Abstract
Cellular infiltration is a classic hallmark of inflammation. Whereas the role of T cells in many types of inflammation is well established, the specific impact of antigen recognition on their migration into the site and on the accumulation of other effector cells are still matters of debate. Using a model of an inflammatory effector phase driven by T-cell receptor (TCR) transgenic T cells, we found (i) that antigen-specific T cells play a crucial role as ‘pioneer cells’ that condition the tissue for enhanced recruitment of further T effector cells and other leucocytes, and (ii) that the infiltration of T cells is not dependent on antigen specificity. We demonstrate that a small number of antigen-specific T cells suffice to initiate a cascade of cellular immigration into the antigen-loaded site. Although antigen drives this process, accumulation of T cells in the first few days of inflammation was not dependent on T-cell reactivity to the antigen. Both transgenic and wild-type T effector cells showed enhanced immigration into the site of antigen challenge after the initial arrival and activation of antigen-specific pioneer cells. This suggests that bystander accumulation of non-specific effector/memory T cells is a general feature in inflammation. Furthermore, tumour necrosis factor (TNF)-α and interferon (IFN)-γ were identified as mediators that contribute to conditioning of the inflammatory site for high-rate accumulation of T effector cells in this T-cell-driven model.
Keywords: antigen-specific recruitment, inflammation, T cells, trafficking
Introduction
T cells play a central role in the initiation and termination of inflammatory reactions. As most non-lymphoid tissues contain few T cells in the non-diseased stage, mechanisms targeting antigen-reactive T cells to the sites of antigen encounters are of major importance. Whereas the molecular pathways of cell migration involving a variety of adhesion molecules and chemokines are well known and were partly addressed in a preceding paper,1 the events triggering T-cell recruitment and the impact of antigen specificity on this recruitment are less clear.
A number of early studies described the disappearance of antigen-specific effector/memory T cells from circulation and their accumulation within antigen-challenged sites2,3 and called this phenomenon ‘antigen-specific recruitment’ or ‘trapping’. Most of these studies involved the accumulation of antigen-specific lymphocytes in antigen-loaded lymphoid tissues; only a few examined peripheral tissues. In some of the latter studies, accumulation of autoantigen-specific T cells, particularly in the organ expressing the autoantigen,4,5 was described, and antigen-specific CD4+ effector cells were found within the lung during experimental influenza infection.6 Furthermore, using a model of antigen application to a skin site, Reinhardt et al.7 showed accumulation of antigen-specific T cells within the antigen-containing skin after the development of effector T cells in vivo. In contrast to these studies, memory CD4+ and CD8+ T cells were found in a wide range of non-lymphoid tissues long after antigen had cleared8–12 and tumour-specific CD8+ T cells infiltrated both antigen-containing and antigen-free tumours.13
Several mechanisms are known that would allow a specific antigen to control either the extravasation of effector T cells or their retention within the tissue. For example, human vascular endothelial cells can present antigen to memory CD4+ and CD8+ T cells and thus could contribute to antigen-specific T effector cell accumulation;14–16 yet in vivo studies have largely failed to detect an impact of antigen presented on endothelial cells on the immigration of specific cells.3 More likely, but still controversial, is the proposal that antigen influences the retention of antigen-specific T cells by inducing long-lasting contacts between cognate T cells and antigen-presenting cells (APCs).17–19 Finally, antigen-induced activation of T cells could control the exit of antigen-specific T cells from tissue by modulation of their expression of adhesion molecules, including receptors involved in chemotaxis.20–22
At later stages of the inflammatory process, which are beyond the scope of this study, local proliferation of the antigen-specific T cells could also contribute to the accumulation of T cells in the inflamed site. The question as to whether T cells are indeed selectively recruited according to antigen reactivity, notably in inflamed non-lymphoid tissues, and, if so, which mechanisms are involved, has not yet been clearly answered.
The aim of this study was to analyse in a well-defined system the role of antigen-specific T cells in the induction of an inflammatory reaction during the effector phase of a secondary immune response. Notably, we wished to clarify whether local antigen results in a preferential accumulation of antigen-reactive T effector cells. We focused on the early phase (i.e. the first few days) of a Th1-driven skin inflammation and showed that T effector cell recruitment into tissue sites during this period is regulated by the presence of activated antigen-specific ‘pioneer T cells’ which, after encountering the locally applied cognate antigen, induce a strongly enhanced recruitment of effector T cells and neutrophils into the challenged skin site. However, T-cell recruitment occurs irrespective of the antigen specificity of the effector T cells, suggesting that local activation of antigen-specific effector T cells modulates the tissue to promote the subsequent recruitment of leucocytes, including T cells. A very small number of antigen-specific T cells are sufficient to induce this cascade of immigration and accumulation, which is at least partly controlled by interferon (IFN)-γ and tumour necrosis factor (TNF)-α.
Materials and methods
Mice
BALB/c and DO11.10 mice were purchased from the Bundesinstitut für Risikobewertung (Berlin, Germany). DO11.10 TCR alpha knockout (k.o.) mice, kindly supplied by K. Bottomly (Yale University, New Haven, CT, USA), were bred under specific pathogen-free conditions in our animal facility. All mice were used at an age of 8–10 weeks for the experiments. Animal experiments were performed in accordance with institutional, state and federal guidelines.
Antibodies, staining and sorting reagents
The following antibodies were produced in our laboratory or the Deutsche Rheumaforschungszentrum (DRFZ; Berlin, Germany): anti-CD8 (Tib105), anti-CD11b (M1/70), anti-FcR II/III (2.4G2), anti-CD25 (PC6.1), anti-CD3 (145.2C11), anti-CD28 (37.51), anti-interleukin (IL)-4 (11B11), fluorescein isothiocyanate (FITC)- and Cy5-labelled anti-CD4 (GK1.5), Cy5-labelled anti-ovalbumin (OVA) T-cell receptor (KJ1.26), FITC-labelled anti-CD4-F(ab) (GK1.5), and FITC-labelled anti-IFN-γ (AN18.17.25). The recombinant P-selectin-human immunoglobulin G (IgG) fusion protein was kindly provided by D. Vestweber (Muenster, Germany). Phycoerythrin (PE)-labelled anti-human IgG antibodies were obtained from Jackson Immuno Research (West Grove, PA). All microbeads were obtained from Miltenyi Biotec (Gladbach, Germany). For in vivo neutralization experiments anti-IFN-γ (clone R4.6.A2, 1 mg/mouse; DRFZ), anti-TNF-α (clone MP6-XT22, 200 μg/mouse; DRFZ,) and control rat IgG (1 mg/mouse; Dunn Labortechnik, Asbach, Germany) were used.
Generation of T effector cells
Lymph nodes (LNs) and spleens were collected from 8–10-week-old mice and minced through a cell strainer. CD4+ T cells were enriched either by panning using anti-CD8, anti-CD11b, anti-FcR II/III and anti-CD25 antibodies or by using anti-CD4-FITC and anti-FITC multisort beads to a purity of 98–99%. Naïve CD4+ CD62L+ T cells were sorted using anti-CD62L microbeads and the magnetic antibody cell sorting (MACS) magnetic separation system (Miltenyi Biotec). For generation of APCs, the CD4-negative fraction of the CD4 sort was depleted of remaining T cells using anti-CD90 microbeads. APCs were irradiated (30 gray) before culture.
To generate Th1 effector cells, naïve CD4+ CD62L+ T cells from DO11.10 or DO11.10 TCR alpha k.o. mice, which carry a transgenic T-cell receptor (TCR) specific for OVA peptide OVA323–339, or from wild-type BALB/c mice were either polyclonally activated for 3 days on plate-bound anti-CD3 (3 μg/ml; clone 145-2c11; DRFZ) and anti-CD28 (5 μg/ml; clone 37.51; DRFZ) or activated with 0·5 μm OVA323–339 peptide (Charité, Berlin, Germany) in the presence of APCs supplemented with 5 μg/ml anti-IL-4 (clone 11B11; DRFZ), 20 ng/ml IFN-γ and 5 ng/ml IL-12 (R&D Systems, Wiesbaden, Germany). We used polyclonal activation of OVA-TCR transgenic and wild-type CD4+ T cells when antigen-specific and non-specific effector T cells with similar activation and differentiation status were desired. In this case, DO11.10 TCR alpha k.o. mice were used, whose CD4+ T cells consist of > 90% OVA-TCR transgenic cells. For generation of antigen-specific pioneer T cells by OVA-specific activation, which in our hands generates higher frequencies of effector cells and which leads to enrichment of antigen-specific T cells, we also used T cells from DO11.10 mice, whose CD4+ T cells consist of only 60–70% OVA-TCR transgenic T cells.
Flow cytometric analysis
Cytometric analysis was performed using a FACSCalibur and cellquest software (BD Bioscience, Franklin Lakes, NJ). For cytometric analysis, dead cells were excluded by staining with 4,6-diamidino-2-phenylindol (DAPI; Sigma-Aldrich, Munich, Germany) or propidium iodide (Sigma-Aldrich). P-selectin binding ligands were detected using the P-selectin-human IgG chimeric protein and the PE-conjugated anti-human IgG antibody as a secondary reagent. Staining was performed in Hanks’ balanced salt solution (HBSS) containing Ca2+ and Mg2+. OVA-TCR transgenic CD4+ T cells were identified using the clonotype-specific antibody KJ1.26.1. For intracellular cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 10 ng/ml) and ionomycin (500 ng/ml) for 4 hr with the addition of Brefeldin A (10 μg/ml; Sigma-Aldrich) for the last 2 hr. After stimulation, cells were stained for OVA-TCR and CD4 expression. Cells were then fixed in 2% paraformaldehyde (PFA), permeabilized with 0·5% saponin and stained for IFN-γ.
Induction of acute skin inflammation
A Th1-driven delayed type hypersensitivity (DTH) was induced as described previously.23 In brief, in vitro generated OVA-TCR transgenic Th1 cells were injected intravenously (i.v.) into BALB/c recipients. Twenty-four hours later, 250 ng of OVA323–339 peptide emulsified in 10 μl of incomplete Freund’s adjuvant (IFA; Sigma-Aldrich) was injected subcutaneously (s.c.) into the left footpad. Phosphate-buffered saline with IFA (PBS/IFA) or the peptide haemagglutinin HA111–119 in IFA was injected into the right footpad as a control. The degree of inflammation was determined by measuring footpad swelling using an Oditest micrometer gauge (Kroeplin Laengenmesstechnik, Schluetern, Germany).
In some experiments, skin inflammation was elicited in shaved hind flanks by s.c. injection of 100 μl of an emulsion of complete Freund’s adjuvant (CFA; Sigma-Aldrich) containing either 100 μg of OVA protein grade V (Sigma) or bovine serum albumin (BSA; Sigma-Aldrich) as a control antigen.
Detection of T-cell accumulation
The homing of Th1 effector cells into organs was studied as described previously.24,25 Th1 cells were labelled with 51chromium (Amersham Buchler, Braunschweig, Germany) at 37° (2 × 107 cells/ml; 20 μCi/ml) or with 111indium (Amersham Buchler) for 20 min at room temperature followed by 2 hr of incubation at 37° in fresh medium and removal of dead cells by gradient centrifugation (17·1% isotonic Nycodenz; Nyegaard, Oslo, Norway). Th1 cells were transferred into recipient animals by i.v. injection. Twenty-four or 48 hr after transfer, animals were killed and the individual tissues were removed. Differential measurement of recovered activity was performed using a γ-counter (Wallac, Turku, Finland). 51Chromium and 111indium differ in their γ-energies and can be distinguished by setting appropriate energy windows in the γ-counter. To compensate for potential (but minimal) isotope-specific artifacts, each cell population was split and one half labelled with one isotope, and one with the other. Then cell type I was mixed with the differentially labelled cell type II and results were averaged.
Histology and immunohistochemistry of tissue sections
For histological evaluation, 4-μm-thick sections of formalin-fixed and paraffin-embedded tissue samples were stained with haematoxylin and eosin (H&E). Neutrophils and macrophages were identified by anti-myeloperoxidase (MP07, clone A0398; Dako, Glostrup, Denmark) or F4/80 (eBioscience, San Diego, CA) staining, as previously described.26 Evaluation and grading of H&E-stained samples was performed by microscopic analysis of five high-power fields (hpfs) per sample by an experienced pathologist. For this, an Olympus AX70 microscope with JVC KY-F70 camera (JVC, Yokohama, Japan) and the diskus software (Carl H. Hilgers, Technisches Büro, Koenigswinter, Germany) were used. Image processing was performed using Adobe Photoshop 7.0 (Adobe, San Jose, CA).
The severity of inflammation was defined using a score evaluating the degree of infiltration and tissue destruction as follows: score grade 0, normal tissue; score grade 1, loose infiltrates in subcutaneous tissue; score grade 2, moderate, predominately phlegmonous infiltrates in subcutaneous tissue; score grade 3, abscess formation in subcutaneous tissue; score grade 4, abscess formation with infiltration of skeletal muscle.
Statistics
Data are presented as mean ± standard deviation (SD). Non-parametric tests (Wilcoxon log rank test and Mann–Whitney U-test) were used for homing experiments and for analysis of footpad swelling. Differences were considered statistically significant at P ≤ 0·05 and highly significant at P ≤ 0·01.
Results
Activation of antigen-specific CD4+ Th1 effector cells within inflamed regions promotes subsequent accumulation of CD4+ effector T cells irrespective of their antigen specificity
To determine the impact of local antigen on the accumulation of CD4+ effector T cells, we elicited acute inflammation in BALB/c recipient mice by subcutaneous injection of OVA protein, emulsified in CFA, into one back flank and BSA in CFA as a control antigen into the contralateral site. Th1 effector cells were generated by activating naïve T cells from TCR transgenic (TG) DO11.10 mice on a TCR alpha-deficient background, which recognize the OVA323–339 peptide of ovalbumin, and from wild-type BALB/c (WT) mice. To generate Th1 cells with defined activation status, stimulation of naïve CD4+ TG and WT T cells was performed by polyclonal activation using anti-CD3 and anti-CD28 antibodies in the presence of IL-12, IFN-γ and anti-IL-4. OVA-TCR transgenic DO11.10 mice on a TCR alpha-deficient background were usually used to ensure a high frequency of transgenic T cells; thus TG Th1 contained > 90% transgenic Th1 cells. TG Th1 or WT Th1 cells, which reflect a population of effector T cells with irrelevant antigen specificity, were labelled with 51chromium and transferred into separate recipient mice 24 hr after antigen challenge.
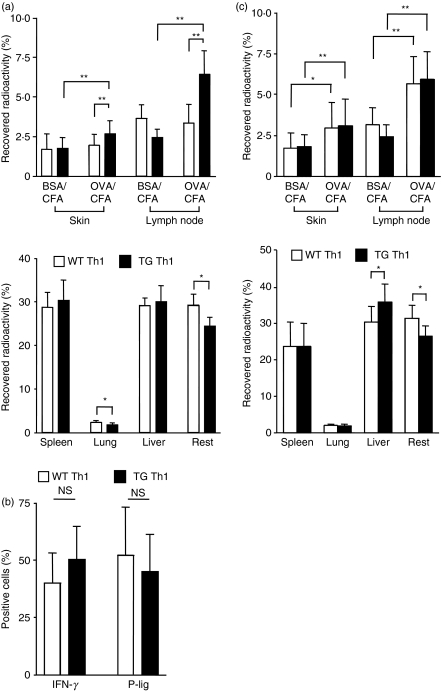
While accumulation of WT Th1 cells did not differ between skin sites containing BSA or OVA, TG Th1 cells accumulated to a significantly greater extent within the OVA-containing skin site and the draining LNs compared with the BSA-containing site and compared with WT Th1 cells recruited to the OVA-containing site (Fig. 1a). To exclude the possibility that differential accumulation of antigen-specific and non-specific T effector cells is attributable to functional differences between the in vitro generated Th1 populations, we determined effector cytokine production and homing molecule expression of the TG and WT Th1 cells. As shown in Fig. 1(b), TG Th1 and WT Th1 cells did not differ in IFN-γ production or P-selectin ligand expression – a homing receptor known to control entry of Th1 effector cells into sites of skin inflammation (Fig. 1b).
Figure 1.
Antigen-specific activation of T helper type 1 (Th1) effector cells promotes subsequent T-cell recruitment irrespective of antigen specificity. (a) Acute inflammation was elicited by subcutaneous (s.c.) injection of complete Freund’s adjuvant (CFA) containing either ovalbumin (OVA) or bovine serum albumin (BSA) into recipient mice. Twenty-four hours later, one group of mice received transgenic (TG) Th1 cells, and a second group received wild-type (WT) Th1 cells (both 51chromium-labelled; n = 16 per group). The percentage of recovered radioactivity within the skin sites and the draining lymph nodes (LNs) (upper panel) and large organs (lower panel) was determined 48 hr after Th1 cell transfer. *P < 0·05, **P < 0·01. (b) The frequency of cells producing interferon (IFN)-γ and that of cells expressing P-selectin ligands (P-lig) among WT Th1 and TG Th1 cells were determined by fluorescence-activated cell sorting (FACS) staining. (c) Acute inflammation was elicited by s.c. injection of OVA/CFA and BSA/CFA in recipient mice which 24 hr later received both TG Th1 and WT Th1 cells (n = 12 per group), differentially labelled with either 51chromium or 111indium, respectively. The percentage of the recovered radioactivity within the skin sites and the draining LNs (upper panel) and large organs (lower panel) was determined 48 hr after T-cell transfer. *P < 0·05, **P < 0·01. NS, non-significant.
These data suggest that antigen-specific T cells do indeed accumulate preferentially within the site containing the specific antigen. However, an alternative explanation would assume that activation of antigen-specific T cells within the tissue results in modulation of the inflamed site, promoting further T effector cell recruitment in a non-specific manner.
To clarify this, we co-transferred TG Th1 and WT Th1 cells into the same recipient mouse injected s.c. with OVA and BSA 24 hr before. To allow simultaneous detection, TG Th1 and WT Th1 cells were differentially labelled with 51chromium and 111indium. To exclude minor influences on homing from differential labelling, we split each cell population and switched labelling. In this setting – co-transfer of antigen-specific with non-specific T cells – we found enhanced recruitment of both TG Th1 and WT Th1 cells within the OVA-containing site and the draining LNs, at both 24 hr (data not shown) and 48 hr after transfer, compared with the site containing BSA as a control antigen (Fig. 1c). This indicates that local activation of antigen-specific T cells promotes an enhanced accumulation of T effector cells irrespective of their antigen specificity.
Characterization of Th1-cell-induced inflammation
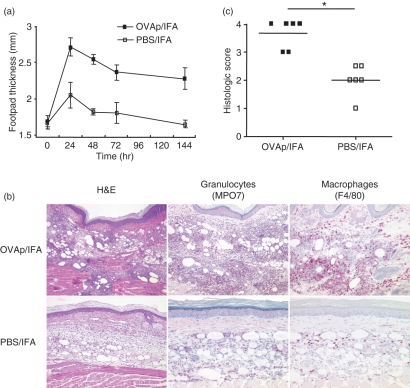
The above data indicated that local T-cell activation drives inflammatory cell recruitment. To further dissect the sequence of events and the kinetics of the inflammatory process, we modified the model by using OVA323–339 peptide emulsified in IFA and, as controls, PBS/IFA or the peptide HA111–119 emulsified in IFA. Use of peptides circumvents a potential delay caused by the processing required for protein antigens; additionally, this protocol (which basically models a DTH reaction) results in greater skin inflammation. OVA-TCR transgenic Th1 effector cells were generated from DO11.10 or DO11.10 TCR alpha k.o. mice by antigen-specific activation using OVA peptide, APCs and cytokine supplements promoting Th1 development. TG Th1 cells (5 × 105 cells) were injected into recipients, which were challenged in the footpads by antigen 24 hr later. This treatment led to a pronounced local inflammation, as indicated by footpad swelling, peaking between 24 and 48 hr after antigen challenge (Fig. 2a). Histological analysis at 24 hr after antigen challenge revealed an infiltrate dominated by neutrophils/macrophages and other signs of inflammation (Fig. 2b).
Figure 2.
Enhanced leucocyte recruitment after antigen-dependent conditioning in a peptide delayed type hypersensitivity (DTH) model. (a) The DTH reaction was induced by transfer of antigen-specific pioneer Th1 cells into recipient mice followed by s.c. injection of ovalbumin (OVA)323–339 (OVAp)/incomplete Freund’s adjuvant (IFA) or phosphate-buffered saline (PBS)/IFA into the footpad. Footpad swelling was determined over time (n = 5). (b) Representative histological staining [haematoxylin and eosin (H&E); myeloperoxidase (MPO) for granulocytes and F4/80 for macrophages; magnification ×200] of OVAp/IFA- and PBS/IFA-injected footpads 24 hr after antigen application. (c) A summary of individual histological evaluations according to the histological score of infiltration and tissue destruction of OVAp/IFA- and PBS/IFA-injected sites (score grade 0, normal tissue; score grade 1, loose infiltrates in subcutaneous tissue; score grade 2, moderate, predominately phlegmonous infiltrates in subcutaneous tissue; score grade 3, abscess formation in subcutaneous tissue; score grade 4, abscess formation with infiltration of skeletal muscle). *P < 0·05.
Injection of adjuvant alone or adjuvant plus control peptide (HA111–119; not shown) at the control site induced a significant cellular infiltration and some swelling, which, however, was much less severe and of shorter duration than that in the antigen-challenged site. The effect of IFA, which is less pronounced than that of CFA (see BSA/CFA in Fig. 1), is most probably attributable to unspecific inflammation, known to be caused by oil-based adjuvants. The usage of adjuvant is, however, required for the local retention of the antigen, as injection of peptide alone does not result in significant swelling (data not shown). An antigen-dependent footpad swelling could also be elicited by dendritic cells (DCs) loaded with peptide antigen; yet slight swelling also occurred at the control site, similar to the IFA protocol, suggesting that injection of isolated DCs alone also causes an unspecific, mild local reaction comparable to the adjuvant effect (data not shown). Thus, only the sustained presence of antigen in combination with antigen-reactive T cells is able to elicit a full-blown inflammatory reaction resulting in strongly enhanced cellular infiltration, as indicated by the significantly increased histological score (Fig. 2c).
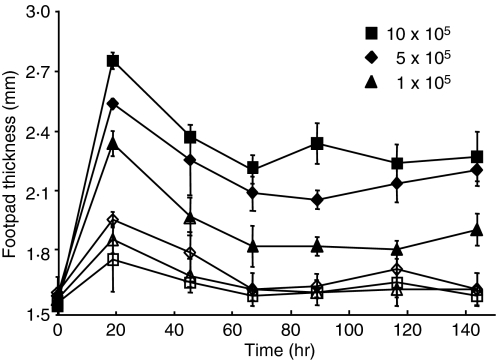
Titration of the adoptively transferred OVA-TCR TG Th1 cells (Fig. 3) demonstrates that the number of antigen-specific T cells fuelling the antigen-induced inflammation is critical for the severity of the inflammation. As few as 1 × 105 OVA-TCR transgenic Th1 cells injected i.v. are sufficient to induce a DTH reaction associated with significant footpad swelling over 1 week.
Figure 3.
Control of the severity of peptide-induced delayed type hypersensitivity (DTH) by the number of antigen-specific pioneer T cells. The peptide-DTH reaction was induced by transfer of the indicated number of antigen-specific T cells into recipients, which were subcutaneously (s.c.) injected with ovalbumin (OVA)323–339 (OVAp)/incomplete Freund’s adjuvant (IFA) or phosphate-buffered saline (PBS)/IFA into the footpads. Footpad swelling was determined at the OVAp/IFA site (filled symbols) and PBS/IFA site (open symbols).
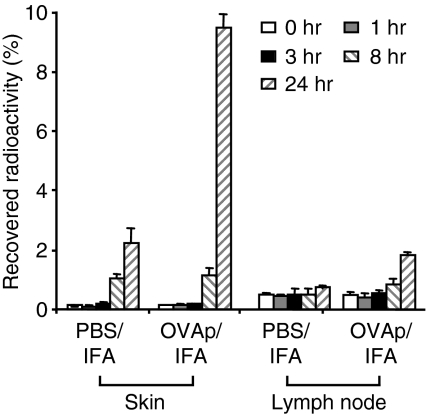
T cells patrol through non-lymphoid tissues under non-inflammatory conditions only at a low rate. It was therefore important to analyse the time-course of T-cell accumulation, which can be seen as a self-amplifying process. TG Th1 effector cells (5 × 105 cells) were radioactively labelled and transferred into recipients, which were challenged by antigen 24 hr later. Before and at sequential time-points after OVA323–339/IFA injection into the footpad, groups of mice were killed and the radioactivity was determined within the antigen-injected and the control sites (PBS/IFA) as well as in the draining LNs. As shown in Fig. 4, the accumulation of antigen-specific T cells within the antigen-containing site was initially as low as in the control site, i.e. < 0·2% of the injected cells within the first 3 hr, followed by an increase to up to 1%at 8 hr after injection. Accordingly, this early phase of recruitment is largely shaped by the unspecific irritation caused by adjuvant. At 24 hr, at the peak of footpad swelling, a much larger fraction (up to 10%) of injected effector T cells was found within the OVA323–339-injected site, reflecting the impact of T-cell reactivity on the amplification of inflammatory infiltration. This suggests that a small number of T cells initiate the high-rate recruitment of T cells and non-T cells by acting as ‘pioneer cells’– a process that could be termed ‘conditioning’ of the tissue. The critical time window for the subsequent phase of self-amplifying, antigen-dependent recruitment is between 8 and 24 hr after challenge.
Figure 4.
Time-course of recruitment of antigen-specific pioneer T cells. Pioneer T cells were radioactively labelled and transferred into recipient mice, which were challenged by ovalbumin (OVA)323–339 (OVAp)/incomplete Freund’s adjuvant (IFA) and phosphate-buffered saline (PBS)/IFA 24 hr later. Before (0 hr) and at sequential time-points after DTH induction, accumulation of pioneer cells was determined within skin sites and draining lymph nodes (LNs) (n = 3–4/time-point).
T-cell recruitment at the peak of inflammation occurs independently of antigen specificity
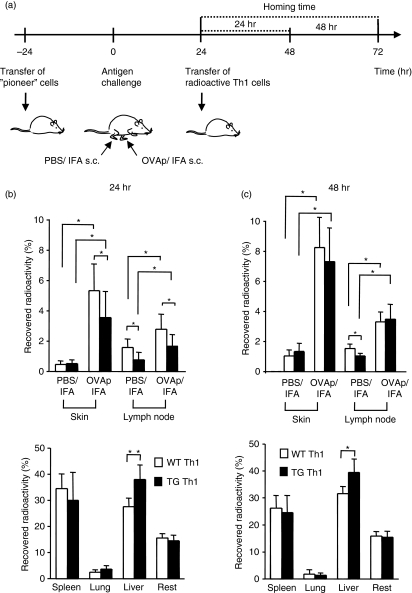
The results of experiments depicted in Fig. 1 suggested that the specificity of a given T cell does not affect proper recruitment. However, in these experiments, the initial induction phase and the recruitment at the peak of inflammation could not be discriminated. We therefore used the above protocol to induce a DTH reaction by injection of antigen-specific pioneer Th1 cells followed by antigen challenge and then analysed the accumulation of antigen-specific versus non-specific effector cells by injection of differentially radiolabelled TG Th1 or WT Th1 cells, as shown in Fig. 5a. For this purpose, TG Th1 and WT Th1 cells were again generated by activation using anti-CD3/anti-CD28 polyclonal activation to ensure similar activation and differentiation statuses of the antigen-specific and non-specific Th1 cells. Mice were killed and the distribution of injected effector Th1 cells was determined after 24 or 48 hr homing time.
Figure 5.
T cells accumulate independently of their antigen specificity within inflamed sites. (a) Experimental set-up for ‘pioneer’ cell-induced peptide-delayed type hypersensitivity (DTH) reaction and detection of effector T-cell accumulation. (b, c) Accumulation of transgenic (TG) T helper type 1 (Th1) or wild-type (WT) Th1 cells (n = 8 per group from two independent experiments) within footpads injected with ovalbumin (OVA)323–339 (OVAp)/incomplete Freund’s adjuvant (IFA) or phosphate-buffered saline (PBS)/IFA. TG Th1 or WT Th1 cells were differentially labelled with 51chromium and 111indium. The percentage of the recovered radioactivity within skin and draining lymph nodes (upper panels) and large organs (lower panels) was determined after 24 hr (b) and 48 hr (c) homing time. *P < 0·05.
Using this approach, we again observed a strongly enhanced accumulation of both TG Th1 and WT Th1 cells within the OVA323–339-containing site compared with the PBS/IFA-containing control site (Fig. 5b,c). Similar results were found when a control peptide (HA111–119) emulsified in IFA was injected into the contralateral site as a control (data not shown). After 24 hr homing time, accumulation of WT Th1 cells was even somewhat higher at the antigen-containing skin site than that of TG Th1 cells. This temporary effect might be attributable to a slightly enhanced trapping of TG Th1 cells within the liver, pointing either to an enhanced activation status of the TG Th1 cells in vivo (i.e. induced by in vivo restimulation) or to a preferential trapping of antigen-specific T cells within the liver by antigen taken up by the liver.
Labelling of TG Th1 and WT Th1 cells with a fluorescent dye and reanalysis by fluorescence-activated cell sorting (FACS) after 24 or 48 hr homing time confirmed these data obtained by radioactive labelling and showed similar accumulation of antigen-specific and non-specific T cells within the antigen-containing site (data not shown). Together, these data show that the recruitment of large numbers of effector T cells occurs independently of their antigen specificity after the conditioning action of a small number of antigen-specific T cells, activated by cognate antigen in the early phase of inflammation.
Blockade of TNF-α and IFN-γ reduces accumulation of T effector cells at early time-points
Activation of effector/memory T cells by antigen results in a rapid release of effector cytokines.27 TNF-α and IFN-γ are the major proinflammatory cytokines of Th1 effector cells, produced in great quantities. Thus, these mediators represent key candidate molecules for the activation of the local recruitment machinery. To test this possibility, we applied TNF inhibitors that are successfully used in the therapy of several inflammatory diseases, or antibodies to TNF and to IFN-γ, in our model. Blockade of TNF-α with Eternacept (Wyeth Pharmaceuticals, Muenster, Germany), a dimerized fusion protein of two p75 TNF-α receptors linked to the Fc portion of human IgG which is cross-reactive with mouse TNF-α,28,29 reduced footpad swelling over a prolonged period in this model (data not shown). IFN-γ blockade reduced only early footpad swelling, within the first 24 hr after DTH induction, which was followed by increased DTH severity, as shown earlier.23
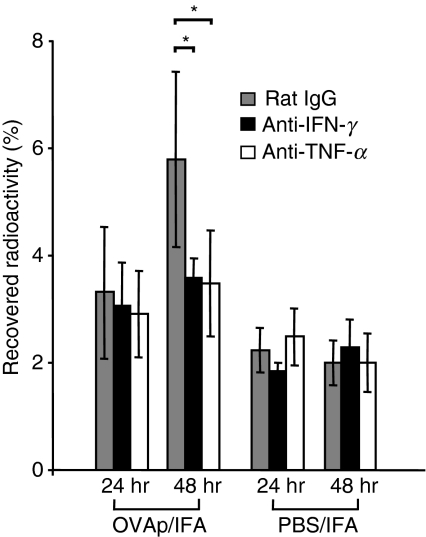
To determine the role of these cytokines in antigen-triggered recruitment, mice were injected intraperitoneally (i.p.) with anti-TNF-α antibody, anti-IFN-γ antibody or control IgG simultaneously with the pioneer cell transfer. Twenty-four hours later, mice were challenged by s.c injection of OVA323–339/IFA. At the same time, radioactively labelled Th1 cells were transferred to mice by i.v. injection. Blockade of either TNF-α or IFN-γ significantly reduced Th1 cell accumulation at the DTH site at 48 hr after DTH induction, suggesting that both cytokines promote local T-cell accumulation (Fig. 6).
Figure 6.
Interferon (IFN)-γ and tumour necrosis factor (TNF)-α blockades affect T effector cell accumulation. Pioneer T cells were transferred into recipients, which were simultaneously treated with anti-IFN-γ, anti-TNF-α or rat immunoglobulin G (IgG). Radioactively labelled transgenic (TG) T helper type 1 (Th1) cells were injected 24 hr later at the time of antigen application [ovalbumin (OVA)323–339 (OVAp)/incomplete Freund’s adjuvant (IFA) and phosphate-buffered saline (PBS)/IFA]. Twenty-four and 48 hr after radioactive cell and antigen application, radioactivity was determined within the skin sites. *P < 0·05 (n = 4/group).
Discussion
Inflammation is the result of complex interactions among different cellular partners reacting to both conserved and antigen-specific signals. While the molecular and cellular processes are well characterized at the level of specific cell populations and general mechanisms, the co-operation of the players in the in vivo context is less well defined. In this study, we sought to highlight the role of antigen-specific T cells in triggering the infiltration of cells into the inflamed tissue and to characterize the early recruitment processes in a Th1-cell-driven inflammation model. Special emphasis was given to the still controversial question of whether the recruitment of the T cells itself is related to their recognition of a specific antigen presented in a peripheral site.
As a result of our study, the following picture of sequential events emerges: (i) an unspecific irritation (in infections induced by pathogen-derived ‘danger’ signals) leads to a low-level infiltration of patrolling T cells, as well as of nonspecific leucocytes, (ii) the local antigen, presented by resident or recruited APCs, initiates the activation of a few antigen-specific T effector cells, (iii) these T cells produce mediators which directly or indirectly induce up-regulation of adhesion molecules on the endothelium and stimulate chemokine-producing cells; and by doing so, the specific T cells act as pioneer cells paving the way for the recruitment of large numbers of effector cells, such as further T effector cells, including bystander memory cells, as well as other leucocytes, and (iv) a full-blown inflammation is finally established as a consequence of high numbers of antigen-specific T cells and of mediator-producing leucocytes acting as secondary effectors and amplifiers, as shown previously.1
The findings of this study demonstrate the crucial role of antigen-specific effector T cells for the elicitation of a vigorous and lasting inflammation driven by both the T cells and other attracted leucocytes. Local effector T-cell activation by cognate antigen results in high-rate recruitment of leucocytes, peaking at 24 hr after antigen application. This includes the recruitment of large numbers of further T effector cells.
Naïve transgenic T cells are not able to induce a sufficient DTH reaction in this setting but require previous in vivo priming.30 It can be assumed that both the requirement for expansion and differentiation into effector T cells in the draining LNs and the 10-fold lower rate of entry of naïve T cells into peripheral inflamed skin sites as compared with Th1 effector cells (A. Hamann, unpublished data) preclude an immediate inflammatory response in this case.
The requirement and the potency of antigen-specific T cells for the induction of the hypersensitivity reaction has already been shown in non-transgenic systems.31,32 Fewer than 10 000 T cells31 or even a single specific T cell,32 using an optimized DTH model with direct injection into the footpad, were reported to trigger a local DTH reaction. Quantification of T-cell accumulation by radioactive labelling, as performed in the present study, confirms this range of required T cells: about 0·2% of the transferred, antigen-specific T cells are recruited non-specifically to the footpad in the first 3 hr after adjuvant + antigen deposition. This corresponds to about 200 Th1 cells in the case of the minimal dose of transferred cells required for significant swelling in our model (1 × 105 TG Th1 cells). Influx increases to up to 1%, i.e. 1000 TG Th1 cells, during the first 8 hr. Considering the time lag between tissue entry and antigen-induced re-activation of the resting effector cells leading to cytokine secretion a few hours later,33 these few hundred pioneer T cells in the footpad appear sufficient to initiate the later massive increase in recruitment and local inflammation in the antigen-injected site.
In support of this, intravital microscopy demonstrated that interactions between T cells and endothelial cells are very rare during the initiation phase of the hypersensitivity reaction.34,35 These few interactions were found to be dependent on selectins,34 consistent with our previous data in this DTH model,1 where we demonstrated that blocking of selectin-dependent adhesion in the early phase abolishes induction of the effector phase. In addition to selectins, a contribution of the complement factor C5a has also been reported for the early recruitment of effector T cells in contact sensitivity reactions.35
Importantly, the data from this study provide evidence that immigration of T cells into a site of inflammation proceeds independently of antigen specificity. Thus, antigen-specific recruitment or trapping of CD4+ effector cells does not occur in the environment of inflamed skin. This does not exclude the possibility that such mechanisms might be applicable in other tissues, such as lymphoid organs,2,3,36 the blood–brain barrier37 and the liver.38 Our data also fail to provide any evidence that exit rates are specifically modulated in antigen-reactive T cells and thereby cause a differential turnover of antigen-specific T cells in the DTH phase studied here. Such mechanisms have been proposed to play a role in the selective retention of antigen-reactive T cells in lymphoid tissues under certain conditions.2,3,36 However, we did not observe a difference between antigen-specific and non-specific effector T-cell numbers in the draining LN. Thus, antigen-reactive effector T cells play a key role as pioneer cells conditioning the local milieu for a high-rate recruitment of effector T cells in the acute phase; yet their accumulation is not affected by their antigen specificity. This might ensure the recruitment of a large pool of effector/memory T cells from the circulation searching for their cognate antigen, as suggested previously.39
The results of an ongoing complementary study (C. Doebis, A. Menning, S. Ghani, U. Lauer, A. Hamann, J. Huehn, and U. Syrbe, in preparation), investigating the time window in which the acute inflammatory process is already in decline, suggest that antigen-specific T cells become enriched during the late phase of inflammation at the site of antigen challenge, similarly to what has been described in a previous report and to what is suggested by accumulation of clonally expanded T cells in chronically inflamed tissues such as the rheumatic joint.7,40 However, our preliminary data, which are consistent with a recent report by Wakim et al.,41 suggest that this is predominantly caused by selective expansion of the antigen-specific T cells at the site of inflammation, and not by selective recruitment or trapping. Our findings therefore suggest that the paradigm of antigen-specific recruitment or retention might deserve a critical reassessment.
Proinflammatory cytokines secreted by antigen-activated T cells are good candidates to boost cell recruitment by inducing up-regulation of adhesion molecules on the endothelium, either directly or via stimulation of resident macrophages or stroma cells releasing additional factors. In particular, mast cells have been described as local amplifiers in inflamed tissues; however, our own studies showed no effect of mast cell depletion in this DTH model (data not shown). In contrast, we and others were able to show the importance of neutrophils as effectors in this cascade of events.1,42
We show here that TNF-α and IFN-γ control T-cell accumulation within the DTH site to a significant extent. IFN-γ acts as a proinflammatory cytokine in the early phase of DTH but has also been shown to play a role as a negative-feedback regulator in Th1-driven inflammation.23 Prevention or modulation of tissue conditioning could be a major mechanism of action of the anti-TNF biologics successfully applied for therapy of chronic inflammatory diseases. Notably, blocking of TNF-α or IFN-γ only affected T-cell accumulation within the antigen-challenged site, suggesting that the basal level of recruitment, induced by adjuvant alone, is triggered by other mediators.
Despite the steep increase in T effector cell recruitment at 24 hr after challenge, blocking effects of anti-TNF or anti-IFNγ were not consistently observed at this early time-point, in contrast to the clear effects at 48 hr. Surprisingly, it has to be concluded that not only unspecific recruitment (adjuvant-induced) but also early T-cell-mediated recruitment are induced by other soluble factors or by surface receptor interactions (such as CD40-CD40L) rather than by TNF or IFN-γ. This suggests that the recruitment mechanisms change over time, which might explain the well-known differences in the kinetics of infiltration of different leucocyte subsets. T cells are less abundant during the early phase but become the dominant cell type after a few days.
In addition, it has to be mentioned that inflammatory sites are not only characterized by increased recruitment but also display a high rate of cell exit via efferent lymph vessels.43 Whether TNF and IFN-γ directly or indirectly control, i.e. prolong, the retention of T cells within the tissue is an interesting question that remains to be addressed.
Acknowledgments
We thank H. Schliemann, H. Hecker and T. Geeske for providing us with monoclonal antibodies, and Simone Spieckermann for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 421, TR 52).
Glossary
Abbreviations:
- APC
antigen-presenting cell
- BSA
bovine serum albumin
- CFA
complete Freund’s adjuvant
- IFA
incomplete Freund’s adjuvant
- OVA
ovalbumin
- OVAp
ovalbumin peptide
- TG
TCR transgenic
- WT
wild type
Disclosures
The authors have no disclosures.
References
- 1.Doebis C, Siegmund K, Loddenkemper C, Lowe JB, Issekutz AC, Hamann A, Huehn J, Syrbe U. Cellular players and role of selectin ligands in leukocyte recruitment in a T-cell-initiated delayed-type hypersensitivity reaction. Am J Pathol. 2008;173:1067–76. doi: 10.2353/ajpath.2008.080052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sprent J. Migration and lifespan of lymphocytes. In: Loor F, Roelants GE, editors. B and T Cells in Immune Response. Basel: John Wiley & Sons; 1977. pp. 59–82. [Google Scholar]
- 3.Ager A, Drayson MT. Lymphocyte migration in the rat. In: Husband AJ, editor. Migration and Homing of Lymphoid Cells. Boca Raton, FL: CRC Press; 1988. p. 1. Vol. 1. [Google Scholar]
- 4.Cross AH, O’Mara T, Raine CS. Chronologic localization of myelin-reactive cells in the lesions of relapsing EAE: implications for the study of multiple sclerosis. Neurology. 1993;43:1028–33. doi: 10.1212/wnl.43.5.1028. [DOI] [PubMed] [Google Scholar]
- 5.Liu CP, Jiang K, Wu CH, Lee WH, Lin WJ. Detection of glutamic acid decarboxylase-activated T cells with I-Ag7 tetramers. Proc Natl Acad Sci USA. 2000;97:14596–601. doi: 10.1073/pnas.250390997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–68. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197:751–62. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 9.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 10.Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. 1999;163:4125–32. [PubMed] [Google Scholar]
- 11.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166:1813–22. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 12.Wiley JA, Hogan RJ, Woodland DL, Harmsen AG. Antigen-specific CD8+ T cells persist in the upper respiratory tract following influenza virus infection. J Immunol. 2001;167:3293–9. doi: 10.4049/jimmunol.167.6.3293. [DOI] [PubMed] [Google Scholar]
- 13.Palmer DC, Balasubramaniam S, Hanada K, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173:7209–16. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pober JS, Kluger MS, Schechner JS. Human endothelial cell presentation of antigen and the homing of memory/effector T cells to skin. Ann N Y Acad Sci. 2001;941:12–25. doi: 10.1111/j.1749-6632.2001.tb03706.x. [DOI] [PubMed] [Google Scholar]
- 15.Mestas J, Hughes CC. Endothelial cell costimulation of T cell activation through CD58-CD2 interactions involves lipid raft aggregation. J Immunol. 2001;167:4378–85. doi: 10.4049/jimmunol.167.8.4378. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Enis DR, Koh KP, Shiao SL, Pober JS. T lymphocyte-endothelial cell interactions. Annu Rev Immunol. 2004;22:683–709. doi: 10.1146/annurev.immunol.22.012703.104639. [DOI] [PubMed] [Google Scholar]
- 17.Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001;2:487–92. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 18.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 19.Westermann J, Bode U, Sahle A, Speck U, Karin N, Bell EB, Kalies K, Gebert A. Naive, effector, and memory T lymphocytes efficiently scan dendritic cells in vivo: contact frequency in T cell zones of secondary lymphoid organs does not depend on LFA-1 expression and facilitates survival of effector T cells. J Immunol. 2005;174:2517–24. doi: 10.4049/jimmunol.174.5.2517. [DOI] [PubMed] [Google Scholar]
- 20.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94:3909–13. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledgerwood LG, Lal G, Zhang N, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 22.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–94. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feuerer M, Eulenburg K, Loddenkemper C, Hamann A, Huehn J. Self-limitation of Th1-mediated inflammation by IFN-{gamma} J Immunol. 2006;176:2857–63. doi: 10.4049/jimmunol.176.5.2857. [DOI] [PubMed] [Google Scholar]
- 24.Butcher EC, Ford WL. Following cellular traffic: methods of labelling lymphocytes and other cells to trace their migration in vivo. In: Weir DM, editor. Handbook of Experimental Immunology. Oxford: Blackwell Scientific Publications; 1986. p. 2. Vol. 2. [Google Scholar]
- 25.Siegmund K, Hamann A. Use of labeled lymphocytes to analyze trafficking in vivo. In: Hamann A, Engelhardt B, editors. Leukocyte Trafficking. Weinheim: Wiley-VCH Verlag GmbH & Co.KGaA; 2005. pp. 497–508. [Google Scholar]
- 26.Heimesaat MM, Fischer A, Siegmund B, et al. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS ONE. 2007;2:e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loehning M, Richter A, Radbruch A. Cytokine memory of T helper lymphocytes. Adv Immunol. 2002;80:115–81. doi: 10.1016/s0065-2776(02)80014-1. [DOI] [PubMed] [Google Scholar]
- 28.Avunduk MC, Avunduk AM, Oztekin E, Baltaci AK, Ozyazgan Y, Mogolkoc R. Etanercept treatment in the endotoxin-induced uveitis of rats. Exp Eye Res. 2004;79:357–65. doi: 10.1016/j.exer.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Christadoss P, Goluszko E. Treatment of experimental autoimmune myasthenia gravis with recombinant human tumor necrosis factor receptor Fc protein. J Neuroimmunol. 2002;2:186–90. doi: 10.1016/s0165-5728(01)00473-8. [DOI] [PubMed] [Google Scholar]
- 30.Blumenthal-Barby F, Schrage A, Eulenburg K, Zeitz M, Hamann A, Klugewitz K. Sustained delayed-type hypersensitivity reaction after in vivo priming but successful induction of unresponsiveness after adoptive transfer of CD4+ effector T cells. Cell Immunol. 2008;2:110–5. doi: 10.1016/j.cellimm.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Bianchi AT, Hooijkaas H, Benner R, Tees R, Nordin AA, Schreier MH. Clones of helper T cells mediate antigen-specific, H-2-restricted DTH. Nature. 1981;290:62–3. doi: 10.1038/290062a0. [DOI] [PubMed] [Google Scholar]
- 32.Marchal G, Seman M, Milon G, Truffa-Bachi P, Zilberfarb V. Local adoptive transfer of skin delayed-type hypersensitivity initiated by a single T lymphocyte. J Immunol. 1982;129:954–8. [PubMed] [Google Scholar]
- 33.Richter A, Lohning M, Radbruch A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J Exp Med. 1999;190:1439–50. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang JM, Yamanouchi J, Santamaria P, Kubes P. A critical temporal window for selectin-dependent CD4+ lymphocyte homing and initiation of late-phase inflammation in contact sensitivity. J Exp Med. 2004;199:1223–34. doi: 10.1084/jem.20032016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norman MU, Hulliger S, Colarusso P, Kubes P. Multichannel fluorescence spinning disk microscopy reveals early endogenous CD4 T cell recruitment in contact sensitivity via complement. J Immunol. 2008;180:510–21. doi: 10.4049/jimmunol.180.1.510. [DOI] [PubMed] [Google Scholar]
- 36.Arnold CN, Butcher EC, Campbell DJ. Antigen-specific lymphocyte sequestration in lymphoid organs: lack of essential roles for alphaL and alpha4 integrin-dependent adhesion or Galphai protein-coupled receptor signaling. J Immunol. 2004;173:866–73. doi: 10.4049/jimmunol.173.2.866. [DOI] [PubMed] [Google Scholar]
- 37.Galea I, Bernardes-Silva M, Forse PA, van Rooijen N, Liblau RS, Perry VH. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204:2023–30. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertolino P, Schrage A, Bowen DG, et al. Early intrahepatic antigen-specific retention of naive CD8+ T cells is predominantly ICAM-1/LFA-1 dependent in mice. Hepatology. 2005;42:1063–71. doi: 10.1002/hep.20885. [DOI] [PubMed] [Google Scholar]
- 39.Ostler T, Pircher H, Ehl S. “Bystander” recruitment of systemic memory T cells delays the immune response to respiratory virus infection. Eur J Immunol. 2003;33:1839–48. doi: 10.1002/eji.200323460. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Quintial R, Baccala R, Pope RM, Theofilopoulos AN. Identification of clonally expanded T cells in rheumatoid arthritis using a sequence enrichment nuclease assay. J Clin Invest. 1996;97:1335–43. doi: 10.1172/JCI118550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakim LM, Gebhardt T, Heath WR, Carbone FR. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol. 2008;181:5837–41. doi: 10.4049/jimmunol.181.9.5837. [DOI] [PubMed] [Google Scholar]
- 42.Kudo C, Yamashita T, Terashita M, Sendo F. Modulation of in vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP-3. II. Inhibition by RP-3 treatment of mononuclear leukocyte recruitment in delayed-type hypersensitivity to sheep red blood cells in rats. J Immunol. 1993;150:3739–46. [PubMed] [Google Scholar]
- 43.Seabrook T, Au B, Dickstein J, Zhang X, Ristevski B, Hay JB. The traffic of resting lymphocytes through delayed hypersensitivity and chronic inflammatory lesions: a dynamic equilibrium. Semin Immunol. 1999;11:115–23. doi: 10.1006/smim.1999.0167. [DOI] [PubMed] [Google Scholar]