Abstract
Ribulose 1, 5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco) is a globally significant biocatalyst that facilitates the removal and sequestration of CO2 from the biosphere. Rubisco-catalyzed CO2 reduction thus provides virtually all the organic carbon utilized by living organisms. Despite catalyzing the rate-limiting step of photosynthetic and chemoautotrophic CO2 assimilation, Rubisco is markedly inefficient as the competition between O2 and CO2 for the same substrate limits the ability of aerobic organisms to obtain maximum amounts of organic carbon for CO2-dependent growth. Random and site-directed mutagenesis procedures were coupled with genetic selection to identify an “oxygen insensitive” mutant cyanobacterial (Synechococcus sp. strain PCC 6301) Rubisco that allowed for CO2-dependent growth of a host bacterium at an oxygen concentration that inhibited growth of the host containing wild-type Synechococcus Rubisco. The mutant substitution, A375V, was identified as an intragenic suppressor of D103V, a negative mutant enzyme incapable of supporting autotrophic growth. Ala-375 (Ala-378 of spinach Rubisco) is a conserved residue in all form I (plant-like) Rubiscos. Structure-function analyses indicate that the A375V substitution decreased the enzyme’s oxygen sensitivity (and not CO2/O2 specificity), possibly by rearranging a network of interactions in a fairly conserved hydrophobic pocket near the active site. These studies point to the potential of engineering plants and other significant aerobic organisms to fix CO2 unfettered by the presence of O2.
The Calvin-Benson-Bassham (CBB) reductive pentose phosphate pathway provides a way for many organisms to reduce carbon dioxide to organic carbon, a process that is vital for life on earth (1). Rubisco is the rate-limiting enzyme in this pathway, catalyzing the initial steps in both autotrophic carbon assimilation (CO2 fixation or carboxylation) and photooxidative metabolism (O2 fixation or oxygenation) via parallel reaction mechanisms that share a common acceptor intermediate, the enediol form of RuBP (2). Thus, in aerobic organisms, the promiscuity of this enediolate for both CO2 and O2 limits autotrophic CO2 assimilation. Severe limitations imposed on the enzyme’s efficiency, primarily due to the competition between CO2 and O2 at the same active site and the poor turnover rate, have prompted numerous studies directed towards improving the enzyme’s net carboxylation efficiency (2). Thus, an increase in the carboxylation catalytic efficiency (Vc/Kc) and a decrease in the oxygenation catalytic efficiency (Vo/Ko) are desirable. The ratio of the two catalytic efficiencies, VcKo/VoKc,, is defined as the specificity factor (Ω). The Ω value varies greatly among Rubisco enzymes from different species (3, 4) and hence could be a potential target for engineering beneficial changes in global CO2 fixation. Moreover, structurally superimposable proteins from different sources, with up to 90 percent sequence identity, often show vastly different catalytic properties (4). Despite an excellent understanding of the details of the reaction mechanism (5), the molecular basis by which such structurally similar proteins exhibit profound differences in kinetic behavior and substrate specificity is unknown.
Naturally occurring Rubisco enzymes are highly divergent based on primary structure alignments; they have been broadly classified into four different forms (forms I, II, III and IV) with the members of each group sharing between 37–85% in-group identity and an overall sequence identity of ~30% (1). However, x-ray crystal structures indicate a striking similarity among these enzymes at the level of secondary and tertiary structures (6, 7). Forms I, II and III represent enzymes with bona fide Rubisco activity, all of which contain an invariant set of active-site residues, a feature that is absent in the form IV enzymes. Form I enzymes, which are present in plants, algae and many phototrophic and chemoautotrophic proteobacteria and cyanobacteria, comprise a unique class that assemble as hexadecamers with eight ~50-kDa large subunits and eight ~15-kDa small subunits (L8S8). A large-subunit dimer comprises the minimum functional unit in all the four forms, with at least 2 active sites per dimeric unit. Every active site is formed by a set of residues from the C-terminal α/β- barrel domain of one large subunit and a few residues from the N-terminal domain of the neighboring large subunit (1, 2, 6).
Ever since it became feasible to obtain fully active recombinant form I enzyme from Escherichia coli (8, 9) and perform structural analyses (10), the form I Rubisco from the cyanobacterium Synechococcus sp. strain PCC6301 has served as a template of choice to study the effect of structural changes on Rubisco function (4, 11). Indeed, this enzyme closely resembles plant Rubisco and any manipulations that result in beneficial changes in the cyanobacterial enzyme might eventually be applied to plant systems. A system was recently designed for biological selection of randomly mutagenized Synechococcus Rubisco genes in which a Rubisco-deletion-mutant of the metabolically versatile photosynthetic bacteria Rhodobacter sphaeroides or R. capsulatus (strain SB I/II−) serves as a surrogate host (12–14). Using this system allows one to monitor the in vivo effects of changes in Rubisco function since growth of the host directly reflects the properties of the Rubisco molecule that is expressed. Subsequently, a similar approach was also used to develop a non-photosynthetic E. coli-based selection system (15, 16). Photoautotrophic CO2-dependent growth of R. capsulatus requires anaerobiosis, i.e., conditions where Rubisco function is uninhibited by the presence of oxygen. However, this organism is also capable of growth with CO2 as sole source of carbon via the CBB pathway under dark aerobic conditions, using the oxidation of molecular H2 to provide the energy to support growth (4). Under such chemoautotrophic growth conditions, oxygen serves as the terminal electron acceptor, thus potentially providing a means to select enzyme variants with an improved ability to support CO2-dependent growth and interact favorably with CO2 in the presence of normal inhibitory levels of O2. Because of the versatile metabolic capacity of R. capsulatus to enable CO2-dependent growth both in the absence or presence of O2, under photosynthetic or nonphotosynthetic autotrophic conditions, respectively, we have undertaken studies to determine whether mutant forms of Rubisco may be isolated that support preferential growth under any of these conditions. In particular, one may easily vary the exogenous CO2 and O2 concentrations, so as to mimic the physiological milieu of heterologously expressed foreign Rubiscos (12, 13, 14, 17, 18).
Recently, a key region was identified in the oxygen-sensitive archaeal form III Rubisco that implicated residues of this region in specific interactions with oxygen (18). Indeed, amino-acid substitutions in this region, at Ser-363 and Met-295 of the Archaeglobus fulgidus enzyme, were shown to directly affect the Ko of this enzyme. In addition, changing Met-295 to an aspartate resulted in a significant increase in Ω. Sequence alignments indicated that Ser-363 of the A. fulgidus form III enzyme is equivalent to a conserved alanine in the form I enzymes (Ala-378 in spinach Rubisco) and an isoleucine in the form II enzymes (Ile-367 in Rhodospirillum rubrum Rubisco). Interestingly, Ser-368, which is adjacent to the Ile-367 in R. rubrum Rubisco, was previously identified as being important for oxygenase activity (19). In the current study, we have found that a substitution in the conserved alanine residue (A375V) in a hydrophobic pocket near the active site of the Synechococcus enzyme was able to suppress the negative phenotype caused by a D103V substitution at the enzyme’s dimer-dimer interface via long-range interactions. Furthermore, the A375V substitution (equivalent to Ala-378 of spinach Rubisco and Ser-363 of the A. fulgidus enzyme) appeared to decrease the oxygen sensitivity of the enzyme, which is manifested as CO2-dependent growth of the host R. capsulatus under aerobic conditions not supported by the wild-type enzyme. These studies suggest that residues within this conserved hydrophobic region might play a key role with respect to oxygen sensitivity by evolutionarily diverse forms of Rubisco.
MATERIALS AND METHODS
Mutagenesis, matings and selection
Random mutagenesis of the Synechococcus wild-type or the D103V-mutant rbcLS genes was achieved through error-prone PCR amplification with Taq DNA polymerase (Invitrogen) in the presence of 0.1 mM MnCl2. The resulting fragment (1.8 kb) was directionally cloned into pRPS-MCS3, a broad-host range vector (12) using T4 DNA ligase (Invitrogen). Site-directed mutagenesis was accomplished using the QuikChange kit from Stratagene (Stratagene, La Jolla, CA). Wild-type and mutant genes that had been cloned into pRPS-MCS3 were expressed in R. capsulatus strain SBI/II− via tri-parental matings involving an E. coli strain that carried a helper plasmid, pRK2013 (20). The trans-complemented R. capsulatus colonies were selected under either chemoheterotrophic or photoheterotrophic conditions as described previously (12, 13, 21).
R. capsulatus growth conditions
Aerobic chemoheterotrophic growth was accomplished on peptone yeast extract plates or in a broth containing Ormerod’s basal salts, 6 g/l peptone, 5 g/l yeast extract, 10 mM NaCl, 3 mM KCl, and 0.1 µg/ml biotin. Both media were supplemented with 1 µg/ml of nicotinic acid and 1 µg/ml of thiamine-hydrochloride (13, 16, 21). The plates were incubated at 30°C for 3 days, when using complex media, and at the same temperature for 1–2 weeks when using defined media. Growth under selective conditions, either in the presence of 0.4% DL-malate (photoheterotrophic) or in the absence of any organic carbon (photoautotrophic growth) was performed using Ormerod’s minimal medium supplemented with 1 µg/ml of nicotinic acid and 1 µg/ml of thiamine-hydrochloride, as described previously (12). For photoautotrophic growth, in the presence of either 5% or 1.5% CO2, the jars were flushed for 15 min with pre-mixed 5% CO2/95% H2 or 1.5% CO2/98.5% H2, respectively. For aerobic chemoautotrophic or mixotrophic growth in a CO2/H2-enriched atmosphere, plates were incubated in the dark in tightly-sealed jars containing air and the CO2/H2-generating kits (Oxoid). To carry out biochemical analysis, cells in the stationary phase (OD660 ~3) were harvested by centrifugation at 8000 × g for 10 min at 25°C, washed with TE buffer (100 mM Tris-HCl (pH 8.0), 1 mM EDTA), and stored at −80°C prior to cell lysis.
Preparation of cell extracts, purification of Rubisco and biochemical analysis
Wild-type and mutant Synechococcus Rubisco enzymes were purified as recombinant proteins after expressing their genes with an N-terminal hexa-histidine tag using the vector pET-28a (Novagen). A two-step purification using affinity (Ni-NTA agarose, Qiagen) and ion-exchange (UnoQ, Bio-Rad) chromatographies resulted in proteins that were >95% pure, as analyzed by SDS-PAGE. The proteins were dialyzed into a Tris buffer (100 mM Tris-HCl, pH 8.0, 1 mM EDTA, 10 mM MgSO4, 1 mM DTT, and 2 mM NaHCO3), mixed with 20% glycerol, and stored as aliquots at −80°C. R. capsulatus cell pellets from phototropically-grown cultures that had been stored at −80°C were thawed on ice and resuspended in TEM buffer (100 mM Tris-HCl, pH 8.0, 1 mM EDTA, and 5 mM β-mercaptoethanol). Cells were disrupted by sonication and low speed supernatants were obtained by microcentrifugation at 16,100 g for 10 min at 4°C. Rubisco activities and protein concentrations in both R. capsulatus crude extracts and purified protein samples were determined as described previously (22, 23). Rubisco purification and the levels of expression in cell-extracts of R. capsulatus cultures were monitored via SDS-PAGE (24) and Western-blot analysis, as described previously (20).
Enzyme assays and kinetic measurements
RuBP carboxylase activity was measured radiometrically via the incorporation of acid-stable 14C from NaH14CO3 (22). The Ω values of purified enzymes (20 µg) were measured from assays performed under saturating (1.23 mM) O2 concentrations (13). [1-3H] RuBP, required for these assays, was synthesized and purified by standard methods (25). The kinetic constants kcat, Kc and Ko were determined from simultaneous assays performed in vials flushed with either 100% N2 or 100% O2, as per the procedure described elsewhere (26) with modifications to the substrate concentration range. The KRuBP values were measured similarly in aerobic assays performed in sealed vials by varying the RuBP concentrations from 5 to 1280 µM. A constant amount of NaHCO3 (10 mM) was used in these assays.
RESULTS
Mutagenesis, suppressor isolation and growth phenotypes
Previous studies had indicated that surface residues involved with large subunit interactions influence the Michaelis constant for CO2 (Kc) of the Synechococcus 6301 enzyme (13, 14). Unlike the wild-type enzyme, an Asp-103-to-Val mutant failed to support CO2-dependent growth of cultures of R. capsulatus strain SBI/II− in a 5% CO2/95 % H2 atmosphere (13). Asp-103 from one dimeric pair interacts with Ser-367 of the second dimeric pair via hydrogen bonding. In order to better understand how Asp-103, which is far from the active site, might influence catalysis and Rubisco-dependent growth, additional rounds of random mutagenesis were instituted. The rationale was that suppressor mutation(s) might be isolated that would overcome the effects of the D103V mutation and would provide further clues as to the interaction of Asp-103 with other residues that might directly or indirectly affect catalysis. This was deemed a highly feasible modus operandi since we could merely select for second-site suppressors of D103V that allowed for CO2-dependent growth in R. capsulatus strain SBI/II−.
With the D103V construct as the starting point, several separate and parallel random mutagenesis and suppressor selection studies were performed in the R. capsulatus strain SBI/II− background under CO2-dependent growth conditions. Using SBI/II− as an indicator strain resulted in mutant enzymes which overcame the negative growth phenotype of the D103V mutant. After screening about 2000 trans-complemented colonies of R capsulatus, isolated from 5 independent mutagenesis experiments, five colonies were able to reproducibly support photoautotrophic growth. Plasmid isolation from R. capsulatus and DNA sequencing confirmed that the suppressor mutation, i.e. a codon change from GCT to GTT, resulted in an alanine to a valine substitution at residue 375 of the large subunit. Whereas all the five plasmids had identical base changes that resulted in the A375V substitution, one of them also carried an additional mutation in the large-subunit gene (rbcL) that would cause phenylalanine at position 97 to be changed to leucine. Site-directed mutagenesis and subsequent screening showed that neither the F97L single mutant nor the F97L/D103V double mutant were able to complement R. capsulatus strain SBI/II− to photoautotrophic growth. These results were consistent with the observation that in four of the five plasmids isolated, a single-mutant substitution at Ala-375 (to a valine) was sufficient to suppress the negative phenotype caused by the D103V substitution. The A375V substitution was capable of suppressing the “negative” effects caused by both the D103V enzyme itself and D103V together with F97L. Thus, the A375V substitution on the same gene as the D103V substitution is, by definition, an intragenic suppressor that restores photoautotrophic growth when the resulting enzyme is used to complement the host R. capsulatus strain, SB I/II− (13). Several subsequent independent mutagenesis and selection experiments over the past six years by three different investigators all resulted in the isolation of the A375V enzyme as a specific suppressor of the D103V mutant enzyme. To further assess the specificity of the A375V suppressor, this alteration was combined with a previously-identified G176D substitution that negatively affects enzyme activity and the ability to allow CO2-dependent growth in R. capsulatus strain SBI/II−. Gly-176 is found in a completely unrelated region of the Rubisco large subunit (14). A375V was unable to suppress the negative phenotype of G176D. The apparent specificity of A375V towards mitigating the effects of D103V suggests structural correlations between Ala-375 and Asp-103 that influence significant aspects of catalysis.
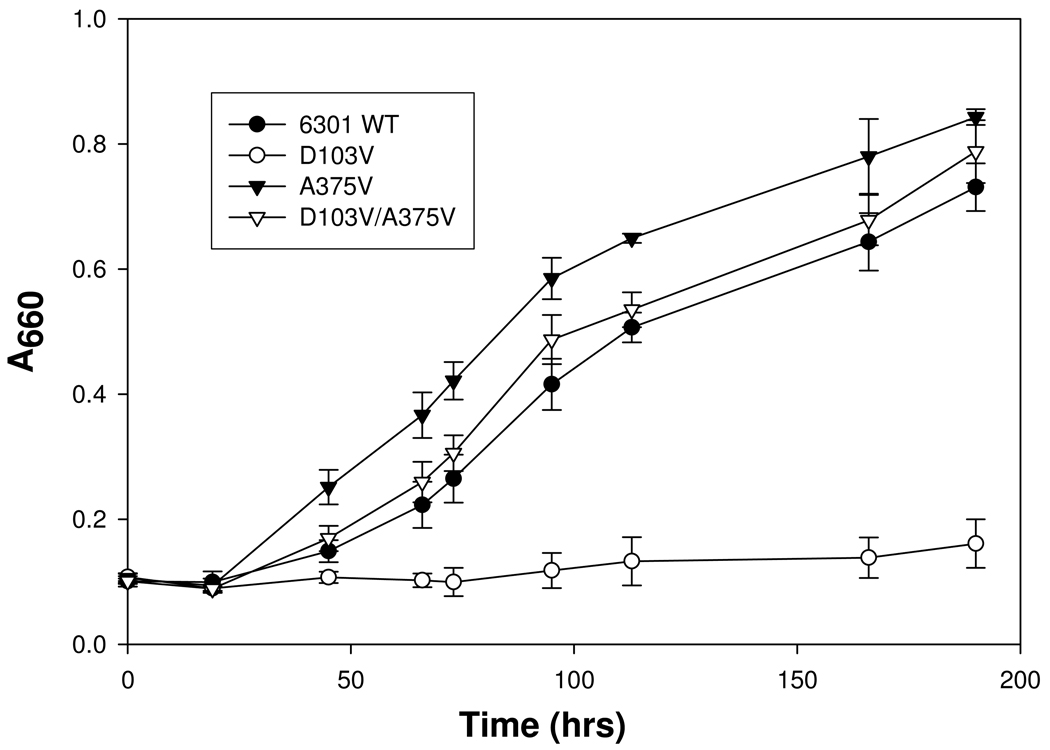
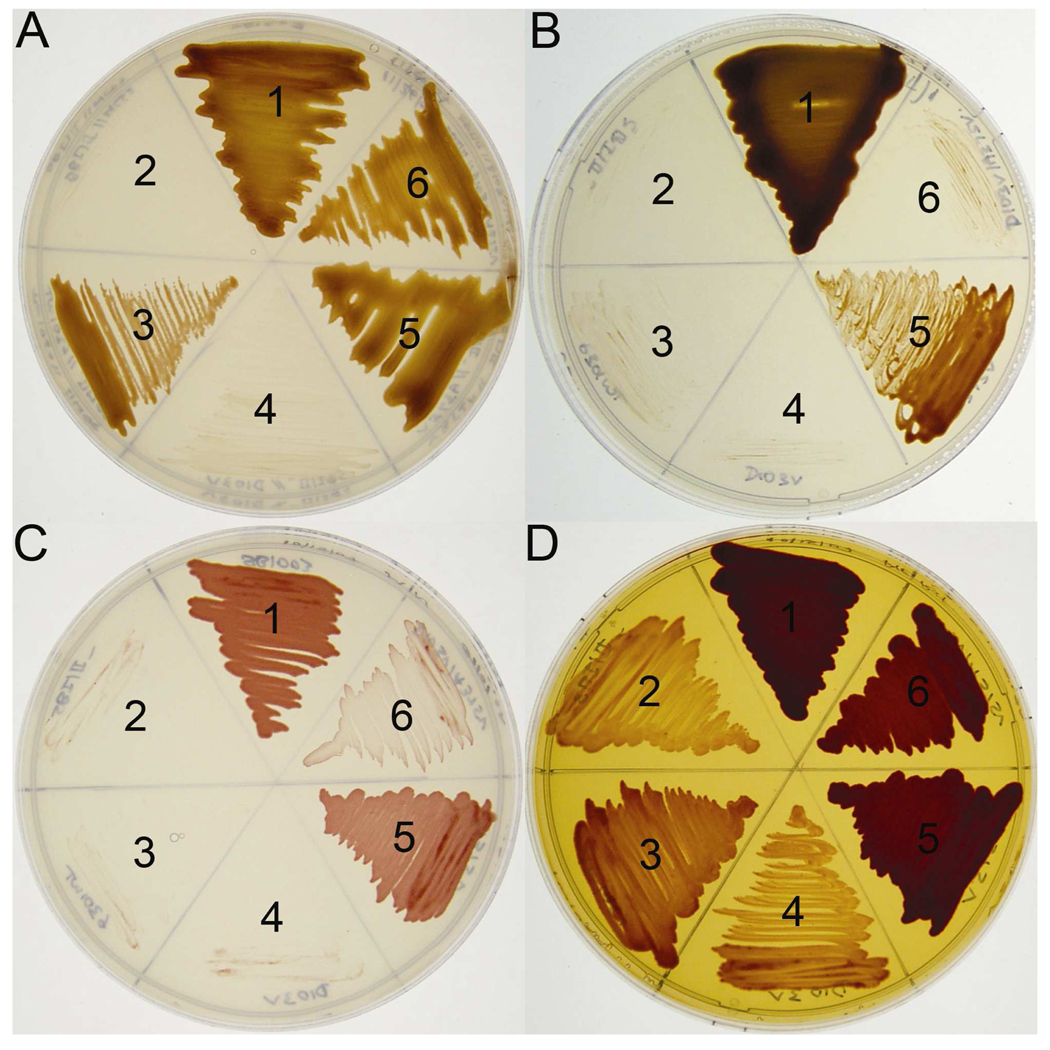
The repeated isolation of the same suppressor (A375V) for D103V from several independent experiments and the strikingly conserved nature of Ala-375 among all form I enzymes, and its conservation as isoleucine in form II and serine in form III enzymes, provided a rationale for the creation of A375V, A375I and A375S single mutant enzymes via site-directed mutagenesis. After construction and verification of the sequences of these site-directed mutants of rbcL, they were trans-complemented along with the wild-type small-subunit gene (rbcS) into R. capsulatus strain SB I/II− (21) via triparental matings and analyzed alongside wild type, D103V, A375V, and D103V/A375V mutant enzymes. Whereas neither A375I nor A375S could support anaerobic photoautotrophic growth, both A375V and D103V/A375V mutants were fully capable of supporting anaerobic phototrophic CO2-dependent growth of the R. capsulatus SBI/II− host strain at high levels of CO2 (Figure 1, Figure 2A). Unlike the wild type Synechococcus enzyme, however, the A375V mutant was able to support anaerobic photoautotrophic growth at a limiting (1.5%) CO2 concentration (Figure 2B). The inability of the wild-type Synechococcus enzyme to support anaerobic photoautotrophic growth at 1.5% CO2 is most likely due to this enzyme’s intrinsically high Kc value, as demonstrated and discussed in several earlier studies (4, 12, 13, 16). The results presented here confirm this conclusion as the wild-type Synechococcus enzyme was perfectly able to support anaerobic CO2-dependent growth at high levels (5 %) of CO2 (Figure 2A). Most intriguing, however, was the unique ability of the A375V enzyme, and to a lesser extent the D103V/A375V suppressor mutant, to support aerobic CO2-dependent chemoautotrophic growth (Figure 2C) and enhance aerobic mixotrophic growth in a complex organic growth media (Figure 2D). Inasmuch as Ala-375 resides in a hydrophobic pocket previously identified to be important for sensitivity to O2 (18, 19), the ability of mutant cyanobacterial enzymes to specifically enable the R. capsulatus indicator strain to grow chemoautotrophically under aerobic conditions not supported by the wild-type Synechococcus enzyme (even at high levels of CO2) suggests that this residue confers reduced sensitivity to oxygen inhibition (Figure 2C). The enhanced growth of both the A375V and D103V/A375V mutants under mixotrophic conditions in a CO2-enriched atmosphere (Figure 2D) is also clearly attributable to the ability of these enzymes to fix and utilize CO2 in aerobic environments. Moreover, the ability of the A375V mutant enzyme to support photoautotrophic growth under limiting CO2 concentrations (Figure 2B) indicated that this mutation is a “positive” change relative to the wild type enzyme.
FIGURE 1.
Wild-type and mutant Synechococcus Rubisco-dependent growth of R. capsulatus strain SBI/II− under CO2-dependent photoautotrophic growth conditions in sealed tubes under an atmosphere of 5–10 % CO2/90–95 % H2. Each data point represents the average ± SD determined from four independent experiments.
FIGURE 2.
Growth phenotypes of R. capsulatus strain SBI/II− complemented with wild-type and mutant Synechococcus Rubisco enzymes. (A) anaerobic CO2-dependent photoautotrophic growth under an atmosphere of 5–10 % CO2/90–95 % H2; (B) anaerobic CO2-dependent photoautotrophic growth under an atmosphere of 1.5 % CO2/98.5 % H2; (C) aerobic CO2-dependent chemoautotrophic growth under an atmosphere of 5–10 % CO2/40–45 % H2/50 % air; and (D) aerobic mixotrophic growth on a complex peptone-yeast extract medium under an atmosphere of 5–10 % CO2/40–45 % H2/50 % air as previously described (1). In all four plates, sector 1 contains wild-type R. capsulatus strain SB1003 with its endogenous form I and form II Rubisco genes that allow growth under all conditions (36); sector 2 contains R. capsulatus strain SBI/II− lacking endogenous functional Rubisco genes; sectors 3 to 6 contain R. capsulatus strain SBI/II− with plasmids containing the wild-type Synechococcus rbcLrbcS genes (sector 3), rbcL-D103V/rbcS (sector 4), rbcL-A375V/rbcS (sector 5), and rbcL-D103V/ A375V/rbcS (sector 6).
Rubisco expression, activity and in vitro stability
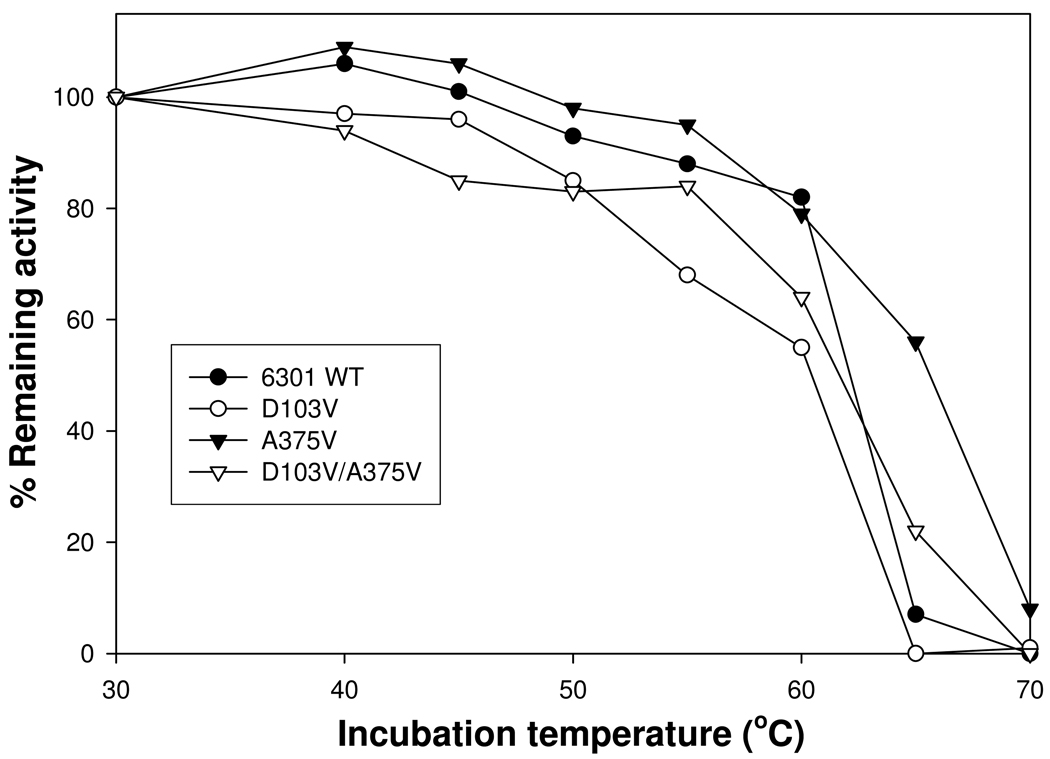
Western immunoblot analysis showed that approximate wild-type levels of holoenzyme were present in R. capsulatus cultures containing D103V, A375V and D103V/A375V mutant proteins (see Figure 1S of the Supporting Information). Furthermore, the Rubisco activities of crude extracts prepared from phototrophically-grown A375V and D103V/A375V R. capsulatus cultures were comparable to the wild type, suggesting that the observed phenotypes arose from subtle changes in protein structure and were not due to major changes in holoenzyme levels in vivo. All the mutant genes were subsequently expressed in E. coli and recombinant enzymes purified for enzymatic analysis. Both A375I and A375S had insignificant levels of Rubisco activity, perhaps because the changes led to the production of unstable enzymes or enzymes that were too severely compromised to be functional and hence were not used for further studies. However, all the other mutant enzymes had significant amounts of Rubisco activity, ranging from 12 % (for A375V) to 63 % (for D103V/A375V) of the wild-type level of activity (Table 1). To further assess the effect of substitutions on the structural stability of purified enzymes in vitro, thermal inactivation assays were carried out. Whereas the wild type, D103V and D103V/A375V mutant enzymes retained comparable amounts of Rubisco activity (0–20%) after a 10-min incubation at 65°C, the A375V single-mutant enzyme consistently retained about 60% of its original activity at this temperature (Figure 3). Thus, the A375V substitution likely had a pronounced effect on both the structure and function of the enzyme, both of which could influence the behavior of the A375V mutant enzyme in vitro (Table 1) and in vivo (Figure 2).
TABLE 1.
Kinetic properties of purified recombinant Rubisco enzymes
| Enzyme | kcat (s−1) | Ω a (VcKo/VoKc) |
Kca (µM CO2) |
Koa (µM O2) |
Ko/Kcb | Vc/Vob | KRuBPa (µ M) |
|---|---|---|---|---|---|---|---|
| Wild type | 4.9 ± 0.9 | 39 ± 2 | 186 ± 13 | 850 ± 66 | 4.6 | 8.5 | 27 ± 3 |
| D103V | 4.9 ± 0.3 | 37 ± 3 | 388 ± 37 | 866 ± 40 | 2.2 | 16.8 | 25 ± 2 |
| A375V | 0.6 ± 0.1 | 32 ± 3 | 143 ± 7 | 1088 ± 43 | 7.6 | 4.2 | 34 ± 3 |
| D103V/A375V | 3.1 ± 0.3 | 30 ± 2 | 294 ± 31 | 1153 ± 142 | 3.9 | 7.7 | 269 ± 33 |
Values are the means ± standard deviation (n−1) of three separate enzyme preparations.
Calculated values.
FIGURE 3.
Thermal inactivation of Synechococcus 6301 wild-type and mutant enzymes. Aliquots of each enzyme were incubated at the respective temperatures for 10 min, cooled on ice and assayed for the remaining amount of carboxylase activity. For each enzyme sample, the amounts of activity corresponding to the different incubation temperatures were normalized against the levels of activity calculated from the same enzyme incubated at 30°C. Data is representative of 2 identical experiments that gave similar results. The A375V mutant enzyme appears to retain at least 55% of the original activity after the 65°C incubation, indicating that the mutation likely results in major structural changes that should contribute to the catalytic properties of the enzyme assayed in vitro.
Catalytic properties of A375V/D103V and A375V mutant enzymes
As previously observed (12), the Kc value of the D103V enzyme is higher than the wild type enzyme, accounting for its “negative” phenotype (Table 1). By contrast, the Kc value of the A375V enzyme is 23% lower than the wild-type enzyme, and this effect is partially manifested in the D103V/A375V suppressor enzyme as well. This may indicate that both Asp-103 and Ala-375 interact with a common structural or functional element that ultimately determines the Michaelis constant for CO2. Thus, the A375V substitution likely causes changes in these structural interactions that ultimately contribute to the enhanced Kc and ability to support anaerobic CO2-dependent phototrophic growth in the R. capsulatus indicator strain. An observation that is unique to the A375V substitution is its ability to cause opposing changes in the Michaelis constants for CO2 and O2. Indeed, the D103V/A375V and the A375V enzymes exhibit 28–36% increases in their Ko values (Table 1). As a result of these opposing changes in the Michaelis constants for CO2 and O2, the Ko/Kc ratio of the A375V enzyme (7.6) is considerably higher than the wild-type enzyme (4.6), while the Ko/Kc ratio of the D103V enzyme (2.2) is only about half of the wild-type value, which is restored to about 85% of the wild-type value in the D103V/A375V suppressor mutant. The inability of wild-type and D103V-mutant enzymes to support aerobic CO2-dependent chemoautotrophic growth (Figure 2C) can be attributable to their low Ko values. The beneficial changes in Kc values, relative to D103V, are likely responsible for conferring photoautotrophic growth competence to the A375V and D103V/A375V mutants (Figure 2A) and more specifically for enabling the A375V mutant to facilitate growth at lower CO2 concentrations compared to the wild-type enzyme (Figure 2B). Much like the wild type enzyme, the higher Kc value of the D103V/A375V mutant enzyme is likely the reason for the inability of this enzyme to support growth at 1.5% ambient CO2 concentrations (Figure 2B). Despite the more favorable Ko/Kc ratio, the Ω (VcKo/VoKc) values of the A375V and D103V/A375V enzymes are slightly lower than the wild-type value (Table 1). It may also be inferred that the calculated Vc/Vo ratios of both the A375V and D103V/A375V enzymes were changed in a seemingly unfavorable direction. Moreover, mutant enzymes containing the A375V substitution showed decreases in kcat and the D103V/A375V suppressor mutant has a pronounced change in its KRuBP value (Table 1). The rate-determining step of Rubisco’s enzymatic mechanism is the addition of either CO2 or O2 to the 2, 3-enediol(ate), rather than the enolization of RuBP that yields the 2,3-enediol(ate) (2). Consequently, an increase in the enzyme’s KRuBP value may also not limit its ability to support growth so long as the physiological levels of RuBP are in excess. Subtle changes in protein expression, not readily discernible by SDS-PAGE and Western-blot analysis, or the differential structural stabilities and regulatory interactions may offset kcat defects. Indeed, diverse Rubiscos or mutant forms of the same Rubisco, with lower kcat values, have been shown to confer photoautotrophic growth of Rhodobacter Rubisco-deletion strains at rates comparable to wild type proteins (12, 13). Thus, it is likely that the opposing changes in Ko and Kc values (and consequent increase in the Ko/Kc ratio) may be the major determinants enabling enzymes containing the A375V substitution to support aerobic CO2-dependent (dark) chemoautotrophic growth under conditions where the wild-type or D103V negative mutant enzymes cannot (Figure 2C).
DISCUSSION
The involvement of a common set of active-site residues for carboxylation and oxygenation has precluded identification of the molecular basis for Rubisco’s differential interaction with its gaseous substrates CO2 and O2 (7). As more divergent Rubisco enzymes with differing Ω values are examined (1, 4), phylogenetic analyses, combined with structure-function studies, should increasingly contribute to delineating the molecular basis for the enzyme’s ability to distinguish between the two competing substrates (27, 28). One might predict that genetic engineering of a virtually “oxygen-insensitive” Rubisco in a photosynthetic host might provide a selective growth advantage under aerobic conditions.
In several previous studies it was noted that photosynthetic bacteria expressing cyanobacterial Rubisco genes exhibit unusual sensitivity to the CO2 levels provided in H2/CO2 gas mixtures used to support anaerobic photoautotrophic growth (12–14). By simply allowing CO2 to accumulate in sealed growth vessels, or by increasing the level of CO2 in H2/CO2 bubbled cultures, it was shown that the limitation in growth was related to the CO2 concentration provided to the cultures. Providing different environmental growth conditions to select for mutant Rubisco proteins is particularly relevant to cyanobacterial Rubisco. This enzyme’s normal habitat is within the carboxysomes of its natural cyanobacterial host, where the enzyme is enriched with high levels of CO2, negating any problem caused by this enzyme’s inherently poor Kc. However, when the cyanobacterial Rubisco genes are expressed in R. capsulatus, where there is no apparent or substantial CO2-concentrating mechanism, CO2-dependent growth is directly tied to the properties of the Rubisco that the organism is made to utilize (4, 12, 13, 14, 16, 17). Indeed, even relatively small differences in the Kc values of cyanobacterial Rubisco appear to be well delineated by the subsequent phenotypic response of R. capulatus (12–14). Such effects on growth provide convenient selection strategies to isolate mutations that affect cyanobacterial Rubisco function. Particularly intriguing for the selection purposes is the inability of the wild-type cyanobacterial Rubisco to support aerobic CO2-dependent chemoautotrophic growth in R. capsulatus strain SBI/II− (21), whereas Rubisco genes from other sources that encode proteins with more favorable properties are able to support aerobic chemoautotrophic growth.
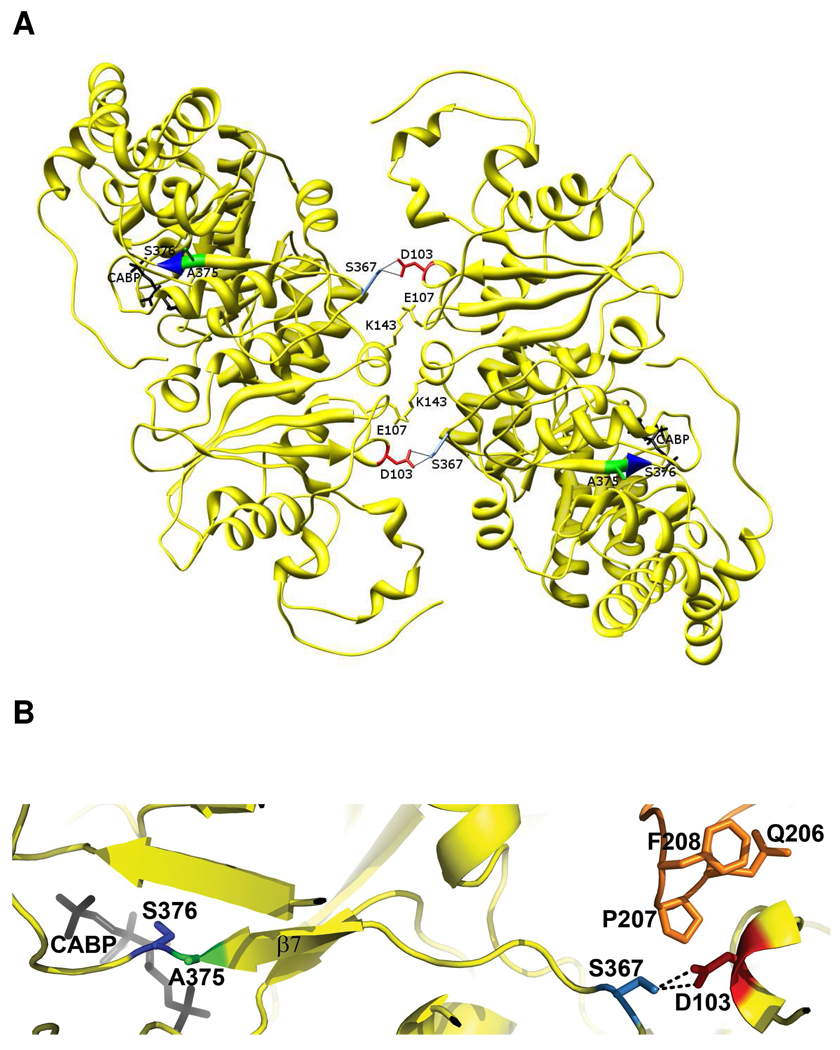
Because Asp-103 of the Synechococcus large subunit is involved in a pair of reciprocal hydrogen-bond interactions with Ser-367 at the interface of large-subunit dimers (Figure 4A), this hydrogen-bond disruption was thought to be responsible for bringing about conformational changes in the holoenzyme that resulted in an increased Kc. Being far away from the active site, it was intriguing that the D103V mutation had a selective effect on the Kc value, with little or no changes in the other kinetic constants (13) (Table 1). A more detailed analysis of the x-ray crystal structure of Synechococcus Rubisco indicates that the D103V substitution at the dimer-dimer interface could very well be affecting the active site in the adjacent large-subunit, because its hydrogen-bond partner, Ser-367, is adjacent to the β-strand (β-7) that leads into the active site. Asp-103 is also within 4Å from residues Gln-206, Pro-207 and Phe-208 of yet another large subunit, which are present in the same strand as the active-site residues Lys-198, Asp-200 and Asp-201 (Lys-201, Asp-203, and Asp-204 in spinach Rubisco) (Figure 4B). Thus, suppressor selection might have been expected to result in the identification of one or more residues in these regions. The identification of A375V as the specific suppressor for D103V is in support of this hypothesis, although it is curious that none of the other residues were selected after random mutagenesis. Perhaps this can be attributed to limitations imposed by the methodology employed; i.e., the requirement for absolutely favorable substitutions (structurally and functionally viable) to occur with an appropriate frequency and among all possible base-changes sampled by error-prone PCR. Statistically, single-base changes best suit a scenario like this, which is the case with suppressor A375V identified in this study.
FIGURE 4.
(A) Interaction of Asp-103 (red) from one monomer of a dimer of Synechococcus Rubisco large subunits with Ser-367 (light blue) of a monomer from a different dimer (1RBL) (37). There are two such interactions for each pair of interacting monomers (yellow). (B) Close-up view of the surface interactions of Asp-103 from one subunit with Ser-367 of another subunit and residues Gln-206, Pro-207 and Phe-208 of yet another large subunit (orange). Ser-367 is on the same strand as Ala-375 (green), which contacts active-site Ser-376 (blue). Residues Gln-206 to Phe-208 are only a few residues away from the active-site residues Lys-198, Asp-200 and Asp-201.
Ala-375 of the Synechococcus enzyme is adjacent to conserved active-site Ser-376 (Ser-379 in spinach form I Rubisco and Ser-368 of R. rubrum form II Rubisco) (Figure 4B). Ser-376, and its equivalent residue in other Rubiscos, forms a hydrogen bond with the C3-hydroxyl group of the substrate RuBP (5). It is thus not surprising that the A375V substitution causes alterations in the KRuBP (Table 1). The proximity to the active-site Ser-376 also explains why all the three substitutions at Ala-375 result in substantial loss of carboxylation activity. Ser-376 is only 9 residues away from Ser-367 and could very well be the link to the active site that ultimately determines the changes in Kc caused by the loss of the D103-S367 hydrogen bond (Figure 4B). In previous studies where various substitutions were made in Ser-376 of the cyanobacterial enzyme, or the equivalent residues in the R. rubrum form II and Chlamydomonas form I enzymes, they all resulted in substantial loss of carboxylase activity and elevated KRuBP values. In each instance the enzymes became more oxygen insensitive (19, 29, 30). Thus, the decreased oxygen sensitivities and kcat values, and the increased KRuBP values that accompany the A375V mutant substitution observed here in either the wild type or the D103V mutants (Table 1) could very well be caused by similar changes in the interactions between active-site Ser-376 and the substrate and/or Rubisco reaction intermediates. A manifestation of these changes in structural interactions could be seen in the form of the increased thermal stability of the A375V mutant enzyme (Figure 3). Notably, the S368A substitution in the R. rubrum (form II) enzyme caused an increase in the Ω value (19), whereas the equivalent substitution in the Chlamydomonas Rubisco (30) or the A375V substitution in the Synechococcus Rubisco (Table 1) result in lower Ω values relative to the wild type. This may be due to subtle differences in the interactions involving these residues in the form I and form II enzymes.
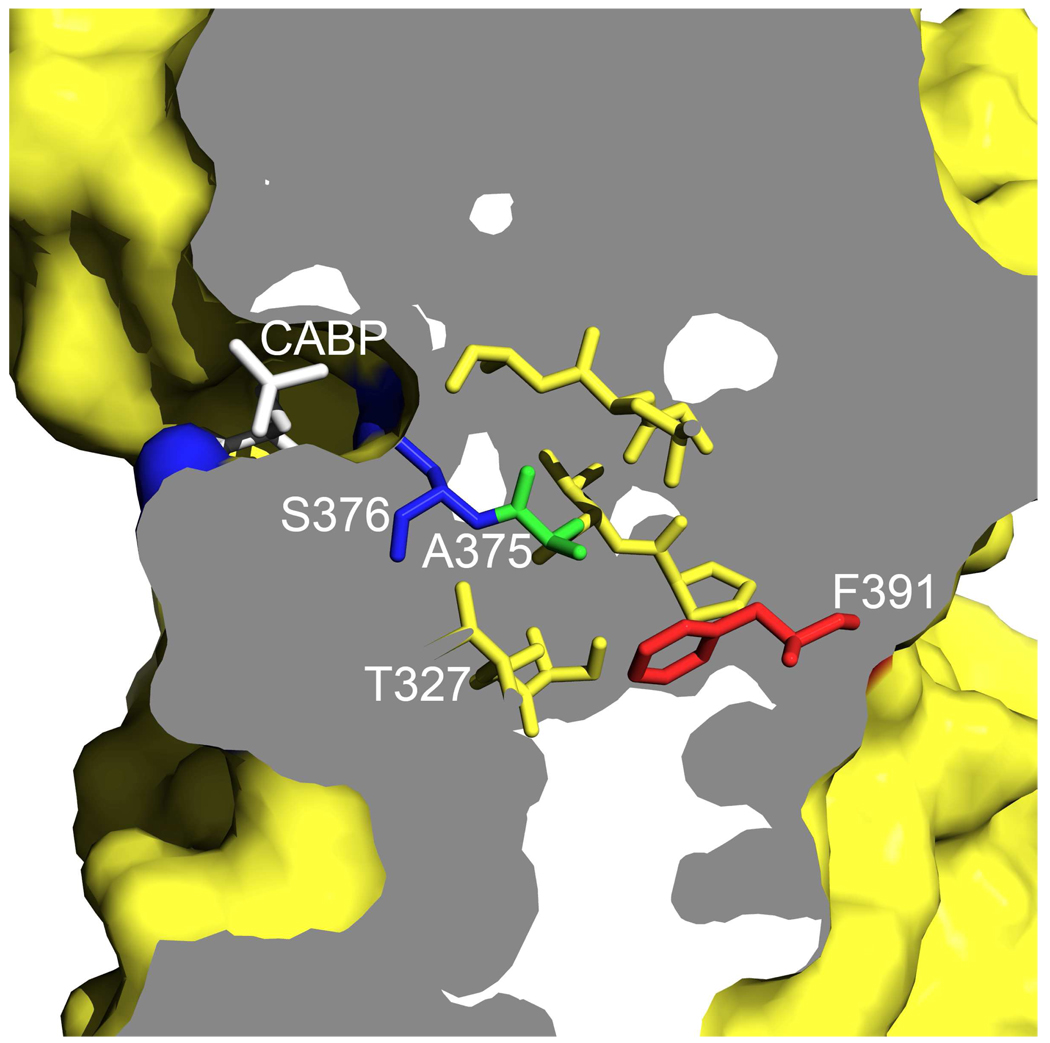
It is apparent that Ala-375 or its equivalent residue in other Rubisco enzymes (Ile or Ser in form II and III, respectively) is surrounded by a hydrophobic pocket of residues, a feature that appears to be common to all Rubisco enzymes. Substitutions (Ile and Val) at the equivalent Ser-363 in the A. fulgidus form III enzyme (18) and an S368A substitution in the R. rubrum form II enzyme (19) also resulted in enzymes that were less sensitive to O2. Because divergent Rubisco enzymes also have dissimilar catalytic properties (2–4), it is thus quite likely that the hydrophobic pockets surrounding Ala-375 (Synechococcus) or the equivalent residues in other Rubiscos might play a role in determining the enzyme’s sensitivity to O2. Further analysis of residues in this region should provide additional insights as to why diverse Rubiscos exhibit differences in their interactions with the substrates CO2 and O2. Certainly, with other proteins that require or use O2 as a substrate, it has been observed that hydrophobic areas or pockets facilitate O2 diffusion to the protein interior (32, 33). With respect to Rubisco, the aforementioned hydrophobic region may influence or facilitate the diffusion of oxygen to the active site (Figure 5). It can be seen in this cross-sectional view of the large-subunit monomer that the substrate analog, CABP, is present on one side of the surface, in a cleft juxtaposed by the active-site residues Lys-331 and Ser-376 (blue). Lys-331 co-ordinates with the carboxyl group of CABP that would be analogous to the incoming CO2. The side chain of residue Ser-376, which seems to be involved in differential O2 sensitivity, is on the other side of the CABP. The hydrophobic pocket of residues extending from Ala-375 leads to residue Phe-391 (red) on the other side of the surface of the protein. One might thus envision that the hydrophobic pocket shown here (Figure 5) might provide a facile diffusion channel to the active site from the opposite surface of the large-subunit monomer.
FIGURE 5.
A hypothetical model for oxygen diffusion into the RubisCO active site via the hydrophobic pocket of residues. The figure shows the cross-sectional view of a surface rendering of the α/β-barrel motif of a single large subunit from the Synechococcus 6301 RubisCO (1RBL) (37). The active site, marked by the presence of CABP (black), is indicated. The active-site residues, loop-6 Lys-331 and Ser-376 (blue), are on either side of the carbon that is attacked by CO2 or O2 in the rate-limiting step of the reaction mechanism. Ser-376 is adjacent to the hydrophobic pocket of residues that have been proposed to play a role in oxygen sensitivity. Residue Phe-391 (red), one of the hydrophobic-pocket residues, appears to be positioned at the surface opposite to the active site.
In summary, we have used the surrogate R. capsulatus system to isolate mutant forms of cyanobacterial Rubisco that greatly improve our perception of residues of the protein that influence oxygen sensitivity and mitigate important kinetic properties. This study clearly illustrates that changing Ala-375 to a more bulky valine residue influences both Ko and Kc of cyanobacterial Rubisco such that the A375V enzyme possesses a relatively high Ko/Kc ratio and this mutant enzyme appears inherently more stable than the wild-type enzyme. These properties, either singly or in combination, or with the mediation of other presently unknown factors, contribute to conferring the A375V mutant enzyme with the unique ability to support aerobic CO2-dependent growth in R. capsulatus. Moreover, it is apparent from the results of these studies that changes in the kcat and/or any perceived trade-offs between kcat and substrate specificity (34) may not be inimical for the construction or selection of Rubisco enzymes capable of substantial CO2 fixation in the presence of high levels of oxygen. The current study represents a further effort to understand aspects of the bifunctionality of Rubisco and could very well form the basis for future genetic engineering of an “oxygen-insensitive” Rubisco.
Supplementary Material
Footnotes
This study was supported by grant GM24497 from the National Institutes of Health and grants DE-FG02-07ER64489 and DE-FG02-91ER20033 the offices of Biological and Environmental Research (Genomics: GTL Program) and Energy Biosciences, respectively, of the U.S. Department of Energy.
Abbreviations: Rubisco, D-ribulose-1,5-bisphosphate carboxylase/oxygenase; RuBP, D-ribulose 1,5-bisphosphate; Vc, Vmax for carboxylation; Vo, Vmax for oxygenation; Kc, Michaelis constant for CO2; Ko, Michaelis constant for O2; Ω, CO2/O2 specificity factor
SUPPORTING INFORMATION AVAILABLE
Western immunoblots (Fig. S1A, B) depicting the levels of wild type and mutant Synechococcus Rubisco proteins in photoheterotrophically and photoautotrophically grown R. capsulatus strain SBI/II−. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Tabita FR, Hanson TE, Li H, Satagopan S, Singh J, Chan S. Function, structure, and evolution of the RubisCO-like proteins and their RubisCO homologs. Microbiol. Mol. Biol. Rev. 2007;71:576–599. doi: 10.1128/MMBR.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spreitzer RJ, Salvucci ME. RubisCO: structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 2002;53:449–475. doi: 10.1146/annurev.arplant.53.100301.135233. [DOI] [PubMed] [Google Scholar]
- 3.Jordan DB, Ogren WL. Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature. 1981;291:513–515. [Google Scholar]
- 4.Tabita FR. Microbial ribulose 1,5-bisphosphate arboxylase/oxygenase: A different perspective. Photosynth. Res. 1999;60:1–28. [Google Scholar]
- 5.Cleland WW, Andrews TJ, Gutteridge S, Hartman FC, Lorimer GH. Mechanism of Rubisco: the carbamate as general base. Chem. Rev. 1998;98:549–561. doi: 10.1021/cr970010r. [DOI] [PubMed] [Google Scholar]
- 6.Andersson I, Taylor TC. Structural framework for catalysis and regulation in ribulose-1, 5-bisphosphate carboxylase/oxygenase. Arch. Biochem. Biophys. 2003;414:130–140. doi: 10.1016/s0003-9861(03)00164-4. [DOI] [PubMed] [Google Scholar]
- 7.Tabita FR, Satagopan S, Hanson TE, Kreel NE, Scott SS. Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J. Exp. Bot. 2008;59:1515–1524. doi: 10.1093/jxb/erm361. [DOI] [PubMed] [Google Scholar]
- 8.Gatenby AA, van der Vies SM, Bradley D. Assembly in E. coli of a functional multi-subunit ribulose bisphosphate carboxylase from a blue-green alga. Nature. 1985;314:617–620. [Google Scholar]
- 9.Tabita FR, Small CS. Expression and assembly of active cyanobacterial ribulose 1, 5-bisphosphate carboxylase/oxygenase in Escherichia coli containing stoichiometric amounts of large and small subunits. Proc. Natl. Acad. Sci. USA. 1985;82:6100–6103. doi: 10.1073/pnas.82.18.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman J, Gutteridge S. The X-ray structure of Synechococcus ribulose-bisphosphate carboxylase/oxygenase-activated quaternary complex at 2.2 Å resolution. J. Biol. Chem. 1993;268:25876–25886. [PubMed] [Google Scholar]
- 11.Parry MAJ, Andralojc PJ, Mitchell RAC, Madgwick PJ, Keys AJ. Manipulation of Rubisco: its amount, activity, function and regulation. J.Exp. Bot. 2003;54:1321–1333. doi: 10.1093/jxb/erg141. [DOI] [PubMed] [Google Scholar]
- 12.Falcone DL, Tabita FR. Expression of endogenous and foreign ribulose 1, 5-bisphosphate carboxylase-oxygenase (RubisCO) genes in a RubisCO deletion mutant of Rhodobacter sphaeroides. J. Bacteriol. 1991;173:2099–2108. doi: 10.1128/jb.173.6.2099-2108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SA, Tabita FR. Positive and negative bioselection of mutant forms of prokaryotic (cyanobacterial) ribulose-1, 5-bisphosphate carboxylase/oxygenase. J. Mol. Biol. 2003;331:557–569. doi: 10.1016/s0022-2836(03)00786-1. [DOI] [PubMed] [Google Scholar]
- 14.Smith SA, Tabita FR. Glycine 176 affects catalytic properties and stability of the Synechococcus sp. strain PCC 6301 ribulose 1, 5-bisphosphate carboxylase/oxygenase. J. Biol. Chem. 2004;279:25632–25637. doi: 10.1074/jbc.M401360200. [DOI] [PubMed] [Google Scholar]
- 15.Parikh MR, Greene DH, Woods KK, Matsumuara I. Directed evolution of Rubisco hypermorphs through genetic selection in engineered E. coli. Prot. Eng. Des. & Sel. 2006;19:113–119. doi: 10.1093/protein/gzj010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DM, Whitney SM, Matsumura I. Artificially evolved PCC6301 Rubisco variants exhibit improvements in folding and catalytic efficiency. Biochem. J. 2007;404:517–524. doi: 10.1042/BJ20070071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn MW, Tabita FR. Synthesis of catalytically active form III ribulose 1,5-bisphosphate carboxylase/oxygenase in archaea. J. Bacteriol. 2003;185:3049–3059. doi: 10.1128/JB.185.10.3049-3059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreel NE, Tabita FR. Substitutions at methionine 295 of Archaeoglobus fulgidus ribulose-1, 5-bisphosphate carboxylase/oxygenase affect oxygen binding and CO2/O2 specificity. J. Biol. Chem. 2007;282:1341–1351. doi: 10.1074/jbc.M609399200. [DOI] [PubMed] [Google Scholar]
- 19.Harpel MR, Hartman FC. Enhanced CO2/O2 specificity of a site-directed mutant of ribulose-bisphosphate carboxylase/oxygenase. J. Biol. Chem. 1992;267:6475–6478. [PubMed] [Google Scholar]
- 20.Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paoli GC, Vichivanives P, Tabita FR. Physiological control and cbb gene regulation in Rhodobacter capsulatus. J. Bacteriol. 1998;180:4258–4269. doi: 10.1128/jb.180.16.4258-4269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitman W, Tabita FR. Inhibition of D-ribulose 1,5-bisphosphate carboxylase by pyridoxal 5’-phosphate. Biochem. Biophys. Res. Commun. 1976;71:1034–1039. doi: 10.1016/0006-291x(76)90758-0. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1971;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Kuehn GD, Hsu TC. Preparative-scale enzymic synthesis of D-[14C]ribulose 1,5-bisphosphate. Biochem. J. 1978;175:909–912. doi: 10.1042/bj1750909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Chastain CJ, Al-Abed SR, Chollet R, Spreitzer RJ. Reduced CO2/O2 specificity of ribulose-1, 5-bisphosphate carboxylase/oxygenase in a temperature-sensitive chloroplast mutant of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 1988;85:4696–4699. doi: 10.1073/pnas.85.13.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du YC, Peddi SR, Spreitzer RJ. Assessment of structural and functional divergence far from the large subunit active site of ribulose-1,5-bisphosphate carboxylase/oxygenase. J. Biol. Chem. 2003;278:49401–49405. doi: 10.1074/jbc.M309993200. [DOI] [PubMed] [Google Scholar]
- 28.Spreitzer RJ, Peddi SR, Satagopan S. Phylogenetic engineering at the interface between large and small subunits impart land-plant kinetic properties to algal Rubisco. Proc. Natl. Acad. Sci. USA. 2005;102:17225–17230. doi: 10.1073/pnas.0508042102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee GJ, McFadden BA. Serine-376 contributes to the binding of substate by ribulose-bisphosphate carboxylase/oxygenase from Anacystis nidulans. Biochemistry. 1992;31:2304–2308. doi: 10.1021/bi00123a014. [DOI] [PubMed] [Google Scholar]
- 30.Zhu G, Spreitzer RJ. Directed mutagenesis of chloroplast ribulosebisphosphate carboxylase/oxygenase: substitutions at large subunit asparagine 123 and serine 379 decrease CO2/O2 specificity. J. Biol. Chem. 1994;269:3952–3956. [PubMed] [Google Scholar]
- 31.Karkehabadi S, Satagopan S, Taylor TC, Spreitzer RJ, Andersson I. Structural analysis of altered large-subunit loop-6/carboxy-terminus interactions that influence catalytic efficiency and CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase. Biochemistry. 2007;46:11080–11089. doi: 10.1021/bi701063f. [DOI] [PubMed] [Google Scholar]
- 32.Fiorucci S, Golebiowski J, Cabrol-Bass D, Antonczak S. Molecular simulations reveal a new entry site in quercetin 2,3-dioxygenase. A pathway for dioxygen? Proteins. 2006;64:845–850. doi: 10.1002/prot.21042. [DOI] [PubMed] [Google Scholar]
- 33.Luna VM, Chen Y, Fee JA, Stout CD. Crystallographic studies of Xe and Kr binding within the large internal cavity of cytochrome ba3 from Thermus thermophilus: Structural analysis and role of oxygen transport channels in the heme-Cu oxidases. Biochemistry. 2008;47:4657–4665. doi: 10.1021/bi800045y. [DOI] [PubMed] [Google Scholar]
- 34.Tcherkez GGB, Farquhar G, Andrews TJ. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases my be perfectly optimized. Proc. Natl. Acad. Sci. USA. 2006;103:7246–7251. doi: 10.1073/pnas.0600605103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogren WL. Photorespiration: pathways, regulation, and modification. Annu. Rev. Plant Physiol. 1984;35:415–442. [Google Scholar]
- 36.Paoli GC, Tabita FR. Aerobic chemolithoautotrophic growth and RubisCO function in Rhodobacter capsulatus and a spontaneous gain of function mutant of Rhodobacter sphaeroides. Arch. Microbiol. 1998;170:8–17. doi: 10.1007/s002030050609. [DOI] [PubMed] [Google Scholar]
- 37.Newman J, Branden CI, Jones TA. Structure determination and refinement of ribulose 1,5-bisphosphate carboxylase/oxygenase from Synechococcus PCC6301. Acta Crystallogr., Sect. D. 1993;49:548–560. doi: 10.1107/S090744499300530X. [DOI] [PubMed] [Google Scholar]
- 38.Lundqvist T, Schneider G. Crystal structure of activated ribulose-1,5-bisphosphate carboxylase complexed with its substrate, ribulose-1,5-bisphosphate. J. Biol. Chem. 1991;266:12604–12611. [PubMed] [Google Scholar]
- 39.Kitano K, Maeda N, Fukui T, Atomi H, Imanaka T, Miki K. Crystal structure of a novel-type archaeal Rubisco with pentagonal symmetry. Structure. 2001;9:473–481. doi: 10.1016/s0969-2126(01)00608-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.