Abstract
Background and Purpose
Migraine with aura is a risk factor for ischemic stroke but the mechanism by which these disorders are associated remains unclear. Both disorders exhibit familial clustering, which may imply a genetic influence on migraine and stroke risk. Genes encoding for endothelial function are promising candidate genes for migraine and stroke susceptibility because of the importance of endothelial function in regulating vascular tone and cerebral blood flow.
Methods
Using data from the Stroke Prevention in Young Women (SPYW) study, a population-based case-control study including 297 women aged 15–49 years with ischemic stroke and 422 women without stroke, we evaluated whether polymorphisms in genes regulating endothelial function, including endothelin-1 (EDN), endothelin receptor type B (EDNRB), and nitric oxide synthase-3 (NOS3), confer susceptibility to migraine and stroke.
Results
EDN SNPs rs1800542 and rs10478723 were associated with increased stroke susceptibility in Caucasians, (OR = 2.1 (95% CI, 1.1 to 4.2) and OR = 2.2 (95% CI, 1.1 to 4.4); p = 0.02 and 0.02, respectively) as were EDNRB SNPs rs4885493 and rs10507875, (OR = 1.7 (95% CI, 1.1 to 2.7) and OR = 2.4 (95% CI, 1.4 to 4.3); p = 0.01 and 0.002, respectively). Only one of the tested SNPs (NOS3 - rs3918166) was associated with both migraine and stroke.
Conclusions
In our study population, variants in EDN and EDNRB were associated with stroke susceptibility in Caucasian but not in African-American women. We found no evidence that these genes mediate the association between migraine and stroke.
Introduction
Several observational studies have shown that migraine is a risk factor for ischemic stroke, specifically among young women,1–4 and at least one study has reported that women with a family history of migraine are at increased risk of stroke, regardless of their personal history of migraine.4 The mechanism by which these disorders are associated remains unclear. However, there appears to be a genetic contribution to both disorders,5–9 which may imply a genetic influence on the association between migraine and stroke in young adults.
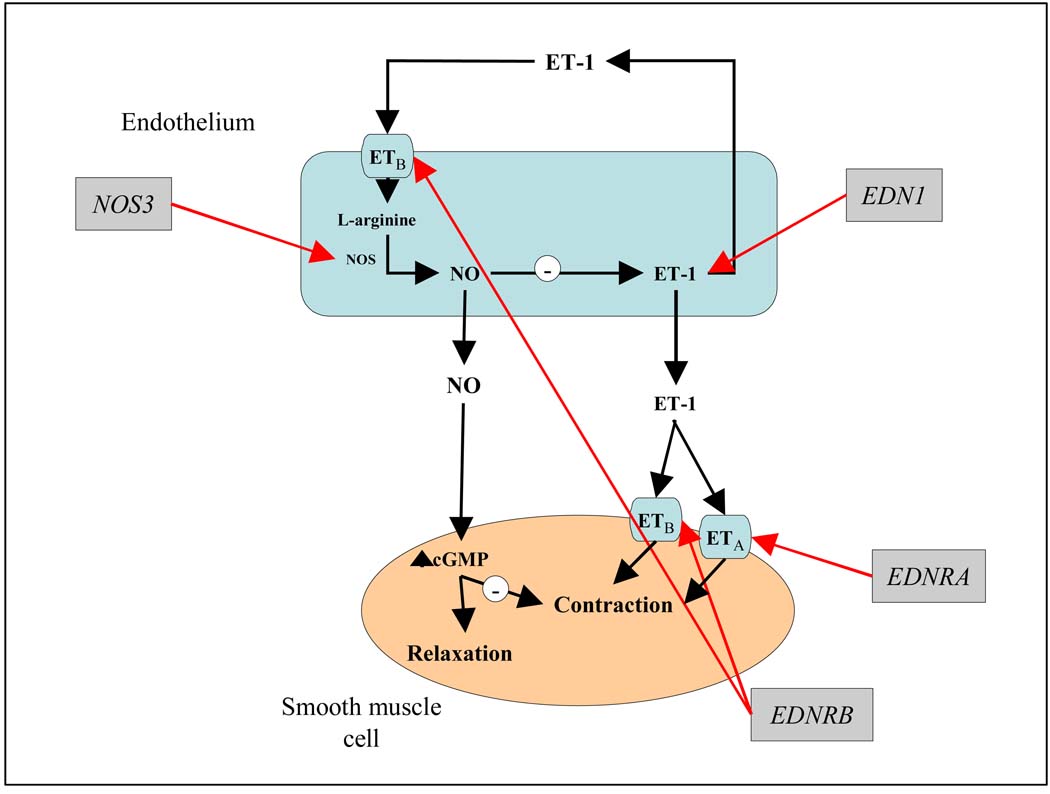
Genes encoding for endothelial function are promising candidate genes for both migraine and stroke susceptibility because of the importance of endothelial function in regulating vasoconstriction and vasodilation of blood vessels and cerebral blood flow. The nitric oxide synthase-3 (NOS3) gene encodes for nitric oxide (NO) synthase, which is responsible for the conversion of L-arginine to NO, a primary vasodilator and regulator of blood flow and vascular tone.10–12 The endothelin-1 (EDN) gene encodes for endothelin-1 (ET-1), a primary vasoconstrictor of vascular smooth muscle cells. Depending on the potency and type of blood vessel, ET-1 may also cause vasodilation via ETB receptors on endothelial cells, (encoded for by the endothelin receptor type B (EDNRB) gene), an action that is mediated by NO.10–12 Therefore, NO and ET-1 released from endothelial cells act reciprocally to maintain vascular tone (Figure 1).
Figure 1.
Candidate genes encoding for endothelial function.
Previous studies have reported on the role of impaired NO dependent vasodilation in the pathophysiology of migraine13 and stroke,10, 14, 15 ET-1 dose-dependent ischemia,16 and associations between ET-1 plasma levels and migraine.17, 18 Consequently, variants in genes that encode for endothelial function have been studied in relation to migraine,19–21 stroke,22–24 and related conditions such as high blood pressure,25, 26 pulse pressure27 and myocardial infarction.28 However a systematic study has not been done to assess the individual or combined contribution of these variants to stroke or to migraine as a risk factor for ischemic stroke. We have sought to evaluate whether polymorphisms in genes regulating endothelial function and vascular tone (NOS3, EDN, and EDNRB) may confer susceptibility to both migraine and stroke in a large biracial population-based case-control study of young onset-stroke carried out in young women. In our analyses we considered risk factor status, and migraine, as well as stroke subtype on an exploratory basis.
Materials and Methods
Study population
The Stroke Prevention in Young Women Study (SPYW) is a population-based case-control study initiated to examine risk factors for ischemic stroke in young women. Study recruitment and data collection occurred in two waves: recruitment for SPYW-1 was conducted between 1992 and 1996, and recruitment for SPYW-2 was conducted between 2001and 2003. In both waves, cases were women hospitalized with a first cerebral infarction identified by discharge surveillance from one of 59 hospitals in the greater Baltimore-Washington area and direct referral from regional neurologists. The methods for discharge surveillance, chart abstraction, case adjudication, and assignment of probable and possible underlying causes have been described elsewhere.29–31 Control subjects were women with no history of stroke identified by random-digit dialing and were matched to cases by age (within ten years) and geographic region of residence in both waves and, additionally matched for race in SPYW-2. SPYW-1 included cases ages 15–44 years recruited within one year of stroke and was designed with a 1:2 case to control ratio. SPYW-2 included cases ages 15–49 recruited within three years of stroke and was designed with a 1:1 case to control ratio. For both study periods, additional cases were recruited after completion of control recruitment.
SPYW participants for whom DNA was available includes 743 subjects (321 cases and 423 controls). Subjects were excluded from the current study if they had genetic or other known specific etiologies for their stroke that would impair detection of new genetic associations. These conditions were: sickle cell disease, sickle thalasemia disease, CNS vasculitis by angiogram and clinical criteria, endocarditis, neurosyphillis, mechanical heart valve, post-radiation arteriopathy, and cocaine use within 48 hours of stroke. These criteria resulted in the exclusion of 24 cases and 1 control, leaving 297 cases and 422 controls in these analyses.
A pair of neurologists evaluated each case to establish ischemic stroke, with disagreements resolved by a third neurologist. Methods for stroke subtype categories have been previously described 29, 31 and include: 1) large artery atherosclerosis, 2) cardioembolism, 3) lacunar stroke, 4) stroke of other determined etiology, and 5) stroke of undetermined etiology. Lifetime headache history was collected from case and control subjects by standardized questionnaire as described previously.32 Briefly, subjects were classified as having migraine with visual aura if they (1) reported ever seeing spots, lines, or flashing lights around the time of their probable migraine or (2) if they reported ever experiencing loss of vision and also reported a frequency of probable migraine with visual aura of at least twice per year. Subjects were identified as having probable migraine without visual aura if they reported no history of visual aura and reported nausea, vomiting, or sensitivity to light during a probable migraine and probable migraine frequency of at least five times per year. Traditional stroke risk factors and other study variables, including age, ethnicity, and history of hypertension, diabetes, myocardial infarction (MI), current smoking status, and current oral contraceptive (OC) use (both defined as use within one month of stroke event for cases and at time of interview for controls), were also collected during standardized interview and were included as covariates in our analyses.
This study was approved by Institutional Review Boards at the University of Maryland, the Centers for Disease Control and Prevention, and at all participating hospitals. Each patient gave written informed consent prior to enrollment.
SNP Selection and Genotyping Methods
SNP selection and genotyping methods for NOS3 have been previously described.24, 33 For EDN, and EDNRB genes, we identified haplotype tag SNPs (htSNPs) from the International HapMap Project European Caucasian and Yoruban data.34 Using the Tagger program, we specified the pairwise tagging method,35 an r2 of 0.8 or greater, and a minor allele frequency of at least 5% from the two populations. The chromosome location, size, number of exons, known SNPs and htSNPs for each candidate gene is shown in Table 1. All alleles for EDN and EDNRB SNPs were determined using the Taqman method developed by Applied Biosystems Inc. (Foster City, CA).
Table 1.
Size, chromosome location, SNPs and tagSNPs of candidate genes
| Gene | Size | Chromosome | Exons | Known SNPs with MAF > 5% |
Number of haplotype bins |
Number of bins tested (AA/CAU) |
|---|---|---|---|---|---|---|
| NOS3 | 23,530 bp | 7 | 26 | 15 / 5 | 13 / 7 | 5 / 2 |
| EDN | 6,119 bp | 6 | 5 | 10 / 5 | 6 / 4 | 6 / 4 |
| EDNRB | 24,288 bp | 13 | 7 | 75 / 66 | 20 / 9 | 11 / 7 |
bp: basepairs; MAF: minor allele frequency; AA: African Americans; CAU: Caucasians
Statistical and Genetic Analyses
We compared risk factor distributions between stroke cases and controls using t-tests for continuous variables and chi-square tests for categorical variables. All polymorphisms were biallelic; therefore frequencies of minor alleles were compared between stroke cases and controls and between women with and without history of migraine and migraine with aura using chi-square. For our primary analyses, we used a race-stratified additive model adjusted for age and geographic region to test the effect of genotype on ischemic stroke. A fully adjusted risk factor model (age, geographic region, smoking, diabetes, hypertension, myocardial infarction, and oral contraceptive use) was also evaluated. Stroke subtype analyses were also performed, however, given the small samples sizes these were considered exploratory or hypothesis generating in nature.
To test whether identified variants mediated the association between migraine and stroke, we modeled the probability of stroke given migraine, with stroke as the response (dependent) variable and history of migraine as the study (independent) variable, with and without the variant present in the model. We compared the outcomes to evaluate whether the association between migraine and stroke remained elevated when variants were controlled for. If the association between migraine and stroke weakened after adjustment for allelic variation, this suggested that the identified htSNP at least partially explains the association between migraine and stroke in our study.
All statistical analyses were performed using SAS version 8.2 software.37 Hardy-Weinberg equilibrium and linkage disequilibrium (LD) were calculated with Haploview version 3.236
Two-tailed p-values of <0.05 were considered statistically significant. In order to test whether the overall difference in allele distribution between cases and controls was greater than might be expected by chance, taking into account multiple comparisons, we used Haploview36 to randomly permute case and control status, simulating 10,000 race-stratified data sets for each candidate gene. A summary chi-square distribution was obtained for the simulated data sets and compared to that of our observed data.
Results
Clinical characteristics of stroke cases (n = 297) and non-stroke controls (n = 422) are shown in Table 2. Cases were slightly older on average compared to controls and were more likely to be of African-American ethnicity. As expected, cases were also more likely than controls to report a history of hypertension, diabetes, and myocardial infarction and were more likely to be current smokers and current OC users. Cumulatively, cases were also more likely than controls to report a history of migraine, including migraine with visual aura.
Table 2.
Demographic and clinical characteristics of stroke cases and controls in the Stroke Prevention in Young Women Study.
| Cases (n = 297) | Controls (n = 422) | p-value | |
|---|---|---|---|
| Age | 40.0 ± 7.9 | 37.9 ± 7.5 | 0.0003 |
| African American | 135 (45%) | 169 (40%) | 0.170 |
| Hyptertension | 108 (36%) | 58 (14%) | <0.0001 |
| Diabetes | 46 (15%) | 21 (5%) | <0.0001 |
| MI or Angina | 38 (13%) | 15 (4%) | <0.0001 |
| Smoking | 138 (47%) | 113 (27%) | <0.0001 |
| Use Oral Contraceptives | 39 (13%) | 33 (8%) | 0.03 |
| Migraine | |||
| No migraine | 152 (51%) | 255 (60%) | 0.02 |
| Migraine no aura | 20 (7%) | 49 (12%) | 0.02 |
| Migraine with aura | 125 (42%) | 118 (28%) | 0.0001 |
SNPs analyzed from the NOS3, EDN, and EDNRB genes and their call rates are shown in Table 3 to Table 5 in order of physical location within the gene. In addition, race-stratified minor allele frequencies and Hardy-Weinberg chi-square and p-values are given. The SNP selection strategy used for NOS3 resulted in the selection of 22 SNPs with an average intra-SNP distance of 2.1kb and a maximum intra-SNP distance of 5kb.33 As mentioned, we used HapMap data to identify htSNPs for EDN and EDNRB. The number of haplotype bins or htSNPs that were identified and the number that we tested from each gene are shown in Table 1. For EDN, we identified six haplotype bins from the Yoruban data and 4 from the European Caucasian data. HtSNPs from each of the identified bins in EDN were tested in our analyses. For EDNRB, 20 haplotype bins were identified in Yorubans and 9 were identified in European Caucasians. Of these, htSNPs from 11 of the Yoruban haplotype bins and 7 of the European Caucasian haplotype bins were tested, resulting in moderate coverage.
Table 3.
SNPs analyzed from NOS3 gene stratified by race and case/control status.
| Marker | Position | Call rate | Allele Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African American | Caucasian | |||||||||||
| Cases | n | Controls | n | HW pvalue | Cases | n | Controls | n | HW pvalue | |||
| rs2373962 (C/G) | 150118625 | 0.90 | 0.12 | 126 | 0.13 | 157 | 0.87 | 0.41 | 129 | 0.39 | 196 | 0.85 |
| rs10277237 (A/G) | 150120992 | 0.92 | 0.48 | 125 | 0.45 | 166 | 0.95 | 0.24 | 129 | 0.22 | 210 | 0.64 |
| rs6946415 (A/G) | 150122196 | 0.93 | 0.10 | 129 | 0.14 | 164 | 0.26 | 0.41 | 133 | 0.38 | 212 | 0.58 |
| rs1800783 (A/T) | 150127045 | 0.96 | 0.40 | 135 | 0.45 | 168 | 0.92 | 0.42 | 135 | 0.41 | 217 | 0.44 |
| rs1800779 (A/G) | 150127591 | 0.96 | 0.14 | 134 | 0.23 | 169 | 0.38 | 0.41 | 135 | 0.40 | 217 | 0.32 |
| rs2070744 (C/T) | 150127727 | 0.92 | 0.13 | 126 | 0.23 | 163 | 0.45 | 0.42 | 133 | 0.40 | 207 | 0.65 |
| rs3918162 (A/G) | 150127934 | 0.91 | 0.04 | 128 | 0.02 | 156 | 1.00 | 0.00 | 133 | 0.00 | 203 | 1.00 |
| rs3918166 (A/G) | 150131204 | 0.95 | 0.07 | 134 | 0.02 | 166 | 1.00 | 0.00 | 132 | 0.00 | 215 | 1.00 |
| rs1799983 (G/T) | 150133759 | 0.95 | 0.14 | 134 | 0.16 | 164 | 0.74 | 0.33 | 132 | 0.31 | 217 | 0.63 |
| rs1800780 (A/G) | 150136527 | 0.94 | 0.45 | 131 | 0.41 | 168 | 0.62 | 0.48 | 133 | 0.45 | 213 | 0.63 |
| rs3918184 (C/T) | 150139867 | 0.95 | 0.40 | 132 | 0.40 | 168 | 0.43 | 0.35 | 135 | 0.40 | 215 | 0.33 |
| rs7792133 (A/G) | 150141894 | 0.94 | 0.00 | 129 | 0.01 | 168 | 1.00 | 0.00 | 134 | 0.00 | 214 | 1.00 |
| rs3730305 (A/C) | 150142048 | 0.96 | 0.20 | 135 | 0.18 | 168 | 0.66 | 0.08 | 135 | 0.11 | 219 | 0.30 |
| rs3918232 (A/G) | 150144288 | 0.95 | 0.00 | 132 | 0.00 | 168 | 1.00 | 0.00 | 133 | 0.00 | 215 | 1.00 |
| rs3918201 (G/T) | 150144992 | 0.91 | 0.02 | 126 | 0.03 | 155 | 0.30 | 0.01 | 133 | 0.00 | 201 | 0.00 |
| rs3918211 (A/G) | 150148555 | 0.96 | 0.15 | 134 | 0.15 | 169 | 0.82 | 0.01 | 135 | 0.00 | 219 | 0.01 |
| rs3918220 (C/G) | 150151104 | 0.95 | 0.05 | 132 | 0.04 | 169 | 1.00 | 0.00 | 135 | 0.00 | 216 | 1.00 |
| rs3800787 (C/G) | 150151284 | 0.90 | 0.11 | 126 | 0.15 | 157 | 0.37 | 0.37 | 130 | 0.39 | 197 | 0.13 |
| rs6464119 (A/G) | 150154801 | 0.94 | 0.16 | 130 | 0.17 | 167 | 1.00 | 0.26 | 135 | 0.27 | 213 | 0.99 |
| rs11769158 (C/T) | 150159815 | 0.95 | 0.03 | 132 | 0.04 | 168 | 1.00 | 0.13 | 133 | 0.12 | 214 | 0.44 |
| rs3763486 (A/G) | 150160913 | 0.91 | 0.23 | 127 | 0.25 | 159 | 1.00 | 0.17 | 133 | 0.19 | 200 | 0.71 |
| rs2303922 (A/C) | 150163370 | 0.94 | 0.46 | 133 | 0.48 | 166 | 0.16 | 0.34 | 134 | 0.37 | 214 | 1.00 |
Table 5.
SNPs analyzed from EDNRB gene stratified by race and case/control status.
| Marker | Position† | Call rate | Allele Frequency* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African American | Caucasian | |||||||||||
| Cases | n | Controls | n | HW pvalue | Cases | n | Controls | n | HW pvalue | |||
| RS4885491 (A/G) | 77368351 | 0.62 | 0.08 | 91 | 0.10 | 99 | 1.00 | 0.16 | 88 | 0.12 | 138 | 0.78 |
| RS12585038 (A/G) | 77378576 | 0.96 | 0.15 | 127 | 0.14 | 140 | 0.12 | 0.11 | 133 | 0.18 | 200 | 0.40 |
| RS3027111 (C/T) | 77379869 | 0.97 | 0.23 | 123 | 0.23 | 145 | 0.16 | 0.11 | 134 | 0.20 | 203 | 0.58 |
| RS4885493 (C/G) | 77381064 | 0.84 | 0.45 | 122 | 0.45 | 131 | 1.00 | 0.19 | 122 | 0.31 | 185 | 0.96 |
| RS7982910 (C/T) | 77382132 | 0.85 | 0.10 | 120 | 0.13 | 138 | 1.00 | 0.47 | 122 | 0.42 | 188 | 0.61 |
| RS3027129 (C/T) | 77384402 | 0.95 | 0.01 | 118 | 0.01 | 142 | 1.00 | 0.10 | 129 | 0.08 | 200 | 0.48 |
| RS9544636 (C/T) | 77393935 | 0.98 | 0.13 | 127 | 0.12 | 146 | 1.00 | 0.09 | 133 | 0.11 | 208 | 0.05 |
| RS9544634 (A/G) | 77397570 | 0.99 | 0.04 | 127 | 0.02 | 147 | 0.40 | 0.08 | 136 | 0.10 | 204 | 0.11 |
| RS17068573 (A/G) | 77399614 | 0.98 | 0.28 | 126 | 0.26 | 146 | 0.88 | 0.10 | 134 | 0.18 | 205 | 0.07 |
| RS12720154 (C/T) | 77401188 | 0.98 | 0.16 | 126 | 0.16 | 145 | 0.22 | 0.10 | 135 | 0.18 | 204 | 0.17 |
| RS11618814 (C/T) | 77402775 | 0.96 | 0.42 | 124 | 0.36 | 144 | 0.00 | 0.46 | 131 | 0.34 | 202 | 0.00 |
| RS9574124 (C/G) | 77403587 | 0.97 | 0.47 | 115 | 0.47 | 122 | 0.00 | 0.31 | 120 | 0.37 | 164 | 0.05 |
| RS10507875 (A/G) | 77415255 | 0.85 | 0.15 | 122 | 0.16 | 137 | 0.78 | 0.09 | 123 | 0.17 | 186 | 0.21 |
| RS1924914 (A/G) | 77420315 | 0.87 | 0.42 | 121 | 0.45 | 138 | 0.72 | 0.26 | 130 | 0.29 | 197 | 0.01 |
| RS7319342 (A/G) | 77421485 | 0.37 | 0.44 | 42 | 0.39 | 46 | 0.00 | 0.36 | 59 | 0.42 | 114 | 0.00 |
| RS1537063 (C/T) | 77426235 | 0.90 | 0.03 | 126 | 0.01 | 139 | 0.17 | 0.08 | 129 | 0.10 | 193 | 1.00 |
| RS11838546 (A/G) | 77429615 | 0.91 | 0.18 | 128 | 0.18 | 147 | 0.65 | 0.11 | 134 | 0.12 | 201 | 0.67 |
| RS4884075 (A/C) | 77439622 | 0.96 | 0.37 | 125 | 0.38 | 143 | 0.25 | 0.11 | 130 | 0.18 | 198 | 0.21 |
As listed by minor allele frequency (bolded in column 1).
Postion refers to NCBI Build 36.2
Associations between NOS3 polymorphisms and stroke risk in our study population have been previously published.24, 33 However, in the current analyses, we stratified these associations by stroke subtype and found that rs2070744 and rs3918166 were both associated with stroke of undetermined etiology in African-Americans, (N= 286, case/control = 123/163, OR = 0.5, 95% CI: 0.2 to 0.9, p= 0.01) and (N= 296, case/control = 130/166, OR = 2.9, 95% CI: 1.0 to 8.0, p= 0.02), respectively but not among Caucasians.
We also found an association between EDN htSNP rs1800542 and increased stroke risk among Caucasian women; those with the minor allele (allele A) had a 2.1-fold increase in the odds of stroke (95% CI: 1.1 to 4.2, p = 0.03) compared to GG homozygotes. This association remained statistically significant in our full model after adjustment for risk factors (Table 6). In exploratory stroke subtype analyses, rs1800542 was significantly associated with cardioembolic stroke in Caucasians (N=211, case/control = 9/202, OR = 6.2, 95% CI: 1.8 to 21.7, p = 0.005).
Table 6.
Effect of EDN and EDNRB polymorphisms on risk of stroke (odds ratios, 95% confidence intervals and p-values).
| Marker | Cases/Controls | Ischemic Stroke | |||
|---|---|---|---|---|---|
| Minimally adjusted model* | Full model† | ||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| EDN | |||||
| rs1800542 | |||||
| African-American | 122/147 | 1.0 (0.7 – 1.5) | 0.970 | 1.0 (0.7 – 1.5) | 0.991 |
| Caucasian | 134/202 | 2.1 (1.1 – 4.2) | 0.030 | 2.3 (1.1 – 4.8) | 0.024 |
| EDNRB | |||||
| rs4885493 | |||||
| African-American | 118/131 | 1.0 (0.7 – 1.4) | 0.972 | 1.1 (0.7 – 1.7) | 0.619 |
| Caucasian | 121/185 | 0.5 (0.3 – 0.8) | 0.002 | 0.6 (0.4 – 0.9) | 0.010 |
| rs10507875 | |||||
| African-American | 118/137 | 0.9 (0.6 – 1.5) | 0.746 | 1.0 (0.6 – 1.7) | 0.872 |
| Caucasian | 123/186 | 0.5 (0.3 – 0.8) | 0.004 | 0.4 (0.2 – 0.7) | 0.002 |
Adjusted for study period, age, and geographic region
Adjusted for study period, age, geographic region, smoking, diabetes, hyptertension, MI, and oral contraceptive use
Two EDNRB htSNPs (rs4885493 and rs10507875) were significantly associated with stroke in Caucasian women (Table 6). Both associations remained statistically significant after adjustment for risk factors in our full model and both were associated with stroke of undetermined etiology in the exploratory stroke subtype analyses after adjustment for risk factors (Table 7).
Table 7.
Effect of EDNRB polymorphisms on risk of stroke of undetermined etiology (odds ratios, 95% confidence intervals and p-values).
| Marker | Cases/Controls | Stroke of Undetermined Etiology | |
|---|---|---|---|
| Full model* | |||
| OR (95% CI) | p-value | ||
| rs4885493 | |||
| African-American | 58/131 | 1.2 (0.7 – 1.9) | 0.478 |
| Caucasian | 72/185 | 0.5 (0.3 – 0.9) | 0.013 |
| rs10507875 | |||
| African-American | 59/137 | 0.8 (0.4 – 1.5) | 0.448 |
| Caucasian | 76/186 | 0.5 (0.2 – 0.9) | 0.018 |
Adjusted for study period, age, geographic region, smoking, diabetes, hyptertension, MI, and oral contraceptive use
Due to the ligand/receptor relationship between ET-1 and ETB, we considered a possible interaction between EDN and EDNRB. We tested two potential interactions in Caucasians, one between rs1800542 from EDN and rs10507875 from EDNRB, and one between rs1800542 and rs4885493 from EDNRB. We did not find an association in either instance (Breslow-Day Test for Homogeneity of the Odds Ratios chi-square = 0.07, p = 0.78 and chi-square = 1.92, p = 0.17 respectively).
With regard to migraine risk, one tested NOS3 SNP showed an association with migraine: the minor allele A in rs3918166 was more common among African-Americans with migraine with visual aura (13%), than without (6%) (x2 = 4.8, p = 0.03). Since rs3918166 was also associated with stroke among African-Americans,33 we considered this SNP as a potential mediator of the association between migraine and stroke in a regression model that included migraine with visual aura as the study variable and stroke as the outcome. However, controlling for rs3918166 had no impact on the association between migraine with visual aura and stroke in our model.
Two EDN htSNPs, rs2070699 and rs1626492, were associated with migraine with visual aura among Caucasian women (x2 = 7.3, p = 0.03 and x2 = 5.1, p = 0.02, respectively), and EDNRB htSNP rs9544636 was significantly associated with the presence of migraine (with and without visual aura) among Caucasians (x2 5.5, p = 0.02). However, none of these htSNPs were also associated with stroke and, therefore, were not considered potential mediators of the association between migraine with aura and stroke.
Results from Haploview permutations of our stroke data indicated that the best observed chi-square for EDN rs1800542 was 4.7 in Caucasians. A measure this extreme was observed in 20% of the 10,000 randomly permuted data sets; therefore the corrected p-value was 0.20 and does not provide strong evidence in favor of rejecting the global null hypothesis that there is no association between allele frequencies in EDN and case or control status. With regard to EDNRB htSNPs, the best observed chi-square was 10.2 (corrected p-value = 0.014) for rs4885493 and the best observed chi-square for rs10507875 was 7.6, corrected p-value = 0.05. Both of these measures provide evidence in favor of rejecting the null hypothesis that there was no association between allele frequencies in EDNRB and case or control status, and therefore support our findings. Further results from permutation analyses of our stroke data, stratified by migraine status indicated no evidence in favor of rejection of the null hypothesis that there is no association between allele frequencies in NOS3, EDN, or ENDRB and case control status among African-American classified as having migraine with visual aura.
Discussion
The endothelium regulates vascular tone and blood flow by secreting vasoconstrictors such as endothelin-1 (ET-1) and vasodilators such as nitric oxide (NO),11, 12 Thus, impaired dilation or exaggerated constriction of blood vessels may reflect reduced production of NO, which can be regarded as an important marker of endothelial cell dysfunction.38 The NOS3 gene encodes for NO synthase; therefore, any polymorphisms in the NOS3 gene leading to decreased function in this gene could lead to NO deficiency. Evidence from experimental animal studies support a role for impaired NO regulation and vasodilation in the pathophysiology of stroke,14, 15 and with migraine because of its role in vasodilation and with the central processing of migraine related pain stimuli.39, 40 A few studies have reported associations between known single polymorphisms in NOS3 and ischemic stroke in humans,22–24 although these associations have not been well replicated.41
As previously reported by members of our research team, there is increased prevalence of the A allele for rs1800779, and increased prevalence of the T allele for rs2070744 in cases compared to controls in our study population.24 While we do not know of any other studies that have reported similar associations with stroke, a recent meta-analysis reported an association between SNP rs2070744 and coronary heart disease in Caucasians, OR = 1.12 (95% CI: 1.01 to 1.24) for T allele.41 Exploratory stroke subtype analyses in our study showed that two of the three SNPs from NOS3 that were associated with overall stroke (rs2070744 and rs3918166) were primarily associated with stroke of undetermined etiology. Although published data on NOS3 and stroke subtypes are limited, one prior study reported an association between the intron4 variant in NOS3 and small vessel ischemic stroke in Caucasian men and women who were 67 years of age on average.23
With regard to migraine, one NOS3 SNP (rs3918166) was significantly associated with migraine with visual aura but this association did not mediate the affect of migraine with visual aura on stroke risk. Borroni et al recently reported that NOS3 polymorphism rs1799983 (Glu298Asp) was a risk factor for migraine with aura,20 however we found no evidence of this association in our analyses.
ET-1, encoded by the EDN1 gene, is the most powerful vasoconstrictor of vascular smooth muscle.11 It increases vascular resistance and blood pressure and may also mediate the vasoconstrictive phase in migraine attacks.18, 42, 43 ET-1 has two actions, it induces vasoconstriction of blood vessels by binding to the endothelin-A (ETA) and endothelin-B (ETB) receptors in smooth muscle cells (encoded by the EDNRA and EDNRB genes, respectively), and it also mediates vasodilatation through acting on ETB receptors in the endothelium (Figure 1). Therefore, NO and ET-1 released from endothelial cells act reciprocally to maintain vascular tone. In most healthy arteries, vasodilation predominates so the net effect should be vasodilation.11 However, if endothelial function or NO activity is impaired, the vasoconstrictor effect of ET-1 on smooth muscle receptors may remain unopposed,44, 45 potentially leading to disorders associated with endothelial dysfunction such as stroke. ET-1 has been associated in animal studies with cerebral blood flow reduction to levels that induce infarction,16 and, in human studies, to increases in plasma, cerebrospinal fluid, and cerebral tissue following an ischemic stroke.46, 47
In this study we observed an association between EDN htSNP rs1800542 and stroke and between two EDNRB htSNPs (rs4885493 and rs10507875) and stroke in Caucasians. None of these associations were present among African-Americans. The exploratory subtype analyses indicated that the increased stroke risk associated with EDN was primarily for cardioembolic stroke while that associated with EDNRB was for strokes of undetermined etiology. We did not find evidence of elevated risk for small-vessel disease associated with EDN or EDNRB in our subtype analysis which is consistent with at least one other study.48 Two SNPs in EDN were associated with migraine but did not mediate the effect of migraine on stroke risk. At least one other study has assessed polymorphisms in EDN and history of migraine in a population based study of men and women who were 69 years of age on average, but did not find any association with EDN; although an association between EDNRA variant (−231 A/G) and migraine was observed.21
Due to the ligand/receptor relation between ET-1 and ETB, we considered a possible interaction between EDN, which encodes for ET-1 and EDNRB, which encodes ETB. We tested rs1800542 from EDN and rs10507875 and rs4885493 from EDNRB for an interaction with stroke risk in Caucasians and did not find any evidence of an interaction with stroke risk in our data. As mentioned above, one reason for this may be that EDN was primarily associated with cardioembolic stroke in subtype analyses and EDNRB was primarily associated with stroke of undetermined etiology.
We observed associations with migraine and stroke in this analysis of polymorphisms from NOS3, EDN and EDNRB. Potential explanations for our results include: direct action of the polymorphism on disease risk; linkage disequilibrium with a nearby disease susceptibility allele; population stratification bias; or chance findings related to multiple testing. To test for population stratification, we genotyped 40 markers with no known association with stroke in our population and evaluated them for systematic differences in allele frequency between cases and controls using the program Structure49 and found no evidence for population stratification within African-Americans or Caucasians in our study.33
In order to test the global null hypothesis that there is no relationship between allele distribution and case status, we performed permutation analyses. The corrected p-values for single SNP analyses for NOS3 and EDNRB were 0.02; therefore with an alpha level of 0.05, we have good evidence in favor of rejecting the null hypothesis. However, the corrected p-value for EDN was 0.2, which is not sufficient evidence to reject the null hypothesis that there is no association between allele distribution and case or control status, also indicating that it is possible that our results for rs1800542 are due to chance. Our sample size posed limitations on our ability to evaluate the relationship between the alleles, migraine, and stroke risk. Our power to detect the reported associations between EDN and EDNRB htSNPs and stroke risk was approximately 80%; however a larger sample size would have been preferable as our power dropped substantially to about 60% after correction for multiple comparisons. Subsequently, stroke subtype analyses were performed on an exploratory or hypothesis generating basis given the small samples sizes for most subtypes.
This study has several strengths. First, we used a population-based design, which is optimal for studying early-onset stroke due to the low incidence of stroke in this age range. Second, we examined multiple polymorphisms and had moderate to good coverage of three of our genes. Third, our study is the first that we know of to evaluate the association between variants in NOS3, EDN, and EDNRB with migraine and stroke in a group of young Caucasian and African-American women.
Endothelial damage, perhaps in combination with a genetic component, has been suggested as a plausible mechanism of association for migraine and stroke or cardiovascular disease.50–52 While we observed separate associations between tested polymorphisms and migraine and stroke in our study, we did not show that the association between migraine and stroke is mediated by the presence of SNPs from our chosen candidate genes. Since this is the first study to test this hypothesis, replication of our findings and inclusion of other genes in this physiologic pathway will be important. Due to the high LD in all of our candidate genes, we could not determine from these analyses whether the functional alleles that may be causing the observed associations with stroke are in our candidate genes or are up or downstream on the chromosomes; therefore regions outside of these genes should be considered in functional studies.
Table 4.
SNPs analyzed from EDN gene stratified by race and case/control status.
| Marker | Position† | Call rate | Allele Frequency* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African American | Caucasian | |||||||||||
| Cases | n | Controls | n | HW pvalue | Cases | n | Controls | n | HW pvalue | |||
| rs1800542 (A/G) | 12400514 | 0.92 | 0.25 | 126 | 0.26 | 147 | 0.45 | 0.08 | 135 | 0.04 | 202 | 0.52 |
| rs2070699 (G/T) | 12400758 | 0.91 | 0.12 | 113 | 0.11 | 136 | 1.00 | 0.46 | 127 | 0.47 | 195 | 0.01 |
| rs9296343 (C/G) | 12401519 | 0.92 | 0.20 | 128 | 0.20 | 147 | 0.37 | 0.07 | 134 | 0.04 | 204 | 0.12 |
| rs1800543 (C/T) | 12402123 | 0.99 | 0.22 | 128 | 0.22 | 147 | 0.18 | 0.23 | 135 | 0.22 | 205 | 0.03 |
| rs10478723 (A/G) | 12403447 | 0.86 | 0.38 | 119 | 0.41 | 138 | 0.28 | 0.08 | 131 | 0.04 | 196 | 0.50 |
| rs1626492 (A/G) | 12403489 | 0.91 | 0.37 | 125 | 0.38 | 145 | 0.51 | 0.28 | 134 | 0.28 | 202 | 0.88 |
| rs6912834 (A/G) | 12403521 | 0.99 | 0.09 | 126 | 0.09 | 146 | 0.38 | 0.12 | 136 | 0.12 | 208 | 0.59 |
| rs2071943 (A/G) | 12403800 | 0.90 | 0.19 | 123 | 0.16 | 145 | 1.00 | 0.21 | 132 | 0.19 | 203 | 0.10 |
| rs1629862 (A/G) | 12403862 | 0.92 | 0.13 | 128 | 0.11 | 147 | 0.47 | 0.09 | 135 | 0.12 | 201 | 0.62 |
| rs5370 (G/T) | 12404241 | 0.87 | 0.19 | 120 | 0.17 | 144 | 0.05 | 0.19 | 120 | 0.21 | 190 | 0.00 |
As listed by minor allele frequency (bolded in column 1).
Postion refers to NCBI Build 36.2
Acknowledgements and Funding
Grant support: This material is based upon work supported in part by the Office of Research and Development, Medical Research Service and the Research Enhancement Award Program in Stroke, the Geriatrics Research, Education, and Clinical Center, Department of Veterans Affairs; a Cooperative Agreement with the Cardiovascular Health Branch, Division of Adult and Community Health, Centers for Disease Control; the National Institute of Neurological Disorders and Stroke (NINDS) and the NIH Office of Research on Women's Health (ORWH) R01 NS45012; the National Institute on Aging (NIA) Pepper Center Grant P60 12583; and the University of Maryland General Clinical Research Center Grant M01 RR 165001, General Clinical Research Centers Program, National Center for Research Resources (NCRR), NIH.
References
- 1.Tzourio C, Iglesias S, Hubert JB, Visy JM, Alperovitch A, Tehindrazanarivelo A, Biousse V, Woimant F, Bousser MG. Migraine and risk of ischaemic stroke: A case-control study. BMJ. 1993;307:289–292. doi: 10.1136/bmj.307.6899.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, Fenton BT, Cheng SH, Stolar MJ, Risch N. Association between migraine and stroke in a large-scale epidemiological study of the united states. Arch Neurol. 1997;54:362–368. doi: 10.1001/archneur.1997.00550160012009. [DOI] [PubMed] [Google Scholar]
- 3.Carolei A, Marini C, De Matteis G. History of migraine and risk of cerebral ischaemia in young adults. The italian national research council study group on stroke in the young. Lancet. 1996;347:1503–1506. doi: 10.1016/s0140-6736(96)90669-8. [DOI] [PubMed] [Google Scholar]
- 4.Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: Case-control study. The world health organisation collaborative study of cardiovascular disease and steroid hormone contraception. BMJ. 1999;318:13–18. doi: 10.1136/bmj.318.7175.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Touze E, Rothwell PM. Heritability of ischaemic stroke in women compared with men: A genetic epidemiological study. Lancet Neurol. 2007;6:125–133. doi: 10.1016/S1474-4422(06)70683-4. [DOI] [PubMed] [Google Scholar]
- 6.Russel M, Hilden J, Sorensen SA, Olesen J. Familial occurrence of migraine without aura and migraine with aura. Neurology. 1993;43:1369–1373. doi: 10.1212/wnl.43.7.1369. [DOI] [PubMed] [Google Scholar]
- 7.Russell MB, Iselius L, Olesen J. Inheritance of migraine investigated by complex segregation analysis. Hum Genet. 1995;96:726–730. doi: 10.1007/BF00210307. [DOI] [PubMed] [Google Scholar]
- 8.Merikangas K. Genetic epidemiology of migraine. In: Sandler M, Collins GM, et al., editors. Migraine: A spectrum of ideas. Oxford: University Press; 1990. pp. 40–47. [Google Scholar]
- 9.MacClellan LR, Mitchell BD, Cole JW, Wozniak MA, Stern BJ, Giles WH, Brown DW, Sparks MJ, Kittner SJ. Familial aggregation of ischemic stroke in young women: The stroke prevention in young women study. Genet Epidemiol. 2006;30:602–608. doi: 10.1002/gepi.20171. [DOI] [PubMed] [Google Scholar]
- 10.Andresen J, Shafi NI, Bryan RM., Jr Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- 11.Houston M. Vascular biology in clinical practice. Philadelphia: Hanley & Belfus, Inc. Medical Publishers; 2002. [Google Scholar]
- 12.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 13.Olesen J, Thomsen LL, Lassen LH, Olesen IJ. The nitric oxide hypothesis of migraine and other vascular headaches. Cephalalgia. 1995;15:94–100. doi: 10.1046/j.1468-2982.1995.015002094.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-l-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Stagliano NE, Dietrich WD, Prado R, Green EJ, Busto R. The role of nitric oxide in the pathophysiology of thromboembolic stroke in the rat. Brain Res. 1997;759:32–40. doi: 10.1016/s0006-8993(97)00200-x. [DOI] [PubMed] [Google Scholar]
- 16.Macrae IM, Robinson MJ, Graham DI, Reid JL, McCulloch J. Endothelin-1-induced reductions in cerebral blood flow: Dose dependency, time course, and neuropathological consequences. J Cereb Blood Flow Metab. 1993;13:276–284. doi: 10.1038/jcbfm.1993.34. [DOI] [PubMed] [Google Scholar]
- 17.Komatsumoto S, Nara M. [lower level of endothelin-1 in migraine with aura] Rinsho Shinkeigaku. 1995;35:1250–1252. [PubMed] [Google Scholar]
- 18.Gallai V, Sarchielli P, Firenze C, Trequattrini A, Paciaroni M, Usai F, Palumbo R. Endothelin 1 in migraine and tension-type headache. Acta Neurol Scand. 1994;89:47–55. doi: 10.1111/j.1600-0404.1994.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths LR, Nyholt DR, Curtain RP, Goadsby PJ, Brimage PJ. Migraine association and linkage studies of an endothelial nitric oxide synthase (nos3) gene polymorphism. Neurology. 1997;49:614–617. doi: 10.1212/wnl.49.2.614. [DOI] [PubMed] [Google Scholar]
- 20.Borroni B, Rao R, Liberini P, Venturelli E, Cossandi M, Archetti S, Caimi L, Padovani A. Endothelial nitric oxide synthase (glu298asp) polymorphism is an independent risk factor for migraine with aura. Headache. 2006;46:1575–1579. doi: 10.1111/j.1526-4610.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 21.Tzourio C, El Amrani M, Poirier O, Nicaud V, Bousser MG, Alperovitch A. Association between migraine and endothelin type a receptor (eta -231 a/g) gene polymorphism. Neurology. 2001;56:1273–1277. doi: 10.1212/wnl.56.10.1273. [DOI] [PubMed] [Google Scholar]
- 22.Elbaz A, Poirier O, Moulin T, Chedru F, Cambien F, Amarenco P. Association between the glu298asp polymorphism in the endothelial constitutive nitric oxide synthase gene and brain infarction. The genic investigators. Stroke. 2000;31:1634–1639. doi: 10.1161/01.str.31.7.1634. [DOI] [PubMed] [Google Scholar]
- 23.Hassan A, Gormley K, O'Sullivan M, Knight J, Sham P, Vallance P, Bamford J, Markus H. Endothelial nitric oxide gene haplotypes and risk of cerebral small-vessel disease. Stroke. 2004;35:654–659. doi: 10.1161/01.STR.0000117238.75736.53. [DOI] [PubMed] [Google Scholar]
- 24.Howard TD, Giles WH, Xu J, Wozniak MA, Malarcher AM, Lange LA, Macko RF, Basehore MJ, Meyers DA, Cole JW, Kittner SJ. Promoter Polymorphisms in the Nitric Oxide Synthase 3 Gene Are Associated With Ischemic Stroke Susceptibility in Young Black Women. Stroke. 2005;36:1848–1851. doi: 10.1161/01.STR.0000177978.97428.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funalot B, Courbon D, Brousseau T, Poirier O, Berr C, Cambien F, Amouyel P, Schwartz JC, Ducimetiere P. Genes encoding endothelin-converting enzyme-1 and endothelin-1 interact to influence blood pressure in women: The eva study. J Hypertens. 2004;22:739–743. doi: 10.1097/00004872-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Tiret L, Poirier O, Hallet V, McDonagh TA, Morrison C, McMurray JJ, Dargie HJ, Arveiler D, Ruidavets JB, Luc G, Evans A, Cambien F. The lys198asn polymorphism in the endothelin-1 gene is associated with blood pressure in overweight people. Hypertension. 1999;33:1169–1174. doi: 10.1161/01.hyp.33.5.1169. [DOI] [PubMed] [Google Scholar]
- 27.Nicaud V, Poirier O, Behague I, Herrmann SM, Mallet C, Troesch A, Bouyer J, Evans A, Luc G, Ruidavets JB, Arveiler D, Bingham A, Tiret L, Cambien F. Polymorphisms of the endothelin-a and -b receptor genes in relation to blood pressure and myocardial infarction: The etude cas-temoins sur l'infarctus du myocarde (ectim) study. Am J Hypertens. 1999;12:304–310. doi: 10.1016/s0895-7061(98)00255-6. [DOI] [PubMed] [Google Scholar]
- 28.Poirier O, Mao C, Mallet C, Nicaud V, Herrmann SM, Evans A, Ruidavets JB, Arveiler D, Luc G, Tiret L, Soubrier F, Cambien F. Polymorphisms of the endothelial nitric oxide synthase gene - no consistent association with myocardial infarction in the ectim study. Eur J Clin Invest. 1999;29:284–290. doi: 10.1046/j.1365-2362.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson CJ, Kittner SJ, McCarter RJ, Sloan MA, Stern BJ, Buchholz D, Price TR. Interrater reliability of an etiologic classification of ischemic stroke. Stroke. 1995;26:46–51. doi: 10.1161/01.str.26.1.46. [DOI] [PubMed] [Google Scholar]
- 30.Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Sloan MA, Wityk RJ, Wozniak MA. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768–774. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kittner SJ, Stern BJ, Wozniak M, Buchholz DW, Earley CJ, Feeser BR, Johnson CJ, Macko RF, McCarter RJ, Price TR, Sherwin R, Sloan MA, Wityk RJ. Cerebral infarction in young adults: The Baltimore-Washington cooperative young stroke study. Neurology. 1998;50:890–894. doi: 10.1212/wnl.50.4.890. [DOI] [PubMed] [Google Scholar]
- 32.MacClellan LG, Cole JW, Wozniak MA, Stern BJ, Mitchell BD, Kittner SJ. Probable migraine with visual aura and risk of ischemic stroke: The stroke prevention in young women study. Stroke. 2007;38:2438–2445. doi: 10.1161/STROKEAHA.107.488395. [DOI] [PubMed] [Google Scholar]
- 33.Howard TD, Yongmei L, Cole JW, Saylor G, Giles WH, Wozniak MA, Gallagher M, Steinberg KK, Macko RF, Stine OC, Kittner SJ. Promoter polymorphisms in the nitric oxide synthase 3 gene are associated with ischemic stroke in a replication population of young african-american women. Stroke. 2007;38:528. [Google Scholar]
- 34.Thorisson GA, Smith AV, Krishnan L, Stein LD. The international hapmap project web site. Genome Res. 2005;15:1592–1593. doi: 10.1101/gr.4413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of ld and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 37.SAS Institute Inc. The SAS system for windows. 1999–2001 [Google Scholar]
- 38.Pepine CJ. The effects of angiotensin-converting enzyme inhibition on endothelial dysfunction: Potential role in myocardial ischemia. Am J Cardiol. 1998;82:23S–27S. doi: 10.1016/s0002-9149(98)00805-4. [DOI] [PubMed] [Google Scholar]
- 39.Meller ST, Gebhart GF. Nitric oxide (no) and nociceptive processing in the spinal cord. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 40.Thomsen LL, Olesen J. Nitric oxide theory of migraine. Clin Neurosci. 1998;5:28–33. [PubMed] [Google Scholar]
- 41.Casas JP, Hingorani AD, Bautista LE, Sharma P. Meta-analysis of genetic studies in ischemic stroke: Thirty-two genes involving approximately 18,000 cases and 58,000 controls. Arch Neurol. 2004;61:1652–1661. doi: 10.1001/archneur.61.11.1652. [DOI] [PubMed] [Google Scholar]
- 42.Kallela M, Farkkila M, Saijonmaa O, Fyhrquist F. Endothelin in migraine patients. Cephalalgia. 1998;18:329–332. doi: 10.1046/j.1468-2982.1998.1806329.x. [DOI] [PubMed] [Google Scholar]
- 43.Hasselblatt M, Kohler J, Volles E, Ehrenreich H. Simultaneous monitoring of endothelin-1 and vasopressin plasma levels in migraine. Neuroreport. 1999;10:423–425. doi: 10.1097/00001756-199902050-00039. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda U, Yamamoto K, Maeda Y, Shimpo M, Kanbe T, Shimada K. Endothelin-1 inhibits nitric oxide synthesis in vascular smooth muscle cells. Hypertension. 1997;29:65–69. doi: 10.1161/01.hyp.29.1.65. [DOI] [PubMed] [Google Scholar]
- 45.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Panza JA. Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension. 2000;35:1237–1241. doi: 10.1161/01.hyp.35.6.1237. [DOI] [PubMed] [Google Scholar]
- 46.Lampl Y, Fleminger G, Gilad R, Galron R, Sarova-Pinhas I, Sokolovsky M. Endothelin in cerebrospinal fluid and plasma of patients in the early stage of ischemic stroke. Stroke. 1997;28:1951–1955. doi: 10.1161/01.str.28.10.1951. [DOI] [PubMed] [Google Scholar]
- 47.Viossat I, Duverger D, Chapelat M, Pirotzky E, Chabrier PE, Braquet P. Elevated tissue endothelin content during focal cerebral ischemia in the rat. J Cardiovasc Pharmacol. 1993;22 Suppl 8:S306–S309. doi: 10.1097/00005344-199322008-00080. [DOI] [PubMed] [Google Scholar]
- 48.Gormley K, Bevan S, Hassan A, Markus HS. Polymorphisms in genes of the endothelin system and cerebral small-vessel disease. Stroke. 2005;36:1656–1660. doi: 10.1161/01.STR.0000173173.38289.69. [DOI] [PubMed] [Google Scholar]
- 49.Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–291. doi: 10.1001/jama.296.3.283. [DOI] [PubMed] [Google Scholar]
- 51.Diener HC, Kurth T. Is migraine a risk factor for stroke? Neurology. 2005;64:1496–1497. doi: 10.1212/01.WNL.0000162488.81911.83. [DOI] [PubMed] [Google Scholar]
- 52.Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD, Launer LJ. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291:427–434. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]