Abstract
Heterogeneity of dendritic cells (DC) is evident in the gut-associated lymphoid tissue and determined, in part, by incompletely understood local environmental factors. Bacterial signalling is likely to be a dominant influence on precursor cells when recruited to the mucosa. We assessed the influence of commensal bacteria on DC differentiation and function. Murine bone marrow progenitors were exposed to Lactobacillus salivarius, Bifidobacterium breve or Bifidobacterium infantis. Differences in cell surface phenotype and function were assessed. Myeloid differentiation factor 88−/− (MyD88) cells were used to determine the influence of Toll-like receptor signalling. While bacterial strains varied in impact, there was a consistent dose-dependent inhibition of DC differentiation with a shift toward a Gr-1+ CD11b+ monocyte-like phenotype. A single bacterium on a per cell basis (1 : 1) was sufficient to alter cell phenotype. The effect was only evident in early precursors. Enhanced interleukin-10 production correlated with increased Forkhead box P3 expression and reduced T-cell proliferation. The bacterial effect on DC differentiation was found to be MyD88-dependent. Signalling by enteric commensals through pattern recognition receptors on precursor cells alters DC differentiation and results in cells that are phenotypically monocyte-like and functionally suppressive. This may account for some of the features of mucosal immune tolerance to the microbiota.
Keywords: bacteria, dendritic cells, monocytes, toll-like receptors
Introduction
Dendritic cells (DCs) are a heterogeneous population of professional antigen-presenting cells that link the innate and adaptive arms of the immune system. They are found throughout the body and have specialized functional characteristics depending on their tissue localization.
The intestine is a unique environment and is home to several distinct phenotypic and functional DC subsets. Despite its heavy burden of over 1014 bacteria consisting of more than 1000 species,1 the default intestinal immune response is tolerance rather than inflammation.2 Several unique functions have been attributed to DCs in the intestine, compared with their splenic equivalents, which may play a role in maintaining intestinal tolerance. The DCs found in the Peyer’s patches induce greater levels of interleukin-10 (IL-10) than splenic DCs3 and are able to promote the induction of forkhead box P3 (FoxP3) and regulatory responses in naïve T cells.4 The DCs from the mesenteric lymph nodes, Peyer’s patches and lamina propria have all been found to induce the gut homing receptors α4β7 and CCR9 on naïve T cells and direct their homing to intestinal tissue.5–7 In addition, intestinal DCs have been implicated in B cell immunoglobulin A class switching which preferentially occurs at mucosal surfaces.8,9
Cross-talk between epithelial cells, bacteria and DCs contributes to the regulation of intestinal immune responses. The intestinal epithelial layer contributes to the maintenance of tolerance by several mechanisms including production of thymic stromal lymphopoietin10,11 and transforming growth factor-β, both of which maintain DCs in an immature state and influence T-cell polarization.12 The scale and diversity of bacteria present in the gut offers an immense pool of antigen and possible immune modulatory molecules capable of manipulating immune responses. Individual bacterial species have been shown to drive diverse responses by DCs and shape subsequent T-cell responses; from robust T helper type 1 responses elicited by some Lactobacillus and Bifidobacterium strains,13 to IL-10 production and regulatory T-cell induction by others.14–16 Commensal bacteria may also contribute to the maintenance of immune homeostasis by inducing inducible nitrogen oxide synthase in Peyer’s patch DCs which is important for initiating B-cell immunoglobulin A class switching.17,18 Through the combined actions of both the epithelial cell layer and the commensal bacteria, the intestinal immune system is regulated to discourage unwarranted inflammatory responses and maintain homeostasis.
Haematopoietic stem cells, found primarily in bone marrow, undergo polarized proliferation to give rise to progenitors of all cells of the innate and adaptive immune system. Downstream of the haematopoietic stem cells, the granulocyte–myeloid progenitor differentiates into the common DC–macrophage progenitor and exits from the bone marrow and enters the circulation. These blood-borne precursors have a short half-life in the bloodstream.19 Recently, these cells have been shown to extravasate into peripheral tissues including skin, lung and small intestine, but not spleen, where they undergo further differentiation into tissue-resident and migratory DCs.20 Interestingly, bone marrow progenitor cells express pattern recognition receptors like Toll-like receptors (TLRs) and can respond to pathogen-associated molecular patterns that are common to both commensal and pathogenic bacteria. While the purpose of TLR expression on haematopoietic progenitor cells has not been conclusively identified, their presence and functionality indicate that innate signals could influence the differentiation of precursor cells.21,22
As precursor cells have recently been shown to enter peripheral tissues like the lamina propria of the small intestine, we sought to determine the possible outcome of interaction with commensal bacteria on these precursor cells using an in vitro coculture system. Here we show that bone marrow cells cocultured in granulocyte–macrophage colony-stimulating factor (GM-CSF) are arrested in the monocytic stage of differentiation following early bacterial exposure. This myeloid differentiation factor 88 (MyD88)-dependent arrest occurs at low levels of bacterial stimulation and imparts an attenuated inflammatory response. Such a response of bone marrow precursors to commensal bacteria may partly explain the refractory nature of lamina propria macrophages and DCs and contribute to intestinal homeostasis.
Materials and methods
Mice
Six- to 16-week-old BALB/c mice were purchased from (Harlan, Oxon, UK). D011.10 mice were purchased from Jackson Laboratory (Bar Harbour, ME). Animals were housed in a conventional animal facility. All procedures in animals were approved by the University College Cork (National University of Ireland) review board. MyD88−/− and MyD88+/− bone marrow was extracted from animals generously donated by Dr Padraic Fallon, Trinity College, Dublin.
Bacteria preparation
Bifidobacterium breve and Bifidobacterium infantis 35624 were grown anaerobically for 48 hr in MRS broth (de Man, Rogosa, Sharpe; Oxoid, Basingstoke, Hampshire, UK) supplemented with 5% cystein (l-cystein HCL; Sigma, Steinheim, Germany) to a concentration of 1 × 109 colony-forming units (CFU)/ml. Lactobacillus salivarius UCC118 was grown anaerobically for 24 hr to a concentration of 1 × 109 CFU/ml in MRS broth. Bacteria were washed twice and resuspended in phosphate-buffered saline (PBS). Bifidobacterium infantis, B. breve and L. salivarius are isolates from the University College Cork and Alimentary Health Ltd. culture collections.
Bone marrow preparation
Bone marrow was prepared as previously described23 with the following modifications. Cells were cultured in complete media containing RPMI-1640 + l-glut, 10% fetal calf serum (FCS), 1% sodium pyruvate, 1% penicillin/streptomycin, 1% vitamins, 1% non-essential amino acids and 0·01% 2-mercaptoethanol (all reagents Invitrogen, Dun Laoghaire, Ireland, except for FCS, which was supplied by Sigma, Irvine, UK) with 200 U/ml (40 ng/ml) recombinant murine GM-CSF (Peprotech, London, UK). Bone marrow cells were plated at 4 × 105 cells/ml in 100-mm Petri dishes for 7 days at 37° in 5% CO2. Media was replenished on day 3 and 5 of culture with fresh GM-CSF. In bacterial coculture experiments, bacteria were added at 4 × 105 CFU/ml of cell culture media (1 : 1 ratio with bone marrow cells), and was replenished (4 × 105 CFU/ml) as indicated with GM-CSF in media changes on day 3 and day 5. At harvest, cells were routinely ≥ 90% viable as determined by trypan blue exclusion (Sigma) and propidium iodide (BD-Pharmingen, San Diego, CA) flow cytometric staining.
Flow cytometry
Using a FACSCalibur (BD Biosciences, Oxfordshire, UK) flow cytometer surface phenotype of cells was determined using four-colour staining with the following antibodies: fluorescein isothiocyanate-conjugated (-FITC) CD11c (HL3), bromodeoxyuridine (BrdU)-FITC (MOPC-21), phycoerythrin-conjugated (-PE) CD4 (L3T4), CD45R/B220-RPE (RA3-6B2), CD273-PE (TY25), CD80-PE (16-10A1), Gr-1-RPE (RB6-8C5), allophycocyanin-conjugated (-APC) CD11b (M1/70) (BD-Pharmingen), F4/80-APC (BM8) (eBioscience, San Diego, CA), CD40-biotin (RM6815), streptavinin-PE-Cy5.5 (Caltag, Carlsbad, CA) and major histocompatibility complex class II (MHC-II)-FITC (M5/114), FoxP3-APC (3G3) (Miltenyi Biotec, Bergisch Gladbach, Germany). Appropriate isotype-matched controls were used for each antibody. FoxP3 was assayed in permeabilized cells following the manufacturer’s instructions.
Cell stimulations
Bone marrow cultures on day 7 were harvested, washed and plated at 1 × 106 cells/ml in complete media for all stimulations. Cells were left unstimulated or were stimulated with 1 μg/ml lipopolysaccharide (LPS; Escherichia coli 0111.B4; Sigma, Steinheim, Germany).
Phagocytosis of FITC-dextran
Bone marrow cultures on day 7 were harvested and incubated with 555 μg/ml FITC-dextran (molecular weight ∼40 000) (Invitrogen, Dun Loaghaire, Ireland) for 4 hr at either 4° or 37° and uptake was determined by flow cytometry.
T-cell cocultures
Bone marrow cultures on day 7 were harvested, washed and incubated for 4 hr with 1 mg/ml albumin from chicken egg white protein (Sigma, Steinheim, Germany). Cells were harvested, washed twice in PBS, and cocultured with a single-cell suspension of lymph node cells from D011.10 mice at a 1 : 50 effector to responder ratio. Cells were cocultured for 5 days before further study.
Proliferation was determined following 6 hr incubation with BrdU. Flow cytometric analysis of BrdU incorporation was performed as described by the manufacturer’s instructions (BD Pharmingen).
Electrochemical enzyme-linked immunosorbent assay
Supernatants from stimulations were collected and analysed for secreted proteins using the mouse pro-inflammatory seven plex tissue culture kit measuring IL-6, IL-1β, IL-10, keratinocyte chemoattractant (KC), interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and IL-12p70 by MesoScale Discovey (Gaithersburg, MD), following the manufacturer’s instructions.
Statistical analysis
Means with SEM are represented in each graph as determined using graphpad prism version 4.0 for Windows (GraphPad Software, San Diego, CA). P-values were calculated by analysis of variance with Tukey post-hoc test. P-values considered as significant are indicated as *≤ 0·05, **≤ 0·01, ***≤ 0·001.
Results
Bacterial exposure inhibits bone marrow to DC differentiation
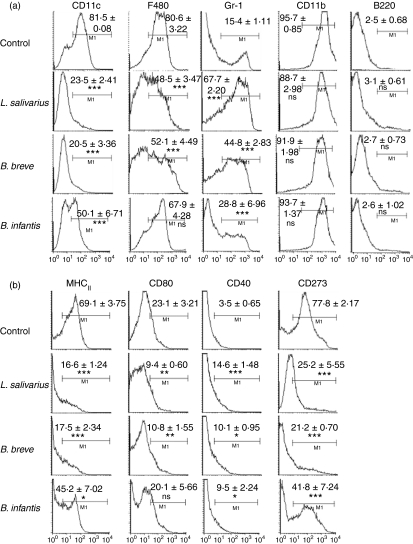
To determine the impact of bacteria exposure on bone marrow differentiation to DCs, bone marrow cells were cultured with GM-CSF in the presence or absence of three commensal bacterial strains (L. salivarius, B. breve and B. infantis) at a 1 : 1 CFU to cell ratio. The phenotype of cells harvested on day 7 of culture was determined by flow cytometry (Fig. 1). Following 7 days of coculture with any of the three bacteria, significantly fewer bone marrow-derived cells expressed the DC marker CD11c than control unexposed cells. In addition, fewer cells expressed F480, the antigen presentation molecule MHC-II and costimulatory molecules CD80 and CD273. Conversely, a greater percentage of cells expressed the monocyte marker Gr-1 following 7-day exposure to bacteria. There was no difference observed among control and bacteria-treated cells in cell viability as determined by trypan blue exclusion, as well as propidium iodide flow cytometric staining (data not shown).
Figure 1.
Exposure of bone marrow cells to bacteria inhibits dendritic cell (DC) differentiation. Bone marrow cells were cultured with non-pathogenic bacteria at a 1 : 1 bacterium : progenitor cell ratio in the presence of granulocyte–macrophage colony-stimulating factor for 7 days and the phenotype of cells was determined by multi-colour flow cytometry for the expression of classical bone marrow-derived DC markers (a). Costimulatory molecule expression was also examined by flow cytometry (b). Values in the upper right indicate percentages of cells falling in the indicated gate. Mean values ± SEM are expressed. n = 7 independent experiments. *P ≤ 0·05, **P≤ 0·01, ***P ≤ 0·001 compared to control cultures.
Although inter-bacterial differences were observed, i.e. there were differences in magnitude, the same trend was maintained. For example, the reduction in CD11c expression compared with control cells was found to be greatest for L. salivarius and B. breve cocultured cells and was statistically significantly greater than the reduction in CD11c observed by B. infantis coculture (P < 0·001). Similarly, enhanced expression of Gr-1 was found to be greatest for L. salivarius and B. breve cocultured cells compared with B. infantis cultured cells (P < 0·001).
Collectively the data show bacteria induced inhibition of DC differentiation and the limited conversion of DC precursors towards cells with a monocyte-like phenotype.
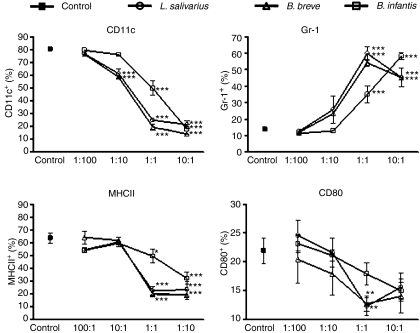
Inhibition of DC differentiation is dependent on dose of bacteria
As the previous experiment demonstrated an inhibition in DC differentiation at a 1 : 1 CFU : bone marrow precursor ratio, we sought to identify the lower limit of bacteria required to mediate DC inhibition. Bacteria were added to bone marrow cultures for 7 days over a range of CFU to cell ratios (1 : 100, 1 : 10, 1 : 1 and 10 : 1 CFU : cell) and the phenotype of the resultant cells was determined by flow cytometry (Fig. 2). A bacteria dose-dependent inhibition of DC differentiation was observed over the range of concentrations examined. A ratio of 1 : 100 CFU : cell was insufficient to inhibit DC differentiation in the majority of cells and these cultures had a phenotype similar to the unexposed control cultures. A ratio of 1 : 10 CFU : cell induced a mixed phenotype of cells with between 0% and 40% reduction in the percentage of cells expressing CD11c and a 5–26% reduction in MHC-II depending on the bacteria. Higher ratios (1 : 1 and 10 : 1 CFU : cell) constituted a strong signal to cells and inhibited DC differentiation. Therefore, encounter with a single bacterium has the ability to alter the response to GM-CSF signalling and block the differentiation of DC precursors at a monocytic stage.
Figure 2.
Altered phenotype is dependent on bacterial dose. Bone marrow cells were cultured in granulocyte–macrophage colony-stimulating factor at a starting ratio of 1 : 100, 1 : 10, 1 : 1 or 10 : 1 bacteria colony-forming units/ml : cell for the entire 7-day culture period. Surface marker expression was determined by flow cytometry. Control cells were not exposed to bacteria. Mean values ± SEM are expressed of n = 4 independent experiments *P ≤ 0·05, **P ≤ 0·01, ***P ≤ 0·001.
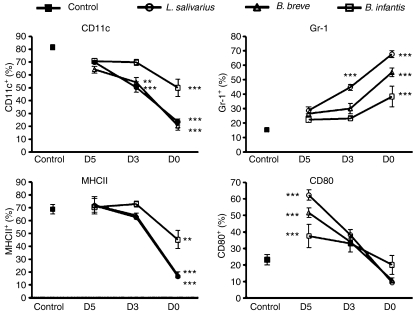
Bacterial inhibition of DC differentiation is an early event
We next investigated the ability of bacteria to inhibit the differentiation of more committed precursors by adding bacteria at various times during culture. Following 3 days of culture in GM-CSF DC precursors began to express CD11c, F480 and MHC-II while maintaining expression of Gr-1 (Figure S1a,b). At this point, addition of L. salivarius for the final 4 days of culture resulted a significant decrease in CD11c expression and a significant increase in Gr-1 expression, but did not impact MHC-II or CD80 expression (Fig. 3). Addition of B. breve resulted in cells with a significant decrease only in CD11c expression, while all other markers were unchanged compared with control. Addition of B. infantis following 3 days of culture did not result in any alteration of phenotype compared with control cells. After 5 days of culture in GM-CSF cells continued to gain expression of CD11c, F480 and MHC-II and to lose expression of Gr-1 (Figure S1a,b). At this point, addition of any of the strains did not alter the phenotype of cells in comparison with control unexposed cells. However at this point, addition of all three bacteria led to a significant increase in the percentage of cells expressing the costimulatory molecules CD80 and CD40 (Fig. 3 and data not shown) indicating that cells are stimulated by exposure and react to the addition of bacteria in the same manner as conventional immature DCs in response to an inflammatory stimulus. Therefore early exposure of bone marrow precursor cells to bacteria is necessary to inhibit differentiation into DCs. Bacterial exposure of more committed precursors does not impair differentiation and in the case of exposure at day 5 of culture, actually results in activated DCs.
Figure 3.
Early bacterial exposure of bone marrow cells is required to inhibit dentritic cell (DC) differentiation. Freshly isolated bone marrow cells were cultured with granulocyte–macrophage colony-stimulating factor for 7 days. Bacteria (1 : 1 colony-forming unit : cell) were added to cultures at the beginning of culture (D0) following 3 days culture in GM-CSF (D3) or following 5 day culture (D5). Control cells were not exposed to bacteria (control). Surface phenotype of cells harvested from cocultures on day 7 was analysed by flow cytometry for the expression of various DC markers. n ≥ 3. Significance is compared with control values. **P ≤ 0·01, ***P ≤ 0·001.
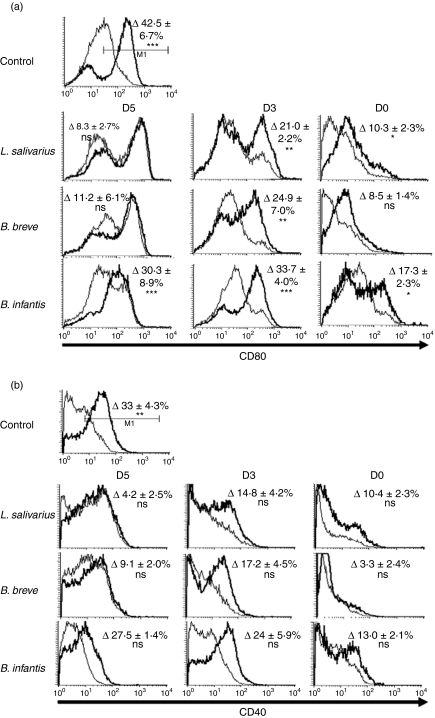
Early bacterial exposure reduces costimulatory molecule up-regulation
As early exposure to bacteria inhibited the expression of DC markers, we next investigated how exposure to bacteria affected up-regulation of costimulatory molecules following LPS stimulation (Fig. 4a,b). Cells exposed to bacteria at day 5 of a 7-day culture had up-regulated baseline CD80 and CD40 expression compared with unexposed controls. As these cells were pre-activated, subsequent overnight exposure to LPS did not induce a distinct up-regulation of the costimulatory molecules CD80 or CD40. Cells exposed to bacteria on day 3 of a 7-day culture, which have a similar phenotype to control cells, responded to LPS by up-regulating CD80 expression, but not CD40 expression, in a manner similar to unexposed control cells. Conversely, cells exposed to bacteria for 7 days modestly up-regulated CD80 in response to LPS; however, the up-regulation was greatly attenuated compared with unexposed control cells (D0 L. salivarius LPS induced up-regulation compared with control up-regulation P < 0·001, similarly B. breve P < 0·001, B. infantis P < 0·01). Under no bacterial coculture condition did overnight LPS stimulation lead to up-regulated CD40 expression. While there were inter-bacterial differences in the degree of up-regulation of CD80, these were not found to be statistically significant.
Figure 4.
Attenuated inflammatory response by cells exposed to bacteria for 7 days. Bone marrow cells were cultured for 7 days in granulocyte–macrophage colony-stimulating factor and exposed to bacteria (1 : 1 ratio) at D0, D3 or D5 of 7-day culture. On day 7, cells were harvested and washed and were either re-plated in complete media or in media with lipopolysaccharide (LPS; 1 μg/ml) for overnight stimulation. Costimulatory molecule CD80 (a) and CD40 (b) expression was determined in bacteria-exposed cells cultured overnight with LPS (bold line) or without LPS (thin line). Non-exposed cells were used as positive control of LPS-induced CD40 and CD80 up-regulation. Values in the upper right corners are the mean difference (± SEM) of the percentage of cells falling in the indicated gate following overnight LPS treatment compared with unstimulated control. n ≥ 3. *P ≤ 0·05, **P ≤ 0·01, ***P≤ 0·001.
Therefore, inhibition of DC differentiation with early, but not late, exposure to bacteria impacts the ability of cells to up-regulate costimulatory molecules in response to LPS.
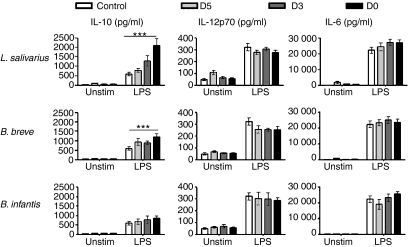
Early exposure to bacteria alters the cytokine secretion profile in response to LPS
We next investigated the impact of early bacterial exposure on cytokine secretion in supernatants from overnight LPS stimulations. While cells exposed to bacteria at any time-point responded similarly to LPS in their secretion of IL-12p70 and IL-6 (as well as IL-1β, IFN-γ, TNF-α and KC; Fig. 5 and data not shown), those cells that had early exposure to L. salivarius and B. breve had significantly increased secretion of IL-10 in response to LPS compared with unexposed control cells. Therefore, stimulation of early bacterially exposed cells results in increased IL-10 secretion and attenuated costimulatory molecule up-regulation indicating that cells have a tempered inflammatory response.
Figure 5.
Altered cytokine production following 7-day exposure to bacteria. Bone marrow cells, cultured for various times with bacteria were harvested and re-plated overnight with or without lipopolysaccharide (LPS) stimulation. Cytokine production from overnight supernatants was determined using electrochemical enzyme-linked immunosorbent assay. ***P≤ 0·001. n ≥ 3.
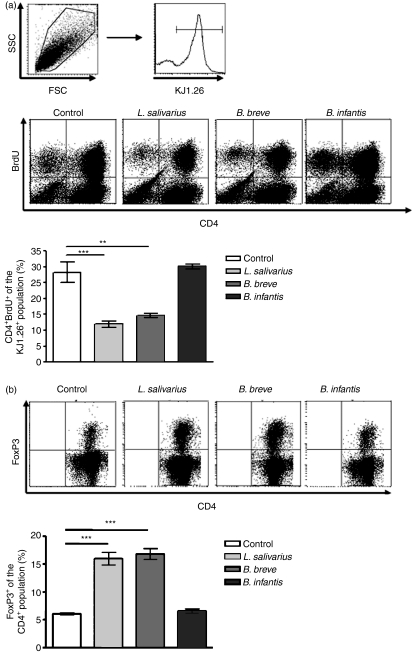
Bacteria-exposed bone marrow cells have altered interactions with T cells
To further explore the functionality of bacteria-exposed bone marrow cells we determined the outcome of their interaction with naïve T cells. Proliferation induced in naïve T cells by presentation of specific antigen by bacteria-exposed bone marrow cells was determined by BrdU incorporation. As seen in Fig. 6(a), cells exposed to L. salivarius and B. breve induced significantly fewer CD4+ cells to proliferate compared with control cells. This reduced proliferative capacity was not the result of unequal antigen loading, as all cells had equivalent ability to uptake FITC-dextran (data not shown). In addition to a reduced proliferative capacity, L. salivarius-exposed and B. breve-exposed cells induced a significant increase in the percentage of CD4+ cells expressing the regulatory cell marker FoxP3 (Fig. 6b). Taken together, these results indicate that the observed phenotypic differences translate into functional differences as bacteria-exposed cells stimulate fewer naive T cells to proliferate and increase their FoxP3 expression.
Figure 6.
Differential T-cell response to bacteria-exposed bone marrow cells. Bacteria-conditioned bone marrow cells were cocultured with ovalbumin (OVA) for 4 hr, washed extensively and then cocultured with lymph node cells from D011.10 mice at a 1 : 50 effector to responder ratio for 5 days. T-cell proliferation was determined by flow cytometric assay of bromodeoxyuridine (BrdU) incorporated during the final 6 hr of culture (a). CD4 and BrdU expression were determined on the KJ1.26+ lymphocytes. Cells harvested from coculture were assessed for expression of CD4 and intracellular FoxP3 by flow cytometry (b). Percentage FoxP3+ was determined in the CD4+ population. n = 3. **P ≤ 0·01, ***P≤ 0·001.
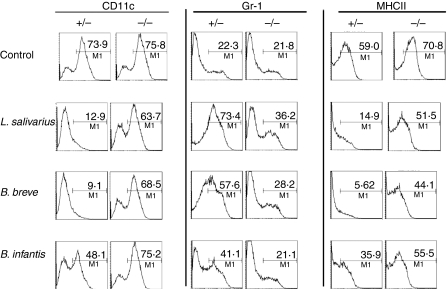
Inhibition of DC differentiation by bacteria is dependent on MyD88 signalling
To determine the possible molecular signalling mechanism for the observed inhibition of DC differentiation we cultured MyD88−/− bone marrow with GM-CSF and bacteria for 7 days and determined the effect on the phenotype of the resultant cells (Fig. 7). In the absence of signalling through the adaptor molecule MyD88 bacteria had no effect on the differentiation of DC precursors into DCs and cells derived from coculture with bacteria and MyD88−/− bone marrow were comparable to unexposed control cells in their expression of CD11c, Gr-1 and MHC-II. Therefore, TLR signalling at early stages of DC differentiation has profound effects on the phenotype and functional properties of cells.
Figure 7.
Modulation of dendritic cell precursors by bacteria is dependent on myeloid differentiation factor 88 (MyD88). Bone marrow cells isolated from MyD88+/− and MyD88−/− mice were exposed to bacteria at D0 of culture in granulocyte–macrophage colony-stimulating factor. Flow cytometric analysis of surface markers CD11c, Gr-1 and major histocompatibilty complex class II (MHC-II) were performed following 7 days of culture. Bone marrow was pooled from six MyD88−/− and six MyD88+/− mice, n = 1.
Discussion
The recent finding that haematopoietic stem cells express TLRs as well as associated accessory molecules and respond to stimulation by TLR agonists has redefined the extent to which innate pattern recognition controls immune responses.21,22 In the present study, coculture of bone marrow progenitors with commensal bacteria inhibited DC differentiation and yielded cells with high Gr-1 and low MHC-II and CD11c expression; a phenotype indicative of both monocytes and myeloid suppressor cells. The bacterial inhibitory effect was found to be MyD88-dependent and was achieved with early precursor exposure to very low levels of bacteria. The resultant monocyte-like cells were refractory to further stimulation, secreted IL-10, inhibited T-cell proliferation and induced FoxP3 expression in naïve T cells.
In contrast to previous studies using single TLR agonists, this work demonstrates that signalling through MyD88 by Gram-positive bacteria inhibits precursors to DC differentiation. Activation of TLRs on bone-marrow-derived cells has previously been investigated and the result, either enhanced or inhibited DC differentiation, was dependent on the TLR engaged.24–31 As Gram-positive bacteria possess ligands for more than one TLR, it is conceivable that the combined signalling through several TLRs functions synergistically to inhibit DC differentiation.
We also demonstrated that a very low dose of bacterial exposure was sufficient to transmit inhibitory signals to early precursors; a single bacterium is sufficient to inhibit DC differentiation on a per cell basis, overriding the signals received by the continued presence of GM-CSF. Responsiveness to low doses of bacteria may allow progenitor cells to acutely sense the presence of danger and more explicitly tailor their differentiation programme to the local environment.
Our results indicated that early encounter of bone marrow cells with bacteria was required to modify differentiation. This is probably linked to the precursor commitment of cells at that point in culture. We found that addition of bacteria following 3 and 5 days in culture with GM-CSF only marginally reduced the recovery of CD11c+ DCs. Three days in culture with GM-CSF may permanently commit cells to DC differentiation, causing them to be refractory to TLR signalling and arrest signals.32,33 While differences exist in the ability of each Gram-positive strain to inhibit DC differentiation, the same general inhibitory trend was observed. Inter-bacterial differences were not the focus of this study so we reduced the confounding variables by excluding Gram-negative bacteria, and focusing only on the general trends elicited by bacterial coculture. Therefore, in the presence of TLR and cytokine signalling, early haematopoietic precursor cells were shown to enhance their potential to differentiate into monocytes – which are innate immune effectors, and inhibit differentiation into dendritic cells – which are adaptive immune regulators. Skewing the immune response away from adaptive immunity towards innate immunity will have an impact on the replenishment of immune cells at the site of inflammation and resolution of immune responses.
Cells generated in this system by early exposure to bacteria are phenotypically and functionally similar to myeloid suppressor cells. Myeloid suppressor cells have previously been described as CD11b+ Gr-1high cells that have the ability to suppress T-cell proliferation and induce T-cell apoptosis. They have been observed in various inflammatory diseases including; experimental autoimmune encephalomyelitis, cancer, autoimmune carditis and traumatic stress.34–38 In our experimental system, we have shown that as well as having a similar phenotype to myeloid suppressor cells, bone marrow cells exposed to bacteria secreted increased levels of IL-10 and reduced naïve T-cell proliferation and increased FoxP3 expression, indicating a possible suppressive role in inflammatory responses.
Recent findings by several groups have indicated that monocyte populations seed not only Langerhans cells in the skin,39 but also DC populations in both lung40 and intestinal lamina propria.38,41 It is tempting to speculate that precursors exiting from bone marrow are recruited to intestinal lamina propria where they encounter bacteria which inhibit their further differentiation into DCs. We have shown that this altered differentiation state leaves cells refractory to LPS-induced costimulatory molecule up-regulation while enhancing IL-10 secretion. It also alters their interaction with T cells, and results in increased FoxP3 expression. Macrophages and monocytes with these characteristics have been described in the intestine.42,43 This strategy of non-responsiveness, immunoregulatory cytokine secretion and regulatory T-cell induction may be important in the maintenance of tolerance in the intestine.
In conclusion, we have shown that exposure of bone marrow to commensal bacteria modifies DC differentiation and results in cells with a monocyte-like phenotype. Phenotypic changes translate into functional differences including; reduced costimulatory molecule up-regulation, secretion of high levels of IL-10, reduced proliferation and increased FoxP3 expression in naïve T cells. The generation of these cells is dependent on the extent of precursor differentiation and MyD88 signalling, and can be generated by very low concentrations of bacteria. Whether these cells are functionally related to intestinal DCs is unclear. However, the ability of precursor cells to shape the direction of haematopoiesis following TLR signalling indicates an added layer of complexity to the influence exerted by the innate system on adaptive immune responses.
Acknowledgments
The authors have been supported in part by Science Foundation Ireland, the Health Research Board of Ireland and the Higher Education Authority of Ireland.
Glossary
Abbreviations:
- BrdU
bromodeoxyuridine
- CFU
colony-forming unit
- DC
dendritic cell
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- FoxP3
forkhead box P3
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IFN-γ
interferon-γ
- IL
interleukin
- KC
keratinocyte chemoattractant
- LPS
lipopolysaccharide
- MHC-II
major histocompatibility complex class II
- MyD88
myeloid differentiation factor 88
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- TLR
Toll-like receptor
- TNF-α
tumour necrosis factor-α
Disclosures
The authors declare no financial or commercial conflict of interest. F.S. has received unrelated grants from Alimentary Health Ltd, GlaxoSmithKline Ltd., and the Procter & Gamble Company, but these facts neither influenced nor constrained the content of this manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Acquisition of dendritic cell (DC) phenotype of bone marrow cells in culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) over a 7-day period. Bone marrow cells were cultured with GM-CSF for the indicated time period. Surface marker (a) and costimulatory molecule (b) expression was examined by flow cytometry. Values at upper right indicate percentage of cells falling in the indicated gate, n = 1.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–48. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–20. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur J Immunol. 2002;32:1445–54. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–9. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 8.Sato A, Hashiguchi M, Toda E, Iwasaki A, Hachimura S, Kaminogawa S. CD11b+ Peyer’s patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003;171:3684–90. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- 9.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–5. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 10.Rimoldi M, Chieppa M, Salucci V, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–14. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 11.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niers LE, Hoekstra MO, Timmerman HM, van Uden NO, de Graaf PM, Smits HH, Kimpen JL, Rijkers GT. Selection of probiotic bacteria for prevention of allergic diseases: immunomodulation of neonatal dendritic cells. Clin Exp Immunol. 2007;149:344–52. doi: 10.1111/j.1365-2249.2007.03421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart AL, Lammers K, Brigidi P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–9. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299–309. doi: 10.1128/IAI.72.6.3299-3309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwabuchi N, Takahashi N, Xiao JZ, Miyaji K, Iwatsuki K. In vitro Th1 cytokine-independent Th2 suppressive effects of bifidobacteria. Microbiol Immunol. 2007;51:649–60. doi: 10.1111/j.1348-0421.2007.tb03953.x. [DOI] [PubMed] [Google Scholar]
- 17.Massacand JC, Kaiser P, Ernst B, Tardivel A, Burki K, Schneider P, Harris NL. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS ONE. 2008;3:e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tezuka H, Abe Y, Iwata M, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–33. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 19.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–83. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 20.Varol C, Landsman L, Fogg DK, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–80. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamb RJ, Capocasale RJ, Duffy KE, Sarisky RT, Mbow ML. Identification and characterization of novel bone marrow myeloid DEC205+Gr-1+ cell subsets that differentially express chemokine and TLRs. J Immunol. 2007;178:7833–9. doi: 10.4049/jimmunol.178.12.7833. [DOI] [PubMed] [Google Scholar]
- 22.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 24.Bartz H, Avalos NM, Baetz A, Heeg K, Dalpke AH. Involvement of suppressors of cytokine signaling in toll-like receptor-mediated block of dendritic cell differentiation. Blood. 2006;108:4102–8. doi: 10.1182/blood-2006-03-008946. [DOI] [PubMed] [Google Scholar]
- 25.Gursel M, Verthelyi D, Klinman DM. CpG oligodeoxynucleotides induce human monocytes to mature into functional dendritic cells. Eur J Immunol. 2002;32:2617–22. doi: 10.1002/1521-4141(200209)32:9<2617::AID-IMMU2617>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Krutzik SR, Tan B, Li H, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–60. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marin-Esteban V, Abdul M, Charron D, Haziot A, Mooney N. Dendritic cells differentiated in the presence of a single-stranded viral RNA sequence conserve their ability to activate CD4 T lymphocytes but lose their capacity for Th1 polarization. Clin Vaccine Immunol. 2008;15:954–62. doi: 10.1128/CVI.00428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Lipopolysaccharide can block the potential of monocytes to differentiate into dendritic cells. J Leukoc Biol. 1999;65:232–40. doi: 10.1002/jlb.65.2.232. [DOI] [PubMed] [Google Scholar]
- 29.Rotta G, Edwards EW, Sangaletti S, et al. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J Exp Med. 2003;198:1253–63. doi: 10.1084/jem.20030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sioud M, Floisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. Eur J Immunol. 2007;37:2834–46. doi: 10.1002/eji.200737112. [DOI] [PubMed] [Google Scholar]
- 31.Sioud M, Floisand Y, Forfang L, Lund-Johansen F. Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J Mol Biol. 2006;364:945–54. doi: 10.1016/j.jmb.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 32.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97:3333–41. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- 33.Terskikh AV, Miyamoto T, Chang C, Diatchenko L, Weissman IL. Gene expression analysis of purified hematopoietic stem cells and committed progenitors. Blood. 2003;102:94–101. doi: 10.1182/blood-2002-08-2509. [DOI] [PubMed] [Google Scholar]
- 34.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–46. [PMC free article] [PubMed] [Google Scholar]
- 35.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–94. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 36.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Valaperti A, Marty RR, Kania G, et al. CD11b+ monocytes abrogate Th17 CD4+ T cell-mediated experimental autoimmune myocarditis. J Immunol. 2008;180:2686–95. doi: 10.4049/jimmunol.180.4.2686. [DOI] [PubMed] [Google Scholar]
- 38.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–37. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 39.Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–73. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103– pulmonary dendritic cell populations. J Immunol. 2008;180:3019–27. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 41.Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J Immunol. 2006;176:4155–62. doi: 10.4049/jimmunol.176.7.4155. [DOI] [PubMed] [Google Scholar]
- 42.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–94. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 43.Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–80. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.