Abstract
Numerous functional defects have been identified in naive T cells from aged mice, including deficiencies in proliferation, cytokine production and signal transduction. It is well documented that the ratio of naïve to memory T cells significantly decreases with age resulting in the majority of T cells from aged hosts expressing activated/memory T-cell markers (CD44hi), yet it is unclear whether T cells with a CD44hi phenotype in aged hosts are functionally equivalent to T cells with a similar phenotype in young hosts. We have identified a population of CD44hi CD8 T cells in old mice that are capable of secreting interferon-γ (IFN-γ) in response to interleukin-12 (IL-12) stimulation. This occurred in the absence of T-cell receptor engagement, a function that was not observed in CD8 T cells from young mice. This phenotype was associated with increased IL-12 receptor β2 gene expression and IL-12 induced signal transducer and activator of transcription 4 (STAT-4) activation, even when CD8 T-cell numbers from young and old mice were normalized for CD44hi expression. Furthermore, we demonstrate that IL-12-induced STAT-4 activation was required for T helper type 1 (Th1) cytokine-induced IFN-γ production in CD8 T cells. These data illustrate that old mice possess a specialized subset of CD44hi CD8 T cells with an enhanced responsiveness to IL-12, enabling these cells to produce substantial amounts of IFN-γ in response to Th1 cytokine stimulation. We have therefore identified a functional difference in the populations of CD44hi CD8 T cells from young and old mice, and believe that understanding age-associated immunological changes is essential for helping the elderly combat deadly diseases.
Keywords: aging, cytokines, signalling, T cell
Introduction
Aging is associated with poor immune function leading to increased susceptibility to infectious diseases and the development of immune-associated disorders.1–6 Specific deficiencies in T-cell function have been identified whereby T cells from old mice produce lower levels of interleukin-2 (IL-2) in response to antigenic stimulus, and have defective T-cell receptor (TCR) -mediated antigen-specific responses compared with young T cells.7–9 Many of the studies on T-cell function in aged hosts have focused on the capacity of naïve cells to respond to challenge, and yet the ratio of naïve to memory T cells significantly decreases with age, resulting in the majority of T cells from aged hosts expressing markers associated with a previous antigenic encounter.10 CD44 expression is commonly used to identify activated/memory T cells because the surface expression of CD44 on CD411 and CD812 T cells has been shown to increase upon TCR ligation in young mice. In old mice, however, the majority of CD4 and CD8 T cells have increased expression of CD44,10 and it is unclear whether this is an indication of antigen-activated cells or a marker of non-thymically derived T cells.13 Regardless, CD44 expression cannot be used as a marker of antigen-specific T-cell activation in old age.14 Very little is known about the functional capacity of CD44hi T cells in aged hosts to respond to challenge with a new infectious agent or antigen. Specifically, it is unclear whether cells possessing a CD44hi phenotype in aged hosts are functionally identical to their phenotypically similar counterparts in younger individuals. Understanding the properties of T cells that express a CD44hi phenotype in aged hosts will provide insight into the ability of elderly individuals to mount immune responses to primary infection, as well as into the efficacy of immunization within this population.
Respiratory infections pose a substantial threat to the elderly population, with the majority of deaths from influenza15 and the recent severe acute respiratory disease epidemic16 occurring in individuals over the age of 65 years. CD8 T cells play a critical role in the protection against viruses, and the primary response of CD8 T cells during viral infection is diminished in old age.17,18 Despite these deficiencies, memory CD8 T-cell responses in old mice are sufficient for viral clearance upon secondary viral infection,18 providing evidence that memory T-cell function remains intact in old age. In old mice, CD8 T cells with a CD44hi phenotype have been shown to secrete interferon-γ (IFN-γ) when stimulated with plate-bound anti-CD3 antibody,19,20 suggesting that their antigen-specific recall capacity remains intact. Perhaps more revealing, however, is our finding that a subset of CD44hi CD8 T cells from the lungs of aged mice could produce IFN-γ in the absence of antigen or TCR cross-linking, a response that was driven by IL-12.21 The TCR-independent IL-12-driven IFN-γ production was increased with the addition of IL-18; however, in contrast to a small population of cytokine responsive CD44hi CD8 T cells in young mice, IL-18 was not required.22 In young mice, and our studies (data not shown), IFN-γ production induced by T helper type 1 (Th1) cytokines (IL-12 and IL-18) was minimal in the CD44hi CD4 T cell subset,22 demonstrating that this specific innate immune function is limited to CD44hi CD8 T cells. Moreover, this population of IFN-γ-secreting CD8 T cells was shown to correlate with the early, non-specific control of infection with Mycobacterium tuberculosis in old mice,23 demonstrating that Th1 cytokine-respondent CD8 T cells in old mice play a role in the innate immune response to an infection with a virulent pathogen. Clearly, CD44hi CD8 T cells in old mice possess different qualities to those in phenotypically similar cells from young mice, and these differences may define the signalling pathways that are relevant to immune-mediated interventions in the elderly.
To further define the molecular requirements for IL-12-driven IFN-γ production in CD8 T cells from old mice, we examined IL-12 receptor (IL-12R) gene expression, IL-12-induced signal transducer and activator of transcription 4 (STAT-4) activation, and Th1 cytokine-induced IFN-γ production in pulmonary and splenic CD8 T cells from young and old mice. We found that IL-12Rβ2 gene expression, IL-12-induced STAT-4 phosphorylation, and IFN-γ production were all enhanced in CD8 T cells from old mice, and occurred predominantly in the CD44hi activated/memory CD8 T-cell subset. Most revealing however, was the discovery that when CD8 T cells from young and old mice were normalized for CD44hi expression, STAT-4 activation remained enhanced in the CD8 T cells from old mice. These data provide evidence that the ability to respond to IL-12 stimulation is not a characteristic of all CD44hi CD8 T cells but is a quality of CD44hi CD8 T cells from old mice, clearly demonstrating that phenotypically similar CD44hi CD8 T cells in young and old mice are not comparable. These data indicate that understanding the immunological changes that occur with age, and accepting them as changes and not deficiencies, is critical for designing better vaccines and immunotherapies for our elderly population.
Materials and methods
Mice
Specific-pathogen-free, female, C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) at 2 months of age (young), or at 18 months of age (old) through a contract with the National Institute On Aging. Mice were housed in a standard vivarium in microisolator cages and were acclimated to the facility for at least 1 week before manipulation. Mice were examined at necropsy and mice with gross lesions were excluded from the study. All procedures were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
Cell isolation
Lungs of young or old mice were perfused with phosphate-buffered saline containing 50 U/ml of heparin through the right ventricle and placed in supplemented Dulbecco’s modified Eagle’s minimal essential medium (DMEM) (500 ml; Mediatech, Herndon, VA) containing 10% heat-inactivated fetal bovine serum (Atlas Biologicals, Fort Collins, CO), 1% HEPES buffer (1 m; Sigma, St Louis, MO), 1%l-glutamine (200 nm; Sigma), 10 ml of a 100 × MEM non-essential amino acid solution (Sigma), and 0·1%β-mercaptoethanol 2-hydroxyethyl-mercaptan (50 mm; Sigma). The lungs were minced into 2-mm2 pieces and digested for 30 min at 37° in 5% CO2 in 4 ml DMEM containing collagenase XI (0·7 mg/ml; Sigma) and type IV bovine pancreatic DNAse (30 μg/ml; Sigma). Ten millilitres of supplemented DMEM was added to stop the enzymatic digestion and the suspension was passed through a 70-μm cell strainer to achieve a single-cell suspension. Residual red blood cells were lysed using Gey’s balanced salt solution (8 mm NH4Cl, 5 mm KHCO3) and resuspended in DMEM. To achieve a single-cell suspension of splenocytes, the spleens of young or old mice were harvested and passed through a 70-μm cell strainer. The red blood cells were subsequently lysed with Gey’s balanced salt solution and the splenocytes were resuspended in DMEM. No significant differences between the total number of cells, or the number of CD8 T cells, in the lung were found between old and young mice.
CD8 purification
Lung cells from two to five young and old mice were placed in a tissue culture grade Petri dish for 1 hr at 37° in 5% CO2. Non-adherent cells were collected and pooled, and CD8+ cells were isolated using CD8a MicroBeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. To obtain maximum purity (over 90% for all samples) using the Miltenyi magnetic cell sorting (MACS) system, cells were passed over two LS columns. CD8+ cells were purified from non-adherent splenocytes from four or five young and old mice using BD™ IMag anti-mouse CD8a particles-DM (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. Supplemented DMEM was used instead of the BD IMag buffer throughout the BD IMag procedure.
In vitrocell culture
Either 5 × 105 cells from individual whole lung cultures or 1 × 105 to 2 × 105 cells from pooled cultures of purified CD8+ cells were plated in 96-well tissue culture plates and, unless otherwise stated, were cultured for 4 hr at 37° in 5% CO2 with supplemented DMEM with or without 10 ng/ml recombinant IL-12 (PeproTech, Rocky Hill, NJ) alone, or in combination with 100 ng/ml IL-2 (R&D Systems, Minneapolis, MN) and 10 ng/ml IL-18 (R&D Systems). Inhibition of the IL-12 signalling pathway was achieved by culturing 5 × 105 purified CD8+ splenocytes with 10 μm Pyridone 6 (P6) (Calbiochem, Gibbstown, NJ) or dimethylsulphoxide in supplemented DMEM for 30 min at 37° in 5% CO2. Then, 100 ng/ml IL-2 (R&D Systems), 10 ng/ml IL-12 (PeproTech) and 10 ng/ml IL-18 (R&D Systems) were added and the cells were cultured for 4 hr at 37° in 5% CO2..
Flow cytometry
All antibodies were obtained from BD Biosciences (San Jose, CA) unless otherwise stated. Intracellular labelling of phosphorylated STAT-4 was carried out in accordance with the BD™ Phosflow Protocol III for mouse splenocytes or thymocytes. Briefly, cells were exposed to 1 × lyse/Fix buffer (BD Biosciences) for 10 min at 37° in 5% CO2, washed, and permeablized with BD Biosciences’ Perm III buffer for 30 min at 4°. Cells were washed and stained concomitantly with 0·3–0·6 μg of anti-CD3-phycoerythrin (PE) or anti-CD3-peridinin chlorophyll protein (PerCP)-Cy5.5, anti-CD8-alexa fluor 647, anti-CD44-PE, anti-IFN-γ-PE-Cy7 or rat immunoglobulin G2aκ (IgG2aκ)-PE-Cy7, and 20 μl anti-phosphorylated STAT-4-alexa fluor 488 or 0·3–0·6 μg mouse IgG2b alexa fluor 488 for 30 min at room temperature. Samples were read on a Becton-Dickinson LSRII and analysed using FACSDiva software (BD Biosciences). Lymphocytes were identified by their characteristic forward and side scatter profiles. Unless otherwise stated, gates were set using unstimulated cells. Non-specific staining determined by isotype control antibodies was consistently less than that found in unstimulated cultures. Specific gating strategies are defined in the figure legends.
Cell sorting
Single-cell suspensions of splenocytes from two young and two old mice were pooled according to age, and stained concurrently with 0·3 μg/1 × 106 cells of anti-CD3-PerCP-Cy5·5, anti-CD8-allophycocyanin and anti-CD44-PE for 20 min at 4°. Cells were washed with 10 ml supplemented DMEM and sorted for CD3+ CD8+ CD44hi and CD3+ CD8+ CD44lo cell populations on a BD FACSAria and analysed using FACSDiva software. Following sorting, 2 × 105 cells were plated and stimulated with IL-12 for 4 hr at 37° 5% CO2 and subsequently fixed and stained following the intracellular staining protocol described above.
IFN-γ ELISpot
CD8+ cells were purified from young and old mice using the Miltenyi MACS system as described previously and 5 × 104 CD8+ cells were plated on a multiscreen-IP filter plate (Millipore, Billerica, MA) that had been pre-coated with IFN-γ capture antibody (ebioscience, San Diego, CA). Cells were cultured in the presence or absence of IL-12 (10 ng/ml) and incubated at 37° in 5% CO2 for 48 hr. The number of IFN-γ-producing cells was determined using the Ready-Set-Go! mouse IFN-γ enzyme linked immunosorbent spot (ELISpot) kit from ebioscience. Spot number was determined by counting the number of spots per well using a dissecting microscope and data were expressed as the number of spots per 50 000 cells. For negative control wells, the spot number did not exceed seven spots per well.
Real-time polymerase chain reaction
Purified CD8+ cells from the spleens of young and old mice were homogenized in 1 ml TRIzol® Reagent (Invitrogen) and frozen at −80°. RNA was isolated using the Qiagen (Valencia, CA) RNeasy kit according to the manufacturer’s instructions, and reverse transcribed using an Omniscript RT kit from Qiagen. Real-time PCR was performed using TaqMan® gene expression assays for 18S and IL-12Rβ2 (Applied Biosystems, Foster City, CA) and analysed with a BioRad IQ5 (BioRad, Hercules, CA). The delta delta cycle threshold method was used for relative quantification of messenger RNA, using 18S as an endogenous normalizer and young mice as a calibrator.
Statistical analysis
Statistical significance was determined using prism 4 software (GraphPad Software, San Diego, CA). The unpaired two-tailed Student t-test was used for comparisons between young and old mice.
Results
IL-12 signalling is enhanced in pulmonary CD8 T cells from old mice
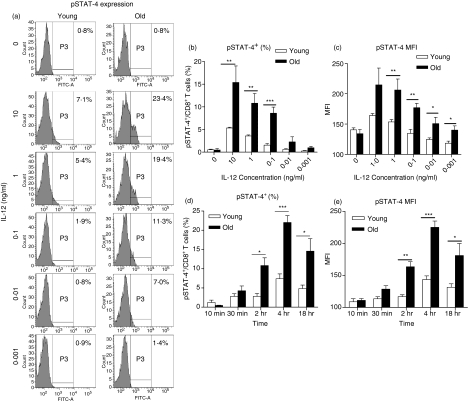
Old mice have significantly more CD8 T cells that can produce IFN-γ in response to Th1 (IL-2, IL-12 and IL-18) cytokine stimulation compared with young, and possess CD8 T cells that produce IFN-γ in response to IL-12 alone.21 We therefore investigated the capacity of CD8 T cells from old mice to respond to IL-12 by determining phosphorylation of STAT-4, a critical component in the IL-12 signalling pathway.24 Lung cells from young and old mice were cultured in the presence or absence of IL-12 and STAT-4 phosphorylation was determined by flow cytometry. Phosphorylated STAT-4 (pSTAT-4) was detected in CD8 T cells isolated from the lungs of young and old mice (Fig. 1). Figure 1(a) shows representative histograms illustrating pSTAT-4 expression in response to varying concentrations of IL-12. Phosphorylation of STAT-4 was dose-dependent in both age groups (Fig. 1a–c), confirming that signalling was specific for IL-12. Furthermore, there was a significantly increased proportion (Fig. 1b) and absolute number (data not shown) of CD8 T cells from old mice that contained phosphorylated STAT-4 compared with young mice, indicating that old mice have more CD8 T cells with the potential to secrete IFN-γ in response to IL-12. Perhaps more significant, determination of the mean fluorescence intensity (MFI) of pSTAT-4 in pulmonary CD8 T cells showed that at each concentration of IL-12 tested, CD8 T cells from old mice contained significantly more pSTAT-4 per cell compared with young CD8 T cells (Fig. 1c). Therefore, at the single-cell level, CD8 T cells from old mice were more responsive to IL-12 than similar cells from young mice. The increase in pSTAT-4 observed in CD8 T cells from old mice was not a result of increased basal levels of pSTAT-4, as pSTAT-4 levels remained equivalent between young and old CD8 T cells in the absence of IL-12 (Fig. 1a–c). Moreover, Fig. 1(d,e) illustrates that age did not affect the kinetics of IL-12 signalling; although expression was always greater in cells from old mice, the timing of STAT-4 phosphorylation was similar in CD8 T cells from young and old mice. We therefore demonstrate for the first time that old mice possess more CD8 T cells within the lung that can phosphorylate STAT-4 in response to IL-12 and, on an individual cell basis, CD8 T cells from the lungs of old mice have enhanced IL-12 signalling compared with their younger counterparts.
Figure 1.
CD8 T cells from old mice have increased phosphorylated signal transducer and activator of transcription 4 (pSTAT-4) levels upon stimulation with interleukin-12 (IL-12). Single-cell suspensions were isolated from the lungs of young (open bars) and old (closed bars) mice and cultured with tissue culture media (TCM) or IL-12 for 4 hr (a–c) or multiple time-points (d,e). Cells were fixed in 1 × lyse/fix buffer, permeabilized with Perm III buffer, and flow cytometry was performed using antibodies against CD3, CD8 and pSTAT-4. Lymphocytes were gated according to their characteristic forward and side scatter and CD8 T cells were identified by CD8+/CD3+ double staining. (a) Representative histograms illustrating pSTAT-4 expression. (b) Percentage of CD8 T cells expressing pSTAT-4 at 4 hr. (c) Mean fluorescence intensity (MFI) of pSTAT-4 in CD8 T cells at 4 hr. An isotype control antibody for pSTAT-4 was used to set the positive gates. Data represent mean ± SE of four or five mice and are representative of two independent experiments. (d) Percentage of CD8 T cells expressing pSTAT-4 at multiple time-points. (e) MFI of pSTAT-4 in CD8 T cells at multiple time-points. Unstimulated cultures were used to set the positive gates. Data represent the mean ± SE of four or five mice and are representative of two independent experiments. Significance was determined by unpaired Student’s t-test *P < 0·05, **P < 0·01, ***P < 0·001.
IL-12 stimulation of CD8 T cells does not act via an intermediate cell or cytokine
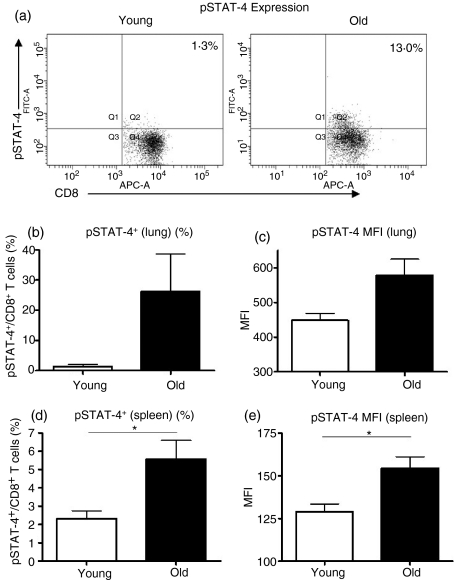
To determine whether IL-12 acted directly on CD8 T cells and not via an intermediate cell or cytokine present in whole lung cultures, and to determine whether increased IL-12 responsiveness observed in pulmonary CD8 T cells from old mice was organ-specific, we purified CD8+ cells from the lungs and spleens of young and old mice, and cultured purified CD8+ cells with IL-12. Very few CD8+ cells can be purified from the lung so we analysed pSTAT-4 expression at a single time-point of 2 hr post-stimulation, when high levels of pSTAT-4 in CD8 T cells could be detected in our previous assays.
Phosphorylation of STAT-4 was detected in purified CD8+ cells from the lungs (Fig. 2a–c) and spleens (Fig. 2d,e) of both young and old mice. These data illustrate that IL-12 can act directly on CD8+ cells and importantly, the proportion of CD8+ cells that could phosphorylate STAT-4 remained greater in lung (Fig. 2a,b) and spleen (Fig. 2d) cultures from old mice. Furthermore, STAT-4 phosphorylation was increased within individual CD8+ cells purified from the lungs (Fig. 2c) and spleens (Fig. 2e) of old mice compared with those purified from young mice. Western blotting was used to verify flow cytometric analysis and showed increased IL-12-induced STAT-4 phosphorylation in splenic CD8+ cells from old mice (data not shown). Furthermore, young and old CD8+ cells expressed similar levels of STAT-4 protein (data not shown). These data provide evidence that increased STAT-4 activation in CD8 T cells from old mice is a function of old age and not the result of the unique environment within the lung.
Figure 2.
Interleukin-12 (IL-12) stimulation leads to signal transducer and activator of transcription 4 (STAT-4) phosphorylation in purified CD8+ cells. CD8+ cells were purified from the lungs (a–c) and spleens (d,e) of young (open bars) and old (closed bars) mice using magnetic cell separation. Purified cells were pooled according to age (a–c), or plated individually (d,e) and cultured with tissue culture medium (TCM) or IL-12 for 2 hr (a–c) or 4 hr (d,e). Cells were fixed, permeabilized and stained with antibodies against CD3, CD8 and pSTAT-4. CD8 T cells were identified by CD8+/CD3+ double staining. (a) Representative dot plots of pSTAT-4 expression in pulmonary CD8 T cells from young and old mice. (b) The percentage of pulmonary CD8 T cells expressing pSTAT-4. (c) Mean fluorescence intensity (MFI) of pSTAT-4 in pulmonary CD8 T cells. Positive gates were set on unstimulated cell cultures. Data represent pools of four or five mice from two independent experiments. Error bars represent the mean ± SE of the two independent experiments. (d) The percentage of splenic CD8 T cells expressing pSTAT-4. (e) MFI of pSTAT-4 in splenic CD8 T cells. Data represent the mean ± SE of four or five mice. Significance was determined by unpaired Student’s t-test. *P < 0·05.
CD8 T cells that phosphorylate STAT-4 in response to IL-12 have increased surface expression of CD44, but CD44hi is not a defining marker for IL-12 responsiveness
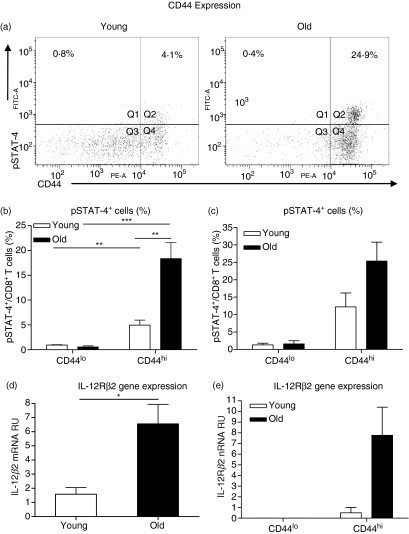
CD8 T cells from young25,26 and old21 mice that produce IFN-γ in response to Th1 cytokines are defined by the bright expression of the cell surface marker CD44. We therefore anticipated that pSTAT-4+ CD8 T cells would also be CD44hi. The representative dot plots in Fig. 3(a) illustrate the known differences in CD44 expression on CD8 T cells between young and old mice, showing that CD8 T cells from young mice displayed a broad range of CD44 expression whereas CD44 expression on CD8 T cells from old mice was more uniform, with the majority of CD8 T cells expressing high levels of CD44.10 Analysis of pSTAT-4+ CD8 T cells confirmed that the majority of pSTAT-4+ cells were of the CD44hi phenotype in both young and old mice, and that old mice had significantly more CD44hi CD8 T cells that could phosphorylate STAT-4 in response to IL-12 stimulation compared with young mice (Fig. 3b). These data confirm that pSTAT-4+ cells have the phenotypic characteristics of activated/memory T cells and a similar phenotype to the population of Th1 cytokine responsive CD8 T cells that have previously been described in young mice.22,25,26
Figure 3.
Phosphorylated signal transducer and activator of transcription 4-positive (pSTAT-4+) CD8 T cells are CD44hi and interleukin-12 receptor β2-positive (IL-12Rβ2+). (a,b) Single-cell suspensions were isolated from the lungs of young (open bars) and old (closed bars) mice and stimulated with IL-12 for 4 hr. Cells were fixed, permeabilized and stained with antibodies against CD3, CD8, CD44 and pSTAT-4. Lymphocytes were gated according to their characteristic forward and side scatter and CD8 T cells were identified by CD8+/CD3+ double staining. (a) Representative dot plots of CD44 and pSTAT-4 expression in pulmonary CD8 T cells from young and old mice. (b) Percentage of CD44hi and CD44lo CD8 T cells expressing pSTAT-4. Data represent the mean ± SE of five mice and are representative of two independent experiments. Significance was determined by unpaired Student’s t-test. **P < 0·01, ***P < 0·001. (c). Single-cell suspensions were isolated from the spleens of two young (open bars) and two old (closed bars) mice and pooled together according to age. Cells were stained with antibodies against CD3, CD8 and CD44. Populations of CD44hi and CD44lo CD8 T cells were purified using high-speed cell sorting and cultured with IL-12 for 4 hr. Cells were fixed, permeabilized and stained for pSTAT-4, and subsequently analysed for the percentage of CD44hi and CD44lo CD8 T cells expressing pSTAT-4. Unstimulated cell cultures were used to set the positive gates. Data represent mean ± SE of the two independent experiments. (d,e) CD8+ cells (d) or CD44hi and CD44lo CD8 T cells (e) were purified using magnetic cell separation (d) or high-speed cell sorting (e), homogenized in 1 ml Trizol, and frozen at −80°. RNA was isolated and reverse transcription polymerase chain reaction was performed for IL-12Rβ2 messenger RNA (mRNA). (d) IL-12Rβ2 relative units (RU) of mRNA. Data represent the mean ± SE of four mice and are representative of two independent experiments. (e) IL-12Rβ2 mRNA RU. Data represent the mean ± SE of two independent experiments of pooled cells from two mice.
To determine whether the increased proportion of pSTAT-4+ CD44hi cells in old mice in response to IL-12 was simply the result of old mice possessing more CD44hi CD8 T cells, we used high-speed cell sorting to purify CD44hi and CD44lo populations of CD8 T cells from the spleens of young and old mice. Purified CD44hi or CD44lo CD8 T cells from young and old mice were normalized for cell number and stimulated with IL-12 for 4 hr. The results shown in Fig. 3(c) confirmed that STAT-4 activation occurred primarily in CD8 T cells expressing high levels of CD44. Most importantly, in cultures with equivalent numbers of CD44hi CD8 T cells, cultures from old mice still had more CD44hi CD8 T cells that could phosphorylate STAT-4 in response to IL-12 (Fig. 3c). These data show that the increased proportion of CD44hi CD8 T cells that phosphorylate STAT-4 in response to IL-12 stimulation is not simply a consequence of old mice having more CD44hi T cells, but is a specific characteristic of CD44hi CD8 T cells in old mice.
We have previously shown that antigen-independent IFN-γ-producing CD8 T cells from old mice have increased surface expression of Th1 cytokine receptors (IL-18R1 and IFN-γR) compared with CD8 T cells from young mice.21 We therefore sought to determine whether IL-12 receptor expression was also increased on CD8 T cells from old mice. As a result of the very low expression of the IL-12 receptor on CD8 T cells, we were unable to reliably detect IL-12Rβ2 surface expression on CD8 T cells using flow cytometry, therefore CD8+ cells were purified from the spleens of young and old mice and analysed for basal IL-12Rβ2 gene expression using real-time–PCR. Expression of the IL-12Rβ2 subunit was analysed because this chain is required for IL-12 signalling and, unlike the β1 subunit, is not shared by any other receptor.27 We were able to detect IL-12Rβ2 messenger RNA in purified CD8+ cells from young and old mice (Fig. 3d), and in populations of purified CD44hi CD8 T cells (Fig. 3e). CD8+ cells from old mice had significantly higher levels of IL-12Rβ2 gene expression compared with young mice (Fig. 3d), and data from the sorted populations illustrate that IL-12Rβ2 gene expression was only detectable in the CD44hi CD8 T-cell population (Fig. 3e). Furthermore, these data show that CD44hi CD8 T cells from old mice had a higher level of IL-12Rβ2 gene expression compared with similar cells from young mice. When combined, our data demonstrate that IL-12Rβ2 expression is exclusively found in the CD44hi CD8 T-cell subset, and that IL-12Rβ2 expression, not CD44hi, is most probably a defining marker for CD8 T cells that can secrete IFN-γ in response to direct cytokine stimulation.
IL-12-driven IFN-γ production in CD8 T cells from old mice is directly linked to STAT-4 phosphorylation
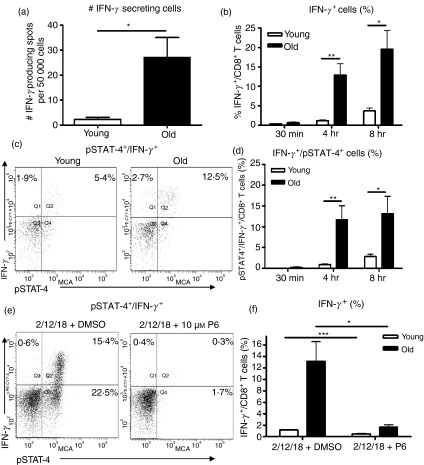
To confirm that the stimulation of purified CD8+ cells with IL-12 directly resulted in IFN-γ production, we stimulated CD8+ cells purified from the lungs of young and old mice for 48 hr with IL-12 and determined the frequency of IFN-γ-secreting cells by ELISpot. Detection of IFN-γ was low, however; we observed more CD8+ cells purified from the lungs of old mice that could produce IFN-γ in response to IL-12 than CD8+ cells purified from the lungs of young mice (Fig. 4a).
Figure 4.
Interleukin-12 (IL-12)-stimulated interferon-γ (IFN-γ) production is increased in CD8 T cells from old mice compared with young mice. (a) CD8+ cells were purified from the lungs of young (open bars) and old (closed bars) mice using magnetic cell separation. Cells were plated on an ELISpot plate that had been pre-coated with IFN-γ capture antibody, and were stimulated with tissue culture medium (TCM) or IL-12 for 48 hr at 37°. IFN-γ-producing cells were determined by ELISpot. Data are expressed as pools of two mice and are the combined result of three independent experiments. Significance was determined by unpaired Student’s t-test. *P < 0·05. (b–d) Single-cell suspensions were isolated from the lungs of young (open bars) and old (closed bars) mice and stimulated with IL-2, IL-12 and IL-18 for 30 min, 4 hr or 8 hr at 37°. Cells were fixed, permeabilized and stained with antibodies against CD3, CD8, phosphorylated signal transducer and activator of transcription 4 (pSTAT-4) and IFN-γ. Lymphocytes were gated according to their characteristic forward and side scatter and CD8 T cells were identified by CD8+/CD3+ double staining. Unstimulated cultures were used to set the positive gates. (b) Percentage of IFN-γ+ CD8 T cells. (c) Representative dot plots of pSTAT-4 and IFN-γ expression in pulmonary CD8 T cells. (d) Percentage of pSTAT-4+/IFN-γ+ CD8 T cells. Data represent the mean ± SE of five mice and are representative of two independent experiments. (e,f) CD8+ cells were purified from the spleens of young (open bars) and old (closed bars) mice using magnetic cell separation. Cells were cultured with 10 μm P6 or dimethylsulphoxide in supplemented Dulbecco’s modified Eagle’s minimal essential medium for 30 min, followed by 4 hr of IL-2, IL-12 and IL-18 stimulation at 37°. Cells were fixed, permeabilized and stained with antibodies against CD3, CD8, pSTAT-4 and IFN-γ. Unstimulated cultures were used to set the positive gates. (e) Representative dot plots showing pSTAT-4 and IFN-γ expression in CD8 T cells from old mice. (f) Percentage of IFN-γ+ CD8 T cells. Data represent the mean ± SE of four mice and are representative of two independent experiments. Significance was determined by unpaired Student’s t-test. *P < 0·05. **P < 0·01, ***P < 0·001.
To verify that STAT-4 activation correlated with IFN-γ production within the same cells, we stimulated whole lung cultures from young and old mice with a combination of IL-2, IL-12 and IL-18 over a time–course and determined the frequency of IFN-γ+ pSTAT-4+ CD8+ T cells using flow cytometric analysis. Interleukin-2 and IL-18 were included in these assays because we have previously shown that the combination of IL-2, IL-12 and IL-18 significantly enhances the production of IFN-γ by CD8 T cells in vitro,21 enabling us to detect intracellular IFN-γ in as little as 4 hr (Fig. 4b). We observed no effect on the phosphorylation of STAT-4 when IL-2 and IL-18 were added to our assays (data not shown), indicating that these cytokines signal independently of STAT-4 and that downstream IL-18 signalling events probably contribute to IFN-γ secretion.28
Figure 4(b) confirms that the lungs of old mice had a higher proportion of CD8 T cells that were capable of producing IFN-γ in response to stimulation with IL-2, IL-12 and IL-18 compared with young mice,21 and that this difference was maintained between 4 and 8 hr. Furthermore, the MFI of IFN-γ was higher in CD8 T cells from old mice (data not shown), suggesting that individual CD8 T cells produce more IFN-γ than young mice upon Th1 cytokine stimulation. Most significant however, was the fact that pSTAT-4 and IFN-γ expression could be detected simultaneously within CD8 T cells from young and old mice (Fig. 4c,d), and by 8 hr the majority of Th1 cytokine stimulated CD8 T cells from both young and old mice that produced IFN-γ+ were also pSTAT-4+ (Fig. 4d). At 4 and 8 hr, however, old mice had a significantly higher proportion of pSTAT-4+ IFN-γ+ CD8 T cells compared with young mice. Using Pearson correlation coefficient analysis we were able to demonstrate a positive correlation between the proportion of pSTAT-4+ CD8 T cells and IFN-γ+ CD8 T cells from young and old mice (r = 0·8400, P < 0·0001; data not shown), providing evidence that increased Th1 cytokine-induced IFN-γ production in CD8 T cells from old mice is the direct effect of increased IL-12 signalling in these cells. To confirm that IL-12 is required for Th1 cytokine-induced IFN-γ production in CD8 T cells, CD8+ splenocytes from young and old mice were cultured with P6, a potent inhibitor of Janus kinases, followed by stimulation with IL-2, IL-12 and IL-18. Figure 4(e,f) demonstrate that P6 significantly abrogated Th1 cytokine-induced STAT-4 activation and IFN-γ production in CD8 T cells from young and old mice, confirming that IL-12 signalling is required for IFN-γ production during Th1 cytokine stimulation. Overall, these data provide evidence that increased IFN-γ production in CD8 T cells from old mice is a result of enhanced IL-12 signalling in these cells.
Discussion
In young mice, high expression of CD44 is considered an indicator of antigen-experienced cells that can respond rapidly to secondary antigenic challenge. Additionally, in both young and old mice a subset of CD44hi CD8 T cells has been identified that can respond directly to cytokine stimulation in the absence of the T-cell receptor.21,22,26 In young mice this population is relatively minor and requires IL-12 and IL-18 to stimulate IFN-γ production.22 An expanded population of CD8 T cells that are reactive to these same cytokines is present in old mice.21 In this study we have determined that CD44hi CD8 T cells from young and old mice, although phenotypically similar, possess functionally different properties. CD8 T cells from old mice were capable of responding to a single cytokine, IL-12, resulting in TCR-independent IFN-γ production. This response was facilitated by increased IL-12Rβ2 message and enhanced STAT-4 signalling. Perhaps most significant however, was that when CD8 T-cell cultures from young and old mice were normalized for CD44hi expression, CD44hi expression did not identify a common cell phenotype between young and old mice in response to cytokine stimulation. CD8 CD44hi T cells from old mice were more responsive to IL-12 stimulation alone, again associated with enhanced pSTAT-4 expression and increased IL-12Rβ2 message. Therefore, a subset of CD44hi CD8 T cells in old mice can be distinguished from CD44hi CD8 T cells in young mice by their capacity to respond to IL-12 alone. This identifies a distinctive population of CD8 T cells in old mice that, although associated with a marker of memory cells, have functional characteristics more akin to cells of the innate immune system.
Our studies are the first to demonstrate that CD8 T cells from old mice have the ability to produce IFN-γ in response to IL-12 stimulation alone, in the absence of TCR activation.21 We hypothesize that the enhanced IL-12 responsiveness observed in old mice is a result of increased IL-12 receptor expression and subsequent STAT-4 activation in the CD44hi CD8 T-cell population of old mice. This is supported by our data demonstrating that CD44hi CD8 T cells from old mice had increased IL-12Rβ2 gene expression and IL-12-induced STAT-4 activation compared with young mice. To confirm that IFN-γ production in CD8 T cells was a direct consequence of IL-12 signalling, we inhibited IL-12 signalling in CD8 T cells from young and old mice and determined Th1 cytokine-induced IFN-γ production. In the presence of a Jak inhibitor, STAT-4 activation and IFN-γ production were significantly reduced in CD8 T cells from young and old mice, confirming that IFN-γ production required IL-12, and further supporting our hypothesis that enhanced IL-12 signalling in CD8 T cells from old mice leads to increased IFN-γ production. Inhibition of the IL-12 signalling cascade was achieved using a potent Jak inhibitor, P6, which has been shown to inhibit the receptor-associated tyrosine kinases, Jak2 and Tyk2,29 both involved in IL-12 signalling.24 Interleukin-2 also signals via the Jak/STAT pathway and would therefore be affected by this inhibitor; however, we do not anticipate this to have a significant impact on IFN-γ production as the inclusion of IL-2 in our assay did not significantly alter IFN-γ production in CD8 T cells (data not shown). More importantly, IL-18 signalling does not involve Jaks30 and therefore remained intact in the presence of P6. Our data illustrate that when IL-2 and IL-12 signalling were inhibited, IL-18 alone was insufficient to induce IFN-γ production in CD8 T cells from young or old mice. Overall, we have shown that old mice have an expanded population of activated CD8 T cells with increased expression of the IL-12 receptor that are capable of responding rapidly and robustly to Th1 cytokine stimulation in the absence of antigenic challenge.
It has been established that CD8 T cells adopt an activated/memory phenotype with age, resulting in the majority of circulating CD8 T cells in old mice possessing a CD44hi phenotype.10 The reason for this expansion is unknown; however, there is evidence that the activated/memory T-cell phenotype associated with age occurs in the absence of antigen recognition,13,31 so representing a non-classically activated population of CD44hi T cells in the aged host. Such activated/memory CD8 T cells in aged hosts have been shown to have several defects relating to the adaptive immune response, including a decreased ability to proliferate, and decreased IL-2 production upon antigen recognition.7 Despite these deficiencies, we have shown here that CD44hi CD8 T cells from old mice have an increased responsiveness to Th1 cytokine stimulation compared with young mice, marked by increased IL-12 signalling capabilities that lead to enhanced IFN-γ production.21 Furthermore, we demonstrated that increased IL-12 responsiveness observed in old mice was not simply the result of an abundance of CD44hi CD8 T cells in old mice because when CD44hi CD8 T-cell subsets were directly compared, IL-12Rβ2 gene expression and IL-12-induced STAT-4 activation were still increased in CD44hi CD8 T cells from old mice. We have therefore demonstrated that IL-12 responsiveness is not a characteristic of all CD44hi CD8 T cells, but old mice have a specialized subset of CD8 T cells within the CD44hi population that are capable of responding to IL-12.
CD44hi CD8 T cells in young mice that can secrete IFN-γ in response to cytokines serve as a population of cells that have the potential to rapidly respond to infection. There is growing evidence that these cells have properties of innate cells that can respond to pathogens such as Listeria monocytogenes and Burkholderia pseudomallei.22,25,32,33 The biological relevance of our findings in old mice can be clearly linked to our studies of infection with the intracellular pathogen M. tuberculosis, where old mice express an early resistance to infection with M. tuberculosis.21,34–37 This early resistance has been shown to correlate with increasing numbers of antigen-independent, IFN-γ-secreting CD44hi CD8 T cells in old mice,23 and is abolished in the absence of either CD8 T cells38 or IFN-γ.36 These data illustrate the importance of CD8 T-cell-derived IFN-γ during the innate immune response of old mice to M. tuberculosis infection. In addition to a decreased bacterial burden, we have demonstrated that old mice have increased levels of IL-12, IL-18 and IFN-γ in the lungs during the first few weeks of M. tuberculosis infection and our current data suggest that IL-12 plays a critical role in the CD8 T-cell-derived IFN-γ-mediated control of M. tuberculosis observed in old mice. We anticipate that our observations are not unique to M. tuberculosis infection, and expect to find a similar early resistance to other intracellular pathogens in aged mice. This appears to be the case for some,39–42 but not all, intracellular pathogens. It will be necessary to dissect the relative contributions of antigen-independent CD8 T-cell responses for each intracellular pathogen early during infection to fully appreciate whether such an enhanced innate response can contribute to the control of infection or alternatively, leads to poor disease outcome. The latter scenario is evident in influenza infection in the elderly, where the presence of clonally expanded CD8 T cells that can secrete IFN-γ has been associated with a failure to secrete influenza-specific antibodies in vaccinated elderly individuals.43 Such data in humans support our observations that CD8 T cells that can be stimulated to produce IFN-γ in an antigen-independent manner also develop in old age in man, but clearly demonstrate that for some infectious models, this response may be detrimental. Regardless of the outcome, CD8 T cells can clearly exhibit an enhanced innate effector function in response to infectious challenge in old age and understanding how IL-12 signalling can promote CD8 T-cell function in vivo may lead to therapies that specifically stimulate, or perhaps inhibit, this population of cells to aid the elderly in the clearance of pulmonary pathogens.
In summary, we have demonstrated that the increased responsiveness of CD8 T cells from old mice to IL-12 is a consequence of increased IL-12Rβ2 gene expression within a population of CD44hi CD8 T cells in old mice, leading to enhanced IL-12 signalling within each individual cell. The net outcome is an expanded population of CD8 T cells that can produce IFN-γ in an antigen-independent manner, which we believe can lead to an enhanced early resistance to infection with M. tuberculosis,36,38 and perhaps to other pathogens. These studies provide evidence that post-exposure therapies or novel vaccine designs that incorporate IL-12 could be developed to reduce the morbidity and mortality associated with infectious diseases in the elderly.
Acknowledgments
We would like to thank Drs Carmine Vacca and Maria Laura Belladona for kindly providing an anti-IL-12Rβ2 antibody for flow cytometry. The project described was supported by Grant Number R01AG-021097 from the National Institute On Aging. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institute On Aging or the National Institute of Health. In addition this work was funded by http://www.PHPID.osu.edu and the Targeted Investment in Excellence Program of the OAA, President and Research Offices at Ohio State.
Acknowledgments
The authors have no financial disclosures to report.
Glossary
Abbreviations:
- DMEM
Dulbecco’s modified Eagle’s minimal essential medium
- IFN-γ
interferon-γ
- IgG
immunoglobulin G
- IL-2
interleukin-2
- IL-12R
interleukin-12 receptor
- MACS
magnetic cell sorting
- MFI
mean fluorescence intensity
- P6
pyridone 6
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein
- pSTAT-4
phosphorylated signal transducer and activator of transcription 4
- TCM
tissue culture media
- TCR
T-cell receptor
- Th1
T helper type 1
References
- 1.Beers NH, Berkow R. The Merck Manual of Geriatrics. 3rd edn. Whitehouse Station, NJ: Merck Research Laboratories; 2000. [Google Scholar]
- 2.Harman D. The aging process. Proc Natl Acad Sci USA. 1981;78:7124–8. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niederman MS, Fein AM. Pneumonia in the elderly. Clin Geriatr Med. 1986;2:241–68. [PubMed] [Google Scholar]
- 4.Orme IM. Aging and immunity to tuberculosis: increased susceptibility of old mice reflects a decreased capacity to generate mediator T lymphocytes. J Immunol. 1987;138:4414–8. [PubMed] [Google Scholar]
- 5.Stead WW, Dutt AK. Tuberculosis in elderly persons. Annu Rev Med. 1991;42:267–76. doi: 10.1146/annurev.me.42.020191.001411. [DOI] [PubMed] [Google Scholar]
- 6.Webster RG. Immunity to influenza in the elderly. Vaccine. 2000;18:1686–9. doi: 10.1016/s0264-410x(99)00507-1. [DOI] [PubMed] [Google Scholar]
- 7.Effros RB, Walford RL. The immune response of aged mice to influenza: diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell Immunol. 1983;81:298–305. doi: 10.1016/0008-8749(83)90237-x. [DOI] [PubMed] [Google Scholar]
- 8.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–24. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linton PJ, Haynes L, Klinman NR, Swain SL. Antigen-independent changes in naive CD4 T cells with aging. J Exp Med. 1996;184:1891–900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerner A, Yamada T, Miller RA. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur J Immunol. 1989;19:977–82. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- 11.Butterfield K, Fathman CG, Budd RC. A subset of memory CD4+ helper T lymphocytes identified by expression of Pgp-1. J Exp Med. 1989;169:1461–6. doi: 10.1084/jem.169.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budd RC, Cerottini JC, Horvath C, Bron C, Pedrazzini T, Howe RC, MacDonald HR. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–9. [PubMed] [Google Scholar]
- 13.Timm JA, Thoman ML. Maturation of CD4+ lymphocytes in the aged microenvironment results in a memory-enriched population. J Immunol. 1999;162:711–7. [PubMed] [Google Scholar]
- 14.Turner J, Orme IM. Identification of altered integrin alpha/beta chain expression on T cells from old mice infected with Mycobacterium tuberculosis. Exp Gerontol. 2002;37:907–16. doi: 10.1016/s0531-5565(02)00026-8. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RN, Smith BL. Deaths: leading causes for 2002. Natl Vital Stat Report. 2005;53 [PubMed] [Google Scholar]
- 16.WHO . Concensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS) Geneva: WHO Department of Communicable Disease Surveillance and Response Epidemic and Alert Response; 2003. [Google Scholar]
- 17.Po JL, Gardner EM, Anaraki F, Katsikis PD, Murasko DM. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123:1167–81. doi: 10.1016/s0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 18.Kapasi ZF, Murali-Krishna K, McRae ML, Ahmed R. Defective generation but normal maintenance of memory T cells in old mice. Eur J Immunol. 2002;32:1567–73. doi: 10.1002/1521-4141(200206)32:6<1567::AID-IMMU1567>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Ernst DN, Weigle WO, Noonan DJ, McQuitty DN, Hobbs MV. The age-associated increase in IFN-gamma synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J Immunol. 1993;151:575–87. [PubMed] [Google Scholar]
- 20.Takayama E, Seki S, Ohkawa T, Ami K, Habu Y, Yamaguchi T, Tadakuma T, Hiraide H. Mouse CD8+ CD122+ T cells with intermediate TCR increasing with age provide a source of early IFN-gamma production. J Immunol. 2000;164:5652–8. doi: 10.4049/jimmunol.164.11.5652. [DOI] [PubMed] [Google Scholar]
- 21.Vesosky B, Flaherty DK, Turner J. Th1 cytokines facilitate CD8-T-cell-mediated early resistance to infection with Mycobacterium tuberculosis in old mice. Infect Immun. 2006;74:3314–24. doi: 10.1128/IAI.01475-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg RE, Cordes CJ, Forman J. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol. 2002;32:2807–16. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Vesosky B, Flaherty DK, Rottinghaus EK, Beamer GL, Turner J. Age dependent increase in early resistance of mice to Mycobacterium tuberculosis is associated with an increase in CD8 T cells that are capable of antigen independent IFN-gamma production. Exp Gerontol. 2006;41:1185–94. doi: 10.1016/j.exger.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–56. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 25.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–93. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambayashi T, Assarsson E, Lukacher AE, Ljunggren HG, Jensen PE. Memory CD8+ T cells provide an early source of IFN-gamma. J Immunol. 2003;170:2399–408. doi: 10.4049/jimmunol.170.5.2399. [DOI] [PubMed] [Google Scholar]
- 27.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 28.Barbulescu K, Becker C, Schlaak JF, Schmitt E, Meyer zum Buschenfelde KH, Neurath MF. IL-12 and IL-18 differentially regulate the transcriptional activity of the human IFN-gamma promoter in primary CD4+ T lymphocytes. J Immunol. 1998;160:3642–7. [PubMed] [Google Scholar]
- 29.Pedranzini L, Dechow T, Berishaj M, Comenzo R, Zhou P, Azare J, Bornmann W, Bromberg J. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res. 2006;66:9714–21. doi: 10.1158/0008-5472.CAN-05-4280. [DOI] [PubMed] [Google Scholar]
- 30.Thomassen E, Bird TA, Renshaw BR, Kennedy MK, Sims JE. Binding of interleukin-18 to the interleukin-1 receptor homologous receptor IL-1Rrp1 leads to activation of signaling pathways similar to those used by interleukin-1. J Interferon Cytokine Res. 1998;18:1077–88. doi: 10.1089/jir.1998.18.1077. [DOI] [PubMed] [Google Scholar]
- 31.Dobber R, Hertogh-Huijbregts A, Rozing J, Bottomly K, Nagelkerken L. The involvement of the intestinal microflora in the expansion of CD4+ T cells with a naive phenotype in the periphery. Dev Immunol. 1992;2:141–50. doi: 10.1155/1992/57057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg RE, Crossley E, Murray S, Forman J. Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes. J Immunol. 2005;175:1751–7. doi: 10.4049/jimmunol.175.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097–105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 34.ALA . Trends in Tuberculosis Morbidity and Mortality. New York: American Lung Association; 2003. [Google Scholar]
- 35.Cooper AM, Callahan JE, Griffin JP, Roberts AD, Orme IM. Old mice are able to control low-dose aerogenic infections with Mycobacterium tuberculosis. Infect Immun. 1995;63:3259–65. doi: 10.1128/iai.63.9.3259-3265.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner J, Orme IM. The expression of early resistance to an infection with Mycobacterium tuberculosis by old mice is dependent on IFN type II (IFN-gamma) but not IFN type I. Mech Ageing Dev. 2004;125:1–9. doi: 10.1016/j.mad.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Vesosky B, Turner J. The influence of age on immunity to infection with Mycobacterium tuberculosis. Immunol Rev. 2005;205:229–43. doi: 10.1111/j.0105-2896.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 38.Turner J, Frank AA, Orme IM. Old mice express a transient early resistance to pulmonary tuberculosis that is mediated by CD8 T cells. Infect Immun. 2002;70:4628–37. doi: 10.1128/IAI.70.8.4628-4637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehrchen J, Sindrilaru A, Grabbe S, Schonlau F, Schlesiger C, Sorg C, Scharffetter-Kochanek K, Sunderkotter C. Senescent BALB/c mice are able to develop resistance to Leishmania major infection. Infect Immun. 2004;72:5106–14. doi: 10.1128/IAI.72.9.5106-5114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gervais F, Patel P, Skamene E. Increased natural resistance to Listeria monocytogenes in senescent mice correlates with enhanced macrophage bactericidal activity. J Gerontol. 1988;43:B152–6. doi: 10.1093/geronj/43.6.b152. [DOI] [PubMed] [Google Scholar]
- 41.Lovik M, North RJ. Effect of aging on antimicrobial immunity: old mice display a normal capacity for generating protective T cells and immunologic memory in response to infection with Listeria monocytogenes. J Immunol. 1985;135:3479–86. [PubMed] [Google Scholar]
- 42.Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A. 2004;61:170–7. doi: 10.1002/cyto.a.20064. [DOI] [PubMed] [Google Scholar]
- 43.Saurwein-Teissl M, Lung TL, Marx F, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–9. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]