Abstract
Astrocyte elevated gene-1 (AEG-1) is induced by human immunodeficiency virus 1 (HIV-1) infection and involved in tumour progression, migration and invasion as a nuclear factor-κB (NF-κB) -dependent gene. The involvement of AEG-1 on lipopolysaccharide (LPS) -induced proinflammatory cytokine production was examined. AEG-1 was induced via NF-κB activation in LPS-stimulated U937 human promonocytic cells. AEG-1 induced by LPS subsequently regulated NF-κB activation. The prevention of AEG-1 expression inhibited LPS-induced tumour necrosis factor-α and prostaglandin E2 production. The AEG-1 activation was not induced by toll-like receptor ligands other than LPS. Therefore, AEG-1 was suggested to be a LPS-responsive gene and involved in LPS-induced inflammatory response.
Keywords: astrocyte elevated gene-1 (AEG-1), lipopolysaccharide, nuclear factor-κB, toll-like receptor 4, tumour necrosis factor-α
Introduction
Astrocyte elevated gene-1 (AEG-1), known as human metadherin, was originally cloned as a human immunodeficiency virus (HIV)-1-inducible transcript in primary human fetal astrocytes by the rapid subtraction hybridization approach.1–3 Activation of AEG-1 is induced by oncogenic Ha-ras, gp120 and tumour necrosis factor-α (TNF-α) as well as by HIV-1 infection.2,4 AEG-1 is ubiquitously expressed in various cell types4 and in particular the expression is augmented in a variety of tumours, such as human malignant glioma and breast cancer.4–7 AEG-1 is reported to be a downstream target of Ha-ras and to be activated through a series of signal sequences consisting of Ras-phosphoinositide 3-kinase (PI3K)-Akt-Myc/Max.8,9 The activation of AEG-1 is involved in tumour progression, migration and invasion as nuclear factor-κB (NF-κB)-dependent gene expression.10,11 However, there is no report concerning the involvement of AEG-1 in the inflammatory response in host defence, although the action of AEG-1 on tumour cells has been extensively studied.
Innate immune cells mediate the inflammatory response to microbial pathogens and products. Lipopolysaccharide (LPS), a Gram-negative bacterial component, is a potent initiator of inflammatory responses. It triggers toll-like receptor 4 (TLR4)-mediated signal pathways including NF-κB, PI3K/Akt and a series of mitogen-activated protein kinases, such as extracellular signal regulated kinase (ERK) 1/2, p38, stress-activated protein kinase/Jun N-terminal kinase (SAPK/JNK).12,13 Subsequently, those signal pathways finally lead to a cellular response, followed by production of proinflammatory cytokines and mediators. AEG-1 is known to participate in the activation of NF-κB10,11 and LPS-induced cytokine production is mainly dependent on NF-κB activation.14–17 However, the interaction between AEG-1 and LPS signalling is not known. This study investigated if and how AEG-1 was involved in LPS-induced TNF-α production in the U937 human promonocytic cell line and in human peripheral blood mononuclear cells. Here we report that AEG-1 may regulate LPS-induced proinflammatory mediator production through its effect on NF-κB activation.
Materials and methods
Materials
Lipopolysaccharide from Escherichia coli O111, phorbol 12-myristate 13-acetate (PMA), polymyxin B sulphate and LY294002 were obtained from Sigma (St Louis, MO). Antibodies to p65, phosphorylated p65 and anti-rabbit immunoglobulin G (IgG) were purchased from Cell Signalling Technology (Beverly, MA). Anti-β-actin antibody and donkey anti-goat IgG antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-metadherin (C-term) antibody was obtained from Invitrogen (Carlsbad, CA). Parthenolide was obtained from Calbiochem (La Jolla, CA).
Cell growth
U937 cells, a human promonocytic cell line, were obtained from the Riken cell bank (Tsukuba, Japan), maintained in RPMI-1640 medium containing 5% heat inactivated fetal calf serum (GIBCO-BRL, Gaithersburg, MD) and antibiotics at 37° under 5% CO2. Human peripheral blood mononuclear cells were isolated from healthy volunteers using density gradient centrifugation (Pharmacia LKB, Uppsala, Sweden) and were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum in tissue culture flasks for 3 hr. The adherent cells were further cultured in fresh medium overnight. The cells were washed vigorously with culture medium and the adherent cells were isolated by gentle detachment with a cell scraper.
Transfection of small interfering RNA
AEG-1-specific siGENOME SMART pool and a non-targeting small interfering RNA (siRNA) were obtained from Dharmacon (Chicago, IL). U937 cells were seeded at a concentration of 2 × 105 cells/well in a 48-well plate in growth medium without antibiotics. Cationic lipid complexes were prepared by incubating 200 nm siRNA with 1 μl Hiperfect transfection reagent (Qiagen, Hilden, Germany) in 50 μl medium without serum and then added to the cells. After 4 hr incubation, the cells were incubated with 200 μl fresh growth medium without antibiotics for at least 18 hr, followed by a second round of siRNA transfection as described above. After 4 hr incubation, the cells were incubated in growth medium without antibiotics. The efficiency of the AEG-1 knockdown was evaluated by immunoblotting and polymerase chain reaction (PCR) 72 and 48 hr, respectively, after the second transfection.
Transfection of dominant negative mutants of IκB-α and Akt
Dominant negative mutants of pCMV-IκB-α M vector and empty vector were purchased from Clontech (Mountain View, CA) and the dominant negative mutant of Akt was obtained from Upstate (Charlottesville, VA). U937 cells were transfected with dominant negative mutants by a FuGene HD transfection reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. In brief, 2.5 × 106 cells were plated in 60-mm plastic dishes 12 hr before transfection in normal growth media without antibiotics. The mixture of plasmid (5 μg) and transfection reagent (20 μl) was added to the culture and the cells were incubated for 4 hr in a 60-mm plastic dish, followed by adding fetal calf serum up to 5%. Two days later, transfected cells were used in the experiments. The transfection efficiency was analysed by immunoblotting with anti-phosphorylated p65 and Akt antibodies.
Determination of TNF-α and prostaglandin E2 (PGE2) production
U937 cells were cultured with or without PMA (100 ng/ml) for 12 hr and then stimulated with LPS at 100 ng/ml for 6 hr. The concentration of TNF-α in the culture supernatant was determined with an enzyme-linked immunosorbent assay kit (BioSource, Camarillo, CA). For PGE2 measurement, U937 cells were cultured with LPS at 1 μg/ml for 24 hr. The concentration of PGE2 in the culture supernatant was determined with an enzyme immunoassay kit (Cayman, Ann Arbor, MI). In some experiments polymyxin B (200 ng/ml) was also added with PMA and then stimulated with LPS.
Reverse transcription (RT)-PCR analysis
The RT-PCR was performed as described previously.18 RNA was extracted from the cells with RNeasy mini kit (Qiagen, Valencia, CA). Semi-quantative RT-PCR was carried out by using the Access Quick RT-PCR system (Promega, Madison, WI). Primers were obtained from Invitrogen with the following sequences: for AEG-1, forward 5′-ACGACCTGGCCTTGCTGAAGAATCT-3′ and reverse 5′-CGGTTGTAAGTTGCTCGGTGGTAA-3′; for TNF-α, forward 5′-ACCACGCTCTTCTGCCTGCT-3′ and reverse 5′-TGATGGCAGAGAGGAGGTTGAC-3′; and for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward 5′-ATGGGGAAGGTGAAGGTCGGAGTC-3′ and reverse 5′-GCTGATGATCTTGAGGCTGTTGTC-3′. The GAPDH was used as an equal loading control. Optimized reverse transcription and PCR conditions were 48° for 45 min followed by 95° for 2 min and 25 cycles at 95° for 45 seconds, 60° for 45 seconds, 72° for 60 seconds. The PCR products were analysed by electrophoresis on 1.5% agarose gel. The gels were stained with CYBR safe DNA gel stain (Molecular Probe, Eugene, OR) and visualized under an ultraviolet transilluminator. The 100 base pair DNA size marker (Invitrogen) was also run to determine the approximate size of the product.
Immunoblotting
Immunoblotting was performed as described elsewhere.7 In brief, the cell lysates were extracted by lysis buffer and were subjected to sodium dodecyl sulphate (SDS)–polyacrylamide gel electrophoresis using a 10% gel. The proteins were electrically transferred to a membrane and the membrane was treated with various antibodies, followed by horseradish peroxidase-conjugated goat anti-rabbit IgG. The protein bands were visualized by a chemiluminescence reagent (Pierce, Rockford, IL). For reprobing, membranes were stripped with the solution containing 2% SDS, 62·5 mm Tris–HCl, pH 6.8, 100 mm 2-mercaptoethanol at 50° for 30 min and treated with corresponding antibodies. The molecular sizes of the antigens were determined by comparison with a prestained protein size marker kit (Invitrogen).
NF-κB-dependent luciferase assay
U937 cells were transfected with siRNA and incubated for 48 hr. The cells were further transfected with 500 ng/well of NF-κB-Taluc luciferase reporter gene (Invitrogen) and an equal amount of pRL-TK plasmid (Promega) by FuGene HD transfection reagent and incubated for 48 hr. The transfected cells were stimulated with LPS (100 ng/ml) for 6 hr. After lysis with a lysis reagent (Promega) the luciferase activity was determined with the dual luciferase assay kit (Promega). The NF-κB-dependent luciferase activity in the cell lysates was determined with a luminometer. The fold increase was calculated based on the untreated control.
Statistical analysis
All the experiments were performed at least three times independently. Experimental data are expressed as the mean of triplicates ± SD in at least three independent experiments. Statistical analysis based on Student’s t-test was carried out for comparisons between two experiments. A value of P< 0·01 was considered statistically significant.
Results
LPS induces the expression of AEG-1 in U937 cells and human monocytes
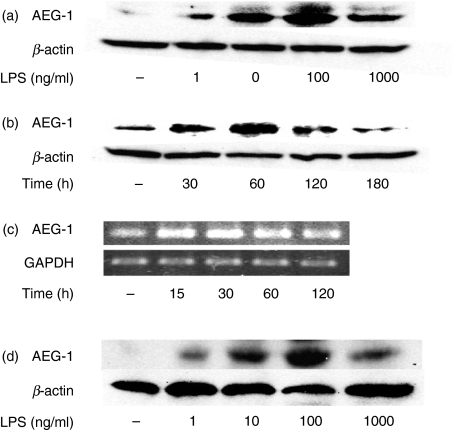
The effect of LPS on the expression of AEG-1 in U937 cells was examined (Fig. 1). U937 cells were stimulated with various concentrations of LPS and the expression of AEG-1 protein and of messenger RNA (mRNA) was determined by immunoblotting and RT-PCR, respectively. AEG-1 was only slightly expressed in non-treated U937 cells. The LPS augmented the AEG-1 expression in a concentration-dependent manner up to 100 ng/ml (Fig. 1a). The enhancing effect of LPS at 1000 ng/ml was less marked than at 100 ng/ml. U937 cells were stimulated with LPS at 100 ng/ml for various times (Fig. 1b). The expression of AEG-1 increased after LPS stimulation, reached a peak at 1 hr, and then declined until 3 hr. The effect of LPS on the expression of AEG-1 mRNA was examined by RT-PCR (Fig. 1c). This showed that LPS enhanced the expression of AEG-1 mRNA 15 min after the stimulation and the enhancement continued for at least 60 min.
Figure 1.
Induction of astrocyte elevated gene-1 (AEG-1) expression by lipopolysaccharide (LPS). (a) U937 cells were cultured with various concentrations of LPS for 1 hr. (b, c) U937 cells were cultured with LPS (100 ng/ml) for various times. (d) Human peripheral blood mononuclear cells were cultured with various concentrations of LPS for 1 hr. The expression of AEG-1 protein and messenger RNA were analysed by immunoblotting (a–c) and reverse transcription–polymerase chain reaction (c).
The effect of LPS on the expression of AEG-1 was examined in human peripheral blood monocytes (Fig. 1d). At 1 ng/ml LPS induced the AEG-1 expression which reached a peak at 100 ng/ml of LPS. A similar concentration-dependent response was seen in human monocytes as well as U937 cells. However, LPS at any concentration did not induce AEG-1 protein and mRNA in RAW 264.7 mouse macrophage-like cells (data not shown). In addition, the AEG-1 expression was not induced by other TLR ligands including poly(I : C), CpG DNA, Pam3CysSK4 and imiquimod R837 in U937 cells (data not shown).
LPS triggers the expression of AEG-1 through NF-κB
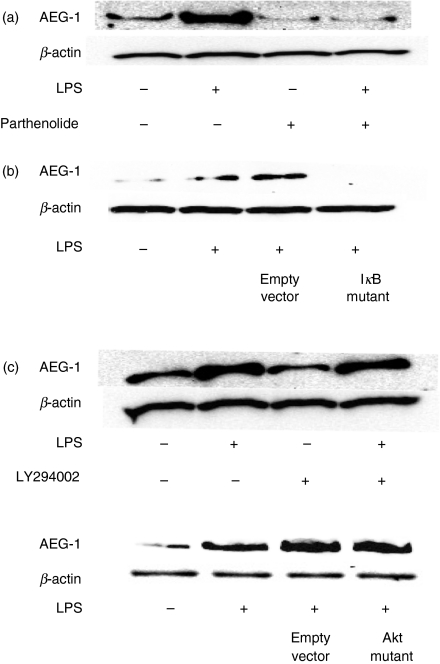
To clarify the signal molecules triggering LPS-induced AEG-1 expression, the effect of parthenolide as a NF-κB inhibitor19 and the IκB dominant negative mutant on LPS-induced AEG-1 expression was examined in U937 cells (Fig. 2a). U937 cells were stimulated with LPS (100 ng/ml) in the presence or absence of parthenolide and the AEG-1 expression was determined by immunoblotting. Parthenolide and IκB dominant negative mutant completely prevented LPS-induced AEG-1 expression. Further, we examined the effect of LY294002 as a PI3K inhibitor8,9 and the Akt dominant negative mutant on LPS-induced AEG-1 expression. Neither LY294002 nor Akt mutant had any effect on AEG-1 expression (Fig. 2b). The effect of a series of pharmacological inhibitors including PD98059 for ERK1/2, SB203580 for p38 and SP600125 for SAPK/JNK on LPS-induced AEG-1 expression was examined. None of them had any effect on it (data not shown).
Figure 2.
Involvement of nuclear factor-κB (NF-κB) activation in lipopolysaccharide (LPS) -induced astrocyte elevated gene 1 (AEG-1) expression. (a) U937 cells were treated with 5 μM parthenolide as a NF-κB inhibitor for 10 hr (top). U937 cells were transfected with IκB dominant negative mutant or empty vector as a negative control and further cultured for 2 days (bottom). (b) U937 cells were pretreated with 25 μm LY294002 as a phosphoinositide 3-kinase (PI3K) inhibitor for 10 hr (top). U937 cells were transfected with Akt dominant negative mutant or empty vector as a negative control and cultured for 2 days (bottom). Those cells were further stimulated with LPS (100 ng/ml) for 1 hr and the expression of AEG-1 was analysed by immunoblotting.
AEG-1 regulates the activation of NF-κB in LPS-stimulated U937 cells
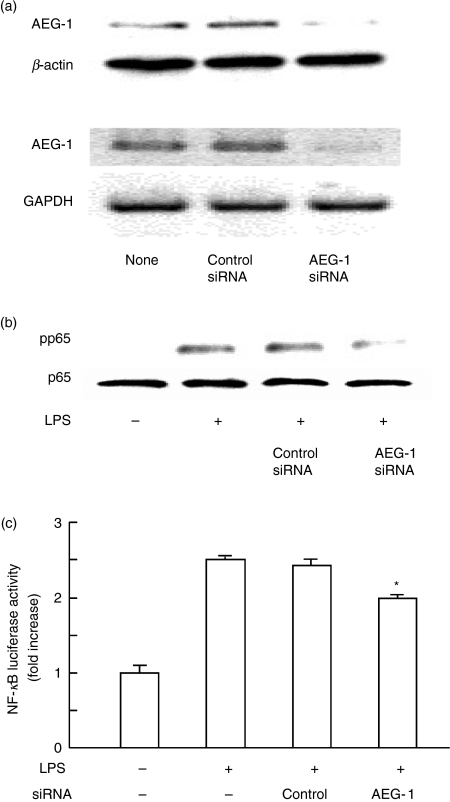
In the preceding section we demonstrated that LPS augmented the expression of AEG-1 via NF-κB activation. On the other hand, AEG-1 is reported to activate NF-κB via IκB degradation and to interact physically with the p65 component of NF-κB.4,10 A possibility was raised that AEG-1 induced by LPS might further affect LPS-induced NF-κB activation. U937 cells were transfected with AEG-1 siRNA and then stimulated with LPS. The phosphorylation of p65 NF-κB was determined in whole cell extract by immunoblotting with anti-phosphorylated p65 (pp65) antibody. First, we confirmed that AEG-1 siRNA significantly inhibited AEG-1 protein and mRNA expression (Fig. 3a), although the control siRNA did not affect it. By using the AEG-1 siRNA, the effect on the phosphorylation of p65 NF-κB was examined (Fig. 3b). The LPS-induced p65 phosphorylation was significantly inhibited by introduction of AEG-1 siRNA into U937 cells, suggesting that AEG-1 up-regulated LPS-induced NF-κB activation. On the other hand, the control siRNA failed to do this. To confirm the regulation of LPS-induced NF-κB activation by AEG-1, U937 cells were transfected with AEG-1 siRNA or control siRNA, transfected with NF-κB luciferase reporter gene with internal control plasmid and stimulated with LPS. The LPS significantly enhanced the NF-κB-dependent reporter gene activity in control siRNA and non-transfected cells. On the other hand, AEG-1 siRNA significantly reduced it (Fig. 3c).
Figure 3.
Inhibitory action of astrocyte elevated gene 1 (AEG-1) small interfering RNA (siRNA) on lipopolysaccharide (LPS)-induced nuclear factor-κB (NF-κB) phosphorylation. (a) U937 cells were transfected with AEG-1 siRNA or negative control siRNA for 72 hr. The expression of AEG-1 protein and of messenger RNA were analysed by immunoblotting (top) and reverse transcription–polymerase chain reaction (bottom). (b) Transfected cells were treated with LPS (100 ng/ml) for 1 hr and the phosphorylation of p65 in whole cell extract was analysed by immunoblotting with anti-phosphorylated p65 (pp65) antibody. (c) The effect of AEG-1 siRNA on LPS-induced NF-κB-dependent luciferase activity. The cells transfected with control or AEG-1 siRNA were stimulated with LPS (100 ng/ml) for 6 hr and the luciferase activity was determined using a luminometer.
AEG-1 regulates the production of TNF-α and PGE2 in LPS-stimulated U937 cells
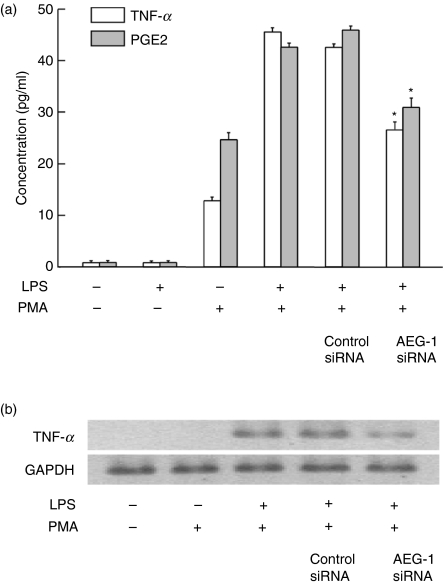
Lipopolysaccharide-induced TNF-α and PGE2 production is mainly dependent on NF-κB activation.14–17 In the preceding section, AEG-1 is shown to regulate LPS-induced NF-κB activation. Therefore, the effect of AEG-1 siRNA on LPS-induced TNF-α and PGE2 production was examined (Fig. 4a). The LPS did not induce TNF-α production in normal U937 cells but did induced it in PMA-treated U937 cells. Therefore, PMA-treated U937 cells were used for the experiment. Introduction of AEG-1 siRNA into U937 cells inhibited the production of TNF-α and PGE2 in response to LPS. Next, the effect of AEG-1 siRNA on LPS-induced TNF-α mRNA expression was examined. AEG-1 siRNA significantly inhibited the expression of TNF-α mRNA in LPS-stimulated U937 cells (Fig. 4b). AEG siRNA also inhibited the expression of cyclo-oxygenase 2 in those cells (data not shown). Polymyxin B (200 ng/ml) as a specific inhibitor of LPS completely abolished the LPS-induced TNF-α and PGE2 production (approximately 100% inhibition), suggesting that LPS as TLR4 ligand itself induced TNF-α and PGE2.
Figure 4.
Inhibitory action of astrocyte elevated gene 1 (AEG-1) small interfering RNA (siRNA) on lipopolysaccharide (LPS)-induced tumour necrosis factor-α (TNF-α) and prostaglandin E2 (PGE2) production. (a) U937 cells were transfected with AEG-1 siRNA or negative control siRNA for 72 hr. Transfected or control cells were treated with phorbol myristate acetate (100 ng/ml) for 12 hr, followed by stimulation with LPS (100 ng/ml) for 6 hr in TNF-α production or with LPS (1 μg/ml) for 24 hr in PGE2 production. The levels of TNF-α and PGE2 in the culture supernatant were determined with enzyme-linked immunosorbent assay and enzyme immunoassay, respectively. *P< 0.01 versus control siRNA. (b) Transfected or control cells were stimulated with LPS (100 ng/ml) for 1 hr. The expression of TNF-α messenger RNA was analysed with reverse transcription–polymerase chain reaction.
Discussion
In the present study we demonstrate for the first time that AEG-1 is a LPS-responsive gene and regulates production of LPS-induced proinflammatory mediators via enhanced NF-κB activation. Based on the finding that AEG-1 is not induced by TLR ligands other than LPS (TLR-4 ligand), AEG-1 may exclusively regulate TLR4-mediated signalling and gene expression. AEG-1 is activated by oncogenic Ha-ras, gp120, TNF-α and HIV-1 infection,2,4 and especially the expression is augmented in a variety of tumours.4–7 Moreover, the activation of AEG-1 is involved in tumour progression, migration and invasion as NF-κB-dependent gene expression.10 Therefore, the present study suggests that AEG-1 may play an important role in the regulation of innate immunity as well as carcinogenesis.
Lipopolysaccharide induced the activation of AEG-1 in a NF-κB-dependent manner. Oncogenic Ha-ras is reported to induce AEG-1 expression via the PI3K signal pathway that augments c-Myc to key E-box elements in the AEG-1 promoter.9 As far as is known, the PI3K signal pathway is only the established signal pathway in AEG-1 activation. In the present study, however, we could not confirm the involvement of the PI3K/Akt pathway in LPS-induced AEG-1 activation. In the case of carcinogenesis the AEG-1 gene is activated over a long period through the PI-3K/Akt signal pathway. On the other hand, in the case of the inflammatory response it might be activated within 30 min through the NF-κB signal pathway. Therefore, various stimuli might utilize different activation pathways for AEG-1 activation.
There is no information on the presence of a κB binding site in AEG-1 promoter. Bioinformatics analysis revealed that the human AEG-1 promoter lacks consensus TATA and CAAT boxes, but contains multiple Sp1 binding motifs and high GC content.4 The Sp1 binding site is observed in numerous genes lacking a TATA box4 and Sp1 has been reported to interact with NF-κB in vitro and in vivo.20–22 The LPS-induced activation of NF-κB can trigger AEG-1 expression via the involvement of Sp1. On the other hand, activated AEG-1 then regulates LPS-induced NF-κB activation. In fact, AEG-1 siRNA inhibited the phosphorylation of p65 NF-κB. It is of interest to characterize the interaction between NF-κB and AEG-1.
Lipopolysaccharide stimulates macrophage and leads to the production of various proinflammatory mediators.23 The pleiotropic effects of LPS are widely accepted as a cause of endotoxin shock, which mainly results from sustained or over-expression of proinflammatory mediators. Our study demonstrates that AEG-1 augments LPS-induced TNF-α and PGE2 production and that AEG-1 regulates LPS-induced, NF-κB-dependent gene expression. AEG-1 might be a target molecule for the therapy of LPS-related diseases, such as sepsis, septic shock and systemic inflammatory response syndrome. In addition to pathogen-stimulated inflammation, many other disease states, such as severe trauma, burns and surgery, induce the NF-κB-dependent production of pro-inflammatory mediators.24–26 The involvement of AEG-1 in such conditions is not unlikely because AEG-1 activation is also triggered by a number of stimuli, Ha-ras, gp120, TNF-α and HIV-1 infection.2,4 The possibility that LPS may induce AEG-1 expression via TNF-α production is unlikely because TNF-α-induced AEG-1 expression takes 3 days.2
Lipopolysaccharide did not augment AEG-1 in RAW 264.7 mouse macrophage-like cells, although anti-metadherin (C-term) antibody specifically recognizes mouse AEG-1 homologue (3D3). Mouse AEG-1 homologue is three amino acids shorter than human AEG-1. Moreover, human AEG-1 protein is located in the cytoplasm and nucleus whereas mouse one is located only in the cytoplasm.4 No AEG-1 augmentation in LPS-stimulated RAW 264.7 cells might be the result of the differences in AEG-1 expression between mouse and human. No augmentation is seen in several human cancer cell lines.4,5 The precise mechanism of no AEG-1 augmentation is still a matter for speculation.
In summary, AEG-1 is a LPS-responsive gene and plays an important role in LPS-induced TNF-α and PGE2 production via NF-κB activation. AEG-1 might be an important regulatory molecule for the control of TLR4 (LPS)-mediated gene expression.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan. We are grateful to Kazuko Takahashi and Akiko Morikawa for their technical assistance.
Glossary
Abbreviations:
- AEG-1
astrocyte elevated gene-1
- ERK
extracellular signal-related kinase
- HIV-1
human immunodeficiency virus type 1
- IgG
immunoglobulin G
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- PBMC
peripheral blood mononuclear cells
- PGE2
prostaglandin E2
- PI3K
phosphoinositide 3-kinase
- PMA
phorbol 12-myristate 13-acetate
- RT-PCR
reverse transcription–polymerase chain reaction
- SAPK/JNK
stress-activated protein kinase/Jun N-terminal kinase
- siRNA
small interfering RNA
- TLR4
toll-like receptor 4
- TNF-α
tumour necrosis factor-α
Disclosures
The authors have no financial conflict of interest.
References
- 1.Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, Fisher PB. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–02. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- 2.Su ZZ, Chen Y, Kang DC, Chao W, Simm M, Volsky DJ, Fisher PB. Customized rapid subtraction hybridization (RaSH) gene microarrays identify overlapping expression changes in human fetal astrocytes resulting from human immunodeficiency virus-1 infection or tumor necrosis factor-α treatment. Gene. 2003;306:67–78. doi: 10.1016/s0378-1119(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 3.Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC, Bruce JN, Volsky DJ, Fisher PB. Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacol Ther. 2007;114:155–70. doi: 10.1016/j.pharmthera.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikuno N, Shiina H, Urakami S, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–55. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–74. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Zhang N, Song LB, et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res. 2008;14:3319–26. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
- 8.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–21. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 9.Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci USA. 2006;103:17390–95. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, Fisher PB. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66:1509–16. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res. 2008;68:1478–84. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- 12.Takada K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 13.Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- 14.Manthey CL, Perera PY, Henricson BE, Hamilton TA, Qureshi N, Vogel SN. Endotoxin-induced early gene expression in C3H/H3J (Lpsd) macrophages. J Immunol. 1994;153:2653–63. [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–25. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 16.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–37. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Sato S, Hemmi H, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;21:324–29. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 18.Hassan F, Islam S, Tumurkhuu G, Naiki Y, Koide N, Mori I, Yoshida T, Yokochi T. Intracellular expression of toll-like receptor 4 in neuroblastoma cells and their unresponsiveness to lipopolysaccharide. BMC Cancer. 2006;8:281–88. doi: 10.1186/1471-2407-6-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chase MA, Wheeler DS, Lierl KM, Hughes VS, Wong HR, Page K. Hsp72 induces inflammation and regulates cytokine production in airway epithelium through a TLR4- and NF-kappaB-dependent mechanism. J Immunol. 2007;179:6318–24. doi: 10.4049/jimmunol.179.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pazin MJ, Sheridan PL, Cannon K, Cao Z, Keck JG, Kadonaga JT, Jones KA. NF-kappa B-mediated chromatin reconfiguration and transcriptional activation of the HIV-1 enhancer in vitro. Genes Dev. 1996;10:37–49. doi: 10.1101/gad.10.1.37. [DOI] [PubMed] [Google Scholar]
- 21.Perkins ND, Edwards NL, Duckett CS, Agranoff AB, Schmid RM, Nabel GJ. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993;12:3551–58. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perkins ND, Agranoff AB, Pascal E, Nabel GJ. An interaction between the DNA-binding domains of RelA (p65) and Sp1 mediates human immunodeficiency virus gene activation. Mol Cell Biol. 1994;14:6570–83. doi: 10.1128/mcb.14.10.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Murphy TJ, Choileain NN, Zang Y, Mannick JA, Lederer JA. CD4− CD25− regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174:2957–63. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 25.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–83. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 26.Ayala A, Chung CS, Lomas JL. Shock-induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Am J Pathol. 2002;161:2283–94. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]