows denote the two electrophoreticaly-distinguishable bands of arylphorin/LSP-1 that appear in the same locations on both gels. The solid-black arrow denotes the single band of LSP-2 which is visible only in protein-fed females directly below the lower arylphorin/LSP-1 band from four to seven days after eclosion. The first lane is a molecular weight marker with sizes labeled to the left in kDa.
The accumulation and disappearance of the two storage proteins differed in response to diet. Levels of the S. crassipalpis arylphorin/LSP-1 homologue started high at eclosion and declined in all treatments. The pattern of decline differed among the treatment groups, producing a significant interaction between feeding treatment and time (Fig. 6a, Table 1b). However, this interaction was due primarily to the fact that the 0-day treatment eclosed with approximately 30% more arylphorin/LSP-1 than any of the other treatments. Because this difference was manifest before the feeding treatments began, and individuals were haphazardly assigned to treatments, this difference was not due to our manipulations and may have been a product of sampling error. Despite starting off slightly higher, levels of arylphorin/LSP-1 in the 0-day treatment quickly declined to match levels in the three protein-fed treatments.
Fig. 6.
The S. crassipalpis LSP-2 homologue showed a different pattern, wherein flies contained no detectable LSP-2 at eclosion and LSP-2 was accumulated with adult feeding. The 0-day treatment did not accumulate noticeable quantities of LSP-2. In contrast, all three protein-fed treatments accumulated LSP-2 during the first few days of adulthood, but accumulation clearly differed among the treatments. The LSP-2 profiles were humped–shaped (Fig. 6b), so we compared both the amount of protein at the peak and the timing of the peak in each treatment (Fig. 6c). At their respective peaks, the 2-day treatment had much less LSP-2 than the 4- and 6-day treatments, which did not differ from each other (Fig. 6c). The timing of maximum LSP-2 accumulation was much later in the 6-day treatment than in the 2-day treatment, and the 4-day treatment was intermediate and not statistically different from either of the two other treatments (Fig. 6c).
Discussion
We found clear evidence of post-threshold plasticity in reproductive timing and output in the flesh fly, Sarcophaga crassipalpis. We also show that the accumulation of one storage protein, but not the other, is associated with post-threshold plasticity in reproductive timing and allotment. This work provides the first linkage between a physiological parameter of nutrient storage and allocation with post-threshold life history plasticity.
POST-THRESHOLD PLASTICITY IN REPRODUCTIVE TIMING AND OUPUT
Individuals in our 2-day treatment took ~30% longer to produce a clutch of fully-matured eggs than individuals in our 4-or 6-day treatments, consistent with our hypothesis that 2-day treatment individuals were expressing a different developmental program with slower oocyte provisioning (Table 1a, Fig. 2). The number of eggs matured did not differ between the 6-day and 4-day treatments; individuals in both treatments produced approximately 25% more mature eggs than individuals in the 2-day treatment (Fig. 3a). Although total egg number was fixed after 4 days of feeding, allocation to egg size remained responsive to resource availability through our 6-day treatment. Six-day treatment individuals had longer eggs than 4-day treatment individuals, which had longer eggs than 2-day treatment individuals. Therefore fecundity reaches its maximum and becomes canalized after 4 days of feeding while egg size remains responsive to additional food intake at least 2 days longer.
Numerous studies have shown that adult food limitation, as well as other types of adult stress, results in later onset of reproduction and lower fecundity, similar to what we observed (Wheeler 1996, Nylin & Gotthard 1998). The majority of these studies manipulate the environment throughout the entire adult period; for example by continuously providing high quality versus low quality nutrition (e.g., Bauerfiend & Fischer 2005, Reim, Teuschel, & Blanckenhorn 2006) or by exposing adults to only warm or cool temperatures (Blanckenhorn & Henseler 2005). However, studies that continuously expose adults to a single treatment regime can not distinguish between pre-threshold plasticity (individuals taking longer to reach the minimum reproductive threshold) and post-threshold plasticity (individuals committed to clutch production but still plastic in the timing or size of the clutch)(see Reznick 1990, Moehrlin & Juliano 1998). The Wilbur and Collins (1973) model specifically predicts post-threshold plasticity (see Fig. 1b). To distinguish between pre- and post-threshold plasticity, an abrupt switch in feeding level (or other environmental parameter) is required (e.g., Moehrlin & Juliano 1998; Shafiei et al 2001). Far fewer studies have manipulated resource availability after animals have reached their minimum threshold for the life history transition (e.g., used a diet-switching approach; Moehrlin & Juliano 1998, Denver et al. 2002). By using a diet-switching approach, we have determined that the feeding threshold for commitment to completing reproduction in flesh flies is less than 2 days of feeding. Hence, our results can be compared to the Wilbur and Collins (1973) model. Strict application of this model to reproduction specifically predicts post-threshold plasticity with delayed clutch production from individuals with high food availability (see Fig. 1b). In contrast, we have demonstrated that post-threshold plasticity in reproduction in flesh flies results in delayed clutch production from individuals with low food availability (see Fig. 1c). Hence, reproduction in flesh flies shows a different pattern of post-threshold plasticity than the most common example of post-threshold plasticity, namely that of amphibian metamorphosis, which follows the predictions of the Wilbur and Collins (1973) model (see Fig. 1b). Nonetheless, the plasticity in flesh fly reproduction clearly suggests a modification in the developmental program, likely moderated by endocrine signaling, after attaining the threshold.
The results of studies on insect reproductive plasticity must be considered in light of the egg provisioning strategies employed by the focal species. Among insect species requiring adult feeding for oogenesis, oocyte development can be either synchronous, wherein animals cyclically mature oocytes in each individual all at once and lay relatively large clutches of eggs, or asynchronous, wherein the provisioning of terminal oocytes among ovarioles is not coordinated so that animals more or less continuously lay eggs (Wheeler 1996, Jervis, Boggs, & Ferns 2007). Clutch-laying species must provision a large number of oocytes all at once, a resource-intensive reproductive event that occurs periodically (similar to metamorphosis). Because asynchronous species can trickle-out just a few eggs at a time according to resource availability, clutch-laying species are likely to have a higher reproductive threshold than species that mature oocytes asynchronously. As a result, effects of diet switching above the reproductive threshold may be less dramatic in trickle-layers than in clutch-layers. Indeed, diet-switching studies in several trickle-laying species of flies and butterflies have shown that removing food after individuals have begun reproduction causes egg-laying rates to decrease compared to constantly-fed controls and eventually stop (Boggs & Ross 1993, Chapman, Trevitt, & Partridge 1994, Fischer & Fiedler 2001, Good & Tatar 2001, Carey et al. 2002).
Although clutch-laying species would likely show clear post-threshold plasticity in egg size or number; we expect that clutch-layers could display post-threshold canalization of reproductive timing due to constraints in the time needed for individuals to ramp up vitellogenesis. Interestingly, a series of studies that were done explicitly in the Wilbur and Collins (1973) framework but used another clutch-laying insect, the lubber grasshopper Romalea microptera, found that reproductive timing became canalized after females reached their reproductive threshold, whereas fecundity remained responsive to food availability for much longer (Moehrlin & Juliano 1998, Juliano et al. 2004).
In contrast, we found that our model the flesh fly S. crassipalpis, a synchronous clutch laying species, showed post-threshold plasticity by delaying reproduction when exposed to post-threshold food limitation (Fig. 1c, Fig. 3a&3b). Although previous studies on reproductive thresholds in flesh-eating flies have not explicitly placed their results into the Wilbur and Collins (1973) framework, several studies have shown that feeding beyond the minimum threshold for reproduction leads to increased fecundity and accelerated clutch provisioning (Browne, Vangerwen, & Williams 1979, Williams, Browne, & Vangerwen 1979, Wall 1993, Yin et al. 1999, Wall et al. 2002). Therefore our findings agree with previous studies on feeding and life history timing in clutch-laying, flesh-eating flies. Two key questions are: 1) how widespread are plastic vs. canalized reproductive strategies across the insects, and 2) what life history traits (e.g., clutch-laying vs. trickle-laying) are associated with plastic vs. canalized strategies for reproductive timing?
Why should flesh flies slow their rates of egg provisioning and delay reproduction when food is restricted after the reproductive threshold is met? Slowing-down reproduction may afford individuals more time to acquire additional high-quality meals and increase fecundity. However, the risk of mortality may eventually outweigh the benefits of delaying reproduction, and the animal should lay a smaller clutch (Day and Rowe 2002). The reproduction vs. longevity trade-off is well described in many invertebrates; by delaying reproduction, our 2-day treatment flesh flies may also be delaying mortality. If true, this would allow more time to locate food resources and produce larger clutches. At the same time, it may simply take longer for individuals in the 2-day treatment to complete oogenesis due to the time needed to mobilize resources from internal stores. Testing these adaptive hypotheses will require determining whether making additional food available to 2-day treatment flies later during their oogenic cycle increases reproductive allotment or affects timing and longevity.
STORAGE PROTEIN: ASSOCIATIONS WITH REPRODUCTION
The most important result of this paper is that a key parameter of nutrient storage and intermediary metabolism, content of the adult-synthesized LSP-2 storage protein, has been associated with post-threshold plasticity in life history timing and reproductive allotment. Further, this correlation identifies LSP-2 as a candidate molecule involved in the mechanisms flesh flies use to assess body condition; clearly a hypothesis necessitating further mechanistic investigation.
We identified two different storage hexamerins in S. crassipalpis adult females; one homologous to the arylphorin/LSP-1 protein and one homologous to the LSP-2 protein observed in other species of higher Diptera. The multiple electrophoretic forms we observed of the arylphorin/LSP-1 homologue were expected because Drosophila melanogaster is known to contain three independent copies of the LSP-1 gene (Roberts et al. 1991). While each of these three proteins in D. melanogaster can be distinguished in nondenaturing-PAGE electrophoresis, there are no apparent functional differences between the three proteins and they can combine with each other to produce the larger functional hexamerins (Roberts et al. 1991). Similarly, the flesh-eating fly Calliphora vicina contains more than ten copies of the arylphorin/LSP-1 gene, none of which show apparent functional differentiation (Schenkel et al. 1985). Our detection of the S. crassipalpis LSP-2 homologue as a single band was also expected because both D. melanogaster and C. vicina contain only one LSP-2 gene and one electrophoretic form of the protein (Benes et al. 1990, Burmester et al. 1998).
Prior to feeding, newly-eclosed S. crassipalpis adult females contained significant arylphorin/LSP-1 reserves that were almost certainly carried over from larval feeding. These arylphorin/LSP-1 reserves were depleted during the first week of adulthood in all treatment groups, including those not fed protein as adults. Our observation that arylphorin/LSP-1 levels decline from eclosion through adulthood are consistent with observations in the other higher flies, D. melanogaster, C. vicina, and Musca domestica, wherein arylphorin/LSP-1 is not synthesized by adults (Schenkel et al. 1985, Benes et al. 1990, Capurro et al. 2000).
In contrast, the pattern of LSP-2 accumulation varied across our feeding treatments. LSP-2 was not detectable in the 0-day treatment, it was detectable only in small quantities in the 2-day treatment, and accumulated to progressively higher levels in the 4- and 6-day treatments. Both LSP-2 content and the timing of the peak of LSP-2 accumulation was associated with the duration of food availability (Fig. 6c). This too is consistent with patterns observed in other species of higher flies, wherein females of D. melanogaster and M. domestica not fed protein do not produce LSP-2 while protein-fed females do (Benes et al. 1990, Capurro et al. 2000). Interestingly, in S. crassipalpis LSP-2 was depleted by the completion of vitellogenesis in both the 2-and 4-day treatments, whereas the 6-day treatment contained substantial LSP-2 stores when we terminated sampling at the completion of vitellogenesis. What then are the functional roles of each of these two storage proteins in adults and how do they relate to post-threshold plasticity in reproductive timing and allotment in S. crassipalpis?
Because adult females in other fly species accumulate LSP-2 whereas males do not, several authors have suggested that LSP-2 plays a key role in providing amino acids for reproduction (Pereira, Marinotti, de Bianchi 1989, Benes et al. 1990). Like S. crassipalpis, M. domestica are clutch-layers, and produce LSP-2 cyclically with reproduction. In this species LSP-2 levels rise during the previtellogenic and vitellogenic periods and fall near the end of vitellogenesis, consistent with LSP-2 serving as an intermediate storage site for amino acids before they are shuttled into yolk proteins (Pereira et al. 1989, Capurro et al. 1997).
If LSP-2 acts as an intermediate amino acid store for vitellogenesis, LSP-2 accumulated in the 2-and 4-day treatments could have served as adult-acquired capital. This protein may have become depleted late in vitellogenesis to support the synthesis of yolk proteins and maximize reproductive provisioning in these protein-limited treatments. In contrast, females in the 6-day treatment would have had greater access to protein income, and LSP-2 retained after the first reproductive bout may be stored to support yolk protein synthesis in future reproductive bouts. Our current hypothesis is that LSP-2 affects reproductive timing and output by acting as an intermediate amino acid storehouse that can be sensed by adults and utilized for current or future reproduction.
Arylphorin/LSP-1 is not accumulated in S. crassipalpis adults and may be acting as a source of amino acid capital derived from larvae. Similarly, while both D. melanogaster and M. domestica utilize most of their larvally-synthesized LSP-1 stores to provide amino acids for building structures like cuticle during pharate adult development, some stores of arylphorin/LSP-1 are carried over from larval life into adulthood, after which this protein disappears from adults within a few days of eclosion (Roberts et al. 1977, de Bianchi et al. 1983). The amino acids derived from arylphorin/LSP-1 as it declines could be allocated to numerous adult functions including reproduction, somatic maintenance, and energy production. Our observation that individuals in the group not fed protein were able to begin provisioning eggs with protein-rich yolk suggests that arylphorin/LSP-1 carried over from larval reserves could be allocated to support adult reproduction, but these reserves are not sufficient to complete reproduction.
Supplementary Material
The following supplementary material is available for this article:
Figure S1 Peptide fingerprint mapping by tandem MS.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2745.XXXX.XXXXX.x
Fig. 5.
Abundance of the two storage hexamerins, (a) LSP-1 and (b) LSP-2, during the first nine days after eclosion in all treatment groups, and (c) the relationship between peak abundance and the timing of the peak across all three fed treatments. In the top two panels, symbols represent the mean and bars the standard error. In the bottom panel symbols represent the mean and bars the 95% C.I.
Acknowledgments
We thank Meghan Brennan, Steve Juliano, and Greg Ragland for statistical advice and useful comments during the course of this study and manuscript preparation. This work was supported by funds from USDA-CSREES 04-35302-745994, NSF-IOS-641505, and the Florida State Agricultural Experiment Station to DH, and NIA AG028512-01 to JH.
References
- Adams TS, Reinecke JP. Reproductive physiology of the screwworm, Cochliomyia hominivorax (Diptera, Calliphoridae).1. Oogenesis. Journal of Medical Entomology. 1979;15:472–483. doi: 10.1093/jmedent/15.5-6.472. [DOI] [PubMed] [Google Scholar]
- Alford RA, Harris RN. Effects of larval growth history on Anuran metamorphosis. American Naturalist. 1988;131:91–106. [Google Scholar]
- Bauerfeind SS, Fischer K. Effects of food stress and density in different life stages on reproduction in a butterfly. Oikos. 2005;111:514–524. [Google Scholar]
- Benes H, Edmondson RG, Fink P, Kejzalarovalepesant J, Lepesant JA, Miles JP, Spivey DW. Adult expression of the Drosophila LSP-2 gene. Developmental Biology. 1990;142:138–146. doi: 10.1016/0012-1606(90)90157-e. [DOI] [PubMed] [Google Scholar]
- Blanckenhorn WU, Henseler C. Temperature-dependent ovariole and testis maturation in the yellow dung fly. Entomologia Experimentalis et Applicata. 2005;116:159–165. [Google Scholar]
- Boggs CL, Ross CL. The effect of adult food limitation on life history traits in Speyeria mormonia (Lepidoptera: Nymphalidae) Ecology. 1997;74:433–441. [Google Scholar]
- Boutin S. Food supplementation experiments with terrestrial vertebrates – patterns, problems, and the future. Canadian Journal of Zoology. 1990;68:203–220. [Google Scholar]
- Bradshaw WE, Johnson K. Initiation of metamorphosis in the pitcher-plant mosquito - effects of larval growth history. Ecology. 1995;76:2055–2065. [Google Scholar]
- Browne LB. Quantitative aspects of the regulation of ovarian development in selected anautogenous Diptera: integration of endocrinology and nutrition. Entomologia Experimentalis et Applicata. 2001;100:137–149. [Google Scholar]
- Browne LB, Vangerwen ACM, Williams KL. Oocyte resorption during ovarian development in the blowfly Lucilia cuprina. Journal of Insect Physiology. 1979;25:147–153. [Google Scholar]
- Burmester T. Evolution and function of the insect hexamerins. European Journal of Entomology. 1999;96:213–225. [Google Scholar]
- Burmester T, Kolling C, Schroer B, Scheller K. Complete sequence, expression, and evolution of the hexamerin LSP-2 of Calliphora vicina. Insect Biochemistry and Molecular Biology. 1998;28:11–22. doi: 10.1016/s0965-1748(97)00054-4. [DOI] [PubMed] [Google Scholar]
- Capurro MD, Marinotti O, Farah CS, James AA, de Bianchi AG. The nonvitellogenic female protein of Musca domestica is an adult-specific hexamerin. Insect Molecular Biology. 1997;6:97–104. doi: 10.1046/j.1365-2583.1997.00162.x. [DOI] [PubMed] [Google Scholar]
- Capurro MD, Moreira-Ferro CK, Marionetti O, James AA, de Bianchi AG. Expression patterns of the larval and adult hexamerin genes of Musca domestica. Insect Molecular Biology. 2000;9:169–177. doi: 10.1046/j.1365-2583.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Harshman L, Liu X, Muller H-G, Partridge L, Wang J-L. Food pulses increase longevity and induce cyclical egg production in Mediterranean fruit flies. Functional Ecology. 2002;16:313–325. [Google Scholar]
- Chapman T, Trevitt S, Partridge L. Remating and male-derived nutrients in Drosophila melanogaster. Journal of Evolutionary Biology. 1994;7:51–69. [Google Scholar]
- Day T, Rowe L. Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. American Naturalist. 2002;159:338–350. doi: 10.1086/338989. [DOI] [PubMed] [Google Scholar]
- de Bianchi AG, Marinotti O, Espinozafuentes FP, Pereira SO. Purification and characterization of Musca domestica storage protein and its developmental profile. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology. 1983;76:861–867. [Google Scholar]
- Denlinger DL. Basis for a skewed sex-ratio in diapause-destined flesh flies. Evolution. 1981;35:1247–1248. doi: 10.1111/j.1558-5646.1981.tb04993.x. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Boorse GC, Glennemeier KA. Endocrinology of complex life cycles: Amphibians. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. Vol. 2. San Diego: Academic Press, Inc; 2002. pp. 469–513. [Google Scholar]
- Ebling FJP. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–683. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- Fischer K, Fiedler K. Effects of adult feeding and temperature regime on fecundity and longevity in the butterfly Lycaena hippothoe (Lycaenidae) Journal of the Lepidopterists’ Society. 2001;54:91–95. [Google Scholar]
- Flanagin VL, Haase SP, Juliano SA. Effects of growth rates on development to metamorphosis in the lubber grasshopper, Romalea microptera. Oecologia. 2000;125:162–169. doi: 10.1007/s004420000441. [DOI] [PubMed] [Google Scholar]
- Gebhardt MD, Stearns SC. Reaction norms for developmental time and weight at eclosion in Drosophila mercatorum. Journal of Evolutionary Biology. 1988;1:335–354. [Google Scholar]
- Good TP, Tatar M. Age-specific mortality and reproduction respond to adult dietary restriction in Drosophila melanogaster. Journal of Insect Physiology. 2001;47:1467–1473. doi: 10.1016/s0022-1910(01)00138-x. [DOI] [PubMed] [Google Scholar]
- Hahn DA, Wheeler DE. Presence of a single abundant storage hexamerin in both larvae and adults of the grasshopper, Schistocerca americana. Journal of Insect Physiology. 2003;49:1189–1197. doi: 10.1016/j.jinsphys.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Borst DW, Eskew MR, Juliano SA. Maximum titers of vitellogenin and total hemolymph protein occur during the canalized phase of grasshopper egg production. Physiological and Biochemical Zoology. 2001;74:885–893. doi: 10.1086/324475. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Borst DW, Juliano SA. Plasticity and canalization in the control of reproduction in the lubber grasshopper. Integrative and Comparative Biology. 2003;43:635–645. doi: 10.1093/icb/43.5.635. [DOI] [PubMed] [Google Scholar]
- Hensley FR. Ontogenic loss of phenotypic plasticity of age at metamorphosis in tadpoles. Ecology. 1993;74:2405–2412. [Google Scholar]
- Hentschel BT, Emlet RB. Metamorphosis of barnacle nauplii: Effects of food variability and a comparison with amphibian models. Ecology. 2000;81:3495–3508. [Google Scholar]
- Jervis MA, Boggs CL, Ferns PN. Egg maturation strategy and survival trade-offs in holometabolous insects: a comparative approach. Biological Journal of the Linnean Society. 2007;90:293–302. [Google Scholar]
- Juliano SA, Olson JR, Murrell EG, Hatle JD. Plasticity and canalization of insect reproduction: Testing alternative models of life history transitions. Ecology. 2004;85:2986–2996. [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebaesold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Analytical Chemistry. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of head of Bacteriophage-T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leips J, Travis J. Metamorphic responses to changing food levels in 2 species of hylid frogs. Ecology. 1994;75:1345–1356. [Google Scholar]
- Meijer T, Drent R. Re-examination of the capital and income dichotomy in breeding birds. Ibis. 1999;141:399–414. [Google Scholar]
- Moehrlin GS, Juliano SA. Plasticity of insect reproduction: testing models of flexible and fixed development in response to different growth rates. Oecologia. 1998;115:492–500. doi: 10.1007/s004420050546. [DOI] [PubMed] [Google Scholar]
- Morey S, Reznick D. A comparative analysis of plasticity in larval development in three species of spadefoot toads. Ecology. 2000;81:1736–1749. [Google Scholar]
- Naumann U, Scheller K. Complete CDNA and gene sequence of the developmentally regulated arylphorin of Calliphora vicina and its homology to insect hemolymph proteins and arthropod hemocyanins. Biochemical and Biophysical Research Communications. 1991;177:963–972. doi: 10.1016/0006-291x(91)90632-h. [DOI] [PubMed] [Google Scholar]
- Nylin S, Gotthard K. Plasticity in life-history traits. Annual Review of Entomology. 1998;43:63–83. doi: 10.1146/annurev.ento.43.1.63. [DOI] [PubMed] [Google Scholar]
- Pan ML, Telfer WH. Methionine-rich hexamerin and arylphorin as precursor reservoirs for reproduction and metamorphosis in female luna moths. Archives of Insect Biochemistry and Physiology. 1996;33:149–162. doi: 10.1002/(SICI)1520-6327(1996)33:2<149::AID-ARCH5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pereira SD, Marinotti O, de Bianchi AG. Nonvitellogenic female protein in Musca domestica. Archives of Insect Biochemistry and Physiology. 1989;11:245–255. [Google Scholar]
- Reim C, Teuschl Y, Blanckenhorn WU. Size-dependent effects of larval and adult food availability on reproductive energy allocation in the Yellow Dung Fly. Functional Ecology. 2006;20:1012–1021. [Google Scholar]
- Roberts DB, Wolfe J, Akam ME. Developmental profiles of 2 major hemolymph proteins from Drosophila melanogaster. Journal of Insect Physiology. 1977;23:871–880. doi: 10.1016/0022-1910(77)90013-0. [DOI] [PubMed] [Google Scholar]
- Roberts DB, Turing JD, Loughlin SAR. The advantages that accrue to Drosophila melanogaster possessing larval serum protein-1. Journal of Insect Physiology. 1991;37:391–399. [Google Scholar]
- Schenkel H, Keizlarovalepesant J, Berreur P, Moreau J, Scheller M, Bregegere F, Lepesant JA. Identification and molecular analysis of a multigene family encoding calliphorin, the major larval serum protein of Calliphorin vicina. Embo Journal. 1985;4:2983–2990. doi: 10.1002/j.1460-2075.1985.tb04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JE. Energy balance and reproduction. Physiology & Behavior. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Bowman R, Reynolds SJ. Food supplementation and possible mechanisms underlying early breeding in the Florida Scrub-Jay (Aphelocoma coerulescens) Hormones and Behavior. 2004;46:565–573. doi: 10.1016/j.yhbeh.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Seo SJ, Kang YJ, Cheon HM, Kim HR. Distribution and accumulation of storage protein-1 in ovary of Hyphantria cunea, Drury. Archives of Insect Biochemistry and Physiology. 1998;37:115–128. doi: 10.1002/(SICI)1520-6327(1998)37:2<115::AID-ARCH1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Shafiei M, Moczek AP, Nijhout HF. Food availability controls the onset of metamorphosis in the dung beetle Onthophagus taurus (Coleoptera: Scarabaeidae) Physiological Entomology. 2001;26:173–180. [Google Scholar]
- Smith DF, McClelland A, White BN, Addison CF, Glover DM. The molecular-cloning of a dispersed set of developmentally regulated genes which encode the major larval serum protein of Drosophila melanogaster. Cell. 1981;23:441–449. doi: 10.1016/0092-8674(81)90139-2. [DOI] [PubMed] [Google Scholar]
- Stearns SC, Koella JC. The evolution of phenotypic plasticity in life-history traits - predictions of reaction norms for age and size at maturity. Evolution. 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Travis J. Anuran size at metamorphosis - experimental test of a model based on intraspecific competition. Ecology. 1984;65:1155–1160. [Google Scholar]
- Twombly S. Timing of metamorphosis in a freshwater crustacean: Comparison with anuran models. Ecology. 1996;77:1855–1866. [Google Scholar]
- Wall R. The reproductive output of the blowfly Lucilia sericata. Journal of Insect Physiology. 1993;39:743–750. [Google Scholar]
- Wall R, Wearmouth VJ, Smith KE. Reproductive allocation by the blow fly Lucilia sericata in response to protein limitation. Physiological Entomology. 2002;27:267–274. [Google Scholar]
- Wheeler D. The role of nourishment in oogenesis. Annual Review of Entomology. 1996;41:407–431. doi: 10.1146/annurev.en.41.010196.002203. [DOI] [PubMed] [Google Scholar]
- Wheeler DE, Buck NA. A role for storage proteins in autogenous reproduction in Aedes atropalpus. Journal of Insect Physiology. 1996;42:961–966. [Google Scholar]
- Wilbur HM, Collins JP. Ecological aspects of amphibian metamorphosis. Science. 1973;182:1305–1314. doi: 10.1126/science.182.4119.1305. [DOI] [PubMed] [Google Scholar]
- Williams KL, Browne LB, Vangerwen ACM. Quantitative relationships between the ingestion of protein-rich material and ovarian development in the Australian sheep blowfly, Lucilia cuprina (Wied) International Journal of Invertebrate Reproduction. 1979;1:75–88. [Google Scholar]
- Yin C-M, Qin W-H, Stoffolano JG. Regulation of mating behavior by nutrition and the corpus allatum in both male and female Phormia regina (Meigen) Journal of Insect Physiology. 1999;45:815–822. doi: 10.1016/s0022-1910(99)00047-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following supplementary material is available for this article:
Figure S1 Peptide fingerprint mapping by tandem MS.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2745.XXXX.XXXXX.x