Abstract
Background
Pharmacogenetics uses genetic variation to predict individual differences in response to medications and holds much promise to improve treatment of addictive disorders.
Objectives
To review how genetic variation affects responses to cocaine, amphetamine, and methamphetamine and how this information may guide pharmacotherapy.
Methods
We performed a cross-referenced literature search on pharmacogenetics, cocaine, amphetamine, and methamphetamine.
Results
We describe functional genetic variants for enzymes dopamine-beta-hydroxylase (DβH), catechol-O-methyltransferase (COMT), and dopamine transporter (DAT1), dopamine D4 receptor, and brain-derived neurotrophic factor (BDNF). A single nucleotide polymorphism (SNP; C-1021T) in the DβH gene is relevant to paranoia associated with disulfiram pharmacotherapy for cocaine addiction. Individuals with variable number tandem repeats (VNTR) of the SLC6A3 gene 3′-untranslated region polymorphism of DAT1 have altered responses to drugs. The 10/10 repeat respond poorly to methylphenidate pharmacotherapy and the 9/9 DAT1 variant show blunted euphoria and physiological response to amphetamine. COMT, D4 receptor, and BDNF polymorphisms are linked to methamphetamine abuse and psychosis.
Conclusions
Disulfiram and methylphenidate pharmacotherapies for cocaine addiction are optimized by considering polymorphisms affecting DβH and DAT1 respectively. Altered subjective effects for amphetamine in DAT1 VNTR variants suggest a ‘protected’ phenotype.
Scientific Significance
Pharmacogenetic-based treatments for psychostimulant addiction are critical for successful treatment.
Keywords: Gene variants, pharmacotherapies, drug therapy, stimulants, individualized therapy, gene-based therapeutics, polymorphisms, genetic variation, subjective effects, drug dependence, addiction psychiatry
INTRODUCTION
New technology in molecular biology, pharmacology, and genomics research has transformed the way pharmacotherapeutic medications are administered in clinical practice. Variability in drug pharmacokinetics and pharmacodynamics are largely influenced by an individual’s genetic makeup (1, 2). Thus, the goal of pharmacogenetics is to correlate drug response with genetic makeup. In theory, individualizing therapy based on genetic profile will optimize responses to therapies and help better define common “polygenetic” diseases into more discrete molecular-genetic disorders (3-6). Indeed, recent pharmacogenetic studies in clinical medicine confirm that individual’s genetic profile is a much better guide for drug choice that leads to improved clinical outcome (3-13). The present challenge however is integrating these advances into mainstream medicine (1, 14).
Initial pharmacotherapy choices for psychiatric diseases fail in 30-40% of patients and a large portion of responders may be due to the placebo effect (15, 16). Taking into account genetic individual variability can improve initial medication choices (17-19). For example, the hepatic cytochrome P450 system metabolizes a majority of psychiatric medications and shows 10% genetic variability (20). Considering this variability can help avoid producing high systemic drug levels that result in serious adverse side effects and noncompliance (18, 20-22). Other adverse side effects such as weight gain and dyskinesias are also linked to gene variants (23, 24).
Recent studies in psychiatry give clear evidence that tailoring drug treatment to a person’s genetic makup is critical to engendering a positive therapeutic response (25), thwarting dangerous side effects due to toxicity (17, 20, 21) and increasing compliance (18). Less attention however has been centered on drug dependence likely because addictions have fewer effective medications so less opportunity has arisen for pharmacogenetic studies. Drug addictions are common polygenic, chronic, relapsing brain disease whose pharmacotherapy would benefit from becoming more optimally defined through molecular-genetic approaches (15, 26-28). Clearly, not all that experiment with drugs of abuse become addicted. This heterogeneity of response to developing addiction can be attributed, in part, to genetically driven inter-individual differences (29, 30). Mutations in genetic code may be responsible for differences in the subjective effects of drugs of abuse making them either more rewarding or aversive depending on the individual’s genotype. Genetically divergent and genetically altered rats, mice (31-33) and human studies support this notion (30, 34, 35).
Gene profiling of individuals who respond divergently to drugs of abuse may help medication development or help guide present pharmacotherapy. For example, genetically driven alterations in enzymes (DβH, COMT), transporters (DAT1) receptors (D4) and neuropeptides (BDNF) alter psychostimulant effects and response to medications aimed at these targets. Thus, this review of pharmacogenetics extends our previous coverage of alcohol and opiates to psychostimulants and follows that review’s goal of identifying possible pharmacogenetic targets (36).
DRUG DEPENDENCE: NEUROBIOLOGICAL SUBSTRATES
The motivating effects of drugs of abuse are mediated through multiple sites and mechanisms in the brain but studies strongly support activation of the mesocorticolimbic dopamine (DA) system as key to mediating their reinforcing effects (32, 36) (33, 37-39). DAergic cell bodies in the ventral tegmental area (VTA) and their projections to the prefrontal cortex (PFC) and nucleus accumbens (NAC) define the core circuitry of this system whereas the amygdala, hippocampus and hypothalamus are associated with emotional memories with drug addiction (40-43). Further, drugs of abuse evoke DA release in the PFC and NAC and lesion studies of these brain areas alters their behavioral effects (44-47).
Figure 1 presents a detailed hypothetical scenario for a DA neuron and its target neuron. DA from terminal regions binds to a number of DA receptor subtypes called D1-like (D1, D5) or D2-like (D2, D3, D4) classified based on molecular and pharmacological attributes. Synaptic DA’s action is terminated by sequestration of the transmitter into the presynaptic neuron through the DA transporter (DAT) (48, 49). DAT levels are high in ventral midbrain and striatum but low in the PFC where the enzyme catechol-O-methyltransferase (COMT) inactivates released DA through enzymatic conversion (50-52). Activation of D1-like receptors leads to increasing amounts of the second messenger cAMP (cyclic adenosine 3′5′-monophosphate) through stimulation of adenylate cyclase via Gs stimulatory G-proteins whereas D2 activation acting through Gi inhibitory G-proteins decreases the formation of cAMP. The formation of cAMP is dependent upon adenylate cyclase and degraded by phosphodiesterase enzymes in the cytoplasm. Increased cAMP levels affect intracellular protein kinase A (PKA). The catalytic subunit of PKA (PKA-Cα) may translocate to the nucleus of the cell where it phosphorylates the transcription factor cAMP-dependent protein kinase (CREB) leading to CRE (cAMP response element)-mediated gene expression (53). PKA-Caα also phosphorylates dopamine and cAMP regulated phosphoprotein 32 kDa (DARPP-32) that has numerous intracellular roles in DAergic neurons and plays a critical role in DA signaling (54). Chronically administered drugs of abuse alter this intracellular cascade (53, 55). Genetically altered components of this system using modified mouse strains or other strategies can influence the behavioral effects of drugs (32). By extension, genetic differences in this system in humans strongly influence how an individual responds to drugs of abuse.
FIG. 1.
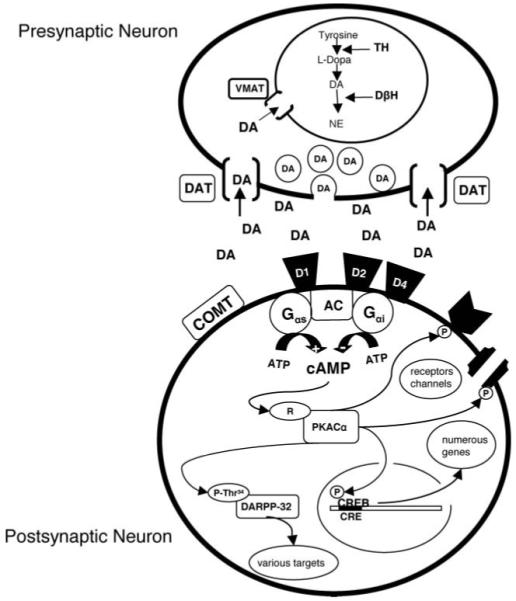
Hypothetical schematic of a DAergic synapse. Presynaptic neuron contains DA precursors leading to vesicular DA fusing with the presynaptic membrane and being released into the synaptic cleft. DA then stimulates DAergic receptors on the postsynaptic cell. DA is inactivated by being sequestered back into the presynaptic neuron by the DA transporter (DAT). DA may also be metabolized extra-synaptically by catechol-O-methyl transferase (COMT). DA present in the cytosol is taken up by the vesicular transporter (VMAT) for degradation and repackaging. Tyrosine hydroxylase is the rate-limiting enzyme in DA synthesis. Dopamine beta-hydroxylase (DβH) is responsible for the formation of norepinephrine (NE). Intracellular mechanisms associated with the cAMP-PKA-CREB pathway through DA D1-like and D2-like receptors are represented in the postsynaptic neuron. D1 stimulates through Gαs whereas D2 receptor activation inhibits cAMP levels via adenylate cyclase (AC) through Gαi G-proteins. cAMP enhances the dissociation of the regulatory subunit of proteins kinase A (PKA) from the catalytic subunit (Cα) causing activation. PKA-Cα may translocate to the nucleus where it phosphorylates cAMP-response element binding protein (CREB) that leads to cAMP response element (CRE)-mediated gene expression. PKA-Cα may phosphorylate other receptors or channels as well. This kinase also phosphorylates dopamine-and cAMP-regulated-phosphoprotein 32 kDa (DARPP-32) at position Thr34, that then leads to regulation of other intracellular proteins and effects on the neuron.
DOPAMINE NEUROTRANSMISSION AND DRUG ABUSE
Drug addiction is a general term encompassing the inability to cease drug-seeking and taking, possible incremental increases in drug intake or tolerance to the drugs pleasurable effects and physiological or psychological dependence. This drug intake continues despite deteriorating physical and mental health and social footing. Elicitation of a withdrawal syndrome involving physiological and/or psychological components upon drug discontinuation also signals drug dependence. Other important pharmacological aspects of drugs of abuse include cross-tolerance or tolerance to one substance that develops also to another substance usually in the same drug class. Similarly, cross-sensitization occurs when sensitization to one substance develops to a different drug also usually in the same class. Psychostimulants readily induce sensitization that is linked to DA and other neurotransmitters such as norepinephrine (NE) (56, 57). Sensitization is considered key in the development of drug dependence (58), can be demonstrated in humans (59) and is linked to craving associated with unconditioned and conditioned drug effects (58) and relapse (60).
Data suggest that DA is associated with ‘euphoria’ elicited by drugs of abuse. DA is also associated with conditioning; it increases the ‘salience’ or attractiveness of external stimuli associated with drug procurement and intake (40). Similar to rodents (33), humans willingly self-administer psychostimulants under controlled conditions (61-63) that activate reward circuitry that is linked to drug-induced euphoria (61, 64, 65). Human imaging studies link DAT and D2 receptor occupancy with cocaine and amphetamine-induced euphoria (66, 67). D1 (68) and D2 receptor antagonism (69, 70) or altering DA metabolism (71) blocks the pleasurable effects of these drugs to some extent.
COCAINE ADDICTION AND ITS PHARMACOTHERAPY
Cocaine addiction is a persistent problem in the United States. The Office of National Drug Control Policy estimates that approximately 3 million individuals chronically use cocaine and years of research and drug development has yielded no indicated treatments (15, 16). Cocaine blocks the re-uptake of a number of neurotransmitters including DA, NE and serotonin (5HT) (72). Yet, DA focused pharmacotherapies including DA antagonists as blocking agents, or direct DA agonists such as cabergoline (73) and bromocriptine (74) have shown limited success (15, 16, 74, 75). However, indirect actions on DA through other neurotransmitters like GABA, such as baclofen (76) and tiagabine (77) show promise. Metabolically altering DA levels (and blocking NE) through enzymatic inhibition may show the best efficacy for the treatment of cocaine addiction (78, 79). Enzymes involved in the production of DA (80) as well as those involved in cocaine metabolism are significantly affected by genetics (81). Most intriguing are recently identified polymorphisms that alter subjective responses to psychostimulants (34, 35). Genetic polymorphisms that are involved in coding DA-related proteins involved in its regulation may guide individualized pharmacotherapy for cocaine addiction. One example discussed below is the DβH inhibitor disulfiram.
COCAINE ADDICTION: DISULFIRAM PHARMACOTHERAPY
Disulfiram (DS) presumably treats alcohol dependence by inhibiting the enzyme aldehyde dehydrogenase leading to increased plasma levels of acetaldehyde upon drinking alcohol, a byproduct of alcohol metabolism that is aversive (82). DS also chelates copper (83) in turn, inhibiting the copper-containing glycoprotein enzyme DβH that catalyzes the conversion of DA to NE in peripheral and central NE-containing neurons (84). DβH inhibition leads to decreases in peripheral and central NE and increases in DA (85, 86). In humans, DS also inhibits plasma and microsomal carboxylesterases and plasma cholinesterase (87, 88) that inactivate cocaine systemically thereby increasing blood levels (88). This effect likely contributes to DS’s efficacy in treating cocaine addiction (89, 90).
Controlled clinical trials support the efficacy of DS for cocaine dependence. DS reduces self-reported cocaine use and positive cocaine urine toxicologies in primary cocaine addicts, alcoholics and opiate dependent subjects who also abuse cocaine, and addicts maintained on methadone or buprenorphine. (91-95). DS’s pharmacological effect is not specifically related to alcohol use (90). A meta-analysis of randomized controlled studies on the effectiveness of DS shows highly significant efficacy (P < 0.001). More recent studies also support DS as a significant treatment in primary cocaine abusers (79) and those with co-dependence (91, 93-96). Carroll et al. conducted a 12-week study and demonstrated that DS with psychotherapy decreased cocaine and alcohol use (91). A one-year follow-up analysis showed DS significantly reduced cocaine, but not alcohol use, suggesting the effect was specific (97). This result for cocaine was confirmed and extended by a more extensive study (79).
Early studies with DS alone showed that massive doses (up to 3 gm loading dose, and up to 1 gm/day for maintenance) induce paranoid psychosis in some patients (98). More recent studies of DS with cocaine showed that DS increases the aversive effects of cocaine such as nervousness that many cocaine abusers describe as “paranoia” (78). A subpopulation of cocaine addicts have a greater susceptibility to developing cocaine-induced paranoia, a characteristic that is genetically mediated (99). Although DS blocks enzymes that metabolize cocaine in the periphery, subjective measures of cocaine craving, ‘high’ or ‘rush’ associated with IV administered cocaine is reduced with DS treatment (78, 95, 100). This suggests that DS’s therapeutic effects may more likely be due to its ability to inhibit DβH (increasing DA levels and decreasing NE) rather than just its pharmacodynamic effects (increasing systemic levels of cocaine).
When DβH levels are low, DA is increased whereas NE is decreased. Mice that lack DβH show increased DA in the PFC and decreased NE. Treatment with disulfiram further increases DA in this region (85). Cocaine potently blocks DA reuptake increasing synaptic levels of the neurotransmitter as does DS via DβH inhibition. Thus, its reasonable to conclude that cocaine/DS combination treatment results in supra-physiological increases in DA. This effect may contribute to the aversive characteristics of the drug as supported by human (88) and rodent studies (57, 86, 101). Under controlled laboratory conditions, Cubells et al. showed cocaine and DS combinations induce intense dysphoric reactions including psychotic symptoms in patients (102) but only in certain individuals suggesting that this effect was due to genetic factors (99). Taken together, evidence supports that DS affects various behavioral effects of cocaine including blunting euphoria or ‘high’, neutralizing craving, or intensifying the dysphoric aspects of cocaine.
FUNCTIONAL SNP C-1021T AND DISULFIRAM ACTION
DβH is a synaptic vesicular enzyme that uses copper as a cofactor and oxygen and ascorbate as co-substrates (103). The enzyme converts DA into NE (104) and is co-released with catecholamines. Individuals genetically deficient in DβH usually perish at birth but those that have less severe forms exhibit physiological abnormalities namely, postural tachycardic syndrome or generalized autonomic failure because of lack of sympathetic tone (105-107). DβH is stable in plasma, correlates with cerebrospinal fluid (CSF) levels (108, 109), and is age-dependent (110). Based on twin and family studies, plasma levels are variable between unrelated individuals (111-113). These genetically driven differences are likely due to polymorphisms in close proximity to the DβH gene. Studies link the C-1021T (-1021C>T) SNP to differences in circulating DβH levels (102, 114-116) The SNP C-1021T is positioned ∼1 kb upstream from the initiation codon of the DβH gene (116). Several studies support the notion that the C-1021T SNP is a functional polymorphism that gives rise to altered transcription and decreased plasma levels of DβH (116-119) but other SNPs may also play a role (80, 120-122).
Decreased DβH attributed to C-1021T is found in diverse populations (European Americans, African Americans, Eastern Indian and Japanese) accounting for up to 52% of overall variation (116, 118, 119, 123). Those individuals that are T allele homozygous have the lowest levels of plasma DβH activity (106, 116, 118, 119, 123). Differences in DβH enzyme levels or activity is linked with a number of psychiatric disorders including schizophrenia (114, 124), psychotic depression (125), nicotine addiction (126), attention deficit hyperactivity disorder (ADHD) (127), post-traumatic stress disorder (128), conduct disorder (129-133), and neurological diseases involving DA neurotransmission such as Parkinson’s Disease (80, 134). The DA metabolite, homovanillic acid (HVA) found in CSF fluid is an indirect measure of monoamine concentration in the brain and is correlated with DβH C-1021T SNP genotype (135).
That C-1021T may be a functional polymorphism leading to decreased basal DβH levels has pharmacogenetic significance for treatment of cocaine dependence with DS. In individuals with already low levels of DβH enzyme, DS would be more effective in increasing DA and decreasing NE (e.g. heterozygous and more so for subjects homozygous for the T allele). Data from DβH deficient mice support this notion in that basal levels of DA in either +/- or -/- homozygous are increased two and five times respectively. DA:NE ratio is significantly reduced (approximately 35%) and DS pretreatment decreases NE and increases DA in wildtype and +/- mice (85). An imbalance in DA:NE in the PFC may underlie increased cocaine-induced paranoia in individuals with low levels of DβH attributed to the C-1021T SNP (80). This theory posits that DA:NE ratios may be elevated at baseline in those homozygous for the T allele. Cocaine, which potently increases DA levels, may lead to supra-physiological levels above that of cocaine alone contributing to cocaine-induced paranoia and psychosis. Consistent with this notion, controlled human experiments utilizing a ‘binge’ type cocaine self-administration paradigm show that subjects homozygous for the T allele (‘very low-activity’) in the DβH gene exhibit greater cocaine-induced paranoia compared to CT or CC genotypes (136, 137). Moreover, cocaine abusers that experience cocaine-induced paranoia (that is extremely aversive) are more prone to develop psychosis and to seek immediate medical treatment (138, 139). Preclinical studies in rats (101) and with genetically deficient DβH mice support this interpretation. Indeed, decreasing DβH through DS treatments or via genetic manipulation enhances the behavioral effects of cocaine and increases anxiety associated with the drug (57, 86). Specifically, cocaine aversion is enhanced in DβH deficient mice and cocaine-induced anxiety eliminated (57); the later effect appears to be mediated through beta-adrenergic receptors. In fact, DβH inhibition with DS facilitates cocaine-induced locomotor sensitization that may reflect enhanced aversiveness of the drug (101).
Taken together, SNP mapping profiles aimed at detecting DβH genotype may help predict whether an individual will respond well to DS treatment for cocaine dependence. That is, DS efficacy may be affected by an individual’s genotype. Individuals harboring the DβH C-1021T SNP TT allele likely would respond better to DS treatments and need less of a dose whereas CT allelic carriers would need an intermediate dose and those with the CC allele may need increased concentrations for maximum therapeutic effectiveness. Evidence to date indicates that DS appears to be a good example of a pharmacogenetic treatment for drug abuse. Future studies are needed to clarify the mechanisms involved, and the contributions of other SNPs implicated in altering DβH levels that may affect treatment outcome.
DOPAMINE RECEPTOR D2 (DRD2)
Literature strongly supports D2 receptor involvement in the reinforcing effects of drugs of abuse. Imaging studies show significantly reduced levels of these receptors in alcoholics (140), cocaine (141), methamphetamine (142), and heroin abusers (143). D2 receptors are also related to drug-induced euphoria (65, 144) and drug craving (145, 146). Individuals with a positive family history of alcoholism—a predisposing factor to developing the disease—that have higher D2 receptor levels, are protected from developing drug dependence (147). Intriguingly, positron emission tomography (PET) imaging studies in drug näive rats show that low D2 receptors in the ventral striatum (which includes the NAc) predicts impulsivity and greater drug self-administration behavior (148). Predetermined rat phenotypes that readily self-administer drugs of abuse suggests genetic factors are involved (31, 149). Inbred rat strains that have a proclivity to self-administer drugs of abuse across classes, have low D2 receptor density (149-151) and altered responses to DAergic agents (33). Similarly, genetic ablation of the D2 receptor in mice significantly decreases drug reward and responsivity (32, 152, 153). Accordingly, deficits in D2 receptors in some human drug abusers persist even after pro-longed periods of abstinence suggesting that these altered levels were not solely drug-induced (154). These data suggest an important role for genetic factors that affect D2 receptor availability and may predict inter-individual responses to drugs of abuse. Pharmacogenetic medications aimed at increasing D2 levels in those individuals genetically predetermined for low levels may prove beneficial.
DOPAMINE TRANSPORTER (DAT) SLC6A3 GENE
The DAT is encoded by the SLC6A3 gene (155) and sequesters DA back into the presynaptic neuron (see Figure 1) to modulate DAergic tone (48, 49). Cocaine strongly inhibits DAT that is related to its reinforcing effects (156, 157). Similarly, other psychostimulants, such as amphetamine and methamphetamine, have action at the DAT enhancing DA neurotransmitter release into the extracellular space potently increasing levels (158-160). Mice genetically deficient in DAT have altered responses to cocaine and amphetamine (161). The cocaine euphoria, or ‘high’ associated with self-administering the drug is correlated with DAT occupancy in humans (66). Thus, genetic alteration in DAT could contribute to altered sensitivity to cocaine in humans.
SLC6A3 comprises 15 exons spanning 60 kb on chromosome 5p15.32. The 3′-untranslated region (UTR) DAT1 polymorphism is a 40 bp VNTR with repeat copy numbers between 3-13 times with the 9 and 10-repeat allele being the most frequently found in the population (162-164). Unique to this polymorphism is that it is located in the untranslated region of the DAT1 gene but has been linked to disease phenotypes involving DA dysregulation. This suggests that it may be in linkage disequilibrium with another mutation that influences either gene expression or functional aspects of the transporter. Preclinical studies suggest that this VNTR alters levels of gene expression, yet it is unknown precisely at what level (165-167).
DAT VNTR AND THERAPEUTIC RESPONSE TO METHYLPHENIDATE: ADHD MODEL
ADHD is a common disorder of childhood and adolescence associated with persistent impairment in academic and social spheres (168, 169). Imaging studies generally show dysregulated DA-rich brain structures, altered presynaptic DA storage and abnormal DAT striatal density (170-175). Individuals with ADHD have a high rate of comorbid psychiatric illness with substance abuse occurring in 1 in 5 patients (168, 176). Methylphenidate (and its various formulations) is the drug of choice for the treatment of ADHD and binds to the DAT similar to cocaine (39, 66, 177).
Some studies (178-181) but not all (166, 182, 183) indicate that the VNTR polymorphism of the DAT1 is a risk factor for psychiatric diseases associated with drug abuse such as ADHD. Haplotype is a combination of alleles at two or more closely linked gene loci on the same chromosome. VNTR polymorphisms located in intron 8 and the other 3′ untranslated region (3′-UTR) of the gene and the 10-repeat 3′ UTR alleles together appear as a common DAT1 haplotype (10/3) (181). Intriguing data from Bellgrove et al. show in normal developing healthy children, the DAT1 genotype—in particular the 10- and 3-repeat allele markers-influences attentional spatial bias and neglect displayed as inattention for left-sided stimuli, characteristics that could be related to the ADHD phenotype (184). Whether this combination haplotype confers risk, or alters pharmacological interventions for cocaine addiction is unknown. One large study showed that alleles of SLC6A3 polymorphism 30 bp VNTR in intron 8 in patients addicted to cocaine and showed compromised function in in vitro reporter assays (185). Replication of this study in different subject populations is warranted.
Some studies show that ADHD individuals with the 10/10 genotype of the DAT1 VNTR show poor response to methylphenidate on clinical and physiological measures (186-191) whereas others have shown no significant response (192, 193) or better response (191, 194, 195) to methylphenidate. Although far from conclusive, VNTR polymorphisms in the DAT1 gene confer clinically relevant alterations that may affect response to methylphenidate, a possible treatment for cocaine addiction.
Methylphenidate treatment is associated with significantly greater frequency of cocaine-free urines compared to placebo controls in cocaine dependent individuals with ADHD (196). Methylphenidate also has been shown to block some of the subjective effects of cocaine and reduce free choice of cocaine over a monetary reward (197). Methylphenidate’s therapeutic action is unknown but likely involves increasing neurotransmission in the orbitofrontal cortex secondary to elevated DA in thalamic brain regions (39). Increased neurotransmission in the orbitofrontal cortex may contribute toward reversing the well-demonstrated finding of hypo-frontality in chronic cocaine dependent subjects (198). Restoring DAergic inhibitory influence from the prefrontal cortex on structures involved in affective drive (199) and executive functioning (200) may underlie normalizing compulsive drug-seeking behaviors.
Properly assessed genetically driven functional changes in the DAT could help determine which patient would benefit most from methylphenidate for cocaine addiction (32). Imaging studies indicate that the 9/9 and 9/10 genotypes are associated with differences in DAT availability in vivo. Some imaging groups find decreased DAT availability of 9/10 allele vs. 10/10 carriers (201) or no difference (202). However, our early collaborating studies using single photon emission tomography (SPECT) and the [123I] beta-CIT ligand highly selective D2 (and D3) receptor ligand noted a 13% increase in striatal binding in the 9/10 repeat heterozygotes compared with the 10 repeat homozygotes in normal non-diseased subjects (203). A more recent study confirmed these findings using the same SPECT ligand in normal subjects. SLC6A3 9 repeat carriers (in this study combined 9/9 and 9/10 genotypes) have significantly higher striatal DAT availability compared to 10 repeat homozygotes (204) suggesting that the SLC6A3 9 repeat genotype leads to increased DAT availability in striatum. Although in vitro studies give conflicting conclusions as to the extent to which variants regulate transcriptional activity or number of DAT proteins (165, 166, 202, 205), it is likely that altered DAT levels contribute to inter-individual differences in the ability of the transporter to clear DA from the synaptic space. Supra-physiological levels of orbitofrontal DA presumably contribute to psychotic symptoms such as paranoid ideation (206). Increased DA levels in this area are also linked to the euphoric effects of cocaine (177, 200). Thus, more DAT in the 9 repeat carriers would presumably sequester greater amounts of synaptic DA in turn reducing the initial pleasurable effects of drugs and perhaps ‘protecting’ the individual from developing drug addiction. This interpretation is consistent with the finding that euphoric effects of amphetamine are significantly decreased (35) in the 9 repeat carriers as is therapeutic response to methylphenidate (178, 195).
Taken together, conflicting results demonstrating altered DAT1 concentrations and/or function are difficult to interpret within the context of methylphenidate treatment for cocaine addiction. Evidence suggests however, that since the VNTR is in an untranslated region and-presently unknown to produce any functional protein-it may be a marker associated with another mutation in linkage disequilibrium that influences either gene expression or functional aspects of the transporter. The presence of ADHD in cocaine dependent subjects in tandem with genotype are important factors to consider in predicting therapeutic response for methylphenidate for cocaine.
AMPHETAMINE: MECHANISMS OF ACTION AND PHAMACOGENETICS
Amphetamine was first used commercially as an over-the-counter inhaler for nasal congestion and asthma (160, 207). Today, amphetamine is used medically for ADHD, narcolepsy with off label use for chronic fatigue syndrome, and adjunct therapy for anhedonia associated with refractory depression. Amphetamine and its derivatives, such as methamphetamine, are widely abused illicit drugs second only to cannabis.
Early studies show that responses vary greatly from one individual to another in response to the same dose of amphetamine (208-210). This heterogeneity of response may be due to a number of factors such as expectancies of the drug (211), psychiatric disease or personality type (212, 213), or gender differences (214). Yet, considerable evidence shows that individual variability in drug response is due to genetics (160). For example, polymorphisms DAT 3′UTR VNTR (35), COMT val158met (215), SLC6A2 NET intronic 36001 C/C (216), 5-HT transporter gene-linked polymorphic region and Intron 2 VNTR (217), adenosine receptor A2a 1976 C/T and 2592 C/Tins (218), casein kinase 1 epsilon (219) and BDNF G196A val66met (220) have all been implicated in altering the subjective effects of amphetamine.
Amphetamine’s primary effects are mediated by action on the DAT. Similar to cocaine and methamphetamine, amphetamine has strong effects on the DAT, reversing the transporter enhancing extraneuronal DA concentrations (158). As mentioned, the SLC6A3 3′-UTR DAT1 polymorphism VNTR, frequently found in the population (162-164), affects gene expression (221), results in altered central DAT levels (166, 201-204), and impacts subjective and therapeutic efficacy of the ADHD drug methylphenidate (178, 195). Similarly, the DAT gene is crucial to individual variability in response to amphetamine (35). Subjects with the 9/9 DAT1 variant report no euphoria, anxiety or “feel drug” responses to increasing doses of amphetamine compared to those with the 9/10 or 10/10 alleles. Homozygous 9/9 repeat allele carriers also have significant decreases in peripheral physiological measures, such as diastolic blood pressure. These data are consistent with studies showing that individuals who have the 9/9 repeat genotype do not respond to the ADHD medication methylphenidate (178, 195).
Initial pleasurable experiences with drugs of abuse predict future use and dependence (222, 223). By extension, subjects with the 9/9 allele experience less positive subjective effects of psychostimulants, in this case amphetamine, and may be “protected” from developing future psychostimulant abuse. Studies in genetically diverse inbred strains Lewis and Fischer 344 rats support this conjecture. Lewis rats (vs. F344 rats) have innate predisposition to self-administer a wide variety of drugs of abuse and have low baseline DAT levels and function (224). Haile et al. (2005) recently showed that the potent and selective DAT inhibitor RTI 336 decreases cocaine-induced activity and self-administration in Lewis but not F344 rats (225). That a highly selective DAT inhibitor was more effective in decreasing cocaine’s behavioral effects in a genotype known to have decreased DAT levels supports this as a possible therapeutic target. Future studies determining if the SLC6A3 3′-UTR DAT1 VNTR polymorphism is a useful phamacogenetic marker for medications to treat amphetamine and cocaine abuse in humans are warranted.
METHAMPHETAMINE
Methamphetamine (METH) is a potent and highly addictive psychostimulant (160). METH efficiently penetrates the CNS (226) evoking the release of high concentrations of monoamines, in particular DA, through multiple mechanisms (158). The half-life of cocaine is 90 minutes whereas METH is upwards of 10 hours (227). Abusers tend to self-administer the drug in “binges” or “runs” lasting 1-3 days or more followed by abstinence then repeated abuse. Because of METH’s lengthy half-life, ‘binge’ administration results in increasing concentrations of the drug within the system that can be toxic particularly because minimal tolerance occurs to its peripheral and central effects (228, 229).
Acute administration of METH produces powerful sympathetic nervous system stimulation. Euphoria, increased energy, heightened attentiveness, hypersexuality, and decreased anxiety are effects of METH intoxication (230). In contrast, depression, anhedonia, irritability, anxiety, fatigue, hypersomnia, poor concentration, intense craving and aggression are associated with METH withdrawal (231-233). Chronic METH ingestion frequently results in severe psychiatric disturbances or METH-induced “psychosis” that resembles schizophrenia (234-236). There are presently no FDA-approved medications indicated for METH addiction but a variety of compounds are being tested (237).
METHAMPHETAMINE’S EFFECTS ON NEUROCHEMISTRY AND BEHAVIOR
METH is similar to cocaine and amphetamine in that it has potent effects on the DAT that likely contribute to its addictive properties. METH greatly enhances DA concentration in reward circuitry, such as the NAC, 1000% over baseline (238) independent of neuron depolarization (239) redistributing cytosolic DA for quick release from the neuron (240). Redistribution of cytosolic DA may be due to METH’s ability to inactivate vesicular monoamine transporter (VMAT2) in monaminergic neurons (241) that is normally responsible for repackaging, storage and re-release of neurotransmitter. METH-induced increases in massive amounts of synaptic DA is auto-oxidized into highly reactive species that inactivates and decreases DAT that further elevates synaptic DA levels (242-244) and neurotoxicity (120, 245-247). DA selective receptor antagonists, DAT antagonists, and DA depletion block METH’s effects on the DAT (248). Likewise, DA D1-like and D2-like antagonists also block METH-induced behavioral sensitization in rats (249, 250). Clozapine, an atypical antipsychotic that blocks a number of receptors, including the D4 receptor subtype (251), antagonizes METH-induced behavioral activation as does a D4 selective antagonist (252). Clozapine pretreatment also blocks METH-induced memory deficits in rodents (253). Neither the non-selective DA blocker haloperidol nor the combination DA D2/5HT blocker risperidone block the subjective effects of METH in humans (254).
Individuals with METH abuse histories have significant decreases in DAT binding in the caudate and putamen as measured with the PET radioligand [11C]WIN-35,428 (255). Other PET studies confirm decreased DAT binding in these areas in current abusers (256), recently detoxified subjects (257), and in subjects drug-free for upwards of 11 months (142). Similar to what has been demonstrated in alcoholics (140), cocaine (141), and heroin (143) abusers, D2 receptor levels are lower in the caudate and putamen of METH abusers, as measured by PET [11C]raclopride (142)(but see (257)).
Research has focused on polymorphisms involving the DAergic system that relates to METH-induced psychosis and vulnerability. The 7-repeat (exon III) DRD4 polymorphism and the functional polymorphism COMT Val158Met are two examples (258-261). Substantive evidence supports an association with the met allele of the COMT enzyme with METH-induced psychosis (262) and as a risk factor for developing prolonged METH-induced psychosis (263). METH-psychosis is associated with proteins linked to DA neurotransmission such as C-kinase-1 (PICK1) that is related to DAT membrane localization and securing a stable protein complex within the membrane (264). Others include the functional polymorphism of glutathione S-transferase involved in oxidative stress protection that results in markedly reduced enzyme (30%) levels possibly potentiating METH’s deleterious effects (265, 266). Organic cation transporter 3 (267), beta-arrestin 2 gene (268), and newly identified SNPs of OPRM1 (269, 270), but not the delta opioid receptor (OPRD1) (271), have also been linked to METH-induced psychosis.
Brain-derived neurotrophic factor (BDNF) is a neurotrophic factor implicated in a number of neurological processes such as cell survival, proliferation, synaptic growth through development, and hippocampal changes associated with learning and conditioned drug reward (272-275). Interestingly, plasma BDNF levels are significantly higher in abstinent METH abusers compared to controls (183) and in multiple substance abusers with schizophrenia (276). Recent association studies have linked an increased incidence of the purported functional BDNF gene polymorphism val66met (277) and substance abuse in males (278). This mutation appears to affect declarative memory in humans (279). Increased incidence of the val66met polymorphism in substance abusers is partly supported by another study (280). It is unknown however, whether the val66met polymorphism alters any of the subjective effects of METH or rather, may be a viable pharmacogenetic target.
Taken together, there has been much progress made in defining polymorphism that may confer susceptibility to abuse and developing psychosis from METH abuse. From a pharmacogenetics perspective, studies have yet to find a target that would improve pharmacotherapeutic decision-making in the setting of variability in response to a drug therapy for this drug of abuse.
CONCLUSIONS
Identifying gene-targets that will improve clinical decision making within the context of variability in response to drug therapy is the principle challenge of pharmacogenetics. Psychiatric pharmacogenetic studies have identified genes implicated in altered responses to psychostimulants and have identified targets for maximizing pharmacotherapeutic response. Studies presented here briefly highlighted therapeutics for drug dependence currently being used but focused mainly on maximizing therapeutic response based on individual’s genetic profile. For example, cocaine pharmacotherapy is influenced by C-1021T SNP of the DβH gene that alters enzyme levels. Disulfiram’s action on this enzyme and DAT gene variable number tandem repeats of the SLC6A3 gene affects treatment response to methylphenidate. DAD4 receptor 77-repeat (exon III) polymorphism may alterclozapine action for the treatment of METH-induced psychosis. Further, the purported functional polymorphism of the BDNF gene val66met may offer a pharmacogenetic target for METH addiction. Val158met may alter COMT enzyme levels and is associated with amphetamine and METH use. Presumed genetically predetermined decreases in DRD2 levels in chronic drug abusers offers another possible pharmacotherapeutic target (see Figure 2).
FIG. 2.
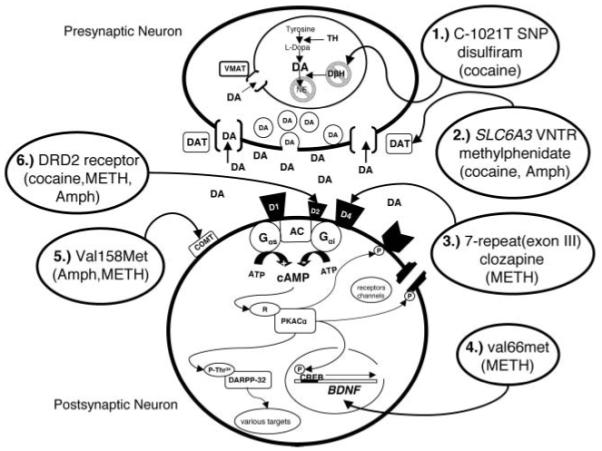
Hypothetical schematic of a DAergic synapse representing various polymorphisms affecting proteins involved in psychostimulant action or altering response to medications for psychostimulant addiction. Cocaine pharmacotherapy is influenced by C-1021T SNP (1) of the DβH gene that alters enzyme levels and disulfiram’s action on this enzyme. DAT gene variable number tandem repeats of the SLC6A3 (2) affects treatment response to methylphenidate and possibly cocaine and amphetamine (AMPH). DAD4 receptor 7-repeat (exon III) polymorphism (3) may alter clozapine action for the treatment of methamphetamine (METH)-induced psychosis. The purported functional polymorphism of the BDNF gene val66met (4) may offer a pharmacogenetic target for METH addiction. Val158met (5) may alter COMT enzyme levels and is associated with AMPH and METH use. Genetically predetermined decreases in DRD2 receptors (6) in chronic drug abusers across classes offers a possible pharmacotherapeutic target.
Individualized treatments based on the presence of SNPs or other gene variants that alter a particular target protein’s function but enhance response to a medication is key. Better-defined contributions of genes already associated with drugs of abuse and how they may alter the subjective effects that contribute to pathological compulsive drug seeking is under way. Future studies should consider gene variants that affect pharmacodynamic, pharmacokinetic and possibly epigenetic factors that could contribute to a positive clinical outcome and response to a particular medication. Information from imaging studies coupled with carefully defined candidate gene variants and their function will also help clarify contributions of candidate genes to phenotype and possibly predict responses to psychostimulants and to medication.
ACKNOWLEDGMENTS
The preparation of this paper was aided by the support of a grant from the National Institute on Alcoholism and Alcohol Abuse (U01-AA013476) and from a grant from the National Institute on Drug Abuse (DA020117) to TA Kosten.
REFERENCES
- 1.Giacomini KM, Brett CM, Altman RB, Benowitz NL, Dolan ME, Flockhart DA, Johnson JA, Hayes DF, Kelein T, Krauss RM, Kroetz DL, McLeod HL, Nguyen AT, Ratain MJ, Relling MV, Reuss V, Roden DM, Schaefer CA, Shuldiner AR, Skaar T, Tantisira KT, Wang L, Weinshilboum RM, Weiss ST, Zineh I, Network PR. The pharmacogenetics research network: from SNP discovery to clinical drug response. Clin Pharmacol Ther. 2007;81(3):328–45. doi: 10.1038/sj.clpt.6100087. R.F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao G, Zhang X, Clark DJ, Peltz G. A genomic “roadmap” to “better” drugs. Drug Metab Rev. 2008;40(2):225–239. doi: 10.1080/03602530801952815. [DOI] [PubMed] [Google Scholar]
- 3.Drolet B, Simard C, Mizoue L, Roden DM. Human cardiac potassium channel DNA polymorphism modulates access to drug-binding site and causes drug resistance. J Clin Invest. 2005;115(8) doi: 10.1172/JCI23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simard C, Drolet B, Yang P, R.B K, Roden DM. Polymorphism screening in the cardiac K+ channel gene KCNA5. Clin Pharmacol Ther. 2005;77(3):138–44. doi: 10.1016/j.clpt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: reponsive variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107(23) doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 6.Tantisira KG, Hwang ES, Raby BA, Silverman ES, Lake SL, Richter BG, Peng SL, Drazen JM, Glimcher LH, Weiss ST. TBX21: a functional variant predicts improvement in asthma with the use of inhaled corticosteroids. Proc Natl Acad Sci USA. 2004;101(52):18099–104. doi: 10.1073/pnas.0408532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JA. Pharmacogenetics: potential for individualized drug therapy through genetics. Trends Genet. 2003;19(11):660–6. doi: 10.1016/j.tig.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Matetzky S, Shenkman B, Guetta V, Shechter M, Bienart R, Goldenberg I, Novikov I, Savion N, Varon D, Hod H. Clopidogrel resistance is associated with inicreased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109(25):3171–5. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 9.Weiss ST, Litonju AA, Lange C, Lazarus R, Liggett SB, Bleecker ER, Tantisira KG. Overview of the pharmacogenetics of asthma treatment. Pharmacogenetics J. 2006;6(5):311–26. doi: 10.1038/sj.tpj.6500387. [DOI] [PubMed] [Google Scholar]
- 10.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 11.Holleman A, Cheok MH, Den Boer ML, Yang W, Veerman AJ, Kazemier KM, Pei D, Cheng C, Pui CH, Relling MV, Janka-Schaub GE, Pieters R, Evans WE. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and reponse to treatment. N Engl J Med. 2004;351(6):601–3. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]
- 12.Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, Desta Z, Perez EA, Ingle JN. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of eficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–8. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 13.Roses AD. Pharmacogenetics and drug development: the path to safer and more effective drugs. Nat Rev Genet. 2004;5(9):645–56. doi: 10.1038/nrg1432. [DOI] [PubMed] [Google Scholar]
- 14.Shields AE, Lerman C. Anticipating clinical intetration of pharmacogenetic treatment strategies for addiction: are primary care physicians ready? Clin Pharmacol Ther. 2008;83(4):635–639. doi: 10.1038/clpt.2008.4. [DOI] [PubMed] [Google Scholar]
- 15.Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opin Investig Drugs. 2006;11(1):91–98. doi: 10.1517/14728214.11.1.91. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162(8):1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra AK, Murphy GMJ, Kennedy JL. Pharmacogenetics of psychotropic drug response. Am J Psychiatry. 2004;161(5):780–96. doi: 10.1176/appi.ajp.161.5.780. [DOI] [PubMed] [Google Scholar]
- 18.Murphy GMJ, Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry. 2003;160(10):1830–5. doi: 10.1176/appi.ajp.160.10.1830. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Jain S, Brahmachari SK, Kukreti R. Pharmacogenetics: a path to predictive medicine for schizophrenia. Pharmacogenomics. 2006;7(1) doi: 10.2217/14622416.7.1.31. [DOI] [PubMed] [Google Scholar]
- 20.deLeon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 and CYP450 2C19. Psychosomatics. 2006;47(1):75–85. doi: 10.1176/appi.psy.47.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Rogers JF, Nafziger AN, Bertino JSJ. Pharmacogenetics affects dosing, efficacy, and toxicity of cytochrome P450-metabolized drugs. Am J Med. 2002;113(9):746–50. doi: 10.1016/s0002-9343(02)01363-3. [DOI] [PubMed] [Google Scholar]
- 22.Scordo MG, Spina E. Cytochrome P450 polymorphisms and response to antipsychotic therapy. Pharmacogenomics. 2002;3(2):201–18. doi: 10.1517/14622416.3.2.201. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XY, Zhou DF, Wu GY, Cao LY, Tan YL, Haile CN, Li J, Lu L, Kosten TA, Kosten TR. BDNF levels and genotype are associated with antipsychotic-induced weight gain in patients with chronic schizophrenia. Neuropharmacology. 2008;33(9):2200–2205. doi: 10.1038/sj.npp.1301619. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XY, Tan YL, Zhou DF, Cao LY, Wu GY, Haile CN, Kosten TA, Kosten TR. Disrupted antioxidant enzyme activity and elevated lipid peroxidation products in schizophrenic patients with tardive dyskinesia. J Clin Psychiatry. 2007;68(5):754–760. doi: 10.4088/jcp.v68n0513. [DOI] [PubMed] [Google Scholar]
- 25.Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney DS, McMahon FJ. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 26.Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278(5335):45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 27.Weisner C, Matzger H, Kaskutas LA. How important is treatment? One-year outcomes of treated and untreated alcohol-dependent individuals. Addiction. 2003;98(7):901–11. doi: 10.1046/j.1360-0443.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- 28.Simpson DD, Joe GW, Broome KM. A national 5-year follow-up of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 2002;59(6):538–65. doi: 10.1001/archpsyc.59.6.538. [DOI] [PubMed] [Google Scholar]
- 29.Goldman D, Oroszi G, Ducci F. The genetics of addictions: un-covering the genes. Nat Rev Genet. 2005;6(7):521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 30.Yacubian J, Sommer T, Schroeder K, Glascher J, Braus DF, Buchel C. Subregions of the ventral striatum show preferential coding of reward magnitude and probability. Neuroimage. 2007;38(3):557–63. doi: 10.1016/j.neuroimage.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Haile CN, Hiroi N, Nestler EJ, Kosten TA. Differential behavioral responses to cocaine are associated with dynamics of mesolimbic dopamine proteins in Lewis and Fischer 344 rats. Synapse. 2001;41:179–190. doi: 10.1002/syn.1073. [DOI] [PubMed] [Google Scholar]
- 32.Haile CN, Kosten TR, Kosten TA. Genetics of dopamine and its contribution to cocaine addiction. Behav Genet. 2007;37(1):119–45. doi: 10.1007/s10519-006-9115-2. [DOI] [PubMed] [Google Scholar]
- 33.Haile CN, Kosten TA. Differential effects of D1- and D2-like compounds on cocaine self-administration in Lewis and Fischer 344 inbred rats. J Pharmacol Exp Ther. 2001;299(2):509–18. [PubMed] [Google Scholar]
- 34.Gabbay FH. Variations in affect following amphetamine and placebo: markers of stimulant drug preference. Exp Clin Psychopharmacol. 2003;11(1):91–101. doi: 10.1037//1064-1297.11.1.91. [DOI] [PubMed] [Google Scholar]
- 35.Lott DC, Kim SJ, Cook EHJ, de Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology. 2005;30(3):602–609. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- 36.Haile CN, Kosten TA, Kosten TR. Pharmacogenetic Treatments for Drug Addiction: Alcohol and Opiates. Am J Drug Alcohol Abuse. 2008;34:355–381. doi: 10.1080/00952990802122564. [DOI] [PubMed] [Google Scholar]
- 37.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 38.Heffner TG, Hartman JA, Seiden LS. Feeding increases dopamine metabolism in the rat brain. Science. 1980;208:1168–1170. doi: 10.1126/science.7375926. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 40.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:75–114. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 41.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 42.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann NY Acad Sci. 2003;985:233–50. [PubMed] [Google Scholar]
- 43.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4(1):23–9. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- 45.DiChiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 47.Zito KA, Vickers G, Roberts DC. Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1985;23:1029–1036. doi: 10.1016/0091-3057(85)90110-8. [DOI] [PubMed] [Google Scholar]
- 48.Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 49.Snyder SH, Coyle JT. Regional differences in H3-norepinephrine and H3-dopamine uptake into rat brain homogenates. JPharmacolExp Ther. 1969;165:78–86. [PubMed] [Google Scholar]
- 50.Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432(1):119–36. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- 51.Mazei MS, Pluto CP, Kirkbride B, Pehek EA. Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res. 2002;936(12):58–67. doi: 10.1016/s0006-8993(02)02542-8. [DOI] [PubMed] [Google Scholar]
- 52.Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22(2):389–95. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 54.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 55.Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- 56.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 57.Schank JR, Liles LC, Weinshenker D. Norepinephrine signaling through beta-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry. 2008;63(11):1007–1012. doi: 10.1016/j.biopsych.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 59.Bartlett E, Hallin A, Chapman B, Angrist B. Selective sensitization to the psychosis-inducing effects of cocaine: a possible marker for addiction relapse vulnerability? Neuropsychopharmacology. 1997;16(1):77–82. doi: 10.1016/S0893-133X(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 60.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43(2):107–13. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 61.Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- 62.Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- 63.Wachtel SR, de Wit H. Subjective and behavioral effects of repeated d-amphetamine in humans. Behav Pharm. 1999;10(3):271–81. doi: 10.1097/00008877-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Vollm BA, deAraujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, Jessard P, Heal RJ, Matthews PM. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology. 2004;29:1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- 65.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156(9):1440–3. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- 66.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–3. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 67.Laruelle M, Gelernter J, Innis RB. D2 receptors binding potential is not affected by Taq1 polymorphisms at the D2 receptor gene. Mol Psychiatry. 1998;3(3):261–5. doi: 10.1038/sj.mp.4000343. [DOI] [PubMed] [Google Scholar]
- 68.Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O’Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arch Gen Psychiatry. 1999;56(12):1101–6. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- 69.Sherer MA, Kumor KM, Jaffe JH. Effects of intravenous cocaine are partially attenuated by haloperidol. Psychiatry Res. 1989;27(2):117–25. doi: 10.1016/0165-1781(89)90127-3. [DOI] [PubMed] [Google Scholar]
- 70.Newton TF, Ling W, Kalechstein AD, Uslaner J, Tervo K. Risperidone pre-treatment reduces the euphoric effects of experimentally administered cocaine. Psychiatry Res. 2001;102(3):227–33. doi: 10.1016/s0165-1781(01)00255-4. [DOI] [PubMed] [Google Scholar]
- 71.Alhassoon OM, Dupont RM, Schweinsburg BC, Taylor MJ, Patterson TL, Grant I. Regional cerebral blood flow in cocaine-versus methamphetamine-dependent patients with a history of alcoholism. Int J Neuropsychopharmacol. 2001;4:105–112. doi: 10.1017/S1461145701002334. [DOI] [PubMed] [Google Scholar]
- 72.Hall FS, Sora I, Drgonova J, Li XF, Goeb M, Uhl GR. Molecular mechanisms underlying the rewarding effects of cocaine. Ann N Y Acad Sci. 2004;1025:47–56. doi: 10.1196/annals.1316.006. [DOI] [PubMed] [Google Scholar]
- 73.Shoptaw S, Watson DW, Reiber C, Rawson RA, Montgomery MA, Majewska MD, Ling W. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST) Addiction. 2005;11(1):78–90. doi: 10.1111/j.1360-0443.2005.00991.x. [DOI] [PubMed] [Google Scholar]
- 74.Kosten TR, George TP, Kosten TA. The potential of dopamine agonists in drug addiction. Expert Opin Investig Drugs. 2002;11(4):491–499. doi: 10.1517/13543784.11.4.491. [DOI] [PubMed] [Google Scholar]
- 75.Kosten TR, Morgan C, Kosten TA. Treatment of heroin addicts using buprenorphine.H. Am J Drug Alcohol Abuse. 1991;17(2):119–28. doi: 10.3109/00952999108992815. [DOI] [PubMed] [Google Scholar]
- 76.Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64(12):1440–1448. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez G, Servarino K, Sofuoglu M, Poling J, Oliveto A, Gonsai K, George TP, Kosten TR. Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: results of a randomized pilot study. Addiction. 2003;98(11):1625–32. doi: 10.1046/j.1360-0443.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- 78.Hameedi FA, Rosen MI, McCance -Kat z EF, McMahon TJ, Price LH, Jatlow PI, Woods SW, Kosten TR. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol Psychiatry. 1995;37(8):560–3. doi: 10.1016/0006-3223(94)00361-6. [DOI] [PubMed] [Google Scholar]
- 79.Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cubells JF, Zabetian CP. Human genetics of plasma dopamine beta-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology. 2004;174(4):463–76. doi: 10.1007/s00213-004-1840-8. [DOI] [PubMed] [Google Scholar]
- 81.Mikami LR, Wieseler S, Souza RL, Schopfter LM, Nachon F, Lockridge O, Chautard-Freire-Maia EA. Five new naturally occurring mutations of the BCHE gene and frequencies of 12 butyryl-cholinesterase alleles in a Brazilian population. Pharmacogenet Genomics. 2008;18(3):213–218. doi: 10.1097/FPC.0b013e3282f5107e. [DOI] [PubMed] [Google Scholar]
- 82.Towell J. F. r., Cho JK, Roh BL, Wang RI. Disulfiram and erythrocyte aldehyde dehydrogenase inhibition. Clin Pharmacol Ther. 1983;33(4):517–21. doi: 10.1038/clpt.1983.70. [DOI] [PubMed] [Google Scholar]
- 83.Johnson SM, Fleming WW. Mechanisms of cellular adaptive sensitivity changes: applications to opioid tolerance and dependence. Pharmacol Rev. 1989;41(4):435–88. [PubMed] [Google Scholar]
- 84.Vaccari A, Saba PL, Ruiu S, Collu M, Devoto P. Disulfiram and diethyldithiocarbamate intoxication affects the storage and release of striatal dopamine. Toxicol Appl Pharmacol. 1996;139(1):102–8. doi: 10.1006/taap.1996.0147. [DOI] [PubMed] [Google Scholar]
- 85.Bourdelat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D. Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology. 2005;183(1):72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- 86.Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006;31(10):2221–30. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- 87.McCance-Katz EF, Kosten TR, Jatlow P. Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biol Psychiatry. 1998;43(7):540–3. doi: 10.1016/S0006-3223(97)00506-4. [DOI] [PubMed] [Google Scholar]
- 88.McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 1998;52(1):27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- 89.Wright C, Moore RD. Disulfiram treatment of alcoholism. Am J Med. 1990;88(6):647–55. doi: 10.1016/0002-9343(90)90534-k. [DOI] [PubMed] [Google Scholar]
- 90.Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. The status of disulfiram: a half of a century later. J Clin Psychopharmacol. 2006;26(3):290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- 91.Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93(5):713–27. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- 92.Carroll KM, Power ME, Bryant K, Rounsaville BJ. One-year follow-up status of treatment-seeking cocaine abusers. Psychopathology and dependence severity as predictors of outcome. J Nerv Ment Dis. 1993;181(2):71–9. doi: 10.1097/00005053-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 93.George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schot tenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine maintained subjects: a preliminary trial. Biol Psychiatry. 2000;47(12):1080–6. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- 94.Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F. Disulfiram therapy in patients abusing cocaine and alcohol. Am J Psychiatry. 1993;150(4):675–6. doi: 10.1176/ajp.150.4.675b. [DOI] [PubMed] [Google Scholar]
- 95.Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Kat z EF, Frankforter TL, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone maintained opioid addicts. Addiction. 2000;95(2):219–28. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- 96.McCance-Katz EF, Price LH, McDougle CJ, Kosten TR, Black JE, Jatlow PI. Concurrent cocaine ethanol ingestion in humans: pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology. 1993;111(1):39–46. doi: 10.1007/BF02257405. [DOI] [PubMed] [Google Scholar]
- 97.Carroll KM, Nich C, Ball SA, McCance E, Frankforter TL, Rounsaville BJ. One year followup of disulfiram and psychotherapy for cocaine alcohol users: sustained effects of treatment. Addiction. 2000;95(9):1335–49. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- 98.Martensen-Larsen O. Psychotic phenomena provoked by tetraethylthiuram disulfide. Q J Stud Alcohol. 1951;12(2):206–16. [PubMed] [Google Scholar]
- 99.Kalayasiri R, Sughondhabirom A, Gueorguie va R, Coric V, L WJ, Morgan PT, Cubells JF, Malison RT. Selfreported paranoia during laboratory “binge” cocaine selfadministration in humans. Pharmacol Biochem Behav. 2006;83(2):249–56. doi: 10.1016/j.pbb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87(23):202–9. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haile CN, During MJ, Jatlow PI, Kosten TR, Kosten TA. Disulfiram facilitates the development and expression of locomotor sensitization to cocaine in rats. Biol Psychiatry. 2003;54:915–921. doi: 10.1016/s0006-3223(03)00241-5. [DOI] [PubMed] [Google Scholar]
- 102.Cubells JF, Kranzler HR, McCance-Katz EF, Anderson GM, Malison RT, Price LH, Gelernter J. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry. 2000;5(1):56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- 103.Stewart LC, Klinman JP. Dopamine beta-hydroxylase of adrenal chromaffin granules: structure and function. Annu Rev Biochem. 1988;57:551–592. doi: 10.1146/annurev.bi.57.070188.003003. [DOI] [PubMed] [Google Scholar]
- 104.Kaufman S, Friedman S. Dopamine-beta-hydroxylase. Pharmacol Rev. 1965;17:71–100. [PubMed] [Google Scholar]
- 105.Senard J-M, Rouet P. Dopamine beta-hydroxylase deficiency. Orphanet J Rare Dis. 2006;1(7):1–4. doi: 10.1186/1750-1172-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deinum J, Steenbergen-Spanjers GC, Jansen M, Boomsma F, Lenders JW, van Ittersum FJ, Hück N, van den Heuvel LP, Wevers RA. DBH gene variants that cause low plasma dopamine beta hydroxylase with or without a severe orthostatic syndrome. A. J Med Genet. 2004;41(4):e38. doi: 10.1136/jmg.2003.009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garland EM, Black BK, Harris PA, D R. Dopamine-beta-hydroxylase in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2007;293:H684–H690. doi: 10.1152/ajpheart.01389.2006. [DOI] [PubMed] [Google Scholar]
- 108.Grzanna R, Coyle JT. Immunochemical studies on the turnover of rat serum dopamine beta-hydroxylase. Mol Pharmacol. 1977;13(5):956–964. [PubMed] [Google Scholar]
- 109.Weinshilboum RM. Serum dopamine beta-hydroxylase. Pharmacol Rev. 1978;30(2):133–66. [PubMed] [Google Scholar]
- 110.Paclt I, Koudelová J. Changes of dopamine-beta-hydroxylase activity during ontogenesis in healthy subjects and in an experimental model (rats) Physiol Res. 2004;53(6):661–7. [PubMed] [Google Scholar]
- 111.Ross SB, Wetterberg L, Myrhed M. Genetic control of plasma dopamine-beta-hydroxylase. Life Sci. 1973;12(12):529–32. doi: 10.1016/0024-3205(73)90056-8. [DOI] [PubMed] [Google Scholar]
- 112.Weinshilboum RM, Raymond FA, Elveback LR, Weidman WH. Serum dopamine-beta-hydroxylase activity: sibling-sibling correlation. Science. 1973;181(103):943–945. doi: 10.1126/science.181.4103.943. [DOI] [PubMed] [Google Scholar]
- 113.Oxenstierna G, Edman G, Iselius L, Oreland L, Ross SB, Sedvall G. Concentrations of monoamine metabolites in the cerebrospinal fluid of twins and unrelated individuals-a genetic study. J Psychiatr Res. 1986;20(1):19–29. doi: 10.1016/0022-3956(86)90020-8. [DOI] [PubMed] [Google Scholar]
- 114.Wei J, Ramchand CN, Hemmings GP. Possible control of dopamine beta-hydroxylase via a codominant mechanism associated with the polymorphic (GT)n repeat at its gene locus in healthy individuals. Hum Genet. 1997;99(1):52–5. doi: 10.1007/s004390050310. [DOI] [PubMed] [Google Scholar]
- 115.Cubells JF, vanKammen DP, Kelley ME, Anderson GM, O’Connor DT, Price LH, Malison RT, Rao PA, Kobayashi K, Nagatsu T, Gelernter J. Dopamine beta-hydroxylase: two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Hum Genet. 1998;102(5):533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- 116.Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, Kim KS, Kim CH, Malison RT, Gelernter J, Cubells JF. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Genet. 2001;68(2):515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zabetian CP, Buxbaum SG, Elston RC, Kohnke MD, Anderson GM, Gelernter J, Cubells JF. The structure of linkage disequilibrium at the DBH locus strongly influences the magnitude of association between diallelic markers and plasma dopamine beta-hydroxylase activity. Am J Genet. 2003;72(6):1389–1400. doi: 10.1086/375499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kohnke MD, Zabetian CP, Anderson GM, Kolb W, Gaertner I, Buchkremer G, Vonthein R, Schick S, Lutz U, Kohnke AM, Cubells JF. A genotype-controlled analysis of plasma dopamine beta-hydroxylase in healthy and alcoholic subjects: evidence for alcohol-related differences in noradrenergic function. Biol Psychiatry. 2002;52(12):1151–1158. doi: 10.1016/s0006-3223(02)01427-0. [DOI] [PubMed] [Google Scholar]
- 119.Bhaduri N, Mukhopadhyay K. Correlation of plasma dopamine beta-hydroxylase activity with polymorphisms in DBH gene: A study on eastern Indian populaion. Cell Mol Neurobiol. 2008;28:343–350. doi: 10.1007/s10571-007-9256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cadet J-L, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17:1775–1788. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- 121.Tang YL, Epstein MP, Anderson GM, Zabetian CP, Cubells JF. Genotypic and haplotypic associations of the DBH gene with plasma dopamine beta-hydroxylase activity in African Americans. Eur J Hum Genet. 2007;15(8):878–83. doi: 10.1038/sj.ejhg.5201838. [DOI] [PubMed] [Google Scholar]
- 122.Guindalini C, Laranjeira R, Collier D, Messas G, Vallada H, Breen C. Dopamine-beta hydroxylase polymorphism and cocaine addiction. Behav Brain Funct. 2008;3(4):1–4. doi: 10.1186/1744-9081-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55(11):967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 124.Wei J, Ramchand CN, Hemmings GP. TaqI polymorphic sites at the human dopamine beta-hydroxylase gene possibly associated with biochemical alterations of the catecholamine pathway in schizophrenia. Psychiatr Genet. 1998;8(1):19–24. doi: 10.1097/00041444-199800810-00003. [DOI] [PubMed] [Google Scholar]
- 125.Cubells JF, Price LH, Meyers BS, Anderson GM, Zabetian CP, Alexopoulos GS, Nelson JC, Sanacora G, Kirwin P, Carpenter L, Malison RT, Gelernter J. Genotype-controlled analysis of plasma dopamine beta-hydroxylase activity in psychotic unipolar major depression. Biol Psychiatry. 2002;51(5):358–64. doi: 10.1016/s0006-3223(01)01349-x. [DOI] [PubMed] [Google Scholar]
- 126.Freire MT, Hutz MH, Bau CH. The DBH -1021 C/T polymorphism is not associated with alcoholism but possibly with patients’ exposure to life events. J Neural Transm. 2005;112(9):1269–74. doi: 10.1007/s00702-005-0339-8. [DOI] [PubMed] [Google Scholar]
- 127.Tang Y, Buxbaum SG, Waldman I, Anderson GM, Zabetian CP, Köhnke MD, Cubells JF. A single nucleotide polymorphism at DBH, possibly associated with attention-deficit/hyperactivity disorder, associates with lower plasma dopamine beta-hydroxylase activity and is in linkage disequilibrium with two putative functional single nucleotide polymorphisms. Biol Psychiatry. 2006;60(10):1034–8. doi: 10.1016/j.biopsych.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 128.Mustapic M, Pivac N, Kozaric-Kovacic D, Dezeljin M, Cubells JF, Muck-Seler D. Dopamine beta-hydroxylase (DBH) activity and - 1021C/T polymorphism of DBH gene in combat-related post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144(8):1087–9. doi: 10.1002/ajmg.b.30526. [DOI] [PubMed] [Google Scholar]
- 129.Rogeness GA, Maas JW, Javors MA, Macedo CA, Harris WR, Hoppe SK. Diagnoses, catecholamine metabolism, and plasma dopamine-beta-hydroxylase. J Am Acad Child Adolesc Psychiatry. 1988;27(1):121–5. doi: 10.1097/00004583-198801000-00019. [DOI] [PubMed] [Google Scholar]
- 130.Rogeness GA, Javors MA, Maas JW, Macedo CA, Fischer C. Plasma dopamine-beta-hydroxylase, HVA, MHPG, and conduct disorder in emotionally disturbed boys. Biol Psychiatry. 1987;22(9):1158–62. doi: 10.1016/0006-3223(87)90058-8. [DOI] [PubMed] [Google Scholar]
- 131.Rogeness GA, Hernandez JM, Macedo CA, Mitchell EL, Amrung SA, Harris WR. Clinical characteristics of emotionally disturbed boys with very low activities of dopamine-beta-hydroxylase. J Am Acad Child Psychiatry. 1984;23(2):203–8.. doi: 10.1097/00004583-198403000-00013. [DOI] [PubMed] [Google Scholar]
- 132.Rogeness GA, Hernandez JM, Macedo CA, Amrung SA, Hoppe SK. Near-zero plasma dopamine-beta-hydroxylase and conduct disorder in emotionally disturbed boys. J Am Acad Child Psychiatry. 1986;25(4):521–7.. doi: 10.1016/s0002-7138(10)60012-x. [DOI] [PubMed] [Google Scholar]
- 133.Galvin M, Shekhar A, Simon J, Stilwell B, Ten Eyck R, Laite G, Karwisch G, Blix S. Low dopamine-beta-hydroxylase: a biological sequela of abuse and neglect? Psychiatry Res. 1991;39(1):1–11. doi: 10.1016/0165-1781(91)90002-7. [DOI] [PubMed] [Google Scholar]
- 134.Healy DG, Abou-Sleiman PM, Ozawa T, Lees AJ, Bhatia K, Ahmadi KR, Wullner U, Berciano J, Moller JC, Kamm C, Burk K, Barone P, Tolosa E, Quinn N, Goldstein DB, Wood NW. A functional polymorphism regulating dopamine beta-hydroxylase influences against Parkinson’s disease. Ann Neurol. 2004;55(3):443–6. doi: 10.1002/ana.20063. [DOI] [PubMed] [Google Scholar]
- 135.Jonsson EG, Bah J, Melke J, Abou Jamra R, Schumacher J, Westberg L, Ivo R, Cichon S, Propping P, Nathen MM, Eriksson E, Sedvall GC. Monoamine related functional gene variants and relationships to monoamine metabolite concentrations in CSF of healthy volunteers. BMC Psychiatry. 2004;4(4) doi: 10.1186/1471-244X-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Lappalainen J, Gelernter J, Cubells JF, Malison RT. Dopamine beta-hydroxylase gene (DbetaH) -1021C-T influences self-reported paranoia during cocaine self-administration. Biol Psychiatry. 2007;61(11):1310–3. doi: 10.1016/j.biopsych.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 137.Sughondhabirom A, Jain D, Gueorguieva R, Corvic V, Berman R, Lynch WJ, Self DW, Jatlow P, R.T M. A paradigm to investigate the self-regulation of cocaine administration in humans. Psychopharmacology. 2005;180(3):436–46. doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- 138.Brady KT, Lydiard RB, Malcolm R, Ballenger JC. Cocaine-induced psychosis. J Clin Psychiatry. 1991;52(12):509–12. [PubMed] [Google Scholar]
- 139.Satel SL, Edell WS. Cocaine-induced paranoia and psychosis proneness. Am J Psychiatry. 1991;148(12):1708–11. doi: 10.1176/ajp.148.12.1708. [DOI] [PubMed] [Google Scholar]
- 140.Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 141.Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D. e. a. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147(6):719–24. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 142.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gately SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- 143.Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropharmacology. 1997;16(2):174–82. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- 144.Volkow ND, Wang GJ, Fowler JS, Thanos P, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replications study. Synapse. 2002;46(2):79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 145.Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2(11):1225–9. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]
- 146.Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser SM, Flor H, Braus DF, Buchholz HG, Gründer G, Schreckenberger M, Smolka MN, Rösch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161(10):1741–2. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- 147.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein RZ, Alexoff D, Thanos P. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63(9):999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 148.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Flores G, Wood GK, Barbeau D, Queirion R, Srivastava l. K. Lewis and Fischer rats: a comparison of dopamine transporter and receptor levels. Brain Res. 1998;814(12):34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- 150.Stefanini E, Frau M, Garau MG, Garau B, Fadda F, Gessa GL. Alcohol-preferring rats have fewer dopamine D2 receptors in the limbic system. Alcohol Alcohol. 1992;27(2):127–30. [PubMed] [Google Scholar]
- 151.McBride WJ, Chernet E, Dyr W, Lumeng L, Li TK. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol. 1993;10(5):387–90. doi: 10.1016/0741-8329(93)90025-j. [DOI] [PubMed] [Google Scholar]
- 152.Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388(6642):586–9. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- 153.Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, Grandy DK, Low MJ. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1(2):610–5. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- 154.Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;31:313–319. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- 155.Kawarai T, Kawakami H, Yamamura Y, Nakamura S. Structure and organization of the gene encoding human dopamine transporter. Gene. 1997;195(1):11–8. doi: 10.1016/s0378-1119(97)00131-5. [DOI] [PubMed] [Google Scholar]
- 156.Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J, Dewey SL, Hitzemann R, Lieberman J. Relationship between psychostimulant-induced “high” and dopamine transporter occupancy. Proc Natl Acad Sci U S A. 1996;93(19):10388–92. doi: 10.1073/pnas.93.19.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 158.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog in Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 159.Liang NY, Rutledge CO. Comparison of the release of [3H]dopamine from isolated corpus striatum by amphetamine, fenfluramine and unlabelled dopamine. Biochem Pharmacol. 1982;31:983–992. doi: 10.1016/0006-2952(82)90332-x. [DOI] [PubMed] [Google Scholar]
- 160.Haile CN. Neurochemical and Neurobehavioral consequences of methamphetamine abuse. In: Karch SB, editor. Drug Abuse Handbook. Second Ed. CRC Press; Boca Raton, FL: 2007. pp. 478–503. 33487. [Google Scholar]
- 161.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 162.Mitchell RJ, Howlett S, Earl L, White NG, McComb J, Schanfield MS, Briceno I, Papiha SS, Osipova L, Livshits G, Leonard WR, Crawford MH. Distribution of the 3′ VNTR polymorphism in the human dopamine transporter gene in world populations. Hum Biol. 2000;72(2):295–304. [PubMed] [Google Scholar]
- 163.Doucette-Stamm LA, B D, Tian J, Mockus S, Mao JI. Population genetic study of the human dopamine transporter gene (DAT1) Genet Epidemiol. 1995;12(3):303–8. doi: 10.1002/gepi.1370120307. [DOI] [PubMed] [Google Scholar]
- 164.Kang AM, Palmatier MA, Kidd KK. Global variation of a 40-bp VNTR in the 3′-untranslated region of the dopamine transporter gene (SLC6A3) Biol Psychiatry. 1999;46(2):151–60. doi: 10.1016/s0006-3223(99)00101-8. [DOI] [PubMed] [Google Scholar]
- 165.Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1(2):152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- 166.Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114(8):975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- 167.VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;27(6):55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Biederman J, Petty CR, Wilens TE, Fraire MG, Purcell CA, Mick E, Monuteaux MC, Faraone SV. Familial risk analyses of attention deficit hyperactivity disorder adn substance use disorders. Am J Psychiatry. 2008;165(1):107–15. doi: 10.1176/appi.ajp.2007.07030419. [DOI] [PubMed] [Google Scholar]
- 169.Barkley RA. Psychosocial treatments for attention-deficit/hyperactivity disorder in children. J Clin Psychiatry. 2002;63(12):36–43. [PubMed] [Google Scholar]
- 170.Aylward EH, Reiss AL, Reader MJ, Singer HS, Brown JE, Denckla MB. Basal ganglia volumes in children with attention-deficit hyperactivity disorder. J Child Neurol. 1996;11(2):112–5. doi: 10.1177/088307389601100210. [DOI] [PubMed] [Google Scholar]
- 171.Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53(7):607–16. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 172.Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18(15):5901–7. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Krause KH, Dresel SH, Krause J, Kung H. F. e. a., Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission tomography. Neurosci Lett. 2000;285(2):107–10. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- 174.Krause KH, Dresel SH, Krause J, Kung H. F. e. a., Tatsc K, Lochmuller H. Elevated striatal dopamine transporter in a drug naive patient with Tourette syndrome adn attention deficit/hyperactivity disorder: positive effect of methylphenidate. J Neurol. 2002;249(8):1116–8. doi: 10.1007/s00415-002-0746-9. [DOI] [PubMed] [Google Scholar]
- 175.Zametkin AJ, Liebenauer LL, Fitzgerald GA, King AC, Minkunas DV, Herscovitch P, Yamada EM, Cohen RM. Brain metabolism in teenagers with attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1993;50(5):333–40. doi: 10.1001/archpsyc.1993.01820170011002. [DOI] [PubMed] [Google Scholar]
- 176.Wilens TE, Upadhyaya HP. Impact of substance use disorder on ADHD and its treatment. J Clin Psychiatry. 2007;68(8):e20. doi: 10.4088/jcp.0807e20. [DOI] [PubMed] [Google Scholar]
- 177.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575–9. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 178.Cook EHJ, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56(4):993–998. [PMC free article] [PubMed] [Google Scholar]
- 179.Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, Cleveland HH, Sanders ML, Gard JM, Stever C. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnositc subtype and severity. Am J Hum Genet. 1998;63(6):1767–76. doi: 10.1086/302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry. 1999;4(2):192–6. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- 181.Brookes KJ, Mill J, Guindalini C, Curran S, Xu X, Knight J, Chen CK, Huang YS, Sethna V, Taylor E, Chen W, Breen G, Asherson P. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Arch Gen Psychiatry. 2006;63(1):74–81. doi: 10.1001/archpsyc.63.1.74. [DOI] [PubMed] [Google Scholar]
- 182.Muglia P, Jain U, Inkster B, Kennedy JL. A quantitative trait locus analysis of the dopamine transporter gene in adults with ADHD. Neuropsychopharmacology. 2002;27(4):655–62. doi: 10.1016/S0893-133X(02)00328-7. [DOI] [PubMed] [Google Scholar]
- 183.Kim DJ, Roh S, Kim YJ, Yoon SJ, Lee HK, Han CS, Kim YK. High concentrations of plasma brain-derived neurotrophic factor in methamphetamine users. Neurosci Lett. 2005;388(2):112–5. doi: 10.1016/j.neulet.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 184.Bellgrove MA, Chambers CD, Johnson KA, Daibhis A, Daly M, Hawi Z, Lambert D, Gill M, Robertson IH. Dopaminergic genotype biases spatial attention in healthy children. Mol Psychiatry. 2007;12(8):786–92. doi: 10.1038/sj.mp.4002022. [DOI] [PubMed] [Google Scholar]
- 185.Guindalini C, Howard M, Haddley K, Laranjeira R, Collier D, Ammar N, Craig I, O’Gara C, Bubb VJ, Greenwood T, Kelsoe J, Asherson P, Murray RM, Castelo A, Quinn JP, Vallada H, Breen G. A dopamine transporter gene functional variant associated with cocaine abuse in a Brazilian sample. Proc Natl Acad Sci U S A. 2006;103(12):4552–7. doi: 10.1073/pnas.0504789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Winsberg BG, Comings DE. Association of the dopamine transporter gene (DAT1) with poor methylphenidate response. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1474–7. doi: 10.1097/00004583-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 187.Roman T, Rohde LA, Hutz MH. Polymorphisms of the dopamine transporter gene: influence on response to methylphenidate in attention deficit-hyperactivity disorder. Am J Pharmacogenomics. 2004;4(2):83–92. doi: 10.2165/00129785-200404020-00003. [DOI] [PubMed] [Google Scholar]
- 188.Cheon KA, Ryu YH, Kim JW, Cho DY. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. Eur Neuropsychopharmacol. 2005;15(1):95–101. doi: 10.1016/j.euroneuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 189.Kooij JS, Boonstra AM, Vermeulen SH, Heister AG, Burger H, Buitelaar JK, Franke B. Response to methylphenidate in adults with ADHD is associated with a polymorphism in SLC6A3 (DAT1) Am J Med Genet B Neuropsychiatr Genet. 2007 doi: 10.1002/ajmg.b.30586. oct 22 epub. [DOI] [PubMed] [Google Scholar]
- 190.Gilbert DL, Wang Z, Sallee FR, Ridel KR, Merhar S, Zhang J, Lipps TD, White C, Badreldin N, Wassermann EM. Dopamine transporter genotype influences the physiological response to medication in ADHD. Brain. 2006;129(8):2038–46. doi: 10.1093/brain/awl147. [DOI] [PubMed] [Google Scholar]
- 191.Joober R, Grizenko N, Sengupta S, Amor LB, Schmitz N, Schwartz G, Karama S, Lageix P, Fathalli F, Torkaman-Zehi A, Ter Stepanian M. Dopamine transporter 3′-UTR VNTR genotype and ADHD: a pharmaco-behavioural genetic study with methylphenidate. Neuropsychopharmacology. 2007;32(6):1370–6. doi: 10.1038/sj.npp.1301240. [DOI] [PubMed] [Google Scholar]
- 192.Mick E, Biederman J, Spencer T, Faraone SV, Sklar P. Absence of association with DAT1 polymorphism and response to methylphenidate in a sample of adults with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2006;141(8):890–4. doi: 10.1002/ajmg.b.30376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Krause J, Dresel SH, Krause KH, La Fougère C, Zill P, Ackenheil M. Striatal dopamine transporter availability and DAT-1 gene in adults with ADHD: no higher DAT availability in patients with homozygosity for the 10-repeat allele. World J Biol Psychiatry. 2006;7(3):152–7. doi: 10.1080/15622970500518444. [DOI] [PubMed] [Google Scholar]
- 194.Kirley A, Lowe N, Hawi Z, Mullins C, Daly G, Waldman I, McCarron M, O’Donnell D, Fitzgerald M, Gill M. Association of the 480 bp DAT1 allele with methylphenidate response in a sample of Irish children with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2003;121(1):50–4. doi: 10.1002/ajmg.b.20071. [DOI] [PubMed] [Google Scholar]
- 195.Stein MA, Waldman ID, Sarampote CS, Seymour KE, Robb AS, Conlon C, Kim SJ, Cook EH. Dopamine transporter genotype and methylphenidate dose response in children with ADHD. Neuropsychopharmacology. 2005;30(7):1374–82. doi: 10.1038/sj.npp.1300718. [DOI] [PubMed] [Google Scholar]
- 196.Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87(1):20–9. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 197.Collins SL, Levin FR, Foltin RW, Kleber HD, Evans SM. Response to cocaine, alone and in combination with methylphenidate, in cocaine abusers with ADHD. Drug Alcohol Depend. 2006;82(2):158–67. doi: 10.1016/j.drugalcdep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 198.Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11(3):184–90. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 199.Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21(11):4090–103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Goldstein RZ, Volkow ND, Chang L, Wang GJ, Fowler JS, Depue RA, Gur RC. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;59(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22(2):133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 202.Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, Innis RB, Laruelle M. The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology. 2001;24(5):553–560. doi: 10.1016/S0893-133X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 203.Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry. 2000;157(10):1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- 204.van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46(5):745–751. [PubMed] [Google Scholar]
- 205.Jonsson EG, Nothen MM, Gustavsson JP, Neidt H, Bunzel R, Propping P, Sedvall GC. Polymorphisms in the dopamine, serotonin, and norepinephrine transporter genes and their relationships to monoamine metabolite concentrations in CSF of healthy volunteers. Psychiatry Res. 1998;79(1):1–9. doi: 10.1016/s0165-1781(98)00027-4. [DOI] [PubMed] [Google Scholar]
- 206.Laruelle M, Abi-Dargham A, VanDyck CH, Rosenblatt W, Zea-Ponce Y, Zonghbi SS, Baldwin RM, Charney DS, Hoffer PB, Kung H. F. e. a. SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med. 1995;36(7):1182–90. [PubMed] [Google Scholar]
- 207.Miller MA, Hughes AL. Epidemiology of amphetamine use in teh United States. In: Cho AK, Segal DS, editors. Amphetamine and its Analogs. Academic Press; San Diego: 1994. p. 503. [Google Scholar]
- 208.Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133(1):130–8. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- 209.Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8(16):1–5. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- 210.Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20(6):RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mitchell SH, Laurent CL, de Wit H. Interaction of expectancy and the pharmacological effects of d-amphetamine: subjective effects and self-administration. Psychopharmacology. 1996;125(4):371–8. doi: 10.1007/BF02246020. [DOI] [PubMed] [Google Scholar]
- 212.Cesarec Z, Nyman AK. Differential response to amphetamine in schizophrenia. Acta Psychiatr Scand. 1985;71(5):523–38. doi: 10.1111/j.1600-0447.1985.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 213.Hutchison KE, Wood MD, Swift R. Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Exp Clin Psychopharmacol. 1999;7(4):493–501. doi: 10.1037//1064-1297.7.4.493. [DOI] [PubMed] [Google Scholar]
- 214.White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73(4):729–41. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- 215.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100(10):6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dlugos A, Freitag C, Hohoff C, McDonald JS, Cook EH, Deckert J, de Wit H. Norepinephrine transporter gene variation modulates acute response to D-amphetamine. Biol Psychiatry. 2007;61(11):1296–305. doi: 10.1016/j.biopsych.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 217.Lott DC, Kim SJ, Cook EH, de Wit H. Serotonin transporter genotype and acute subjective response to amphetamine. Am J Addict. 2006;15(5):327–35. doi: 10.1080/10550490600859868. [DOI] [PubMed] [Google Scholar]
- 218.Hohoff C, McDonald JM, Baune BT, Cook EH, Deckert J, de Wit H. Interindividual variation in anxiety response to amphetamine: possible role for adenosine A2A receptor gene variants. Am J Med Genet B Neuropsychiatr Genet. 2005;139(1):42–4. doi: 10.1002/ajmg.b.30228. [DOI] [PubMed] [Google Scholar]
- 219.Veenstra-VanderWeele J, Qaadir A, Palmer AA, Cook EHJ, de Wit H. Association between the casein kinase 1 epsilon gene region and subjective response to Damphetamine. Neuropsychopharmacology. 2006;31(5):1056–63. doi: 10.1038/sj.npp.1300936. [DOI] [PubMed] [Google Scholar]
- 220.Flanagin BA, Cook EHJ, de Wit H. An association study of the brain-derived neurotrophic factor Val66Met polymorphism and amphetamine response. Am J Med Genet B Neuropsychiatr Genet. 2006;141(6):576–83. doi: 10.1002/ajmg.b.30327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14(4):1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- 222.Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend. 1983;11(2):147–165. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- 223.Fergusson DM, Horwood LJ, Lynskey MT, Madden PA. Early reactions to cannabis predict later dependence. Arch Gen Psychiatry. 2003;60(10):1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- 224.Gulley JM, Everett CV, Zahniser NR. Inbred Lewis and Fischer 344 rat strains differ not only in novelty- and amphetamine-induced behaviors, but also in dopamine transporter activity in vivo. Brain Res. 2007;1151(2):32–45. doi: 10.1016/j.brainres.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Haile CN, Zhang XY, Carroll FI, Kosten TA. Cocaine self-administration and locomotor activity are altered in Lewis and F344 inbred rats by RTI 336, a 3-phenyltropane analog that binds to the dopamine transporter. Brain Res. 2005;1055(12):186–95. doi: 10.1016/j.brainres.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 226.Lake CR, Quirk RS. CNS stimulants and the look-alike drugs. Psychiatr Clin North Am. 1984;7:689–701. [PubMed] [Google Scholar]
- 227.Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos. 1993;21:717–723. [PubMed] [Google Scholar]
- 228.Fischman MW, Schuster CR. Tolerance development to chronic methampetamine intoxication in the rhesus monkey. Pharmacol Biochem Behav. 1974;2:503–508. doi: 10.1016/0091-3057(74)90010-0. [DOI] [PubMed] [Google Scholar]
- 229.Perez-Reyes M, White WR, McDonald SA, Hicks RE, Jeffcoat AR, Hill JM, Cook CE. Clinical effects of daily methamphetamine administration. Clin Neuropharmacol. 1991;14:352–358. doi: 10.1097/00002826-199108000-00007. [DOI] [PubMed] [Google Scholar]
- 230.Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–248. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 231.Gawin FH, Ellinwood EHJ. Cocaine and other stimulants. Actions, abuse, and treatment. N Engl J Med. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- 232.Cretzmeyer M, Sarrazin MV, Huber DL, Block RI, Hall JA. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24:267–277. doi: 10.1016/s0740-5472(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 233.Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- 234.Srisurapanont M, Ali R, Marsden J, Sunga A, Wada K, Monteiro M. Psychotic symptoms in methamphetamine psychotic in-patients. Int J Neuropsychopharmacol. 2003;6:347–352. doi: 10.1017/S1461145703003675. [DOI] [PubMed] [Google Scholar]
- 235.Yui K, Ikemoto S, Ishiguro T, Goto K. Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci. 2000;914:1–12. doi: 10.1111/j.1749-6632.2000.tb05178.x. [DOI] [PubMed] [Google Scholar]
- 236.Akiyama K, Kanzaki A, Tsuchida K, Ujike H. Methamphetamine-induced behavioral sensitization and its implications for relapse of schizophrenia. Schizophrenia Res. 1994;12:251–257. doi: 10.1016/0920-9964(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 237.Rose ME, Grant JE. Pharmacotherapy for methamaphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions. Ann Clin Psychiatry. 2008;20(3):145–155. doi: 10.1080/10401230802177656. [DOI] [PubMed] [Google Scholar]
- 238.Zetterstrom T, Sharp T, Collin AK, Ungerstedt U. In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. Eur J Pharmacol. 1988;148:327–334. doi: 10.1016/0014-2999(88)90110-0. [DOI] [PubMed] [Google Scholar]
- 239.Fischer J, Cho A. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- 240.Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14(4):2260–71. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu Y, Edwards RH. The role of vesicular transport porteins in synaptic transmission and neural degeneration. Annu Rev Neurosci. 1997;20:125–156. doi: 10.1146/annurev.neuro.20.1.125. [DOI] [PubMed] [Google Scholar]
- 242.Berman SB, Zigmond MJ, Hastings TG. Modification of dopamine transporter function: effect of reactive oxygen species and dopamine. J Neurochem. 1996;67:593–600. doi: 10.1046/j.1471-4159.1996.67020593.x. [DOI] [PubMed] [Google Scholar]
- 243.Fleckenstein AE, Metzger RR, Beyeler ML, Gibb JW, Hanson GR. Oxygen radicals diminish dopamine transporter function in rat striatum. Eur J Pharmacol. 1997;334:111–114. doi: 10.1016/s0014-2999(97)01175-8. [DOI] [PubMed] [Google Scholar]
- 244.Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur J Pharmacol. 1998;361:269–275. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- 245.Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 246.Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- 247.Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacol Sci. 2003;92:178–195. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- 248.Metzger RR, Haughey HM, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid decrease in dopamine transporter function: role of dopamine and hyperthermia. J Pharmacol Exp Ther. 2000;295:1077–1085. [PubMed] [Google Scholar]
- 249.Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S. Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology. 1989;98:89–92. doi: 10.1007/BF00442011. [DOI] [PubMed] [Google Scholar]
- 250.Witkin JM, Savtchenko N, Mashkovsky M, Beekman M, Munzar P, Gasior M, Goldberg SR, Ungard JT, Kim J, Shippenberg T, Chefer V. Behavioral, toxic, and neurochemical effects of sydnocarb, a novel psychomotor stimulant: comparisons with methamphetamine. J Pharmacol Exp Ther. 1999;288:1298–1310. [PubMed] [Google Scholar]
- 251.Wong AHC, VanTol HHM. The dopamine D4 receptors and mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1091–1099. doi: 10.1016/j.pnpbp.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 252.Okuyama S, Kawashima N, Chaki S, Yoshikawa R, Funakoshi T, Ogawa SI, Suzuki Y, Ideda Y, Kumagai T, Nakazato A, Nagamine M, Tomisawa K. A selective dopamine D4 receptor antagonist, NRA0160: a preclinical neuropharmacological profile. Life Sci. 1999;65:2109–2125. doi: 10.1016/s0024-3205(99)00476-2. [DOI] [PubMed] [Google Scholar]
- 253.Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, Kobayashi K, Yoshida S, Maeda K, Takuma K, Nabeshima T, Yamada K. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol Psychiatry. 2006;59(1):75–84. doi: 10.1016/j.biopsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 254.Wachtel SR, Ortengren A, de Wit H. The effects of acute haloperidol or risperidone on subjective responses to methamphetamine in healthy volunteers. Drug Alcohol Depend. 2002;68(1):23–33. doi: 10.1016/s0376-8716(02)00104-7. [DOI] [PubMed] [Google Scholar]
- 255.McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;58:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 257.Iyo M, Sekine Y, Mori N. Neuromechanism of developing methamphetamine psychosis: a neuroimaging study. Ann N Y Acad Sci. 2004;1025:288–295. doi: 10.1196/annals.1316.036. [DOI] [PubMed] [Google Scholar]
- 258.Chen CK, Hu X, Lin SK, Sham PC, el-W L, Li T, Murray RM, Ball DM. Association analysis of dopamine D2-like receptor genes and methamphetamine abuse. Psychiatr Genet. 2004;14(4):223–6. doi: 10.1097/00041444-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 259.Liu HC, Lin SK, Liu SK, Chen SL, Hu CJ, Chang JG, Leu SJ. DAT polymorphism and diverse clinical manifestations in methamphetamine abusers. Psychiatr Genet. 2004;14(1):33–7. doi: 10.1097/00041444-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 260.Vandenbergh DJ, Rodriguez LA, Hivert E, Schiller JH, Villareal G, Pugh EW, Lachman H, Uhl GR. Long forms of the dopamine receptor (DRD4) gene VNTR are more prevalent in substance abusers: no interaction with functional alleles of the catechol-o-methyltransferase (COMT) gene. Am J Med Genet. 2000;96(5):678–83. doi: 10.1002/1096-8628(20001009)96:5<678::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 261.Hosák L, Libiger J, Cizek J, Beránek M, Cermáková E. The COMT Val158Met polymorphism is associated with novelty seeking in Czech methamphetamine abusers: preliminary results. Neuro Endocrinol Lett. 2006;27(6):799–802. [PubMed] [Google Scholar]
- 262.Suzuki A, Nakamura K, Sekine Y, Minabe Y, Takei N, Suzuki K, Iwata Y, Kawai M, Takebayashi K, Matsuzaki H, Iyo M, Ozaki N, Inada T, Iwata N, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Mori N. An association study between catechol-O-methyl transferase gene polymorphism and methamphetamine psychotic disorder. Psychiatr Genet. 2006;16(4):133–8. doi: 10.1097/01.ypg.0000218613.35139.cd. [DOI] [PubMed] [Google Scholar]
- 263.Ujike H, Harano M, Inada T, Yamada M, Komiyama T, Sekine Y, Sora I, Iyo M, Katsu T, Nomura A, Nakata K, Ozaki N. Nine- or fewer repeat alleles in VNTR polymorphism of the dopamine transporter gene is a strong risk factor for prolonged methamphetamine psychosis. Pharmacogenetics J. 2003;3(4):242–247. doi: 10.1038/sj.tpj.6500189. [DOI] [PubMed] [Google Scholar]
- 264.Matsuzawa D, Hashimoto K, Miyatake R, Shirayama Y, Shimizu E, Maeda K, Suzuki Y, Mashimo Y, Sekine Y, Inada T, Ozaki N, Iwata N, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Hata A, Sawa A, Iyo M. Identification of functional polymorphisms in the promoter region of the human PICK1 gene and their association with methamphetamine psychosis. Am J Psychiatry. 2007;164(7):1105–14. doi: 10.1176/ajp.2007.164.7.1105. [DOI] [PubMed] [Google Scholar]
- 265.Zimniak P, Nanduri B, Pikua S, Bandorowicz-Piku3a J, Singhal SS, Srivastava SK, Awasthi S, Awasthi YC. Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. Eur J Biochem. 1994;224(3):893–9. doi: 10.1111/j.1432-1033.1994.00893.x. [DOI] [PubMed] [Google Scholar]
- 266.Hashimoto T, Hashimoto K, Matsuzawa D, Shimizu E, Sekine Y, Inada T, Ozaki N, Iwata N, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Iyo M. A functional glutathione S-transferase P1 gene polymorphism is associated with methamphetamine-induced psychosis in Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2005;135(1):5–9. doi: 10.1002/ajmg.b.30164. [DOI] [PubMed] [Google Scholar]
- 267.Aoyama N, Takahashi N, Kitaichi K, Ishihara R, Saito S, Maeno N, Ji X, Takagi K, Sekine Y, Iyo M, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Iwata N, Inada T, Ozaki N. Association between gene polymorphisms of SLC22A3 and methamphetamine use disorder. Alcohol Clin Exp Res. 2006;30(10):1644–9. doi: 10.1111/j.1530-0277.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 268.Ikeda M, Ozaki N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Kishi T, Sekine Y, Iyo M, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Inada T, Iwata N. Possible association of beta-arrestin 2 gene with methamphetamine use disorder, but not schizophrenia. Genes Brain Behav. 2007;6(1):107–12. doi: 10.1111/j.1601-183X.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 269.Ide S, Kobayashi H, Tanaka K, Ujike H, Sekine Y, Ozaki N, Inada T, Harano M, Komiyama T, Yamada M, Iyo M, Ikeda K, Sora I. Gene polymorphisms of the mu opioid receptor in methamphetamine abusers. Ann N Y Acad Sci. 2004 Oct;1025:316–24. doi: 10.1196/annals.1316.039. [DOI] [PubMed] [Google Scholar]
- 270.Ide S, Kobayashi H, Ujike H, Ozaki N, Sekine Y, Inada T, Harano M, Komiyama T, Yamada M, Iyo M, Iwata N, Tanaka K, Shen H, Iwahashi K, Itokawa M, Minami M, Satoh M, Ikeda K, Sora I. Linkage disequilibrium and association with methamphetamine dependence/psychosis of muopioid receptor gene polymorphisms. Pharmacogenomics J. 2006;6(3):179–88. doi: 10.1038/sj.tpj.6500355. [DOI] [PubMed] [Google Scholar]
- 271.Kobayashi H, Hata H, Ujike H, Harano M, Inada T, Komiyama T, Yamada M, Sekine Y, Iwata N, Iyo M, Ozaki N, Itokawa M, Naka M, Ide S, Ikeda K, Numachi Y, Sora I. Association analysis of delta-opioid receptor gene polymorphisms in methamphetamine dependence/psychosis. Am J Med Genet B Neuropsychiatr Genet. 2006;141(5):482–6. doi: 10.1002/ajmg.b.30337. [DOI] [PubMed] [Google Scholar]
- 272.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 273.Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9(5):224–37. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19(10):4110–22. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5(1):69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 276.Jockers-Scherübl MC, Danker-Hopfe H, Mahlberg R, Selig F, Rentzsch J, Schürer F, Lang UE, Hellweg R. Brain-derived neurotrophic factor serum concentrations are increased in drug-naive schizophrenic patients with chronic cannabis abuse and multiple substance abuse. Neurosci Lett. 2004;371(1):79–83. doi: 10.1016/j.neulet.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 277.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 278.Cheng CY, Hong CJ, Yu YW, Chen TJ, Wu HC, Tsai SJ. Brain-derived neurotrophic factor (Val66Met) genetic polymorphism is associated with substance abuse in males. Brain Res Mol Brain Res. 2005;140(12):86–90. doi: 10.1016/j.molbrainres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 279.Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23(17):6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Liu QR, Walther D, Drgon T, Polesskaya O, Lesnick TG, Strain KJ, de Andrade M, Bower JH, Maraganore DM, Uhl GR. Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson’s Disease. Am J Med Genet B Neuropsychiatr Genet. 2005;134(1):93–103. doi: 10.1002/ajmg.b.30109. [DOI] [PubMed] [Google Scholar]


