Abstract
Berylliosis is driven by the accumulation in the lung of beryllium-specific T helper type 1 (Th1) cells recognizing beryllium as antigen when presented principally by human leucocyte antigen DP molecules carrying a glutamate at position β69 (HLA-DPGlu69). This study was designed to clarify the precise role of peptides in beryllium binding to the HLA-DP groove’s pocket 4 and to identify peptides with higher affinity for pocket 4 that might prevent beryllium presentation and T-cell stimulation. Beryllium/HLA-DP interactions were analysed by the ability of beryllium to compete with CLIP and CLIP-derived peptides to HLA-DPGlu69 soluble molecule. The CLIP-derived low-affinity peptide CLIP-AA, could not outcompete beryllium; while the CLIP-derived high-affinity peptides CLIP-YY, CLIP-QY and CLIP-RF were only marginally influenced by the presence of beryllium in the competition assay. The effect of these CLIP-derived high-affinity peptides on beryllium presentation was determined by measuring interferon-γ (IFN-γ) release upon beryllium stimulation of peripheral blood mononuclear cells obtained from beryllium-hypersensitive subjects. CLIP-YY did inhibit beryllium presentation and T-cell activation, while CLIP-QY and CLIP-RF markedly enhanced the IFN-γ response to beryllium. Anti-HLA-DP monoclonal antibody blocked the beryllium-induced IFN-γ release in the presence of CLIP-QY (88%) and CLIP-RF (76%). A similar effect was observed for CLIP-YY capability to block IFN-γ release by beryllium stimulation in the presence of CLIP-QY (79%) and CLIP-RF (76%). Overall, these data support the proposal that HLA-DP high-affinity peptides might be used as a model for specific berylliosis therapy.
Keywords: antigen presentation, berylliosis, beryllium, CLIP, human leucocyte antigen, human leucocyte antigen-DP, immunogenetic, peptide-based therapy, T cells
Introduction
Berylliosis is a chronic granulomatous disorder of the lung maintained by the exaggerated accumulation in the lower respiratory tract of activated, CD4+ effector memory T cells recognizing beryllium as a specific antigen/hapten;1–4 it affects 1–16% of beryllium-exposed individuals.4 Berylliosis is associated with the human leucocyte antigen DP supratypic variant HLA-DPGlu695–13 and, in HLA-DPGlu69-negative subjects, with the HLA-DR variant HLA-DRPhe47,13 where genetic and exposure factors interact in a supramultiplicative manner in the determination of susceptibility.6 The HLA-DPGlu69 molecules are responsible for antigen presentation of beryllium to T cells and for the higher T-cell proliferation rates of HLA-DPGlu69-expressing subjects in response to beryllium.14–17 As HLA-DPGlu69 is expressed in over 80% of berylliosis patients, these molecules are responsible for the reaction to beryllium in the large majority of affected subjects.2,5–13
As a result of the properties of the HLA-DP pocket 4, which carries an electron donor residue in the polymorphic position 69 (Glu69) and in the invariant positions 13 (Gln), 14 (Glu), 27 (Arg) and 28 (Tyr) of the β chain, beryllium binds to HLA-DPGlu69 molecules with significantly higher affinity than to HLA-DP-Lys69 molecules,18 the more frequent supratypic variant of HLA-DP.19 In the HLA-DP2 molecular model, the distances between the Glu69, Gln13, Glu14, Arg27 and Tyr28 residues span from 0·31 to 0·64 nm.20 Hence, they can be reasonably considered in sufficiently close proximity to co-ordinate directly the positively charged beryllium ion.21 Furthermore, as pocket 4 of the HLA-DPGlu69 molecule preferentially binds electron-donor amino acids such as Arg, Asn, Gln, His, Lys, Trp and Tyr,20,22,23 it is reasonable to hypothesize that the amino acid residues of peptides capable of locking into the pocket of an HLA-DPGlu69 molecule might contribute to beryllium binding in a similar way to that seen in the nickel model for the binding of nickel to invariant residues of the HLA-DR β-chain molecule.24,25
It has been debated whether beryllium binds directly to the HLA-DP or HLA-DR molecule or binds to protein peptides, either outside or inside the HLA groove’s pockets, hitherto forming a ‘beryllium antigen’26 suitable for binding by the HLA molecule and for presentation to T cells.
This study was designed to identify peptides capable of preferentially binding to the HLA-DP pocket 4 and to interfere with the binding of beryllium, or of a beryllium/peptide antigen, making it unavailable for antigen presentation and T-cell stimulation.
Materials and methods
Study population
Thirteen individuals with beryllium hypersensitivity were enrolled in the study after obtaining their informed consent and the approval of the Cleveland Clinic IRB. They comprised 11 males and two females, all Caucasians, with a mean age of 39 ± 6 years and an average time of employment of 9 ± 6 years. Ten of the 13 were HLA-DPGlu69-positive (Table 1) and were used to determine the effect of high-affinity peptides upon beryllium presentation. As controls, five normal unexposed subjects, four male and one female, all Caucasians, with a mean age of 31 ± 3 years were enrolled.
Table 1.
Beryllium hypersensitivity status and HLA class II typing of the study population
| HLA-class II typing |
||||||
|---|---|---|---|---|---|---|
| No. | Patient ID | Status | HLA-DR | HLA-DP | HLA-DQ | HLA-DPGlu69 |
| 1 | M.L. | Beryllosis | *01; *11 | *0201; *0401 | *02; *06 | Positive |
| 2 | H.P. | Berylliosis | *01; *03 | *0101; *0901 | *02; *05 | Positive |
| 3 | D.W. | Berylliosis | *07 | *0401; *1701 | *02 | Positive |
| 4 | A.C. | Berylliosis | *01 | *0201; *0301 | *05 | Positive |
| 5 | M.H. | Berylliosis | *03; *04 | *0201; *0401 | *02; *03 | Positive |
| 6 | W.Q. | Be-sensitized | *01; *13 | *0401; *0402 | *03; *05 | Negative |
| 7 | S.E. | Be-sensitized | *04; *11 | *0401 | *03 | Negative |
| 8 | J.C. | Be-sensitized | *15; *16 | *0401; *1001 | *02; *03 | Positive |
| 9 | M.L. | Be-sensitized | *13 | *0402; *1901 | *06 | Positive |
| 10 | R.S. | Berylliosis | *01 | *0301; *1001 | *05 | Positive |
| 11 | E.D. | Be-sensitized | *07; *15 | *0601; *1401 | *02; *03 | Positive |
| 12 | D.W. | Be-sensitized | *03; *04 | *0401 | *02; *03 | Negative |
| 13 | T.C. | Be-sensitized | *04; *13 | *0201; *0401 | *03; *06 | Positive |
HLA typing
HLA-class II typing was carried out in all patients and controls as previously described.8,13
Reagents
Monoclonal antibodies (mAb) directed against HLA-DR (L243),13 HLA-DP (B7/21),13 HLA-DQ (L2),13 HLA-class I (W6/32)13 and the 19 000 molecular weight Mycobacterium tuberculosis protein (HYT6)13 were purified from culture supernatants.
Invariant chain-derived peptide, CLIP, and its variants used in the study (Table 2), either with free amino acid termini or in biotinylated form, were obtained at > 90% purity from Advanced Biotech (Bergamo, Italy). Peptides were designed based on our previous study20 for obtaining peptides presenting different affinities for pocket 4 and 6 of HLA-DPGlu69 molecules. In particular, one peptide with reduced affinity (CLIP-AA) and three peptides with increased affinity (CLIP-QY, CLIP-RF and CLIP-YY) for HLA-DPGlu69 were used.
Table 2.
Characteristics of the peptides designed for the study
| Name | Sequence | Details and use in the study |
|---|---|---|
| Bt-CLIP | Bt-PKPPKPVSKMRMATPLLMQA | Ii 82–101, reference biotinylated CLIP peptide |
| Bt-CLIP-AA | Bt-PKPPKPVSKMRMATALLMQA | Reference biotinylated CLIP-AA peptide |
| Bt-CLIP-RF | Bt-PKPPKPVSKMRMRTFLLMQA | Reference biotinylated CLIP-RF peptide |
| Bt-CLIP-QY | Bt-PKPPKPVSKMRMQTYLLMQA | Reference biotinylated CLIP-QY peptide |
| Bt-CLIP-YY | Bt-PKPPKPVSKMRMYTYLLMQA | Reference biotinylated CLIP-YY peptide |
| CLIP | SKMRMATPLLMQA | CLIP peptide |
| CLIP-AA | SKMRMATALLMQA | Mutated (P6: Pro→Ala) CLIP peptide with reduced affinity for HLA-DPGlu69 molecules |
| CLIP-RF | SKMRMRTFLLMQA | Mutated (P4: Ala→Arg; P6 Pro→Phe) CLIP peptide with higher affinity for HLA-DP DPGlu69 molecules |
| CLIP-QY | SKMRMQTYLLMQA | Mutated (P4: Ala→Gln; P6 Pro→Tyr) CLIP peptide with higher affinity for HLA-DPGlu69 molecules |
| CLIP-YY | SKMRMYTYLLMQA | Mutated (P4: Ala→Tyr; P6 Pro→Tyr) CLIP peptide with higher affinity for HLA-DPGlu69 molecules |
| TT30 | FNNFTVSFWLRVPKVSASHLE | Tetanus toxoid 947–967; HLA-DP stabilizing peptide |
Bt, biotin.
Soluble HLA-DP2 and HLA-DP2K69 molecules were produced in soluble form in Drosophila melanogaster Schneider 2 as previously described.18,20
Beryllium-peptide competition tests
Competition assays using HLA-DP soluble molecules were performed as previously described.18,20 Briefly, 0·5 μg soluble HLA-DP was incubated overnight with biotinylated peptide in the presence of increasing amounts of the appropriate non-biotinylated competitor peptide, of CLIP peptide or of BeSO4 at 0·3–300 mm in 20 mm sodium acetate/150 mm NaCl pH 5·0 or 20 mm phosphate buffer/150 mm NaCl pH 7·5, containing 1 mm phenylmethylsulphonyl fluoride. To terminate the assays, 50 ml 2 × phosphate-buffered saline containing 3% bovine serum albumin was added to each reaction tube.
The amount of biotinylated peptide bound on the HLA-DP molecules at the end of the competition assays was determined as already described18,20 using an enzyme-linked immunosorbent assay (ELISA) using the HLA-DP specific mAb B7/21 as capture agent. Values of 50% inhibitory concentration (IC50), i.e. the concentrations of the competitor peptide or of beryllium required to compete for 50% of maximum binding of the biotinylated peptide, were calculated using the least squares fit methods of the titration data with the Graph Pad Prism software (GraphPad Software Inc., San Diego, CA).
Lymphocyte proliferation blocking assays
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by density centrifugation on a Ficoll–Hypaque gradient. The PBMCs (0·5 × 105 cells/well) were then plated in 96-well round-bottomed microtitre plates in RPMI-1640 tissue culture medium [2 mm l-glutamine, 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin in the presence of beryllium sulphate (BeSO4*4H2O) at 2, 10 and 50 μm (all reagents form Sigma Co., St Louis, MO)] as previously described.13 Purified mAb were used at increasing concentrations (10, 20 and 50 μg/ml) to inhibit lymphocyte proliferation and cytokine production as previously described.13
Free amino acid termini peptides designed for competing with beryllium in antigen presentation to beryllium-specific T lymphocytes were used at increasing concentrations (0·4, 2, 10, 50, 250 μm).
The levels of interferon-γ (IFN-γ) released in the culture supernatants of beryllium-stimulated PBMCs were measured in triplicate on supernatants collected after 5 days and frozen at −80° with commercially available solid-phase, two-site ELISA kits (Pierce-Endogen, Woburn, MA) and the results were expressed as the means of triplicate cultures.
Statistical analysis
All the data are expressed as mean ± standard deviation of the mean (SD). Comparisons between groups were made using Student’s t-test.
Molecular modelling
Modelling of the HLA-DP molecule(s) with bound beryllium and peptides was carried out using InsightII/Discover (Accelrys, San Diego, CA) as previously described with minor modifications.20,22
Results
Competition assays in the HLA-DP soluble molecule system
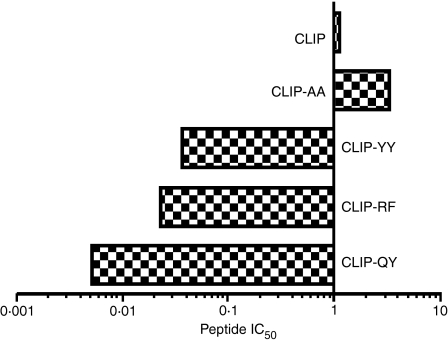
The interaction between the HLA-DPGlu69 molecule, beryllium and peptides presenting variable affinities for pocket 4 of HLA-DPGlu69 molecules, was analysed by measuring the ability of beryllium to compete with peptides for HLA-DP binding at pH 5·0, i.e. the acidic microenvironment where peptides are loaded onto HLA class II molecules. To this end, a panel of CLIP-derived peptides [CLIP-AA (presenting a Pro to Ala modification in P6), CLIP-YY (presenting an Ala to Tyr modification in P4 and a Pro to Tyr modification in P6), CLIP-RF (presenting an Ala to Arg modification in P4 and a Pro to Phe modification in P6) and CLIP-QY (presenting an Ala to Gln modification in P4 and a Pro to Tyr modification in P6)] was used, designed to display varying affinities for pockets 4 and 6 of the HLA-DPGlu69 molecule. As expected,20,22,23 CLIP-AA (IC50 versus CLIP = 3·50) showed decreased affinity compared to the CLIP peptide while CLIP-YY (IC50 versus CLIP = 0·035), CLIP-RF (IC50 versus CLIP = 0·022) and CLIP-QY (IC50 versus CLIP = 0·005) showed increasing affinity for HLA-DP2 (Fig. 1).
Figure 1.
Competition assay between biotinylated CLIP and CLIP-derived peptides (CLIP-AA, CLIP-YY, CLIP-QY and CLIP-RF) for the HLA-DPGlu69 soluble molecules. The IC50 values are shown for each peptide as μm values on the abscissa of each panel. The ordinate is drawn through the point of equimolar competition with the biotinylated CLIP peptide (1 μm). The bars extending to the left of the vertical axis therefore represent CLIP mutated peptides that bind with higher affinity than biotinylated CLIP; bars extending to the right represent CLIP mutated peptides that bind with lower affinity than biotinylated CLIP. The IC50 values obtained in the competition assays tests at pH 5·0 with an increased amount of CLIP-derived peptides (CLIP-AA, CLIP-YY, CLIP-QY, CLIP-RF) with respect to a fixed amount of biotinylated-CLIP peptide on HLA-DP2-soluble molecule are shown.
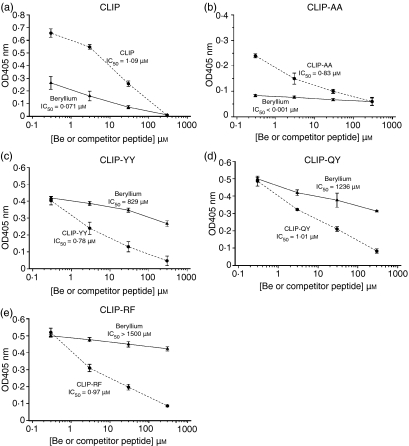
To assess the ability of CLIP-derived peptides to interfere with the binding of beryllium to the HLA-DP molecule, soluble HLA-DP molecules were incubated in the presence of a concentration of biotinylated CLIP-derived peptide capable of providing 90% of the maximum binding at pH 5·0 and in the presence of increasing amounts of either the appropriate non-biotinylated CLIP-derived peptide or of BeSO4 (0·3–300 μm) as competitors. As already shown,18 BeSO4 was capable of competing with biotinylated-CLIP (10 μm) for the binding to sHLA-DPGlu69 because it displaced CLIP from sHLA-DPGlu69 (BeSO4, IC50 versus CLIP = 0·07 μm; non-biotinylated CLIP, IC50 versus CLIP = 1·09 μm; Fig. 2a). Similarly, beryllium was capable of competing with the CLIP-derived, low-affinity peptide CLIP-AA (50 μm) for HLA-DPGlu69 binding (BeSO4, IC50 versus CLIP-AA < 0·001 μm; non-biotinylated CLIP-AA, IC50 versus CLIP-AA = 0·83 μm; Fig. 2b). In contrast, the binding of the CLIP-derived high-affinity peptides CLIP-YY (BeSO4, IC50 versus CLIP YY = 829 μm; non-biotinylated CLIP-YY, IC50 versus CLIP-YY = 0·78 μm; Fig. 2c), CLIP-QY (BeSO4, IC50 versus CLIP-QY = 1236 μm; non-biotinylated CLIP-QY, IC50 versus CLIP-QY = 1·01 μm; Fig. 2d) and CLIP-RF (BeSO4, IC50 versus CLIP-RF > 1500 μm; non-biotinylated CLIP-RF, IC50 versus CLIP-RF = 0·97 μm; Fig. 2e) were only marginally influenced by the presence of beryllium as indicated by the 11 843-fold to > 21 429-fold greater affinities for the HLA-DP molecules than the CLIP peptide. When the competition assays were replicated using the soluble HLA-DP2Lys69 molecule, i.e. the allelic counterpart of HLA-DPGlu69, no beryllium interference with the binding of the CLIP or the CLIP-derived peptides was observed (data not shown).
Figure 2.
Analysis of the ability of CLIP derived high-affinity peptides to compete for soluble HLA-DPGlu69 with beryllium in vitro. Peptide and BeSO4 competition curves generated in the presence of fixed amounts of biotinylated CLIP, CLIP-AA, CLIP-YY, CLIP-QY and CLIP-RF are shown (a–e). Data points represent the mean (± SD) of three separate experiments. In each graph, BeSO4 competitions are represented using triangles and a continuous line, while CLIP (or CLIP-derived) peptide competitions are represented using circles and a dashed line.
Interference of CLIP-derived peptides with beryllium-stimulated T-cell activation
To assess the functional consequences of the ability of the high-affinity CLIP-derived peptides to compete with beryllium for HLA-DP binding, it was important to determine whether the same peptides could interfere with beryllium binding to antigen-presenting cells expressing the HLA-DPGlu69 molecule and modulate the ensuing beryllium-stimulated T-cell activation and proliferation in berylliosis-affected subjects carrying HLA-DPGlu69 (PBMCs baseline response: BeSO4 2 μm: 59·14 ± 11·32 IFN-γ pg/ml, BeSO4 10 μm: 138·30 ± 13·9 IFN-γ pg/ml, BeSO4 50 μm: 107·24 ± 45·6 IFN-γ pg/ml, and no stimulus: 10·8 ± 7·2 IFN-γ pg/ml).
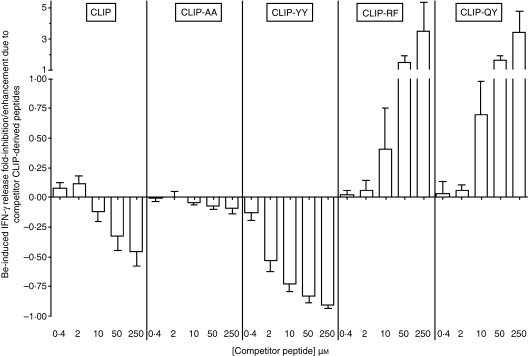
For each of the mononuclear cell samples, the effect of peptides upon antigen presentation was evaluated at the optimal BeSO4 concentration (varying from 10 to 50 μm for the study mononuclear cell samples) in the presence of five peptide concentrations (0·4, 2, 10, 50 and 250 μm). Consistent with peptide-binding data, CLIP-AA did not exert any modulatory activity upon beryllium-stimulated T-cell activation, while CLIP exerted only minimal inhibitory activity at the highest doses (Fig. 3).
Figure 3.
Analysis of the ability of CLIP-derived peptides to interfere with T-cell stimulation by beryllium presented in the context of the HLA-DPGlu69 molecule using fresh blood mononuclear cells from individuals with berylliosis. The interference [inhibition or augmentation of beryllium-stimulated interferon-γ (IFN-γ)release] curves obtained with the CLIP, CLIP-AA, CLIP-YY, CLIP-QY and CLIP-RF peptides are shown. Each bar represents the mean (± SD) of triplicates generated from each of five study subjects, using the individual’s optimal BeSO4 concentration.
In contrast, the high-affinity CLIP-derived CLIP-YY peptide markedly inhibited beryllium-stimulated T-cell activation from 0·13-fold to 0·94-fold in a dose-dependent manner (Fig. 3).
Strikingly, the CLIP-derived peptides endowed with the higher affinities for HLA-DPGlu69 molecules, CLIP-QY and CLIP-RF, markedly enhanced the IFN-γ response to beryllium (from 0·13-fold to 7·51-fold for CLIP-QY and from 0·08-fold to 10·68-fold for CLIP-RF) at doses that were equimolar with those of the stimulant and, importantly, they did so in a dose-related manner (Fig. 3).
Specificity of the interference with T-cell activation of CLIP-derived peptides with or without beryllium stimulation
To assess whether CLIP-QY and CLIP-RF possessed either superantigenic or mitogenic properties, blood mononuclear cells from five beryllium-unexposed control donors were tested for T-cell activation in response to beryllium, CLIP-QY or CLIP-RF and in response to the combination of beryllium and peptides. In none of the control mononuclear cell samples, could IFN-γ release be induced either by BeSO4 alone (12·1 ± 4·3 IFN-γ pg/ml; no stimulus, 11·3 ± 7·0 IFN-γ pg/ml), by any of the peptides (CLIP 13·4 ± 2·2 IFN-γ pg/ml, CLIP-AA 15·1 ± 7·9 IFN-γ pg/ml, CLIP-YY 14·9 ± 6·3 IFN-γ pg/ml, CLIP-QY 12·3 ± 3·2 IFN-γ pg/ml and CLIP-RF 11·1 ± 2·7 IFN-γ pg/ml) or by BeSO4 in combination with either CLIP (14·1 ± 4·6 IFN-γ pg/ml), CLIP-AA (11·9±6·2 IFN-γ pg/ml), CLIP-YY (12·4 ± 4·1 IFN-γ pg/ml), CLIP-QY (11·4 ± 5·6 IFN-γ pg/ml) or CLIP-RF (12·8 ± 3·2 IFN-γ pg/ml).
In addition, to determine whether CLIP-QY and CLIP-RF enhanced beryllium-stimulated T-cell activation in the context of antigen presentation by HLA-DPGlu69, HLA isotype-specific mAbs were used in an inhibition assay as previously described.13,14 Strikingly, beryllium-stimulated (10 and 50 μm) IFN-γ release in the presence of the CLIP-QY (10 and 50 μm) or in the presence of the CLIP-RF (10 and 50 μm) peptides was inhibited by 88·2 ± 9·4% and by 75·4 ± 6·0%, respectively, by the anti-HLA-DP mAb B7/21. Minimal or no inhibitions were observed with mAbs against anti-HLA-DR (Be/CLIP-QY: 8·5 ± 3·9%; Be/CLIP-RF: 4·5 ± 3·9%, P = 0·012 compared to HLA-DP), anti-HLA-DQ (Be/CLIP-QY: 1·0 ± 9·1%; Be/CLIP-RF: 0·1 ± 7·6%, P = 0·006 compared to HLA-DP), anti-HLA-class I (Be/CLIP-QY: 2·6 ± 5·1%; Be/CLIP-RF: 4·1 ± 3·8%, P = 0·009 compared to HLA-DP) or the anti-MTB (Be/CLIP-QY: 0·1 ± 4·4%; Be/CLIP-RF:0·3 ± 4·5%, P = 0·005 compared to HLA-DP) (Fig. 4).
Figure 4.
Analysis of the HLA-DP restriction of beryllium-stimulated T-cell release of interferon-γ (IFN-γ) in the presence of the stimulation-augmenting peptides CLIP-QY and CLIP-RF. Cells were stimulated with CLIP-QY and CLIP-RF peptides at an optimal concentration of beryllium and peptide in the presence of monoclonal antibodies specifically reacting against HLA-DR, HLA-DP, HLA-DQ, HLA-class I and the Mycobacterium tuberculosis (Mtb) 19 000 molecular weight protein. Inhibition of IFN-γ release from beryllium-stimulated T cells in the absence of monoclonal antibody is shown.
Collectively, the data suggest that beryllium binding to HLA-DPGlu69 and presentation to T cells may require the presence of peptides carrying residues specifically interacting with beryllium and the electron donor amino acid residues lining pockets 4 and 6 of the HLA-DP molecules, thereby forming an antigenic beryllium/peptide complex.
Inhibition of beryllium-stimulated T-cell activation by the high-affinity CLIP-derived peptide CLIP-YY
Contrary to the CLIP-QY and CLIP-RF peptides, the high-affinity CLIP-derived CLIP-YY peptide markedly inhibited beryllium-stimulated T-cell activation. CLIP-YY inhibited beryllium-stimulated (10 and 50 μm) IFN-γ release at a peptide concentration below the molar concentration of beryllium (13·5 + 12·2% inhibition at 0·2 μm concentration; 53·0 + 18·7% inhibition at 2 μm concentration, 72·4 + 14·2% at 10 μm concentration and 83·1 + 11·1% at 50 μm concentration) (Fig. 3).
Furthermore, CLIP-YY was capable of blocking the presentation of beryllium/peptide complexes Be/CLIP-QY and Be/CLIP-RF irrelevant of the timing of coincubation. Preincubation with CLIP-YY followed by stimulation with optimal beryllium concentrations (10 μm) together with equimolar concentration of CLIP-QY or CLIP-RF inhibited T-cell activation by 3·8 ± 4·4% and 5·0 ± 3·5% at 0·4 μm CLIP-YY, 33·1 ± 19·1% and 38·0 ± 6·2% at 2 μm CLIP-YY, 57·2 ± 8·9% and 55·3 ± 14·2% at 10 μm CLIP-YY and 72·0 ± 9·4% and 73·1 ± 12·4% at 50 μm CLIP-YY, respectively. Strikingly, coincubation with the same concentrations of the CLIP-YY (10 μm) peptide inhibited T-cell activation induced by stimulation with beryllium (10 μm) and either CLIP-QY (10 μm) or CLIP-RF (10 μm) by 63·4 ± 19·6% and 62·4 ± 11·5% respectively (Fig. 5).
Figure 5.
Analysis of the ability of the CLIP-YY peptide to block the augmentation of beryllium-stimulated T-cell release of interferon-γ (IFN-γ) in the presence of the high-affinity peptides CLIP-QY and CLIP-RF. The graph shows the inhibition curves of IFN-γ release from T cells stimulated with BeSO4 (10 μm), CLIP-QY and CLIP-RF (10 μm) in the presence of different concentrations of CLIP-YY peptide. Each bar represents the mean (± SD) of triplicates generated from each study subject at the individual’s optimal concentration of BeSO4.
Discussion
Structure/function studies have indicated residue Glu69 of the HLA-DP molecule as the key site of beryllium binding and have suggested that beryllium might bind to HLA-DPGlu69 molecules by interacting with the glutamate residue in position 69 of the β chain.13–15,17,18 This residue could co-ordinate the bivalent beryllium cation together with the electron donor residues Asnα60, Hisβ9, Glnβ13, Gluβ14, Tyrβ28, Argβ27 and Argβ75, which all make up the inside of pocket 4 of HLA-DP. In an in silico simulation of beryllium-binding, the most stable beryllium/HLA-DPGlu69 complex was obtained by co-ordination of beryllium by residues Glnβ13, Gluβ14, Argβ27 and Gluβ69 in pocket 4 (Fig. 6a). However, neither computer modelling nor peptide competition assays could precisely determine whether beryllium binds directly, and exclusively, with the above amino acid residues in the HLA-DP pocket 4. It is therefore reasonable to hypothesize that peptides that express amino acid residues carrying electron donor groups in position P4, i.e. the position locking into pocket 4, may participate in the co-ordination of the beryllium ion by the glutamate residue in position 69 within pocket 4, hence stabilizing the beryllium/HLA-DPGlu69 complex, when bound to the HLA-DP groove.
Figure 6.
(a) Analysis of the HLA-DPGlu69 molecule and beryllium interaction in pocket 4 of HLA-DP2 molecule. The figure shown is the most stable HLA-DPGlu69/beryllium complex model among all the possible interaction models evaluated between HLA-DP groove electron donor groups and beryllium as ion. Specifically, the peptide-binding pocket 4 of HLA-DP2 is capable of co-ordinating beryllium by using its residues Glnβ13, Gluβ14, Argβ27 and Gluβ69. The HLA-DP α chain backbone is reported in grey coloured ribbon style, while the HLA-DP β-chain backbone is reported in light blue coloured ribbon. Amino acids are reported in stick style and coloured by atom (C: grey; O: red; N: blue; H: white). Beryllium is shown as van der Walls radius and coloured green. (b) Analysis of the HLA-DPGlu69 molecule and CLIP-RF interaction in pocket 4 of the HLA-DP2 molecule. The figure shows the Arg94 of the CLIP-RF peptide buried in pocket 4 of HLA-DP2. This amino acid does not form an H-bond with the Gluβ14 or Glnβ13 and together with Argβ27 and Gluβ69, which are interacting with H-bonds, are representing an electron donor environment that could determine beryllium co-ordination. The CLIP-RF backbone is shown in green. The HLA-DP surface is shown as semi-transparent and the electron donor amino acids in pocket 4 are shown in stick style coloured by atom (C: grey; O: red; N: blue; H: white). The H-bonds are shown in green. (c) Analysis of the HLA-DPGlu69 molecule and CLIP-YY interaction in pocket 4 of the HLA-DP2 molecule. The figure shows the Tyr94 of the CLIP-YY peptide deeply buried in pocket 4 of HLA-DP2 interacting by H-bond with the Gluβ14. In this way both these electron donor amino acids are unavailable for interacting with beryllium and the Glnβ13 is too far from Argβ27 and Gluβ69 to co-ordinate beryllium. Argβ27 and Gluβ69 are interacting with H-bond. The CLIP-YY backbone is shown in green. The HLA-DP surface is shown as semi-transparent and electron donor amino acids in pocket 4 are shown in stick style coloured by atom (C: grey; O: red; N: blue; H: white). H-bonds are shown in green.
Current concepts of the pathogenesis of metal hypersensitivity are that metals bind to HLA molecules through interaction with peptides endowed with high affinity both for the metal and the HLA peptide binding groove.24–26 Consistent with this notion and with the observed ability of ferritin to augment beryllium binding and presentation to blood mononuclear cells from beryllium-sensitized individuals,27 the above hypothesis is strongly supported by the finding that the CLIP-QY and the CLIP-RF peptides, carrying strong electron-donor residues in P4, can augment beryllium presentation. In particular, CLIP-RF, which carries an alanine to arginine (a stronger electron donor group) residue substitution in P4, can significantly augment beryllium presentation, lprobably through increased binding affinity of beryllium for the pocket 4 of HLA-DPGlu69 as outlined in Fig. 6(b).
Further support for this notion is lent by the ability of the CLIP-YY peptide to inhibit antigen presentation of beryllium to sensitized T cells: the alanine to tyrosine (a weaker electron donor than the arginine of CLIP-RF and the glutamine of CLIP-QY) substitution in pocket 4 of CLIP-YY endows the modified peptide with higher affinity for pocket 4 while lowering its electron donor capability. Importantly, the CLIP-YY peptide, by entering deeply into pocket 4 where it can interpose between the electron donor residues (Argβ27, Glnβ13, Gluβ14 and Gluβ69) can prevent beryllium binding and block beryllium presentation (Fig. 6c). As a result, CLIP-YY can block T-cell activation at doses 100-fold lower than those required from unmodified CLIP, even in the presence of peptides with a similar affinity for pocket 4.19,20,22
Altogether, as these findings suggest that the inhibition of beryllium presentation observed with the CLIP-YY peptide is the likely consequence of the displacement of the bound peptide co-ordinating beryllium within the pocket 4 of HLA-DP, the data support the concept that beryllium presentation may be mediated by selected peptides expressing amino acid residues with high affinity for pocket 4 of the HLA-DP molecule and with high electron donor capability.
These finding have potential clinical implications. Treatment of berylliosis relies today upon the use of corticosteroids and, eventually, lung transplantation. Recent studies, confirming the pivotal functional role of the disease-associated HLA-DPGlu69 molecule have suggested that the ability of anti-HLA-DP antibodies to block the disease process in vivo could eventually be exploited for treatment purposes, as with antibodies against the accessory molecule lymphocyte function-associated antigen 3.28 Chelation of beryllium has also been proposed for blocking presentation of beryllium to T cells and for preventing T-cell activation and the ensuing inflammatory damage.21 Although clinical experience with therapeutic peptides is limited to the glatiramer acetate in multiple sclerosis29 and to cat and bee venom allergy models,30 it is conceivable that blocking peptides may work with greater specificity than chelating agents and greater versatility than anti-major histocompatibility complex antibodies, hence they may represent an interesting treatment model.
Acknowledgments
This study was supported in part by the US Department of Energy (DoE) grants DE-FG02-93ER61714 and DE-FG02-ER63416, by the MIUR-PRIN (Italy) and by a grant from ‘I Guzzini’. F.B. was supported by an ‘I Guzzini’ foundation postdoctoral fellowship.
References
- 1.Saltini C, Kirby M, Trapnell BC, Tamura N, Crystal RG. Biased accumulation of T lymphocytes with “memory”-type CD45 leukocyte common antigen gene expression on the epithelial surface of the human lung. J Exp Med. 1990;171:1123–30. doi: 10.1084/jem.171.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saltini C, Winestock K, Kirby M, Pinkston P, Crystal RG. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T cells. N Engl J Med. 1989;320:1103–9. doi: 10.1056/NEJM198904273201702. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot AP, Canavera SJ, Gharavi L, Newman LS, Kotzin BL. Target organ localization of memory CD4(+) T cells in patients with chronic beryllium disease. J Clin Invest. 2002;110:1473–82. doi: 10.1172/JCI15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreiss K, Miller F, Newman L, Ojo-Amaize EA, Rossman M, Saltini C. Chronic beryllium disease. From the workplace to cellular immunology, molecular immunogenetics, and back. Clin Immunol Immunopath. 1994;71:123–9. doi: 10.1006/clin.1994.1061. [DOI] [PubMed] [Google Scholar]
- 5.Richeldi L, Sorrentino R, Saltini C. HLA-DPB1 glutamate 69: a genetic marker of beryllium disease. Science. 1993;262:242–4. doi: 10.1126/science.8105536. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, Kreiss K, Mroz MM, Zhen B, Tartoni P, Saltini C. Interaction of genetic and exposure factors in the prevalence of berylliosis. Am J Ind Med. 1997;32:337–40. doi: 10.1002/(sici)1097-0274(199710)32:4<337::aid-ajim3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, White PS, Petrovic M, et al. Differential susceptibilities to chronic beryllium disease contributed by different Glu69 HLA-DPB1 and -DPA1 alleles. J Immunol. 1999;163:1647–53. [PubMed] [Google Scholar]
- 8.Saltini C, Richeldi L, Losi M, et al. Major histocompatibility locus genetic markers of beryllium sensitization and disease. Eur Respir J. 2001;18:677–84. doi: 10.1183/09031936.01.00106201. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Farris GM, Newman LS, Shou Y, Maier LA, Smith HN. Beryllium sensitivity is linked to HLA-DP genotype. Toxicology. 2001;165:27–38. doi: 10.1016/s0300-483x(01)00410-3. [DOI] [PubMed] [Google Scholar]
- 10.Rossman MD, Stubbs J, Lee CW, Argyris E, Magira E, Monos D. Human leukocyte antigen Class II amino acid epitopes: susceptibility and progression markers for beryllium hypersensitivity. Am J Respir Crit Care Med. 2002;165:788–94. doi: 10.1164/ajrccm.165.6.2104002. [DOI] [PubMed] [Google Scholar]
- 11.Maier LA, McGrath DS, Sato H, et al. Influence of MHC class II in susceptibility to beryllium sensitization and chronic beryllium disease. J Immunol. 2003;171:6910–8. doi: 10.4049/jimmunol.171.12.6910. [DOI] [PubMed] [Google Scholar]
- 12.McCanlies EC, Ensey JS, Schuler CR, Kreiss K, Weston A. The association between HLA-DPB1Glu69 and chronic beryllium disease and beryllium sensitization. Am J Ind Med. 2004;46:95–103. doi: 10.1002/ajim.20045. [DOI] [PubMed] [Google Scholar]
- 13.Amicosante M, Berretta F, Rossman M, et al. Identification of HLA-DRPhebeta47 as the susceptibility marker of hypersensitivity to beryllium in individuals lacking the berylliosis-associated supratypic marker HLA-DPGlubeta69. Respir Res. 2005;6:94. doi: 10.1186/1465-9921-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lombardi G, Germain C, Uren J, et al. HLA-DP allele-specific T cell responses to beryllium account for DP-associated susceptibility to chronic beryllium disease. J Immunol. 2001;166:3549–55. doi: 10.4049/jimmunol.166.5.3549. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot AP, Torres M, Marshall WH, Newman LS, Kotzin BL. Beryllium presentation to CD4+ T cells underlies disease-susceptibility HLA-DP alleles in chronic beryllium disease. Proc Natl Acad Sci USA. 2000;97:12717–22. doi: 10.1073/pnas.220430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amicosante M, Deubner D, Saltini C. Role of the berylliosis-associated HLA-DPGlu69 supratypic variant in determining the response to beryllium in a blood T-cells beryllium-stimulated proliferation test. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:175–9. [PubMed] [Google Scholar]
- 17.Bill JR, Mack DG, Falta MT, et al. Beryllium presentation to CD4+ T cells is dependent on a single amino acid residue of the MHC class II beta-chain. J Immunol. 2005;175:7029–37. doi: 10.4049/jimmunol.175.10.7029. [DOI] [PubMed] [Google Scholar]
- 18.Amicosante M, Sanarico N, Berretta F, et al. Beryllium binding to HLA-DP molecule carrying the marker of susceptibility to berylliosis glutamate beta 69. Hum Immunol. 2001;62:686–93. doi: 10.1016/s0198-8859(01)00261-0. [DOI] [PubMed] [Google Scholar]
- 19.Castelli FA, Buhot C, Sanson A, et al. HLA-DP4, the most frequent HLA II molecule, defines a new supertype of peptide-binding specificity. J Immunol. 2002;169:6928–34. doi: 10.4049/jimmunol.169.12.6928. [DOI] [PubMed] [Google Scholar]
- 20.Berretta F, Butler RH, Diaz G, et al. Detailed analysis of the effects of Glu/Lys beta69 human leukocyte antigen-DP polymorphism on peptide-binding specificity. Tissue Antigens. 2003;62:459–71. doi: 10.1046/j.1399-0039.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- 21.Keizer TS, Sauer NN, McCleskey TM. Beryllium binding at neutral pH: the importance of the Be-O-Be motif. J Inorg Biochem. 2005;99:1174–81. doi: 10.1016/j.jinorgbio.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Diaz G, Amicosante M, Jaraquemada D, et al. Functional analysis of HLA-DP polymorphism: a crucial role for DPbeta residues 9, 11, 35, 55, 56, 69 and 84–87 in T cell allorecognition and peptide binding. Int Immunol. 2003;15:565–76. doi: 10.1093/intimm/dxg057. [DOI] [PubMed] [Google Scholar]
- 23.Diaz G, Canas B, Vazquez J, Nombela C, Arroyo J. Characterization of natural peptide ligands from HLA-DP2: new insights into HLA-DP peptide-binding motifs. Immunogenetics. 2005;56:754–9. doi: 10.1007/s00251-004-0735-5. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, Vollmer J, Moulon C, Weltzien HU, Marrack P, Kappler J. Components of the ligand for a Ni++ reactive human T cell clone. J Exp Med. 2003;197:567–74. doi: 10.1084/jem.20021762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamerdinger K, Moulon C, Karp DR, et al. A new type of metal recognition by human T cells: contact residues for peptide-independent bridging of T cell receptor and major histocompatibility complex by nickel. J Exp Med. 2003;197:1345–53. doi: 10.1084/jem.20030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amicosante M, Fontenot AP. T-cell recognition in chronic beryllium disease. Clin Immunol. 2006;121:134–43. doi: 10.1016/j.clim.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Sawyer RT, Day BJ, Fadok VA, et al. Beryllium-ferritin: lymphocyte proliferation and macrophage apoptosis in chronic beryllium disease. Am J Respir Cell Mol Biol. 2004;31:470–7. doi: 10.1165/rcmb.2004-0090OC. [DOI] [PubMed] [Google Scholar]
- 28.Chou YK, Edwards DM, Weinberg AD, et al. Activation pathways implicate anti-HLA-DP and anti-LFA-1 antibodies as lead candidates for intervention in chronic berylliosis. J Immunol. 2005;174:4316–24. doi: 10.4049/jimmunol.174.7.4316. [DOI] [PubMed] [Google Scholar]
- 29.Farina C, Weber MS, Meinl M, Wekerle H, Hohlfeld R. Glatiramer acetate in multiple sclerosis: update on potential mechanisms of action. Lancet Neurol. 2005;4:567–75. doi: 10.1016/S1474-4422(05)70167-8. [DOI] [PubMed] [Google Scholar]
- 30.Ali FR, Larche L. Peptide-based immunotherapy: a novel strategy for allergic disease. Expert Rev Vaccines. 2005;4:881–9. doi: 10.1586/14760584.4.6.881. [DOI] [PubMed] [Google Scholar]