Abstract
Background and Purpose
Intraventricular hemorrhage (IVH) is a common complication of prematurity that results in neurologic sequelae including cerebral palsy, post-hemorrhagic hydrocephalus and cognitive deficits. Despite this, there is no standardized animal model exhibiting neurological consequences of IVH in prematurely delivered animals. We asked whether induction of moderate-to-severe IVH in premature rabbit pups would produce long-term sequelae of cerebral palsy, posthemorrhagic hydrocephalus, reduced myelination and gliosis.
Methods
The premature rabbit pups, delivered by C-section, were treated with intraperitoneal glycerol at 2h postnatal age to induce IVH. The development of IVH was diagnosed by head ultrasound at 24h age. Neuro-behavioral, histological and ultrastructural evaluation and diffusion tensor imaging (DTI) studies were performed at 2-week age.
Results
While 25% IVH pups developed motor impairment with hypertonia and 42% developed post-hemorrhagic hydrocephalus, pups without IVH (non-IVH) were unremarkable. Immunolabeling revealed reduced myelination in the white matter of IVH pups compared to saline- and glycerol-treated non-IVH controls. Reduced myelination was confirmed by Western blot analysis. There was evidence of gliosis in IVH pups. Ultrastructural studies in IVH pups showed that myelinated and unmyelinated fibers were relatively preserved except for focal axonal injury. DTI showed reduction in fractional anisotropy and white matter volume confirming white matter injury in IVH pups.
Conclusion
The rabbit pups with IVH displayed post-hemorrhagic hydrocephalus, gliosis, reduced myelination and motor deficits, like humans. The study highlights an instructive animal model of the neurologic consequences of IVH, which can be used to evaluate strategies in the prevention and treatment of post-hemorrhagic complications.
Keywords: Germinal matrix hemorrhage-intraventricular hemorrhage, white matter, myelin, gliosis, ventriculomegaly neurobehavioral testing
Introduction
Germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH) is a major problem of premature infants as both preterm birth rate and neonatal survival have substantially increased over the last two decades.1 The major neurologic consequences of IVH are cerebral palsy, post-hemorrhagic hydrocephalus and cognitive deficits.2 As GMH-IVH is not preventable and since clinical treatments are inadequate, it is crucial to develop treatment strategies to prevent or minimize the neurologic consequences. Therefore, a suitable animal model of the consequences of GMH-IVH is necessary to test new modalities of prevention and treatment.
GMH-IVH has been induced in several animal species, including rat, mouse, dog, sheep and pig, either by needle injection of blood into the ventricle or by altering hemodynamic parameters including blood pressure, circulating blood volume, serum osmolarity, pCO2 or O2 levels.3,4 However, these animal models do not closely resemble premature infants with IVH with respect to etiology, pathology and clinical consequences.5–7 Needle insertion in the brain for infusion of blood has an inherent disadvantage of producing direct injury to brain; and changing hemodynamic factors, such as induction of hypercarbia, hypoxia or hypervolumia, confounds the IVH-induced brain pathology. A typical periventricular white matter (WM) injury or hemorrhagic infarction, often noted in infants with IVH, does not develop in these models. The incidence of post-hemorrhagic hydrocephalus in the blood infusion model is high relative to preterm neonates, and the animals do not exhibit motor impairments (hypertonic cerebral palsy) similar to premature infants.8–10 Of note, hypertonic cerebral palsy has not been produced in rodent pups; and full-term piglets are resistant to WM injury due to advanced neurologic development. Another limitation of the existing models is failure to use prematurely delivered animals to model IVH because this requires special expertise to rear them. Hence, there is lack of an animal model of neurologic consequences of IVH that naturally mimics preterm survivors of IVH.
We selected premature rabbit pups to model consequences of IVH because of their close resemblance to humans in several aspects.11 First, rabbit pups have a gyrencephalic brain, abundant germinal matrix and perinatal brain growth, unlike rodents. Second, hypertonic cerebral palsy has been successfully produced in rabbit pups, but not in other species.12 Third, premature rabbit pups, like premature infants, are at risk of spontaneous GMH (10%), which is substantially increased with intraperitoneal glycerol (80%) administration.11 Glycerol treatment results in dehydration and high serum osmolarity, which is attended by intracranial hypotension and selective rupture of the germinal matrix vasculature.14 Fourth, hemorrhage in this model, as we recently reported, leads to inflammatory changes around the lateral ventricle, just as in preterm infants.13 Fifth, the model is not confounded by significant toxicity of glycerol upon brain, kidney, lung or other organs.13 Therefore, we chose to evaluate the neurologic consequences of IVH in this rabbit pup model. We asked whether induction of moderate-to-severe IVH in the brain of premature rabbit pups would produce cerebral palsy and posthemorrhagic hydrocephalus and whether the neurologic sequelae were associated with reduced myelination of WM, neuronal loss, and gliosis. In this study, we found that premature pups with IVH displayed post-hemorrhagic hydrocephalus, motor impairment with hypertonia, gliosis and reduced myelination of WM, similar to humans.
Materials and Methods
Animal experiment
Institutional Animal Care and Use Committee of New York Medical College approved the animal protocol. The details of acute brain injuries in the first 3 days of life in our model of glycerol-induced IVH have been previously established and published.13 We obtained timed pregnant New Zealand rabbits from Charles River Laboratories, Inc. (USA). We delivered the pups prematurely by C-section at 29 days of gestational age (full-term=32 days). Pups were immediately dried and kept in an infant incubator pre-warmed to a temperature of 35°C. Pups were fed 1ml rabbit milk at 4-hour age and then ~2 ml every 12 hours (100ml/kg/day) for the first 2 days using 3.5 French feeding tube. After day-2, we used kitten milk formula (KMR, PETAG Inc. IL) and advanced feeds to 125,150,200,250 and 280 ml/kg on postnatal days 3,5,7,10 and 14 respectively.
At 2h age, the pups alternatively received 50% glycerol (6.5 gm/kg) or saline treatment intraperitoneally. Head ultrasound was performed at 24h age to assess for the presence and severity of IVH using Acuson Sequoia C256 (Siemens) ultrasound machine. There was no difference in the presence and grading of IVH between 6, 24h and 72h age in glycerol-treated pups on head ultrasound (selected samples). As described before, IVH was classified as a) mild, no gross hemorrhage and hemorrhage detected on microscopy of H&E stained brain sections; b) moderate, gross hemorrhage into lateral ventricles without significant ventricular enlargement (2 separate lateral ventricles discerned); or c) severe, IVH with significant ventricular enlargement (fusion of ventricles into a common chamber) and/or intraparenchymal hemorrhage.14 Since, microscopic IVH cannot be diagnosed by head ultrasound, we followed pups with moderate and severe IVH for 2-week period to evaluate neurological consequences. We included both glycerol-treated and saline-treated non-IVH pups as controls.
Rabbit Tissue Collection and Processing
Tissue processing was done as described.13 The brain slices were immersion-fixed in 4% paraformaldehyde and cryoprotected into sucrose. Tissues were frozen into optimum cutting temperature compound (Sakura, Japan). Frozen coronal blocks were cut into 12μ sections using cryostat.
Immunohistochemistry and Nissl’s staining, quantification of myelination, gliosis and neuronal density
We have described them in supplementary methods.
Western Blot Analyses and Electron microscopy
The techniques are illustrated in supplementary methods.
Neuro-behavioral examination
We performed neuro-behavioral testing at postnatal day-14 based on a modification of neurobehavioral scoring protocol described elsewhere.12,13 The testing was performed by 2 blinded physicians. We evaluated cranial nerves by testing smell (aversive response to ethanol), sucking and swallowing (formula was given with a plastic pipette). The responses were graded on a scale of 0–3, 0 being the worst response and 3 the best response. Motor examination included tone (modified Ashworth’s scale), motor activity, locomotion at 30° angle, righting reflex and gait. Tone was assessed by active flexion and extension of forelegs and hindlegs (score 0–3). The righting reflex was evaluated by their ability and rapidity to turn prone when placed in supine position. Sensory examination was limited to touch on face (touching face with cotton swab) and extremities as well as pain on limbs (mild pin-prick). Grading of tone, gait and locomotion at 30° angle are described in footnote of Table 1. To assess coordination and muscle strength of extremities, we evaluated the ability of the pups to hold their position at 60°slope. The test was conducted on a rectangular surface (18×6inch) placed at 60° inclination. We placed the pup at upper end of the surface and measured the latency to slip down the slope. To assess vision we preformed visual cliff test. The test scored whether pups stopped at the edge of an apparent cliff. All animals could detect the cliff.
Table 1.
Neurobehavioral evaluation of premature pups (E29) at day-14 postnatal age
| System | Test | Glycerol (+) IVH (+) n=20 |
Glycerol (+) IVH (−) n=17 |
Glycerol (−) IVH (−) n=15 |
|---|---|---|---|---|
| Cranial nerve | Aversive response to alcohol | 3(3,3) | 3(3,3) | 3(3,3) |
| Sucking and swallowing | 3(3,3) | 3(3,3) | 3(3,3) | |
| Vision | 3(3,3) | 3(3,3) | 3(3,3) | |
| Motor | Motor activity | |||
| Head | 3(3,3) | 3(3,3) | 3(3,3) | |
| Forelegs | 3(2,3) | 3(3,3) | 3(3,3) | |
| Hind legs | 3(2.7,3) | 3(3,3) | 3(3,3) | |
| Righting reflex* | 5(4,5) # | 5(5,5) | 5(5,5) | |
| Locomotion on 30° inclination† | 3(3,3) | 3(3,3) | 3(3,3) | |
| Tone‡ fore-limb | 0(0,0) | 0(0,0) | 0(0,0) | |
| hind-limb | 0(0.25,0) | 0(0,0) | 0(0,0) | |
| Hold their position at 60° inclination (latency to slip down the slope in seconds) | 6.7### | 12.1 | 11.5 | |
| Distance walked in 60 seconds (in inches) | 61.5# | 104 | 94 | |
| Gait § | 3(2.75,3)### | 4(4,4) | 4(4,4) | |
| Motor impairment | Weakness in extremities (%) | 25%# | 0% | 0% |
| Sensory | Facial touch | 3(3,3) | 3(3,3) | 3(3,3) |
| Pain | 3(3,3) | 3(3,3) | 3(3,3) | |
Values are median and interquartile range. 0 is the worst response and 3 is the best response.
indicates score (range 1–5): Number of times turns prone within 2 seconds when placed in supine out of 5 tries.
indicates score (range 0–3) 0, Does not walk; 1, takes a few steps (less than 8″); 2, Walks for 9–18 inches; 3, walks very well beyond 18 inches.
indicates score (range 1–3): 0, no increase in tone;1, slight increase in tone; 2, considerable increase in tone and 3, Limb rigid in flexion or extension.
Gait was graded as 0 ( no locomotion), 1 ( crawls with trunk touching the ground for few steps and then rolls over), 2 (walks taking alternate steps, trunk low and cannot walk on inclined surface), 3 (walks taking alternate steps, cannot propel its body using synchronously the hind legs, but walks on 30° inclined surface), 4 (walks, runs and jumps without restriction, propels the body using synchronously the back legs, but limitation in speed, balance and co-ordination manifesting as clumsiness in gait) or 5 (normal walking).
P<0.05,
P<0.001 for the comparison between pups with IVH and saline- as well glycerol-treated controls. Fisher exact test used to compare incidence of motor impairment and Mann Whitney U test for other parameters.
Diffusion Tensor Imaging (DTI)
Premature rabbit pups with glycerol-induced IVH and glycerol-treated non-IVH controls of 2-week postnatal age were anesthetized and transcardially perfused with 0.01M PBS followed by 4% paraformaldehyde. The brains were harvested and immersion fixed in 4% paraformaldehyde. Before MRI, the brains were washed into PBS and placed into home-built MRI compatible tube. The tube was filled with Fluorinert (Sigma), an MRI susceptibility matching fluid. Imaging was conducted on a 9.4-T horizontal bore magnet (Bruker, Billerica, USA) with custom-made cosine 1H radio frequency coil (14mm diameter). The technical details of DTI experiments are described in supplementary methods
Statistics and analysis
We compared cross-sectional areas (ventricle, cortex and forebrain), neuronal count, astrocyte density, ratio of myelinated to unmyelinated area and myelin basic protein (MBP) levels between IVH pups and saline- as well as glycerol-treated controls (non-IVH). We used Mann-Whitney U test (non-parametric variables) or t-test (parametric variable) to perform pairwise comparison and ANOVA to compare multiple groups. A p value of <0.05 was considered significant.
RESULTS
Survival and growth of rabbit pups
As premature rabbit pups are not common animal model and since they die with minor insult, we evaluated their survival in our laboratory. The pups were hand-fed as the dams were sacrificed after C-section. Fifteen percent of the glycerol-treated pups with IVH died within 72h and another 15% by day-14 postnatal age (n=20), whereas 19.1% of glycerol-treated (n=15) and 18% of saline-treated (n=17) controls (non-IVH) died by day-14. Among IVH pups, the cause of death was either episodes of prolonged seizures or aspiration of formula during feeding within the first three days. The deaths after three days in this group were attributed to increase in intracranial pressure (neck retraction and opisthotonus) or aspiration of formula. Among non-IVH pups, feeding related issues were the predominant cause of death. We next compared the weight of four groups of pups--glycerol-induced IVH, glycerol-treated controls (non-IVH), saline-treated controls (non-IVH) and full-term pups (Fig. 1A). Full term pups were reared by the dam and preterm pups were hand-fed. The four groups of pups had comparable weight at each epoch. Together, IVH pups had a survival of ~70% at day-14 compared to ~80% in non-IVH controls; and the weight of hand-fed premature pups was comparable to dam-fed term pups.
Figure 1.
A) Growth chart: Growth chart of a) premature pups with glycerol-induced IVH, saline-treated controls and glycerol-treated controls (non-IVH) and b) full-term pups. Data are mean±SEM (n=8 each). The 4 groups of premature pups had comparable weight. B) Healthy and paralyzed pup: Note the difference in posture of 2 pups. Healthy pup (green label) is upright on the bench, while paralyzed pup (red label) is unable to stand and is lying on the bench with stiff extremities. C) Coronal section through normal and IVH brain at 24h age. Note ventricular dilation and fusion of the ventricles (block arrows) in IVH while normal brain has slit-like ventricle (arrowhead). Scale bar=1cm. D). Coronal section through forebrain of non-IVH pup at 2-week age. Note small ventricle (arrowhead). E) Coronal section through forebrain of IVH pup with ventriculomegaly (arrow) at 2-week age. Scale bar=0.5cm. F) Nissl’s staining of brain sections from IVH (lower-panel) and non-IVH (upper-panel) pup. The sections were taken at 2 levels---mid-septal nucleus (left-panel) and ventro-lateral nucleus of thalamus (right-panel). Sections show ventricle, cortex and WM.
Clinical consequences of IVH in rabbit pups
To determine the motor and sensory capabilities of IVH pups compared to non-IVH controls, we performed neuro-behavioral examination at day-14 (Table 1). We found weakness in extremities of 25% pups (n=20) with IVH---3 pups with hind-leg weakness, one pup with predominantly fore-leg weakness and one pup with complete paralysis of both fore- and hind-legs (Fig. 1B). In contrast, non-IVH pups—saline- and glycerol-treated controls—did not manifest motor weakness in the extremities. The quadriparesis and diplegia in pups were diagnosed by the presence of abnormal gait and limitation in the speed of walking. The gait in pups without motor weakness typically consisted of walking, jumping and running with synchronous use of the hind-legs. In contrast, pups with motor impairment presented with clumsiness in walking, asymmetrical gait, walking with alternate steps, inability to synchronously use the hind-legs or complete inability to walk. The scores for gait were significantly lower in IVH pups compared to controls (P<0.001). Furthermore, there was significant limitation in the speed of locomotion in IVH pups compared to controls (P<0.05). We also noted that scores of righting reflex were significantly lower in IVH pups compared to controls (P<0.05). The latency to slip down the slope was significantly reduced in IVH pups relative to controls (P<0.001). Of note, among pups with motor impairment, we observed slight increase in tone (score=1) in the legs of 4 pups and considerable increase in tone (score=2) in extremities of one pup. No visual or sensory impairment was found in any pup.
Ventriculomegaly, stretching and thinning of cerebral cortex, but no cortical atrophy in IVH pups
We performed gross and histopathological evaluation of the brains at day-14 (Fig. 1C through F). The cross-sectional areas of the ventricle, whole forebrain and cerebral cortex were measured in Nissl-stained brain sections (at 2 coronal levels: midseptal-nucleus and ventral posterolateral nucleus of the thalamus) of IVH and non-IVH pups. Data were plotted as box and whisker plot (Fig. 2A&B). We found larger ventricular size in IVH pups compared to controls at both midseptal nucleus and thalamus levels (P<0.05 each). Of note, cross-sectional area of the cerebral cortex or whole forebrain (excluding ventricles) was comparable between IVH pups and non-IVH controls.
Figure 2. Measurement of cross-sectional areas of ventricle, cortex and whole forebrain.
(A, B): Nissl’s stained cryosections at 2 coronal levels---mid-septal nucleus (upper panel) and ventro-lateral nucleus of thalamus (lower panel): —were evaluated. The cross-sectional areas of ventricle, cortex and whole forebrain were compared. Data are shown as box-and-whisker plot that depicts median, 10th,25th,75th and 90th percentile as vertical boxes with error bars (n=12 each). Ventricular area was significantly greater in IVH pups than saline- and glycerol-treated non-IVH controls at both the coronal levels. However, cerebral cortex and forebrain cross-sectional area were similar between the 3 groups.*P<0.05 for IVH pups vs. glycerol controls. #P<0.05 for IVH pups vs. saline controls. Greater gliosis in pups with IVH than controls (C,D): C) Representative immuno-fluorescence of cryosections labeled with GFAP antibody. Hypertrophic astrocytes (arrowhead) were abundant forming dense network in IVH pups, while non-pups had fewer astrocytes. D) Bar graph shows mean ± s.e.m.(n=6pups). Astrocyte count revealed significantly higher density in the periventricular zone (PVZ) and superficial WM of pups with IVH compared to glycerol- and saline-treated non-IVH controls, but not in the cerebral cortex. *P<0.05 for IVH pups vs. glycerol-treated non-IVH pups. #P<0.05 for IVH pups vs. saline-treated non-IVH pups.
We defined ventriculomegaly as a ventricular area that measures more than three standard deviations above the mean for age in non-IVH pups. Thus, at 2-week age, a ventricular area of ≥ 9mm2 and 12.4mm2 (mean+3SD) at the level of midseptal nucleus and ventral posterolateral thalamus respectively was considered to be ventriculomegaly. While 42% of IVH pups had ventriculomegaly (Fig. 1E), none of the glycerol- and saline-treated controls had ventricular dilation. All the pups with ventriculomegaly exhibited palpable anterior fontanel and separation of cranial sutures, unlike controls. Predictably, all pups with severe IVH (n=3) developed ventriculomegaly, while only 22% of pups with moderate IVH had ventricular dilation (n=9).
We next measured cortical thickness and circumference of the brain section. We found significant reduction in cortical thickness in the forebrain of IVH pups (both with and without ventriculomegaly) compared to non-IVH controls (Supplemental Fig. 1). The forebrain circumference was significantly greater in IVH pups with ventriculomegaly compared to non IVH controls, but not in IVH pups without ventriculomegaly.
We next performed neuronal count in the cerebral cortex of IVH pups compared to glycerol- and saline-treated controls. Importantly, we found no significant difference in the neuronal density in IVH pups compared to non-IVH controls (data not shown). Together, IVH pups at day-14 exhibited ventriculomegaly and thinning as well as stretching of cortical mantle, but there was no evidence of cortical atrophy.
Reactive gliosis in IVH
We immuno-labeled coronal brain sections with GFAP antibody and performed astrocyte count in region around the ventricle (periventricular zone), superficial WM (corona radiata and internal capsule) and cerebral cortex. We observed abundant hypertrophic astrocytes--with large cell body and numerous processes making a dense network–in the periventricular zone and WM of IVH pups, in contrast to glycerol- and saline-treated non-IVH controls (Fig. 2C,D). Accordingly, astrocyte count was significantly higher in the periventricular zone and WM of IVH pups compared to controls (P<0.05 each), but not in the cerebral cortex. Hence, IVH resulted in periventricular astrogliosis in premature pups.
Reduced Myelination in IVH
As periventricular leukomalacia is associated with IVH, we next evaluated myelination in IVH pups compared to non-IVH controls. We double-labeled cryosections with MBP and pan-axonal filament specific antibodies and measured ratio of myelinated (MBP) and unmyelinated fibers (pan-axonal filament, n=10). We found that the expression of MBP in corona radiata, corpus callosum and internal capsule was significantly lesser in IVH pups compared to both glycerol- and saline-treated controls (P<0.05 each; Fig. 3A,B). Further, MBP expression was similar in IVH pups with and without ventriculomegaly (n=5each, data not shown). Of note, regional comparison within the WM areas revealed that MBP level was lower in the corona radiata and corpus callosum than in the internal capsule (P=0.016, 0.014 respectively) in IVH pups. To further confirm our finding, we quantified MBP in the brain homogenates by Western blot analysis. Consistent with immunostaining data, we found that MBP protein level was significantly lower in IVH pups than controls (Fig. 3C,D). In conclusion, development of IVH is attended by reduced expression of MBP.
Figure 3. Reduced myelination in IVH pups than non-IVH controls.
(A,B) Representative immunofluorescence of cryosections labeled with MBP and pan-axonal filament specific antibodies. The ratio of myelinated to unmyelinated fibers was measured in corpus callosum (CC), corona radiata (CR) and internal capsule (IC) using Metamorph-software. Bar graph shows mean±s.e.m (n=10 each). Reduced myelination in IVH pups was noted in all the three WM regions—CR, CC and IC—compared to glycerol- and saline-treated controls. Scale bar=20μm. (C,D) Representative Western blot analysis of MBP on brain homogenates. The bar graph shows mean±s.e.m. (n=6 pups). MBP levels were normalized to β-actin levels. Reduced myelination in IVH pups was noted relative to saline- and glycerol-treated non-IVH controls. There was no statistical difference between saline and glycerol controls. *P<0.05 for IVH pups vs. glycerol-treated non-IVH pups. #P<0.05 for IVH pups vs. saline-treated non-IVH pups.
Ultrastructural studies
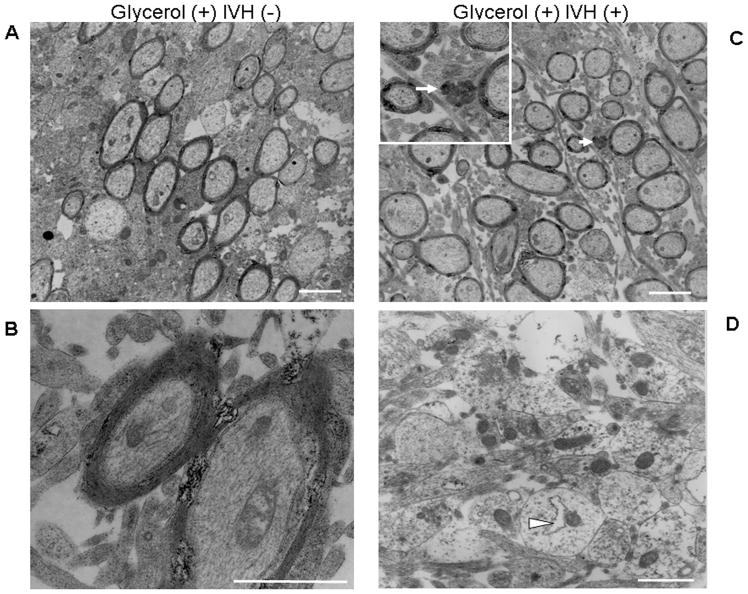
Ultrastructural evaluation did not show major differences in axonal and myelin morphology in the WM regions--corona radiata, corpus callosum and internal capsule--of IVH pups compared to non-IVH controls at day-14 (Fig. 4). Overall, the myelinated and unmyelinated fibers were well organized, and preserved in IVH pups, similar to non-IVH controls. However, a few axons in IVH pups showed features of axonal degeneration including intra-axonal vacuoles and autophagosomes, which was not seen in non-IVH pups. We did not observe thinning of myelin, hypermyelination, Wallerian-like axonal degeneration or the presence of inflammatory cells in IVH pups. In addition, remyelinating axons and very small axons representing regenerating sprouts were not identified.
Figure 4. Ultrastructural evaluation of white matter.
A) Representative electron micrograph of corona radiata in non-IVH pup. Myelinated and unmyelinated fibers show no pathologic changes. B) High-power view of myelinated axons of a non-IVH pup showing unremarkable myelin and axon. C) Representative electron micrograph of corona radiata in a IVH pup. Myelinated and unmyelinated fibers are organized just as non-IVH controls. Note intra-axonal autophagosome (arrow) within unmyelinated fiber suggesting axonal degeneration. Inset showing high-power view of autophagosome. D) High-power view of unmyelinated axons in IVH pup. Note regular and uniform size of axons. Note intra-axonal vesicle (arrowhead), indicating axonal degeneration. Scale bar=2μm (Upper-panel) and 1μm (lower-panel).
DTI in IVH pups
As MRI is highly sensitive in quantifying WM injury, we performed ex vivo DTI on fixed brain of IVH pups and glycerol-treated non-IVH controls (n=4each). We used maps of apparent diffusion coefficient (ADC) and fractional anisotropy (FA) to evaluate changes in the WM. ADC maps showed ventriculomegaly in IVH pups, while ventricles were slit-like in non-IVH controls (Fig. 5A). Ventriculomegaly was bilateral and symmetrical in the lateral ventricles. FA, a directionally invariant index of diffusion anisotropy, depicts variance among the three eigenvalues of diffusion tensor. Directionally encoded color (DEC) maps were employed to reflect orientation-specific anisotropies in the medial–lateral, dorsal–ventral, and anterior–posterior directions with red, green, and blue colors respectively. The WM region of interest including corona radiata, corpus callosum, internal capsule and fimbria-fornix were evaluated (Figs. 5B–E). In IVH pups, the FA was significantly decreased in the corpus callosum, corona radiata and fimbria-fornix compared to controls (P<0.05 each), but not in the internal capsule. The FA changes in corpus callosum and fimbria-fornix were dominant in the medial-lateral direction, whereas it was dominant in the dorsal-ventral direction within the corona radiata. As observed in Fig. 5B, specific WM areas in the corpus callosum, fimbria fornix, corona radiata were significantly reduced in size in IVH pups compared to controls, but not in the internal capsule (Supplemental Table 1).
Figure 5. Diffusion tensor imaging of rabbit pup brain.
(A) Coronal ADC maps at the level of mid-septal nucleus and ventral postero-lateral nucleus of thalamus show larger ventricles in IVH pup than non-IVH control. B) FA maps of contiguous coronal slices of pups (day-14) with IVH and without IVH as indicated. WM structures--corpus callosum (CC), corona radiata (CR) and fimbria fornix (FF)--show decreased FA in IVH pups compared to glycerol-treated control. (C, D&E) We analyzed FA in the dominant orientation coordinates in medial-lateral(R), dorsal-ventral(G) and anterior-posterior(B) directions. FA was significantly decreased in the CC(P<0.05), CR(P<0.03) and FF(P<0.05) in IVH pups compared to glycerol-treated controls. The FA changes in CC and FF were dominant in the medial-lateral direction, whereas it was dominant in the dorsal-ventral direction within the CR. * P<0.05 for FA of IVH pups vs. glycerol-treated non-IVH pups.
DISCUSSION
GMH-IVH continues to be a major problem of modern neonatal intensive care units worldwide. In this study, we evaluated the neurologic consequences of IVH in premature rabbit pups, in which IVH was induced by intraperitoneal glycerol at 2-hour postnatal age. We found that premature pups with IVH developed motor impairment with hypertonia (25%) and ventriculomegaly (42%) at 2-week postnatal age. Accordingly, the pups showed histological and radiological evidence of reduced myelination and gliosis. The study underscores a novel animal model that can be used to evaluate strategies in the prevention and treatment of the consequences of IVH.
We have recently reported characterization of acute brain injury (<72h) in our rabbit pup model of IVH;13 and here, we are describing relatively long-term outcomes (2-week) of IVH in rabbit pups. Our model mimics human conditions and has a number of merits. First, development and progression of IVH in this model is morphologically similar to IVH in premature infants because hemorrhage initiated by rupture of germinal matrix vasculature progresses to IVH. Second, induction of IVH in our model neither causes direct injury to the brain by needle stab nor confounds the model with unwanted metabolic-changes in the neural cells by hypoxia-ischemia or hypercapnia, as used in several studies.3, 4 Third, there are a number of inherent benefits of using rabbit that have been described in the introduction. Fourth, rabbit pups with IVH displayed neurologic complications of motor impairment, ventricular dilation and reduced myelination, just as in premature infants. However, there are some limitations of our model. We delivered pups by C-section, euthanized the dams and nursed orphan pups in an infant incubator. These pups were hand-fed using feeding tubes, which was labor intensive and requiring technical expertise and experience. Even though glycerol does not produce major systemic adverse effects, this can potentially open the blood-brain barrier and exert metabolic changes in the brain, similarly to mannitol,15 Together, our model of neurologic consequences of IVH in premature rabbit pups mimics premature infants with post-hemorrhagic complications.
To our knowledge, this is the first animal model depicting neurologic consequences of IVH in a prematurely delivered animal. As our rabbit pups (E29) are 3–4 days premature (term=32d; E29=87–90% gestation), they are equivalent to premature infants of ~33-week gestational age. However, upon linking cortical and non-cortical development of rabbit with human, the neurodevelopment of E29 rabbits equates to 18 weeks and P11 rabbits (postnatal day-14 for E29 pups) to 29 weeks of gestational age in humans.16 Furthermore, myelination begins at P4–7 in rabbits and in second trimester of pregnancy in humans. Based on these considerations, our d14 pups are comparable to premature human infants of 29–35 weeks gestation; and postnatal care of 2-week duration provided to E29 rabbits is equivalent to 14–18 weeks of neonatal care given to premature infants. However, these comparisons have obvious limitations as human neurodevelopment is more complex and intricate compared to those of rabbits or rodents.
The most important and novel observation made in the model was that premature pups displayed clinical evidence of hypertonia with motor impairment and ventriculomegaly, similar to premature infants. We found that 25% pups with IVH exhibited signs of cerebral palsy including hypertonia, abnormal gait, limitation in locomotion and poor ability to hold their position at 60° inclination without slipping. We observed only slight increase in tone among pups with motor impairment, except for one pup that showed considerable increase in tone. Consistent with our findings, several studies have shown that IVH in premature infants have higher occurrence of cerebral palsy and other neurological sequelae compared to premature infants without IVH.17,18 A prospective study has reported that 24% of infants with grade III (moderate) and IV (severe) IVH have abnormal neurological diagnoses, including cerebral palsy and cognitive deficits at 5-year age.8 Importantly, premature infants with IVH or other brain injuries, who sustain WM damage, may not manifest with significant spasticity or other signs of cerebral palsy immediately at birth, however, neurological manifestations may appear at later age and progress over weeks or months.19 In contrast to motor impairment, we did not observe any apparent sensory involvement in preterm rabbits, just as preterm infants.20 Another important clinical manifestation in our model was the development of post-hemorrhagic ventricular dilation in 42% of pups with IVH, but not in controls. In a neonatal rat model of hydrocephalus, 65% of pups (P7) injected with blood into the cerebral ventricle developed hydrocephalus, whereas 50% of pups injected with artificial CSF also developed hydrocephalus.10 However, clinical studies in premature infants with IVH have reported hydrocephalus in 9%, 36% and 47% survivors of mild, moderate and severe IVH respectively, similar to our animal model.9 We also noted reduced cortical thickness and increased circumference of the forebrain of IVH pups compared to saline and glycerol-treated non-IVH controls. However, cerebral cortical area was comparable between IVH pups and non-IVH controls. This suggests stretching and thinning of cortical mantle, yet preserving the cross sectional cortical area, in IVH pups. Together, motor deficits and ventriculomegaly in our model mimic premature infants with IVH in a number of aspects, suggesting the clinical relevance of the model.
Another key observation was reduced myelination in IVH pups relative to non-IVH controls. In addition, there was no difference in myelination between IVH pups with and without hydrocephalus. Consistent with the findings in our model, an association between IVH and WM injury in premature infants has been reported by many investigators.21,22 Notably, in one old series of premature infants who died with IVH, periventricular leukomalacia of some degree has been observed in 75% cases.23 The possible mechanisms of underlying WM injury in IVH include: 1) destruction of germinal matrix and periventricular WM, 2) concomitant reperfusion injury of the ischemic region around the area of hemorrhage,243) neutrophil and macrophage infiltration as well as apoptosis of neural cells,13 and 4) post-hemorrhagic hydrocephalus.2 Importantly, IVH pups exhibited preservation of myelinated and unmyelinated fibers except for focal axonal degeneration (Fig. 4). Our previous study on acute brain injuries in IVH has revealed evidence of axonal damage in the pups with IVH during the first 48h of life.13 Thus, it seems likely that most of the pathological changes in axonal morphology were transient and were repaired over the next 2-week period. In conclusion, the present animal model of IVH, exhibiting hypomyelination without major axonal degeneration, is similar to preterm survivors of IVH.25
We used DTI to quantify and visualize WM patterns in IVH pups compared to non-IVH controls. Consistent with histologic data and Western blot analyses, we found reduced FA in corpus callosum, fimbria-fornix and corona radiata of IVH pups compared to non-IVH controls. However, FA and area of internal capsule in IVH pups was similar to non-IVH controls. It seems that internal capsule sustained smaller damage than corona radiata and corpus callosum because internal capsule is located at a greater distance from the ventricle compared to corona radiata and corpus callosum. Indeed, immunohistochemistry revealed lesser myelination in the corona radiata and corpus callosum than the internal capsule in IVH pups (Fig. 3). Of note, ex vivo MRI on fixed brain provides very high spatial resolution (eg. 78×94×94μm) because of high signal-to-noise ratio arising from time available for signal averaging.26 Other advantages of MRI are that it is three-dimensional, free of tissue distortion and sectioning artifacts, and that it complements histological immuno-staining methods. Together, our MRI study has established applicability of performing DTI in our model for further mechanistic and therapeutic studies.
In conclusion, the present study describes a novel rabbit pup model of neurologic consequences of GMH-IVH, which displays manifestations of motor deficits and ventricular dilation, similar to human premature infants. In accordance with the clinical features, histological analysis revealed reduced myelination of the forebrain, which was further supported by DTI. This model seems to be an attractive tool for evaluating therapeutic strategies in the prevention and treatment of posthemorrhagic complications in premature infants.
Supplementary Material
Supplementary Fig. 1: Reduced cortical thickness and greater circumference in the forebrain of pups with IVH compared to pups without IVH. A. Nissl’s stained cryosections at 2 coronal levels---mid-septal nucleus (upper panel: A, C) and ventro-lateral nucleus of thalamus (lower panel: B,D): —were evaluated. The cortical thickness and circumference of the coronal brain section were compared. Data are shown as box-and-whisker plot that depicts median,10th, 25th, 75th and 90th percentile as vertical boxes with error bars (n=6–8). (A,B) Cortical thickness of IVH pups (both with and without ventriculomegaly) was significantly reduced compared to saline- and glycerol-treated non-IVH controls. ###P<0.001 for IVH pups with ventriculomegaly vs. glycerol controls. ***P<0.001 for IVH pups with ventriculomegaly vs. saline controls. †P<0.05 for IVH pups without ventriculomegaly vs. glycerol controls. §§P<0.01 for IVH pups without ventriculomegaly vs. saline controls. (C,D) Forebrain circumference of IVH pups with ventriculomegaly was significantly greater compared to saline- and glycerol-treated non-IVH controls. However, forebrain circumference of IVH pups without ventricle dilation was comparable to non-IVH controls. ##P<0.001 for IVH pups with ventriculomegaly vs. glycerol controls. ***P<0.001 for IVH pups with ventriculomegaly vs. saline controls.
Supplemental Table 1: WM size (measured by DTI) in pups with IVH and glycerol-treated non-IVH
Acknowledgments
Supported by Pfizer (PB), NIH/NINDS NS050586 (PB) and NS052519 (FH) grants (PB), Children’s Hospital Foundation at Maria Fareri Children’s Hospital (PB) and Juvenile Diabetes Research Foundation (FH). We thank Joanne Abrahams for technical assistance.
References
- 1.Shennan AH, Bewley S. Why should preterm births be rising? BMJ. 2006;332:924–925. doi: 10.1136/bmj.332.7547.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volpe JJ. Intracranial hemorrhage:Germinal matrix hemorrhage. Neurology of newborn. 2008:517–588. [Google Scholar]
- 3.Balasubramaniam J, Del Bigio MR. Animal models of germinal matrix hemorrhage. J Child Neurol. 2006;21:365–371. doi: 10.1177/08830738060210050201. [DOI] [PubMed] [Google Scholar]
- 4.Goddard J, Lewis RM, Armstrong DL, Zeller RS. Moderate, rapidly induced hypertension as a cause of intraventricular hemorrhage in the newborn beagle model. J Pediatr. 1980;96:1057–1060. doi: 10.1016/s0022-3476(80)80641-x. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramaniam J, Xue M, Buist RJ, Ivanco TL, Natuik S, Del Bigio MR. Persistent motor deficit following infusion of autologous blood into the periventricular region of neonatal rats. Exp Neurol. 2006;197:122–132. doi: 10.1016/j.expneurol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Aquilina KHC, Cherian S, Tucker A, Porter H, Whitelaw A, Thoresen M. A neonatal piglet model of intraventricular hemorrhage and posthemorrhagic ventricular dilation. J Neurosurg (2 Supple Pediatrics) 2007;107:126–136. doi: 10.3171/PED-07/08/126. [DOI] [PubMed] [Google Scholar]
- 7.Cherian S, Whitelaw A, Thoresen M, Love S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 2004;14:305–311. doi: 10.1111/j.1750-3639.2004.tb00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vohr B, Garcia Coll C, Flanagan P, Oh W. Effects of intraventricular hemorrhage and socioeconomic status on perceptual, cognitive, and neurologic status of low birth weight infants at 5 years of age. J Pediatr. 1992;121:280–285. doi: 10.1016/s0022-3476(05)81204-1. [DOI] [PubMed] [Google Scholar]
- 9.Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: Natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87:F37–41. doi: 10.1136/fn.87.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherian S, Thoresen M, Silver IA, Whitelaw A, Love S. Transforming growth factor-betas in a rat model of neonatal posthaemorrhagic hydrocephalus. Neuropathol Appl Neurobiol. 2004;30:585–600. doi: 10.1111/j.1365-2990.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo AV, Welch K, Conner S. Spontaneous germinal matrix and intraventricular hemorrhage in prematurely born rabbits. J Neurosurg. 1982;56:404–410. doi: 10.3171/jns.1982.56.3.0404. [DOI] [PubMed] [Google Scholar]
- 12.Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, Gladson CL, Beardsley DJ, Murdoch G, Back SA, Tan S. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: A model for human cerebral palsy? J Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgiadis PXH, Chua C, Hu F, Collins L, Huynh C, LaGamma EF, Ballabh P. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 2008;39:3378–88. doi: 10.1161/STROKEAHA.107.510883. [DOI] [PubMed] [Google Scholar]
- 14.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier:An overview:Structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Rapoport SI. Osmotic opening of the blood-brain barrier:Principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20:217–230. doi: 10.1023/a:1007049806660. [DOI] [PubMed] [Google Scholar]
- 16.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 17.Bassan H, Limperopoulos C, Visconti K, Mayer DL, Feldman HA, Avery L, Benson CB, Stewart J, Ringer SA, Soul JS, Volpe JJ, duPlessis AJ. Neurodevelopmental outcome in survivors of periventricular hemorrhagic infarction. Pediatrics. 2007;120:785–792. doi: 10.1542/peds.2007-0211. [DOI] [PubMed] [Google Scholar]
- 18.Futagi Y, Toribe Y, Ogawa K, Suzuki Y. Neurodevelopmental outcome in children with intraventricular hemorrhage. Pediatr Neurol. 2006;34:219–224. doi: 10.1016/j.pediatrneurol.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Ohgi S, Akiyama T, Fukuda M. Neurobehavioural profile of low-birthweight infants with cystic periventricular leukomalacia. Dev Med Child Neurol. 2005;47:221–228. doi: 10.1017/s0012162205000447. [DOI] [PubMed] [Google Scholar]
- 20.Spittle AJ, Boyd RN, Inder TE, Doyle LW. Predicting motor development in very preterm infants at 12 months’ corrected age: The role of qualitative magnetic resonance imaging and general movements assessments. Pediatrics. 2009;123:512–517. doi: 10.1542/peds.2008-0590. [DOI] [PubMed] [Google Scholar]
- 21.Larroque B, Marret S, Ancel PY, Arnaud C, Marpeau L, Supernant K, Pierrat V, Roze JC, Matis J, Cambonie G, Burguet A, Andre M, Kaminski M, Breart G. White matter damage and intraventricular hemorrhage in very preterm infants: The epipage study. J Pediatr. 2003;143:477–483. doi: 10.1067/S0022-3476(03)00417-7. [DOI] [PubMed] [Google Scholar]
- 22.Rushton DI, Preston PR, Durbin GM. Structure and evolution of echo dense lesions in the neonatal brain. A combined ultrasound and necropsy study. Arch Dis Child. 1985;60:798–808. doi: 10.1136/adc.60.9.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong DL, Sauls CD, Goddard-Finegold J. Neuropathologic findings in short-term survivors of intraventricular hemorrhage. Am J Dis Child. 1987;141:617–621. doi: 10.1001/archpedi.1987.04460060035027. [DOI] [PubMed] [Google Scholar]
- 24.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: Experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 25.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 26.Chahboune H, Ment LR, Stewart WB, Ma X, Rothman DL, Hyder F. Neurodevelopment of c57b/l6 mouse brain assessed by in vivo diffusion tensor imaging. NMR Biomed. 2007;20:375–382. doi: 10.1002/nbm.1130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: Reduced cortical thickness and greater circumference in the forebrain of pups with IVH compared to pups without IVH. A. Nissl’s stained cryosections at 2 coronal levels---mid-septal nucleus (upper panel: A, C) and ventro-lateral nucleus of thalamus (lower panel: B,D): —were evaluated. The cortical thickness and circumference of the coronal brain section were compared. Data are shown as box-and-whisker plot that depicts median,10th, 25th, 75th and 90th percentile as vertical boxes with error bars (n=6–8). (A,B) Cortical thickness of IVH pups (both with and without ventriculomegaly) was significantly reduced compared to saline- and glycerol-treated non-IVH controls. ###P<0.001 for IVH pups with ventriculomegaly vs. glycerol controls. ***P<0.001 for IVH pups with ventriculomegaly vs. saline controls. †P<0.05 for IVH pups without ventriculomegaly vs. glycerol controls. §§P<0.01 for IVH pups without ventriculomegaly vs. saline controls. (C,D) Forebrain circumference of IVH pups with ventriculomegaly was significantly greater compared to saline- and glycerol-treated non-IVH controls. However, forebrain circumference of IVH pups without ventricle dilation was comparable to non-IVH controls. ##P<0.001 for IVH pups with ventriculomegaly vs. glycerol controls. ***P<0.001 for IVH pups with ventriculomegaly vs. saline controls.
Supplemental Table 1: WM size (measured by DTI) in pups with IVH and glycerol-treated non-IVH