Abstract
A rising tide of obesity and type 2 diabetes has resulted from the development of technologies that have made inexpensive high calorie foods readily available and exercise unnecessary for many people. Obesity and the metabolic syndrome (insulin resistance, visceral adiposity and dyslipidemia) wreak havoc on cells throughout the body thereby promoting cardiovascular and kidney disease, and degenerative diseases of the brain and body. Obesity and insulin resistance promote disease by increasing oxidative damage to proteins, lipids and DNA as the result of a combination of increased free radical production and an impaired ability of cells to detoxify the radicals and repair damaged molecules. By covalently modifying membrane-associated proteins, the membrane lipid peroxidation product 4-hydroxynonenal (HNE) may play particularly sinister roles in the metabolic syndrome and associated disease processes. HNE can damage pancreatic β cells and can impair the ability of muscle and liver cells to respond to insulin. HNE may promote atherosclerosis by modifying lipoproteins and can cause cardiac cell damage by impairing metabolic enzymes. An adverse role for HNE in the brain in obesity and the metabolic syndrome is suggested by studies showing that HNE levels are increased in brain cells with aging and Alzheimer’s disease. HNE can cause the dysfunction and degeneration of neurons by modifying membrane-associated glucose and glutamate transporters, ion-motive ATPases, enzymes involved in amyloid metabolism, and cytoskeletal proteins. Exercise and dietary energy restriction reduce HNE production and may also increase cellular systems for HNE detoxification including glutathione and oxidoreductases. The recent development of low molecular weight molecules that scavenge HNE suggests that HNE can be targeted in the design of drugs for the treatment of obesity, the metabolic syndrome, and associated disorders.
Keywords: Alzheimer’s disease, atherosclerosis, diabetes, insulin resistance, lipid peroxidation, metabolic syndrome
The Metabolic Syndrome
Obese individuals are at increased risk for diabetes, cardiovascular disease and cancers, and usually die prematurely (Barness et al., 2007). Overweight people often exhibit perturbed energy and lipid metabolism characterized by elevated levels of glucose, triglycerides and low density lipoproteins (LDL) in their blood (Howard et al., 2003). But individuals who are not considered obese may also exhibit physiological alterations similar to those seen in obesity including insulin resistance (resulting in impaired glucose tolerance), visceral adiposity and dyslipidemia (elevated levels triglycerides and LDL cholesterol, and decreased levels of HDL). The later phenotype, which has been dubbed the “metabolic syndrome” (MS), predisposes to several major age-related diseases including diabetes (Bakris et al., 2008), cardiovascular disease (Aggoun, 2007), stroke (Gil-Nunez, 2007) and possibly cognitive impairment and Alzheimer’s disease (Pasinetti and Eberstein, 2008; Stranahan et al., 2008). The prevalence of MS and obesity have increased rapidly in the past 30 years, tripling in adolescents between 1970 and 2000 (Harrell et al., 2006), heightening the importance of understanding the cellular and molecular mechanisms by which these conditions promote disease.
Two systemic alterations involved in obesity and the MS are increased oxidative damage to cellular constituents (proteins, lipids and DNA) and increased inflammation as indicated by elevated levels of tumor necrosis factor (TNF), interleukin-1β and other pro-inflammatory cytokines (Sutherland et al., 2004; Pennathur and Heinecke, 2007; Grattagliano et al., 2008). Oxidative stress and inflammation go hand-in-hand in the many tissues that are affected because oxidative stress induces the production of inflammatory cytokines, and the cytokines in turn induce free radical production. In this regard, MS and obesity accelerate processes that are generally considered fundamental to the aging process and may in this way hasten the onset of many different age-related disorders (Chung et al., 2009). One specific facet of oxidative stress and inflammation, membrane lipid peroxidation, is the focus of this review article. Lipid peroxidation (LP) is an autocatalytic process initiated by free radical attack on the unsaturated (double) bonds of membrane fatty acids (Fig. 1). Five reactive oxygen species (ROS) are most commonly involved in the initiation of LP: superoxide anion radical (O2-.), hydrogen peroxide (H2O2), hydroxyl radical (OH.), nitric oxide (NO.) and peroxynitrite (ONOO.). The major source of superoxide in most cells is that produced in mitochondria as “by-product” of oxidative phosphorylation in the electron transport chain. Much of the superoxide is rapidly converted to hydrogen peroxide by mitochondrial superoxide dismutase (SOD2) and cytoplasmic superoxide dismutase (SOD1). Hydroxyl radical can then be produced by a process called the Fenton reaction in which Fe2+ or Cu+ interact with hydrogen peroxide. Nitric oxide is generated in response to elevations of intracellular Ca2+ levels; Ca2+ binds calmodulin, which then activates nitric oxide synthase which catalyzes the conversion of arginine to citrulline and nitric oxide. Nitric oxide may then interact with superoxide to generate peroxynitrite. Among the different ROS, hydroxyl radical and peroxynitrite are particularly aggressive inducers of LP.
Figure 1.
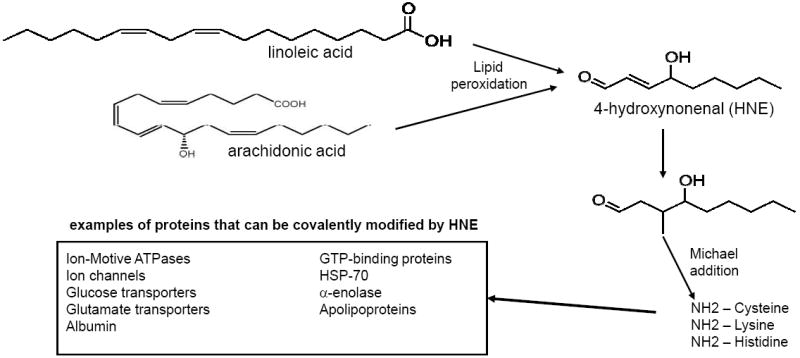
Mechanisms involved in the generation of HNE from membrane lipids, and protein modification by HNE. HNE is generated primarily from linoleic and arachidonic acid in membranes as the result of free radical (hydroxyl radical and peroxynitrite) attack on the unsaturated bonds in these lipids. HNE can covalently modify proteins on lysine, histidine and cysteine residues in a process called Michael addition. Modification by HNE typically impairs the function of the proteins; examples of proteins known to be modified and functionally altered by HNE in the metabolic syndrome and associated diseases are listed (Mark et al., 1997a, 1997b; Keller et al., 1997; Blanc et al., 1997; Kelly et al., 1996; Perluigi et al., 2005).
The process of lipid peroxidation is initiated by interaction of hydroxyl radical or peroxynitrite with unsaturated lipids which triggers chain peroxidation by abstracting allylic hydrogens (Girotti, 1998). The resulting lipid radicals rapidly interact with oxygen, thereby propagating the reaction via peroxyl radical intermediates; this process simultaneously generates lipid hydroperoxides and aldehydes of various chain lengths. Lipid peroxidation can be terminated by so-called chain-breaking antioxidants such as vitamin E. One specific aldehydic product of LP called 4-hydroxynonenal (HNE) is increasingly recognized as a particularly important mediator and marker of cellular dysfunction and degeneration in a range of disorders including cardiovascular disease (Leonarduzzi et al., 2005), stroke (Tang et al., 2007), Alzheimer’s disease (Lovell et al., 1997; Bruce-Keller et al., 1998), arthritis (Morquette et al., 2006) and asthma (Johnson et al., 2007). In this review article, I describe evidence supporting a contributory role for HNE in the pathogenesis of obesity, the MS and associated diseases based upon the ability of this aldehyde to adversely affect membrane-associated proteins involved in the regulation of cellular ion and energy homeostasis.
The Production, Chemistry and Metabolism of HNE
HNE is a 9-carbon amphiphilic lipid formed when n-6-polyunsaturated fatty acids such as arachidonic acid and linoleic acid are attacked by peroxidative free radicals (Fig. 1). The specific non-enzymatic pathways by which HNE is formed during the process of LP is not fully understood, but likely involve dimerized and oligomerized fatty acid derivatives as key intermediates (Schneider et al., 2008). In addition, there is evidence for an enzymatic mechanism for HNE production which may be particularly prominent in microsomes (Esterbauer et al., 1986). The lipophilic property of HNE makes it remain associated with the membranes where it is generated, but HNE is also capable of moving within and between cellular compartments.
When HNE encounters proteins, it can interact with thiol (SH) and amino (NH2) groups of cysteine, lysine and histidine residues via a process called Michael addition resulting in a covalent bond between HNE and the amino acid. Numerous proteins have been shown to be modified by HNE (Fig. 1) including: plasma membrane ion and nutrient transporters; receptors for growth factors and neurotransmitters; mitochondrial electron transport chain proteins; protein chaperones; proteasomal proteins; and cytoskeletal proteins (Petersen and Doorn, 2004; Poli et al., 2008). In many cases the function of the protein to which HNE binds is impaired. Accordingly, if HNE levels are increased excessively, cellular function can be compromised and damage and death of the cell (apoptosis) may occur. For example, studies of experimental models of Alzheimer’s disease have shown that modification of ion-motive ATPases (Na+ and Ca2+ pumps), and glucose and glutamate transporters, can disrupt cellular ion and energy homeostasis in brain cells resulting in the dysfunction and death of neurons (Keller et al., 1997; Mark et al., 1997a, 1997b). However, there are cases in which HNE modification activate signaling cascades (Grimsrud et al., 2008). For example, HNE can activate the epidermal growth factor receptor tyrosine kinase in the absence of a ligand (Liu et al., 1999) and HNE can increase the activity of ASK1 kinase resulting in activation of jun-N-terminal kinase (Soh et al., 2000), suggesting specific mechanisms by which HNE might affect cell proliferation and survival.
Cells have several mechanisms to detoxify/remove HNE and thereby prevent its potentially damaging actions. Perhaps the most important defense against HNE is glutathione (GSH), a tripeptide that includes a cysteine residue. Indeed, data suggest that intracellular concentrations of HNE are regulated by glutathione-S-transferases, GSH and a protein called RLIP76 that catalyzes the ATP-dependent transport of HNE out of cells (Singhal et al., 2009). In addition to glutathione, the dipeptide carnosine (β-alanyl-L-histidine) can quench HNE via intra-molecular Michael addition (Guiotto et al., 2005). Proteins present in high amounts within or outside of cells may also play important roles in binding and thereby quenching HNE; examples include albumin (Aldini et al., 2008) and apolipoproteins (Hoff et al., 1989; Pedersen et al., 2000).
Involvement of HNE in Obesity and the Metabolic Syndrome
Accumulating evidence suggests that HNE may play important roles in pathogenic cellular changes that cause insulin resistance and other abnormalities in the MS, and HNE may also mediate disease processes promoted by the MS. Levels of HNE are increased in the blood and muscle tissue of obese subjects compared to normal weight subjects (Vincent et al., 2001; Russell et al., 2003). Improved insulin sensitivity in muscle cells resulting from exercise or dietary energy restriction is associated with reduced levels of HNE and other LP products (Johnson et al., 2007; Vincent et al., 2007; Morris et al., 2008). Levels of HNE are also increased in adipocytes in obesity where it may impair the function of proteins that play important roles in lipid anabolism (Grimsrud et al., 2007) and suppression of inflammation (Zarrouki et al., 2007). Pancreatic beta cells are vulnerable to oxidative stress and LP, and there is evidence that LP (and by inference HNE) contributes to the dysfunction and death of pancreatic beta cells in diabetes (Lenzen, 2008) and alcohol-induced pancreatic damage (Aleynik et al., 1999). Excessive production of HNE might be sufficient to cause obesity and the MS. When the gene encoding the HNE-conjugating enzyme mGSTA4-4 is disrupted in mice, HNE accumulates in multiple tissues and the mice develop obesity and insulin resistance (Singh et al., 2008). Additional findings in the latter study suggest that HNE causes fat accumulation by promoting fatty acid synthesis and suppressing fatty acid beta-oxidation.
In a study of human subjects the level of HNE in the plasma increased significantly and rapidly (within minutes to hours) after consumption of an energy-dense high-fat “fast food” diet (Devaraj et al., 2008). This is the kind of diet that promotes the MS and obesity when consumed regularly, suggesting a role for HNE very early the development of MS and obesity. On the other hand, when moderately obese subjects were maintained on an alternate day calorie restriction diet, their levels of circulating HNE and markers of inflammation decreased, and multiple health indicators improved (Johnson et al., 2007), demonstrating that membrane LP (and its detrimental effects on health) can be reversed by dietary restriction.
HNE and MS-Related Diseases
During the past several decades the results of epidemiological, clinical and animal studies have provided compelling evidence that the MS adversely affects many different organ systems and is a risk factor for major diseases that cause morbidity and premature deaths. Thus, MS increases the risk of cardiovascular disease (Cersosimo and DeFronzo, 2006), stroke (Towfighi and Ovbiagele, 2008), kidney disease (Lastra et al., 2006; Sarafidis and Ruilope, 2006), and Alzheimer’s disease (Pasinetti and Eberstein, 2008). Increased oxidative stress plays a role in all of these MS-related disorders. The remainder of this article describes some of the evidence for the involvement of HNE in three of the most prominent disorders for which the MS is an important risk factor, namely, cardiovascular disease, stroke and Alzheimer’s disease.
Cardiovascular Disease
Levels of HNE are elevated in atherosclerotic lesions of human subjects and in animal models of cardiovascular disease (Ylä-Herttuala et al., 1989). HNE covalently modifies LDL, and this modification activates macrophages which may contribute to the vascular inflammation that occurs in atherosclerotic lesions (Hoff et al., 1989). LDL has been shown to bind many molecules of HNE via covalent modification of lysine and histidine residues (Annangudi et al., 2008). Interestingly, the apolipoprotein E4 isoform binds less HNE than do the E2 and E3 isoforms (Pedersen et al., 2000) which may contribute to the lesser effectiveness of apoE4 in protecting against oxidative vascular damage and atherosclerosis. More recent findings provide evidence that HNE promotes macrophage foam cell formation by increasing the production of class A scavenger receptors at the translational level (Yun et al., 2008). In addition, HNE may promote the adhesion of pro-inflammatory immune cells (macrophages and lymphocytes) to the vascular endothelium in the early stages of atherosclerosis (Go et al., 2007). Moreover, HNE can directly impair the barrier function of vascular endothelial cells and may even trigger apoptosis of these cells (Herbst et al., 1999). Even the hyper-proliferation of vascular smooth muscle cells that occurs in cardiovascular disease may, at least in part, be mediated by HNE. Thus, exposure to HNE resulted in the activation of mitogen-activated protein kinases and increased proliferation in cultured vascular smooth muscle cells (Kakishita and Hattori, 2001). However, the correlation in humans between high-fat diet, HNE level in blood and obesity/metabolic syndrome is suggestive, but it does not prove causality. Nevertheless, studies of experimental models have shown that exposure to HNE can induce excessive fat accumulation (Wonisch et al., 2001; Singh et al., 2009).
Myocardial infarction (MI) is an all too common, and often fatal, result of atherosclerotic lesions in the coronary arteries that supply blood to heart muscle cells. The extent of damage and death of cardiac myocytes is directly related to consequent morbidity and mortality (Abbate et al., 2003). In addition to its roles in the process of atherosclerosis, HNE may contribute to the damage to cardiac myocytes that occurs in myocardial infarction. HNE accumulates in myocardial cells during reperfusion injury (Blasig et al., 1995). HNE may cause the dysfunction and death of cardiac myocytes by a mechanism involving disruption of the actin cytoskeleton and dysregulation of cellular calcium homeostasis (VanWinkle et al., 1994). Arrhythmia is a common occurrence leading to mortality after an MI. HNE may promote cardiac arrhythmia by inhibiting potassium channels resulting in membrane depolarization and action potential prolongation (Bhatnagar et al., 1995). Survivors of a MI may experience long-term cardiac insufficiency associated with remodeling and hypertrophy of the ventricles. It has been suggested that HNE contributes to cardiac hypertrophy by inhibiting the mitochondrial energy-regulating enzyme NADP+-isocitrate dehydrogenase (Benderdour et al., 2003). Cellular mechanisms that detoxify HNE may play important roles in protecting heart cells against MI-induced damage. For example, glutathione transferase (Ishikawa et al., 1986) and oxidoreductases (Srivastava et al., 1998) can quench HNE in cardiac myocytes. Finally, dietary energy restriction, which reduces levels of LP and HNE, protects the heart against cellular damage and dysfunction in animal models of myocardial infarction (Ahmet et al., 2005; Mattson and Wan, 2005).
Stroke
Stroke is a major cause of morbidity and mortality, and the MS and obesity greatly increase one’s risk for a stroke (Singleton and Smith, 2006). As described above for coronary artery atherosclerosis, HNE likely plays roles in the atherosclerotic process in the cerebral blood vessels that are occluded by a clot or rupture during a stroke. Studies of postmortem brain tissues from patients and control subjects have documented increased levels of lipid peroxidation/HNE associated with neurons and inflammatory glial cells (Takizawa et al., 1994; McKracken et al., 2001). But the strongest evidence that HNE actually contributes to the dysfunction and death of neurons in stroke comes from studies of animal models. Levels of HNE are increased in hippocampal neurons prior to their degeneration after transient global forebrain ischemia in a gerbil model of cardiac arrest (Urabe et al., 2000). Arachidonic acid, a major source of HNE, is generated in response to phospholipase A2 activation after transient cerebral ischemia, and inhibiting phospholipase A2 activation can reduce ischemic brain damage (Adibhatla et al., 2003). HNE increases the vulnerability of neurons to excitotoxicity and apoptosis by impairing the function of proteins involved in the regulation of cellular ion homeostasis and removal of damaged proteins. Thus, HNE impairs the function of the plasma membrane Na+/K+- and Ca2+-ATPases, glucose transporter and glutamate transporter, while enhancing Ca2+ influx through voltage-dependent Ca2+ channels (Mark et al., 1997a, 1997b; Keller et al., 1997; Lu et al., 2002).
Evidence that HNE plays a pivotal role in neuronal degeneration after a stroke is provided by a recent study in which a histidine analog (histidyl hydrazide) that quenches HNE was shown to reduce brain damage and improve functional outcome in a middle cerebral artery occlusion – reperfusion mouse stroke model (Tang et al., 2007). Interestingly, intraventricular administration of apoE protected neurons against degeneration after global cerebral ischemia in a mouse model (Horsburgh et al., 2000), consistent with the possibility of an underlying mechanism involving scavenging of HNE by ApoE (Pedersen et al., 2000). Another study showed that overexpression of mitochondrial manganese superoxide dismutase suppresses LP and HNE production, and reduces neuronal dysfunction and death in a mouse model of focal ischemia/reperfusion injury (Keller et al., 1998). Other agents that reduce HNE production by inhibiting LP, including alpha-Phenyl-n-tert-butyl-nitrone (Lin et al., 2004), the organotellurium compound AS101 (Okun et al., 2007) and ebselen (Pawlas and Malecki, 2007) have been reported effective in experimental models of stroke.
Neurodegenerative Disorders
As an increasing number of people reach their 7th, 8th decades of life, the number of those suffering from neurodegenerative disorders of aging – mainly Alzheimer’s and Parkinson’s diseases – is rapidly increasing. Because of the long-term (many years) 24/7 care required, these diseases impose costs beyond that of any other major diseases. Approximately 5 million Americans currently suffer from Alzheimer’s disease and the cost for their care is approximately 150 billion dollars per year (www.alz.org). In the case of Parkinson’s disease more than 1 million Americans have the disease at a total yearly cost of approximately 40 billion dollars (Huse et al., 2005). Less common neurodegenerative disorders that are, nevertheless, equally devastating to the patient and their family and friends include amyotrophic lateral sclerosis (Sejvar et al., 2005) and Huntington’s disease (Martin et al., 2008a). Oxidative stress occurs in the neurons affected in all of these neurodegenerative disorders (Mattson, 2004, 2006; Boillée et al., 2006; Mattson and Magnus, 2006) and membrane LP and HNE are believed to contribute to the dysfunction and death of the neurons and the patient. However, the evidence that the MS is an important factor in these neurodegenerative disorders is strongest for Alzheimer’s disease.
Alzheimer’s disease is characterized by the degeneration of neurons in brain regions involved in cognition (hippocampus, entorhinal cortex, frontal cortex and associated structures) and emotional behaviors (amygdala, prefrontal cortex, hypothalamus and others). The abnormal production and aggregation of amyloid β-peptide (Aβ) is believed to be a pivotal event in the disease process (Mattson, 2004). An important role for HNE in Alzheimer’s disease is suggested by data from human patients, and animal and cell culture models. Biochemical and immunohistochemical analysis of brain tissue samples from Alzheimer’s disease patients and age-matched neurologically normal control subjects have documented elevated levels of HNE in association with Aβ plaques and neurofibrillary tangles (degenerating neurons with intracellular aggregates of the microtubule-associated protein tau) (Sayre et al., 1997; Markesbery and Lovell, 1998; Williams et al., 2006). Moreover, HNE levels are increased in the cerebrospinal fluid in Alzheimer’s patients demonstrating a robust ongoing LP process, and also suggesting HNE as a potential biomarker of the disease (Lovell et al., 1997).
Exposure of cultured neurons to Aβ results in LP and HNE production (Fig. 2), and HNE impairs the function of membrane ion-motive ATPases and glutamate and glucose transporters which renders neurons vulnerable to excitotoxicity and apoptosis (Mark et al., 1997a, 1997b; Keller et al., 1997; Kruman et al., 1997). Inflammatory processes involving activation of Toll-like receptors (part of the innate immune system) may mediate the death of neurons downstream of Aβ and HNE (Tang et al., 2008). In the early stages of Alzheimer’s disease HNE may impair synaptic communication between neurons as suggested by studies showing that Aβ and HNE impair coupling of muscarinic acetylcholine receptors to the GTP-binding protein Gq11 (Kelly et al., 1996; Blanc et al., 1997). Additional studies have shown that infusion of HNE into the brains of rats can damage cholinergic neurons and impair their learning and memory ability demonstrating that HNE production is indeed sufficient to cause cognitive impairment (Bruce-Keller et al., 1998). Treatments that suppress LP can protect neurons against dysfunction and degeneration in animal and cell culture models of Alzheimer’s disease (Butterfield et al., 2004; Goodman and Mattson, 1994; Cutler et al., 2004; Bruce et al., 2006; Nishida et al., 2006). Moreover, scavenging of HNE with either glutathione or ApoE2/ApoE3 can protect neurons against Aβ toxicity (Mark et al., 1997b; Pedersen et al., 2000; Lauderback et al., 2002). Emerging findings suggest that, in addition to acting downstream of Aβ production, HNE plays a role in increasing Aβ production by affecting the expression and enzymatic activities of β- and γ-secretases (Jo et al., 2008; Chen et al., 2008; Tamagno et al., 2008).
Figure 2.
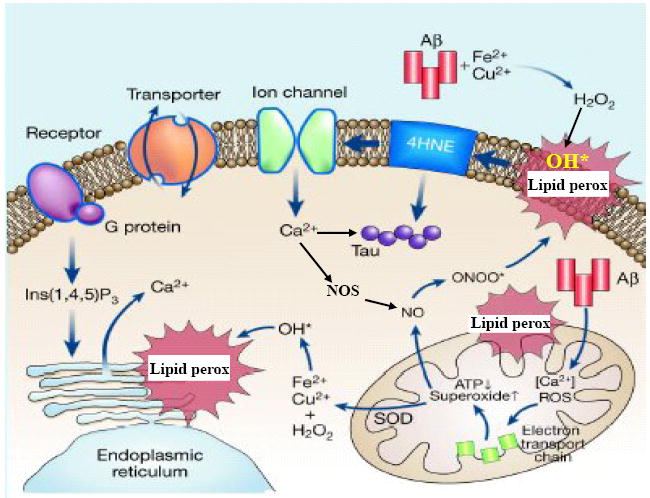
Model showing the role of membrane lipid peroxidation and HNE in the pathogenesis of neuronal dysfunction and degeneration in Alzheimer’s disease. Neurons suffer from increased amounts of oxidative stress in Alzheimer’s disease, probably due to changes occurring during the aging process in combination with the production and aggregation of amyloid β- peptide (Aβ). In the presence of trace amounts of Fe2+ or Cu+, Aβ aggregates and in the process generates hydrogen peroxide (H2O2) which is in turn converted to hydroxyl radical (OH*). Hydroxyl radical induces membrane lipid peroxidation and the production of HNE; lipid peroxidation and HNE production can also be induced by peroxynitrite (ONOO*) which is produced when superoxide interacts with nitric oxide (NO). By binding to and impairing the function of membrane ion (Na+ and Ca2+) transporters, HNE causes membrane depolarization and Ca2+ influx through membrane channels. Lipid peroxidation in membranes of mitochondria and endoplasmic reticulum may further disrupt cellular Ca2+ homeostasis. HNE and Ca2+ may also play pivotal roles in the aggregation of the microtubule-associated protein tau, thereby forming intracellular neurofibrillary tangles. Thus, HNE may be involved in the early changes in synaptic function, as well as the subsequent degeneration and death of neurons in Alzheimer’s disease. Modified from Figure 2 in Mattson (2004).
The possibility that dietary and lifestyle factors that promote MS and obesity, particularly excessive energy intake and a sedentary lifestyle, may increase the risk of Alzheimer’s disease is suggested by the results of epidemiological studies (Pope et al., 2003; Mattson et al., 2004). Findings from studies in which the diet or amount of exercise are varied in mouse models of Alzheimer’s disease suggest that, indeed, the disease process can be modified by conditions that promote or protect against MS. Transgenic mice that express mutant forms of human amyloid precursor protein (APP), presenilin-1 and/or tau exhibit amyloid/tau pathology, synaptic dysfunction and cognitive impairment (Gotz and Ittner, 2008). When placed on a high (saturated) fat diet (Refolo et al., 2000) or a refined sugar diet (Cao et al., 2007), APP mutant mice exhibit accelerated deposition of amyloid and cognitive impairment. Conversely, when placed on a low calorie diet, APP/presenilin-1/tau triple-mutant mice exhibit lower levels of amyloid and tau pathologies and improved cognitive function (Halagappa et al., 2007). Interestingly, the latter study also showed that an alternate day fasting diet improves cognitive function in the triple-mutant Alzheimer’s disease mice, but do not exhibit reduced amyloid or tau pathology, suggesting that this particular (anti-diabetic) diet can protect neurons downstream of Aβ production and tau aggregation. Diabetes has been shown to impair hippocampal plasticity (synaptic potentiation, neurogenesis, and learning and memory) even in the absence of Alzheimer’s disease-like pathology (Stranahan et al., 2008) suggesting that obesity and the MS may cause cognitive impairment even prior to the development of Alzheimer’s disease. Diabetic animals exhibit increased HNE levels in their brains (Lyn-Cook et al., 2009), whereas dietary energy restriction results in reduced HNE levels (Zhu et al., 1999; Dasuri et al., 2009). Exercise, which can prevent and reverse MS and obesity, may also protect the brain against Alzheimer’s disease. APP mutant mice that exercise exhibit reduced amyloid pathology and improved cognitive performance compared to their sedentary counterparts (Adlard et al., 2005; Lazarov et al., 2005; Parachikova et al., 2008). Exercise (and dietary energy restriction) can also counteract the adverse effects of MS/diabetes on synaptic plasticity and cognitive function (Stranahan et al., 2009).
Targeting HNE to Prevent and Treat Metabolic Syndrome and Associated Diseases
Because HNE appears to play important roles in the development of obesity and the MS on the one hand, and in the pathogenesis of MS-related diseases, it seems prudent to develop drugs and other treatments that target HNE. Two general strategies to accomplish this goal would be to reduce the production of HNE by suppressing LP or to inhibit/detoxify HNE downstream of LP (Table 1). Due to space limitations I will only provide a few selected examples and references. Antioxidants that inhibit membrane LP, and that have been reported effective in attenuating biomarkers and symptoms in MS, obesity and one or more associated disorders include α-lipoic acid (Kocak et al., 2000; Baydas et al., 2004), α-tocopherol (Sena et al., 2008), and coenzyme Q10 (Sena et al., 2008). Molecules that bind and remove/detoxify HNE include glutathione (Kruman et al., 1997; Mark et al., 1997b), histidine analogs similar to carnosine (Guiotto et al., 2005; Tang et al., 2007), N-acetylcysteine (Haber et al., 2003; El Midaoui et al., 2008) and taurine (Haber et al., 2003; Xiao et al., 2008). More recently, carnosine derivatives that are resistant to carnosinase, and that have increased HNE-quenching efficacy, have been developed (Vistoli et al., 2009). Such agents may eventually be used to treat a range of disorders in which lipid peroxidation and HNE play major roles.
Table 1.
Potential therapeutic interventions for MS/obesity and associated disorders that target 4-hydroxynonenal.
| Treatment | Mechanism of Action | References |
|---|---|---|
| α-lipoic acid | inhibits lipid peroxidation | Kocak et al., 2000; Baydas et al., 2004 |
| α-tocopherol | inhibits lipid peroxidation | Mark et al., 1997a; Sena et al., 2008 |
| Coenzyme Q10 | inhibits lipid peroxidation | Sena et al., 2008 |
| Mn-SOD mimetics | inhibit lipid peroxidation | Bruce et al., 1996; Ceradini et al., 2008 |
| Glutathione | binds and detoxifies HNE | Mark et al., 1997b; |
| Histidine analogs | bind and detoxify HNE | Guiotto et al., 2005; Tang et al., 2007) |
| N-acetylcysteine | binds and detoxifies HNE | Haber et al., 2003; El Midaoui et al., 2008 |
| Taurine | binds and detoxifies HNE | Haber et al., 2003; Xiao et al., 2008 |
| Carnosine analogs | bind and detoxify HNE | Vistoli et al., 2009 |
taurine with HNE is via taurine’s amino group
Another approach would be to enhance intrinsic antioxidant and detoxification systems by activating signaling pathways that upregulate the expression of antioxidant and phase-2 detoxifying enzymes. Increasing evidence suggests that some dietary phytochemicals that improve health may act via such a process called hormesis in which adaptive cellular stress responses are activated (Mattson, 2008a, 2008b). One such pathway involves the transcription factor Nrf-2 which binds to the antioxidant response element sequence upstream of genes encoding glutathione-S-transferases and the antioxidant enzymes NQO1 and heme oxygenase (Surh et al., 2008). Nrf-2 can be activated by sulforaphane and curcumin, which are phytochemicals that have been reported to have beneficial effects in animal models of diabetes, cardiovascular disease and neurodegenerative disorders (Mattson and Cheng, 2006; Siow et al., 2007). Other adaptive stress response pathways that are amenable to activation by natural products or synthetic drugs include those involving the transcription factor NF-κB (Camandola and Mattson, 2007), the sirtuin – FOXO pathway (Jiang, 2008) and the calcium/cyclic AMP – CREB pathway (Ichiki, 2006).
While efforts to develop drugs or dietary supplements that reduce HNE production or block its adverse actions should be pursued, it should also be recognized that dietary and lifestyle factors that protect against and reverse obesity and the MS also reduce LP and HNE production. Regular moderate exercise and dietary energy restriction, if practiced consistently, are very effective preventative and therapeutic interventions for MS and many of its associated diseases. Reductions in HNE production and enhanced HNE detoxification may contribute to the health benefits of exercise and dietary moderation. For example, exercise training enhanced SOD2 protein levels in the hearts of both young and old rats (Lawler et al., 2009). It was reported that alternate day caloric restriction reduces levels of HNE in serum samples from overweight patients with asthma, and this is associated with improved asthma symptoms and reductions in levels of triglycerides, cholesterol and insulin levels (Johnson et al., 2007). A study in rats showed that levels of HNE in the kidney increase during aging, and that the age-related increase in HNE accumulation is prevented by caloric restriction (Lee et al., 2004). Dietary energy restriction may also reduce HNE production and enhance HNE detoxification in brain cells as suggested by studies of rodents showing that dietary energy restriction enhances protein chaperone production and plasma membrane redox system activity in brain tissue samples (Guo et al., 2000; Hyun et al., 2006). A better understanding of the molecular mechanisms by which diet and exercise affect HNE metabolism will likely foster the development of novel and complementary approaches for preventing and treating the MS and its associated diseases, many of which are age-related.
Acknowledgments
I thank KC Alexander for editorial assistance. This work was supported by the Intramural Research Program of the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbate A, Biondi-Zoccai GG, Bussani R, Dobrina A, Camilot D, Feroce F, Rossiello R, Baldi F, Silvestri F, Biasucci LM, Baldi A. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41:753–760. doi: 10.1016/s0735-1097(02)02959-5. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Dempsey RJ. Phospholipase A2, hydroxyl radicals, and lipid peroxidation in transient cerebral ischemia. Antioxid Redox Signal. 2003;5:647–654. doi: 10.1089/152308603770310329. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggoun Y. Obesity, metabolic syndrome, and cardiovascular disease. Pediatr Res. 2007;61:653–659. doi: 10.1203/pdr.0b013e31805d8a8c. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Aldini G, Vistoli G, Regazzoni L, Gamberoni L, Facino RM, Yamaguchi S, Uchida K, Carini M. Albumin is the main nucleophilic target of human plasma: a protective role against proatherogenic electrophilic reactive carbonyl species? Chem Res Toxicol. 2008;21:824–835. doi: 10.1021/tx700349r. [DOI] [PubMed] [Google Scholar]
- Aleynik SI, Leo MA, Aleynik MK, Lieber CS. Alcohol-induced pancreatic oxidative stress: protection by phospholipid repletion. Free Radic Biol Med. 1999;26:609–619. doi: 10.1016/s0891-5849(98)00246-9. [DOI] [PubMed] [Google Scholar]
- Annangudi SP, Deng Y, Gu X, Zhang W, Crabb JW, Salomon RG. Low-density lipoprotein has an enormous capacity to bind E-4-hydroxynon-2-enal HNE: detection and characterization of lysyl and histidyl adducts containing multiple molecules of HNE. Chem Res Toxicol. 2008;21:1384–1395. doi: 10.1021/tx8000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakris G, Stockert J, Molitch M, Zhou Q, Champion A, Bacher P, Sowers J, on behalf of the STAR Investigators Risk factor assessment for new onset diabetes: literature review. Diabetes Obes Metab. 2008 Jun 16; doi: 10.1111/j.1463-1326.2008.00925.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A. 2007;143A:3016–3034. doi: 10.1002/ajmg.a.32035. [DOI] [PubMed] [Google Scholar]
- Baydas G, Donder E, Kiliboz M, Sonkaya E, Tuzcu M, Yasar A, Nedzvetskii VS. Neuroprotection by alpha-lipoic acid in streptozotocin-induced diabetes. Biochemistry (Mosc) 2004;69:1001–1005. doi: 10.1023/b:biry.0000043542.39691.95. [DOI] [PubMed] [Google Scholar]
- Benderdour M, Charron G, DeBlois D, Comte B, Des Rosiers C. Cardiac mitochondrial NADP+-isocitrate dehydrogenase is inactivated through 4-hydroxynonenal adduct formation: an event that precedes hypertrophy development. J Biol Chem. 2003;278:45154–45159. doi: 10.1074/jbc.M306285200. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. Electrophysiological effects of 4-hydroxynonenal, an aldehydic product of lipid peroxidation, on isolated rat ventricular myocytes. Circ Res. 1995;76:293–304. doi: 10.1161/01.res.76.2.293. [DOI] [PubMed] [Google Scholar]
- Blanc EM, Kelly JF, Mark RJ, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of lipid peroxidation, impairs signal transduction associated with muscarinic acetylcholine and metabotropic glutamate receptors: possible action on G alpha(q/11) J Neurochem. 1997;69:570–580. doi: 10.1046/j.1471-4159.1997.69020570.x. [DOI] [PubMed] [Google Scholar]
- Blasig IE, Grune T, Schönheit K, Rohde E, Jakstadt M, Haseloff RF, Siems WG. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. Am J Physiol. 1995;269:H14–22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- Boillée S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Malfroy B, Baudry M. beta-Amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci U S A. 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Li YJ, Lovell MA, Kraemer PJ, Gary DS, Brown RR, Markesbery WR, Mattson MP. 4-Hydroxynonenal, a product of lipid peroxidation, damages cholinergic neurons and impairs visuospatial memory in rats. J Neuropathol Exp Neurol. 1998;57:257–267. doi: 10.1097/00005072-199803000-00007. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Hensley K, Harris M, Mattson M, Carney J. beta-Amyloid peptide free radical fragments initiate synaptosomal lipoperoxidation in a sequence-specific fashion: implications to Alzheimer’s disease. Biochem Biophys Res Commun. 1994;200:710–715. doi: 10.1006/bbrc.1994.1508. [DOI] [PubMed] [Google Scholar]
- Camandola S, Mattson MP. NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007;11:123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- CDC. Diabetes 2008: disabling disease to double by 2050. Atlanta, GA: US Department of Health and Human Services, CDC; 2008. Available at http://www.cdc.gov/nccdphp/publications/aag/pdf/diabetes.pdf. [Google Scholar]
- Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006;22:423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- Chen L, Na R, Gu M, Richardson A, Ran Q. Lipid peroxidation up-regulates BACE1 expression in vivo: a possible early event of amyloidogenesis in Alzheimer’s disease. J Neurochem. 2008;107:197–207. doi: 10.1111/j.1471-4159.2008.05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasuri K, Nguyen A, Zhang L, Fernandez-Kim OS, Bruce-Keller AJ, Blalock BA, Cabo RD, Keller JN. Comparison of rat liver and brain proteasomes for oxidative stress-induced inactivation: Influence of ageing and dietary restriction. Free Radic Res. 2009;43:28–36. doi: 10.1080/10715760802534812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Wang-Polagruto J, Polagruto J, Keen CL, Jialal I. High-fat, energy-dense, fast-food-style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism. 2008;57:867–870. doi: 10.1016/j.metabol.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Midaoui A, Ismael MA, Lu H, Fantus IG, de Champlain J, Couture R. Comparative effects of N-acetyl-L-cysteine and ramipril on arterial hypertension, insulin resistance, and oxidative stress in chronically glucose-fed rats. Can J Physiol Pharmacol. 2008;86:752–760. doi: 10.1139/Y08-090. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Benedetti A, Lang J, Fulceri R, Fauler G, Comporti M. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation. Biochim Biophys Acta. 1986;876:154–166. doi: 10.1016/0005-2760(86)90329-2. [DOI] [PubMed] [Google Scholar]
- Gil-Núñez A. The metabolic syndrome and cerebrovascular disease: suspicion and evidence. Cerebrovasc Dis. 2007;24:S64–75. doi: 10.1159/000107380. [DOI] [PubMed] [Google Scholar]
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- Go YM, Halvey PJ, Hansen JM, Reed M, Pohl J, Jones DP. Reactive aldehyde modification of thioredoxin-1 activates early steps of inflammation and cell adhesion. Am J Pathol. 2007;171:1670–1681. doi: 10.2353/ajpath.2007.070218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman Y, Mattson MP. Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Exp Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- Götz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Grimsrud PA, Picklo MJ, Sr, Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6:624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- Guiotto A, Calderan A, Ruzza P, Borin G. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12:2293–2315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- Guo Z, Ersoz A, Butterfield DA, Mattson MP. Beneficial effects of dietary restriction on cerebral cortical synaptic terminals: preservation of glucose and glutamate transport and mitochondrial function after exposure to amyloid beta-peptide, iron, and 3-nitropropionic acid. J Neurochem. 2000;75:314–320. doi: 10.1046/j.1471-4159.2000.0750314.x. [DOI] [PubMed] [Google Scholar]
- Haber CA, Lam TK, Yu Z, Gupta N, Goh T, Bogdanovic E, Giacca A, Fantus IG. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. Am J Physiol Endocrinol Metab. 2003;285:E744–753. doi: 10.1152/ajpendo.00355.2002. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Harrell JS, Jessup A, Greene N. Changing our future: obesity and the metabolic syndrome in children and adolescents. J Cardiovasc Nurs. 2006;21:322–330. doi: 10.1097/00005082-200607000-00014. [DOI] [PubMed] [Google Scholar]
- Herbst U, Toborek M, Kaiser S, Mattson MP, Hennig B. 4-Hydroxynonenal induces dysfunction and apoptosis of cultured endothelial cells. J Cell Physiol. 1999;181:295–303. doi: 10.1002/(SICI)1097-4652(199911)181:2<295::AID-JCP11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Hoff HF, O’Neil J, Chisolm GM, 3rd, Cole TB, Quehenberger O, Esterbauer H, Jürgens G. Modification of low density lipoprotein with 4-hydroxynonenal induces uptake by macrophages. Arteriosclerosis. 1989;9:538–549. doi: 10.1161/01.atv.9.4.538. [DOI] [PubMed] [Google Scholar]
- Horsburgh K, McCulloch J, Nilsen M, McCracken E, Large C, Roses AD, Nicoll JA. Intraventricular infusion of apolipoprotein E ameliorates acute neuronal damage after global cerebral ischemia in mice. J Cereb Blood Flow Metab. 2000;20:458–462. doi: 10.1097/00004647-200003000-00003. [DOI] [PubMed] [Google Scholar]
- Howard BV, Ruotolo G, Robbins DC. Obesity and dyslipidemia. Endocrinol Metab Clin North Am. 2003;32:855–867. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]
- Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S, Lenhart G. Burden of illness in Parkinson’s disease. Mov Disord. 2005;20:1449–1454. doi: 10.1002/mds.20609. [DOI] [PubMed] [Google Scholar]
- Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci U S A. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiki T. Role of cAMP response element binding protein in cardiovascular remodeling: good, bad, or both? Arterioscler Thromb Vasc Biol. 2006;26:449–455. doi: 10.1161/01.ATV.0000196747.79349.d1. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Esterbauer H, Sies H. Role of cardiac glutathione transferase and of the glutathione S-conjugate export system in biotransformation of 4-hydroxynonenal in the heart. J Biol Chem. 1986;261:1576–1581. [PubMed] [Google Scholar]
- Jiang WJ. Sirtuins: novel targets for metabolic disease in drug development. Biochem Biophys Res Commun. 2008;373:341–344. doi: 10.1016/j.bbrc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- Jo DG, Arumugam TV, Woo HN, Park JS, Tang SC, Mughal M, Hyun DH, Park JH, Choi YH, Gwon AR, Camandola S, Cheng A, Cai H, Song W, Markesbery WR, Mattson MP. Evidence that gamma-secretase mediates oxidative stress-induced beta-secretase expression in Alzheimer’s disease. Neurobiol Aging. 2008 Aug 5; doi: 10.1016/j.neurobiolaging.2008.07.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakishita H, Hattori Y. Vascular smooth muscle cell activation and growth by 4-hydroxynonenal. Life Sci. 2001;69:689–697. doi: 10.1016/s0024-3205(01)01166-3. [DOI] [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Furukawa K, Barger SW, Rengen MR, Mark RJ, Blanc EM, Roth GS, Mattson MP. Amyloid beta-peptide disrupts carbachol-induced muscarinic cholinergic signal transduction in cortical neurons. Proc Natl Acad Sci U S A. 1996;93:6753–6758. doi: 10.1073/pnas.93.13.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koçak G, Aktan F, Canbolat O, Ozoğul C, Elbeğ S, Yildizoglu-Ari N, Karasu C, ADIC Study Group--Antioxidants in Diabetes-Induced Complications Alpha-lipoic acid treatment ameliorates metabolic parameters, blood pressure, vascular reactivity and morphology of vessels already damaged by streptozotocin-diabetes. Diabetes Nutr Metab. 2000;13:308–318. [PubMed] [Google Scholar]
- Kruman I, Bruce-Keller AJ, Bredesen D, Waeg G, Mattson MP. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci. 1997;17:5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastra G, Manrique C, Sowers JR. Obesity, cardiometabolic syndrome, and chronic kidney disease: the weight of the evidence. Adv Chronic Kidney Dis. 2006;13:365–373. doi: 10.1053/j.ackd.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Kanski J, Hackett JM, Maeda N, Kindy MS, Butterfield DA. Apolipoprotein E modulates Alzheimer’s Abeta(1-42)-induced oxidative damage to synaptosomes in an allele-specific manner. Brain Res. 2002;924:90–97. doi: 10.1016/s0006-8993(01)03228-0. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Kwak HB, Kim JH, Suk MH. Exercise training inducibility of MnSOD protein expression and activity is retained while reducing prooxidant signaling in the heart of senescent rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1496–502. doi: 10.1152/ajpregu.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jung KJ, Kim JW, Kim HJ, Yu BP, Chung HY. Suppression of apoptosis by calorie restriction in aged kidney. Exp Gerontol. 2004;39:1361–1368. doi: 10.1016/j.exger.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Leonarduzzi G, Chiarpotto E, Biasi F, Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 2005;49:1044–1049. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008;36:343–347. doi: 10.1042/BST0360343. [DOI] [PubMed] [Google Scholar]
- Lin S, Rhodes PG, Lei M, Zhang F, Cai Z. alpha-Phenyl-n-tert-butyl-nitrone attenuates hypoxic-ischemic white matter injury in the neonatal rat brain. Brain Res. 2004;1007:132–141. doi: 10.1016/j.brainres.2004.01.074. [DOI] [PubMed] [Google Scholar]
- Liu W, Akhand AA, Kato M, Yokoyama I, Miyata T, Kurokawa K, Uchida K, Nakashima I. 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J Cell Sci. 1999;112:2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Ehmann WD, Mattson MP, Markesbery WR. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol Aging. 1997;18:457–461. doi: 10.1016/s0197-4580(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Lu C, Chan SL, Fu W, Mattson MP. The lipid peroxidation product 4-hydroxynonenal facilitates opening of voltage-dependent Ca2+ channels in neurons by increasing protein tyrosine phosphorylation. J Biol Chem. 2002;277:24368–24375. doi: 10.1074/jbc.M201924200. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- Lyn-Cook LE, Jr, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, Mark P, Wands JR, Xu H, de la Monte SM. Hepatic Ceramide May Mediate Brain Insulin Resistance and Neurodegeneration in Type 2 Diabetes and Non-alcoholic Steatohepatitis. J Alzheimers Dis. 2009;16:715–729. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997a;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997b;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Martin B, Golden E, Keselman A, Stone M, Mattson MP, Egan JM, Maudsley S. Therapeutic perspectives for the treatment of Huntington’s disease: treating the whole body. Histol Histopathol. 2008a;23:237–250. doi: 10.14670/hh-23.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, Frank BP, Thomas S, Chadwick WA, Greig NH, Bates GP, Sathasivam K, Bernier M, Maudsley S, Mattson MP, Egan JM. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies and extends survival in a mouse model of Huntington’s disease. Diabetes. 2008b Nov 4; doi: 10.2337/db08-0799. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx (Neurotherapeutics) 2004;1:111–116. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal. 2006;8:1997–2006. doi: 10.1089/ars.2006.8.1997. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Awareness of hormesis will enhance future research in basic and applied neuroscience. Crit Rev Toxicol. 2008a;38:633–639. doi: 10.1080/10408440802026406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008b;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKracken E, Graham DI, Nilsen M, Stewart J, Nicoll JA, Horsburgh K. 4-Hydroxynonenal immunoreactivity is increased in human hippocampus after global ischemia. Brain Pathol. 2001;11:414–421. doi: 10.1111/j.1750-3639.2001.tb00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquette B, Shi Q, Lavigne P, Ranger P, Fernandes JC, Benderdour M. Production of lipid peroxidation products in osteoarthritic tissues: new evidence linking 4-hydroxynonenal to cartilage degradation. Arthritis Rheum. 2006;54:271–281. doi: 10.1002/art.21559. [DOI] [PubMed] [Google Scholar]
- Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol. 2008;104:708–715. doi: 10.1152/japplphysiol.01034.2007. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Yokota T, Takahashi T, Uchihara T, Jishage K, Mizusawa H. Deletion of vitamin E enhances phenotype of Alzheimer disease model mouse. Biochem Biophys Res Commun. 2006;350:530–536. doi: 10.1016/j.bbrc.2006.09.083. [DOI] [PubMed] [Google Scholar]
- Okun E, Arumugam TV, Tang SC, Gleichmann M, Albeck M, Sredni B, Mattson MP. The organotellurium compound ammonium trichloro(dioxoethylene-0,0’) tellurate enhances neuronal survival and improves functional outcome in an ischemic stroke model in mice. J Neurochem. 2007;102:1232–1241. doi: 10.1111/j.1471-4159.2007.04615.x. [DOI] [PubMed] [Google Scholar]
- Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30:121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, Eberstein JA. Metabolic syndrome and the role of dietary lifestyles in Alzheimer’s disease. J Neurochem. 2008;106:1503–1514. doi: 10.1111/j.1471-4159.2008.05454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlas N, Małecki A. Effects of ebselen on glutathione level in neurons exposed to arachidonic acid and 4-hydroxynonenal during simulated ischemia in vitro. Pharmacol Rep. 2007;59:708–714. [PubMed] [Google Scholar]
- Pedersen WA, Chan SL, Mattson MP. A mechanism for the neuroprotective effect of apolipoprotein E: isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 2000;74:1426–1433. doi: 10.1046/j.1471-4159.2000.0741426.x. [DOI] [PubMed] [Google Scholar]
- Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal. 2007;9:955–969. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Fai Poon H, Hensley K, Pierce WM, Klein JB, Calabrese V, De Marco C, Butterfield DA. Proteomic analysis of 4-hydroxy-2-nonenal-modified proteins in G93A-SOD1 transgenic mice--a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2005;38:960–968. doi: 10.1016/j.freeradbiomed.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med Res Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- Pope SK, Shue VM, Beck C. Will a healthy lifestyle help prevent Alzheimer’s disease? Annu Rev Public Health. 2003;24:111–132. doi: 10.1146/annurev.publhealth.24.100901.141015. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Russell AP, Gastaldi G, Bobbioni-Harsch E, Arboit P, Gobelet C, Dériaz O, Golay A, Witztum JL, Giacobino JP. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? FEBS Lett. 2003;551:104–106. doi: 10.1016/s0014-5793(03)00875-5. [DOI] [PubMed] [Google Scholar]
- Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. 2006;26:232–244. doi: 10.1159/000093632. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem. 2008;283:15539–15543. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ, Holman RC, Bresee JS, Kochanek KD, Schonberger LB. Amyotrophic lateral sclerosis mortality in the United States, 1979-2001. Neuroepidemiology. 2005;25:144–152. doi: 10.1159/000086679. [DOI] [PubMed] [Google Scholar]
- Sena CM, Nunes E, Gomes A, Santos MS, Proença T, Martins MI, Seiça RM. Supplementation of coenzyme Q10 and alpha-tocopherol lowers glycated hemoglobin level and lipid peroxidation in pancreas of diabetic rats. Nutr Res. 2008;28:113–121. doi: 10.1016/j.nutres.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Singh SP, Niemczyk M, Saini D, Awasthi YC, Zimniak L, Zimniak P. Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry. 2008;47:3900–3911. doi: 10.1021/bi702124u. [DOI] [PubMed] [Google Scholar]
- Singh SP, Niemczyk M, Zimniak L, Zimniak P. Fat accumulation in Caenorhabditis elegans triggered by the electrophilic lipid peroxidation product 4-hydroxynonenal (4-HNE) Aging. 2009;1:68–80. doi: 10.18632/aging.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Yadav S, Roth C, Singhal J. RLIP76: A novel glutathione-conjugate and multi-drug transporter. Biochem Pharmacol. 2009;77:761–769. doi: 10.1016/j.bcp.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton JR, Smith AG. Therapy insight: neurological complications of prediabetes. Nat Clin Pract Neurol. 2006;2:276–282. doi: 10.1038/ncpneuro0172. [DOI] [PubMed] [Google Scholar]
- Siow RC, Ishii T, Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–15. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- Soh Y, Jeong KS, Lee IJ, Bae MA, Kim YC, Song BJ. Selective activation of the c-Jun N-terminal protein kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12 cells. Mol Pharmacol. 2000;58:535–541. doi: 10.1124/mol.58.3.535. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Chandra A, Ansari NH, Srivastava SK, Bhatnagar A. Identification of cardiac oxidoreductases involved in the metabolism of the lipid peroxidation-derived aldehyde-4-hydroxynonenal. Biochem J. 1998;329:469–475. doi: 10.1042/bj3290469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009 Mar 11; doi: 10.1002/hipo.20577. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;2:82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- Takizawa S, Matsushima K, Shinohara Y, Ogawa S, Komatsu N, Utsunomiya H, Watanabe K. Immunohistochemical localization of glutathione peroxidase in infarcted human brain. J Neurol Sci. 1994;122:66–73. doi: 10.1016/0022-510x(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo DG, Mattson MP, Tabaton M. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104:683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Cutler RG, Jo DG, Magnus T, Chan SL, Mughal MR, Telljohann RS, Nassar M, Ouyang X, Calderan A, Ruzza P, Guiotto A, Mattson MP. Neuroprotective actions of a histidine analogue in models of ischemic stroke. J Neurochem. 2007;101:729–736. doi: 10.1111/j.1471-4159.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- Tang SC, Lathia JD, Selvaraj PK, Jo DG, Mughal MR, Cheng A, Siler DA, Markesbery WR, Arumugam TV, Mattson MP. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol. 2008;213:114–121. doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi A, Ovbiagele B. Metabolic syndrome and stroke. Curr Diab Rep. 2008;8:37–41. doi: 10.1007/s11892-008-0008-z. [DOI] [PubMed] [Google Scholar]
- Urabe T, Yamasaki Y, Hattori N, Yoshikawa M, Uchida K, Mizuno Y. Accumulation of 4-hydroxynonenal-modified proteins in hippocampal CA1 pyramidal neurons precedes delayed neuronal damage in the gerbil brain. Neuroscience. 2000;100:241–250. doi: 10.1016/s0306-4522(00)00264-5. [DOI] [PubMed] [Google Scholar]
- VanWinkle WB, Snuggs M, Miller JC, Buja LM. Cytoskeletal alterations in cultured cardiomyocytes following exposure to the lipid peroxidation product, 4-hydroxynonenal. Cell Motil Cytoskeleton. 1994;28:119–134. doi: 10.1002/cm.970280204. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Powers SK, Dirks AJ, Scarpace PJ. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord. 2001;25:378–388. doi: 10.1038/sj.ijo.0801536. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- Vistoli G, Orioli M, Pedretti A, Regazzoni L, Canevotti R, Negrisoli G, Carini M, Aldini G. Design, synthesis, and evaluation of carnosine derivatives as selective and efficient sequestering agents of cytotoxic reactive carbonyl species. ChemMedChem. 2009;4:967–975. doi: 10.1002/cmdc.200800433. [DOI] [PubMed] [Google Scholar]
- Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Wonisch W, Zellnig G, Kohlwein SD, Schaur RJ, Bilinski T, Tatzber F, Esterbauer H. Ultrastructural analysis of HNE-treated Saccharomyces cerevisiae cells reveals fragmentation of the vacuole and an accumulation of lipids in the cytosol. Cell Biochem Funct. 2001;19:59–64. doi: 10.1002/cbf.888. [DOI] [PubMed] [Google Scholar]
- Xiao C, Giacca A, Lewis GF. Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia. 2008;51:139–146. doi: 10.1007/s00125-007-0859-x. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MR, Im DS, Lee SJ, Woo JW, Hong KW, Bae SS, Kim CD. 4-hydroxynonenal contributes to macrophage foam cell formation through increased expression of class A scavenger receptor at the level of translation. Free Radic Biol Med. 2008;45:177–183. doi: 10.1016/j.freeradbiomed.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Zarrouki B, Soares AF, Guichardant M, Lagarde M, Géloën A. The lipid peroxidation end-product 4-HNE induces COX-2 expression through p38MAPK activation in 3T3-L1 adipose cell. FEBS Lett. 2007;581:2394–2400. doi: 10.1016/j.febslet.2007.04.048. [DOI] [PubMed] [Google Scholar]
- Zhu H, Guo Q, Mattson MP. Dietary restriction protects hippocampal neurons against the death-promoting action of a presenilin-1 mutation. Brain Res. 1999;842:224–229. doi: 10.1016/s0006-8993(99)01827-2. [DOI] [PubMed] [Google Scholar]


