Abstract
Fve is a fungal protein isolated from the golden needle mushroom Flammulina velutipes and has previously been reported to trigger immunological responses in both mouse and human lymphocytes. In this study, we evaluated the potential application of Fve as an adjuvant for tumour immunotherapy and examined the underlying mechanism(s). When the human papillomavirus (HPV)-16 E7 oncoprotein was used as a model antigen, mice coimmunized with HPV-16 E7 and Fve showed enhanced production of HPV-16 E7-specific antibodies as well as expansion of HPV-16 E7-specific interferon (IFN)-γ-producing CD4+ and CD8+ T cells as compared with mice immunized with HPV-16 E7 alone. Tumour protection assays showed that 60% of mice coimmunized with HPV-16 E7 plus Fve, as compared with 20% of those immunized only with HPV-16 E7, remained tumour-free for up to 167 days after challenge with the tumour cells. Tumour therapeutic assays showed that HPV-16 E7 plus Fve treatment significantly prolonged the survival of tumour-bearing mice as compared with those treated only with HPV-16 E7. In vivo cell depletion and adoptive T-cell transfer assays showed that CD4+ and CD8+ T cells and IFN-γ played critical roles in conferring the antitumour effects. Interestingly, Fve could stimulate the maturation of splenic dendritic cells in vivo and induce antigen-specific CD8+ T-cell immune responses. In summary, Fve has potent adjuvant properties that enhance T helper type 1 antigen-specific humoral and cellular immune responses which confer strong antitumour effects. The use of Fve as an adjuvant could be an attractive alternative to the current vaccination strategy for cancer immunotherapy.
Keywords: adjuvant, dendritic cells, fungal immunomodulatory protein Fve, human papillomavirus-16 E7, immunotherapy
Introduction
Cancer immunotherapy is an attractive alternative for the treatment of patients with cancer. When immunotherapy is used in the absence of radiotherapy or chemotherapy, there are fewer side effects than found with classical antitumour chemotherapeutics. The identification and cloning of genes encoding tumour-associated antigens recognized by T cells have renewed hopes that it may be possible to develop cures for some cancers.1 Although several treatments using tumour-associated antigens have been approved for some types of cancer, their efficacies have been variable and generally insufficient. One important factor contributing to insufficient and variable responses to immunotherapy is the poor immunogenicity of most tumour antigens, which results in insufficient activation of tumour antigen-specific CD8+ T cells.
Cervical cancer is the second highest cause of cancer deaths in women and kills approximately 274 000 women worldwide each year.2,3 Epidemiological and laboratory studies strongly support a crucial role for persistent human papillomavirus (HPV) infection and transcription in cervical carcinogenesis.4,5 Among over 100 HPV genotypes, HPV type 16 is the most common and is responsible for more than 50% of all cervical cancers.6 HPV-16 E7, one of its oncoproteins, is essential for the induction and maintenance of cellular transformation.5 Thus, HPV-16 E7 has been a major target of many prophylactic HPV vaccines for the prevention of HPV infections as well as many therapeutic HPV vaccines for the control of existing HPV infections and HPV-associated lesions, such as squamous intraepithelial lesions and cervical cancer. However, the antigen-specific immune responses and antitumour effects generated by HPV-16 E7 alone are weak and insufficient to control tumour growth. Several strategies have been developed to increase the potency of the HPV-16 E7 vaccine; for example, various immune modulators, such as cytokines,7,8 heat shock proteins,9,10 non-toxic bacterial toxins,11 and CpG12 have been incorporated as adjuvants to enhance HPV-16 E7-specific immunity. These have proved to be effective in animal models, but their potency in humans has yet to be assessed and active research to develop novel adjuvant molecules is still ongoing.
Fve, or fungal immunomodulatory protein (FIP)-fve, is a major fruiting body protein isolated from the edible golden needle mushroom, Flammulina velutipes.13–15 It belongs to an FIP family, sharing sequence similarity with LinZhi-8 from Ganoderma lucidum,16 Fip-gts from Ganoderma tsugae17 and Fip-vvo from Volvariella volvacea.18 The Fve protein is an acetylated protein consisting of 114 amino acid residues with an estimated molecular weight of 12.7 kDa.13 Fve has been shown to have the ability to trigger the proliferation of mouse splenocytes and human peripheral mononuclear cells and to enhance the production of interleukin (IL)-2, IFN-γ and tumour necrosis factor (TNF)-α.13,19 Moreover, it has been suggested that coadministration of Fve with antigen may drive strong T helper type 1 (Th1)-skewed immune responses.20
In this study we explored the potential role of Fve as an adjuvant for cancer immunotherapy. Using HPV type 16 E7 as a model tumour antigen and the TC-1 cell-induced tumour model, we carried out a series of proof-of-concept studies to show the effectiveness of Fve as an adjuvant to enhance both humoral and cellular responses to antitumour therapy in vivo.
Materials and methods
Mice and the tumour cell line
Six- to eight-week-old female C57BL/6 mice were purchased from the Laboratory Animal Center (Sembawang, Singapore). Breeding pairs of OT-I and OT-II mice with transgenic Vα2Vβ5 T-cell receptors (TCRs) specific for the ovalbumin (OVA)257–264 epitope in the context of H-2Kb and the OVA323–339 epitope in the context of I-Ab were originally acquired from The Jackson Laboratory (Bar Harbor, ME) and were maintained and bred in the Animal Holding Unit of the National University of Singapore. All animal procedures were performed according to approved protocols and in accordance with the Institutional Animal Care and Use Committee of The National University of Singapore. The maintenance of TC-1 cells (kindly provided by Dr T. C. Wu, Johns Hopkins University, Baltimore, MD) has been described previously.21 On the day of tumour challenge, TC-1 cells were harvested by trypsinization and washed three times with phosphate-buffered saline (PBS), and the designated numbers of cells for tumour inoculation were resuspended in 200 μl of PBS.
Reagents
All antibodies were purchased from BD PharMingen (San Diego, CA) unless otherwise stated. For the detection of antigen-specific immunoglobulin G1 (IgG1) in mouse sera, the rat anti-mouse Igκ light chain (clone 187.1) and biotin-conjugated rat anti-mouse IgG1 monoclonal antibody (mAb) (clone LO-MG1-2; Serotec Ltd., Oxford, UK) were used. The purified mouse IgG1 (clone 107.3) was used as the standard. For the cytokine enzyme-linked immunosorbent assay (ELISA), mAbs of rat anti-mouse IFN-γ (clone R4-6A2), biotin-conjugated rat anti-mouse IFN-γ (clone XMG1.2), rat anti-mouse IL-2 (clone JES6-IA20), biotin-conjugated rat anti-mouse IL-2 (JES6-5H4), rat anti-mouse IL-4 (clone, BVD6-24G2) and biotin-conjugated rat anti-mouse IL-4 (clone BVD4-1D11) were used. Rat anti-mouse TNF-α (clone AF-410-NA) and biotinylated rat anti-mouse TNF-α (clone BAF410) were purchased from R&D Systems (Minneapolis, MN). Rat anti-mouse CD3ε (clone 145-2C11) and CD28 (clone 37.51) mAbs and recombinant mouse IL-2 were used for stimulation of T cells in vitro. Fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8β (clone 53-5.8), biotin-conjugated rat anti-mouse CD4 (clone GK1.5), streptavidin-PerCP and allophycocyanin (APC)-conjugated rat-anti-mouse IFN-γ (clone XMG-2.1) were used for intracellular cytokine staining. Rat anti-mouse CD16/CD32 (clone D34-485), biotin-conjugated rat anti-mouse CD8α (clone 53-6.7), APC-conjugated rat anti-mouse CD4 (clone, RM4-5), PE-conjugated rat anti-mouse CD11c (clone HL3), FITC-conjugated anti-I-Ab (clone AF6-120.1), FITC-conjugated anti-CD86 (clone GL1) and streptavidin-conjugated PerCP were used for surface marker staining for splenic dendritic cells (DCs).
Monoclonal anti-CD4 (clone, GK1.5), anti-CD8 (clone, 2.43) and anti-IFN-γ (clone, R4-6A2) antibodies for the in vivo depletion experiment were purified from the supernatants of hybridoma cells (American Type Culture Collection, Bethesa, MD) by passage through protein G columns (Amersham Biosciences AB, Uppsala, Sweden).
Production of Fve and recombinant HPV-16 E7 proteins
The purification of Fve protein from crude extracts of F. velutipes (golden needle mushroom) has been described previously.13,15 The purified Fve was treated with the polymyxin B agarose and the endotoxin level of the Fve protein was determined using the LAL assay kit according to the manufacturer’s instructions (BioWhittaker, Walkersville, MD). There was no detectable level of endotoxin in the purified Fve protein.
The cDNA of HPV-16 E7 (a gift kindly provided by Dr S. W. Chan, Institute of Molecular and Cellular Biology, ASTAR, Singapore) was subcloned into the pGEX-4T1 expression vector (Amersham Biosciences AB). The open reading frame of HPV-16 E7 was amplified by polymerase chain reaction using a set of primers: E7-F 5′-TTGTTGGATCCCATGGAGATACACCTACATTG-3′ and E7-R 5′-TTACTGAATTCTTATGGTTTCTGAGAACAGATG-3′. The amplified DNA was digested with BamHI and EcoRI, and the resulting fragment was then cloned into the BamHI and EcoRI sites of the pGEX-4T1 vector. The pGEX-HPV-16 E7 recombinant plasmid was transformed into Escherichia coli TG-1 for protein expression. The HPV-16 E7 protein was purified from GST-HPV-16 E7 fusion proteins after thrombin treatment.
Preparation of DCs
Bone marrow-derived dendritic cells (BM-DCs) were generated with granulocyte–macrophage colony-stimulating factor (GM-CSF) according to a method previously described.22 In brief, bone marrow cells were harvested from femurs and tibias of normal C57BL/6 mice and washed with PBS. The cells (4 × 106 to 6 × 106) were resuspended in complete RPMI-1640 medium containing recombinant mouse GM-CSF (20 ng/ml; BD PharMingen) and cultured in 100-mm-diameter Petri dishes. On day 3 of culture, half of the medium was replaced with fresh medium supplemented with GM-CSF (10 ng/ml). On day 5 of culture, immature DCs were harvested for purification.
The splenic DCs were purified as previously described23 with some modifications. Spleens (from eight mice) were minced with scissors and digested in 10 ml of Hanks’ balanced salt solution (HBSS) with Ca2+ and Mg2+ (Sigma-Aldrich, St Louis, MO) containing collagenase D (400 U/ml; Roche Molecular Biochemicals, Mannheim, Germany) for 30 min at 37°. Next, 1 ml of 0.1 m ethylenediaminetetraacetic acid (EDTA) was added at room temperature for 5 min to disrupt cell adhesion. The digested tissue samples were filtered through a 40-μm nylon mesh to remove undigested fibrous material. All subsequent steps were performed at room temperature using HBSS without Ca2+ and Mg2+ (Sigma-Aldrich). Cells in the filtrates were recovered by centrifugation, resuspended in 1·068 g/cm3 OptiPrep® density gradient medium (Sigma-Aldrich) and centrifuged at 600 g for 15 min. The low-density fraction was collected (2–4% of the total) and resuspended in magnetic antibody cell sorting (MACS) running buffer [PBS with 0.5% bovine serum albumin (BSA) and 2 mm EDTA] for subsequent purification.
Purification of DCs and T cells
CD11c (N418), CD90.2 (Thy1.2), CD4 (L3T4) and CD8 (Ly-2) microbeads were used for the isolation of the splenic DCs, BM-DCs from cell cultures, Thy1.2+ T cells, and CD4+ and CD8+ T cells from spleens, respectively, according to the manufacturer’s instructions (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Briefly, cells were labelled with 10 μl of microbeads per 1 × 107 cells at 4° for 20 min and washed twice. The labelled cells were subsequently separated using an autoMACS™ separator (Miltenyi Biotec GmbH). The purities of the various cell populations were determined by flow cytometry analysis. The purity of DCs and Thy1.2+ T cells was above 97% and 98%, respectively. The purity of OT-II CD4+ T cells and OT-I CD8+ T cells was above 95% and 85%, respectively (data not shown).
In vitro cell proliferation and cytokine production assays
Thy1.2+ T cells were purified from spleen cells of C57BL/6 mice by magnetic cell sorting. The purity of Thy1.2+ T cells was above 95%. The purified Thy1.2+ T cells were seeded in triplicate (1 × 105 per well) into a 96-well U-bottom plate in the presence or absence of 2 × 104 BM-DCs treated with mitomycin C (Roche Diagnostics GmbH, Mannherim, Germany). BM-DCs alone were used as a control. All cells were treated with or without Fve (20 μg/ml) and pulsed with [3H]-labelled thymidine for the last 18 hr of cultures. The cells were harvested and [3H]thymidine incorporation was measured by liquid scintillation counting at 72 hr. Supernatants were collected and cytokine production was measured by ELISA.
ELISA for anti-HPV-16 E7 antibodies
Mice were subcutaneously immunized with PBS, 20 μg of HPV-16 E7 or 20 μg of HPV-16 E7 plus 20 μg of Fve at days 0, 14 and 28. Sera were collected weekly for antibody analysis by ELISA. For IgG1 analysis, a 96-well plate was coated with HPV-16 E7 protein (5 μg per well) and incubated at 4° overnight. The wells were then blocked with blocking buffer (PBS containing 0.05% Tween-20 and 1% BSA). Diluted sera were added and incubated at 4° overnight. The plate was then incubated with biotin-conjugated anti-mouse IgG1 for 1 hr, followed by the addition of ExtrAvidin®-alkaline phosphatase (Sigma-Aldrich) for another hour. The signal was developed by adding p-nitrophenyphosphate substrate (Sigma-Aldrich) and read with a microplate reader at 405 nm (Tecan Group Ltd, Männedorf, Switzerland). It has been well established that C57BL/6 mice express the Igh1-b gene, which encodes the IgG2c isotype rather than IgG2a.24 Thus, the levels of antigen-specific IgG2c in the mouse sera were detected using an ELISA kit (Bethyl Laboratories, Montgomery, TX) according to the manufacturer’s protocol.
Intracellular IFN-γ production in T cells of Fve-immunized mice
To investigate the HPV-16 E7-specific cellular immune response, mice were subcutaneously immunized with PBS, 20 μg of HPV-16 E7, or 20 μg of HPV-16 E7 plus 20 μg of Fve at days 0 and 14 and splenocytes were collected at day 28. To determine the cytokine production profiles of splenocytes in primary cultures, 5 × 105 splenocytes per well were stimulated with HPV-16 E7 protein for 72 hr and culture supernatants were collected for cytokine assays by ELISA. To determine the cytokine production profiles of T subsets by intracellular staining, splenocytes were first cultured with 10 μg/ml HPV-16 E7 for 9 days, followed by secondary stimulation with anti-CD3 and anti-CD28 antibodies prior to intracellular staining with cytokine-specific antibodies. Recombinant mouse IL-2 was added to the primary splenocyte cultures at days 3 and 6 to a final concentration of 10 U/ml to maintain the cells up to 9 days; these cultured cells from mice within the same group were then pooled to set up secondary cultures. Briefly, 2 × l05 cells were restimulated with 5 μg/ml anti-mouse CD3 and 2 μg/ml anti-mouse CD28 mAbs for 12 hr. Monensin (Sigma-Aldrich) was added 6 hr before harvesting. Cells were surface-stained with anti-CD8β and anti-CD4 mAbs, fixed with paraformaldehyde, permeabilized with saponin (Sigma-Aldrich) and then stained intracellularly with anti-mouse IFN-γ mAb. Flow cytometric analysis was performed using a FACSCalibur with cell quest software (BD Biosciences, San Jose, CA).
In vivo tumour protection and depletion assays
Mice were subcutaneously immunized with PBS, 20 μg of HPV-16 E7, 20 μg of Fve, or 20 μg of HPV-16 E7 plus 20 μg of Fve at days 0, 14 and 28. TC-1 cells (5 × l04) were inoculated into the right flank of a mice at day 30. To deplete CD4+ T cells, CD8+ T cells or IFN-γ, mice were intraperitoneally injected with 800 μg of anti-CD4, 500 μg of anti-CD8 or 500 μg of anti-IFN-γ mAbs respectively at days −4, −1, 6, 13, 20, 27, 34, 41 and 48. Tumour size was measured every 2 days in two perpendicular dimensions and expressed as length × width (mm2) and the survival was monitored. The depletion of CD4+ and CD8+ T cells was assessed at day 6 after the first immunization, and the depletion in the spleen was > 95% as determined by flow cytometric analysis. Scientists performing the analyses of tumour size and survival rates were blinded to the treatments.
In vivo tumour therapeutic assay
Mice were subcutaneously inoculated with 5 × l04 TC-1 cells in the right flank at day 0. Mice were then subcutaneously immunized with PBS, 20 μg of HPV-16 E7, 20 μg of Fve, or 20 μg of HPV-16 E7 plus 20 μg of Fve at days 3, 10 and 17. For tumour metastasis therapeutic assay, mice were intravenously injected with 2 × l04 TC-1 cells in the tail vein at day 0. Mice were then subcutaneously immunized with the same regimen at days 3, 10 and 17. The survival rates were monitored daily.
T-cell adoptive transfer
Splenocytes from immunized mice were collected and Thy 1.2+ T cells were isolated using Thy1.2 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) for cell transfer experiments. The purity of T cells was above 98% as determined by flow cytometric analysis. Freshly purified Thy1.2+ T cells (8 × 106) were adoptively transferred into recipient mice at days −1, 3, 6 and 9. Recipient mice were inoculated subcutaneously in the right flank with 5 × 104 TC-1 cells at day 0. The tumour size was measured on alternate days from day 10 onwards.
Analysis of in vivo activation of DCs
Mice were intravenously injected with PBS or 20 μg of Fve. Twelve hours later, enriched splenic DCs (1 × 106 cells in 100 μl) were blocked with anti-CD16/CD32 mAb at 4° for 20 min prior to incubation with mAbs against CD4, CD8, CD11c and I-Ab and with mAbs against CD4, CD8, CD11c and CD86 at 4° for 25 min. Flow cytometric analysis was performed using a FACSCalibur with cell quest software (BD Biosciences).
Analysis of DC-directed CD4+ and CD8+ T-cell activation
Naïve C57 BL/6 mice were intravenously injected with PBS, 100 μg of ovalbumin (OVA), 20 μg of Fve, or OVA (100 μg) plus Fve (20 μg) 24 hr prior to isolation of the CD11c+ DCs. Purified CD11c+ DCs were pulsed with 1 μm of OVA323–339 peptide or 1 μm of OVA257–264 peptide, respectively (AnaSpec, Inc., San Jose, CA), for 2 hr at 37° and then washed extensively. Naïve CD4+ and CD8+ cells were purified from OT-II and OT-I transgenic mice, respectively. DCs (5 × 103 cells) were incubated with 5 × 104 CD4+ T cells or 5 × 104 CD8+ T cells, respectively, in U-bottom 96-well plates in 200 μl of complete RPMI-1640 medium in triplicate for 72 hr. Supernatants were collected and cytokine production was determined by ELISA.
Statistical analysis
Results for tumour sizes are presented as mean ± standard error of the mean (SEM) and were analysed by one-way analysis of variance (anova). Differences in antibody and cytokine production between groups were analysed by Student’s t-test. In tumour protection, therapeutic and adoptive transfer experiments, the tumour-free and survival analyses were carried out using the Kaplan–Meier analysis and the log-rank test. P< 0·05 was considered as statistically significant.
Results
Fve stimulated mouse T-cell proliferation and IFN-γ production in vitro
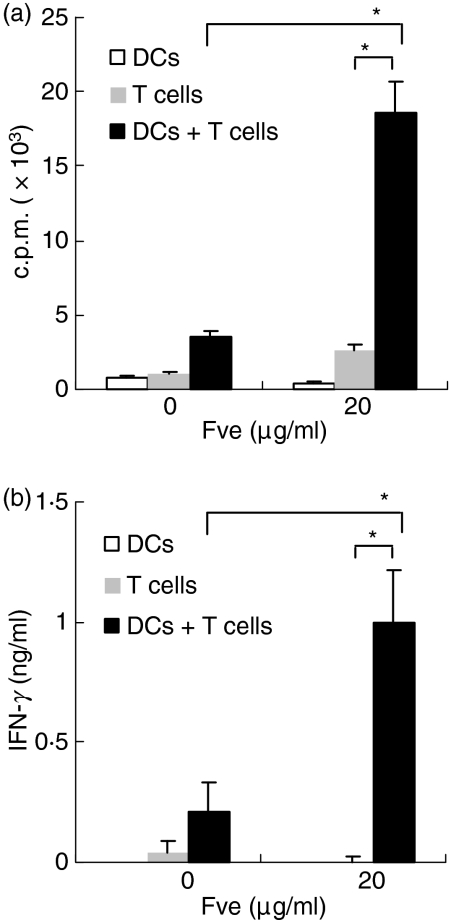
To examine the immunostimulatory effect of Fve in vitro, mouse splenocytes were cultured in the presence of increasing concentrations of the Fve protein. As shown in Fig. S1 (a and b), Fve induced the proliferation of splenocytes in a dose-dependent manner and significant production of IFN-γ and TNF-α. Fve predominately stimulated the proliferation of T cells in the splenocytes as cellular proliferation was drastically reduced in T-cell-depleted splenocytes (data not shown). Fve-induced T-cell proliferation and IFN-γ production were accessory-cell-dependent (Fig. 1 and Fig. S1c).
Figure 1.
Fve stimulates mouse splenic T-cell activation in an accessory-cell-dependent manner. Purified CD90+ T cells (1 × 105 cells/well) were co-cultured with mitomycin C-treated bone marrow-derived dendritic cells (BM-DCs) (2 × 104 cells/well) in the presence or absence of Fve (20 μg/ml) in triplicate using the 96-well plate. The DCs and CD90+ T cells alone were included for comparison. The cultures were pulsed with [3H]thymidine for the last 18 hr of the co-culture. The cells were harvested and thymidine incorporation was measured by liquid scintillation counting at 72 hr (a). The culture supernatants were collected at 72 hr and interferon (IFN)-γ production was measured by enzyme-linked immunosorbent assay (ELISA) (b). *P < 0·05. c.p.m., counts per minute.
Coadministration of HPV-16 E7 plus Fve increased HPV-16 E7-specific B-cell and T-cell activities
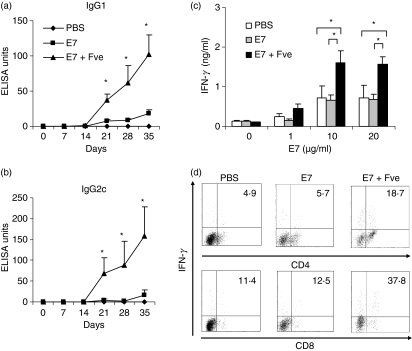
We next investigated the effects of Fve on the induction of HPV-16 E7-specific immunity. Firstly, we measured HPV-16 E7-specific antibodies in the sera of the various groups of differentially immunized mice. As shown in Fig. 2a,b, mice immunized with HPV-16 E7 plus Fve produced significantly higher levels of IgG1 and IgG2c as compared with those immunized with HPV-16 E7 alone. HPV-16 E7-specific IgG1 and IgG2c in mice immunized with HPV-16 E7 plus Fve were 7-fold and 33-fold higher, respectively, than those of mice immunized with HPV-16 E7 alone at day 28 (Fig. 2a,b). These results indicate that Fve could enhance strong HPV-16 E7-specific humoral immune responses.
Figure 2.
Fve enhanced human papillomavirus (HPV)-16 E7-specific antibody production and up-regulated interferon (IFN)-γ production of in vitro HPV-16 E7-stimulated T cells. For the HPV-16 E7-specific humoral immune response, mice (n = 5 per group) were immunized with phosphate-buffered saline (PBS) (♦), HPV-16 E7 (▪) or HPV-16 E7 plus Fve (▴) at days 0, 14 and 28. Sera were collected weekly for the measurement of HPV-16 E7-specific (a) immunoglobulin G1 (IgG1) and (b) IgG2c by enzyme-linked immunosorbent assay (ELISA). For the T-cell immune response, mice (n = 5 per group) were immunized with PBS (♦), HPV-16 E7 (▪), and HPV-16 E7 plus Fve (▴) at days 0 and 14. Splenocytes were collected at day 28 and cultured with 1, 10 or 20 μg/ml HPV-16 E7. Supernatants were collected at 72 hr and IFN-γ levels were measured by ELISA (c). For intracellular staining, the HPV-16 E7 cultured primary T cells were restimulated with anti-CD3 and anti-CD28 antibodies for 12 hr. The numbers in the upper right quadrant indicate the percentage of IFN-γ+ cells among the gated CD4+ cells and CD8+ cells, respectively (d). Data are representative of three independent experiments. Error bars represent the standard error of the mean. *P< 0·05.
Consistently, IFN-γ production was significantly enhanced in splenocytes from mice immunized with HPV-16 E7 plus Fve as compared with those from mice immunized with HPV-16 E7 or PBS (Fig. 2c and Fig. S3). In order to elucidate the IFN-γ-producing T subsets, the HPV-16 E7 cultured primary cells were restimulated with anti-CD3 and anti-CD28 antibodies for 12 hr and cytokine production was examined by intracellular staining. Flow cytometry analysis revealed that 18·7% of IFN-γ-producing cells within the CD4+ T subset were induced in the HPV-16 E7 plus Fve co-immunized mice, whereas only 4·9% and 5·7% of IFN-γ+ cells were induced in HPV-16 E7 and PBS mice, respectively (Fig. 2d, upper panel). Similarly, 37·8% of the CD8+ T cells in the HPV-16 E7 plus Fve co-immunized mice were IFN-γ+ cells, whereas only 11·4% and 12·5% of IFN-γ+ CD8+ T cells were detected in HPV-16 E7 and PBS mice, respectively (Fig. 2d, lower panel). These results indicate that Fve could significantly increase HPV-16 E7-specific IFN-γ-secreting CD4+ and CD8+ T cells.
Coadministration of HPV-16 E7 and Fve enhanced protection of mice against tumour growth
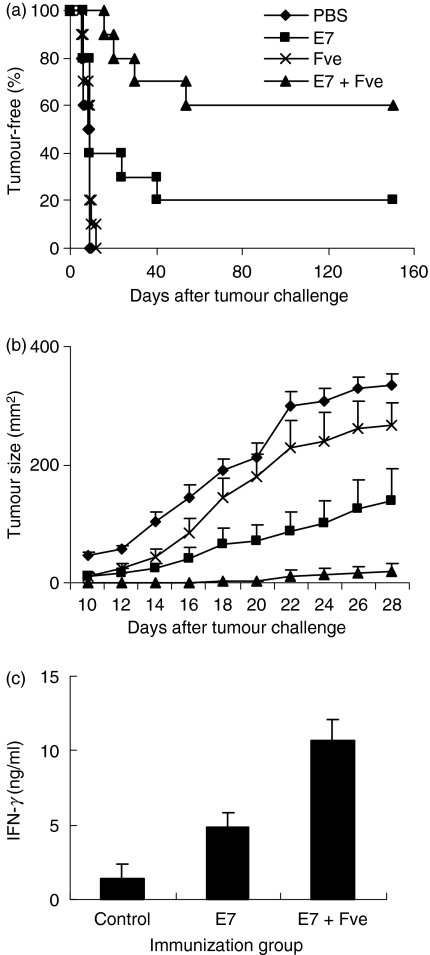
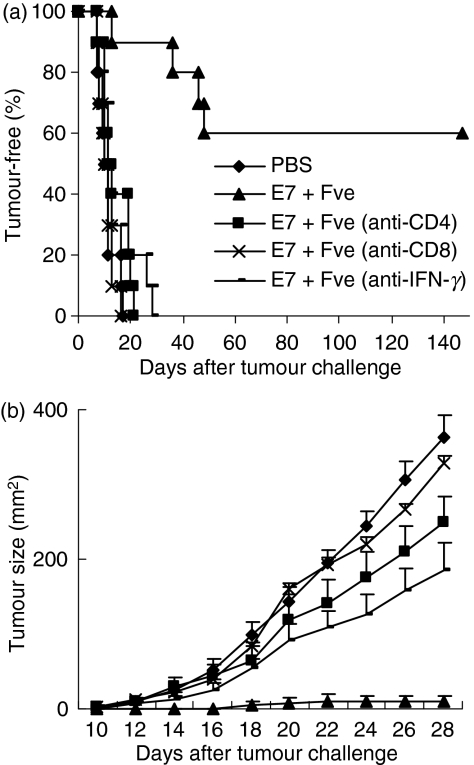
The finding that immunization with HPV-16 E7 plus Fve led to enhanced HPV-16 E7-specific immunity prompted us to explore the antitumour potential of such an immunization regimen in vivo. Mice immunized with PBS, HPV-16 E7, Fve, or HPV-16 E7 plus Fve were challenged with TC-1 cells subcutaneously and tumour growth was monitored. Results showed that 60% of mice co-immunized with HPV-16 E7 plus Fve remained tumour-free for up to 167 days after the tumour challenge, whereas only 20% of mice remained tumour-free (P< 0·05) in the group immunized with HPV-16 E7 (Fig. 3a). Mice immunized with PBS or Fve alone developed tumours rapidly within 10 and 15 days after tumour challenge, respectively (Fig. 3a). Interestingly, mice immunized with Fve alone generally showed a reduction in tumour size as compared with the PBS control mice (Fig. 3b). This suggests that Fve protein alone could confer partial suppression of tumour growth. However, such a suppressive effect was insufficient to protect mice against tumour formation in the absence of HPV-16 E7-specific immune responses as only mice in the group immunized with HPV-16 E7 plus Fve exhibited a low tumour burden or remained tumour-free over the total duration of this study.
Figure 3.
Co-immunization with human papillomavirus (HPV)-16 E7 and Fve enhanced protection against the growth of TC-1 tumours. Mice (n= 10 per group) were immunized with phosphate-buffered saline (PBS) (♦), 20 μg of HPV-16 E7 (▪), 20 μg of Fve (×) or 20 μg of HPV-16 E7 plus 20 μg of Fve (▴) at days 0, 14 and 28 and then inoculated subcutaneously with TC-1 cells at day 30. The mice were monitored daily for tumour growth by palpation (a) and the tumour size was measured every 2 days (b). One hundred and sixty-seven days after tumour challenge, splenocytes were collected from the tumour-free mice and cultured with HPV-16 E7 protein in vitro. Supernatants were collected at 72 hr and interferon (IFN)-γ levels were analysed (c).
To determine whether long-term HPV-16 E7-specific immunity could be established in the immunized mice, splenocytes from tumour-free mice (HPV-16 E7 alone or HPV-16 E7 plus Fve groups) were collected and stimulated with HPV-16 E7 protein in vitro 167 days after tumour challenge. Splenocytes from naïve mice were used as the negative control. Results showed that, upon HPV-16 E7 antigen stimulation, splenocytes from HPV-16 E7 plus Fve co-immunized mice still produced higher levels of IFN-γ as compared with those from HPV-16 E7-immunized mice (Fig. 3c). Hence, Fve-enhanced HPV-16 E7-specific immunity persisted in the co-immunized mice and may account for the long-term protection against tumour formation. Taken together, these data indicate that immunization with Fve plus HPV-16 E7 is not only more effective than immunization with HPV-16 E7 alone; it is also able to confer long-term protection against tumour formation in the co-immunized mice.
Therapeutic immunization of HPV-16 E7 and Fve suppressed tumour growth and prolonged the survival of tumour-bearing mice
We also determined whether coadministration of HPV-16 E7 and Fve was equally effective at suppressing the growth of the established tumour. In this set of experiments, TC-1 cells were inoculated into the left flanks of mice 3 days prior to regular treatments with PBS, HPV-16 E7, Fve, and HPV-16 E7 plus Fve, respectively. Results showed that mice treated with HPV-16 E7 plus Fve had the highest tumour survival rate (Fig. 4a).
Figure 4.
Human papillomavirus (HPV)-16 E7 plus Fve co-immunization therapeutically extended the survival of tumour-bearing mice. For the therapeutic assay (a), mice (n= 10 per group) were inoculated subcutaneously with TC-1 cells on day 0 and then treated with phosphate-buffered saline (PBS) (♦), HPV-16 E7 (▪), Fve (×) or HPV-16 E7 plus Fve (▴) at days 3, 10 and 17. For the therapeutic metastasis assay (b), mice (n= 5 per group) were intravenously injected with TC-1 cells. Their survival was monitored and analysed by log-rank test.
Further investigations were then carried out in a tumour metastasis model established by injecting TC-1 cells intraveneously into the tail vein of each mouse at day 0. Mice were then treated with same regimens. As shown in Fig. 4b, none of the mice in the groups immunized with PBS, Fve or HPV-16 E7 alone survived beyond 55 days, while mice treated with HPV-16 E7 plus Fve showed significantly prolonged survival for up to 120 days. These results indicate that Fve could significantly enhance HPV-16 E7-specific antitumour activity therapeutically.
In summary, the results of these series of proof-of-principle experiments strongly support the notion that Fve could enhance an antigen-specific immune response that not only confers long-term protection against tumour growth but also retards tumour growth at the early and advanced stages of tumour development.
Both CD4+ and CD8+ T-cell subsets and IFN-γ were essential for protection against tumours
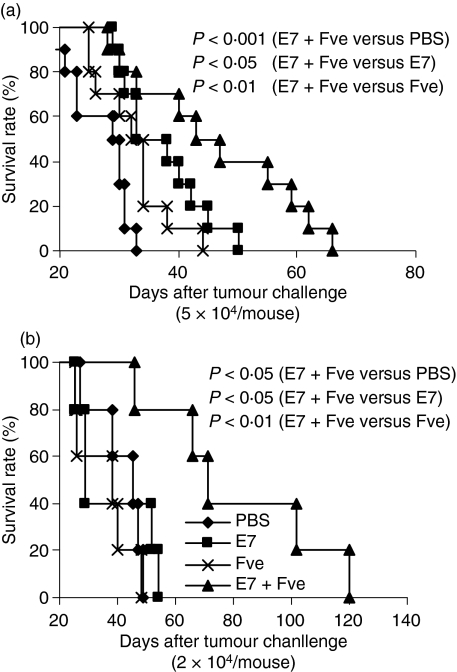
As Fve significantly increased IFN-γ-secreting T cells and enhanced HPV-16 E7-specific antitumour immunity, we next performed in vivo antibody depletion assays to determine the roles of the T-cell subsets and IFN-γ in the antitumour effects induced by combined vaccination. As expected, 60% of the HPV-16 E7 plus Fve co-immunized mice without any depletions remained tumour-free throughout the duration of this part of the study (Fig. 5a), while the tumours that developed in the remaining 40% of these mice were dramatically reduced in size (Fig. 5b). In contrast, all the mice depleted of CD4+ T cells, CD8+ T cells or IFN-γ developed tumours within 27 days. Interestingly, mice depleted of CD8+ T cells had similar tumour sizes to the PBS control, whereas tumour growth was retarded in the mice depleted of CD4+ T cells and of IFN-γ (Fig. 5b). These data suggested that, while CD8+ T cells, CD4+ T cells and IFN-γ are essential for the antitumour protection generated in mice co-immunized with HPV-16 E7 plus Fve, the CD8+ T subset plays a more dominant role in these antitumour effects.
Figure 5.
CD4+ and CD8+ T cells and interferon (IFN)-γ were essential for protection against tumours in mice immunized with human papillomavirus (HPV)-16 E7 plus Fve. Mice (n= 10 per group) were immunized with phosphate-buffered saline (PBS) or 20 μg of HPV-16 E7 plus 20 μg of Fve at days 0, 14 and 28. TC-1 cells (5 × l04) were inoculated subcutaneously into the right flanks of mice at day 30. To deplete CD4+ T cells (▪), CD8+ T cells (×) or IFN-γ (-), mice immunized with HPV-16 E7 plus Fve were injected intraperitoneally with anti-CD4, anti-CD8, or anti-IFN-γ monoclonal antibodies (mAbs), respectively, at days −4, −1, 6, 13, 20, 27, 34, 41 and 48. Mice immunized with PBS (♦) and HPV-16 E7 plus Fve (▴) without depletion were included as the control groups. The mice were monitored daily for tumour growth by palpation (a) and the tumour size was measured every 2 days (b). Error bars represent the standard error of the mean.
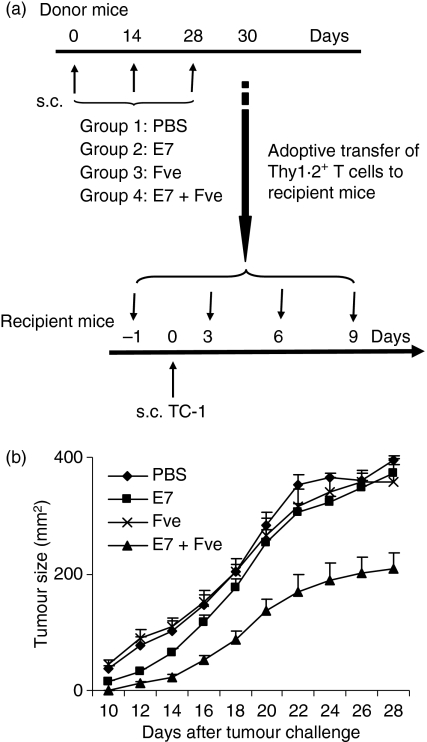
To further investigate the roles of T cells in the antitumour effects seen in the HPV-16 E7 plus Fve co-immunized mice, a T-cell adoptive transfer experiment was set up. The immunization and tumour challenge regimen was as detailed in Fig. 6a. Mice that received T cells from co-immunized mice showed a significant reduction in tumour growth (Fig. 6b) compared with those receiving T cells adoptively transferred from donor mice immunized with HPV-16 E7, Fve and PBS, respectively. These data support the notion that T cells play a pivotal role in therapeutic antitumour effects and such effects are probably directly correlated to the efficacy and magnitude of the HPV-16 E7-specific T-cell responses, as demonstrated in Fig. 2c,d.
Figure 6.
Adoptive transfer of T cells from mice immunized with human papillomavirus (HPV)-16 E7 plus Fve retarded tumour growth and prolonged survival. Eight million T cells purified from mice immunized with phosphate-buffered saline (PBS) (♦), HPV-16 E7 (▪), Fve (×) or HPV-16 E7 plus Fve (▴) were adoptively transferred to recipient mice at days −1, 3, 6 and 9. Recipient mice (n= 10 per group) were inoculated subcutaneously with 5 × 104 TC-1 cells at day 0 (a). Ten days after the tumour challenge, the size of the tumour formed was measured on alternate days. Data are presented as mean ± standard error of the mean (b).
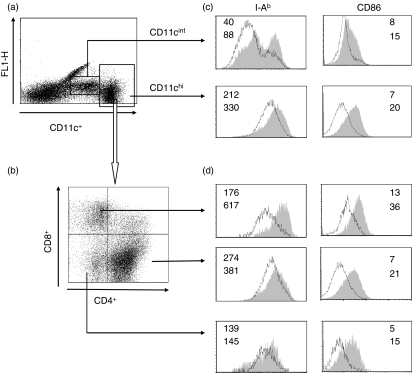
Fve stimulated phenotypic maturation of splenic DCs in vivo and enhanced CD8+ T-cell activation
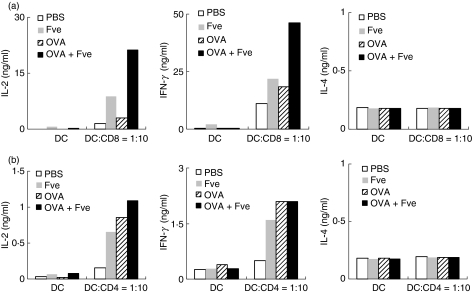
To elucidate the mechanism of the adjuvant action of Fve, we further analysed the effect of Fve on DCs which are responsible for the priming of the specific immune response.25,26 To this end, splenic CD11c+ DCs were enriched from spleens of mice which were intravenously injected with Fve prior to harvest and analysed for surface expression of major histocompatibility complex (MHC) class II and CD86. As shown in Fig. 7c, DCs isolated from Fve-treated mice up-regulated MHC-II and CD86 molecules in both CD11chi and CD11cint subsets as compared with those from control mice. CD11chi DCs can be further subdivided into CD4+, CD8α+ and CD4− CD8α− subtypes (Fig. 7b). Up-regulation of MHC-II and CD86 was seen in all three subtypes of CD11chi DCs (Fig. 7d), especially in the CD8α+ DCs. This indicates that Fve stimulates phenotypic maturation of splenic DCs in vivo. Subsequently, the in vitro functional assays for these Fve-stimulated DCs were performed using OVA-specific CD8+ and CD4+ T cells from OT-I and OT-II mice, respectively. Analysis of culture supernatants of OVA-specific CD8+ T cells co-cultured with OVA-laden DCs isolated from OVA plus Fve co-injected mice showed that production of IFN-γ and IL-2 was greatly increased compared with those from all the other experimental control groups (Fig. 8a). Such a marked enhancement of IFN-γ and IL-2 production by OVA-specific CD4+ T cells was not observed in similar parallel functional assays performed using CD4+ T cells from OT-II mice (Fig. 8b). There was no increased induction of IL-4, the signature cytokine for Th2 responses, by either CD4+ or CD8+ OVA-specific T cells. These data suggest that Fve can efficiently enhance the OVA-specific CD8+ T-cell immune response, probably by modifying the ability of DCs to present antigen.
Figure 7.
Fve induced splenic dendritic cell (DC) phenotypic maturation in vivo. Mice (n= 8 mice/group) were intraveneously injected with phosphate-buffered saline (PBS) or Fve 12 hr prior to cell harvest. DCs were enriched from spleens for staining of major histocompatibility complex (MHC) class II and CD86. Splenic DCs were separated into CD11cint and CD11chi populations (a) and CD11chi DCs were further divided into CD4+, CD8α+ and CD4− CD8α− subpopulations (b). MHC class II and CD86 up-regulation could be seen in both CD11cint and CD11chi populations (c) and the three CD11chi subpopulations of DCs (d) after stimulation with Fve in vivo. Open histograms delineated with a dark line represent PBS-stimulated DCs, and filled grey histograms represent Fve-stimulated DCs. The numbers in the histograms indicate the mean fluorescent intensities (MFIs) of DCs stimulated with PBS (upper number) and Fve (lower number), respectively.
Figure 8.
Fve greatly enhanced antigen-specific responses of CD8+ T cells. Mice (n= 8 mice/group) were intraveneously injected with phosphate-buffered saline (PBS), ovalbumin (OVA), Fve or OVA plus Fve and the dendritic cells (DCs) were isolated from the pool of eight spleens harvested from each group of mice 24 hr later. Purified DCs were pulsed with 1 μm OVA257–264 peptide or 1 μm OVA323–367 peptide for 2 hr at 37° and washed three times before co-culturing with CD8+ T cells from OT-I mice or CD4+ T cells from OT-II mice, respectively, for 72 hr. Supernatants were collected for cytokine assays by enzyme-linked immunosorbent assay (ELISA). The experiments were performed three times and representative data from one of these experiments are presented. DC, dendritic cells alone; DC:CD8 = 1 : 10, the ratio of DCs to CD8+ T cells from OT-I mice is 1 : 10; DC:CD4 = 1 : 10, the ratio of DCs to CD4+ T cells from OT-II mice is 1 : 10.
Discussion
Antigen-specific immunotherapy is a promising strategy to eradicate systemic tumours at multiple sites while conferring the advantage of specific discrimination between neoplastic and non-neoplastic cells. However, a major hurdle for the development of such vaccines for treatment and prevention of cancer is the poor immunogenicity of tumour-associated antigens. An attractive strategy to overcome this problem is the use of an immune modulator as an adjuvant to boost antigen-specific immunity and enhance the efficacy of tumour vaccines.27
Our in vivo tumour protection results showed that 60% of the mice co-immunized with HPV-16 E7 plus Fve remained tumour-free after tumour challenge as compared with only 20% of mice immunized with HPV-16 E7 alone (Fig. 3). More importantly, the enhanced antitumour effects induced by HPV-16 E7 plus Fve co-immunization were also observed in the therapeutic tumour model (Fig. 4). These data indicate that HPV-16 E7 plus Fve co-immunization is more efficacious for the protection of mice against tumour challenge and the eradication of established tumours, and that Fve enhances antitumour effects. It is conceivable that the enhanced HPV-16 E7-specific T-cell immunity and increased IFN-γ production by these T cells induced by Fve may contribute, at least in part, to the enhanced antitumour effects observed in this study.
It is well known that antigen-specific T-cell immunity plays a critical role in tumour immunotherapy.28 Previous studies using other adjuvants such as heat shock protein 65,9 bacteria exotoxin,11 IL-12,7 CpG12 and 3-O-deacylated monophosphoryl lipid A (MPL) mixed with a purified Quillala saponaria saponin immunologic adjuvant (QS21)29 found that CD4+ and/or CD8+ cells play major roles in protecting animals from challenge with HPV-16 E7-expressing TC-1 cells. In this study, we performed in vivo T-cell subset depletion assays to elucidate the contribution of CD4+ and CD8+ T cells to antitumour activity. We found that both T-cell subsets are essential for the inhibition of tumour growth and CD8+ T cells appear to play a more dominant role in protection against tumours. These results concur well with the conventional dogma that CD8+ T cells are pivotal and highly specialized for cytolytic function and thus have been the main focus of cancer immunotherapy, whereas CD4+ T cells confer helper functions in the antitumour effect by providing activation signals to CD8+ T cells30,31 and contributing to the survival maintenance of CD8+ T memory cells.32–36 Recent studies, however, found that tumour-specific CD4+ T cell were able to eliminate a wide variety of tumours that were resistant to CD8-mediated rejection,37,38 providing new supporting evidence for the hypothesis that CD4+ T cells may play a broader role in antitumour responses. Moreover, we found that tumour-free mice protected by immunization with HPV-16 E7 plus Fve had higher levels of IFN-γ production at 167 days after tumour inoculation (Fig. 3c), indicating that Fve enhances HPV-16 E7-specific memory immunity to protect mice against tumour growth. These findings are important because the capacity to elicit an effective long-term memory immune response is essential for the success of a vaccination strategy.39–41
We also adoptively transferred total T cells from HPV-16 E7 plus Fve immunized mice into tumour-bearing recipient mice to address the importance of T cells in mediating the antitumour effects seen in our study. Results indicated that T cells from mice immunized with HPV-16 E7 plus Fve were more efficacious in suppressing tumour growth than those from mice immunized with HPV-16 E7 alone (Fig. 6b). This enhanced efficacy was probably correlated to the increased number of HPV-16 E7-specific effector T cells induced by co-immunization. However, other possibilities, such as increased HPV-16 E7-specific T-cell avidity, cannot be excluded as antigen-specific CD8+ T cells with high avidity are known to produce stronger antitumour effects in vaccinated mice than low-avidity CD8+ T cells.42,43 In recent years, adoptive transfer of antigen-specific T cells into patients has emerged as a promising new approach to cancer treatment.44–46 Our T-cell transfer data suggest that the Fve protein may be a good immunotherapeutic vaccine adjuvant to enhance the antitumour immunity mediated by tumour antigen-specific T cells, thereby providing a promising new strategy to improve the efficacy of the adoptive cell therapy approach.
In addition, we found that HPV-16 E7 plus Fve co-immunization significantly up-regulated HPV-16 E7-specific IgG1 and IgG2c. The enhanced production of IgG2c in C57BL/6 (Fig. 2b) and IgG2a in BALB/cJ mice (Fig. S2b) was consistent with the previous finding that Fve can increase the Th1-skewed humoral OVA-specific immune response.20 Previous studies using the NY-ESO-1 tumour antigen as a model antigen have shown that antigen-specific IgG2a antibodies contribute to DC maturation and cross-priming of CD8+ T cells, probably through a mechanism mediated by antigen–antibody immune complexes.47,48 Although there is no direct evidence that antibody-mediated responses play an important role in controlling HPV-associated malignancies, a possible role of increased production of HPV-16 E7-specific antibody, which may improve antitumour activity, cannot be ruled out and warrants further study.
IFN-γ has been shown to inhibit tumour growth in vivo by up-regulation of MHC class I molecules, inducing inflammation at tumour sites as well as eliciting an angiostatic effect.49–54 Our study also demonstrated that IFN-γ was critical for generating potent antitumour effects against TC-1 tumour challenge. We found that the tumour protection effect of the HPV-16 E7 plus Fve vaccine was significantly attenuated in IFN-γ-depleted mice (Fig. 5). These results are consistent with previous studies demonstrating the important role of IFN-γ in the antitumour effect.51,53,55,56 Interestingly, we found that the mean tumour size of HPV-16 E7 plus Fve-immunized mice with IFN-γ depletion was smaller than that of PBS-immunized mice (Fig. 5b), suggesting that additional IFN-γ-independent mechanisms may also contribute to the suppression of tumour growth found.
It is worth noting that the Fve protein alone conferred some antitumour effects compared with the effects of PBS (Fig. 3b). This interesting observation may be explained by the fact that the Fve protein mitogenically expands the T-cell pool and induces high levels of IFN-γ production (Fig. 1), creating a microenvironment to confer partial antitumour effects. This may also represent an additional beneficial effect of using Fve as an adjuvant for antitumour immunotherapeutic vaccines.
It is well known that activation of innate immunity is a prerequisite for an adjuvant function.57 The roles of DCs in the priming and differentiation of naïve T cells are well recognized.26 The majority of adjuvants are microbial products that activate innate responses through pattern recognition receptors, such as Toll-like receptors (TLRs) on DCs. For example, monophosphoryl lipid A, imiquimod and CpG motifs, which are agonists for TLR4, TLR7 and TLR9, respectively, have been developed as vaccine adjuvants for the treatment of cancer.58,59 In this study, we found that Fve induced maturation of DCs in vivo and, notably, it appears that Fve preferentially, although not exclusively, drives maturation of the CD8+ DC subset. Moreover, in vitro functional assays using OVA-specific CD8+ T cells from OT-I mice clearly showed that DCs from mice co-immunized with Fve and OVA greatly enhanced the activation of antigen-specific CD8+ T cells (Fig. 8a). It is well known that there are at least three distinct subsets (CD4+ CD8−, CD4− CD8+ and CD4− CD8−) of DCs found in the mouse spleen. Previous studies have shown that these subsets exhibit intrinsic differential capacities to present soluble antigen and activate antigen-specific CD4+ and CD8+ T subsets. The CD8− subsets show the greatest ability to stimulate antigen-specific MHC class II-restricted CD4+ T cells, whereas the CD8+ DCs are much more efficient at cross-presenting antigen and stimulating MHC class I-restricted CD8+ T cells.60,61 Taking these findings together, it is reasonable to propose that Fve modifies the ability of DCs (for example, by up-regulating the CD8+ DC subset to enhance CD8+ T cells via the cross-presentation pathway) to generate robust and long-lasting HPV-16 E7-specific CD8+ T-cell immune responses for antitumour effects. This may be a unique feature of the adjuvant effects of Fve and therefore further work is warranted to validate this notion. Thus, our future research will focus on elucidation of the effects of Fve on the various DC subsets and the subsequent priming and polarization of antigen-specific T-cell subsets.
In summary, our study has demonstrated for the first time that an immunomodulatory protein, Fve, exhibited effective adjuvant effects to enhance robust and long-lasting adaptive antigen-specific immune responses that conferred strong prophylactic and therapeutic antitumour effects. Notably, it appears that, by targeting CD8+ DCs, Fve can effectively enhance antigen-specific CD8+ T-cell immune responses. For HPV-associated malignancies, prophylactic vaccines which aim to induce neutralizing antibodies have been successfully used in clinical applications.62,63 However, the challenge for the future is to combine the prophylactic approach with therapeutic immunization, for example, combining E7 with the HPV capsid protein L1 or L2 to develop chimeric vaccines.64–66 We envisage that Fve could potentially be an effective adjuvant not only for such HPV chimeric vaccines; it could also be generally exploited to develop other efficacious anticancer or antiviral vaccines.
Acknowledgments
We would like to thank Dr Tzyy-Choou Wu for the TC-1 tumour cell line, Dr Siew-Wee Chan for the HPV-16 E7 cDNA clone, Drs Bee Wah Lee and Nge Cheong for their critical reading of the manuscript and Ms Hongmei Wen for technical assistance. This research is supported by a grant (NMRC/1016/2005) from The National Medical Research Council Singapore and K.Y.C. is the main recipient of this grant. There is no financial or commercial conflict of interest to be disclosed.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Supplementary materials and methods
Figure S1. Fve stimulates mouse splenic T-cell proliferation in an accessory-cell-dependent manner.
Figure S2. Fve enhanced human papillomavirus (HPV)-16 E7-specific antibody production in BALB/cJ mice.
Figure S3. Fve enhanced interferon (IFN)-γ production in C57BL/6 mice co-immunized with human papillomavirus (HPV)-16 E7 plus Fve.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than about missing material) should be directed to the corresponding author for the article.
References
- 1.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006;6:753–63. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Munoz N. The viral etiology of cervical cancer. Virus Res. 2002;89:183–90. doi: 10.1016/s0168-1702(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 6.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 7.Ahn WS, Bae SM, Kim TY, Kim TG, Lee JM, Namkoong SE, Kim CK, Sin JI. A therapy modality using recombinant IL-12 adenovirus plus E7 protein in a human papillomavirus 16 E6/E7-associated cervical cancer animal model. Hum Gene Ther. 2003;14:1389–99. doi: 10.1089/104303403769211619. [DOI] [PubMed] [Google Scholar]
- 8.Bermudez-Humaran LG, Cortes-Perez NG, Lefevre F, et al. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J Immunol. 2005;175:7297–302. doi: 10.4049/jimmunol.175.11.7297. [DOI] [PubMed] [Google Scholar]
- 9.Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI, Mizzen LA. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16 E7. Clin Exp Immunol. 2000;121:216–25. doi: 10.1046/j.1365-2249.2000.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, Wu TC. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–42. [PubMed] [Google Scholar]
- 11.Liao CW, Chen CA, Lee CN, Su YN, Chang MC, Syu MH, Hsieh CY, Cheng WF. Fusion protein vaccine by domains of bacterial exotoxin linked with a tumor antigen generates potent immunologic responses and antitumor effects. Cancer Res. 2005;65:9089–98. doi: 10.1158/0008-5472.CAN-05-0958. [DOI] [PubMed] [Google Scholar]
- 12.Kim TY, Myoung HJ, Kim JH, Moon IS, Kim TG, Ahn WS, Sin JI. Both E7 and CpG-oligodeoxynucleotide are required for protective immunity against challenge with human papillomavirus 16 (E6/E7) immortalized tumor cells: involvement of CD4+ and CD8+ T cells in protection. Cancer Res. 2002;62:7234–40. [PubMed] [Google Scholar]
- 13.Ko JL, Hsu CI, Lin RH, Kao CL, Lin JY. A new fungal immunomodulatory protein, FIP-fve isolated from the edible mushroom, Flammulina velutipes and its complete amino acid sequence. Eur J Biochem. 1995;228:244–9. [PubMed] [Google Scholar]
- 14.Seow SV, Kuo IC, Paaventhan P, Kolatkar PR, Chua KY. Crystallization and preliminary X-ray crystallographic studies on the fungal immunomodulatory protein Fve from the golden needle mushroom (Flammulina velutipes) Acta Crystallogr D Biol Crystallogr. 2003;59:1487–9. doi: 10.1107/s0907444903011879. [DOI] [PubMed] [Google Scholar]
- 15.Paaventhan P, Joseph JS, Seow SV, Vaday S, Robinson H, Chua KY, Kolatkar PR. A 1.7A structure of Fve, a member of the new fungal immunomodulatory protein family. J Mol Biol. 2003;332:461–70. doi: 10.1016/s0022-2836(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 16.Haak-Frendscho M, Kino K, Sone T, Jardieu P. Ling Zhi-8: a novel T cell mitogen induces cytokine production and upregulation of ICAM-1 expression. Cell Immunol. 1993;150:101–13. doi: 10.1006/cimm.1993.1182. [DOI] [PubMed] [Google Scholar]
- 17.Lin WH, Hung CH, Hsu CI, Lin JY. Dimerization of the N-terminal amphipathic alpha-helix domain of the fungal immunomodulatory protein from Ganoderma tsugae (Fip-gts) defined by a yeast two-hybrid system and site-directed mutagenesis. J Biol Chem. 1997;272:20044–8. doi: 10.1074/jbc.272.32.20044. [DOI] [PubMed] [Google Scholar]
- 18.Hsu HC, Hsu CI, Lin RH, Kao CL, Lin JY. Fip-vvo, a new fungal immunomodulatory protein isolated from Volvariella volvacea. Biochem J. 1997;323(Pt 2):557–65. doi: 10.1042/bj3230557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang PH, Hsu CI, Tang SC, Huang YL, Lin JY, Ko JL. Fungal immunomodulatory protein from Flammulina velutipes induces interferon-gamma production through p38 mitogen-activated protein kinase signaling pathway. J Agric Food Chem. 2004;52:2721–5. doi: 10.1021/jf034556s. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh KY, Hsu CI, Lin JY, Tsai CC, Lin RH. Oral administration of an edible-mushroom-derived protein inhibits the development of food-allergic reactions in mice. Clin Exp Allergy. 2003;33:1595–602. doi: 10.1046/j.1365-2222.2003.01790.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 22.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 23.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–86. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 24.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–92. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 26.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 27.Singh M, O’Hagan D. Advances in vaccine adjuvants. Nat Biotechnol. 1999;17:1075–81. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda H, Chamoto K, Tsuji T, Suzuki Y, Wakita D, Takeshima T, Nishimura T. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 2004;95:697–703. doi: 10.1111/j.1349-7006.2004.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerard CM, Baudson N, Kraemer K, Bruck C, Garcon N, Paterson Y, Pan ZK, Pardoll D. Therapeutic potential of protein and adjuvant vaccinations on tumour growth. Vaccine. 2001;19:2583–9. doi: 10.1016/s0264-410x(00)00486-2. [DOI] [PubMed] [Google Scholar]
- 30.Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 31.Marzo AL, Lake RA, Robinson BW, Scott B. T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res. 1999;59:1071–9. [PubMed] [Google Scholar]
- 32.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 33.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 34.Gao FG, Khammanivong V, Liu WJ, Leggatt GR, Frazer IH, Fernando GJ. Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. Cancer Res. 2002;62:6438–41. [PubMed] [Google Scholar]
- 35.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–81. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 36.Lin CT, Chang TC, Shaw SW, Cheng PJ, Huang CT, Chao A, Soong YK, Lai CH. Maintenance of CD8 effector T cells by CD4 helper T cells eradicates growing tumors and promotes long-term tumor immunity. Vaccine. 2006;24:6199–207. doi: 10.1016/j.vaccine.2006.05.108. [DOI] [PubMed] [Google Scholar]
- 37.Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–83. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–54. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foulds KE, Wu CY, Seder RA. Th1 memory: implications for vaccine development. Immunol Rev. 2006;211:58–66. doi: 10.1111/j.0105-2896.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 40.Salerno-Goncalves R, Sztein MB. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 2006;14:536–42. doi: 10.1016/j.tim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–41. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 42.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–7. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S, Linette GP, Longerich S, Haluska FG. Antimelanoma activity of CTL generated from peripheral blood mononuclear cells after stimulation with autologous dendritic cells pulsed with melanoma gp100 peptide G209-2M is correlated to TCR avidity. J Immunol. 2002;169:531–9. doi: 10.4049/jimmunol.169.1.531. [DOI] [PubMed] [Google Scholar]
- 44.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valmori D, Souleimanian NE, Tosello V, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA. 2007;104:8947–52. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnurr M, Chen Q, Shin A, et al. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105:2465–72. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- 49.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 50.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 51.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 52.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–8. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 53.Dominiecki ME, Beatty GL, Pan ZK, Neeson P, Paterson Y. Tumor sensitivity to IFN-gamma is required for successful antigen-specific immunotherapy of a transplantable mouse tumor model for HPV-transformed tumors. Cancer Immunol Immunother. 2005;54:477–88. doi: 10.1007/s00262-004-0610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol. 2001;166:2276–82. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 55.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–56. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 56.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–72. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–8. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 58.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seya T, Akazawa T, Tsujita T, Matsumoto M. Role of Toll-like receptors in adjuvant-augmented immune therapies. Evid Based Complement Alternat Med. 2006;3:31–8. doi: 10.1093/ecam/nek010. discussion 133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166:5327–30. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 61.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 62.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 63.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 64.Greenstone HL, Nieland JD, de Visser KE, et al. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–5. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jochmus I, Schafer K, Faath S, Muller M, Gissmann L. Chimeric virus-like particles of the human papillomavirus type 16 (HPV 16) as a prophylactic and therapeutic vaccine. Arch Med Res. 1999;30:269–74. doi: 10.1016/s0188-0128(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 66.de Jong A, O’Neill T, Khan AY, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20:3456–64. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.