Abstract
The fundamental mechanisms that underlie endotoxin tolerance remain to be elucidated, and the clinical significance of endotoxin tolerance in the context of active systemic infection remains in question. We hypothesized that the endotoxin tolerance phenotype would result in decreased inflammation at the expense of altered bacterial clearance and, thus, higher mortality in a murine model of polymicrobial sepsis induced by cecal ligation and puncture (CLP). Endotoxin tolerance was induced in C57Bl/6 mice with 5 mg/kg LPS or vehicle 18 h before subsequent CLP. Lung tissue, peritoneal fluid, and blood were collected at 1, 3, 6, and 18 h after surgery for subsequent analysis. Peritoneal macrophages were isolated for ex vivo phagocytosis assay. In separate experiments, mice were allowed to recover, and survival was monitored for 7 days. Endotoxin tolerance attenuated plasma TNF-α and IL-6 at 6 h after CLP. Peritoneal fluid cytokines were significantly attenuated as well. Endotoxin tolerance significantly improved bacterial clearance in both blood and peritoneal fluid after CLP. Similarly, ex vivo phagocytosis by primary peritoneal macrophages and RAW264.7 murine peritoneal macrophages was significantly improved after induction of the endotoxin tolerance phenotype. Contrary to our original hypothesis, we conclude that endotoxin tolerance significantly attenuates the host inflammatory response, augments bacterial clearance, and improves survival in this murine model of polymicrobial sepsis.
Keywords: Rodent, LPS, inflammation, phagocytosis, tolerance
INTRODUCTION
All cells respond to stress through the activation of primitive, evolutionarily conserved genetic programs that maintain homeostasis and ensure cell survival. Stress adaptation, which is known by a myriad of terms, including tolerance, desensitization, conditioning, and reprogramming, is a common paradigm found throughout nature in which a primary exposure of a cell or organism to a stressful stimulus results in an adaptive response by which repeated exposure to the same stimulus produces a minimal response. For example, primary exposure of a cell or organism to LPS, a cell wall component of gram-negative bacteria, produces a change in phenotype whereby a second exposure to LPS produces a minimal response—a phenomenon known as endotoxin tolerance (1). Although LPS induces a dramatic increase in TNF-α gene expression in human peripheral blood monocytes (PBMCs), a second exposure produces a markedly attenuated response with decreased TNF-α gene expression (2). The common assertion that endotoxin tolerance represents a global down-regulation of proinflammatory gene expression, however, is perhaps incomplete and not entirely accurate. Whereas TNF-α production is significantly diminished, production of other proinflammatory cytokines such as IL-1β and IL-6 may be increased, decreased, or unchanged (3).
Although the basic concept has been known for decades, the fundamental mechanisms occurring at the molecular level that lead to endotoxin tolerance remain to be fully elucidated. More importantly, the physiological significance and clinical relevance of endotoxin tolerance, especially in the context of a live bacterial infection, remain in question (4–6). Here, we sought to determine the effects of the endotoxin tolerance phenotype in a clinically relevant model of sepsis that involves live bacterial infection, rather than bolus injection of LPS.
The cecal ligation and puncture (CLP) model of polymicrobial sepsis is a well-characterized model that closely mimics the sepsis syndrome in humans (7). Although the cytokine profile induced by CLP is similar to that after bolus injection of LPS, the time course over which this inflammatory response develops is quite distinct. Compared with the fulminant and relatively self-limited course induced by bolus injection of LPS, the response after CLP is slower to develop and is characterized, at least initially, by a state of hyperdynamic vaso-dilatory shock for approximately 12 h, followed by decreased cardiac output and vasoplegia thereafter. Cecal ligation and puncture therefore closely mimics the clinical sepsis syndrome induced by intestinal perforation with polymicrobial peritonitis (8, 9). An intact innate immune response is essential to combat this live bacterial challenge. In direct contrast to bolus injection of LPS, a compensatory anti-inflammatory response or the administration of anti-inflammatory agents such as IL-10, IL-1 receptor antagonist, or anti–TNF-α antibodies may actually hasten mortality in the CLP model by impairing the host’s ability to clear the bacterial challenge (10–12). Therefore, given the effects of LPS preconditioning on the innate immune response, we hypothesized that LPS preconditioning would result in decreased inflammation at the expense of altered bacterial clearance and, thus, higher mortality in this model of polymicrobial sepsis (4, 13–16).
MATERIALS AND METHODS
CLP of polymicrobial sepsis
All experiments were conducted in accordance with the National Institutes of Health’s Guidelines for the Use of Laboratory Animals (National Institutes of Health Publication 85–23, revised 1996) and with approval of the Institutional Animal Care and Use Committee, Cincinnati Children’s Research Foundation. Animals were acclimatized for 7 days before surgical manipulation and maintained on 12-h light-dark cycles with access to food and water ad libitum. Male C57Bl/6 mice (Charles River Laboratories, Wilmington, Mass) weighing 25 to 30 g were used in all in vivo experiments. Cecal ligation and puncture was performed as previously described (7). Briefly, mice were preconditioned with LPS (Escherichia coli 0111:B4; Sigma-Aldrich, St. Louis, Mo), 5 mg/kg body weight, or vehicle administered via intraperitoneal injection and were returned to their cages. This LPS preconditioning regimen has been shown previously by our laboratory to reduce the levels of circulating cytokines and protect against LPS-mediated death (17). LPS preconditioning occurred at the same time of day for all experiments. After 18 h, mice were anesthetized with administration of thiopentone sodium (400 μg/10 g body weight, i.p.). Once adequate anesthesia was achieved, the mice were placed in a supine position, and the lower abdomen was shaved and prepared with alcohol. The cecum was exteriorized via a small abdominal incision and then ligated with a 3–0 silk suture placed approximately 5 mm distal to the ileocecal valve. The ligated cecum was punctured twice with a 21-gauge needle and gently squeezed to extrude a small amount of fecal content. The cecum was replaced in the abdomen, and the incision was closed in two layers with 4–0 silk sutures and cyanoacrylate adhesive. One milliliter of 0.9% isotonic sodium chloride solution was injected subcutaneously to compensate for third-space fluid loss that occurred during the procedure, and mice were subsequently placed on a heating pad and allowed to recover from anesthesia. Sham mice were treated identically, except that the cecum was neither ligated nor punctured. Lung tissue, peritoneal fluid, and blood samples were collected at 1, 3, 6, and 18 h after CLP. In separate experiments, mice were returned to their cages and monitored closely for survival for 7 days.
Measurement of plasma and peritoneal fluid concentrations of cytokines
Plasma and peritoneal fluid concentrations of TNF-α, IL-6, and IL-10 were measured via commercially available enzyme-linked immunosorbent assay kits (Biosource International, Camarillo, Calif) using the protocols recommended by the manufacturer.
Measurement of white blood cell counts
Standard techniques of clinical pathology were used to determine white blood cell count (WBC) in both plasma and peritoneal fluid, as previously described (18).
Subcellular fractionation and protein extraction
Lung tissue samples were homogenized with a Polytron homogenizer (Brinkmann Instruments, West Orange, NY) in a buffer containing 0.32 M sucrose, 10 mM Tris-HCl (pH 7.4), 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 10 mM 2-ME, 20 μM leupeptin, 0.15 μM pepstatin A, 0.2 mM phenyl-methylsulfonyl fluoride, 50 mM NaF, 1 mM sodium orthovanadate, and 0.4 nM microcystin. The homogenates were centrifuged (1,000 × g, 10 min), and the supernatant (cytosol + membrane extract) was collected for evaluation of IkB kinase (IKK) activity as described below.
IKK kinase assay
IkB kinase activity was determined by immune complex kinase assay and was estimated as the ability to phosphorylate glutathion-S-transferase–IκBα as previously described (19). After immunoprecipitation of lysate with specific antibody directed to IKK-γ (Santa Cruz Biotechnology, Santa Cruz, Calif), the immunoprecipitate was incubated for 30 min at 30°C in 40 μL of reaction buffer (25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.6; 20 mM MgCl2; 20 mM glycerolphosphate; 0.1 mM sodium orthovanadate; 2 mM dithiothreitol; 25 μM adenosine triphosphate; and 5 μCi of [γ-32P] adenosine triphosphate. Glutathion-S-transferase–IκBα (1 μg) was used as substrates for IKK complex. Reaction products were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by autoradiography. Densitometric analysis was performed using ImageQuant (Molecular Dynamics, Sunnyvale, Calif).
Bacterial clearance
In separate experiments, mice were killed under isoflurane 24 h after CLP. Blood samples were obtained via direct cardiac puncture using sterile technique. Using a sterile 22-gauge needle, 1 mL of sterile phosphate-buffered saline was injected through the fascia into the peritoneal cavity and gently aspirated. Serial dilutions of blood and peritoneal fluid lavage were plated on nutrient agar and incubated overnight at 37°C. Colony counts were performed to assess bacterial burden.
Cell culture
RAW264.7 murine peritoneal macrophages (American Type Culture Collection, Bethesda, Md) were maintained in Dulbecco’s Modified Eagle’s Medium (Gibco BRL, Grand Island, NY) containing 10% fetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, and 2.2 g/L sodium bicarbonate (Sigma) at 37°C in a room air/5% CO2 tissue culture incubator. Cells were used between passages 3 and 5.
Isolation of murine peritoneal macrophages
In separate experiments, C57Bl/6 mice (Charles River Laboratories) were preconditoned with either LPS (5 mg/kg, i.p.) or vehicle 18 h before isolation of primary peritoneal macrophages via peritoneal lavage. Briefly, mice were anesthetized with isoflurane, and the peritoneal fascia was exposed by dissection. Using a sterile 22-gauge needle, 3 mL of sterile phosphate-buffered saline was injected through the fascia into the peritoneal cavity. Peritoneal fluid was then withdrawn through the fascia with an 18-gauge needle. The recovered peritoneal fluid was centrifuged at 1,500 rpm × 10 min, and the pellet was isolated, resuspended in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum, and plated on a 96-well plate. Peritoneal macrophages were allowed to adhere for 1 h at 37°C. Cytospin of the sample was performed to confirm the percentage of macrophages present, which was consistently greater than 90% of the cells in the sample.
Phagocytosis assay
Phagocytic function of RAW264.7 cells or primary peritoneal macrophages was analyzed using a Vybrant phagocytosis assay kit (V-6694; Molecular Probes, Eugene, Ore) according to the manufacturer’s protocol. Using this method, the process of phagocytosis can be quantified by following the internalization of fluorescently labeled bacterial particles. The protocol takes advantage of the detectability of the intracellular fluorescence emitted by the engulfed bacteria and the effective fluorescence quenching of remaining extracellular probe by trypan blue. Briefly, 100,000 cells/mL of RAW264.7 cells or primary peritoneal macrophages obtained from vehicle- or LPS-preconditioned mice were plated on a 96-well plate. The endotoxin tolerance phenotype was induced in RAW264.7 via preconditioning with LPS (E. coli 0111:B4; Sigma-Aldrich; 10 ng/mL) or vehicle × 18 h at 37°C. Both cell types were then treated with fluorescein-labeled E. coli or Staphylococcus aureus (Sigma) and incubated in the dark at 37°C × 2 h. The bacterial suspension was removed after 2 h. The cells were treated with trypan blue to quench any remaining extracellular probe. The trypan blue was removed, and the 96-well plates were analyzed by a fluorometer, with phagocytosis expressed as the percentage increase over baseline, with baseline phagocytosis being that of the untreated RAW 264.7 cells or vehicle-treated primary peritoneal macrophages, respectively.
Data analysis
All values in the figures and text are expressed as mean ± SEM of n observations. The results were examined by ANOVA, followed by a Bonferroni correction post hoc t test. The log-rank method was used to compare differences in survival rates between groups. A P value less than 0.05 was considered significant.
RESULTS
LPS preconditioning attenuates the systemic inflammatory response after CLP
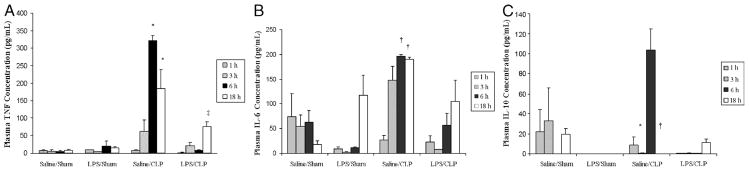
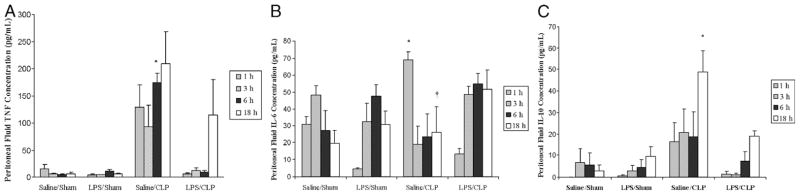
LPS preconditioning alone did not elicit clinical signs of sepsis during the 18 h before CLP or sham surgery. However, by 4 to 6 h after CLP, all of the mice developed early clinical signs of sepsis, including lethargy, piloerection, and diarrhea. By 6 h after CLP, there was a significant increase in both plasma (Fig. 1) and peritoneal fluid (Fig. 2) TNF-α, IL-6, and IL-10 concentrations. In contrast, plasma and peritoneal fluid TNF-α, IL-6, and IL-10 concentrations were significantly decreased in LPS-preconditioned mice, providing evidence that LPS preconditioning attenuated the host inflammatory response in this model.
Fig. 1. LPS preconditioning attenuates the host systemic inflammatory response after CLP.
C57Bl/6 mice (n = 3 at each time point per group) were preconditioned with either 5 mg/kg LPS or vehicle (0.9% saline) × 18 h before subsequent CLP or sham surgery. Plasma cytokines (A, TNF-α; B, IL-6; C, IL-10) were measured at 1, 3, 6, and 18 h after CLP. *P < 0.05 saline/CLP vs. saline/sham and LPS/CLP; †P < 0.05 saline/CLP vs. saline/sham only; ‡P < 0.05 LPS/CLP vs. LPS/sham.
Fig. 2. LPS preconditioning attenuates the host systemic inflammatory response after CLP.
C57Bl/6 mice (n = 3 at each time point per group) were preconditioned with either 5 mg/kg LPS or vehicle (0.9% saline) × 18 h before subsequent CLP or sham surgery. Peritoneal fluid cytokines (A, TNF-α; B, IL-6; C, IL-10) were measured at 1, 3, 6, and 18 h after CLP. *P < 0.05 saline/CLP vs. saline/sham and LPS/CLP; †P < 0.05 saline/CLP vs. saline/sham only; ‡P < 0.05 LPS/CLP vs. LPS/sham.
LPS preconditioning inhibits IKK activation after CLP
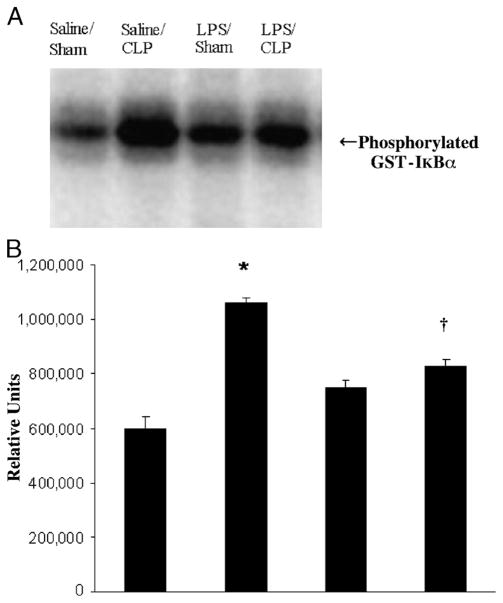
Nuclear factor (NF)–κB is a pluripotent transcription factor that functions as a master switch or control point for the expression of a large number of proinflammatory genes thought to be involved in the pathophysiology of sepsis. A common pathway and rate-limiting step for the activation of NF-kB occurs when its inhibitor IkB is phosphorylated by IKK, specifically at the serine-32 and serine-36 residues of IkBα (20, 21). IkB kinase activation in the lung was significantly increased at 18 h after CLP. LPS preconditioning significantly attenuated IKK kinase activity after CLP compared with mice preconditioned with vehicle alone (Fig. 3). These data demonstrate that LPS preconditioning attenuates the host innate immune response after CLP, at least partially through a mechanism involving inhibition of IKK activation.
Fig. 3. LPS preconditioning inhibits IKK kinase activity in the lung after CLP.
C57Bl/6 mice (n = 3 per group) were preconditioned with either 5 mg/kg LPS or saline × 18 h before subsequent CLP or sham surgery. Lung tissue was harvested at 18 h after CLP, and IKK kinase activity was determined via kinase assay. A representative assay is shown (A) with image quantitative analysis of all mice (B). *P < 0.05 saline/CLP vs. saline/sham; †P < 0.05 saline/CLP vs. LPS/CLP.
LPS preconditioning improves bacterial clearance after CLP
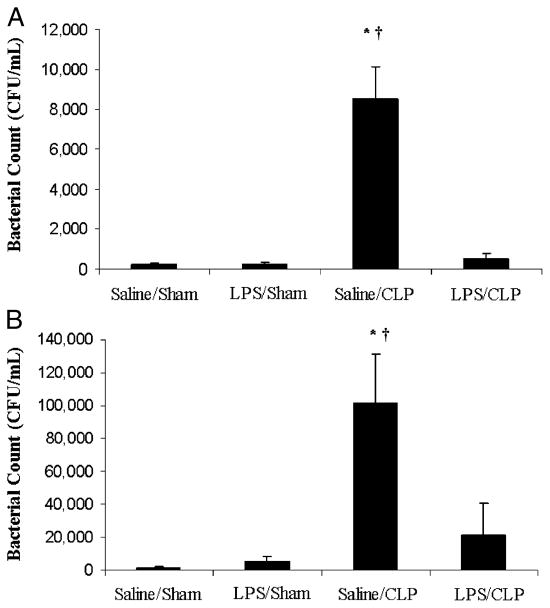
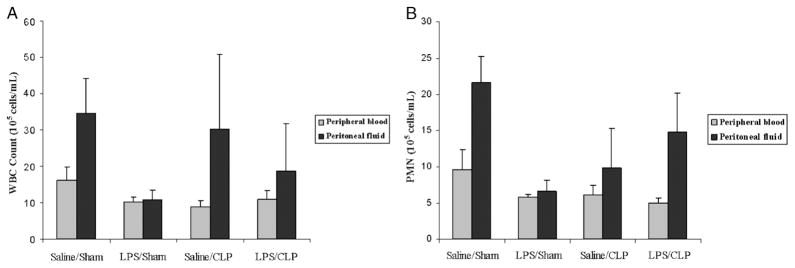
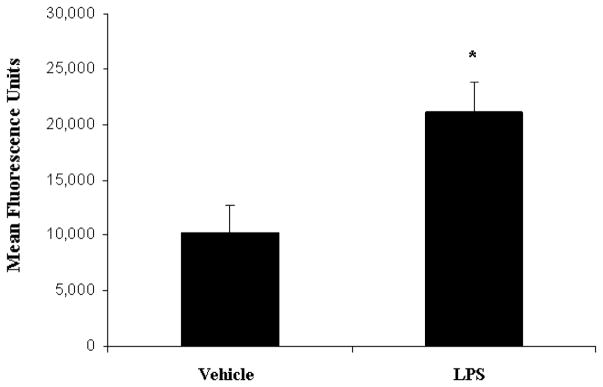
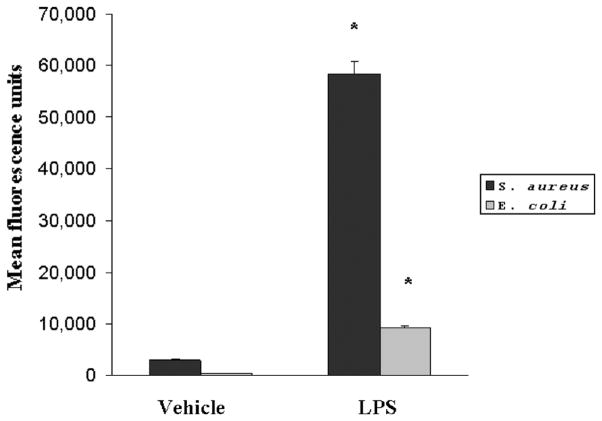
An intact, functional innate immune response is required to combat the polymicrobial infection that occurs after CLP (10–12). The inhibitory effects of LPS preconditioning on the innate immune response can therefore adversely affect bacterial clearance. However, LPS preconditioning was associated with significantly lower quantitative bacterial counts in both the peripheral blood and peritoneal fluid after CLP (Fig. 4). Importantly, the peripheral blood and peritoneal fluid WBC and polymorphonuclear counts did not significantly differ between LPS-preconditioned mice and vehicle-preconditioned mice (Fig. 5). Collectively, these data suggest that LPS preconditioning may have direct effects on bacterial phagocytosis by resident WBCs. To directly test the hypothesis that LPS preconditioning modulates phagocytosis by resident WBCs, primary peritoneal macrophages were isolated at 18 h after in vivo LPS or vehicle preconditioning and exposed to fluoresce in-tagged bacteria ex vivo. LPS preconditioning significantly augmented phagocytosis of both fluorescein-tagged gram-negative bacteria (E. coli) and gram-positive bacteria (S. aureus) (Fig. 6). In separate experiments, RAW264.7 mouse peritoneal macrophages were preconditioned with LPS (10 ng/mL × 18 h) before exposure to either fluorescein-tagged S. aureus or E. coli. This preconditioning regimen consistently induces the endotoxin tolerance phenotype in RAW264.7 macrophages, as determined by a significant reduction in TNF-α expression (data not shown). Similar to the primary peritoneal macrophages, however, LPS preconditioning significantly increased phagocytosis of both gram-negative and gram-positive bacteria in RAW264.7 macrophages (Fig. 7). Collectively, these data demonstrate that LPS preconditioning augments bacterial clearance through increased phagocytosis by resident macrophages.
Fig. 4. LPS preconditioning is associated with lower bacterial counts in the peripheral blood (A) and peritoneal fluid (B).
C57Bl/6 mice (n = 5 per group) were preconditioned with either 5 mg/kg LPS or vehicle (0.9% saline) × 18 h before subsequent CLP or sham surgery. Peripheral blood and peritoneal fluid samples were obtained 24 h after CLP and cultured in nutrient agar at 37°C for 24 h. *P < 0.05 saline/CLP vs. saline/sham; †P < 0.05 saline/CLP vs. LPS/CLP.
Fig. 5. LPS preconditioning does not significantly decrease peripheral blood or peritoneal WBC counts after CLP.
C57Bl/6 mice (n = 8 per group) were preconditioned with either 5 mg/kg LPS or vehicle (0.9% saline) × 18 h before subsequent CLP or sham surgery. Peripheral blood and peritoneal fluid samples were obtained 24 h after CLP, and WBC counts (A) and PMN (B) were measured using Coulter counter.
Fig. 6. LPS preconditioning significantly increases bacterial clearance by augmenting phagocytosis.
Ex vivo phagocytosis was performed using primary peritoneal macrophages cultured from mice at 18 h after preconditioning with either LPS (5 mg/kg) or vehicle (0.9% saline). Peritoneal macrophages were then treated with fluorescein-tagged S. aureus and E. coli. *P < 0.05 compared with vehicle.
Fig. 7. LPS preconditioning significantly increases bacterial clearance by augmenting phagocytosis.
RAW264.7 mouse peritoneal macrophages were preconditioned with either LPS (10 ng/mL) or vehicle (0.9% saline) for 18 h before subsequent treatment with either fluorescein-tagged S. aureus or E. coli. *P < 0.05 compared with vehicle.
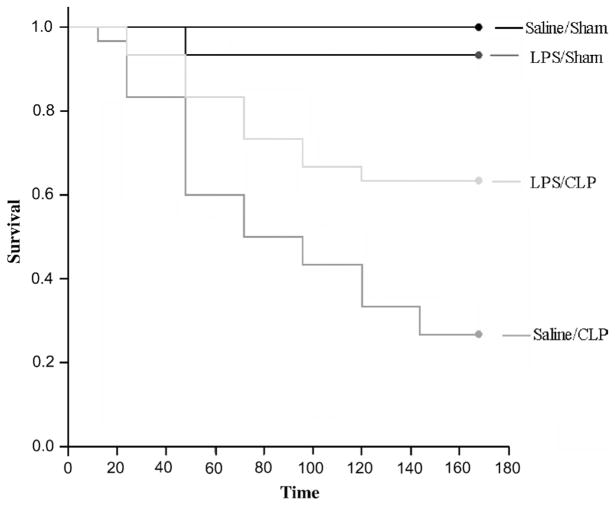
LPS preconditioning improves survival after CLP
LPS preconditioning significantly improved survival after CLP when compared with mice that were preconditioned with vehicle alone (Fig. 8).
Fig. 8. Kaplan-Meier survival curves after CLP.
C57Bl/6 mice (n = 30 per group) were preconditioned with either LPS (5 mg/kg, i.p.) or vehicle 18 h before CLP or sham surgery. Survival was monitored for 7 days. LPS preconditioning significantly improved survival after CLP (P < 0.01).
DISCUSSION
Beeson (22) first described the phenomenon of endotoxin tolerance more than 60 years ago by demonstrating that the febrile response of rabbits diminished with consecutive administration of endotoxin. However, whereas the basic concept has been known for decades, the underlying mechanisms and clinical relevance of endotoxin tolerance remain in question. Several different methods of in vivo LPS preconditioning have been described in the literature, all of which use a low, sublethal dose of LPS that usually does not elicit a detectable host inflammatory response. Our laboratory has previously shown that single administration of a sublethal dose of LPS (5 mg/kg) significantly attenuates TNF-α expression and improves survival after administration of a lethal (60 mg/kg) dose of LPS. These effects are at least in part mediated through inhibition of the NF-κB pathway (17). However, compared with the fulminant and relatively self-limited course induced by bolus injection of LPS, the response after CLP is slower to develop and more closely mimics the sepsis syndrome in humans (7). Furthermore, the effects of LPS preconditioning on the innate immune response in the context of an ongoing live polymicrobial infection may not be predictable based on the classic endotoxin tolerance paradigm. For example, it may be possible that endotoxin tolerance–mediated attenuation of the host inflammatory response will worsen the outcome after live bacterial infection due to a relative inability to respond to the infectious challenge. In contrast to our original hypothesis, here, we show that LPS preconditioning (also known as pretreatment, preexposure, priming, or reprogramming) attenuated the host inflammatory response but augmented bacterial clearance in a murine model of polymicrobial, septic peritonitis. These effects were also associated with markedly improved survival.
Several studies have noted that PBMC isolated from both children and adults with the systemic inflammatory response syndrome (after surgery, trauma, cardiopulmonary bypass, cardiac arrest and resuscitation, etc.) and septic shock show strikingly similar alterations in intracellular signal transduction pathways that are consistent with the endotoxin tolerance phenotype (3, 4, 6, 23). For example, impaired ex vivo cytokine production after LPS stimulation has been demonstrated in PBMCs obtained from adults with sepsis and trauma. Furthermore, low levels of LPS-mediated ex vivo cytokine production from these patients have been associated with poor clinical outcome. These patients are seemingly anergic and are thought to have a markedly increased risk of secondary, noscomial infections (3, 4, 6, 23).
Sepsis is initially characterized by a dramatic increase in proinflammatory cytokines, which is often followed paradoxically by a period of relative immunosuppression characterized by monocyte dysfunction that has been termed immunoparalysis (24). Several authors have suggested an association between the endotoxin tolerance phenotype and immunoparalysis (2–5, 23, 25–30). However, from a conceptual standpoint, immunoparalysis seems to be strikingly different from classic endotoxin tolerance in one regard. Early studies suggested that preconditioning macrophages and mononuclear cells with small doses of LPS (i.e., classic endotoxin tolerance) resulted in both (1) a diminished proinflammatory response to a subsequent dose of LPS in vitro and in vivo and (2) increased phagocytosis in vitro (the latter not being consistent with the concept of immunoparalysis). The results of the current in vivo study are consistent with these early in vitro studies (31) and, in stark contrast with in vivo studies showing that LPS preconditioning significantly inhibited bacterial clearance and phagocytosis, resulting in increased mortality (13, 14). The results of the current study are further supported by the recent finding that LPS preconditioning augments bacterial clearance in a model using a single live bacterial challenge with Pseudomonas aeruginosa (32, 33).
The phenomenon of endotoxin tolerance may have quite different effects in various animal models of sepsis. For example, LPS preconditioning followed by treatment with heat-killed S. aureus results in significantly higher concentrations of TNF-α—a priming effect, rather than tolerance (34, 35). The CLP model is polymicrobial in nature and more clinically relevant to critically ill patients who develop fecal peritonitis in that gram-positive bacteria, gram-negative bacteria, and fungi may be cultured from the blood and peritoneal fluid of these animals (18, 19). Our results further strengthen the assertion that LPS preconditioning in vivo reduces the inflammatory response, augments bacterial clearance, and improves survival from a mixed gram-positive and gram-negative bacterial infection. Collectively, these data suggest that the endotoxin tolerance phenotype may not be an ideal model to study the immunosuppression or immunoparalysis that follows sepsis or traumatic injury.
Several important questions remain to be addressed. The fundamental mechanisms occurring at the molecular level that lead to endotoxin tolerance remain to be elucidated. For example, the mechanism by which LPS preconditioning increases phagocytic activity is still not known and remains an active area of investigation in many laboratories, including our own. Several laboratories have independently shown that other agents may induce the endotoxin tolerance phenotype, including TNF-α (36), IL-1β (37, 38), lipoteichoic acid (39), CpG DNA (40), and other bacterial peptides (14, 15). In addition, several endogenous danger signals, including heat shock proteins (41, 42) and hyaluronan (43), have recently been shown to induce the endotoxin tolerance phenotype. Whether these agents also induce tolerance in vivo, as well as augmenting bacterial clearance and improving survival in the context of an active, ongoing infection are important questions that must be answered.
In conclusion, we show that LPS preconditioning attenuates the host inflammatory response, improves bacterial clearance possibly by increasing phagocytic activity of peritoneal macrophages, and improves survival in a poly-microbial model of murine sepsis induced by CLP. The collective evidence strongly suggests that endotoxin tolerance and immunoparalysis are conceptually distinct. From a clinical standpoint, a therapy that attenuates the host inflammatory response, although improving bacterial clearance, has obvious interest, and a thorough mechanistic understanding of endotoxin tolerance is warranted and necessary.
Acknowledgments
This study was supported by the Cincinnati Children’s Research Foundation and the National Institutes of Health (grant nos. R01 GM61723 and R01 GM064619 to H.R.W., R01 GM067202 to B.Z., and K08 GM077432-01 to D.S.W.).
References
- 1.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 2.Wolk K, Docke W, von Baehr V, Volk H, Sabat R. Comparison of monocyte functions after LPS- or IL-10–induced reorientation: importance in clinical immunoparalysis. Pathobiology. 1999;67:253–256. doi: 10.1159/000028104. [DOI] [PubMed] [Google Scholar]
- 3.West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]
- 4.Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Endotoxin tolerance: is there a clinical relevance? J Endotoxin Res. 2003;9:101–107. doi: 10.1179/096805103125001487. [DOI] [PubMed] [Google Scholar]
- 5.Van Epps HL. Ignoring endotoxin. J Exp Med. 2006;203:1137. doi: 10.1084/jem.2035fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard W, Choudry M, Schwacha M, Kerby J, Rue L, Bland K, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24:52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 9.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 10.Remick D, Manohar P, Bolgos G, Rodriguez J, Moldawer L, Wollenberg G. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock. 1995;4:89–95. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Remick DG, Garg SJ, Newcomb DE, Wollenberg G, Huie TK, Bolgos GL. Exogenous interleukin-10 fails to decrease the mortality or morbidity of sepsis. Crit Care Med. 1998;26:895–904. doi: 10.1097/00003246-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Ashare A, Powers LS, Butler NS, Doerschug KC, Monick MM, Hunninghake GW. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L633–L640. doi: 10.1152/ajplung.00231.2004. [DOI] [PubMed] [Google Scholar]
- 13.Mason CM, Dobard E, Summer WR, Nelson S. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J Infect Dis. 1997;176:1293–1302. doi: 10.1086/514125. [DOI] [PubMed] [Google Scholar]
- 14.Wang JH, Doyle M, Manning BJ, Blankon S, Wu QD, Power C, Cahill R, Redmond HP. Cutting edge: bacterial lipoprotein induces endotoxin-independent tolerance to septic shock. J Immunol. 2003;170:14–18. doi: 10.4049/jimmunol.170.1.14. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien GC, Wang JH, Redmond HP. Bacterial lipoprotein induces resistance to gram-negative sepsis in TLR4-deficient mice via enhanced bacterial clearance. J Immunol. 2005;174:1020–1026. doi: 10.4049/jimmunol.174.2.1020. [DOI] [PubMed] [Google Scholar]
- 16.Yadavilli GK, Auletta JJ, Gould MP, Salata RA, Lee JH, Heinzel FP. Deactivation of the innate cellular immune response following endotoxic and surgical injury. Exp Mol Pathol. 2001;71:209–221. doi: 10.1006/exmp.2001.2387. [DOI] [PubMed] [Google Scholar]
- 17.Zingarelli B, Hake PW, Cook JA. Inducible nitric oxide synthase is not required in the development of endotoxin tolerance in mice. Shock. 2002;17:478–484. doi: 10.1097/00024382-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Zingarelli B, Sheehan M, Hake PW, O’Connor M, Denenberg A, Cook JA. Peroxisome proliferator activator receptor-gamma ligands 15-deoxy-Delta (12,14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol. 2003;171:6827–6837. doi: 10.4049/jimmunol.171.12.6827. [DOI] [PubMed] [Google Scholar]
- 19.Zingarelli B, Hake PW, Denenberg A, Wong HR. Sesquiterpene lactone parthenolide, an inhibitor of IkappaB kinase complex and nuclear factor-kappaB, exerts beneficial effects in myocardial reperfusion injury. Shock. 2002;17:127–134. doi: 10.1097/00024382-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, et al. IKK-1 and IKK-2: cytokine-activated IkB kinases essential for NF-kB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 21.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkB kinase that activates the transcription factor NF-kB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 22.Beeson PB. Development of tolerance to typhoidal bacterial pyrogen and its abolition by reticulo-endothelial blockade. Proc Soc Exp Biol Med. 1946;61:248–259. doi: 10.3181/00379727-61-15291p. [DOI] [PubMed] [Google Scholar]
- 23.Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Reprogramming of circulatory cells in sepsis and SIRS. J Endotoxin Res. 2005;11:311–320. doi: 10.1179/096805105X58733. [DOI] [PubMed] [Google Scholar]
- 24.Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller JM, Docke WD, Kox WJ. Monocyte deactivation—rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;4:S474–S481. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 25.Heagy W, Hansen C, Nieman K, Cohen M, Richardson C, Rodriguez JL, West MA. Impaired ex vivo lipopolysaccharide-stimulated whole blood tumor necrosis factor production may identify “septic” intensive care unit patients. Shock. 2000;14:271–276. doi: 10.1097/00024382-200014030-00005. Discussion 276–277. [DOI] [PubMed] [Google Scholar]
- 26.Heagy W, Hansen C, Nieman K, Rodriguez JL, West MA. Impaired mitogen-activated protein kinase activation and altered cytokine secretion in endotoxin-tolerant human monocytes. J Trauma. 2000;49:806–814. doi: 10.1097/00005373-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Wilson CS, Seatter SC, Rodriguez JL, Bellingham J, Clair L, West MA. In vivo endotoxin tolerance: impaired LPS-stimulated TNF release of monocytes from patients with sepsis, but not SIRS. J Surg Res. 1997;69:101–106. doi: 10.1006/jsre.1997.5040. [DOI] [PubMed] [Google Scholar]
- 28.Heagy W, Nieman K, Hansen C, Cohen M, Danielson D, West MA. Lower levels of whole blood LPS–stimulated cytokine release are associated with poorer clinical outcomes in surgical ICU patients. Surg Infect (Larchmt) 2003;4:171–180. doi: 10.1089/109629603766956960. [DOI] [PubMed] [Google Scholar]
- 29.Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, Dhainaut JF, Cavaillon JM. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000;162:1877–1883. doi: 10.1164/ajrccm.162.5.2003058. [DOI] [PubMed] [Google Scholar]
- 30.Wolk K, Docke WD, von Baehr V, Volk HD, Sabat R. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood. 2000;96:218–223. [PubMed] [Google Scholar]
- 31.Lehner MD, Hartung T. Endotoxin tolerance—mechanisms and beneficial effects in bacterial infection. Rev Physiol Biochem Pharmacol. 2002;144:95–141. doi: 10.1007/BFb0116586. [DOI] [PubMed] [Google Scholar]
- 32.Varma TK, Durham M, Murphey ED, Cui W, Huang Z, Lin CY, Toliver-Kinsky T, Sherwood ER. Endotoxin priming improves clearance of Pseudomonas aeruginosa in wild-type and interleukin-10 knockout mice. Infect Immun. 2005;73:7340–7347. doi: 10.1128/IAI.73.11.7340-7347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphey ED, Fang G, Varma TK, Sherwood ER. Improved bacterial clearance and decreased mortality can be induced by LPS tolerance and is not dependent upon IFN-gamma. Shock. 2007;27:289–295. doi: 10.1097/01.shk.0000245024.93740.28. [DOI] [PubMed] [Google Scholar]
- 34.Peck OM, Williams DL, Breuel KF, Kalbfleisch JH, Fan H, Tempel GE, Teti G, Cook JA. Differential regulation of cytokine and chemokine production in lipopolysaccharide-induced tolerance and priming. Cytokine. 2004;26:202–208. doi: 10.1016/j.cyto.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Peck OM, Fan H, Tempel GE, Teti G, Halushka PV, Cook JA. Staphylococcus aureus and lipopolysaccharide induce homologous tolerance but heterologous priming: role of interferon-gamma. Shock. 2004;21:254–260. doi: 10.1097/01.shk.0000111662.09279.59. [DOI] [PubMed] [Google Scholar]
- 36.Murphey ED, Traber DL. Protective effect of tumor necrosis factor-alpha against subsequent endotoxemia in mice is mediated, in part, by interleukin-10. Crit Care Med. 2001;29:1761–1766. doi: 10.1097/00003246-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Ferlito M, Romanenko OG, Ashton S, Squadrito F, Halushka PV, Cook JA. Effect of cross-tolerance between endotoxin and TNF-alpha or IL-1beta on cellular signaling and mediator production. J Leukoc Biol. 2001;70:821–829. [PubMed] [Google Scholar]
- 38.Alves-Rosa F, Vulcano M, Beigier-Bompadre M, Fernandez G, Palermo M, Isturiz MA. Interleukin-1beta induces in vivo tolerance to lipopolysaccharide in mice. Clin Exp Immunol. 2002;128:221–228. doi: 10.1046/j.1365-2249.2002.01828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166:5161–5167. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- 40.Yeo SJ, Yoon JG, Hong SC, Yi AK. CpG DNA induces self and cross-hyporesponsiveness of RAW264.7 cells in response to CpG DNA and lipopolysaccharide: alterations in IL-1 receptor–associated kinase expression. J Immunol. 2003;170:1052–1061. doi: 10.4049/jimmunol.170.2.1052. [DOI] [PubMed] [Google Scholar]
- 41.Kilmartin B, Reen DJ. HSP60 induces self-tolerance to repeated HSP60 stimulation and cross-tolerance to other pro-inflammatory stimuli. Eur J Immunol. 2004;34:2041–2051. doi: 10.1002/eji.200425108. [DOI] [PubMed] [Google Scholar]
- 42.Aneja R, Odoms K, Dunsmore K, Shanley TP, Wong HR. Extracellular heat shock protein–70 induces endotoxin tolerance in THP-1 cells. J Immunol. 2006;177:7184–7192. doi: 10.4049/jimmunol.177.10.7184. [DOI] [PubMed] [Google Scholar]
- 43.del Fresno C, Otero K, Gomez-Garcia L, Gonzalez-Leon MC, Soler-Ranger L, Fuentes-Prior P, Escoll P, Baos R, Caveda L, Garcia F, et al. Tumor cells deactivate human monocytes by upregulating IL-1 receptor associated kinase M expression via CD44 and TLR4. J Immunol. 2005;174:3032–3040. doi: 10.4049/jimmunol.174.5.3032. [DOI] [PubMed] [Google Scholar]