Abstract
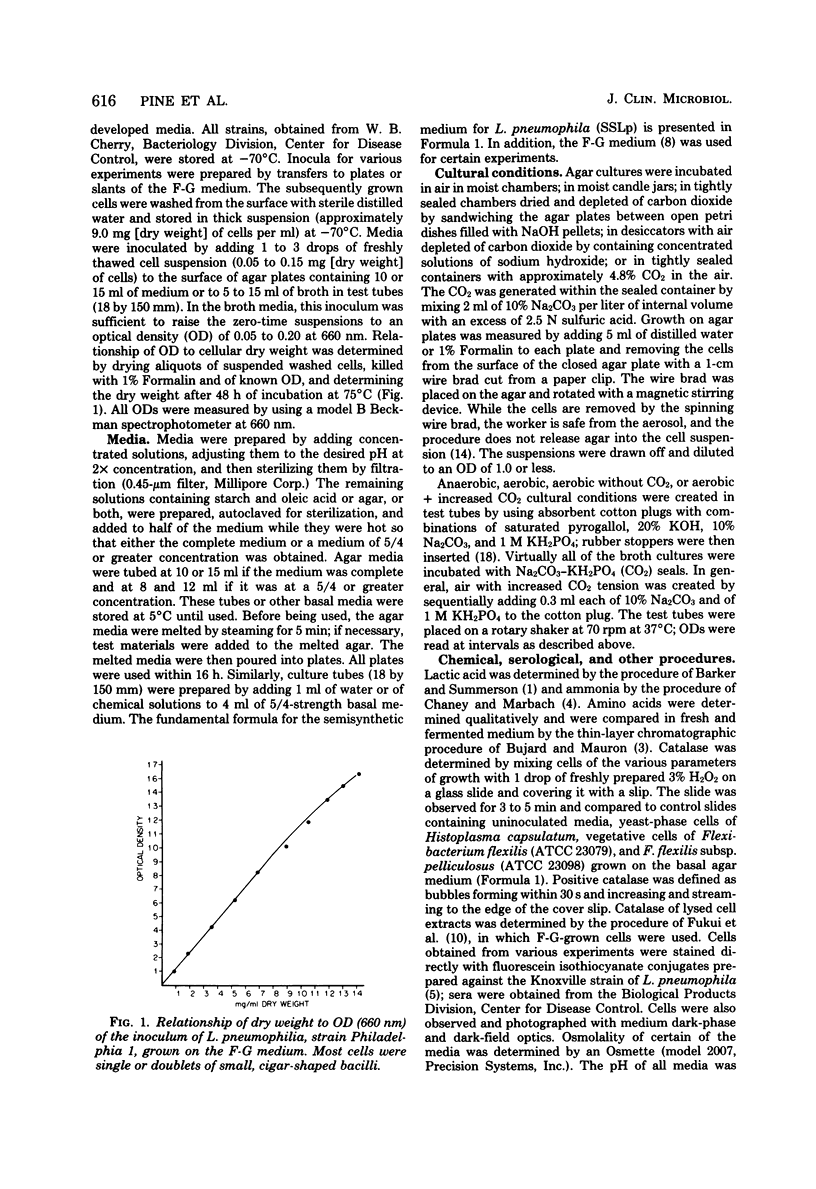
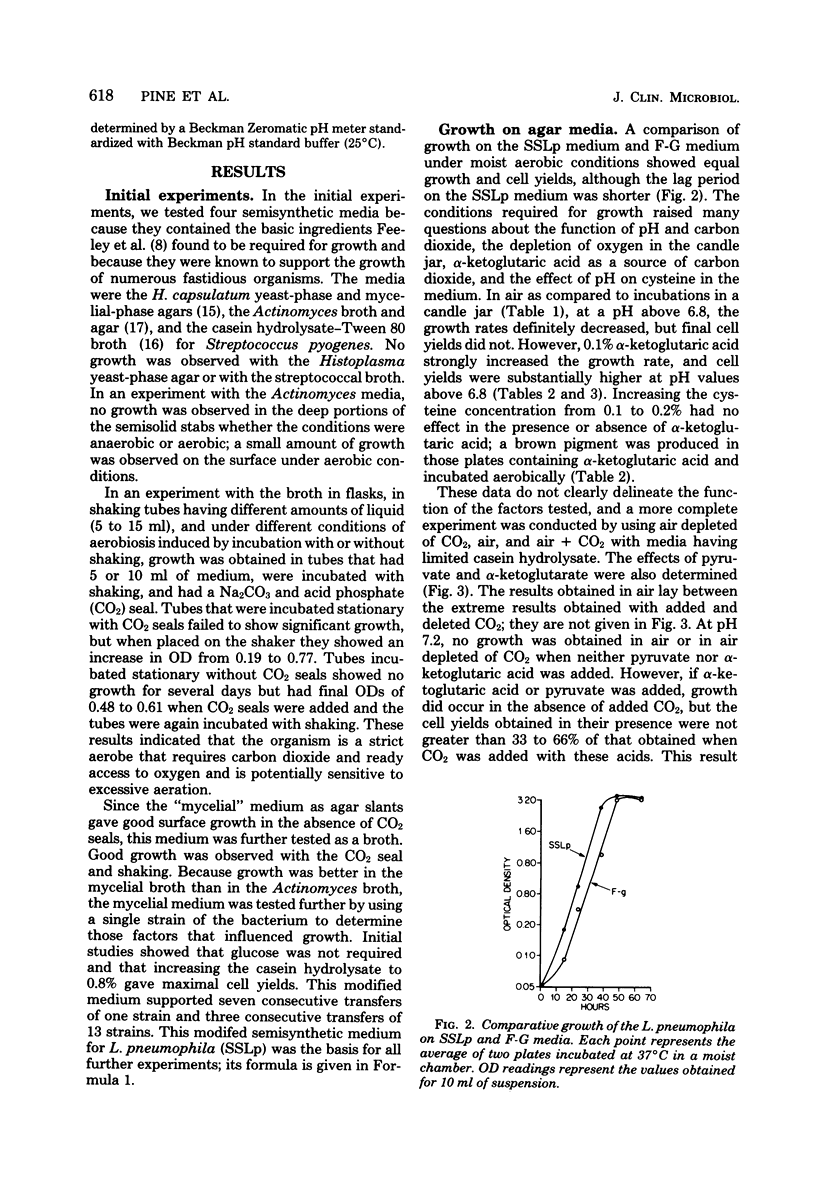
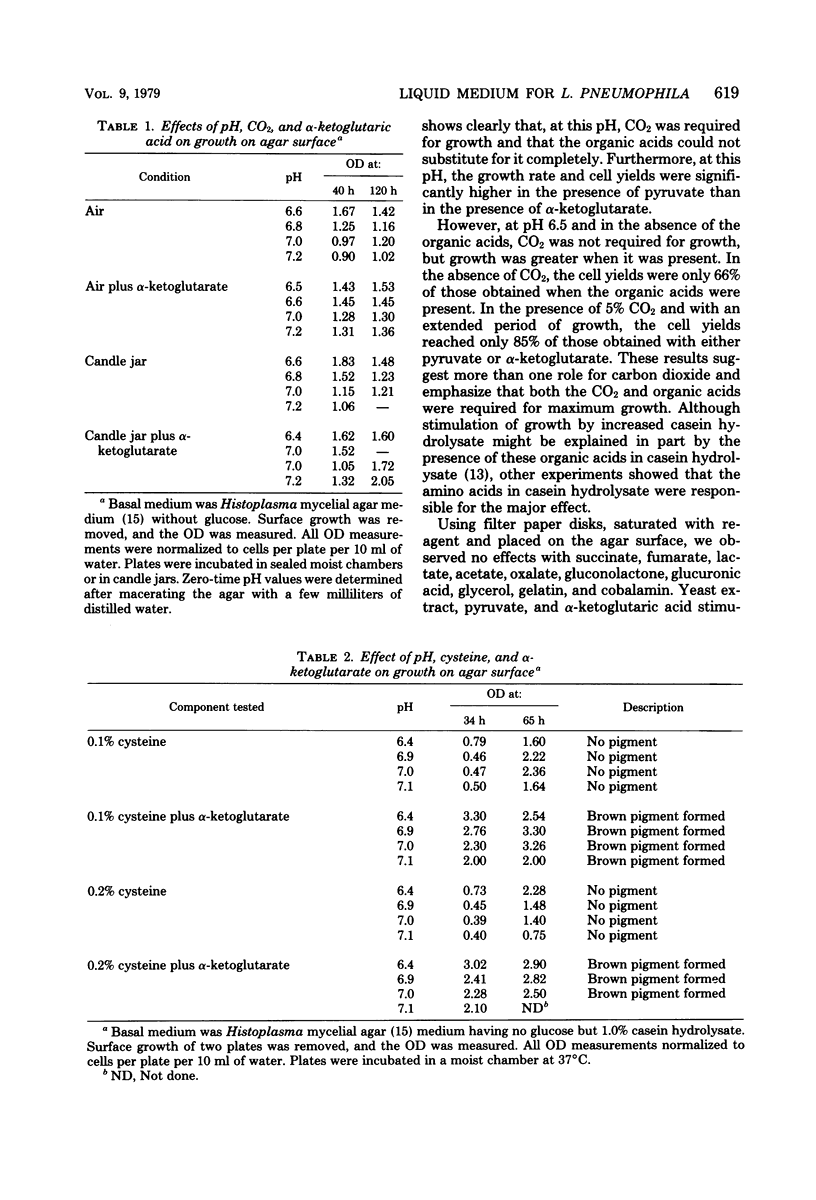
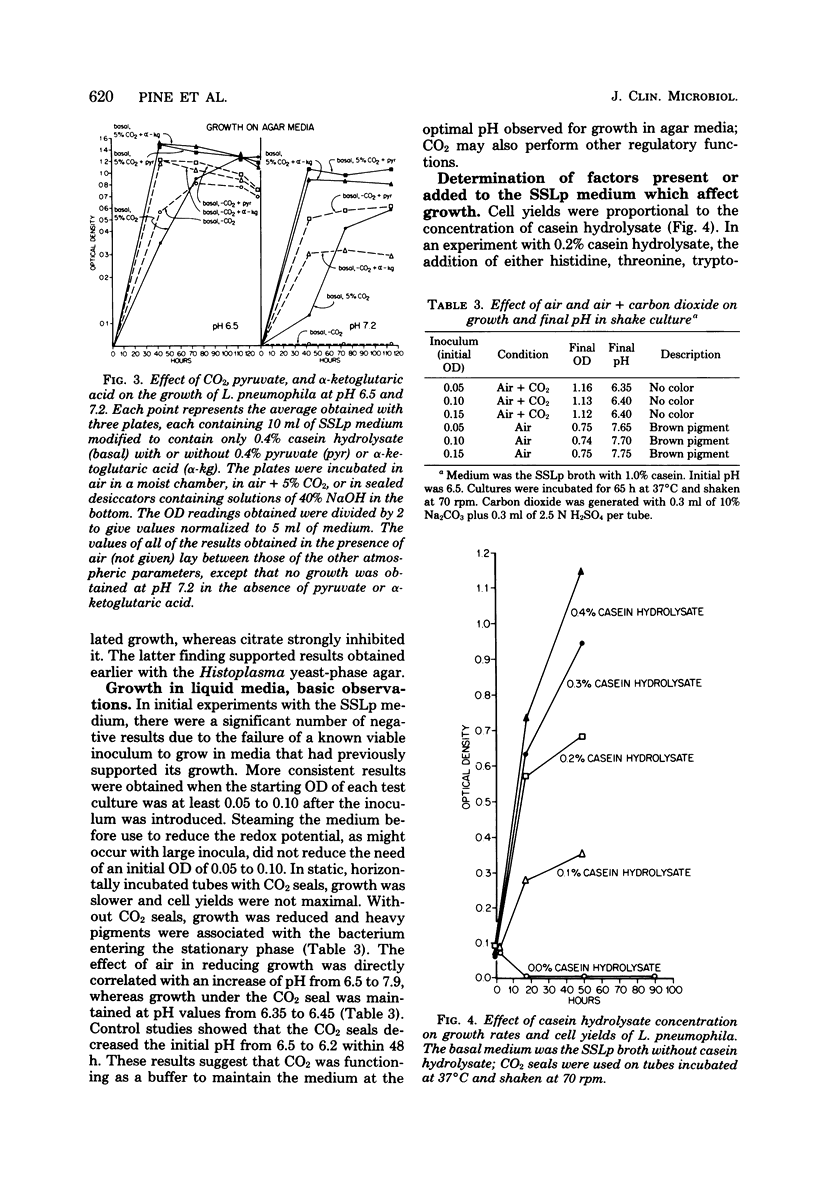
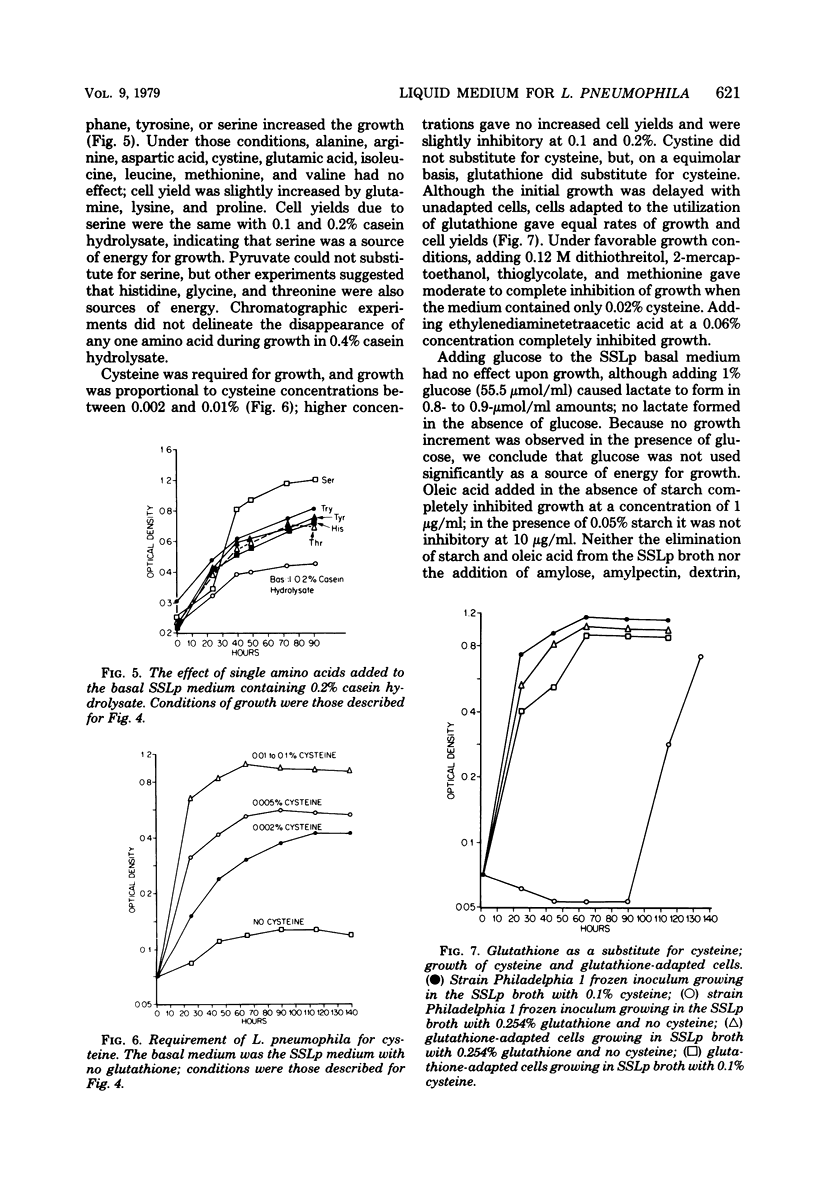
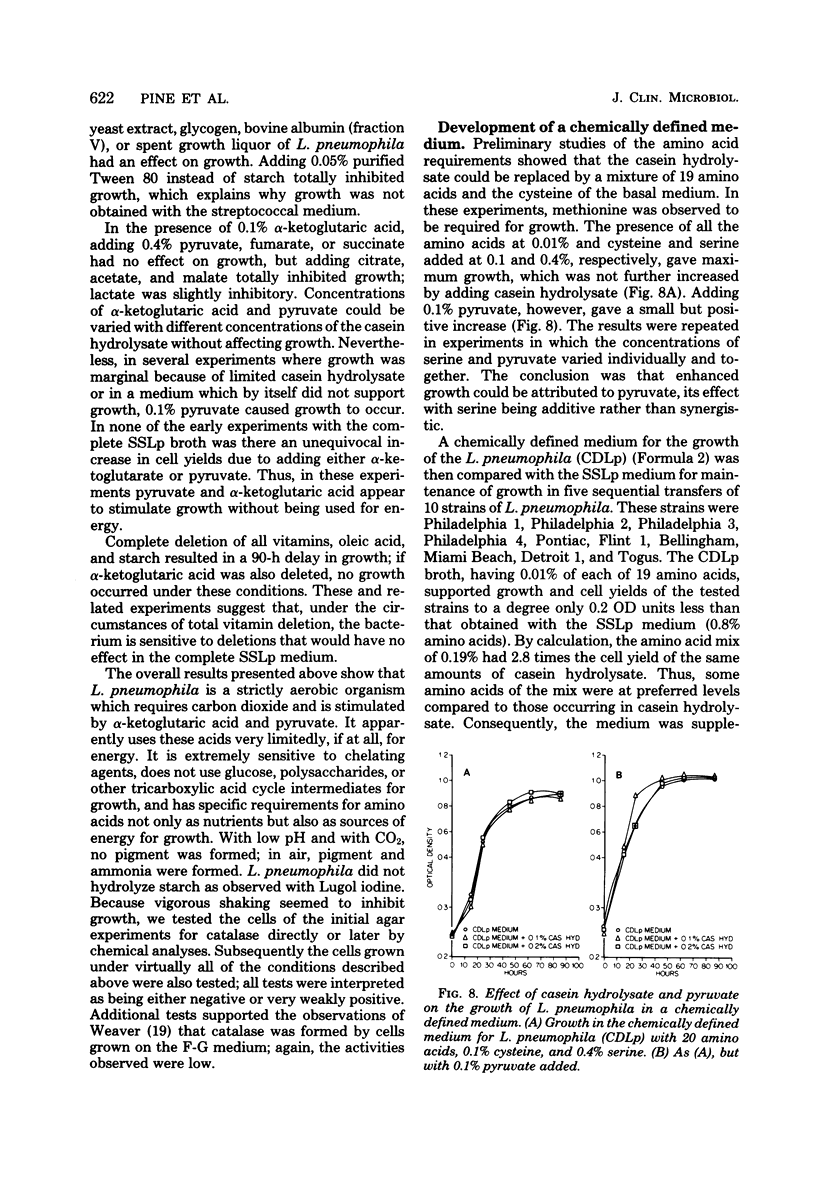
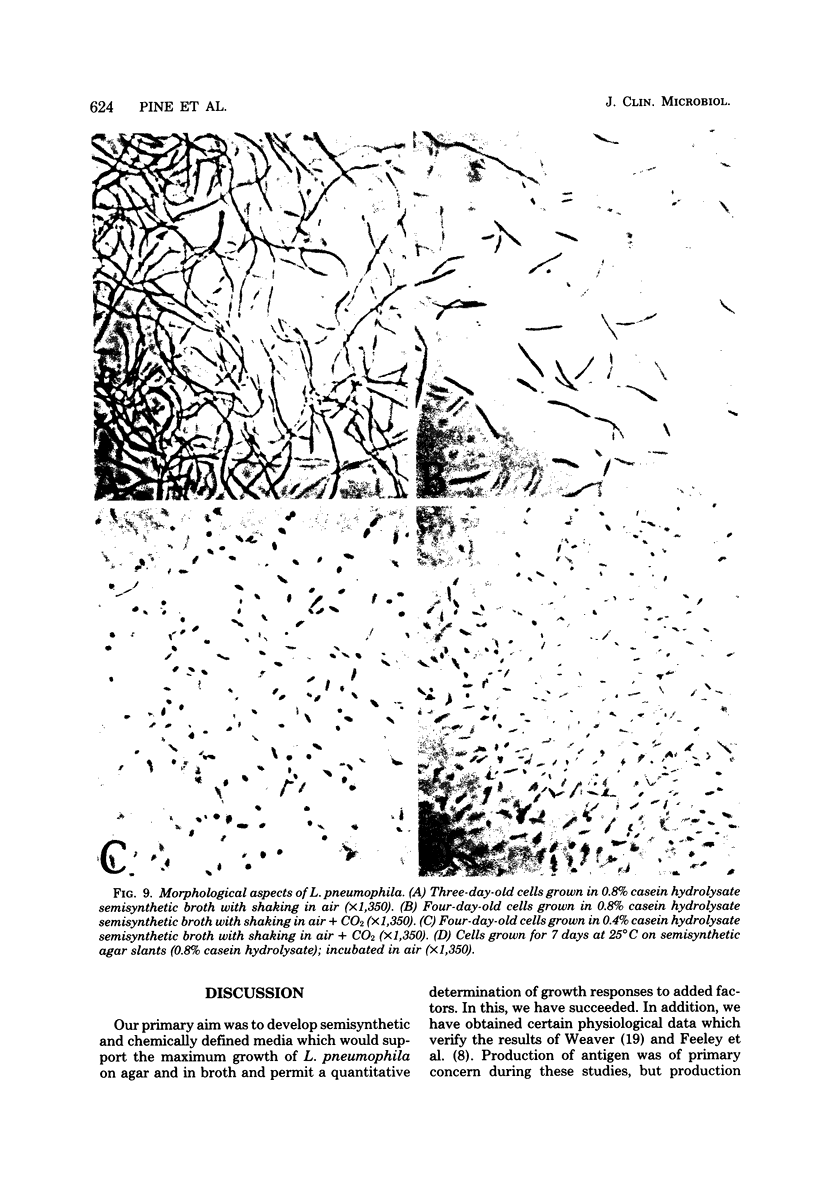
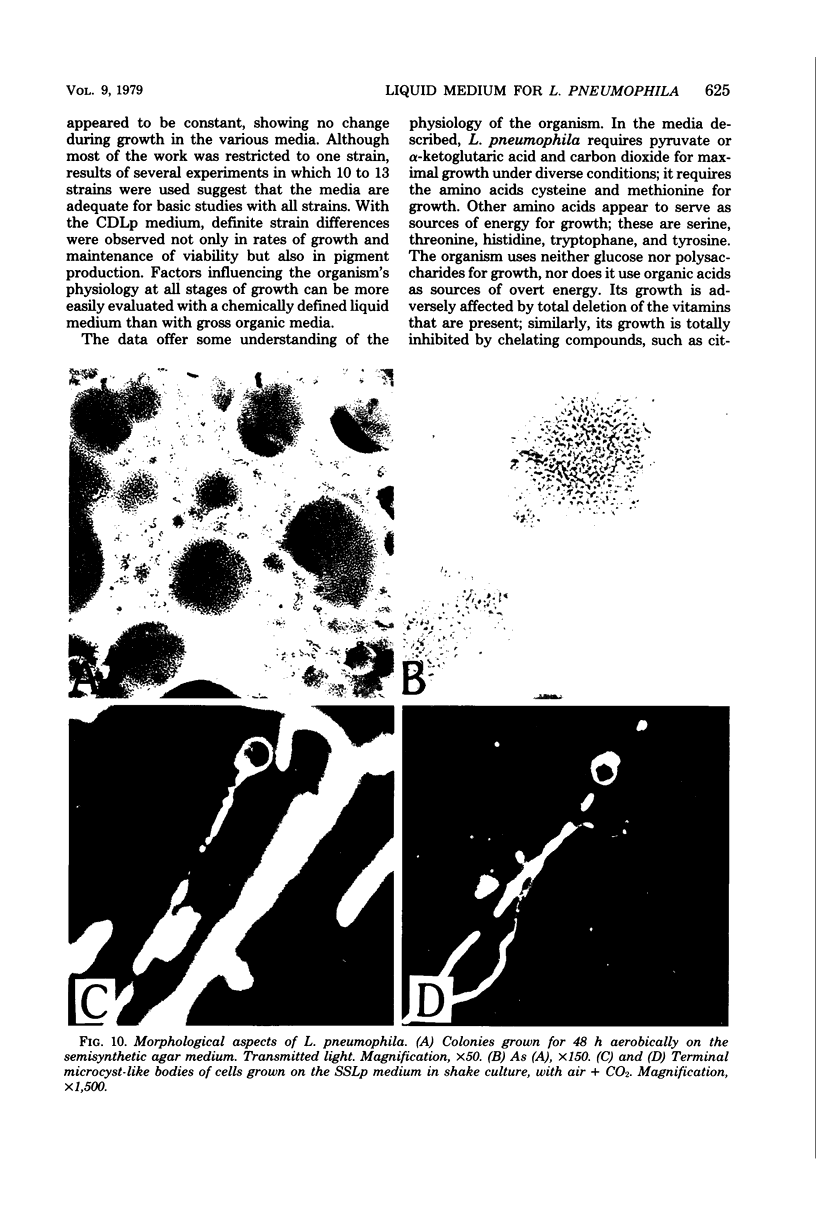
A chemically defined liquid medium has been developed for the study of the physiology and antigen production of the Legionnaires disease bacterium. The medium contains basal salts, vitamins, alpha-ketoglutaric acid, pyruvate, 0.05% l-cysteine, 0.05% glutathione, and a mixture of 20 additional amino acids, each of 0.01% final concentration, except serine, which was at 0.1%. The medium in shake culture at 37 degrees C with increased CO2 at pH 6.5, supports the maximum rate of growth, the highest cell yields, and the maximum cell surface antigen as distinguished by specific fluorescein isothiocyanate-conjugated antibody. Studies during the development of this medium showed that CO2, pyruvate, and alpha-ketoglutarate strongly stimulated growth; that cysteine and methionine were required for growth; and that serine, threonine, histidine, tyrosine, and tryptophane were energy sources. Glutathione substituted for cysteine, but cystine did not. The organisms did not use glucose and polysaccharides, as judged by cell yields when these carbohydrates were present or absent. The chelators malate, citrate, and ethylenediaminetetraacetic acid totally inhibited growth. Beta-mercaptoethanol, thioglycolate, dithiothreitol, and Tween 80 (0.05%) inhibited growth strongly or completely. Catalase activity was extremely weak or absent. Morphology varied, depending upon conditions and phases of growth. In general, filamentous forms became chains of cigar-shaped bacilli fragmenting to pairs and becoming coccoidal in the late stationary pha-e of growth. The organism grew at 25, 30, and 37 degrees C. Although they varied in their growth characteristics, 10 isolates were passed for five transfers in the chemically defined broth, giving maximum rates of growth, cell yields, and antigen production.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bujard E., Mauron J. A two-dimensional separation of acid, neutral and basic amino acids by thin layer chromatography on cellulose. J Chromatogr. 1966 Jan;21(1):19–26. doi: 10.1016/s0021-9673(01)91255-5. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Cherry W. B., Pittman B., Harris P. P., Hebert G. A., Thomason B. M., Thacker L., Weaver R. E. Detection of Legionnaires disease bacteria by direct immunofluorescent staining. J Clin Microbiol. 1978 Sep;8(3):329–338. doi: 10.1128/jcm.8.3.329-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. Biology of the myxobacteria. Annu Rev Microbiol. 1966;20:75–106. doi: 10.1146/annurev.mi.20.100166.000451. [DOI] [PubMed] [Google Scholar]
- Fager E. W. Recurrent group analysis in the classification of flexibacteria. J Gen Microbiol. 1969 Oct;58(2):179–187. doi: 10.1099/00221287-58-2-179. [DOI] [PubMed] [Google Scholar]
- Feeley J. C., Gorman G. W., Weaver R. E., Mackel D. C., Smith H. W. Primary isolation media for Legionnaires disease bacterium. J Clin Microbiol. 1978 Sep;8(3):320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. W., Tsai T. R., Orenstein W., Parkin W. E., Beecham H. J., Sharrar R. G., Harris J., Mallison G. F., Martin S. M., McDade J. E. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 1977 Dec 1;297(22):1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- Fukui S., Tanaka A., Kawamoto S., Yasuhara S., Teranishi Y., Osumi M. Ultrastructure of methanol-utilizing yeast cells: appearance of microbodies in relation to high catalase activity. J Bacteriol. 1975 Jul;123(1):317–328. doi: 10.1128/jb.123.1.317-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R. A. A classification of flexibacteria. J Gen Microbiol. 1969 Oct;58(2):189–206. doi: 10.1099/00221287-58-2-189. [DOI] [PubMed] [Google Scholar]
- MACLEOD P., MORGAN M. E. Alpha-keto acids in vitamin-free casein hydrolyzates (acid). Science. 1959 Aug 28;130(3374):505–506. doi: 10.1126/science.130.3374.505. [DOI] [PubMed] [Google Scholar]
- Mandel M., Lewin R. A. Deoxyribonucleic acid base composition of flexibacteria. J Gen Microbiol. 1969 Oct;58(2):171–178. doi: 10.1099/00221287-58-2-171. [DOI] [PubMed] [Google Scholar]
- PINE L. Studies on the growth of Histoplasma capsulatum. II. Growth of the yeast phase on agar media. J Bacteriol. 1955 Oct;70(4):375–381. doi: 10.1128/jb.70.4.375-381.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE L., WATSON S. J. Evaluation of an isolation and maintenance medium for Actinomyces species and related organisms. J Lab Clin Med. 1959 Jul;54(1):107–114. [PubMed] [Google Scholar]
- Pine L. Growth of Histoplasma capsulatum. VI. Maintenance of the mycelial phase. Appl Microbiol. 1970 Mar;19(3):413–420. doi: 10.1128/am.19.3.413-420.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L. Variation of M protein with sequential transfer of group A streptococci in semisynthetic media. Microbios. 1976;16(65-66):153–168. [PubMed] [Google Scholar]




