Abstract
Cervical cytobrush sampling is a relatively non-invasive method for obtaining mucosal cells from the female genital tract. To define mucosal immune cells sampled by cervical cytobrushing and to validate this approach for local immunity studies, we investigated the impact of human immunodeficiency virus (HIV) status and inflammation on the yield and composition of cervical cytobrush specimens. Cervical cytobrush samples were obtained from 89 chronically HIV-infected and 46 HIV-negative women. The HIV-infected women had significantly higher yields of CD3+, CD45+, CD19+, CD14+, Langerin+ and CD24+ cells than the uninfected women. While cytobrush-derived T cells from uninfected women were predominantly CD4+ (4·2 CD4 : 1 CD8), CD8+ T cells were predominant in HIV-infected women (0·6 CD4 : 1 CD8). The majority of CD4+ and CD8+ T cells from HIV-infected and uninfected women were of the effector memory (CD45RA− CCR7− CD27−) phenotype. HIV-infected women had significantly elevated levels of interleukin (IL)-1β, IL-6 and IL-8 in cervical supernatants compared with uninfected women. We observed a significant positive correlation between T-cell counts and IL-1β, tumour necrosis factor (TNF)-α and IL-12 concentrations. Neutrophil counts correlated significantly with cervical concentrations of IL-1β, TNF-α, IL-8, IL-6 and IL-10. Antigen-presenting cell numbers correlated significantly with TNF-α and IL-12 concentrations. HIV-infected women on antiretroviral therapy had similar levels of cervical lymphocyte infiltration and inflammation to women naïve to therapy. In conclusion, we suggest that inflammation at the cervix and HIV infection are likely to be key determinants in the absolute number of mucosal immune cells recovered by cervical cytobrushing.
Keywords: cervix, genital, HIV, inflammation, T cell
Introduction
Women are at a significantly higher risk of acquiring human immunodeficiency virus (HIV)-1 infection heterosexually than men.1 The female genital tract has become a site of great interest and focus in the fight against HIV/AIDS. Although mucosal surfaces in the female genital tract are not organized secondary lymphoid structures, genital tissue is considered to be a part of the common mucosal immune system, which includes the respiratory and intestinal tracts.2 Understanding the cervicovaginal environment and its role in innate and adaptive immunity in HIV infection is important in preventing HIV infection in women.
Although there is a clear need for reliable, validated non-invasive methods for investigating mucosal immune responses in the female genital tract, progress has been limited by technical challenges associated with obtaining genital tissue. Invasive biopsy approaches to isolating lymphocytes from rectal and gastrointestinal mucosal tissue dominate the literature3–7 and fewer approaches are available for sampling mucosal tissue from the female genital tract, such as cervical lavages and cervical cytobrushes.8–10 Cervical cytobrushing is a relatively non-invasive approach that has proved useful for obtaining viable lymphoid cells from the intraepithelial layer of the cervix that can be used in functional T-cell assays.8–10 Although cervical cytobrushing is well tolerated, there have been few reports on the composition of cervical cytobrush specimens11–14 and the impact of genital inflammation, HIV infection and antiretroviral (ARV) therapy on this.13,14
A number of lymphocyte subsets have been identified at the cervix of healthy women including T cells, macrophages and B cells.11,12 Sexually transmitted infections (STIs) and genital inflammation are known to modulate the recruitment of these subsets to the cervix.15,16 Pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α in the genital tract have been associated directly with enhanced HIV replication and indirectly with recruitment, differentiation and activation of the immune cells which act as targets of HIV infection.17–20
The aim of this study was to clearly define the mucosal immune cells derived by cervical cytobrushing and to validate cytobrushing as a useful non-invasive sampling modality for genital mucosal immunity studies. Investigating the impact of HIV infection, ARV therapy and genital tract inflammation on the cellular composition of cervical cytobrush specimens will give useful information for future studies based on this mucosal sampling method. We show that women infected with HIV tend to have significantly higher numbers of cervical cytobrush-derived T cells, neutrophils, B cells, Langerhans cells, and monocytes than uninfected women. We show here that HIV infection is also associated with higher cervicovaginal concentrations of IL-6, IL-1β and IL-8. Irrespective of HIV status, IL-1β, TNF-α and IL-12 concentrations in the genital tract are significantly correlated with T-cell numbers at the cervix. However, ARV therapy in HIV-infected women was not detectably associated with reduced levels of either cervical lymphocyte infiltration or inflammation. Together, these findings suggest that inflammation at the cervix and HIV infection are likely to be key determinants in the absolute number of mucosal cells recovered by cervical cytobrushing.
Materials and methods
Study participants and specimen collection
Eighty-nine consecutive chronically HIV-infected women attending the Nyanga Day Hospital, Cape Town, South Africa were recruited for this study between January and August 2007. Of these patients, 41 of 89 (46·1%) were using ARV therapy and 48 of 89 (53·9%) were therapy naïve. Forty-six HIV-negative women from the University of Cape Town Empilisweni Centre, Cape Town, South Africa also formed part of the study. Women who had CD4 counts ≤ 300 cells/μl, were menstruating at the time of sampling, were post-menopausal, or had undergone a hysterectomy were excluded from the study. Because STIs (associated with visible discharge, ulceration or macroscopic cervical changes) are likely to have an impact on the quality and composition of cytobrushes, we excluded women with any visible evidence of STIs. The study was approved by the Research Ethics Committee of the University of Cape Town, South Africa. Written informed consent was obtained from all volunteers before initiation of the study. Two cytobrushes were taken from each woman; the first Digene (Digene Corporation, Gaithersburg, MD) cervical sample was taken to obtain cervical cells from the endocervix for direct ex vivo analysis, and a second Digene cervical sample was taken for detection of human papilloma virus (HPV) infection using Digene HC2.
Collection and processing of cervical specimens
Cervical lymphocytes were collected using a Digene cervical sampler according to a previously described method.21 Briefly, under speculum examination, the cervix was swabbed using sterile gauze to remove most of the mucus. The Digene cervical sampler was inserted into the endocervical canal and cervical cells were collected using a single gentle 360° rotation of a cytobrush at the cervical os. The cytobrush was placed in 3 ml of transport medium [R10, RPMI-1640 medium supplemented with 5 mm glutamine, fungin, penicillin-streptomycin and 10% fetal calf serum (FCS)]. Processing was performed within 4 hr of collection to maximize recoverable yields and maintain viability. Cervical samples that had visible red blood cell contamination were discarded. Each cytobrush was flushed ∼30 times with R10 in the collection tube using a Pasteur pipette. The cell suspension was then transferred to a clean 15-ml tube and centrifuged at 2300 rpm (1000 g) for 10 min. The supernatant containing soluble factors from the cervical brush and associated cervical mucus was aliquoted equally into cryotubes and frozen at −80° for assessment of cervical inflammatory cytokine levels and HIV shedding. The pelleted cells were resuspended in 1·2 ml of R10 prior to processing for (i) counting and phenotyping using an automated Guava cell counter [200 μl of cells (16% of sample); Guava Technologies, Hayward, CA], (ii) performing absolute counts of CD4 and CD8 T cells [500 μl of cells (42% of sample); MultiTest on BD FACS Caliber; Becton Dickinson (BD) Biosciences, San Jose, CA] and (iii) memory marker analysis by flow cytometry [500 μl of cells (42% of sample); LSRII; BD Biosciences].
Isolated mucosal cells were quantified with phenotypic markers, including CD3-phycoerythrin (PE) (T cells; Guava Technologies), CD45-PE (common leucocyte antigen; Guava Technologies), CD19-PE (B cells; BD Biosciences), Langerin (CD207)-PE (Langerhans cells; Coulter Immunotech, Miami, FL), CD24-PE (neutrophils; BD Biosciences) and CD14-PE (monocytes; Guava Technologies) using Guava Automated Cell counting technology. Briefly, cells (25 μl/tube) were stained with pre-titrated monoclonal antibodies and incubated at 4° for 30 min. Cells were washed with 1 ml of wash buffer ([1% FCS in phosphate-buffered saline (PBS)]) and centrifuged at 1500 rpm (437 g) for 5 min. The supernatant was discarded and a volume of 200 μl of Cell Fix (BD Biosciences) was added to each tube. Samples were acquired and analysed using Cytosoft® software (Guava Technologies). At least 2000 events were captured per analysis. Automated cell counting was performed in duplicate for the first 15 samples to ensure reproducibility (data not shown). We have compared the accuracy of 2000 events per analysis with 10 000, 8000 and 4000 events captured for three mucosal specimens and have found concordance among results generated at all thresholds of events captured with a coefficient of variation of 9·2% (Fig. S1).
The MultiTEST CD3 fluorescein isothiocyanate (FITC)/CD8 PE/CD45 PerCP/CD4 allophycocyanin (APC) Reagent and TruCOUNT tubes (BD Biosciences) were used to determine the absolute counts and percentages of CD8+ CD3+ T cells and CD4+ CD3+ T cells, according to the manufacturer’s instructions. Samples were acquired and analysed on a FACS Caliber (BD Biosciences) using MultiSET software (BD Biosciences). For all absolute CD4 and CD8 counts performed on the FACS Caliber, 95% of the cervical cytobrush-derived cells were captured.
To measure the viability of cervical cytobrush-derived T cells, we used differential staining of Annexin V and propidium iodide (PI). Freshly isolated cervical cytobrush-derived cells were washed twice with 2 ml of cold PBS [1500 rpm (437 g) for 5 min] and stained with Annexin and PI (BD Biosciences Cell Viability Kit) according to the manufacturer’s instructions. The cells were acquired immediately on a FACS Caliber (BD Biosciences). Data were subsequently analysed using FlowJo® software (Treestar, Ashland, OR).
The maturational stage of CD8+ and CD8− T cells was assessed by staining peripheral blood mononuclear cells (PBMC) and cervical cells with the pre-titrated phenotypic markers Pacific blue-labelled anti-CD3 (BD Biosciences), PercPCy5·5-labelled anti-CD8 (BD Biosciences), APC-labelled anti-CCR7 (R&D Systems Inc., Minneapolis, MN), PE-labelled anti-CD27 (BD Biosciences) and Cy7.PE-labelled anti-CD45RA (BD Biosciences) for 1 hr at 4°. Cells were washed by adding 2 ml of 10% FCS PBS (containing 0·01% NaN3), centrifuged (5 min at 300 g) and fixed with BD Cell Fix. Cell fluorescence was measured by flow cytometry using a nine-colour LSRII (BD Biosciences), and FlowJo software (Tree Star) was used for colour compensation and data analysis.
Inflammatory cytokines at the cervix
Inflammatory cytokine (TNF-α, IL-10, IL-1β, IL-6, IL-8 and IL-12p70) concentrations in cervical supernatants were determined using a Human Inflammation Cytometric Bead Array (CBA) kit (BD Biosciences Pharmingen, San Diego, CA) according to the manufacturer’s instructions. The limit of detection of this assay ranged between 1·9 and 7·2 pg/ml (average 3·6 pg/ml). Cytokine concentrations have been expressed as pg/ml of cervical supernatant. Cytokine values below the assay’s limit of detection were reported as the mid-point between the lowest reading and zero.
Determination of viral load in cervical supernatant and plasma
Viral load was determined in cervical supernatants and plasma samples using Nuclisens Easyq HIV 1 Version 1.2 (BioMerieux, Lyon, France). The detection limit of this assay was 50 copies/ml. The cervical supernatant fraction was obtained following flushing of the cervical cytobrush 30 times with 3 ml of the transport medium and removal of cells by centrifugation (250 g for 10 min). Plasma was obtained from acid dextrose citrate (ACD) anti-coagulated whole blood following Ficoll density gradient centrifugation.
Detection of cervical HPV infection by Digene HC2
Infection at the cervix with ‘high-risk’ HPV types was evaluated using Digene HC2 as previously described.22 Digene HC2 detects 13 high-risk HPV types including HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59 and -68.
Statistical analysis
The Mann–Whitney U-test for unmatched samples was performed for independent sample comparisons; a paired t-test was performed for dependent sample comparisons, a χ2 test was performed to compare proportions and Spearman rank tests were applied for correlations, using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA). All tests were two-tailed and P-values of P ≤ 0·05 were considered significant.
Results
The composition of cervical cytobrush samples from 46 HIV-negative and 89 chronically HIV-infected women was investigated. Of the HIV-infected women, 48 of 89 (53·9%) were ARV naïve and 41 of 89 (46·1%) were on ARV therapy. Table 1 compares the clinical status of the HIV-infected women included in this study. Therapy-naïve HIV-infected women had significantly lower CD4 counts (P= 0·01) and significantly higher plasma viral loads (P ≤ 0·0001) and were shedding significantly more HIV at the genital mucosa than HIV-infected women on ARVs (P ≤ 0·0001; Table 1). All HIV-infected women on ARVs had undetectable plasma virus, although 20% of them where shedding virus (Table 1). More therapy-naïve HIV-infected women were also infected with high-risk HPVs than women on ARVs, although this was not significant (Table 1). Table 2 summarizes the ARV drug regimes of the women on therapy. The majority of HIV-infected women taking ARVs were on lamivudine (3TC)/stavudine (d4T)/efavirenz (EFV) triple therapy (26·8%; 11 of 41) or Combivir (3TC/zidovudine)/EFV triple therapy (22·0%; nine of 41).
Table 1.
Clinical details of human immunodeficiency virus (HIV)-infected women included in the study
| HIV-infected women |
|||
|---|---|---|---|
| Characteristic | Not on ARV | On ARV | P-value |
| n | 48 | 41 | |
| CD4 count [cells/μl; median (IQR)] | 395 (314–527) | 481 (415–605) | 0·011 |
| Plasma HIV load [copies/μl; median (IQR)] | 4950 (720–10 450) | 0 (0–0) | < 0·0001 |
| Genital HIV load [copies/μl; median (IQR)] | 93 (0–498) | 0 (0–22) | < 0·00011 |
| Number of women with detectable HIV in genital tract [n/total (%)] | 25/34 (73·5) | 8/40 (20·0) | < 0·00012 |
| HR HPV prevalence [n/total (%)] | 18/48 (37·5) | 8/42 (18·2) | 0·062 |
| Genital HPV load [RLU/control; median (IQR)] | 279 (4–1130) | 10 (2–162) | 0·071 |
P-value calculated using Mann–Whitney U-test.
P-value calculated using χ2 test.
ARV, antiretroviral; IQR, interquartile range; HPV, human papilloma virus; HR, high risk; RLU, relative light units.
Table 2.
Details of antiretroviral regimes followed by human immunodeficiency virus (HIV)-infected women on therapy
| Antiretroviral regime | n/total | % |
|---|---|---|
| Lamivudine/stavudine/efavirenz | 11/41 | 26·8 |
| Combivir (lamivudine/zidovudine)/efavirenz | 9/41 | 22·0 |
| Lamivudine/zidovudine/efavirenz | 4/41 | 9·8 |
| Combivir (lamivudine/zidovudine)/nevirapine | 4/41 | 9·8 |
| Lamivudine/nevirapine | 3/41 | 7·3 |
| Lamivudine/nevirapine/zidovudine | 2/41 | 4·9 |
| Kaletra (lopinavir/ritonavir)/efavirenz | 2/41 | 4·9 |
| Lamivudine/stavudine/nevirapine | 1/41 | 2·4 |
| Kaletra (lopinavir/ritonavir)/zidovudine | 1/41 | 2·4 |
| Kaletra (lopinavir/ritonavir)/nevirapine | 1/41 | 2·4 |
| Efavirenz/zidovudine | 1/41 | 2·4 |
| Efavirenz/stavudine | 1/41 | 2·4 |
| Nevirapine/zidovudine | 1/41 | 2·4 |
Impact of HIV infection on recruitment of cervical leucocytes
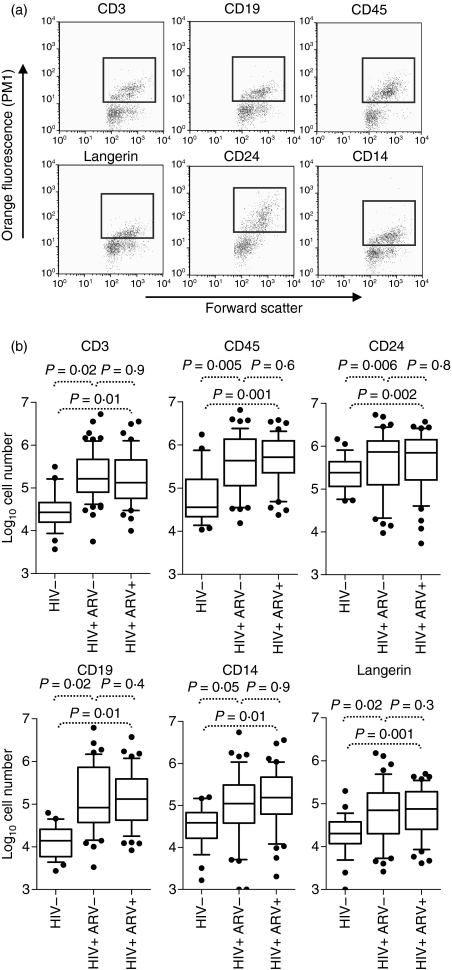
The absolute numbers of T cells (CD3+), leucocytes (CD45+), B cells (CD19+), neutrophils (CD24+), monocytes (CD14+) and Langerhans cells (Langerin+) recovered from the cervix of uninfected and HIV-infected women were compared (Fig. 1). Compared with uninfected women, those infected with HIV had significantly elevated numbers of all subsets (Fig. 1; 9·2-fold for CD3+ cells, P= 0·02; 5·4-fold for CD45+ cells, P= 0·005; 3·7-fold for CD24+ cells, P = 0·006; 26·2-fold for CD19+ cells, P = 0·02; 9·1-fold for CD14+ cells, P = 0·05; and 6·2-fold for Langerin+ cells, P = 0·02; Mann–Whitney U-test). We found no significant differences in the numbers of any immune subsets at the cervix of HIV-infected women on ARV compared with therapy-naïve women (Fig. 1). When we investigated the impact of coinfection with high-risk HPV on mucosal cell recruitment in women infected with HIV, we observed no significant difference in any of the cell subsets studied in women with and without HPV coinfection (Table S1).
Figure 1.
Impact of human immunodeficiency virus (HIV) infection on ex vivo cervical cytobrush yields. (a) Representative plots of ex vivo cervical CD3+, CD19+, CD45+, Langerin+, CD24+, and CD14+ populations quantified using a Guava automated cell counter. Red dots indicate cells that were considered positive for each phenotypic marker while green dots represent cells considered negative. (b) Summary of absolute immune cell counts isolated from ex vivo cervical cytobrushes determined using a Guava automated cell counter from 46 HIV-negative women (HIV−), 48 therapy-naive chronically HIV-infected women (HIV+ ARV−) and 41 HIV-infected women on ARV (HIV+ ARV+). Box and whisker plots indicate the median and the 25th and 75th percentiles. Dots represent outliers. The Mann–Whitney U-test was applied to compare cervical counts in HIV-negative versus HIV-positive women. P-values ≤0·05 were considered significant.
Neutrophils (CD24+) were the most dominant cytobrush cell type isolated from both HIV-infected and uninfected women, making up 46·5% and 73·2% of the cells evaluated, respectively. In HIV-infected women, CD3+ T cells, CD19+ B cells and CD14+ monocytic cells were present in almost identical proportions, accounting for 17·4%, 18·5% and 17·6% of the cells evaluated at the cervix, respectively. In uninfected women, CD3+ T cells and CD14+ antigen-presenting cells were present at a 1 : 1 ratio, making up 11·2% and 11·4% of evaluated cervical cells, while B cells (CD19+) were notably scarce in HIV-negative women, making up only 4·2% of the cells evaluated.
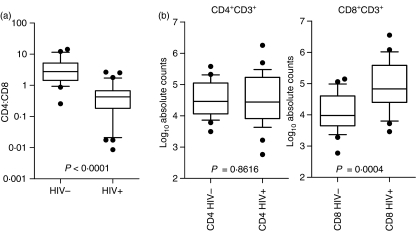
The median yield of CD3+ T cells derived from a single cervical cytobrush was significantly higher from HIV-infected women compared with HIV-negative women [163 920 (interquartile range (IQR) 79 409–465 140) compared with 27 017 (IQR 15 744–45 274), respectively; Fig. 1 and Table 3]. In uninfected women, the majority of CD3+ cells were CD4+ (CD4:CD8 ratio of 2·7 : 1; Fig. 2a and Table 3). In contrast, in HIV-infected women, the majority of CD3+ cells were CD8+ (CD4:CD8 ratio of 0·4 : 1). Previous studies have demonstrated selective depletion of CD4+ T cells from gut- and rectum-associated lymphoid tissues23,24 during HIV infection. Whereas we found CD4 absolute counts were similar in HIV-positive and HIV-negative women (Fig. 2b and Table 3), absolute numbers of CD8 T cells were significantly different between the two groups, with those of the HIV-positive women being sevenfold higher (Fig. 2b and Table 3). While the shift to CD8 dominance at the cervix is clear during HIV infection (Fig. 2 and Table 3), we were unable to distinguish whether this was attributable to a selective influx of CD8+ T cells, depletion of cervical CD4+ T cells or a combination of T-cell recruitment and CD4 depletion.
Table 3.
Comparison between absolute CD4 and CD8 T-cell counts and CD4:CD8 ratios in human immunodeficiency virus (HIV)-infected and uninfected women
| HIV− Median (IQR) | HIV+ Median (IQR) | P-value1 | |
|---|---|---|---|
| Absolute CD45+ cell counts | 36 041 (21 649–159 085) | 431 580 (112 932–1 372 000) | 0·005 |
| Absolute CD3+ cell counts | 27 017 (15 744–45 274) | 163 920 (79 409–465 140) | < 0·0001 |
| CD4:CD8 ratio | 2·7 (1·4–5·2) | 0·4 (0·2–0·7) | < 0·0001 |
| Absolute CD4+ CD3+ cell counts | 29 097 (11 549–112 458) | 27 587 (8211–169 627) | 0·9 |
| Absolute CD8+ CD3+ cell counts | 9579 (4462–40 338) | 67 732 (25 044–383 083) | 0·0004 |
P-value calculated using the Mann–Whitney U-test.
IQR, interquartile range.
Figure 2.
CD4:CD8 ratio and absolute CD4 and CD8 T-cell counts in cervical cytobrush samples from uninfected and human immunodeficiency virus (HIV)-1-infected women. (a) CD4:CD8 T-cell ratios were determined by flow cytometry from ex vivo derived cervical cytobrush samples stained with CD3, CD4 and CD8. (b) Absolute CD4 and CD8 T-cell counts were determined using ratios inferred by flow cytometry and absolute CD3 counts were determined using Guava automated cell counting of CD3+ cells. Box and whisker plots indicate the median and 25th and 75th percentiles. Dots represent outliers. The Mann–Whitney U-test was applied to compare cervical counts in HIV-negative versus HIV-positive women. P-values ≤0·05 were considered significant.
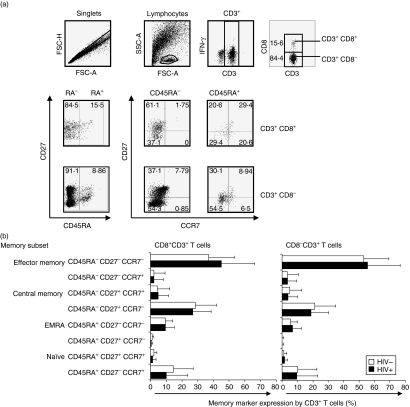
Based on differential expression of CD45RA, CD27 and CCR7 phenotypic markers, cervical CD3+ T cells from HIV-infected and uninfected women were defined as either naïve cells (CD45RA+ CD27+ CCR7+), EMRA (effector memory cells expressing RA; CD45RA+ CD27− CCR7−), effector memory cells (CD45RA− CD27− CCR7−), central memory cells (CD45RA− CD27+ CCR7+) or CD45RA− CD27+ CCR7− T cells (Fig. 3). In both HIV-infected and uninfected women, the majority of CD3+ cells were effector memory T cells (CD45RA− CD27− CCR7−; Fig. 3b) followed by a CD45RA− CD27+ CCR7−-expressing memory subset. Naïve T cells were almost absent from cervical cytobrush specimens while central memory (CD45RA− CD27+ CCR7+) accounted for < 10% of total cervical T cells. In uninfected women, significantly more CD8− T cells were effector memory CD45RA− CD27− CCR7− than CD8+ cells (53·0 ± 16·5% versus 37·0 ± 16·0%, respectively; P = 0·0003; unpaired t-test). Other than this, we observed similar distributions of memory subsets in HIV-infected and uninfected women (Fig. 3b).
Figure 3.
Memory subset composition of ex vivo cervical cytobrush-derived T cells. (a) Representative plots of staining combinations used to differentiate memory subsets of cervical T cells based on differential expression of CD3, CD8, CD45RA, CCR7 and CD27. (b) CD45RA+ CD27+ CCR7+ (naïve), CD45RA+ CD27− CCR7+, CD45RA+ CD27+ CCR7−, CD45RA+ CD27− CCR7− (effector memory cells expressing RA (EMRA)), CD45RA− CD27+ CCR7−, CD45RA− CD27+ CCR7+ (central memory), CD45RA− CD27− CCR7+ and CD45RA− CD27− CCR7− (effector memory) CD8+ (left panel) and CD8− (right panel) T-cell frequencies in cervical cytobrush samples derived from HIV-infected (black bars) and uninfected (clear bars) women. Each bar represents the mean percentage memory marker expression by CD8− and CD8+ T cells (± standard deviation). The unpaired Student’s t-test was applied to compare cervical counts in HIV-negative versus HIV-positive women. P-values ≤0·05 were considered significant.
Differential staining with Annexin V and PI was used to investigate the viability of CD3+ T cells isolated from cervical cytobrush specimens (data not shown). The average viability of cervical cytobrush-derived CD3+ cells was found to be 60·7% (± 23·2%; mean value ± standard deviation; Annexin− PI−). While dead cells (Annexin− PI+) made up only 2·2% (± 3·2%) of CD3+ cervical cells, the remaining cells were either early (Annexin+ PI−; 10·9 ± 8·1%) or late apoptotic (Annexin+ PI+; 26·2 ± 24·0%).
Cervical inflammatory cytokines and yield of cervical leucocytes
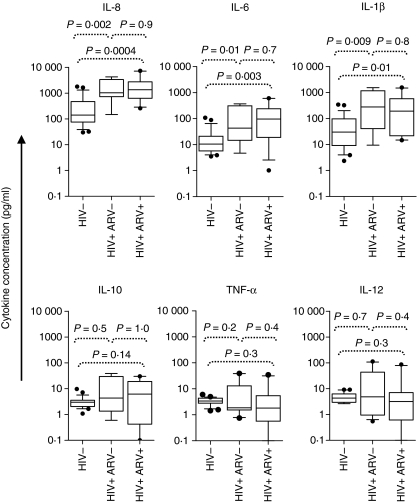
We next investigated the impact of chronic HIV-1 infection on inflammatory cytokine concentrations in the female genital tract. Cytokine levels were measured in cervical supernatants from women with chronic HIV-1 infection (ARV− and ARV+) and compared with those of HIV-uninfected women (Fig. 4). Levels of IL-8 (P= 0·002 for ARV− and P= 0·0004 for ARV+), IL-6 (P= 0·01 for ARV− and P= 0·003 for ARV+), and IL-1β (P= 0·009 for ARV− and P = 0·01 for ARV+) were significantly higher in both ARV− and ARV+ HIV-infected women, compared with uninfected women. HIV-infected women who were on ARV therapy had similar levels of inflammatory cytokines at the cervix to women who were therapy naïve. We found that HPV coinfection did not have a significant impact on cervical IL-8, IL-6, IL-1β, IL-12 or TNF-α concentrations in HIV-infected women (Table S2). However, there was a trend towards lower cervical IL-10 levels in HPV+ HIV+ women compared with HPV− HIV+ women (Table S2), indicating that coinfection with HPV may be associated with reduced IL-10 levels at the genital mucosa.
Figure 4.
Relationship between HIV infection and inflammatory cytokine concentrations in the female genital tract. Interleukin (IL)-8, IL-6, IL-1β, IL-10, tumour necrosis factor (TNF)-α and IL-12 p70 concentrations at the cervix of uninfected women (HIV−), therapy-naïve HIV-infected women (HIV+ ARV−) and HIV-infected women on antiretroviral (ARV) therapy (HIV+ ARV+) were measured. The Mann–Whitney U-test was applied to compare cervical cytokine concentrations in HIV-negative versus HIV-positive women. P-values ≤0·05 were considered significant.
Elevated levels of certain inflammatory cytokines were found to correlate with increased yields of the cervical cell subsets evaluated (Table 4), indicating either cytokine-mediated recruitment of immune cells or induction of cytokine expression by the cells themselves. Cervical CD3+ T-lymphocyte yields were found to correlate with IL-1β (P = 0·02), TNF-α (P= 0·02) and IL-12 levels (P= 0·01; Table 4). The yield of antigen-presenting cell subsets (CD14+ monocytes and Langerin+ Langerhans cells) correlated with cervical levels of TNF-α (P = 0·006 for CD14+ and P = 0·02 for Langerin+ cells) and IL-12 levels (P = 0·004 for CD14+ and P= 0·02 for Langerin+ cells). CD24+ neutrophil yields correlated with cervical concentrations of IL-1β (P= 0·002), TNF-α (0·03), IL-8 (P= 0·0003), IL-6 (P= 0·03) and IL-10 (P= 0·03). Similarly, absolute numbers of CD19+ B cells correlated with IL-1β (P= 0·004), TNF-α (P= 0·01), IL-12 (P= 0·004) and IL-10 (P= 0·02).
Table 4.
Impact of cervical inflammatory cytokine concentrations and absolute cell numbers in women with chronic human immunodeficiency virus (HIV) infection
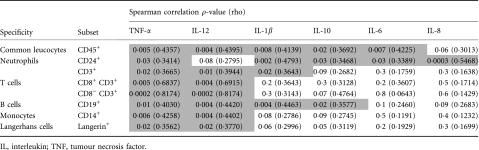 |
Discussion
In this study, we investigated the impact of HIV infection and mucosal inflammation on the composition and yield of cervical cytobrush-derived immune cells. We found that cytobrush samples from HIV-infected women yielded significantly more CD3+ T cells, CD45+ leucocytes, CD19+ B cells, CD14+ antigen-presenting cells, Langerin+ Langerhans cells and CD24+ neutrophils than those from HIV-uninfected women. We observed a significantly lower CD4:CD8 ratio at the cervix of HIV-infected women compared with HIV-negative women. The dominant memory phenotype of cervical CD8+ and CD4+ T cells in both uninfected and HIV-infected women was found to be that of effector memory (CD45RA− CCR7− CD27−). Levels of IL-1β, IL-6 and IL-8 were significantly elevated in cervical supernatants of HIV-infected compared with uninfected women and we observed a correlation between the level of inflammatory cytokines at the cervix and cervical lymphocyte yields. These results provide a link between HIV infection, local mucosal inflammation and immune cell recruitment to the cervix.
HIV infection has previously been associated with increased inflammation both in plasma and at the cervical mucosa.17,18,21,23,24 Mucosal inflammation has been linked both directly to increased local HIV replication and indirectly to mucosal recruitment, differentiation and activation of various immune cells that are targeted by HIV.17–19 Several studies have also shown a significant correlation between certain STIs and genital tract inflammation.14,17,25 Although some studies have suggested that HIV infection alone may result in increased local genital tract inflammation,26 others have shown that women infected with HIV-1 are significantly more susceptible to STIs and bacterial vaginosis (BV) and that this may account for the increased genital inflammation observed during HIV infection.27–29 Mitchell et al30 found that concomitant bacterial vaginosis and not HIV infection per se is responsible for elevated IL-1β levels detectable in the vaginal fluid of HIV-infected women. Lipopolysaccharides (LPSs) released from these opportunistic pathogens at the cervical mucosa may lead to the production of pro-inflammatory cytokines and, subsequently, cause the activation and proliferation of T cells.31 Inflammatory responses induced by STIs may be particularly relevant to HIV-1 transmission in sub-Saharan Africa where HIV-1 subtype C isolates are dominant. HIV subtype C isolates have been reported to have enhanced responsiveness to TNF-α-mediated activation of long terminal repeat (LTR) as a result of increased numbers of NF-κB binding sites.32 While we have not screened for STIs other than HPV in this study, we propose that the elevated numbers of certain cervical immune subsets and inflammatory cytokines that we have measured in HIV-infected women compared with uninfected women are likely to be the result of both immunosuppression caused by HIV infection and the associated increased prevalence of bacterial vaginosis or STIs. Because we excluded women with macroscopic evidence of genital infection from the study, we acknowledge that this may bias our analysis towards reduced genital inflammation and cellular recruitment at the cervix.
We found that HIV-infected women who were on ARV therapy had similar levels of inflammatory cytokines and immune cell numbers at the cervix compared with women who were therapy naïve. All of the HIV-infected women on therapy in this study had undetectable plasma viraemia and were not shedding HIV in the genital tract. Because effective ARV therapy is associated with immune reconstitution and a reduced incidence of opportunistic infections in blood, we hypothesized that women on therapy may have a lower incidence of STIs and therefore have less genital inflammation. There is currently no evidence for immune reconstitution in the genital tract. While some studies have found that the prevalence of certain STIs is reduced following initiation of ARV therapy,33 others have found that the introduction of ARV therapy is associated with increased risk behaviour34–36 and, as a result, increased STI prevalence rates.34,35,37 In addition, even in people taking ARV drugs, the concentrations of these chemicals in genital tissues vary considerably with anatomic site and efficacy (dependent on factors such as drug regime), with the result that individuals on ARV therapy show substantial variability in HIV loads in genital secretions.38–40 We did not test for STIs other than HPV but found no evidence of HIV shedding in the genital tract of women on ARVs as well as a trend towards reduced HPV infection and HPV viral load in women on ARVs compared with therapy-naïve participants. This suggests that the HIV-infected women in this study on ARVs were effectively HIV-suppressed both systemically and in the female genital tract. Increased risk behaviour and concomitant increased STI prevalence34–37 may therefore account for the similar levels of inflammation we observed in the two groups of HIV-infected women on and not on ARV therapy.
During inflammatory responses, neutrophils are the first subset to be recruited to sites of infection where they generate chemotactic responses involved in recruitment of other components of the immune response to the site of challenge.41,42 In both HIV-infected and uninfected women, we identified neutrophils as the predominant immune cell subset at the cervix. We observed a significant positive correlation between cervical cytobrush neutrophil numbers and concentrations of IL-1β, TNF-α, IL-8, IL-6 and IL-10. Similarly, we observed a significant correlation with cytobrush neutrophil yields and each of the other cell subsets assessed, indicating that their recruitment may be commonly regulated or interdependent. We cannot, however, differentiate the temporal relationships among inflammation, neutrophil infiltration and recruitment of other immune subsets in this study because of the cross-sectional nature of the analysis. However, our results are broadly in agreement with those of Belec et al.,17 who similarly showed that IL-β, IL-6 and TNF-α levels and the proportion of inflammatory cells were elevated in the genital tract during chronic activation.
The central role of inflammatory mediators during recruitment was also observed for cervical CD3+ T cells where genital levels of IL-1β, TNF-α and IL-12 correlated with T-cell recruitment. While IL-1β and TNF-α levels in the female genital tract have previously been associated with T-cell recruitment in HIV infection,43 we add IL-12 to the list of cytokines potentially mediating this function. We and others have shown that CD4+ T cells predominate at the cervix of healthy HIV-negative women,11,44 with CD4+ T cells generally outnumbering CD8+ T cells 4 : 1 at the genital mucosa. We show in this study, however, that the ratio of CD4:CD8 T cells at the mucosa shifts significantly in HIV-infected women, with CD8+ T cells becoming the dominant T-cell subset and outnumbering CD4+ T cells by approximately 1·8 to 1. Several studies have similarly documented either lower CD4+15 or higher CD8+ T-cell yields at the cervix during HIV infection.8,16,45
While the shift to CD8 dominance at the cervix is clear during HIV infection, we were unable to distinguish whether this was attributable to a selective influx of CD8+ T cells, depletion of cervical CD4+ T cells or a combination of T-cell recruitment and CD4 depletion. There is a precedent for the latter from studies of sustained and massive gut CD4 depletion during HIV infection.46,47 While systemic CD4+ T-cell reconstitution is clearly observed in HIV-infected individuals following initiation of ARV therapy,48,49 we observed no significant difference in the absolute counts of cytobrush-derived CD4+ T cells at the cervix in women who where naïve to therapy or who were taking ARV drugs. Data on the impact of ARV therapy on gut CD4 reconstitution are limited, but some studies have demonstrated that near-complete CD4+ T-cell restoration at the gut may be achieved if therapy is initiated during primary HIV infection.50 In comparison, it has been shown that, during chronic HIV infection, gut mucosal CD4+ T-cell restoration is slow and incomplete,5 possibly as a result of anatomically variable drug availability at mucosal surfaces.38,39
Cervical cytobrush-derived CD4+ and CD8+ T cells from both HIV-infected and uninfected women predominantly had an effector memory phenotype (CD45RA− CD27− CCR7−). Effector memory cells have the capacity to reside long term at inflamed peripheral tissues and epithelial surfaces, where they may allow for rapid control of invading pathogens at sites of entry, preventing systemic infections and unwarranted immune responses.51 We showed that central memory (CD45RA− CD27+ CCR7+) T cells were present but at lower numbers at the cervix of both HIV-infected and uninfected women. It has been suggested that, whereas these cells probably home to T-cell areas of secondary lymphoid organs and have little or no effector function, they readily proliferate and differentiate to effector cells in response to antigenic stimulation.52 While Effector memory T cells become exhausted quickly,51 central memory T cells have the capacity to differentiate into very potent and possibly longer living effectors.52
In summary, we have shown that HIV infections are associated with increased female genital tract inflammation and lymphocyte recruitment. Specifically, we found that women with the highest yields of cervical lymphocytes were most likely to have elevated inflammatory cytokine concentrations at the cervix. As elevated inflammatory genital cytokines during HIV infection have also been associated with concomitant STI or BV,30,43 this may be an important confounder when assessing mucosal cellular responses from the cervical cytobrush-derived samples of HIV-infected women.
Acknowledgments
We thank the women from the Nyanga Day Hospital and the Uluntu Centre who kindly participated in the study, Janine Jones for collecting the specimens, Dr Darren Martin for reviewing the manuscript and for his constructive comments, and Dr Heather Jaspan for her constructive discussions. This study was supported in part by grants from the Doris Duke Charitable Foundation HIV Pathogenesis Program, the Wellcome Trust, the South African AIDS Vaccine Initiative (SAAVI MRC South Africa) and the Center for HIV/AIDS Vaccine Immunology (CHAVI) (NIH/NIAID grant AI64518). NN, WB and JP received training in the USA as part of the Columbia University-Southern African Fogarty AITRP Program. NN is funded by the National Research Foundation (South Africa) African Scholarships Program. WH is funded by the NIH (RO1-AI065653), EDCTP, Aeras, Dana and Gates Foundations. JP is funded by the Wellcome Trust on an Intermediate Fellowship in Infectious Diseases.
Disclosures
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Comparison of the number of events captured using the Guava automated cell counter and the calculated absolute number of CD3+ cells recovered from mucosal specimens.
Table S1. Impact of HPV co-infection on absolute cervical cytobrush cell numbers in women with HIV infection.
Table S2. Impact of HPV co-infection on cervical inflammatory cytokines in HIV infected women.
Please note: Wiley-Blackwell is not responsible for the content of functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.UNAIDS . AIDS epidemic update. 2007. URL http://data.unaids.org/pub/FactSheet/2008/(epi07_fs_regionalsummary_subsafrica_en.pdf) [accessed on 08 April 2009] [Google Scholar]
- 2.Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–277. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musey L, Ding Y, Cao J, Lee J, Galloway C, Yuen A, Jerome KR, McElrath MJ. Ontogeny and specificities of mucosal and blood human immunodeficiency virus type 1-specific CD8(+) cytotoxic T lymphocytes. J Virol. 2003;77:291–300. doi: 10.1128/JVI.77.1.291-300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton PA, Elliott J, Poles MA, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000;14:1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 5.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shacklett BL, Yang O, Hausner MA, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods. 2003;279:17–31. doi: 10.1016/s0022-1759(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 7.Critchfield JW, Lemongello D, Walker DH, Garcia JC, Asmuth DM, Pollard RB, Shacklett BL. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J Virol. 2007;81:5460–5471. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musey L, Hu Y, Eckert L, Christensen M, Karchmer T, McElrath MJ. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med. 1997;185:293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shacklett BL, Cu-Uvin S, Beadle TJ, et al. Quantification of HIV-1-specific T-cell responses at the mucosal cervicovaginal surface. AIDS. 2000;14:1911–1915. doi: 10.1097/00002030-200009080-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kaul R, Plummer FA, Kimani J, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1- resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 11.Prakash M, Patterson S, Kapembwa MS. Evaluation of the cervical cytobrush sampling technique for the preparation of CD45+ mononuclear cells from the human cervix. J Immunol Methods. 2001;258:37–46. doi: 10.1016/s0022-1759(01)00464-1. [DOI] [PubMed] [Google Scholar]
- 12.Quayle AJ, Kourtis AP, Cu-Uvin S, et al. T-lymphocyte profile and total and virus-specific immunoglobulin concentrations in the cervix of HIV-1-infected women. J Acquir Immune Defic Syndr. 2007;44:292–298. doi: 10.1097/QAI.0b013e31802c5b3a. [DOI] [PubMed] [Google Scholar]
- 13.Nicol AF, Frenandes ATG, Grinsztejn B, et al. Distribution of immune cell subsets and cytokine-producing cells in the uterine cervix of HPV-infected women: Influence of HIV-1 coinfection. Diagn Mol Pathol. 2005;14:39–47. doi: 10.1097/01.pas.0000143309.81183.6c. [DOI] [PubMed] [Google Scholar]
- 14.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between HSV-2 and HIV in the female genital tract. AIDS. 2007;21:589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 15.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 16.Olaitan A, Johnson MA, MacLean A, Poulter LW. The distribution of immunocompetent cells in the genital tract of HIV-positive women. AIDS. 1996;10:759–764. doi: 10.1097/00002030-199606001-00010. [DOI] [PubMed] [Google Scholar]
- 17.Belec L, Gherardi R, Payan C, Prazuck T, Malkin JE, Tevi-Benissan C, Pillot J. Proinflammatory cytokine expression in cervicovaginal secretions of normal and HIV-infected women. Cytokine. 1995;7:568–574. doi: 10.1006/cyto.1995.0077. [DOI] [PubMed] [Google Scholar]
- 18.Crowley-Nowick PA, Ellenberg JH, Vermund SH, Douglas SD, Holland CA, Moscicki AB. Cytokine profile in genital tract secretions from female adolescents: impact of human immunodeficiency virus, human papillomavirus, and other sexually transmitted pathogens. J Infect Dis. 2000;181:939–945. doi: 10.1086/315311. [DOI] [PubMed] [Google Scholar]
- 19.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahrbach KM, Barry SM, Ayehunie S, Lamore S, Klausner M, Hope TJ. Activated CD34-derived Langerhans cells mediate traninfection with Human Immunodeficiency Virus. J Virol. 2007;81:6858–6868. doi: 10.1128/JVI.02472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gumbi PP, Nkwanyana NN, Bere A, et al. Impact of mucosal inflammation on cervical HIV-1-specific CD8 T cell responses in the female genital tract during chronic HIV infection. J Virol. 2008;82:8529–8536. doi: 10.1128/JVI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passmore JS, Milner M, Denny L, et al. Mucosal T cell responses to HPV type 16 in women with HPV-associated CIN. Immunology. 2006;119:507–14. doi: 10.1111/j.1365-2567.2006.02465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thea DM, Porat R, Nagimbi K, Baangi M, St Louis ME, Kaplan G, Dinarello CA, Keusch GT. Plasma cytokines, cytokine antagonists, and disease progression in African women infected with HIV-1. Ann Intern Med. 1996;124:757–762. doi: 10.7326/0003-4819-124-8-199604150-00009. [DOI] [PubMed] [Google Scholar]
- 24.Zara F, Nappi RE, Brerra R, Migliavacca R, Maserati R, Spinillo A. Markers of local immunity in cervico-vaginal secretions of HIV infected women: implications for HIV shedding. Sex Transm Infect. 2004;80:108–112. doi: 10.1136/sti.2003.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen CR, Plummer FA, Mugo N, et al. Increased interleukin-10 in the the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS. 1999;13:327–332. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 26.Bebell LM, Passmore JS, Williamson C, Mlisana C, Iriogbe I, Loggerenberg F, Abdool Karim Q, Abdool Karim S. Impact of elevated inflammatory cytokines in the female genital tract on CD4 destruction during acute HIV-1 infection. J Infect Dis. 2008;198:710–14. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 27.Cu-Uvin S, Hogan JW, Warren D, et al. Prevalence of lower genital tract infections among human immunodeficiency virus (HIV)-seropositive and high-risk HIV-seronegative women. HIV Epidemiology Research Study Group. Clin Infect Dis. 1999;29:1145–1150. doi: 10.1086/313434. [DOI] [PubMed] [Google Scholar]
- 28.Moodley P, Connolly C, Sturm AW. Interrelationships among HIV-1 infection, bacterial vaginosis, trichomoniasis and the presence of yeasts. J Infect Dis. 2002;185:69–73. doi: 10.1086/338027. [DOI] [PubMed] [Google Scholar]
- 29.Sobel JD. Gynecologic infections in HIV-infected women. Clin Infect Dis. 2000;31:1225–1233. doi: 10.1086/317436. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell CM, Balkus J, Agnew KJ, Cohn S, Luque A, Lawler R, Coombs RW, Hitti JE. Bacterial vaginosis, not HIV, is primarily responsible for increased vaginal concentrations of proinflammatory cytokines. AIDS Res Hum Retroviruses. 2008;24:667–671. doi: 10.1089/aid.2007.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tough DF, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J Exp Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montano MA, Nixon CP, Ndung’u T, Bussmann H, Novitsky VA, Dickman D, Essex M. Elevated tumor necrosis factor-alpha activation of human immunodeficiency virus type 1 subtype C in Southern Africa is associated with an NF-kappaB enhancer gain-of-function. J Infect Dis. 2000;181:76–81. doi: 10.1086/315185. [DOI] [PubMed] [Google Scholar]
- 33.Bunnell R, Ekwaru JP, Solberg P, et al. Changes in sexual behaviour and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20:85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- 34.Wilson TE. Sexual and reproductive behaviour of women with HIV infection. Clin Obstet Gynaecol. 2001;44:289–299. doi: 10.1097/00003081-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Boily MC, Bastos FI, Desai K, Masse B. Changes in the transmission dynamics of the HIV epidemic after the wide-scale use of antiretroviral therapy could explain increases in sexually transmitted infection. Sex Transm Dis. 2004;31:100–113. doi: 10.1097/01.OLQ.0000112721.21285.A2. [DOI] [PubMed] [Google Scholar]
- 36.McClelland RS, Baeton JM, Richardson BA, Layreys L, Emery S, Mandaliya K, Ndinya-Achola JO, Overbaugh J. Comparison of genital HIV-1 shedding and sexual risk behaviour among Kenyan women based on eligibility for initiation of HAART according to WHO guidelines. J Acquir Immune Defic Syndr. 2006;41:611–615. doi: 10.1097/01.qai.0000191284.62707.b7. [DOI] [PubMed] [Google Scholar]
- 37.Scheer S, Chu PL, Klausner JD, Katz MH, Schwarcz SK. Effect of highly active antiretroviral therapy on diagnoses of sexually transmitted diseases in people with AIDS. Lancet. 2001;357:432–435. doi: 10.1016/S0140-6736(00)04007-1. [DOI] [PubMed] [Google Scholar]
- 38.Taylor S, Pereira AS. Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex Transm Infect. 2001;77:4–11. doi: 10.1136/sti.77.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lalani T, Hicks C. Does antiretroviral therapy prevent HIV transmission to sexual partners? Curr HIV/AIDS Rep. 2007;4:80–85. doi: 10.1007/s11904-007-0012-y. [DOI] [PubMed] [Google Scholar]
- 40.Mayer KH, Anderson DJ. Heterosexual HIV transmission. Infect Agents Dis. 1995;4:273–284. [PubMed] [Google Scholar]
- 41.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 42.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 43.Decrion AZ, Dichamp I, Varin A, Herbein G. HIV and inflammation. Curr HIV Res. 2005;3:243–259. doi: 10.2174/1570162054368057. [DOI] [PubMed] [Google Scholar]
- 44.Poppe WA, Drijkoningen M, Ide PS, Lauweryns JM, Van Assche FA. Lymphocytes and dendritic cells in the normal uterine cervix. An immunohistochemical study. Eur J Obstet Gynecol Reprod Biol. 1998;81:277–282. doi: 10.1016/s0301-2115(98)00202-4. [DOI] [PubMed] [Google Scholar]
- 45.Iqbal SM, Ball TB, Kimani J, Kiama P, Thottingal P, Embree JE, Fowke KR, Plummer FA. Elevated T cell counts and RANTES expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. J Infect Dis. 2005;192:728–738. doi: 10.1086/432482. [DOI] [PubMed] [Google Scholar]
- 46.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 47.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 49.Altfeld M, Rosenberg ES, Shankarappa R, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheroutre H, Madakamutil L. Mucosal effector memory T cells: the other side of the coin. Cell Mol Life Sci. 2005;62:2853–2866. doi: 10.1007/s00018-005-5232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.