Abstract
A new proinflammatory cytokine interleukin-32 (IL-32) has six isoforms. Although IL-32 can be detected in sera from patients suffering from Crohn’s disease and rheumatoid arthritis, it is unclear which isoforms are involved. To this end, we investigated the functions of the most abundant IL-32β by generating K562-IL-32β stable cell lines. This report confirms, using IL-32 small interfering RNA, that IL-32β induces an anti-inflammatory cytokine IL-10 in K562-IL-32β cells and U937 promonocytic cells, which express endogenous IL-32β upon phorbol 12-myristate 13-acetate (PMA) treatment, and monocyte-derived dendritic cells (DC) upon lipopolysaccharide (LPS) treatment. Interleukin-32β was induced in monocyte-derived macrophages by LPS and in monocyte-derived DC by LPS, poly(I:C), or anti-CD40 antibody, but was not induced by PMA. We showed that IL-32β expression was increased in a time-dependent manner in monocyte-derived DC upon LPS treatment and peaked at 24 hr. Production of IL-10 was exactly coincident with IL-32β expression, but IL-1β and tumour necrosis factor-α production peaked at 6 hr after LPS treatment, then steeply declined. Interleukin-12 p40 was induced at 9 hr and gradually increased until 48 hr, at which time IL-32β and IL-10 were no longer increased. Knock-down of IL-32β by IL-32 small interfering RNA led to the decrease of IL-10, but the increase of IL-12 in monocyte-derived DC, which means that IL-32β promotes IL-10 production, but limits IL-12 production. We also showed that IL-10 neutralization increases IL-12, IL-1β and tumour necrosis factor-α production, which implies that IL-10 suppresses such proinflammatory cytokines. Taken together, our results suggest that IL-32β upregulates the production of an anti-inflammatory cytokine IL-10, and then IL-10 suppresses proinflammatory cytokines.
Keywords: cytokine, dendritic cell, inflammation, interleukin-10, interleukin-32
Introduction
Interleukin-32 (IL-32), originally reported as natural killer (NK) transcript 4, which was the IL-32γ isoform, is produced by activated T cells and NK cells and was thought to be secreted because it contains an internal signal sequence and lacks a transmembrane region.1 However, the function of IL-32 has been unclear. Recently, IL-32 was defined as a proinflammatory cytokine that is induced in epithelial cells and monocytes by interferon-γ (IFN-γ) stimulation in a time-dependent manner.2–4 Moreover, proteinase 3 has been identified as an IL-32α binding protein that produces the active form of IL-32α. Cleavage of IL-32α by proteinase 3 induces macrophage inflammatory protein-2 in mouse Raw264.7 cells and IL-8 in human peripheral blood mononuclear cells (PBMC).5 These observations suggest that IL-32 belongs to a proinflammatory cytokine group. Six alternatively spliced isoforms of IL-32 have been reported,3,6,7 but the functional differences among the isoforms have not been elucidated.
Interleukin-10 is a well-known anti-inflammatory cytokine that suppresses immune responses by inhibiting the production of inflammatory cytokines such as tumour necrosis factor-α (TNF-α), IL-1 and the cell surface expression of major histocompatibility complex class II.8 Interleukin-10 was originally known as a cytokine synthesis inhibitory factor (i.e. CSIF) that is produced by T helper type 2 cells upon stimulation with concanavalin A or antigen.9 Myeloid cells are major sources of IL-10.10 Myeloid cells, including antigen-presenting cells, are stimulated by pathogen-associated molecular patterns [e.g. lipopolysaccharide (LPS)] to produce proinflammatory cytokines such as IL-12, TNF-α, chemokines, prostaglandin and nitric oxide, whereas IL-10 prevents the induction of these inflammatory mediators.8,11
When infection with a pathogen occurs, proinflammatory responses must be triggered to regulate the proper immune responses. However, the inflammatory process must be resolved to prevent damage to the host.12 The proinflammatory cytokine IL-12 has been reported to induce IL-10 and IFN-γ, but suppress IL-4 in BALB/c mice infected with the intramacrophage parasite Leishmania major and in infected severe combined immunodeficient (SCID) mice.13,14 Another proinflammatory cytokine, TNF-α, is also known to induce IL-10 in THP-1 cells.15 Through these regulatory loops, the host maintains a homeostatic immune balance. In this study, we demonstrate that IL-32β induces the anti-inflammatory cytokine IL-10, which could be another regulatory loop of the innate immune response.
Materials and methods
Reagents and cell culture
The human erythroleukaemia K562 cell line and the promonocytic U937 cell line were grown in RPMI-1640 culture medium supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (Hyclone, Logan, UT). Recombinant human granulocyte–macrophage colony-stimulating factor (rhGM-CSF) and rhIL-4 (Endogen, Woburn, MA) were used for the primary cultures. Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma (St Louis, MO). Small interfering (si) RNA for IL-32 and non-targeting control siRNA were purchased from Dharmacon (Lafayette, CO).
Cloning and construction of stable cell lines
Human IL-32α and IL-32β complementary DNA (cDNA) were amplified by polymerase chain reaction (PCR) and cloned into pcDNA3.1+ using EcoRI and XhoI. Stable clones were constructed by transfecting K562 cells with pcDNA3.1+, pcDNA3.1+IL-32α or pcDNA3.1+IL-32β plasmids using a nucleofection kit V (Amaxa, Cologne, Germany) and then G-418 (1 mg/ml) resistant clones were selected for 3 weeks.
Preparations of dendritic cells and macrophages from monocytes isolated with immunomagnetic beads
Dendritic cells (DC) and macrophages were obtained as previously described.16 Briefly, human PBMC from healthy donors (Red Cross Blood Centre, Daejeon, Korea) were isolated by density centrifugation on Histopaque 1077 (Sigma). The Red Cross Blood Centre Committee (Seoul, South Korea) approved the use of these cells. Monocytes were purified by negatively depleting T, B and NK cells, erythrocytes, and granulocytes using mouse antibody-reactive immunomagnetic beads (Dynal, Oslo, Norway). Briefly, anti-CD2, -CD7, -CD16, -CD19, -CD56 and -CD235a-labelled PBMC were incubated with immunomagnetic beads for 30 min at 4° with gentle rotation, and positive cells were removed using a Dynal magnet. Purified monocytes were found to be > 90% positive for the CD14 marker. Immature DC were generated by culturing monocytes in human DC medium containing 400 ng/ml rhGM-CSF and 20 ng/ml rhIL-4 or macrophage medium containing 10 ng/ml rhM-CSF (R&D Systems, Minneapolis, MN) in a 12-well plate at a concentration of 5 × 105 cells per well.
Transfections
K562-IL-32β and U937 cells were transfected with 1 μg IL-32 siRNA and non-targeting control siRNA using a nucleofection kit V (Amaxa) as described. The siRNA for IL-32 or control siRNA were introduced into monocytes using a monocyte nucleofection kit (Amaxa) according to the manufacturer’s instructions and then differentiated into DC by culturing in DC medium containing rhIL-4 and rhGM-CSF for 6 days. pcDNA3.1+-IL-32β and pcDNA3.1+ empty vector were introduced into U937 cells by electroporation for ectopic overexpression.
Reverse transcription-PCR and Western blot analysis
Total RNA from K562-vector, K562-IL-32β, U937 or DC was extracted using an easy-BLUE kit (iNtRON, Seongnam, South Korea). First-strand synthesis was performed using AMV reverse transcriptase (Promega, Madison, WI) according to the manufacturer’s instructions then PCR-amplified using iTag polymerase (iNtRON). The following primers were used: IL-32 sense primer: 5′-ATGTGCTTCCCGAAGGTCCTC-3′; IL-32 antisense primer: 5′-TCATTTTGAAGGATTGGGGTTC-3′; IL-10 sense primer: 5′-AACCTGCCTAACATGCTTCGA-3′; and IL-10 antisense primer: 5′-CTCATGGCTTTGTAGATGCCT. Cells were harvested and lysed in RIPA buffer (20 mm Tris–HCl, pH 7·4, 150 mm NaCl, 0·5% nonidet P-40, 0·1% sodium deoxycholic acid, 0·05% sodium dodecyl sulphate, 10 mmβ-phosphoglycerate, 10 mm sodium pyrophosphate, 1 mm sodium vanadate and protease inhibitor cocktail). Interleukin-32 was detected using a monoclonal antibody (mAb), KU32-52, as previously described.7
Enzyme-linked immunosorbent assays
The IL-32 enzyme-linked immunosorbent assay (ELISA) system was previously described.7 Briefly, cell culture supernatants were incubated with mAb KU32-56-precoated on 96-well plates for 1 hr at room temperature. After washing three times with 0·05% Tween-20 in phosphate-buffered saline (PBST), wells were incubated with the biotinylated KU32-52 in 3% skim milk for 1 hr at room temperature, washed three times with PBST, then incubated with streptavidin peroxidase in 3% skim milk, followed by three washes with PBST. SureBlue TMB peroxidase substrate (KPL, Gaithersburg, MD) was added and the reaction was stopped by adding 50 μl 1·25 m sulphuric acid. Enzyme activity was measured at 450 nm using an ELISA reader (Photoread, Berthold, Germany). Interleukin-10, IL-1β, TNF-α, IL-12 p70 and IL-12 p40 were measured in culture supernatants using the appropriate sandwich ELISA kits.
IL-10 neutralization
Monocyte-derived DC of 1·5 × 106 cells/ml were treated with 10 μg/ml IL-10 neutralizing mAb (R&D Systems) in the presence or absence of 1 μg/ml LPS for 24 hr. Cells were harvested for Western blotting, and culture media were collected and assayed for IL-10, IL-12 p70, IL-12 p40, IL-1β and TNF-α using the appropriate ELISA kits (R&D Systems).
Results
IL-10 transcripts are induced in IL-32β-expressing K562 cells upon PMA stimulation
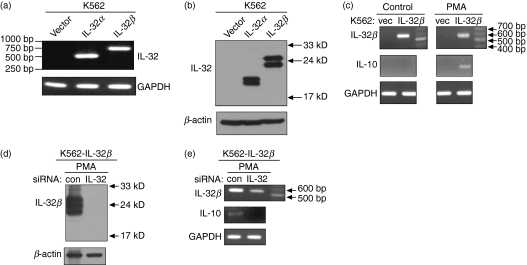
We subcloned the full-length cDNA for human IL-32α or human IL-32β into the expression vector pcDNA3.1+, then transfected these into the human erythroleukaemia cell line K562 to generate a constitutive expression system for IL-32α or IL-32β. Strong expression of IL-32α or IL-32β was detected by both reverse transcription (RT) -PCR and Western blotting (Fig. 1a,b). Although not clearly understood, the multiband pattern of IL-32 on the Western blot is thought to be caused by modifications of the IL-32 peptide according to sequence analysis.3 In addition, we could not detect any secreted IL-32 in the culture medium using Western blotting (data not shown). We investigated the genes that were modulated by IL-32 using these stable cell lines. One of the genes that was upregulated by IL-32β after PMA treatment was that for IL-10. The IL-10 transcripts were increased in IL-32β-expressing K562 cells upon PMA stimulation compared with mock vector-transfected K562 cells (Fig. 1c). The effect of IL-32α on IL-10 induction was not clear (data not shown). However, we could not detect IL-10 by ELISA or Western blotting. This may be the result of an effect of PMA-induced differentiation on translation because PMA causes erythroleukaemia K562 cells to differentiate into megakaryocytic cells.17–19 To confirm that IL-32β induces IL-10, we transfected IL-32β-expressing K562 cells with IL-32 siRNA. Non-targeting control siRNA was included in experiments as a control. Interleukin-32 silencing caused the specific decrease of IL-32 expression as well as a concomitant reduction in IL-10 transcripts (Fig. 1e). The extent of IL-32 silencing was more drastic at the protein level than at the level of transcription (Fig. 1d,e).
Figure 1.
Construction of interleukin-32α (IL-32α) or IL-32β stable cell lines in K562 cells, and IL-10 induction by IL-32β upon phorbol 12-myristate 13-acetate (PMA) treatment. Stable cell lines for IL-32α or IL-32β were constructed in K562 cells and confirmed by reverse transcription–polymerase chain reaction (RT-PCR) (a) and Western blotting (b). K562-IL-32β cells were treated with 50 nm PMA for 24 hr, then total RNA was extracted for RT-PCR. Interleukin-10 transcripts were induced upon PMA stimulation of IL-32β expressing cells (c). A concomitant reduction in IL-10 transcripts with IL-32 knock-down by small interfering RNA transfection was detected (e). IL-32β suppression was confirmed by Western blotting (d).
Silencing of IL-32β induces downregulation of IL-10 in U937 cells
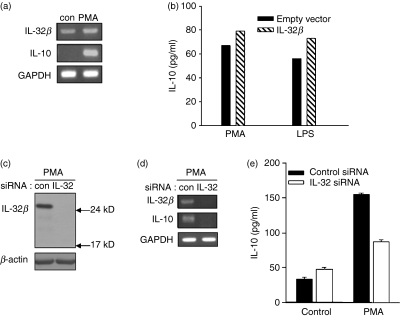
When we performed RT-PCR for IL-32 with total RNA from the promyelomonocytic cell line U937, the putative IL-32β band (567 base pairs for cDNA size) was observed (Fig. 2a). We cloned this band into a sequencing vector and confirmed its identity as IL-32β by sequencing (data not shown). Interleukin-32β is very abundant in U937; U937 cells have been used as a model system for monocyte or macrophage-like cells that produces IL-10 upon stimulation.20–24 We also observed that IL-10 transcription was induced by PMA treatment (Fig. 2a). Therefore, we tried to determine the relevance of IL-32β in the induction of IL-10 in U937 cells treated with PMA and LPS. Production of IL-10 was further increased by ectopic overexpression of IL-32β in U937 cells (Fig. 2b). We speculated that IL-32β might have a positive role in IL-10 induction. To test this, we tried to knock-down IL-32β by transfecting U937 cells with IL-32 siRNA. With IL-32β suppression, PMA did not induce IL-10 transcription (Fig. 2d). We confirmed that the IL-32 siRNA worked effectively (Fig. 2c). We further confirmed the effect of IL-32β on IL-10 induction using ELISA. When U937 cells were transfected with IL-32 siRNA then treated with PMA for 24 hr, a significant reduction in IL-10 production compared with control cells was observed (Fig. 2e). These observations imply that IL-32β upregulates IL-10 production.
Figure 2.
Regulation of interleukin-10 (IL-10) expression by IL-32β in U937 cells. U937 cells expressing endogenous IL-32β were treated with 50 nm phorbol 12-myristate 13-acetate (PMA) for 24 hr, then IL-10 transcription was measured (a). U937 cells were transfected with 1 μg pcDNA3.1+-IL-32β and pcDNA3.1+ empty vector by electroporation followed by PMA treatment for 24 hr. Additional increase of IL-10 by ectopic expression of IL-32β was analysed by enzyme-linked immunosorbent assay (ELISA) (b). This experiment was performed three times in duplicate. Mean values of duplicate are presented. U937 cells were transfected with 1 μg of IL-32 small interfering (si) iRNA or control siRNA. Twenty-four hours later, cells were treated with 50 nm PMA for 20 hr. A decrease in IL-10 expression by IL-32 suppression was detected by reverse transcription–polymerase chain reaction (d). Knock-down of IL-32β protein was checked by Western blotting (c). A reduction in secreted IL-10 after IL-32β silencing was measured using an ELISA kit (e). All values of this experiment are shown as the mean ± SEM of duplicates.
IL-32β is induced in both monocyte-derived macrophages and monocyte-derived DC by LPS, poly(I:C) and anti-CD40 antibody treatment, but not by PMA treatment
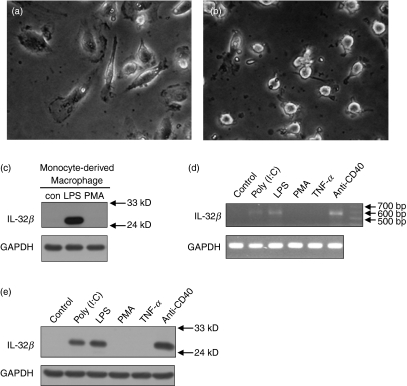
Interleukin-32 is abundantly expressed in the spleen.3 We investigated the expression pattern of IL-32 in myeloid lineage cells from human PBMC. Monocytes were purified from human PBMC, and then differentiated into macrophages or DC for 6 days. The phenotypes of differentiated cells were ensured by detecting cell-type-specific surface markers like CD1a, CD14, CD80 and CD86 (data not shown), and the cells were photographed (Fig. 3a,b). We detected IL-32β by Western blotting, which was induced in monocyte-derived macrophages by LPS treatment but not by PMA treatment (Fig. 3c). Interleukin-32β was strongly induced in monocyte-derived DC upon treatment with LPS, poly(I:C) and the anti-CD40 antibody. There was little effect of PMA or TNF-α on IL-32β induction (Fig. 3d,e). These data indicate that IL-32β is induced by microbial products that activate the Toll-like receptor 3 (TLR3) or TLR4 signalling pathways. The bands in Fig. 3(d) were confirmed as IL-32β cDNA by sequencing but we did not detect IL-32β in the culture medium by ELISA (data not shown).
Figure 3.
Interleukin-32β (IL-32β) induction in monocyte-derived macrophages or monocyte-derived dendritic cells (DC). Phase contrast images of monocyte-derived macrophages (a) and monocyte-derived DC (b) obtained from human peripheral blood mononuclear cells. Macrophages were treated with 1 μg/ml lipopolysaccharide (LPS) or 20 nm phorbol 12-myristate 13-acetate (PMA) for 24 hr, then analysed by Western blotting for IL-32 (c). Monocyte-derived DC were treated with 10 μg/ml poly(I:C), 1 μg/ml LPS, 20 nm PMA, 100 ng/ml tumour necrosis factor-α (TNF-α), or 1 μg/ml of anti-CD40 for 24 hr, then total RNA or cell lysates were prepared for reverse transcription–polymerase chain reaction (RT-PCR) (d) or Western blotting (e), respectively. Untreated negative controls were analysed simultaneously (c–e).
IL-10 production coincides with IL-32β expression in a time-dependent manner
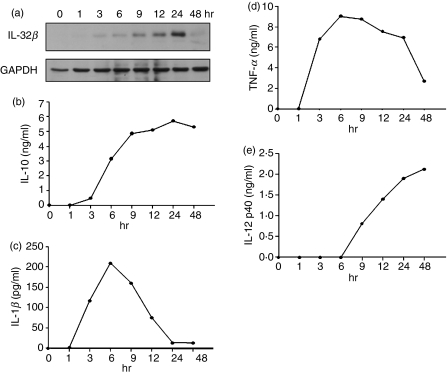
We tried to reveal the relationship between IL-32β and IL-10, or other proinflammatory cytokines, in primary cells using human PBMC. Monocytes were purified from human PBMC and differentiated into DC. To analyse the time–course expression patterns of IL-32β, IL-10 and proinflammatory cytokines, LPS-treated monocyte-derived DC and culture media were prepared at the indicated time-points (Fig. 4). Expression of IL-32β gradually increased until 24 hr, then decreased (Fig. 4a). The production pattern of IL-10 was identical to that of IL-32β, which was peaked at 24 hr (Fig. 4b). On the other hand, IL-1β and TNF-α induction peaked at 6 hr, and then declined steeply (Fig. 4c,d). This implies that IL-1β and TNF-α were induced earlier, before IL-10 was available in the culture medium, then those cytokines decreased sharply as IL-10 was increased. Interleukin-12 p40 was induced at around 9 hr, and continued to be mildly increased until 48 hr, at which time IL-32β and IL-10 production was no longer increased (Fig. 4e). Much later induction of IL-12 p40 than of IL-1β and TNF-α might be the result of IL-10, which is already effective enough at this time. The expression pattern of IL-12 p40 did not correspond to the pattern of IL-32β expression. It means that IL-32β is irrelevant to IL-12 induction. These results suggest that IL-10 production is promoted by IL-32β, but other proinflammatory cytokines are suppressed.
Figure 4.
Time–course expression patterns of interleukin-10 (IL-10) and proinflammatory cytokines. Monocytes purified from human peripheral blood mononuclear cells (PBMC) were differentiated into dendritic cells (DC). Cells and culture media were prepared at the indicated time-points. Seventy micrograms of cell lysates were used for Western blotting for IL-32β (a) and culture supernatants were analysed for IL-10, IL-1β, tumour necrosis factor-α (TNF-α) and IL-12 p40 using the respective enzyme-linked immunosorbent assay (ELISA) kits (b, c, d, e). The ELISA were performed twice in duplicate with two independent sources of PBMC and the mean values of duplicate were presented.
IL-32β increases IL-10, but decreases IL-12 in monocyte-derived DC
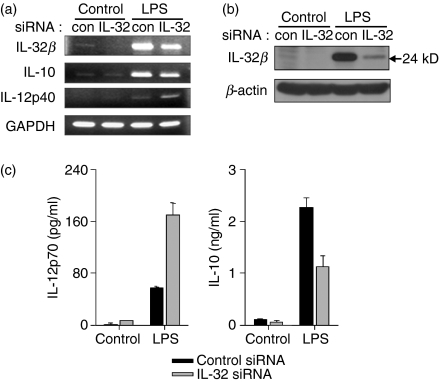
We tried to confirm the relationship between IL-32β and IL-10 in monocyte-derived DC. Monocytes purified from human PBMC were transfected with IL-32 siRNA and differentiated into DC by incubation with rhIL-4 and rhGM-CSF for 6 days. In contrast to the observed reduction in IL-10 transcription, IL-12 p40 transcription was increased by LPS treatment when IL-32β was suppressed (Fig. 5a). We verified, using Western blotting, that IL-32 siRNA worked effectively in the transfected monocytes (Fig. 5b). The protein levels of IL-10 and IL-12 p70 in the culture medium were measured using ELISA kits. Consistent with our transcriptional analysis, we observed a substantial reduction in IL-10 and an increase in IL-12 p70 as a result of IL-32β silencing (Fig. 5c). These observations strongly suggest that IL-32β induces IL-10 production, but suppresses IL-12 production. Alternatively, the inhibitory effect of IL-32β on IL-12 induction may be mediated by the increased IL-10, because IL-10 has been shown to limit the inflammatory response by inhibiting IL-12 production.13
Figure 5.
Interleukin-10 (IL-10) induction by IL-32β upon lipopolysaccharide (LPS) stimulation in monocyte-derived dendritic cells (DC). Non-targeting control small interfering (si) RNA or IL-32 siRNA was transfected into monocytes, which were then differentiated into DC. Cells were treated with 1 μg/ml LPS for 24 hr, then total RNA was purified, cell lysates were prepared, and the culture medium was collected. Interleukin-10 transcripts were induced by IL-32β, but IL-12 p40 transcription was decreased (a). IL-32β expression was drastically reduced by IL-32 siRNA transfection (a, b). Culture media were assayed for IL-12 p70 and IL-10 by enzyme-linked immunosorbent assay (c). All values represent the mean ± SEM of duplicates.
IL-10 neutralization increases proinflammatory cytokines production such as IL-12 p40, IL-1β and TNF-α
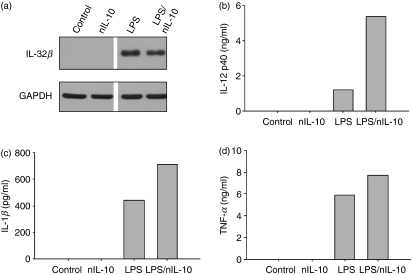
Previous data showed that IL-32β promotes IL-10 production but suppresses proinflammatory cytokines such as IL-1β, TNF-α and IL-12. However, it is ambiguous whether the suppression of proinflammatory cytokines is by IL-32β or IL-10. Therefore, we performed an IL-10 neutralization experiment to analyse whether the suppression of proinflammatory cytokines is the result of the induction of IL-10 or not. Interleukin-10 neutralizing antibody was administered to monocyte-derived DC in the presence or absence of LPS for 24 hr. Expression of IL-32β and the levels of proinflammatory cytokines were analysed. Interleukin-32β was expressed in the LPS-treated cells (Fig. 6a); IL-12 p40, IL-1β and TNF-α production were increased in the IL-10 neutralizing antibody-treated cells even though IL-32β was expressed in those cells (Fig. 6b–d). Our results suggest that the suppression of proinflammatory cytokines was the result of IL-10 induction.
Figure 6.
The effect of interleukin-10 (IL-10) neutralization on proinflammatory cytokine production; 1·5 × 106 monocyte-derived dendritic cells were treated with 10 μg IL-10 neutralizing antibody (nIL-10) in the absence or presence of 1 μg/ml lipopolysaccharide (LPS) for 24 hr, then cells and culture media were prepared and analysed for IL-32β by Western blotting (a) and proinflammatory cytokines by enzyme-linked immunosorbent assay (ELISA). Interleukin-10 neutralization increases the IL-12 p40, IL-1β and tumour necrosis factor-α (TNF-α) (b–d). The ELISA were performed twice in duplicate with two independent sources of peripheral blood mononuclear cells and the mean values of duplicates were presented.
Discussion
When confronted with an infectious agent, a host organism mounts inflammatory responses that initiate an adequate immune response. However, this inflammatory immune response must be limited to avoid harmful effects to the host. There must be mediators that balance the pro- and anti-inflammatory responses. In fact, the proinflammatory cytokines IL-12 and TNF-α are known to induce the anti-inflammatory cytokines IL-10 and IFN-γ. Interleukin-10 inhibits the induction of proinflammatory cytokines, including IL-12, and as a result protects the host from excessive inflammatory reactions.25
Interleukin-32 is a proinflammatory cytokine the function of which has recently been redefined. Unlike most cytokines, IL-32 has six isoforms as a result of alternative splicing and the cell surface receptor for IL-32 has not yet been elucidated, despite considerable effort. Moreover, IL-32β seems to function intracellularly rather than as a soluble factor because we could not detect IL-32β by Western blotting with K562-IL-32β culture medium before or after treatment with PMA (data not shown). The culture media of U937 cells or DC did not contain IL-32β, either. Interleukin-32β was also produced by T cells but was not secreted into the culture medium,6 and intracellular accumulation of IL-32α protein was detected in human pancreatic periacinar myofibroblasts but was not detected in culture supernatants.4 It is possible that the pathway for IL-10 induction includes IL-32β-interacting molecules. The issues regarding IL-32 secretion are still controversial.
Interestingly, IL-32β was not induced by PMA in monocyte-derived macrophages or monocyte-derived DC. This, however, does not necessarily mean that IL-10 is not induced by PMA in these cells. It implies that there is another pathway for IL-10 induction mediated by IL-32β. Although we confirmed IL-32β cDNA by sequencing, there may be roles for other isoforms because we also identified IL-32γ cDNA in U937 cells (data not shown).
There are several reports showing the kinetics of cytokine production in THP-1 promonocytic cells. Anti-inflammatory cytokine IL-10 production precedes IL-12 and IL-6 production upon treatment with bacterial lipoprotein, whereas the concentration of TNF-α reached its peak 2 hr after stimulation then declined sharply.15,26 We confirmed IL-32β expression in THP-1 cells by Western blotting and cDNA sequencing after RT-PCR, as in U937 promonocytic cells (data not shown). This suggests that the endogenous IL-32β might induce IL-10 earlier than IL-12 or IL-6 in THP-1 cells. It would appear that TNF-α is produced much earlier, before IL-10 can be available in the culture medium.26
We demonstrated that IL-32β was induced by poly(I:C) or LPS, indicating that the TLR3 and TLR4 signalling pathways induce IL-32β. Therefore, we hypothesize that microbial products induce some isoforms of IL-32 which then trigger proinflammatory responses. However, other isoforms, including IL-32β, which may be induced with time gaps from the induction of proinflammatory isoforms, can suppress inflammation by inducing anti-inflammatory cytokines as a regulatory loop.
Acknowledgments
This study was supported by grant no. R01-2006-000-10145-0 from the Korea Science and Engineering Foundation and by a basic research grant (2006-E0119) from the Korea Research Foundation. We thank Dr Cheol-Heui Yun, Faculty of the Department of Food and Animal Biotechnology, Seoul National University, for supporting the primary cell culture.
References
- 1.Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992;148:597–603. [PubMed] [Google Scholar]
- 2.Dinarello CA, Kim SH. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis. 2006;65(Suppl. 3):iii61–4. doi: 10.1136/ard.2006.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–42. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Nishida A, Andoh A, Shioya M, Kim-Mitsuyama S, Takayanagi A, Fujiyama Y. Phosphatidylinositol 3-kinase/Akt signaling mediates interleukin-32α induction in human pancreatic periacinar myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2008;294:G831–8. doi: 10.1152/ajpgi.00535.2007. [DOI] [PubMed] [Google Scholar]
- 5.Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarllo CA, Kim SH. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci USA. 2006;103:3316–21. doi: 10.1073/pnas.0511206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, Izuhara K. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–40. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 7.Kim KH, Shim JH, Seo EH, et al. Interleukin-32 monoclonal antibodies for immunohistochemistry, Western blotting, and ELISA. J Immunol Methods. 2008;333:38–50. doi: 10.1016/j.jim.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 8.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 11.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- 13.Ma X, Trinchieri G. Regulation of interleukin-12 production in antigen-presenting cells. Adv Immunol. 2001;79:55–92. doi: 10.1016/s0065-2776(01)79002-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZE, Zheng S, Corry DB, Dalton DK, Seder RA, Reiner SL, Locksley RM. Interferon gamma-independent effects of interleukin 12 administered during acute or established infection due to Leishmania major. Proc Natl Acad Sci USA. 1994;91:12932–6. doi: 10.1073/pnas.91.26.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giambartolomei GH, Dennis VA, Lasater BL, Murthy PK, Philipp MT. Autocrine and exocrine regulation of interleukin-10 production in THP-1 cells stimulated with Borrelia burgdorferi lipoproteins. Infect Immun. 2002;70:1881–8. doi: 10.1128/IAI.70.4.1881-1888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SC, Kim KD, Kim JT, et al. Expression of human NDRG2 by myeloid dendritic cells inhibits down-regulation of activated leukocyte cell adhesion molecule (ALCAM) and contributes to maintenance of T cell stimulatory activity. J Leukoc Biol. 2008;83:89–98. doi: 10.1189/jlb.0507300. [DOI] [PubMed] [Google Scholar]
- 17.Whalen AM, Galasinski SC, Shapiro PS, Nahreini TS, Ahn NG. Megakaryocytic differentiation induced by constitutive activation of mitogen-activated protein kinase kinase. Mol Cell Biol. 1997;17:1947–58. doi: 10.1128/mcb.17.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shelly C, Petruzzelli L, Herrera R. K562 cells resistant to phorbol 12-myristate 13-acetate-induced growth arrest: dissociation of mitogen-activated protein kinase activation and Egr-1 expression from megakaryocyte differentiation. Cell Growth Differ. 2000;11:501–6. [PubMed] [Google Scholar]
- 19.Jacquel A, Herrant M, Defamie V, et al. A survey of the signaling pathways involved in megakaryocytic differentiation of the human K562 leukemia cell line by molecular and c-DNA array analysis. Oncogene. 2006;25:781–94. doi: 10.1038/sj.onc.1209119. [DOI] [PubMed] [Google Scholar]
- 20.Brigino E, Haraguchi S, Koutsonikolis A, Cianciolo GJ, Owens U, Good RA, Day NK. Interleukin 10 is induced by recombinant HIV-1 Nef protein involving the calcium/calmodulin-dependent phosphodiesterase signal transduction pathway. Proc Natl Acad Sci USA. 1997;94:3178–82. doi: 10.1073/pnas.94.7.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrelds IM, van Hal PT, Haakmat RC, Hoogsteden HC, Saxena PR, Zijlstra FJ. Time dependent production of cytokines and eicosanoids by human monocytic leukaemia U937 cells; effects of glucocorticosteroids. Mediators Inflamm. 1999;8:229–35. doi: 10.1080/09629359990397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naldini A, Bernini C, Pucci A, Carraro F. Thrombin-mediated IL-10 up-regulation involves protease-activated receptor (PAR)-1 expression in human mononuclear leukocytes. J Leukoc Biol. 2005;78:736–44. doi: 10.1189/jlb.0205082. [DOI] [PubMed] [Google Scholar]
- 23.Slezak K, Guzik K, Rokita H. Regulation of interleukin 12 and interleukin 10 expression in vaccinia virus-infected human monocytes and U-937 cell line. Cytokine. 2000;12:900–8. doi: 10.1006/cyto.1999.0646. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi Y, Kuroki M, Imakiire T, Abe H, Uchida H, Beppu R, Yamashita Y, Shirakusa T. Thrombospondin-1 differentially regulates release of IL-6 and IL-10 by human monocytic cell line U937. Biochem Biophys Res Commun. 2002;290:1551–7. doi: 10.1006/bbrc.2002.6386. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol. 2004;24:2385–96. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murthy PK, Dennis VA, Lasater BL, Philipp MT. Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with Borrelia burgdorferi lipoproteins. Infect Immun. 2000;68:6663–9. doi: 10.1128/iai.68.12.6663-6669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]