Abstract
Estradiol is the most potent and ubiquitous member of a class of steroid hormones called estrogens. Fetuses and newborns are exposed to estradiol derived from their mother, their own gonads, and synthesized locally in their brains. Receptors for estradiol are nuclear transcription factors that regulate gene expression but also have actions at the membrane, including activation of signal transduction pathways. The developing brain expresses high levels of receptors for estradiol. The actions of estradiol on developing brain are generally permanent and range from establishment of sex differences to pervasive trophic and neuroprotective effects. Cellular end points mediated by estradiol include the following: 1) apoptosis, with estradiol preventing it in some regions but promoting it in others; 2) synaptogenesis, again estradiol promotes in some regions and inhibits in others; and 3) morphometry of neurons and astrocytes. Estradiol also impacts cellular physiology by modulating calcium handling, immediate-early-gene expression, and kinase activity. The specific mechanisms of estradiol action permanently impacting the brain are regionally specific and often involve neuronal/glial cross-talk. The introduction of endocrine disrupting compounds into the environment that mimic or alter the actions of estradiol has generated considerable concern, and the developing brain is a particularly sensitive target. Prostaglandins, glutamate, GABA, granulin, and focal adhesion kinase are among the signaling molecules co-opted by estradiol to differentiate male from female brains, but much remains to be learned. Only by understanding completely the mechanisms and impact of estradiol action on the developing brain can we also understand when these processes go awry.
I. INTRODUCTION
Estradiol is the most biologically prevalent and active compound of a class of steroids called estrogens, and it exerts potent and wide-ranging effects on the developing brain. It is a well-established but rarely celebrated fact that estradiol, and activity of the enzyme responsible for estradiol synthesis, P-450 aromatase, as well as estrogen receptors, are all at their highest levels in the brain either prenatally or during the first few days of life and then gradually decline to adult levels. The challenge is discerning the functional significance of estradiol to the developing brain and the mechanisms by which such function is achieved. But what does that mean, exactly? Some readers will assume the topic is sex differences in the brain and how they are established by gonadal hormones during development. To others, increasing concern over the potential impact of endocrine disrupters in the environment, particularly the so-called estrogen mimetics, and how they derail normal brain development, might be the incentive for reading on. Still others may be captivated by the promise of estrogens as potent neuroprotective agents in adult models of brain damage and the potential that similar positive effects could provide therapeutic benefit for pediatric brain damage. All are correct, and the goal of this review is to take a broad-based view of how estradiol impacts on normal brain development in both males and females.
The majority of what we know about the impact of estradiol on brain development comes from rodent models, predominantly rat and mouse but including hamsters, voles, and guinea pigs. Birds, in particular zebra finches and Japanese quail, have also provided novel insights into the myriad of ways estradiol can alter brain development. In primates, both human and otherwise, we know a great deal more about what estradiol is not doing than what it is doing. There is a clear need for more information about this potent steroid and how it affects the developing primate brain.
The purpose of restricting the discussion to the immature brain is to highlight that development is different, and thus so are the effects of estradiol. We cannot take what we know about estradiol action in the adult brain and extrapolate it to the perinatal brain. Many fundamental principles established in the adult simply do not apply to the neonate, and even more importantly, many unique parameters found only in the developing brain act to guide or restrict the potential actions of estradiol during the early phase of life. It is these actions that are particularly of interest as they can have life-long impacts by influencing neuronal survival, axonal projections, dendritic branching, and synaptic patterning; in other words, all of the things we think of as mattering to variability in brain function. By studying the impact of estradiol on these critical end points of brain development, we have an experimental tool that provides leverage for understanding how they normally occur. Moreover, the magnitude of estradiol-induced changes in the developing brain are often far greater than those in the adult and are not confounded by experience or reproductive status. In many ways the study of cellular mechanisms of estradiol induction of dendritic spine synapses, cell birth and death, or dendritic branching is far easier in the immature brain than the adult. The problem is that the same mechanism cannot be assumed to be equally engaged during the different life phases, requiring that all effects observed early cannot be generalized to the adult, and vice versa. With these caveats in mind, the current state of the art of the mechanistic basis of estradiol action on the developing brain will be reviewed.
II. ESTRADIOL CONTENT AND ESTROGEN RECEPTORS IN DEVELOPING BRAIN
Before considering the mechanisms and functional outcomes of estradiol action in the developing brain, it seems important to know if there is any, if so where, and if so how much? Most effects of estradiol require a receptor, so the levels and distribution of these critical proteins are of central interest. Steroids are distinct from peptides in that they are synthesized on demand, as opposed to stored, and for the most part they act on targets at a distance from the place of origin, although this concept has recently been challenged (19). Estradiol is derived from testosterone following aromatization of the A ring via the p450 enzyme aromatase, also called estradiol synthase. The regulation and anatomical distribution of this enzyme is discussed in some detail in section IVC. Information on estradiol content in the brain has been relatively sparse because it is so difficult to measure due to the high signal-to-noise ratio when dealing with a liophilic molecule, estradiol, in a lipid-rich environment, the brain. Radioimmunoassay remains the technique of choice for quantitative determination of steroid levels due to its relative ease and cost effectiveness in measuring large numbers of samples. Early studies focused on circulating levels of the androgen precursors to estradiol, with only one report of estradiol content in the brain itself, and this was limited to the hypothalamus (179). Newborn males had two to three times more than females, as expected based on the aromatization hypothesis (see below), and this fundamental fact was replicated 20 years later (3). Surprisingly, however, in a first foray into measuring estradiol content in brain regions outside the hypothalamus, the same relationship did not apply. Estradiol content was detected in the cortex and hippocampus of the newborn female, which in the absence of the androgen precursor available to males was hard to explain. Accumulating evidence suggests the brain can serve as its own gonad and make estradiol de novo from cholesterol (97, 188), but this emerging concept requires greater validation by more precise techniques for quantifying estradiol. A point highlighted by the recent detection of high levels of 17α-estradiol in the developing brain by gas chromatography/mass spectrometry (214). The 17α-isomer of estradiol was generally considered inactive due to its poor binding to the classic estrogen receptor (ER), but this view is being reconsidered in light of the potential for a novel membrane-associated receptor, referred to as ER-X (218), and evidence of neuroprotective effects.
The difficulty in precisely measuring the amount of estradiol is exactly opposite to the ease of measuring its receptor, a fact well reflected in the number of studies on the distribution and quantification of ER in the developing brain. Detailed maps have been constructed based on immunocytochemistry for the ER protein, autoradiography for binding of radiolabeled ligand, and in situ hybridization histochemistry for the mRNA (18, 60, 61, 73, 78, 84, 120, 138, 192, 246). The broad brush strokes of estrogen receptor distribution in the brain indicate it is tightly conserved across a wide range of species. High levels of expression are found in the diencephalon of reptiles, amphibians, birds, and mammals. Greater variance is found in cortical brain regions, but this in large part reflects the differences in degree of brain development. Likewise, there are some sex differences (246) in ER expression in the developing brain, but these are generally small in magnitude, restricted to specific subregions, and to date have not proven informative as to the basis of sex differences in brain development. Such evidence may be coming, however, based on a recent report that the degree of social behavior expressed by males of several vole species was correlated with ER levels in brain areas known to regulate this response (53).
The neonatal brain is also capable of metabolizing estradiol, although at a lesser rate than that of the pituitary (175). Little attention has been given to the potential for sex differences in estradiol metabolism or to its role as a physiological regulator of hormone action in the developing brain.
III. MULTIPLE MECHANISMS OF ESTRADIOL ACTION
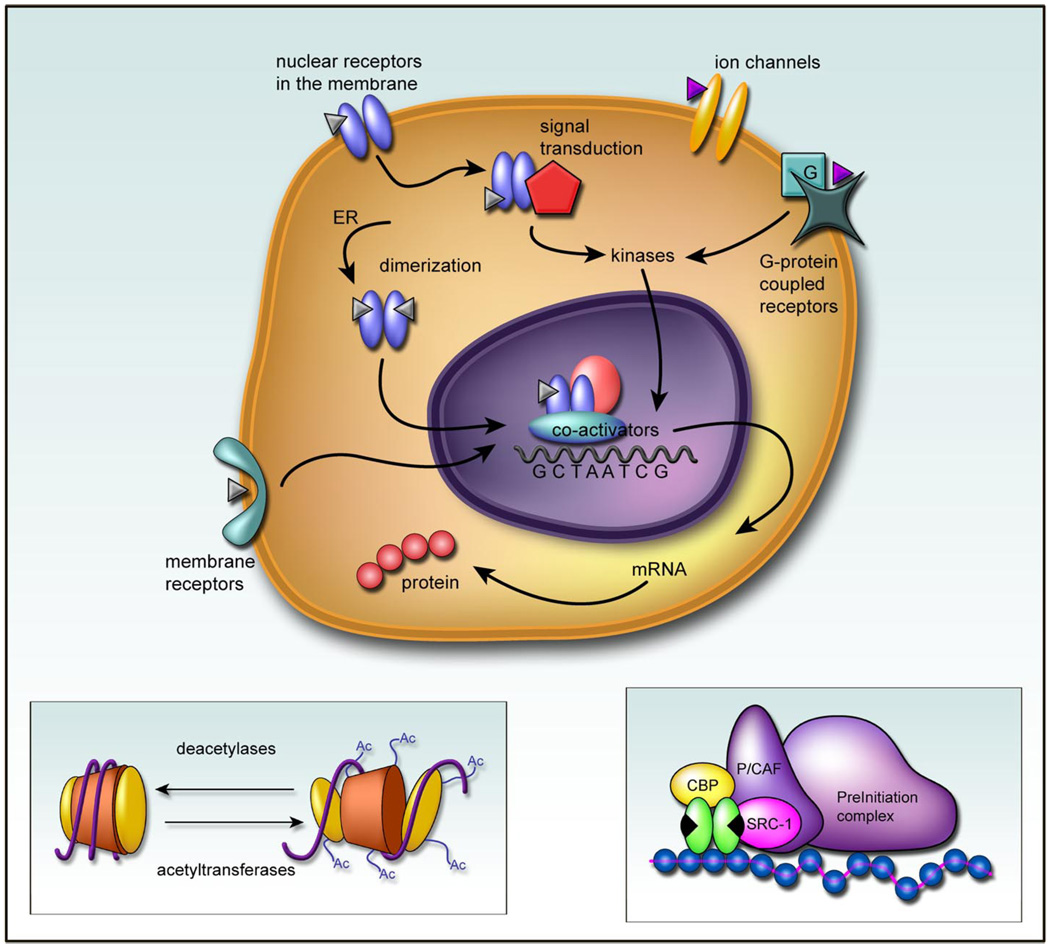
Concepts of how estradiol can impact on cell function continue to evolve. Traditional views of ligand-activated receptors directly initiating transcription have been complimented by revelations that the same protein can also activate cytoplasmic signal transduction pathways and may operate independent of ligand. The notion of membrane receptors for estradiol in the brain has persisted in the literature despite the inability to isolate, clone, or in anyway characterize the beast (with the pos sible exception of ER-X). A new 7-trans-membrane domain receptor, GPR30, has emerged as a potential candidate (172) but does not appear to be on the plasma membrane and has not been well characterized in brain (72). Membrane effects of estradiol have not been considered in the context of brain development. Nonetheless, it is clear that estradiol action extends beyond the nuclear transcription factor induction of gene transcription. These actions can be loosely divided along three lines: 1) “classic” ER activation involving homodimerization, translocation to the nucleus and transcription; 2) “nouveau” actions that still involve the nuclear receptor only acting in novel ways outside the nucleus; and 3) estradiol effects that apparently do not involve any receptor for estradiol but instead are mediated at other receptors, such as those for neurotransmitters (Fig. 1).
FIG 1.
A multiplicity of estradiol actions. Receptors for estradiol are members of a nuclear targeted transcription factor superfam-ily. Upon binding, the receptors dimerize and are translocated to the nucleus where they associate with coactivators such as steroid receptor coactivator 1 (SRC-1) and CREB binding protein (CBP) and form part of a preinitiation complex for inducing transcription. A critical function of this complex is the acetylation of histones on the chromatin to relax the DNA helix and allow for association with the palindromic sequences that constitute the estrogen response element (ERE). In addition to this long-term genomic effect of estradiol, the ER has been associated with the cell membrane of neurons where it can interact directly with signal transduction pathways such as that involving mitogen-activated protein kinase. Estradiol has also been reported to directly affect ion channels and G proteincoupled receptors, independent of the estrogen receptor (ER), and there may be a novel membrane-bound receptor for estradiol distinct from the classic ER’s, but this remains unsettled.
A. Nuclear Receptors and Transcription
ERs are members of a superfamily of nuclear transcription factors, all of which are characterized by the presence of a central DNA-binding domain that targets the receptor to a hormone responsive element (HRE). For ER, the DNA binding site contains two palindromic hexanucleotide repeats that bind an ER homodimer. It is referred to as an estrogen response element, or ERE. All steroid receptors also possess a ligand binding domain that when occupied alters the conformation and hence function of the receptor. In 1999, the Nuclear Receptors Nomenclature Committee (http://www.ens-lyon.fr/LBMC/LAUDET/nomenc.html) adapted a unified nomenclature based on the system developed for the P-450 gene superfamily. Receptors for steroids and steroid hormonelike receptors were designated as NR3. There are currently two recognized receptors for estradiol, ERα and ERβ, identified as NR3A1 and NR3A2, respectively, and two estrogen-related orphan receptors (ERRα/ERR1 and ERRβ/ERR2) designated NR3B1 and NR3B2 (28).
The distinctions in distribution and function of ERα versus ERβ continue to be actively investigated. The two receptors have only 56% homology in the ligand binding domain, suggesting a high degree of selectivity, yet the residues that line the binding cavity are highly conserved, resulting in relatively similar affinities for 17β-estradiol. Recently developed ligands that are relatively selective are based on single amino acid differences between the two isoforms (109) and are beginning to illuminate the differential functions of each form. Even more useful but still lacking is development of selective antagonists for each isoform, leaving knockout mice and all their attendant caveats as a dominant source from which to base conclusions. Nonetheless, the knockout mouse model has provided several new insights regarding estradiol action in the developing brain, mostly in regard to the role of ERβ, while proving generally confirmatory regarding ERα.
In the absence of ligand, ERs, like other nuclear receptors, exist in a multiprotein complex of heat shock proteins that render them inactive but available for binding. Upon binding, the heat shock proteins are shed in response to conformational change, receptors homo- or heterodimerize, and a new set of proteins is recruited to form a transcriptionally active complex. In most tissues, the majority of ER resides in the nucleus, but there is active energy-dependent shuttling of the receptor from the cytoplasm into the nuclear compartment where receptors cluster into transcriptionally active or inactive foci (56). In the brain, the complex morphology of neurons and glia has revealed ER in sites far distant from the nucleus (33, 34, 132), so far in fact that shuttling from cytoplasm to nucleus seems unlikely. This has contributed to the interest in nontranscriptional effects of ER in the nervous system and may be a particularly useful strategy when dealing with cells that can project to sites at considerable distance.
B. Coactivators and Corepressors
A component of the transcriptional ability of nuclear receptors is the involvement of numerous auxiliary proteins that can either augment or inhibit normal activity. The large variety of coproteins available, their interactions, and their cellular specificity provide for a far greater and more nuanced complexity to steroid action than that afforded by simpler ligand/receptor systems. Coactivators bind to an LXXLL motif embedded in the ligand binding domain and then recruit acetyl-transferases, such as p300/CBP, that induce conformational changes in the chromatin essential for access to EREs. The SRC grouping, or steroid receptor coactivators, were the first identified and probably most fundamental for augmenting ligand-dependent transcription of ER. The earliest discovered coactivator, SRC-1, is involved in establishment of sex differences in the brain determined by estradiol (14). Full activation of ER requires SRC or other coactivator binding at two separate activator sites on the receptor. An additional complexity in ER action is its ability to influence transcription of genes even in the absence of an ERE. For instance, ERs modulate transcription at AP-1 by associating with Fos/Jun complexes and thereby not interacting directly with the DNA (225). The same is true for SP-1 sites, and ER can interact directly with nuclear factor κB pathway (105). This has precluded the ability to a priori qualify whether a particular gene is estrogen responsive based on the presence or absence of an ERE in the promoter region.
The counterparts to coactivators are the corepressors, although these seem to be generally more important to thyroid receptor and retinoic acid receptor function than that of steroid hormone receptors. Nonetheless, overexpression of nuclear receptor co-repressor (N-CoR) and silencing mediator for retinoid and thyroid hormone receptor (SMRT) can impact on the agonist versus antagonist response of ER to mixed ligands, such as tamoxifen, suggesting the potential for some physiological role for these proteins in estradiol action (80, 198, 236).
C. Extranuclear Receptors and Signal Transduction
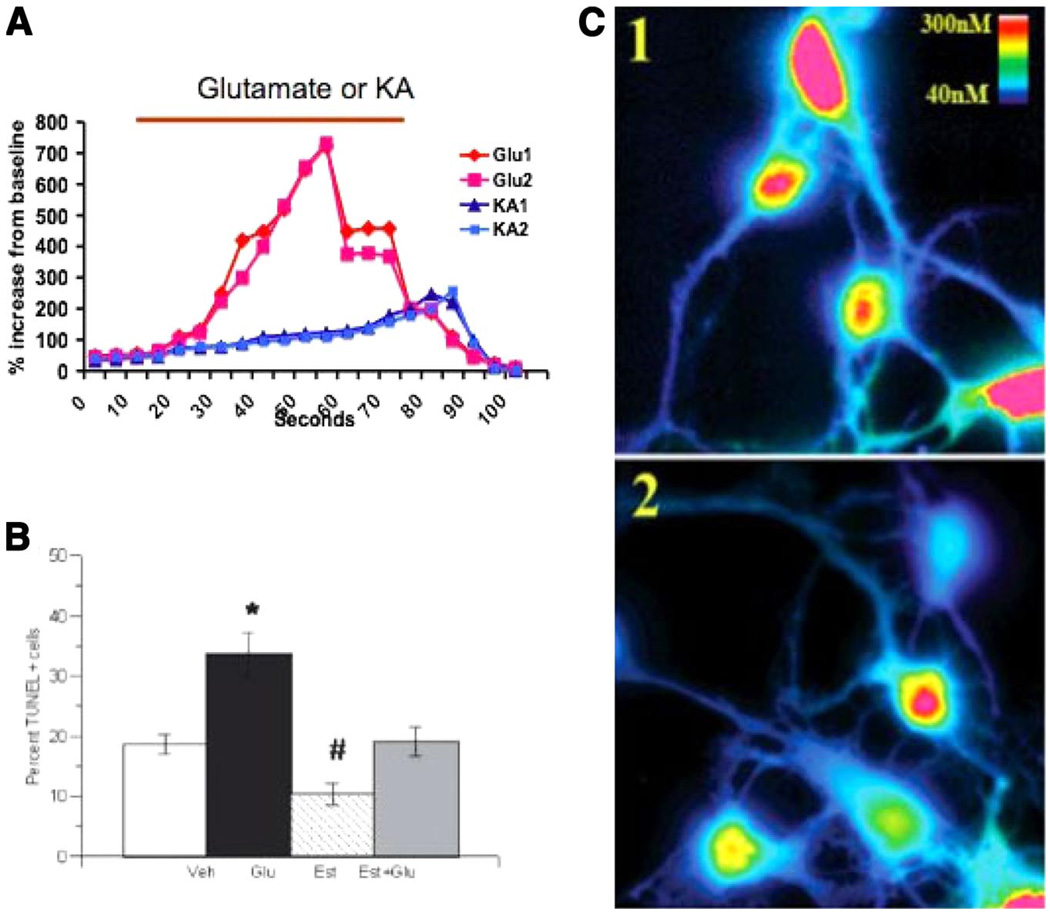
A major revelation in the revolution of steroid hormone actions is the discovery that the superfamily of nuclear transcription factors actually harbors secret ki-nase activation capacity. In some way that remains poorly understood, but is unequivocally established, steroid hormone receptors, such as ER, interact with and activate kinases, such as mitogen-activated protein (MAP) kinase, resulting in the phosphorylation of ERK or CREB or AKT and perhaps other signal transduction proteins not yet discovered, that then lead to the nucleus and transcription (214, 243). Reassuring thoughts that such effects are peripheral to the main (i.e., traditional) pathway of steroid action are rudely interrupted by findings that ER mediates phosphorylation of CREB and ERK in such reproductively relevant and sexually dimorphic brain regions as the preoptic area (1, 2). Functional significance of the clandestine relationship between ER and MAP kinase is found in the neuroprotection arena where estradiol-mediated reductions in cell death following excitotoxic insult are apparently dependent on this pathway (139, 196).
D. Membrane Effects and Receptors
The question of membrane effects and putative membrane receptors for estradiol is open and long standing (31). Separating physiologically meaningful effects from pharmacological artifacts continues to be a challenge that has not yet been met. Reports of membrane effects/receptors for estradiol date back 25 years (85, 165), but convincing evidence of a bona fide receptor that has the requisite saturability, binding affinity, and a definitive kilodalton weight proves elusive. The discovery that nuclear receptors, now known to be extra nuclear as well, could also intercalate into the membrane (174), provided a new twist by suggesting that all along there has been only one receptor but in addition to its role in the nucleus, it can also masquerade as a membrane receptor, although this finding has not gone unchallenged (235). Moreover, not all estrogen effects could be explained by this model, and recent discovery of GPR30, a G protein-coupled membrane receptor unrelated to nuclear ERs and localized to the endoplasmic reticulum, provided yet another potential mechanism for rapid estrogen signaling (177, 207). Activation of GPR30 by estradiol elicits a sustained mobilization of intracellular calcium, suggesting direct physiological relevance, although this finding is also not without its critics (89). Still unexplained are effects of estradiol that occur rapidly and predominantly at the plasma membrane of neurons (83). Whether these genuinely involve a receptor or are “membrane effects” remains unresolved.
When discussing the question of physiological significance of rapid estradiol effects, the elephant in the room is whether changes in the level of the ligand are conducive to allowing such rapid effects to occur. There are no active release mechanisms for steroids, nor are there any currently identified rapid degradation or inactivation pathways, although there is some functional evidence for the latter (64). In the serum, estradiol levels can vary dramatically, but these changes occur gradually over a matter of days, not minutes; thus it is hard to imagine how a rapid effect is compatible with this profile. But perhaps it doesn’t need to be. Emerging evidence for de novo synthesis of estradiol by neurons and observation of subcellular localization of the aromatase enzyme in synaptic terminals has led to the proposal that estradiol might also be able to act in a manner analogous to a neurotransmitter (19). This intriguing idea is supported empirically by behavioral data on rapid modulation of nociception and sexual behavior in response to local changes in aromatase activity at the spinal cord or preoptic area (21, 52).
Estradiol effects on the developing brain have generally not been viewed under the lens of unorthodoxed mechanisms of action. Steroid effects at this time of life are by and large permanent, resulting in substantial morphological differences by determining life and death, dendritic branching, and synaptic patterning. It is naturally assumed that these effects involve traditional transcriptionally mediated effects of estradiol. But is this assumption warranted? Our research group has explored the potential of rapid effects of estradiol in immature neurons by administering a large dose in a quick release vehicle and monitoring a well-established response, dendritic spine formation. In the adult brain, estradiol can induce the formation of dendritic spines in as little as 30 min (121), but we found that in the immature brain a similar dose of estradiol requires over 4 h to impact formation of spines, and this effect is blocked by the ER antagonist ICI 182,780. In other instances, mature neurons exhibit rapid changes in calcium influx (245), but we have found no such rapid effects on calcium influx induced by GABA or glutamate in immature hypothalamic or hippocampal neurons. In one of the few cases where rapid membraneassociated effects were compared in immature and mature cells, estradiol increased free intracellular calcium to promote progesterone synthesis in postpubertal hypothalamic astrocytes but not in neonatal astrocytes (136). These observations have led us to speculate that the developing brain might be immune to rapid effects of estradiol, to prevent the permanent organizational effects this steroids can have when acting during a sensitive period. This is particularly relevant in the arena of sexual differentiation, as it is critical that brain sex coincide with gonadal sex for successful coordination of physiology and behavior.
IV. ESTRADIOL AND THE ESTABLISHMENT OF SEX DIFFERENCES IN THE BRAIN
Sex differences in the brain are widespread and vary in magnitude and impact depending on the brain region and functional end point being modulated. The majority of sex differences in the brain are permanently established during a restricted developmental window by the actions of gonadal steroids on a bipotential substrate. By and large, brain sex differences that are robust and reliable are also those that are most relevant to reproduction. Nonreproduction-related sex differences, be they behavioral or anatomical, tend to be small and highly variable, with the exception of aggression, which one could argue is actually reproduction related (i.e., competition for mates and resources, maternal aggression, etc.). Sex differences in cognition, stress and anxiety, food preferences, locomotion, and even visual acuity are all documented and genuine. But, we know very little about the neural substrates and mechanistic basis of these sex differences. Moreover, these sex differences tend to be allomorphic, meaning they vary along a continuum with a great deal of overlap between the sexes, as opposed to those sex differences which are truly of two forms, or dimorphic and usually directly relevant to reproduction.
Structurally, sex differences in the brain can be categorized as volumetric, indicating a region is larger in one sex versus the other, or connective, meaning the type or amount of synapses or size of a particular projection differs between males versus females. Physiologically, sex differences in the brain are those related to the amount of neurochemicals or neurotransmitters, or the intrinsic excitability of particular classes of neurons. In the adult there is considerable interest in the effects of estradiol on membrane excitability, receptor density, and levels of neurotransmitters. Less attention has been given to the neonate, but examples of such effects do exist and include changes in calcium binding proteins, GABA, and glutamate levels, enzymatic activity, and so on. Developmental estradiol-mediated changes in physiology are the likely mechanistic basis for establishment of many permanent morphological sex differences, but such causal connections are rarely made.
A. Sex Determination Versus Sex Differentiation
In 2001, the Institute of Medicine, an arm of the National Academy of Sciences charged with examining policy matters related to human health, published a report on “Exploring the Biological Contributions to Human Health: Does Sex Matter?” (242). One of the first challenges facing the committee was to define sex as opposed to gender. Sex is the classification of living things according to their reproductive organs, which are in turn determined by the chromosomal complement. Gender is a person’s self-representation as male or female and how that person is responded to by social institutions (which is why the term gender should not be used in reference to subjects in an animal study). Gender is shaped by environment and experience. Sex is not.
From a historical perspective, the discovery of the genetic basis of sex is shockingly new. That males have an X and a Y chromosome and females two X chromosomes has only been known for a little over 50 years, and it wasn’t until 1990 that the critical gene on the Y chromosome, SRY, for the differentiation of testis from the bipotential gonad, was finally isolated and sequenced (111). The expression of SRY is initiated within the first days or weeks of pregnancy (rodent versus human), and with this single event, the ultimate state of the organism’s sex is determined. In the absence of SRY, regardless of how much else of the Y chromosome is present, the gonad will develop into an ovary. Thus the female developmental pathway is the default, but the establishment of sex as female is no less determinant than the establishment of sex as male. Once gonadal sex is established it will guide the formation of the appropriate urogenital tract. The Wolffian duct system will survive and become the vas deferens and associated secretory glands of the male reproductive system while actively suppressing the formation of the female system. Conversely, if the gonad becomes an ovary, the male system will degenerate due to insufficient androgen and the Mullerian duct system will develop into the female reproductive tract. Differentiation of external genitalia and secondary sex characteristics are then progressively established by gonadal steroid synthesis at the appropriate time in life. In humans, the process of sex determination and formation of external genitalia is complete by the 13th week of gestation, a remarkably early event in the 40-wk process.
Sex determination is therefore the process leading to formation of all things we generally associate with gender but really are a reflection of sex. Sex differentiation of the brain, by contrast, is a separate process that is largely driven by gonadal steroids during a later developmental period and in humans may relate to self-perception of gender. The impact of steroids is restricted to a sensitive window, which is mid to late gestation in primates, as best we can tell, and just before and after birth in rodents. This process is multi-faceted, impacting on reproductive behavior and physiology as well as many non-reproductionrelated processes via distinct mechanisms in separate brain areas and often with varying sensitive periods. Of the many contributing variables to the complex process of brain sex differentiation, one of the most potent and prevalent is estradiol.
B. The Organizational/Activational Hypothesis of Brain Sex Differentiation
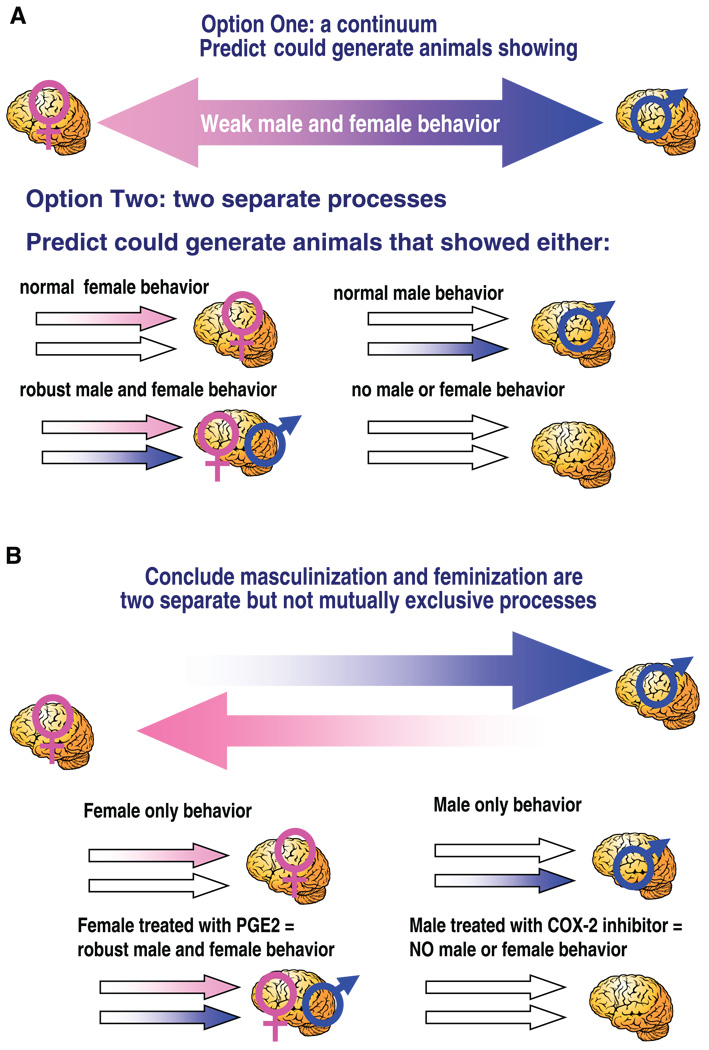
The notion that steroids act early in development to dictate adult sexual behavior and brain morphology is now one of the most well-accepted tenets in behavioral neuroendocrinology; so well-accepted in fact that it is honored as a dogma worthy of challenge (9). With a few exceptions that prove the rule, the dogma generally stands, as long as it is restricted in its application to reproductively relevant outcomes [see McCarthy and Konkle (129a) for further discussion]. There are two distinct and readily quantified reproductive end points subject to this organizational/activational process: 1) sexual behavior and 2) cyclical versus pulsatile gonadotropin secretion. In males, there is a requirement for gonadal steroid action early in development in order for adult steroids to effectively induce male sex behavior. In females, a lack of early exposure to high levels of gonadal steroids is essential for both sexual behavior and the ovulatory surge of the gonadotropin luteinizing hormone (LH). If a developing female is inadvertently exposed to gonadal steroid hormones or mimetic agents, as an adult she will not only be lacking in sexual receptivity, she will also be sterile due to an inability to ovulate (24, 25). Moreover, if as an adult she is treated with male levels of androgen, she will exhibit the male pattern of sexual behavior when presented with a sexually receptive female (26, 238). In other words, the brain sex of a female is converted to that of a male by administration of exogenous steroids during a critical perinatal window (Fig. 2).
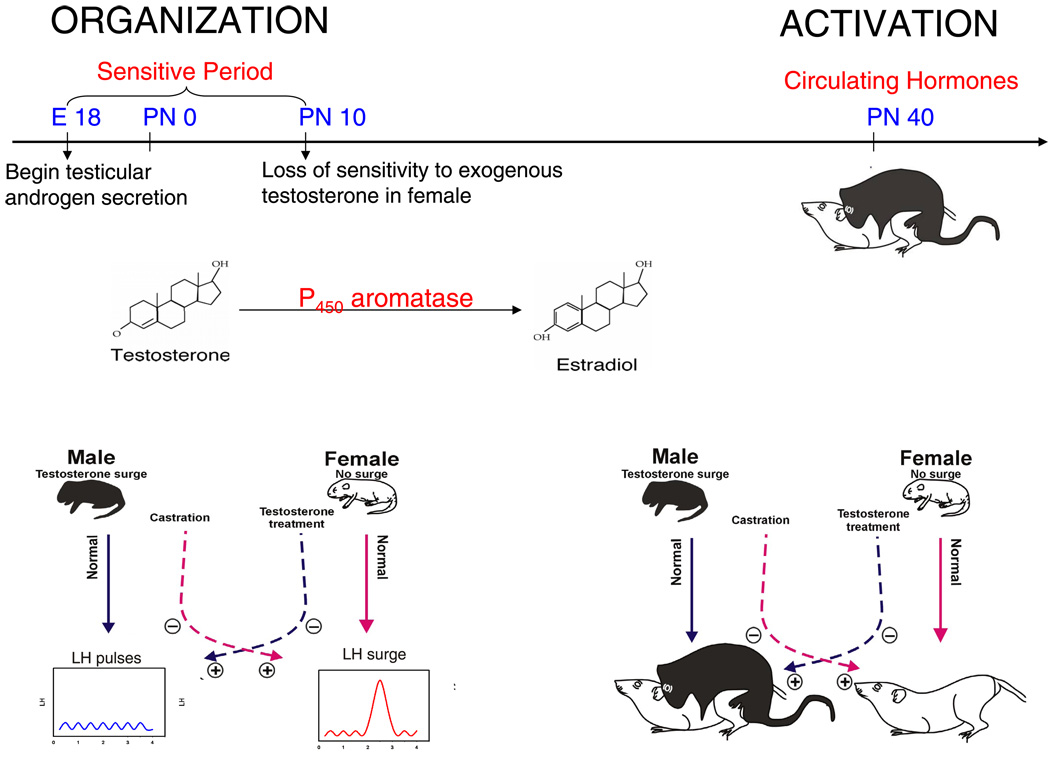
FIG 2.
The organizational/activational hypothesis of estradiol action on the developing brain. Originally proposed in 1959 by Phoenix et al. (164), the organizational/activational hypothesis codified the principle that early hormone effects organize the brain such that adult hormonal effects are constrained by that prior exposure. The establishment of sex differences in physiology and behavior is a function of differential gonadal steroid synthesis during a perinatal sensitive period. In rats, the production of testicular androgens begins prenatally, around embryonic day 18, and defines the onset of the sensitive period. The female ovary remains quiescent, and a lack of exposure to androgens, and the aromatized product estradiol, is essential for normal female brain development. Treatment of females with exogenous testosterone results in its aromatization to estradiol and masculinization of adult brain and behavior. The developmental time point in which the female becomes insensitive to the masculinizing effects of exogenous testosterone operationally defines the end of the sensitive period. As adults, males show only pulsatile release of the gonadotropin luteinizing hormone (LH) from the pituitary, while females exhibit a large surge in LH release to induce ovulation at the midpoint of the estrus cycle. Likewise, only adult males exhibit the masculine pattern of sexual behavior of mounting a female, while only females adopt the sexually receptive posture termed lordosis. Exposure of developing females to testosterone, which is aromatized to estradiol, during the sensitive period will render them both sterile and sexually unreceptive. Both the male and female adult patterns are determined by hormonal organization during development but are dependent on adult sex-specific hormones to be activated in the adult.
As with most dogmas, however, its easy to forget the previously existing morass of observations that provided no clarity in the absence of a unifying hypothesis. Early studies of hormonal modulation of behavior refer only tangentially to sex as a potential variable explaining why adult male and female chickens, dogs, guinea pigs, rats, or mice responded differently to injections of estradiol when assayed for reproductive behavior. Adding to the confusion was the observation that injections of estradiol increased masculine sexual behavior in males, yet it was so clearly a female hormone. In fact, giving estradiol to females also resulted in female sexual behavior. Treating females with testosterone, the male hormone, could induce male behavior, but only when given at extremely high doses for long duration, and even then, the behavior induced was qualitatively and quantitatively inferior to that seen in males (27). Clarification of these observations was delayed until two seminal studies. The first was the formulation of the organizational/activational hypothesis of hormone effects on the brain, which codified the concept that gonadal steroids act during a perinatal sensitive period to permanently alter the neural architecture of the brain and thereby restrain the response profile of the adult brain when exposed to a particular hormone (164). This study was conducted in guinea pigs, an animal since abandoned in sex differentiation research due to its sensitive period being entirely prenatal and thereby precluding postnatal manipulations. Nonetheless, this study unequivocally established that adult reproductive behavior was entirely a function of developmental exposure to gonadal steroids combined with an adult hormonal milieu appropriate (homotypic) for that sex (i.e., estradiol and progesterone in females and testosterone in males). Both sexes are equal in their requirement for activation of reproductive behavior in adulthood, but central to the hypothesis is that organization is driven by steroids of gonadal origin in males only, whereas female brain development is the default, i.e., occurs in the absence of gonadal steroids. Some early chaffing over the notion of the female brain as a passive process is now replaced by the conundrum that while surely female brain development is organized, we have no idea by what. Identifying the (presumably) genetic variables directing female brain development is one of the great unmet challenges of neuroen-docrinology.
C. The Aromatization Hypothesis in Brain Sex Differentiation
At approximately the same time that morphometric sex differences were being described in the avian and mammalian brain, another mystery of sexual differentiation was also being resolved. In early studies on the organizational/activational hypothesis in rats, testosterone was administered to newborn females to masculinize the brain. As a control for the steroid injection, newborn females were also treated with estradiol. To everyone’s surprise, estradiol was not only effective at masculinizing the brain, it was more effective than testosterone. Administering the nonaromatizable androgen dihydrotestosterone (DHT), which potently activates androgen receptors, was largely ineffective (239). These results were difficult to reconcile with two things: first was the notion that activation of the male testis during perinatal development was the basis for masculinization, and second was that during pregnancy estradiol production by the placenta results in maternal levels of estradiol so high that there would clearly be exposure of all of the fetuses, precluding any differential exposure in one sex over the other. The latter problem could be explained by the presence of α-fetoprotein, a circulating binding globulin found in late-gestation fetuses and early postnatal pups that has a high affinity for estradiol and thereby sequesters the steroid in the bloodstream, preventing it from masculinizing the brain. The former issue, of what is the role of testosterone, was explained by the discovery that the brain is a major producer of the P-450 enzyme aromatase, also called estradiol synthase as it converts testosterone into estradiol (115). Not only is aromatase expressed by neurons in the brain, it is expressed at its highest level in the sexually dimorphic regions of the preoptic area and hypothalamus during the perinatal sensitive period (77, 104, 113, 185, 227, 228). Aromatase is also found in the telencephalon, but at much lower levels than the diencephalon (122). Together, these observations formed the basis of the aromatization hypothesis; perinatal fetuses and pups are protected by maternal estradiol via the binding capacity of the α-fetoprotein in their circulation, and testicularly derived testosterone diffuses into the male brain where it is locally aromatized to estradiol; estradiol then initiates the process of masculinization. The aromatization hypothesis was first proposed by Naftolin in 1975 (145) and expanded on by others (133, 226).
There have been many challenges to the aromatization hypothesis the most notable being the observation of α-fetoprotein within neurons and the suggestion that rather than a nondiscriminating barrier it might actually be a selective delivery system (212, 215). This interesting notion has neither been proven nor categorically refuted for the entire brain. However, the importance of α-fetoprotein in protecting female fetuses from behavioral masculinization and infertility at the hands of maternal estrogens is definitively established by observation of both those end points in female neonates with a null mutation rendering them incapable of α-fetoprotein production (15). There are also periodic but persistent reports of impaired female brain development by disrupting estradiol synthesis and/or receptor binding, suggesting a role for this steroid in female organization (59, 131). Subtleties in aromatase effects continue to be revealed with modern techniques. For example, the male aromatase knockout mouse is capable of normal male copulation if supplied with exogenous estrogens, but appears to be lacking in motivation and sexual preference (16). Both of the latter effects appear to be due to estradiol-mediated effects on development of the main olfactory system (17). That male mice developmentally deprived of estradiol continue to copulate is in contrast to the situation in rats and highlights that mounting by mice, which also occurs at very high rates in normal female mice, is a distinct component of sexual behavior not subject to the same organizational constraints as other aspects of the behavior.
Female littermates of male aromtase knockout mice also have severely impaired sexual behavior, further suggesting an active role for estradiol in feminization of the mouse brain (17). The observations on α-fetoprotein knockout mice and aromatase knockout mice appear contradictory, one indicating the female must be protected from estradiol and one indicating she requires it for normal development. The basis of the discrepancy could easily be the source of the estradiol, maternal in the case of α-fetoprotein and fetal in the aromatase knockout. Thus the potential that highly regulated locally synthesized estradiol within the female brain is importantly contributing to her development is a possibility worth considering. A recent regionally specific analysis of estradiol levels in the hypothalamus, hippocampus, and cortex of male and female rats from embryonic life through to adulthood reveals there are far fewer sex differences than would be predicted. Moreover, the levels in brain are not clearly reflective of those in the circulation, suggesting brain steroidogenesis may be critical to both male and female brain development (A. Konkle and M. McCarthy, unpublished observations). Alternatively, the behavioral discrepancy between α -fetoprotein and aromatase null mutant mice could simply be the result of differences in the timing of estradiol action. The sequestering effect of α-fetoprotein is predominantly in the prenatal and early postnatal period and then becomes substantially less important. Conversely, animals lacking aromatase are deprived of estradiol throughout the postnatal sensitive period.
A definitive role for early estradiol determining the sexual phenotype of the adult rodent brain is established, but what about humans? The issue of human brain sexual differentiation is one fraught with political, religious, and cultural bear traps. Debates on the biological basis of partner preference, same-sex marriage, and the scientific aptitudes of men versus women continue to rage, and the potential for a resolution anytime soon seems unlikely. In regard to hormonal influences on the developing human brain, we are constrained to correlative data or so-called “naturally occurring experiments” in which fetuses or newborn infants have been systematically exposed to hormones or synthetic analogs in a dose or duration that would not normally occur. The most celebrated and intensely studied of these is a genetic disorder of steroidogenesis in the adrenal gland that results in elevated androgens in female fetuses, a condition known as congenital adrenal hyperplasia (CAH). Diagnosis is usually at birth when the presence of partially masculinized genitalia is revealed. Studies of CAH girls in England and Europe, Japan, and North American, while differing in details and magnitude of the effects, consistently conclude there is some masculinization of the brain consequent to fetal exposure to androgens (see Ref. 49 for review). But these studies speak to the issue of androgens, and the focus of this review is estrogens. From the 1940s until 1971, a common medical practice for maintaining a healthy pregnancy was the treatment of pregnant women with the highly potent estradiol analog diethylstilbesterol (DES). The practice was discontinued when it became evident that daughters of DES-treated mothers had an increased incidence of clear cell adenocarcinoma of the vagina and cervix. Interest in the psychosexual effects of DES exposure in women followed the reports of estradiol being the masculinizing factor of the male rodent brain. Early reports of psychosis (107) and lack of interest in parenting (63) in DES-exposed girls were not replicated in subsequent larger studies (118, 146), including ones by the same group of scientists. In hindsight, the lack of effect of DES on brain differentiation of human females is consistent with empirical results generated in primates in which most results indicate no important role for estrogens in masculinization, this function instead being performed by prenatal androgens combined with social context and rearing conditions (232). What if any role estrogens might be playing in primate brain development remains to be determined, but evidence for an important role for aromatase persists (137).
D. Estradiol Regulates Apoptosis to Establish Volumetric Sex Differences
There are three plausible mechanisms by which estradiol can permanently influence the size of a particular brain region or subnuclei during development: 1) changes in cell proliferation, 2) changes in cell survival, or 3) changes in migration to a particular loci. All of these possibilities have been examined in at least some brain regions. In mammals there is little evidence that estradiol impacts on neuronal or glial proliferation in developing brain, and there is some clear evidence that it definitely does not (105, 159, 234), but the possibility cannot be entirely ruled out. The same is true for a potential contribution of neuronal migration to sex differences in the mammalian brain: no evidence in favor and definite evidence against (105, 159). While there is no evidence that estradiol impacts on neuronal migration, there is a suggestion that ERβ is required for normal development of the cerebral cortex by regulating the health of the radial glia along which the neuronal precursors must migrate (234). This novel observation has not been linked to estradiol and may reflect a nonhormonally regulated function of ERβ. With proliferation and migration largely ruled out, this leaves differential survival as the most likely variable mediating volumetric sex differences. In song birds, another excellent model for sexual differentiation of the brain, most volumetric differences also appear to be due to estradiol’s impact on survival, and not proliferation or migration, with the notable exception of the higher vocal center (HVC), a brain region critical for song learning and production. Here it is the greater incorporation of new neurons into the HVC of males during the critical period for sexual differentiation that leads to a much larger nucleus volume compared with females (40). Why the rules are different for this particular song nucleus is unclear but highlights the principle that there are many ways to achieve the same end point of a larger structure in one sex versus the other.
Estradiol modulation of naturally occurring cell death in the developing brain is limited to very specific regions and can have opposing effects, sometimes promoting cell survival, and sometimes orchestrating a cell’s demise.
1. Sexually dimorphic nucleus of the preoptic area
In rats, the celebrated sexually dimorphic nucleus (SDN) is the poster child for sex differences in the brain. It is five to seven times larger in males than females and situated right in the heart of male-sex-behavior-central, the preoptic area (134). The SDN has been extensively studied and exhaustively reviewed, yet its true function remains an enigma (see Refs. 54, 98, 209). The SDN both sends and receives a wide-ranging array of inputs, suggesting it serves as integrative node for variables regulating expression of male sex behavior, but such a function is hard to demonstrate. Nonetheless, for this discussion, it serves the didactic purpose of illustrating one of the fundamental principles by which estradiol modulates brain development, the control of apoptosis. The SDN is a dense collection of neurons in the medial preoptic nucleus. The volume of the SDN can be quantified by sectioning and staining the brain, tracing the area consisting of dense cells and reconstructing the area based on slice thickness and magnification. The density of cells does not differ in males and females, but the number and therefore the area occupied does. Males and females start out with the same number of neurons destined to become part of the SDN, but beginning about postnatal day 3 and peaking on postnatal day 7, cells in the female die at a prolific rate. By 10 days of life, it’s game over, the final volume of the SDN is forever established. If females are treated with either estradiol or aromatizable androgen sufficiently early, the cells will not die, and despite the absence of estradiol later, the female will always have a male-sized SDN (178). Unfortunately, this valuable model system of modulation of naturally occurring cell death has never been sufficiently exploited to reveal the secret of how estradiol saves the cells. Other than being distinguished by their high levels of calbindin expression (193), a calcium binding protein, SDN neurons are not particularly different from those in the surround, yet they are preferentially saved by estradiol. The unfortunate fact that most mouse strains do not have an SDN further precludes the opportunity for unraveling this mystery.
2. Anteroventral periventricular nucleus
The anteroventral periventricular nucleus (AVPV) is notably in contrast to the SDN in that this nucleus is larger in females, and this is entirely due to the ability of estradiol to kill off cells (195). The AVPV also offers several advantages over the SDN, with the two most important being that it is present in mice and its functional significance is clear, it is a critical node in the control of the surge of gonadotropin secretion required for ovulation. Moreover, the AVPV is part of a well-defined sexually dimorphic circuit. A substantial portion of the neurons in the AVPV are dopaminergic, and it is their survival that is primarily undermined by estradiol. The use of single- and double-knockout mice suggests that both isoforms of ER are required for the full male AVPV phenotype to be achieved (35), an interesting contrast to the SDN which uses only ERα to regulate cell death. The functional involvement of both isoforms is confirmed with the use of ERα and ERβ selective agonists, either of which when given neonatally to females will reduce AVPV volume and impart infertility due to impaired cyclicity (160). That roughly identical results are found in rats and mice and the unusual nature of the estradiol action (i.e., killing cells, or at least preventing them from surviving) and the requirement for both receptor isoforms, should provide a good hook from which to begin to investigate the specific cellular mechanisms by which estradiol is acting.
Other sex differences in the volume of particular subnuclei or brain regions are found, but they are much smaller in magnitude and have not been investigated on a mechanistic level or specifically in the context of estradiol action. Androgens also regulate cell survival, particularly in the spinal cord via a complex regulation of growth and survival factors, and the interested reader is referred to an excellent recent review by Forger (67). Lastly, we have recently found that androgens impact on cell proliferation in the developing hippocampus and may thereby contribute to the larger volume of this structure in males (L.-M. Zhang, S. Zup, A. Konkle, and M. McCarthy, unpublished data).
E. Estradiol Promotes Neurite Growth
Some of the earliest and most spectacular reports on estradiol effects on the developing brain were the landmark studies of Dominique Toran-Allerand and co-workers (216, 217, 219) on the profound induction of neurite outgrowth from organotypic explant cultures of the preoptic area, hypothalamus, and cerebral cortex. Vivid photos of little plugs of tissue that sprouted neurites like hair on a Chia Pet when watered with estradiol, left little doubt that this hormone is a potent regulator of brain development. But unfortunately, studying neurite development in the actual brain turns out to be an extremely difficult problem, and progress has been relatively slow given the obvious importance of the end points. Adding to the complexity is the nature of the estradiol action itself, which has been less than straightforward. Estradiol provided in the dish directly activates neurite growth, and at the time these discoveries were made, the late 1970s and early 1980s, the assumption was that ER acting at EREs would transcribe specific genes that would then direct neurite growth. Growth factors, such as nerve growth factor (NGF) and brain-derived nerve growth factor (BDNF), and structural proteins involved in neurite extension, such as growth-associated protein 43 (GAP-43) and microtubule-associated protein (MAP-2), were obvious candidates for upregulation by ER, and this does indeed occur (76, 220). But, the model of ER acting on an ERE in the promoter region of said growth factors did not fit the data. For example, the MAP kinase signaling pathway was also activated by estradiol in cortical neurons (124, 197). The preliminary assumption that this was secondary to estradiol-induced increases in NGF was negated by the observation that phosphorylation of ERK occurred independently of activation of trk receptors. This raised the intriguing possibility that ER was interacting directly with the MAP kinase signaling pathway in developing neurons. The MAP kinase activating effect of estradiol was not blocked by the ER antagonists tamoxifen or ICI 182,780, but parsimony still suggested the effects were mediated by ER, probably the α-isoform but also possibly via the β-isoform (197). The exclusive developmental expression of ERα in neocortex suggested this receptor was the mediating agent. But again, the data did not fit, including the observation that the rapid activation of the MAP kinase pathway persisted in mice with a null mutation for ERα. Similar observations were made of hypothalamic neurons where estradiol conjugated to BSA (and therefore not able to penetrate into cells) was effective at inducing axonal growth in an ICI/tamoxifenresistant manner (42) that required the trkB receptor (39). Thus was born ER-X, a novel membrane-associated receptor for estradiol with a kilodalton weight between that of the 67 kDa ERα and the 60 kDa ERβ. Several other features distinguish this novel receptor. It is exclusively associated with caveolar-like microdomains (CLM). CLM serve the same purpose in neurons that caveoli do in other cell types, which is to provide a cholesterol-enriched scaffolding for anchoring and associating signal transduction proteins. By associating with CLM, ER-X is presumably brought into direct contact with MAP kinase and its associated proteins. ER-X shows an unusual developmental pattern in expression, peaking between postnatal days 7 and 10 before becoming undetectable in the adult (218). An unusual ligand-affinity profile also distinguishes the receptor from ERα and -β, in particular its high affinity for the naturally occurring 17α-estradiol stereoisomer of 17β-estradiol. In fact, ER-X has an equal affinity for the α- and β-stereoisomers (as opposed to the 100-fold lower affinity of ERα for 17α-estradiol), and 17α-estradiol is even more effective than 17β at activating MAP kinase in cortical neurons. The story is brought full circle with the evidence that 17α-estradiol levels are notably elevated in the developing brain and that this previously considered inactive enantiomer is synthesized locally in the brain (221). This provocative series of findings challenges many of the preconceived notions of steroid action in the developing brain, highlighting the need for more careful research into this fascinating area of neuroendocrinology.
Considerable traction towards understanding the neurite growth-promoting effects of estradiol is found in the neural circuitry regulating sexually differentiated gonadotropin secretion. Neurons of the AVPV provide a critical source of afferent input to LHRH neurons for initiating the LH surge required for ovulation. The AVPV, in turn, receives a sexually differentiated afferent input from the principle nucleus of the bed nuclei of the stria terminalis (BSTp). In a series of elegant and technically challenging studies, Polston and Simerly (169) determined a profile of sexually dimorphic projections from the BSTp, with a greater galanin projection to the AVPV in males and a greater substance P projection to the preoptic area in females. Even more striking is the presence of a GABAergic projection from BSTp to AVPV that is ~10-fold greater in males than females (168). Changes in circulating steroid levels in adults have no impact on the dimorphism in innervation, providing proof of principle of the organizational impact of steroids during development. Given the AVPV is significantly smaller in males, the larger BSTp projection provides a substantial and permanent inhibitory clamp. ERs can be found in neurons of both the AVPV and the BSTp, making either a likely target for estradiol regulation of the projection. Combined explant cultures of neonatal AVPV and BSTp determined that a diffusible target-derived factor from the AVPV, induced by estradiol, directs the growth of neurites towards itself (103). Identification of the factor(s) awaits further study, but the results to date are noteworthy for their clear functional significance and context within a well-defined neural circuit (Fig. 3).
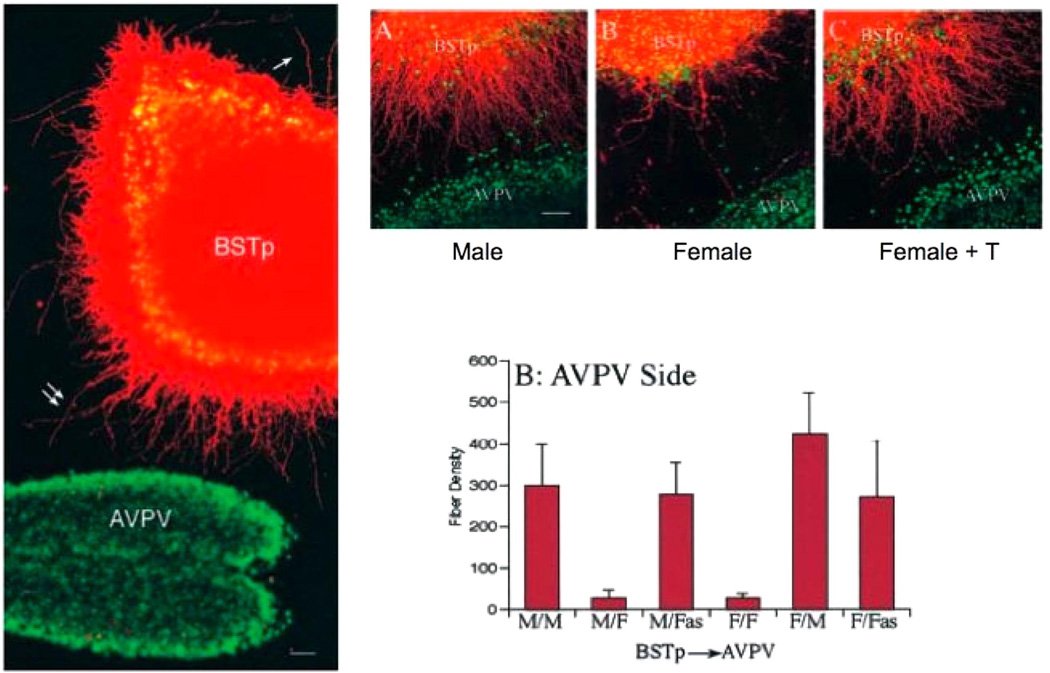
FIG 3.
Mechanisms of estradiol action for establishing a sexually dimorphic projection from the principle nucleus bed nucleus of the stria terminals (BSTp) to the anteroventral periventricular nucleus (AVPV). The AVPV is notable both for its central role in the control of the female-specific LH surge and for being larger in females than males. There is also a substantive sex difference in the size of the afferent projection from the BSTp to the AVPV, being up to 10-fold larger in males. To distinguish whether this sex difference in innervation arises in the BSTp or the AVPV, Simerly and colleagues (103)developed mixed-sex cocultures of the two nuclei. The BSTp is labeled with DiI (pseudo-colored red), and the AVPV is visualized with a Hoeschst stain (visualized here as green). Note the neuronal processes originating from the BSTp explant and extending toward the AVPV (left panel). The use of mixed sex cultures is illustrated with representative photomicrographs in the top panel and graphically by fiber density in the bottom panel. When a male-derived BSTp was paired with a male-derived AVPV (M/M), there were significantly more neurites extending toward the AVPV compared with a male-derived BSTp cocultured with a female-derived AVPV (M/F), and was not different from female-female cocultures (F/F). A female-derived BSTp innervated a male-derived AVPV (F/M) to the same degree as a M/M coculture, and treatment of females with testosterone before coculturing with a male BSTp resulted in a neurite growth rate identical to that of M/M cocultures. These data demonstrate that a hormonally determined target derived factor in the AVPV directs the innervation by the BSTp to produce a sexually dimorphic neural circuit. [From Ibanez et al. (103), copyright 2001 by Society for Neuroscience.]
Estradiol also promotes axon growth of fetal neurons derived from the ventromedial nucleus (VMN) of the hypothalamus and cultured in vitro. The embryonic day on which the neurons are cultured impacts on the magnitude of the effect, whether there is a response in both sexes versus only males and the requirement for glia in the culture. The younger the neurons when plated, the more responsive and less demanding they seem to be. In concordance with ER-X, the axonal growth-promoting effects of estradiol on male hypothalamic neurons are reported to be mediated at the membrane and involve the MAP kinase pathway (45). Reducing levels of estradiol-induced trkB receptor prevents the axonal growth as well, but if and how the MAP kinase pathway and growth factor pathways are intersecting to promote axonal growth is not clear, although the principle of a target-derived factor induced by estradiol appears to apply here as well (43).
A class of signaling molecules originally identified for their role in metastatic cancer, the focal adhesion complex family of proteins, are important regulators of neurite growth by controlling interaction with the extracellular matrix via the integrins. Focal adhesion kinase (FAK) and its closely associated protein, paxillin, are both higher in the neonatal female hypothalamus, and in contrast to most other proteins are actually reduced by estradiol (199). This has led to speculation that FAK and paxillin may be important to feminization of the brain, making them the first such potential regulators identified. FAK expression is highly enriched in growth cones, and FAK expression in the brain peaks on postnatal day 0 (reviewed in Ref. 81). Similarly, paxillin is highly phosphorylated during embryonic development in the rat (223). FAK is necessary in netrin-mediated chemoattraction and chemorepulsion of axons (117, 176), and it may be required for growth cone turning (184). Hippocampal neurons deficient for FAK demonstrate increased axon length and branching in vitro (180). Neuroblastoma cells overexpressing paxillin demonstrate enhanced cell spreading and growth factor-induced lamellopodia formation (224). Dendrites of female hypothalamic neurons branch less frequently than those of females (140), and estradiol promotes branching of cultured hypothalamic neurons (199). Since estradiol is a masculinizing hormone, both responses are consistent with elevated FAK in females reducing dendritic branching, but a causal relationship is not yet established.
Similar to its opposing effects on apoptosis in different subnuclei, estradiol can also have opposing effects on neurite growth. Neurite growth on serotoninergic neurons derived from the embryonic mesencephalon is inhibited by physiological levels of estradiol (119). This is consistent with the perinatally established sex difference in the distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus where males exhibit a lower density of fibers than females (194).
F. Estradiol Regulates Synaptic Patterning
Equally important as the number of neurons in a particular brain region is the amount and nature of the connections they receive and establish. Complex behavioral and physiological responses, such as those associated with reproduction, require neural networks capable of coordinating diverse amounts of information, both external and internal. Essential data include olfactory profiles of conspecifics, time of day, internal endocrine milieu, and reproductive state. These variables must be assessed and integrated for execution of the appropriate appetitive, i.e., motivational, and consummatory components of reproductive behavior. As discussed above, one of the principle actions of estradiol on the developing rodent brain is to precondition the neural network so that adult hormonal secretions can activate the correct response. To do so, estradiol is a potent modulator of the formation of dendritic spines, the major site of excitatory glutamatergic synapses. Depending on the brain region, estradiol can either increase or decrease the density and/or number of dendritic spines. Estradiol is also capable of modulating dendritic spine formation in the adult brain, and this has generated considerable interest, particularly in the hippocampus where spine formation is synonymous with plasticity associated with learning and memory. But there are several important and perhaps informative differences between estradiol induction of dendritic spine synapses in the adult versus the neonate. First is the magnitude of the effect. In adult female hippocampus, estradiol induces at maximum a 30% increase in the density of dendritic spines (244), and in the hypothalamus, the effect is even smaller (41). In contrast, in the neonatal preoptic area or hypothalamus, estradiol increases dendritic spines by 200–300%. Second is that estradiol-induced spines in the adult are transient, when the estradiol disappears so do the spines, but in the neonate, the density of spines that are formed within the first few days after birth will persist into adulthood (5, 127). These differences suggest that both the mechanism and the function of estradiol-induced spine synapse formation are fundamentally different in the developing as opposed to mature brain. Information we have to date would support this view, although no direct empirical evidence has been generated, reflecting more the separation of investigator focus than technical or biological limitations.
One of the most striking things regarding estradiol regulation of spines is that although the end point may be exactly the same, i.e., a two- to threefold increase in the density of spines, the mechanism for reaching that end point can be markedly different in different brain regions. The current state of understanding for each region investigated is reviewed here.
1. Arcuate nucleus
The arcuate is a hypothalamic nucleus and is a small bilateral structure just above the median eminence and adjacent to the third ventricle. It contains dopaminergic, enkephalinergic, and GABAergic neurons, many of which coexpress releasing peptides such as corticotrophin releasing hormone (CRH), growth hormone releasing hormone (GHRH), and thyrotropin releasing hormone (TRH). Neurons of the arcuate exert regulatory control over the anterior pituitary as well as other hypothalamic nuclei and thereby play a central role in reproduction, feeding, and stress responding. Quantitative electron microscopy (EM) of tissue sections through the arcuate nucleus of adult rats treated neonatally with gonadal steroids reveals a marked and permanent hormonally determined sexual dimorphism in the density of axodendritic spine synapses as well as axosomatic synapses, but there is no difference in the density of axodendritic shaft synapses (127). Males have two- to threefold more axosomatic synapses than females, who have an equally large bias in the same direction for axodendritic spine synapses. The pattern is reversed in males castrated as neonates or females treated with testosterone, which is readily aromatized to estradiol in the neonatal brain. Treatment is within the first few days of life, but synapses are quantified on postnatal day 60, attesting to the permanency of the treatment effects. Axodendritic spines are the major site of excitatory glutamatergic synapses, whereas axosomatic synapses tend to be inhibitory GABAergic synapses. Thus a sex difference in the relative amount of axosomatic versus axodendritic synapses has a profound impact on the excitability as well as potential source of afferent input to arcuate neurons in males versus females. Quantitative EM is a labor-intensive approach and not practical for comparing the effect of multiple experimental variables. Axodendritic spine synapses can be more easily quantified in Golgi impregnated tissue, providing information on number, density, and cellular location (i.e., distal or proximal to the soma), and an approximate measure of dendritic spine measures can be gained by Western blot analysis of a protein specific to spines, sphinophilin (82). Unfortunately, there is no readily available marker for axosomatic synapses, leaving these important inhibitory synapse structures much less investigated than their excitatory counterparts on dendritic spines.
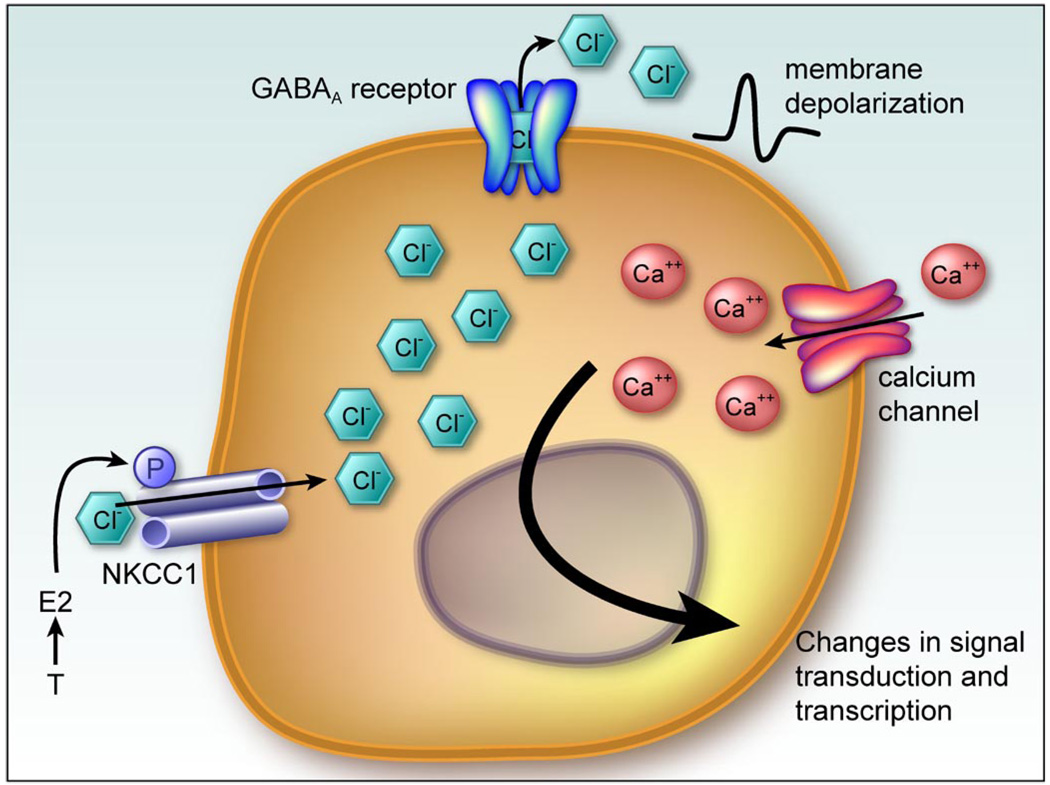
Work by our group confirmed the induction of dendritic spines by estradiol in the developing arcuate, using both EM and analysis of Golgi-Cox impregnated tissue. The hormonally induced change in dendritic spines was correlated with a marked sex difference in the morphology of protoplasmic astrocytes in the same brain region (141, 142). This sex difference is also determined by estradiol acting within the first few days of life and results in male astrocytes that are more complex in terms of the number of primary processes and the frequency with which they branch. In other words, the male astrocytes have a more stellate appearance as opposed to the bipolar appearance of female astrocytes. Thus there is an inverse relationship between the density of dendritic spines and the complexity of astrocytes within the arcuate, creating the temptation to invoke a causal relationship. Tantamount to establishing a causal link is determining the primary site of estradiol action. Is it acting on the neurons, the astrocytes, or both? Attempts to localize ER in arcuate astrocytes were unsuccessful (143), and more importantly, activation of the GABAA receptor was found to be a critical component of estradiol-induced increases in astrocyte stellation (144). The rate-limiting enzyme in GABA synthesis, GAD, is found only in neurons, thus establishing this cell type as the likely primary site of estradiol action where it increases the activity of GAD, and thereby the synthesis of GABA, which is released from neurons to act on neighboring astrocytes to induce stellation. Astrocytes express GABAA receptors and, due to relatively high intracellular chloride, respond with membrane depolarization and an influx of calcium. Activation of GABAA receptors on immature cortical astrocytes induces differentiation and stellation (69, 147), an effect consistent with that observed in the arcuate nucleus. This still leaves unanswered the question of how estradiol downregulates dendritic spines on the neurons. The possibility of a physical barrier to spine formation created by the highly stellate male astrocytes has been proposed but remains untested (143). In the adult arcuate nucleus, the same population of astrocytes as studied in the neonate is capable of physically stripping synapses and allowing for reestablishment later. This feature is unique to females and is a component of the remodeling that occurs across the estrous cycle (75). Whether there is an analogous developmental process in which astrocytes are permanently differentiated by the actions of estradiol acting via GABA, to suppress the formation of dendritic spines, remains unknown (Fig. 4).
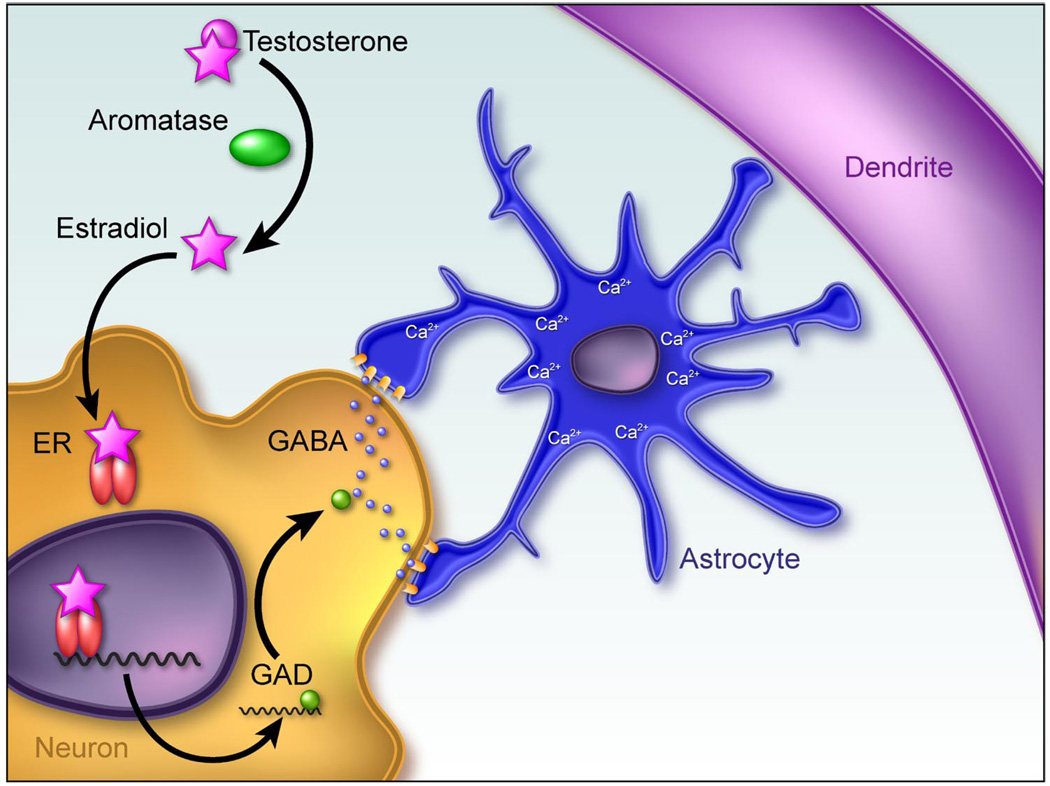
FIG 4.
Working model of estradiol action establishing sexually dimorphic astrocyte morphology and synaptic patterning in the arcuate nucleus. The arcuate nucleus is located in the dorsomedial hypothalamus and exerts a regulatory subluence over the anterior pituitary and adjacent hypothalamic nuclei to regulate such diverse functions as feeding, growth, stress responding, and reproduction. There are half as many dendritic spine synapses on neurons in the neonatal male arcuate as there are on female dendrites (126). This sex difference is entirely dependent on elevated estradiol in the male brain during the perinatal sensitive period. Conversely, the astrocytes in the male arcuate are far more stellate, with more processes that frequently branch, compared with females. Mong et al. (144) determined that estradiol increases the synthesis of the inhibitory neurotransmitter GABA by neurons, which then acts on neighboring astrocytes to induce stellation. It is speculated that the increased complexity of the astrocytes in males suppresses the formation of dendritic spine synapses, but the mechanism of how that is achieved is currently unknown.
2. The preoptic area
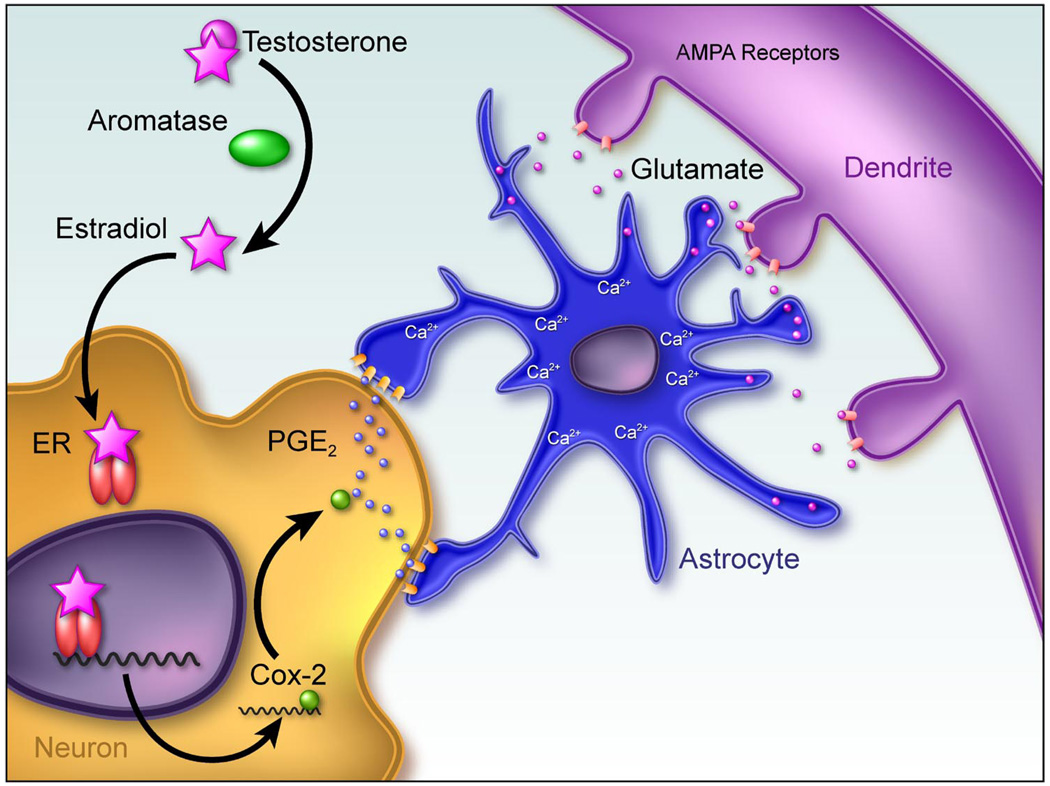
The medial preoptic area (POA) is the major brain site controlling adult male sexual behavior and female maternal behavior and is thus not surprising as a site of major sex differences in morphometry. The SDN has already been discussed, but in addition to this remarkable volumetric difference, the same region also boasts a sex difference in dendritic spine synapses, with males having two- to threefold higher levels than females (7, 209). Again, as in the arcuate nucleus, the induction of spines is permanent, with the pattern established in the first few days persisting until at least 90 days of age (5). There is also a sex difference in the morphometry of astrocytes in the POA, with again males having more complex, stellate astrocytes than females (6). But, the relationship between POA astrocyte morphology and dendritic spine patterning is the precise opposite of that seen in the arcuate, precluding the potential for establishing general principles about estradiol mediation of astrocytic/neuronal crosstalk. Moreover, when the mechanism of estradiol induction of dendritic spines is explored in greater depth, there is no role for GABA, but instead, there appears to be a requirement for activation of glutamatergic AMPA receptors. However, glutamate receptor activation is just one step in a complex process that begins with estradiol upregulation of the enzyme cyclooxygenase-2, or COX-2, the inducible form of COX and a nodal point in the production of prostaglandins and the thromboxanes (95). Induction of COX-2 is strongly yoked with an inducible form of prostaglandin E2 synthase, mPGEs, leading to the preferential production of PGE2 over other prostanoids. Estradiol treatment of neonatal female rats increases PGE2 levels in the POA by sevenfold, and this appears to be a direct result of estradiol induction of COX-2 gene transcription (5). The estradiol-induced increase in females mimics the naturally occurring process in males where COX-2 and PGE2 levels are higher than those of females due to the endogenous production of estradiol in the male newborn brain, using his own testicular testosterone as precursor. A current working model proposes that estradiol acts first in the neuron to increase COX-2 levels and activity, thereby increasing PGE2 which is released from the neuron to act on neighboring astrocytes. There are two effects of PGE2 on astrocytes: increased stellation (6) and release of glutamate (32, 187). The glutamate released by the astrocytes in response to PGE2 is speculated to then activate AMPA receptors on the neighboring (or originating) neuron to induce formation of dendritic spines (Fig. 5). The functional impact of PGE2 production and formation of spines in the newborn male brain will be discussed below.
FIG 5.
Working model of mechanism of estradiol action establishing sexually dimorphic synaptic patterning in the preoptic area. The preoptic area (POA) is the critical brain region controlling expression of male sexual behavior and exhibits some of the most robust sex differences in the brain. In addition to the sexually dimorphic nucleus, male POA neurons have about twice as many dendritic spines as females, and this level of spines can be induced in females by treatment with estradiol during the perinatal sensitive period. Dendritic spines are the primary site for excitatory synapses. Astrocytes are also more complex in the male POA, with longer and more frequently branching processes. Both of these morphological sex differences are the result of estradiol action in the neonatal brain (4). The initiating event is the induction of COX-2, a pivotal enzyme in the production of prostanoids and specifically linked to an increased synthesis and release of prostaglandin E2 (PGE2). Receptors for PGE2 are G protein linked and can be found on astrocytes. Activation of EP receptors can induce glutamate release from astrocytes in a calcium-dependent manner, and glutamate induces the formation of dendritic spines. In this system, application of PGE2 induces a 2- to 3-fold increase in the density of dendritic spines on POA dendrites, and this effect can be blocked by antagonists to the glutamate AMPA receptor. Thus, in this model, a critical neuronal/astrocytic cross-talk is believed to be essential for PGE2 to induce a sexually dimorphic synaptic pattern determined by estradiol.
The discovery that the prostaglandin PGE2, a compound normally associated with inflammation and fever, is a potent regulator of normal male development was not only a surprise, but it also illustrated the point of how little we know about brain development and how comparing and contrasting the male and female brain at this highly dynamic time point can provide novel insights into previously unconsidered mechanisms.
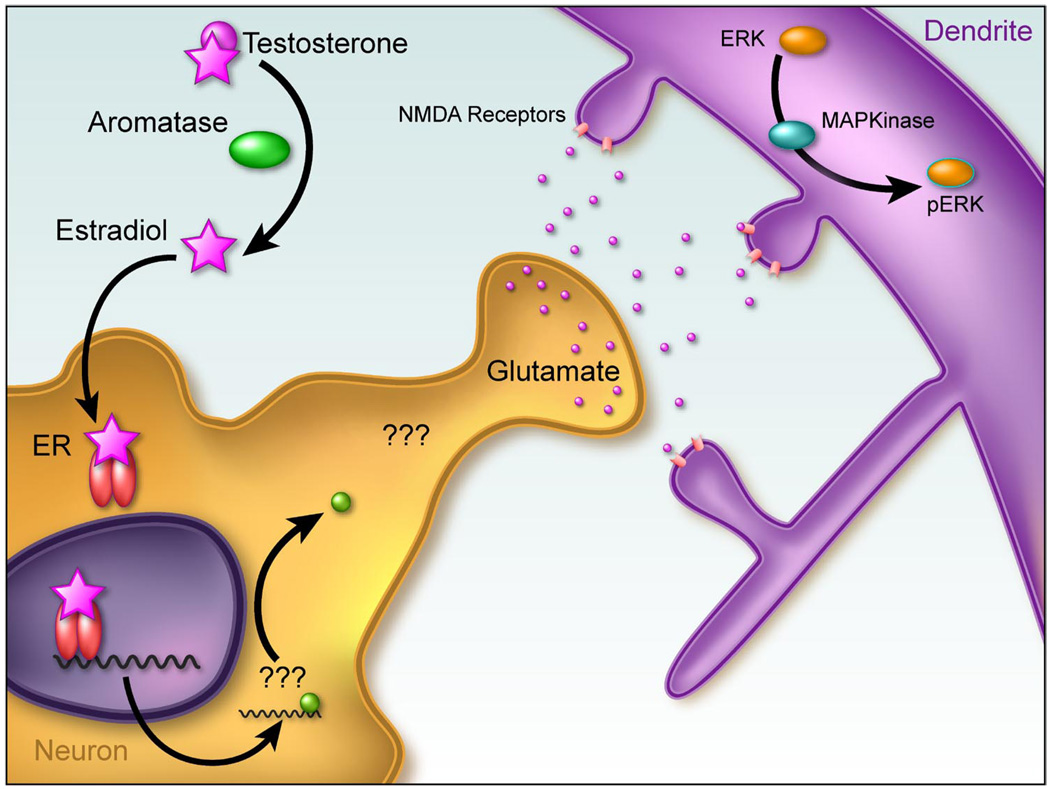
3. VMN of the mediobasal hypothalamus
What the POA is to male sex behavior, the VMN is to female sex behavior. Lesions of this hypothalamic nucleus eliminate lordosis responding in the female rat, and implanting estradiol directly into the VMN induces the behavior. Neurons in the ventrolateral subdivision express high levels of ER and project to the midbrain central gray, forming an essential link in the neural circuitry controlling female sex behavior. The clear connection to a behavioral output that is hormonally dependent (female sexual behavior) has made the VMN a target of intensive study, but much remains unresolved. Early ultrastructural analysis indicated no sex differences in numerical density of dendritic spine or shaft synapses at postnatal day 5 (126), but a subsequent stereological analysis reported that the number of both types of synapses was greater in males at this same developmental time point and persisted throughout life. Treatment of newborn males with an ER antagonist reduced levels to those of females, confirming this as a classic estradiol-mediated organizational masculinization event (170). But, a recent study reports the opposite sexual dimorphism in the adult, with males having more axosomatic synapses and females more axodendritic synapses, particularly during the proestrus portion of the cycle (186). Admittedly, contradictory results are found even within the same laboratory. Based on Golgi impregnation, we reported in 1999 (140) that there was no sexual dimorphism in the density of dendritic spines in the VMN of postnatal day 3 rat pups, but that the dendrites of males branched more frequently than those of females. To our surprise, when we recently measured a protein associated with dendritic spines, spinophilin, there was more in the mediobasal hypothalamus of males than females. A reanalysis of the original Golgi-impregnated tissue revealed that males do indeed have more dendritic spines, and these appear to be a consequence of the greater number of branches per dendrite (210). Thus males have more spines overall, but not at a higher density than in females. Whether this sex dimorphism persists into adulthood is unknown but highlights the complexity of hormonally induced synaptic patterning changes in this nucleus and our need for additional information.
Advances have been made in elucidating the cellular mechanisms of estradiol action in this nucleus, and not surprisingly, they differ fundamentally from those in the POA and arcuate nucleus. Both PGE2 and GABA have been ruled out as critical mediators of estradiol induction of spines in the VMN (210). Furthermore, unlike the POA and arcuate, astrocytes are not part of the cell-to-cell communication required for estradiol induction of spines in the VMN, but there is a central role for glutamate. Moreover, the mechanism of estradiol action is trans-synaptic, involving enhanced glutamate release from presynaptic terminals acting on postsynaptic NMDA and AMPA receptors to activate MAP kinase and increase levels of the protein spinophilin. As discussed above, estradiol acting at ERs can directly activate MAP kinase, an effect which occurs rapidly and is mediated at the membrane. However, in this scenario, the effects of estradiol on activation of MAP kinase are indirect, being secondary to the calcium influx induced by opening of NMDA receptors (Fig. 6). This constitutes a novel action of estradiol in developing brain, enhancement of glutamate release at synaptic terminals to build permanent sex differences. How estradiol achieves this end is unknown, but that the ER is required is established by the ability of pretreatment with the ER antagonist ICI 182,780, to completely prevent estradiol-enhanced glutamate release and formation of postsynaptic dendritic spines (J.A. Schwarz, S.M. Thompson and M.M. McCarthy, unpublished observations).
FIG 6.
Working model of mechanism of estradiol action establishing sexually dimorphic dendritic morphology in the ventromedial nucleus (VMN) of the hypothalamus. The VMN is the critical brain region controlling expression of female sexual behavior. Dendrites on neurons in this nucleus branch more frequently in males, and also have an overall greater number of spine synapses (210). This sex difference is established during the perinatal sensitive period and is a function of estradiol action in the male brain. The estradiol-induced increase in dendritic spines can be blocked by antagonism of the NMDA glutamate receptor and mimicked by application of an NMDA receptor-specific agonist. Downstream to activation of glutamate receptors is activation of the mitogen-activated protein kinase pathway, leading to spine formation (Schwarz et al., unpublished observations) and possibly dendritic branching. The fundamental effect of estradiol is to promote the release of glutamate from nerve terminals. Unlike the arcuate nucleus and the POA, no definitive role for astrocytes has been established in this system. However, since the primary effect of estradiol is presynaptic, there is a requirement for cell-to-cell communication to permanently organize this brain region into the masculine phenotype.
G. Behavioral Masculinization, Defeminization, and Feminization
A major challenge in developmental neuroendocrinology is establishing causal connections between neonatally determined neuroanatomical sex differences and adult physiology and behavior. Fortunately, there is a robust and reliable end point: sexual behavior in the albino laboratory rat, which is determined by neonatal hormone action. Whether an adult rat responds to sexual advances from another adult rat with a male response (mounting, intromitting, and ejaculating) or a female response (lordosis) is entirely dependent on two variables: 1) the hormonal milieu during a perinatal sensitive window and 2) the hormonal milieu of the adult. As discussed in section IVB, for normal male sexual behavior the neonate must be exposed to a critical level of neuronal estradiol matched with a critical level of circulating testosterone as an adult. For female behavior, the neonate must not be exposed to a critical level of neuronal estradiol matched with a threshold level of estradiol sequential with progesterone in the adult. Male sexual behavior is opportunistic and readily expressed whenever a receptive female is present. Female sexual behavior is physiologically constrained to be expressed only in temporal proximity to ovulation. A fascinating but mechanistically unexplained distinction in male and female sexual behavior is the impact of experience. Males improve with practice, and if testosterone is removed (via castration) will continue to exhibit high levels of copulatory behavior that only gradually extinguishes over a period of months. Females, in contrast, get it right the first time and every time but will only exhibit lordosis under the proper hormonal umbrella. If steroids are eliminated, so is female sex behavior. In terms of ultimate causation, the adaptive basis for the distinction is obvious; there is no benefit and possible cost to a female that mates outside the window of opportunity for conception, whereas males maximize fitness by never missing an opportunity to share the wealth. Proximately, however, this dichotomy in the nature of the plasticity attendant to both behaviors is suggestive of distinct and separate neuronal circuitries, be they physically or merely functionally so.
So how does the brain mediate these distinct strategies in males and females? A useful framework for investigating mechanistic questions of sexual differentiation is the operationally defined and distinct processes of masculinization, feminization, and defeminization. Masculinization refers to an active developmental process initiated by gonadal steroids, in particular estradiol derived from testicular androgen, during the perinatal sensitive period followed by expression of normal male copulatory behavior in adulthood. Feminization is essentially what happens in the absence of masculinization, meaning it is the developmental program that will be executed in the absence of estradiol and will construct a brain predisposed to expression of lordosis under the proper hormonal conditions in adulthood. Defeminization is distinct from but normally occurs in tandem with masculinization and refers to the process whereby the ability to express female sexual behavior is lost.
Studies attempting to identify cellular mechanisms of estradiol-induced masculinization have by and large focused on neurotransmitters (55, 99, 130). While much information was gained, a definitive demonstration of the cellular mechanism was lacking as evidenced by the inability to induce behavioral masculinization in the absence of exogenous steroid treatment. As discussed in section IVF2, a novel new regulator of estradiol-mediated changes in synaptic patterning is the prostaglandin PGE2 (4, 5). The synthesis of prostanoids begins with the oxygenative cyclization of arachidonic acid by cyclooxygenase. The inducible isoform of cyclooxygenase, COX-2, is an immediate early gene responsive to a variety of stimuli including fever, injury, and stimuli associated with neuronal plasticity (44, 79, 95). The POA is considered the brain region where sensory and hormonal stimuli are integrated for male sexual behavior (51), and we therefore investigated whether PGE2-induced permanent developmental changes in synaptic profile of this brain region were correlated with behavior. We found that males treated with a COX-2 inhibitor, the nodal point in PGE2 synthesis, during the first 2 days of life have permanently reduced numbers of POA dendritic spines compared with normal males and as adults exhibit little to no male sexual behavior despite normal circulating testosterone levels. Moreover, newborn female pups treated with PGE2 for 2 days have permanently elevated POA dendritic spines compared with normal females, and most importantly, as adults, if provided with exogenous testosterone, will behave as if they are males towards receptive females. These PGE2 masculinized females will pursue and engage in vigorous mounting of receptive females and appear exactly as normal males. Thus PGE2 is both necessary and sufficient for full masculinization of sex behavior. This surprising finding also provided a new tool for investigation of sex differentiation of the brain, since it allowed for the first time the ability to masculinize the brain without steroids. This in turn allowed for asking the question, Are the processes of masculinization and defeminization two sides of the same coin? In other words, if a brain is masculinized, is defeminization an irrevocable byproduct? Or, alternatively, are masculinization and defeminization two separate processes that can be manipulated independently of each other?
To answer this question, males that had been treated with a COX-2 inhibitor and therefore showed no male sex behavior were treated with a compliment of female hormones and then experimentally assayed for female sex behavior. The results were strikingly clear: males treated with COX-2 inhibitors as neonates displayed neither male nor female sexual behavior as adults; they were asexual. Conversely, females treated with PGE2 as neonates that displayed robust male sex behavior as adults were also capable of robust and perfectly normal female sexual receptivity when under the correct hormonal milieu (209). From this we conclude that masculinization and defeminization are separate and distinct processes (Fig. 7), thereby raising the question, What are the cellular mechanisms of defeminization?
FIG 7.
Masculinization, defeminization, and feminization of sex behavior. The embryonic brain is bipotential in its ability to adopt a masculine or feminine phenotype, but this potentiality is lost after a perinatal sensitive period. The reproductive behavior of the laboratory rodent provides a valuable model system for fundamental questions about how a sex phenotype is permanently imprinted on the immature brain. In this context, masculinization refers to the organization of the brain such that it will support the expression of male sexual behavior in adulthood. Defeminization is the removal of the default phenotype, the feminine. Defeminization is an active hormonally driven process that organizes the brain to preclude the expression of female sexual behavior in adulthood. Both masculinization and defeminization are induced by estradiol. Feminization occurs in the absence of high levels of estradiol. A: there are two opposing views of how these processes could be codified in the brain. Option one is a continuum; masculinization and feminization are opposite ends of the spectrum, and defeminization is an obligate component of masculinization. In this scenario, one could experimentally manipulate animals during the sensitive period such that in adulthood they could fall anywhere along the spectrum. In option two, masculinization and defeminization are viewed as two separate processes. In this scenario, it should be possible to create animals that show robust male and female sex behavior and animals that are essentially asexual, exhibiting neither male nor female sexual behavior. B: the discovery that PGE2 is a fundamental determinant of behavioral masculinization allowed for a new examination of these two options by circumventing the need for estradiol to induce masculinization. By inducing masculinization with PGE2, it could be determined if defeminization was also affected. Treatment of neonatal females with PGE2 resulted in animals that would show either male or female sexual behavior as adults if provided the appropriate activational hormonal milieu. Likewise, treatment of newborn males with a cyclooxygenase (COX)-2 inhibitor to block the endogenous production of PGE2 eliminated all sex behavior, regardless of the adult hormonal milieu. From these observations it is concluded that masculinization and defeminization are mechanistically distinct processes regulated by estradiol. How this steroid hormone mediates defeminization has yet to be determined.
Defeminization is an estradiol-mediated active process that either prevents the formation of the feminine circuit or somehow overrides it, resulting in a permanent suppression or inhibition of female sex behavior. There is some suggestion that the developmental timing of masculinization and defeminization is slightly different, with masculinization beginning prenatally and completing early postnatally, whereas defeminization is entirely postnatal (233). But this is not a prerequisite since a female rat can be fully masculinized and defeminized by postnatal treatment with estradiol (209). One of the principle questions that require answering is, What is the neural circuit of defeminization? This merely begs the question, What is the neural circuit of feminization? One would presume the neuronal addresses are the same, or at least on the same block. It is well known that the VMN of the hypothalamus is a central site in the control of female sexual receptivity, i.e., lordosis, and thus the VMN is the female analog to the male POA. The dendrites of male VMN neurons are longer, branch more frequently, and have more dendritic spines (143, 209). Thus the degree and perhaps source of afferent input to the male VMN may be fundamentally different than that of the female.
Mice bearing null mutations for both the α- and β-form of the ER have been exploited for their potential to provide new insights into the process of sexual differentiation. The ERKO mouse, which lacks the α-form of the receptor, fails to exhibit normal male sexual behavior when treated with testosterone as an adult, consistent with the concept of estrogen action being required for masculinization and establishing that it is the α-form of the receptor that is needed for both organizational and activational effects of estradiol on reproductive behavior (182; but see Refs. 156, 157). Additional careful behavioral assessment of the BERKO, a knockout mouse strain lacking functional ERβ, reveals a potential role for this isoform of the receptor in defeminization. Males lacking ERβ are more easily induced to exhibit female sexual behavior than their wild-type littermates when both are treated with a female hormonal profile as adults. Importantly, these males exhibit perfectly normal male sexual behavior that does not differ from males possessing Erβ throughout life (112). This observation could provide an inroad into determining the cellular mechanisms of defeminization, which remains unknown other than that it is not the opposite of masculinization.
There are some advances being made into the mechanism(s) of estradiol-induced defeminization, with at least two potential cellular events identified. The first relates to formation of spines and involves enhancement of glutamate release leading to postsynaptic activation of AMPA and NMDA receptors, followed by activation of MAP kinase and production of spinophilin, as reviewed above. This is a novel action of estradiol for establishment of sex differences in the brain and occurs on a relatively rapid time scale, within 6 h, but is nonetheless mediated by the classic ER, presumably ERα. The second involves the focal adhesion complex of proteins, also mentioned above. Estradiol reduces both FAK and paxillin in the newborn brain, and expression of both of these proteins is positively associated with a reduction in neurite growth and branching (199). Thus estradiol can promote dendritic extension by reducing FAK and paxillin. This is also a novel action for estradiol in the immature brain, the actual reduction of signaling proteins. There are no other clear examples in which an important class of signaling molecules is actively suppressed by estradiol during the sensitive period, making the focal adhesion complex of proteins a unique candidate for mediating normal female brain development. Establishing that this is actually true will be far more challenging, as the female brain is the default pathway and therefore lacks the trigger (i.e., estradiol) that initiates the male pathway.
H. Gonadotropin Secretion
Neurons expressing gonadotropin releasing hormone (GnRH; also referred to as luteinizing hormone releasing hormone or LHRH) are diffusely located throughout the preoptic area and bed nucleus of the stria terminalis in rodents, and also in the arcuate nucleus of primates and guinea pigs. Given the central, actually essential, role of these neurons in reproduction, two things are particularly notable. First, there are no obvious sex differences in the number, distribution, or morphology of GnRH neurons. Second, despite the importance of estradiol in gonadotropin secretion, GnRH neurons do not express ERα and have very little ERβ (241). Yet these neurons preside over the most marked of sexually dimorphic responses, the pattern of LH release from the anterior pituitary. In males, LH is pulsatile and regular, with a peak every 30 min to a few hours depending on the species. In females, this pulsatile pattern is disrupted by a mid-cycle surge in LH that is required for ovulation. In the absence of this surge, there is no softening of the stigmata on the Graffian follicle, and the ova is not expelled, regardless of how high estradiol levels rise. The cyclic release of LH is an inherent property of the female brain and is permanently erased by perinatal exposure to estradiol. Perinatal females exposed to high levels of testosterone, or estradiol, are sterile as adults due to lack of ovulation. Androgenized females (although really they are estrogenized) are characterized by lack of cyclicity and persistent vaginal estrus as a result of growth of follicles to the preovulatory state where they remain, in essence, stuck. Of interest is the observation that low-dose exposure to androgens or estrogens neonatally will impact fertility later in adulthood, after a period of apparently normal fertility (86). There is no mechanistic explanation for this phenomenon, which in many ways parallels normal aging but at an accelerated rate.
An important distinction in the sensitive period for control of gonadotropin secretion is its duration, which seems to extend beyond that for differentiation of reproductive behavior. In a systematic comparison of the two responses, Diaz et al. (58) reported that sensitivity to steroid-mediated defeminization ended abruptly on postnatal day 7, whereas disruption of gonadotropin secretion extended beyond day 9 (58). Others report that female rats exposed to estradiol prior to 5 days of life are masculinized, but after that time the same dose will decrease the interpulse interval for LH release and advance the onset of puberty (125). Changes in the glutamatergic input to the GnRH neurons are implicated in these effects, but precisely where and how estradiol is acting remains unknown. The same is true for the masculinizing effects of early exposure to estradiol on the LH surge; the cellular mechanism is essentially completely unknown. In fairness, however, so is the cellular mechanisms of positive feedback of estradiol in the adult female that leads to the LH surge. This is certainly not from lack of trying, as the control of gonadotropin secretion in the adult female has been, and continues to be, a topic of enormous interest, but apparently also of enormous complexity (62, 88, 123).
I. Growth Hormone and Prolactin Secretion
At the time of the mid-cycle LH surge in an adult female, there is an accompanying but slightly offset prolactin surge, and this too is subject to elimination by early exposure to estradiol. Moreover, adults rendered anovulatory due to neonatal androgen or estrogen exposure are hyperprolactinemic, but interestingly the relative impact of drugs that modify dopaminergic versus serotoninergic effects on prolactin secretion vary depending on whether the sterility was induced by testosterone or estradiol (50). In embryonic culture, two to three times as many prolactin-expressing neurons from female hypothalamus survive as those derived from male hypothalamus (30), but here the authors conclude the difference is independent of gonadal steroids. There is also neonatal sex differentiation of the response to elevated prolactin in adults; females increase food intake but males do not (87), but again, what role if any neonatal gonadal steroids play in this distinction is unclear.
In contrast to more prolactin expressing neurons in females, there are more growth hormone releasing hormone (GHRH) neurons in the arcuate nucleus of males, but not appearing until as late as postnatal day 40 and possibly as a secondary effect of changes in somatostatin neurons (155). More important, however, are the patterns of GH secretion that are markedly different in males versus females, with males having more frequent higher amplitude pulses than females (106, 149). This sex difference is determined by gonadal secretions but has not been definitively linked to estrogens versus androgens. Functionally, the sex difference has important implications for liver function and body weight. About 75–85% of the genes in the liver exhibit a sex difference in expression, although the magnitude of the difference is small, ~10% (181). Interestingly, when 86 genes expressed at differential rates in male versus female liver were examined, the sex difference was found to be driven by the sex difference in GH secretion pattern for a large majority. This is secondary to the activation of STAT5B, which is favored by the rapid cyclic pattern of GH secretion characteristic of males. Thus the sex differentiation of the secretion pattern of this anterior pituitary peptide has life-long consequences for the entire body. Mechanistically, the basis of the hormone-induced sexual differentiation of this system at the level of the brain is entirely unknown. Our knowledge of sexual differentiation of prolactin secretion is equally primitive, and in both instances, there has been little to no investigation of the neuronal basis of these fascinating and obviously functionally significant sex differences.
V. ESTRADIOL REGULATES THE PHYSIOLOGY OF DEVELOPING NEURONS
Morphometry is a wonderful thing. Differences in form are tangible and intuitively appealing. Often form follows function, but many functions that can’t be traced back to form might be related to physiology. In the developing brain, the effects of estradiol on physiology are relatively unexplored. The hypothalamus, the major site of estradiol action, does not readily lend itself to electrophysiological analysis even in the adult, and the technical challenges in the neonate have proven prohibitive, although recent advances have been made (245). Assessing the physiological state of neurons can be done indirectly via immunocytochemical or in situ hybridization detection of mRNAs or proteins that reflect a change in activity. The two most celebrated and widely used of these are the immediate early gene c-fos, which can be detected at the protein or mRNA level, and the phosphorylated (i.e., activated) form of cAMP response element binding (CREB) protein. Recent technical advances in the use of fluorescent dyes that change their emission profile upon binding calcium provide a different view point of cellular physiology and provide novel insights into how estradiol impacts on the physiology of developing neurons.
A. c-fos
Detection of the immediate early gene c-fos has been used with great effect in the adult brain, helping to map the neural circuitry underlying sexual behavior, maternal behavior, stress and anxiety responding, and olfaction just to name a few. In the neonatal rat, there is little behavior to speak of, making this activated protein of limited use for that purpose. There is a sex difference in levels of fos protein detected by Western immunoblot in the immature hypothalamus during the first few days of life (158), but whether this sex difference is due to differential estradiol levels in males versus females has not been determined. There is also an induction of c-fos in the POA of neonates following maternal licking and grooming of the pups, but there is no sex difference and no apparent role for steroids in this response (129). In many ways this is not surprising given that much of the evidence in the adult brain indicates that the estradiol does not regulate c-fos expression per se, but instead alters the probability of specific neural pathways being activated in response to a salient stimulus. The change in probability may be in part achieved by ER interacting with AP-1 sites via fos/jun dimmers, but whether this is occurring in the developing brain is unknown.
B. CREB, CBP, and ERK
CREB is a prevalent transcription factor that is activated by phosphorylation of specific residues, an event that can be detected with the use of phospho-specific CREB antibodies. There are baseline sex differences in the level of pCREB in reproductively relevant ER-rich regions of the neonatal rat brain, being higher in males. There is also a dramatic sex difference in the induction of pCREB following depolarizing GABA activity on the day of birth (11, 13). GABA is a ubiquitous and dominant inhibitory neurotransmitter in the adult brain. The GABAA receptor is a chloride-permeant ionophore that upon opening hyperpolarizes neuronal membranes via influx of this negative ion and further inhibits excitation via shunting inhibition due to decreased membrane resistance. In contrast, in immature neurons, the transmembrane chloride concentration gradient favors an efflux of chloride out of the cell upon GABAA receptor opening, resulting in membrane depolarization sufficient to activate voltagegated calcium channels (128). Administration of the GABAA agonist muscimol to neonatal males results in a massive induction of pCREB in response to the influx of calcium via L-type voltage-gated calcium channels. Interestingly, female littermates treated in an identical manner not only do not display an induction of pCREB, they actually show a significant reduction, indicating a divergence in signal transduction pathways activated in male versus female brains. This divergence is a function of estradiol, which augments the depolarizing action of GABA on neonatal hypothalamic neurons by increasing the activity of the cotransporter that maintains high intracellular chloride (162). This is also true in the developing hippocampus and will be discussed in greater detail below.
CREB binding protein (CBP) is another ubiquitous protein important to transcriptional events, and in addition to its obvious ability to bind CREB, it also interacts directly with ERs, acting as a coactivator. Newborn male rats have more CBP in brain regions either destined to become sexually dimorphic or critical to the control of sex-typic behaviors or physiology (12), and it is assumed this sex difference is due to elevated estradiol in males.
C. Depolarizing GABA
Views on GABA action have changed considerably over the past decade. Once considered the primary source of inhibition in the brain, we now know it to be a principle source of excitation via depolarization-induced calcium influx through voltage-sensitive calcium channels. This action is most prominent developmentally and appears to be present throughout the brain, although it is not completely ubiquitous. The calcium influx induced consequent to depolarizing GABA action places it squarely in the camp of being a trophic factor, although many of the aspects of its potential trophic action have not been well explored. The excitatory effects of GABA are mediated by the GABAA receptor, a chloride ionophore, and the relative transmembrane chloride gradient. Whether GABAA receptor activation results in chloride influx or efflux is determined by the transmembrane chloride concentration gradient, which is in turn determined by the activity and expression of chloride cotransporters (57). During the neonatal period, the reversal potential for chloride (ECl) is positive relative to the resting membrane potential (22), resulting in a net outward driving force on chloride when GABAA receptors open and membrane depolarization sufficient to open voltage-sensitive calcium channels, primarily of the L-type (114). As development progresses, ECl becomes negative relative to the resting membrane potential, shifting the driving force on chloride inward and leading to GABAA receptor-mediated hyperpolarization, the primary basis for synaptic inhibition in the mature brain.
The gradual developmental shift from GABA-mediated excitation to inhibition is controlled by changes in chloride cotransporter expression and/or activity, including NKCC1 and KCC2, along with chloride channels such as ClC2 (190). NKCC1 promotes chloride transport into the cell along with sodium and potassium, and its expression is high in neonatal brain but declines with advancing age. Conversely, KCC2 promotes chloride efflux, and its expression is low neonatally but increases as development progresses such that by the end of the second postnatal week, KCC2 levels are elevated, and NKCC1 levels are significantly decreased (166, 167). Consequently, around this time, GABAA receptor activation results in chloride influx and membrane hyperpolarization (201).
Estradiol enhances the depolarizing action of GABA in developing hypothalamic neurons by increasing the magnitude of the calcium transient with each depolarization, increasing the number of neurons that respond to GABAA receptor activation with a calcium transient, and extending the developmental duration of depolarizing GABA action (162). Similar findings of estradiol enhancing and extending the duration of depolarizing GABA have been made for hippocampal neurons (152), and in both cases estradiol appears to upregulate the activity of the chloride cotranporter NKCC1, which transports chloride into cells and maintains a depolarizing gradient (Fig. 8). In neurons of the substantia nigra, there is a sex difference in the response to GABA agonist, with a depolarizing effect maintained in males while females have the more mature hyperpolarizing response. Here, estradiol downregulates KCC2, the cotransporter that sends chloride out of cells, and in this way also maintains a gradient that favors depolarization (74).
FIG 8.
Estradiol enhancement of depolarizing GABA action. The normally inhibitory transmitter GABA is predominantly excitatory in the developing brain due to a shift in the reversal potential for chloride so that activation of GABAA receptors results in chloride efflux and membrane depolarization as opposed to hyperpolarization. The membrane depolarization induced by GABA is sufficient to activate voltage-gated calcium channels and promote calcium influx. This excitatory effect of GABA is linked to trophic actions and promotes the maturation of synapses. Estradiol enhances the excitatory actions of depolarizing GABA by increasing the magnitude of the calcium influx, increasing the number of neurons that respond to GABA as depolarizing and extending the developmental duration of depolarizing GABA. The principle action of estradiol is to increase the amount and activity of the sodiumpotassium-chloride cotransporter NKCC1 that maintains intracellular chloride. This transporter is expressed at high levels in mature neurons but gradually declines as development proceeds and is superceded by another transporter, KCC2, which transports chloride out of the cell. A potential deleterious consequence of the estradiol-induced enhancement of depolarizing GABA is a lowering of the threshold to excitotoxicity in the immature brain.
In the substantia nigra, the sex differences in depolarizing GABA action are speculated to underlie sex differences in the threshold to epileptic seizure, which is lower in developing males than females (74). In the hippocampus and hypothalamus, however, the functional significance of depolarizing GABA remains unclear. One difficulty in establishing the functional significance of estradiol enhancement of depolarizing GABA is that at this time, the only way to enhance depolarizing GABA is by estradiol administration. Administering GABAA agonists to females in an attempt to mimic the normal state of males will not work, since in the absence of estradiol, GABAA receptor activation is fundamentally different.
D. Progesterone Receptor
One of the principle genes transcribed by activated ER is progesterone receptor (PR). In fact, many brain regions show no PR expression unless estradiol has been in the neighborhood during the previous days. The preoptic area is one of those regions, and during development the male has robust expression of PR, whereas the female has little to none. This is a classic ERE-genomically mediated effect and contributes to the argument that estradiol’s effects on the developing brain are of the traditional variety (173, 230). The same sex difference is found in rats and mice, and in both cases is regulated by ERα, but there are some subtle species differences that may speak to the not-so-subtle species differences in male sexual behavior. The induction of PR by estradiol is independent of the SRY gene, and thereby the gonads (231), making it a quintessential example of hormonally mediated sex differentiation. The next challenge is to determine what functional role estradiol-induced PR is playing in sex-specific brain development (229).
E. Granulin
The ER is fundamentally a transcription factor. Developmentally there is a sensitive window for permanent organizational effects imprinted on the brain by estradiol. Administering estradiol to females appears to fully mimic the effects of endogenous estradiol aromatized from testicular androgens in males (the sequestering effects of α-fetoprotein are overcome by using large doses of estradiol). If ever there was a situation that is perfect for a microarray gene chip-type analysis to identify novel genes induced by estradiol, this would seem to be it. Subtractive hybridization was used effectively in this regard and identified granulin as a gene strongly enriched in the hypothalamus of male and androgenized female 5-day-old rat pups. Transcription of the granulin gene by estradiol was confirmed (206), and its functional significance to masculinization was also confirmed with the use of antisense oligonucleotides to block translation of the mRNA into protein. Males or androgenized females administered the granulin antisense oligos during the first few days of life had compromised male sexual behavior as adults (204). Granulin promotes the growth of epithelial cells in the periphery and in the brain is largely associated with tumor growth. In what capacity granulin contributes to normal masculinization of the brain remains unknown at this time (205).
VI. ESTRADIOL IS SYNTHESIZED DE NOVO BY THE DEVELOPING BRAIN
Every field has its studies that become iconoclastic. Distinguishable from dogma by not postulating any theory or guiding principle, these studies are statements of fact, experimental findings that are cited over and over again as fundamental. In developmental neuroendocrinology, the iconoclasts are a small handful of studies measuring serum testosterone and hypothalamic estradiol in the neonatal rat (179, 237). These have provided essential supporting evidence to the organizational/activational hypothesis, as well as the aromatization hypothesis, by establishing that neonatal males do indeed have high circulating testosterone and elevated hypothalamic estradiol compared with females. These findings have stood the test of time and are not in question. The risk of iconoclasim, however, is that facts that are true for one place and circumstance are often inappropriately generalized to other places and circumstances.
We recently embarked on a major effort to quantify the concentration of estradiol and testosterone in multiple brain regions of the rat, beginning prenatally and extending into adulthood. The fundamental principle that males have more androgen, and hence more estrogen, in the preoptic area and hypothalamus, during a restricted perinatal period, was generally upheld. However, there were some notable discrepancies. First, the period of elevated androgens and estrogens in the hypothalamus extended beyond that previously reported and beyond what is reported for the period of elevated gonadal synthesis. More importantly, levels were elevated in both males and females, although more so in males, before declining in both sexes just prior to postnatal day 10. Second, levels of estradiol and testosterone in the hippocampus and cortex changed across development in a pattern that could not be explained as originating from the gonads, and that was at times different in males and females. For instance, cortical levels of estradiol and testosterone peaked in females on postnatal day 6. On this same developmental day, levels were precipitously dropping in the hippocampus in both males and females. Third, removal of both the gonads and adrenal glands at birth did not impact on brain estradiol or testosterone concentrations (Konkle and McCarthy, unpublished observations). These findings illustrate two general principles: 1) steroid levels in different brain regions are largely independent of each other, and 2) these levels do not depend on gonadally derived precursors. The great challenge now is determining where these steroids are coming from, and more importantly, what they are doing.
The de novo (from cholesterol) synthesis of estradiol in the avian brain is well established (97, 188) and has recently been reported for the mammalian hippocampus as well (96, 171). Steroidogenesis requires the transport of cholesterol into the inner mitochondrial membrane by steroidogenic acute regulatory protein (StAR), and the subsequent conversion to pregnenolone by the P-450scc enzyme CYP11. Both of these have been detected at high levels in hippocampal pyramidal neurons, along with picomolar levels of pregnenolone and its sulfated derivative (108). Conversion of pregnenolone to progesterone, as well as dehydroepiandrosterone (DHEA), requires 3β-hy-droxysteroid dehydrogenase (3β-HSD). Developmentally, the expression of 3β-HSD is at its highest level in newborn hippocampus and declines with increasing age. Concentrations of hippocampal pregnenolone and progesterone measured by gas chromatography/mass spectrometry were also at their highest on the day of birth and exceeded those found in plasma, suggesting local synthesis (102). Other studies confirm the presence of 3β-HSD as well as 5α-reductase and 21-hydroxylase (102, 135, 189, 202). Allopregnanolone, a 5α-reduced metabolite of dihydroprogesterone, as well as DHEA have also been detected at low levels in the brains of adult adrenalectomized and gonadectomized male rats (47, 183). Cultured astrocytes and neurons from neonatal rodent cerebral cortex are capable of synthesizing DHEA and subsequently converting the steroid to testosterone and ultimately estradiol (248). In situ hybridization detection of CYP19 (aromatase) mRNA reveals high levels in POA, hypothalamus, and amygdala, with moderate levels in the hippocampus (227, 228). Activity assays reveal the same pattern in explants or cultured neurons of neonatal rat and mouse brain. The highest levels of estradiol production from [3H]androstenedione are found in the diencephalic brain regions, but the neonatal hippocampus appears to make up to half that seen in these sexually dimorphic brain regions (122). Interestingly, the hippocampus and cortex are distinct from sexually dimorphic brain regions in not exhibiting aromatase activity until shortly after parturition (122). A critical enzyme, CYP17, required for the conversion of pregnenolone to DHEA had long eluded detection in the adult brain, despite measurable levels of DHEA, until a recent report detecting it in adult male hippocampus (96). Previous reports of this enzyme had been limited to the developing brain (135, 248). Thus all of the requisite machinery appears to be in place for the de novo synthesis of estradiol by the brain.
Given one accepts the developing brain is making its own estradiol, the next obvious question is, Why? There must be important functional benefits if such an energetically expensive process is maintained. In the bird, male brain-derived estrogens are both necessary and sufficient for establishment of the song control circuit, a sexually dimorphic behavioral response (97). In the mammalian brain, what little we know about de novo estradiol synthesis during development suggests that it is often the same in both sexes, or at least the resultant estradiol concentration is the same. Estradiol has potent neuroprotective effects in the adult brain, and this is true in some instances in the developing brain as well (see below). Neuroprotection is an attribute of benefit to both males and females; thus this may be the primary ultimate causation for estradiol synthesis in the developing telencephalon of both males and females, but how this is achieved proximately is not known.
VII. ESTRADIOL ALTERS THE IMPACT OF NEONATAL BRAIN DAMAGE
A. Estradiol Is Protective
The enormous interest in estradiol as a neuroprotective agent in animal models of adult stroke, hypoxia, or blunt trauma has not translated to the field of pediatric brain damage. This is likely due to two factors. One is that a substantial amount of interest in the adult is based on the relative merits or faults of hormone replacement therapy in aging woman. The second reason may be a combination of the technical difficulties associated with studying ischemia in neonatal rodents, the overall lower level of interest in development, and the widespread but false perception that mechanisms established in the adult generalize to the neonate only on a smaller scale. Hypoxic/ischemic brain injury occurs in 2–4 full-term neonates per 1,000 births and up to 50% of premature babies suffer some form of brain damage in the United States (www.MarchofDimes.com/peristats; Ref. 208). There is a 50% mortality rate in infants experiencing severe hypoxic/ ischemic injury and a complication rate of 80% in survivors, including long-term cognitive and behavioral impairment, with deficits in attention span, short-term recall, and problem solving (68). Thus there are compelling reasons for exploring the potential beneficial effects of estradiol in models of pediatric brain damage.
In the adult brain, hypoxia/ischemia induces massive release of glutamate into the extracellular space, initiating an excitotoxic cascade due to excessive influx of calcium through NMDA receptors and voltage-gated calcium channels (29, 48). The same is true for the immature brain in regards to excessive release of glutamate, but the effects of glutamate are not the same in the immature brain as in the adult. This appears to be due to both the population and functionality of glutamate receptors being distinctly different in the developing brain.
The emphasis in hypoxia/ischemia research on the neonate has been on the 1-wk-old rat pup. This is in large part due the practical fact that administering glutamate analogs to pups younger than 7 days is generally without effect. Initial interpretations that the imperviousness of the neonatal brain to glutamate was a function of no glutamate receptors was revised based on clear evidence of both the presence and activity of glutamate receptors. The conclusion that the newborn rat is not sensitive to glutamate excitotoxicity also requires revisiting, with attention given to the subregions of the hippocampus and the sex of the animals being investigated. We found that administering kainic acid to newborn males and females results in selective damage to the dentate gyrus exclusively in females (94). The CA1 and CA2/3 regions are largely unharmed by this treatment protocol, but there is up to a 40% cell loss in the dentate of females. Pretreating females with estradiol is partially protective against kainic acid-induced damage (92), but the precise mechanism remains unclear. Kainic acid is a selective glutamate receptor agonist that binds primarily to AMPA and kainate receptors and activates NMDA receptors only secondary to membrane depolarization. The AMPA receptor is generally impermeable to calcium due to the presence of the GluR2 subunit that renders the pore too small for this large cation. Early in development, many AMPA receptors do not possess a GluR2 subunit and as a result will freely flux calcium into the cell upon activation (100). Postnatal day 3 males and females have equal levels of GluR2 protein and mRNA when quantified by Western blot or quantitative PCR, respectively, but estradiol treatment increases levels in both sexes, and to the same degree in both sexes. This is correlated with reduced calcium influx following AMPA receptor activation by kainic acid in cultured hippocampal neurons visualized with fura 2-AM (90). But estradiol regulation of the GluR2 subunit does not explain why males exhibit no hippocampal damage in response to kainic acid, since there is no sex difference in the levels of GluR2, nor is there a sex difference in endogenous hippocampal estradiol during this developmental time point.
In addition to the ionotropic AMPA/kainate and NMDA receptors, the neonatal hippocampus expresses metabotropic receptors that are activated directly by glutamate. In an in vitro model of glutamate-induced excitotoxicity in immature hippocampal neurons, there was marked cell death induced by direct glutamate administration, and this correlated with a massive increase in intracellular calcium (Fig. 9). Surprisingly, the source of the calcium was not extracellular but was instead released from internal stores following activation of type I metabotropic glutamate receptors. When cultured hippocampal neurons were pretreated with physiological levels of estradiol, there was a complete protection against glutamate-mediated excitotoxicity. Estradiol treatment was observed to reduce the levels of mGluR1 by two- to threefold. The reduction in metabotropic receptors is presumably responsible for the bulk of the neuroprotective effect but likely only one of multiple mechanisms (93). Estradiol levels are elevated in the hippocampus at birth, gradually declining over the first week of life. A potential but untested source of the relative imperviousness of the neonatal hippocampus to glutamate-mediated excitotoxicity could be this endogenous neuroprotective steroid. Put in a different way, the ultimate causation for the de novo synthesis of estradiol by the developing hippocampus might be for purposes of endogenous neuroprotection, an interesting but as yet untested hypothesis.
FIG 9.
Estradiol provides neuroprotection from glutamate excitotoxicity in immature brain. Excessive calcium influx following overactivation of glutamate receptors is the gateway to adult neuronal excitotoxicity. NMDA and AMPA receptors are primary routes for calcium influx into mature neurons. In immature hippocampal neurons, glutamate-mediated excitotoxicity is determined by the mGluR class of glutamate receptors and involves release of calcium from internal stores. A: neurons imaged with the calcium-sensitive dye fura 2-AM can be visualized over time to reveal the dynamics of calcium handling. Bath application of kanic acid (KA) versus glutamate (Glu) reflects the differential sources of calcium. KA activates AMPA, and then NMDA receptors and calcium influx into the cell with a slow upward slope that terminates gradually. In contrast, glutamate activates metabotropic glutamate receptors which release calcium from internal stores in a rapid surge that quickly depletes the reserve before the drug application is complete. B: treatment of cultured immature hippocampal neurons with glutamate significantly increases cell death, as indicated by TUNEL assay; pretreatment with estradiol completely prevents the glutamate-induced cell death and is neuroprotective against spontaneous cell death as well. C: pseudo-colored image of neurons being imaged for free calcium shows the control condition in panel 1, revealing a high level of calcium, whereas in panel 2, a decrease in the peak amplitude of the calcium is observed following pretreatment with estradiol. This reduction in the amplitude of the calcium transient correlates with an observed decrease in the amount of metabotropic glutamate receptor following estradiol treatment. [From Hilton et al. (93), copyright 2006 Wiley-Blackwell Publishing.]
B. Estradiol Is Damaging
Too much of a good thing is bad, and estradiol at very high doses in young animals has been known for a long time to be toxic, in particular to the arcuate nucleus where there is substantial cell death, resulting in polycystic ovaries and anovulation in the adult (38). The selective vulnerability of the arcuate is further selective to β-endorphin-expressing neurons and appears to involve the conversion of estradiol to catechol estrogens (37).
In the neonate, estradiol can also impact on damage caused by other means. Returning to pediatric brain damage, in addition to the excitotoxic glutamate released extracellularly during hypoxic, there is also a massive increase in extracellular GABA (8). As reviewed above, the action of GABA in the developing brain is fundamentally different from that in the adult, being depolarizing and excitatory as opposed to hyperpolarizing and inhibitory. This led us to speculate that excessive depolarization induced by GABA would mimic the excitotoxic effects of glutamate seen in more mature brains. This hypothesis was tested and confirmed by the administration of muscimol, a highly selective GABAA agonist, to newborn pups on the day of birth and 1 day after, with cell death assessed at 1 wk of age. Unlike the damage induced by glutamate, excessive activation of GABAA receptors induced widespread cell death in the hippocampus in both males and females, although the damage was slightly but significantly greater in males (150, 151). The damage induced was enduring and compromised performance on cognitive tasks later in life. Cell death was correlated with expression of genes associated with the apoptotic cascade, such as caspase-3 and BCl2, and was prevented by pretreatment with an L-type voltage-gated channel blocker, thus confirming the classic excessive calcium-induced excitotoxic pathway of cell death (153, 154).
Estradiol is a potent regulator of the depolarizing actions of GABA, increasing the magnitude of the calcium transient induced and the number of neurons that respond to GABA as depolarizing in both the hippocampus and hypothalamus. In the hippocampus, estradiol appears to delay the natural progression of depolarizing to hyperpolarizing GABA that is normally complete by ~2 wk postnatally (152). When muscimol administration is combined with estradiol treatment, the amount of cell death in the hippocampus is significantly increased in both in vivo and in vitro models. There are at least two mechanisms by which estradiol increases the calcium transient consequent to depolarizing GABA: 1) an increase in the activity of NKCC1, the sodium-potassium-chloride cotransporter that pumps chloride into the cell and thereby maintains a chloride gradient conducive to depolarizing the membrane upon opening of the GABAA receptor, and 2) an increase in the α1c-subunit of the L-type voltage-gated calcium channel.
Thus there appear to be opposing actions of estradiol on neonatal brain damage induced by glutamate- versus GABA-mediated excitotoxicity, being protective against glutamate but exacerbating damage against GABA. This contradiction makes it difficult to justify any attempts at translating this research into clinical practice at this time. Moreover, since both GABA and glutamate are released following hypoxia/ischemia in neonates, the appropriate model would seem to be exogenous administration of a combination of both of these excitotoxic agents. To our surprise, we found that combined muscimol and kainic acid to neonates was not damaging, yet either one alone was, and the lack of damage in response to the combination was correlated with a marked upregulation of the calcium binding protein calbindin (91). Estradiol regulates calbindin levels in adult brain (116, 203), and there are sex differences in calbindin levels within the first few days of life (36), but there have been no careful studies of estradiol effects on calcium binding proteins in the neonatal brain. This is just one of many missing pieces of information regarding the effects of estradiol on the developing brain at the mechanistic level, both in the context of normal development and in response to injury or environmental insult.
VIII. ESTRADIOL MIMETICS AND ENDOCRINE DISRUPTION OF THE DEVELOPING BRAIN
The lay book Our Stolen Future was published in 1996. The lead author, Dr. Theo Colborn, is a senior scientist at the World Wildlife Fund and a recognized expert on endocrine-disrupting chemicals. The book was heralded as the modern day equivalent to Rachel Carson’s Silent Spring, and the analogy seemed appropriate given the response by Congress mandating the Environmental Protection Administration (EPA) to establish the Endocrine Disrupting Screening and Testing Advisory Committee. This was subsequently replaced by the Endocrine Disrupter Methods Validation Advisory Committee, which reported its progress to the United States Congress in 2000 and 2002. In September of 2005, the EPA presented notice to the Federal Registrar of their intended strategy for selecting the initial group of 50–100 chemicals to be screened under Tier 1 of the Agency’s Endocrine Disruptor Screening Program. It has been 10 years to get this far, meaning no federal oversight of potentially endocrine disrupting chemicals in the environment. This in large part reflects the tremendous complexity of the issue, beginning with what is the definition of endocrine disruption (66). Should something that mimics estradiol be considered endocrine disrupting? Moreover, chemicals used as pesticides or in plastics have multiple actions on the nervous system, only one of which might be interacting with steroid receptors, and this interaction can be highly complex, having mixed agonist/antagonist actions depending on the tissue, stage of development, route of exposure, and endogenous endocrine milieu. That synthetic chemicals in the environment can wreak havoc on the endocrine system of animals is irrefutable when considering aquatic species. Exposure from pesticide runoff, industrial waste, and oral contraceptives in effluent of water treatment plants are the primary sources, with fish, amphibians and aquatic reptiles literally swimming in the stuff, and illustrating the price with abnormal genitalia, increased incidences of intrasex individuals, and infertility (101).
The majority of research on endocrine disrupting compounds has focused on reproductive abnormalities, a natural bias given the visual impact and obvious importance of males with ovotestis or hemipenises. But there has been persistent interest in the impact of endocrine disrupting compounds on the nervous system. The potential for disruption of estradiol action altering normal brain development was recognized early on, but actually demonstrating this has been less simple.
In some instances, the effects of an endocrine disrupting compound are straightforward and easily interpretable as mimicking estrogen action. A systematic comparison of the alkylphenol 4-tert-octylphenol, commonly used in paints, pesticides, herbicides, detergents and plastics, with the synthetic estrogen DES, found equal magnitude effects on preventing the ability to exhibit an LH surge and induce ovulation in rats (240). A similar parallel effect was observed between neonatal DES and two naturally occurring xenoestrogens: 1) genistein, an isoflavin found in many grains, and 2) zearalenone, a mycotoxin produced by the cereal pathogen Fusarium graminearum. Both compounds increased the volume of the SDN when administered to neonatal females and altered the responsiveness of the pituitary to GnRH of adult males and females (65). When genistein was examined for its effects on the AVPV, a sexually dimorphic brain region that is larger in females than males, it did not mimic the effect of estradiol in females and exerted a demasculinizing effect on males, counter to what would be expected of an estrogen-mimicking compound. If another parameter, colocalization of ER with tyrosine hydroxylase, was used as the end point, genistein as well as the plasticizer bisphenol-A (BPA) both behaved as estrogen mimetics (161). Interest in BPA is particularly high given its pervasiveness in the environment and conflicting evidence regarding its disease-promoting potential. Administration of BPA to pregnant and lactating rats in their drinking water prevented the development of a sex difference in CRH-expressing neurons in one region of rat brain (71). This is an unusual and potentially important effect of BPA on the developing brain but is hard to interpret in the context of endocrine disruption, since the hormonal basis of the sex difference in CRH expression is not established. A similar caveat can be applied to prenatal BPA effects on “depression-like behavior” in which a sex difference was eliminated or prevented from forming (70). These are indeed important observations, but are they endocrine disruption? There are many ways in which such a sex difference could be prevented without having a clear endocrinedisrupting effect.
IX. FUTURE CHALLENGES
Tremendous progress has been made in elucidating the effects of estrogens on the developing brain, and the cellular and molecular mechanisms by which those effects are achieved, but much remains to be learned. The discovery that the brain itself can make estradiol confounds our ability to draw simple conclusions about the level of hormone exposure between males and females. Emerging evidence that highly localized on-demand synthesis of estradiol, making this steroid more akin to a neurotransmitter (19), further comprises our ability to mimic or fully antagonize the actions of this ubiquitous and potent signaling molecule. There is a need for a methodology that provides highly sensitive precise quantification of steroids in the brain. There is also a great need for imaging techniques that illuminate where precisely estradiol is acting: at the membrane, at the nucleus, on dendritic spines, at the axon terminal, or in vesicles? Clarification of how much estradiol and where will go a long way in helping to focus our attention on the how. Moreover, little consideration is given to the potential role of metabolism of estradiol as a variable regulating its biological activity, although there is evidence for regional variation and regulation of the degradation pathway (20, 175). There is also the tantalizing idea that α-fetoprotein may serve as a selective delivery system for estradiol to neurons (212), but definitive proof and regional specificity have not been demonstrated. Thus there are serious challenges in gaining a clear understanding of estradiol action on the developing brain, and this is before even considering the perhaps far more complicated issue of the receptors actually transducing the hormone signal.
From a conceptual standpoint, clarifying when estradiol is acting in the developing brain as a mediator of sexual differentiation, as opposed to a general trophic factor or neuroprotective agent, is essential for establishing mechanism. Endocrine disruption by man-made chemicals can only be understood in the context of knowing the natural course of events. Male and female brains differ in profound ways, and many of these differences are determined by perinatal estradiol action, but not all of them. Moreover, sometimes males and females do not really differ at all, or achieve the same end point via different routes (see Ref. 129a). Empirical evidence reviewed here suggests estradiol exerts broad influences throughout the developing brain, but these influences vary widely in magnitude and by mechanism. We need to develop a more unified view of the developing brain that integrates where estradiol is acting, how it is acting, and when it is acting. Each of these might vary in important ways between males and females given the genetic background of every male neuron is different from that of every female neuron (10).
Equally important is to consider that estradiol effects on the developing brain do not occur in an otherwise steroid-free environment. Estradiol is the end product of a complex steroidogenic pathway beginning with cholesterol and progressing through multiple potential branch points. All steroids, including progestins, androgens, glucocorticoids, and mineralocorticoids, are derived from the same precursors. As reviewed above, the number and variety of steroidogenic enzymes detected in the brain is a large and growing list. Many steroid receptors interact with similar domains on the genome, with each other, or with membrane-bound signaling pathways such as MAP kinase or phosphatidylinositol 3-kinase. These interactions can be synergistic or antagonistic depending on the steroid, the receptor, or the cell type. To truly understand the physiology of estradiol actions on the developing brain will require a working model that incorporates these additional modulating forces.
Finally, a truly synthetic view of steroid action on the brain requires a shifting of emphasis to the neuroscience in neuroendocrinology, and shifting away from the endocrinology. The approach taken in this review has been to catalog the many different effects of estradiol and to distinguish the various mechanisms of action between brain regions and how they differ from those in the adult. But the brain is an integrated organ, events in the preoptic area are not independent of those in the hypothalamus, and so on, and a greater attention to the building of neuroanatomical circuits and how these ultimately impact on function is required. Moreover, modern approaches in neuroscience, such as functional MRI and multielectrode arrays, have increased our appreciation for how the relative rates of activity in particular brain regions are essential to the integration of experience and expectation in the execution of behavior. Questions of how early gonadal steroid exposure can influence these integrated functions have not yet begun to be asked but are essential for further progress.
The ultimate goal in understanding estradiol action in the developing brain is to use that information for benefit in the study of the many tangentially related aspects of brain development. Therapeutic approaches to neonatal brain damage are extremely limited and of marginal value, and the sex of the child is never considered as an important variable. Equally important but far more challenging is to gain insight into the etiology of mental health disorders that originate in development and show strong sex biases. These include the much higher incidence of autism or autism spectrum disorder in males (23, 163, 222) and the severity and age of onset of schizophrenia, both of which are greater and earlier in males (46, 148, 200). The mechanisms of estradiol action on the developing brain, and aberrations in those mechanisms, are likely important variables in the vulnerability to later life disabilities.
REFERENCES
- 1.Abraham IM, Han S, Todman M, Korach KS, Herbison AE. Estrogen recptor β mediates rapid estrogen action on gonapotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 3.Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 4.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amateau SK, McCarthy MM. Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 6.Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol. 2002;14:904–910. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RH, Fleming DE, Rhees RW, Kinghorn E. Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Res. 1986;370:1–10. doi: 10.1016/0006-8993(86)91098-x. [DOI] [PubMed] [Google Scholar]
- 8.Andine P, Orwar O, Jacobson I, Sandberg M, Hagberg H. Changes in extracellular amino acids and spontaneous neuronal activity during ischemia and extended reflow in the CA1 of the rat hippocampus. J Neurochem. 1991;57:222–229. doi: 10.1111/j.1471-4159.1991.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 9.Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 10.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Auger A, Hexter DP, McCarthy MM. Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res. 2001;890:110–117. doi: 10.1016/s0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]
- 12.Auger AP, Perrot-Sinal TS, Auger CJ, El A, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding proteins, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auger AP, Perrot-Sinal TS, McCarthy MM. Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain. Proc Natl Acad Sci USA. 2001;98:8059–8064. doi: 10.1073/pnas.131016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci USA. 2002;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 16.Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Horm Behav. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- 17.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Ann NY Acad Sci. 2003;1007:251–262. doi: 10.1196/annals.1286.024. [DOI] [PubMed] [Google Scholar]
- 18.Bakker J, Pool CW, Sonnemans M, van Leeuwen FW, Slob AK. Quantitative estimation of estrogen and androgen receptorimmunoreactive cells in the forebrain of neonatally estrogen-deprived male rats. Neuroscience. 1997;77:911–919. doi: 10.1016/s0306-4522(96)00423-x. [DOI] [PubMed] [Google Scholar]
- 19.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase) Trends Neurosci. 1998;21:243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- 21.Balthazart J, Cornil CA, Taziaux M, Charlier TD, Baillien M, Ball GF. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2006;138:783–791. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Barna B, Kuhnt U, Siklos L. Chloride distribution in the CA1 region of newborn and adult hippocampus by light microscopic histochemistry. Histochem Cell Biol. 2001;115:105–116. doi: 10.1007/s004180000230. [DOI] [PubMed] [Google Scholar]
- 23.Baron-Cohen S, Knickmeyer RC, Balmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- 24.Barraclough CA. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. doi: 10.1210/endo-68-1-62. [DOI] [PubMed] [Google Scholar]
- 25.Barraclough CA, Gorski RA. Evidence that the hypothalamus is responsible for androgen-induced sterility in the female rat. Endocrinology. 1961;68:68–79. doi: 10.1210/endo-68-1-68. [DOI] [PubMed] [Google Scholar]
- 26.Baum MJ. Differentiation of coital behavior in mammals: a comparative analysis. Neurosci Neurobehav Rev. 1979;3:265–285. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- 27.Beach FA. Hormones and Behavior. New York: Hoeber; 1948. [Google Scholar]
- 28.Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 29.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 30.Beyer C, Eusterschulte B, Pilgrim C, Reisert I. Sex steroids do not alter sex differences in tyrosine hydroxylase activity of dopaminergic neurons in vitro. Cell Tissue Res. 1992;270:547–552. doi: 10.1007/BF00645057. [DOI] [PubMed] [Google Scholar]
- 31.Beyer C, Pawlak J, Karolczak M. Membrane receptors for oestrogen in the brain. J Neurochem. 2003;87:545–550. doi: 10.1046/j.1471-4159.2003.02042.x. [DOI] [PubMed] [Google Scholar]
- 32.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 33.Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- 34.Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–290. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- 35.Bodo C, Kudwa AE, Rissman EF. Both estrogen receptor-alpha and-beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology. 2006;147:415–420. doi: 10.1210/en.2005-0834. [DOI] [PubMed] [Google Scholar]
- 36.Brager D, Sickel MJ, McCarthy MM. Developmental sex differences in calbindin-D28K and calretinin immunoreactivity in the neonatal rat hypothalamus. J Neurobiol. 2000;42:315–322. [PubMed] [Google Scholar]
- 37.Brawer JR, Beaudet A, Desjardins GC, Schipper HM. Pathologic effect of estradiol on the hypothalamus. Biol Reprod. 1993;49:647–652. doi: 10.1095/biolreprod49.4.647. [DOI] [PubMed] [Google Scholar]
- 38.Brawer JR, Naftolin F, Martin J, Sonnenschein C. Effects of a single injection of estradiol valerate on the hypothalamic arcuate nucleus and on reproductive function in the female rat. Endocrinology. 1978;103:501–512. doi: 10.1210/endo-103-2-501. [DOI] [PubMed] [Google Scholar]
- 39.Brito VI, Carrer HF, Cambiasso MJ. Inhibition of tyrosine kinase receptor type B synthesis blocks axogenic effect of estradiol on rat hypothalamic neurones in vitro. Eur J Neurosci. 2004;20:331–337. doi: 10.1111/j.1460-9568.2004.03485.x. [DOI] [PubMed] [Google Scholar]
- 40.Burek MJ, Nordeen KW, Nordeen EJ. Sexually dimorphic neuron addition to an avian song-control region is not accounted for by sex differences in cell death. J Neurobiol. 1997;33:61–71. [PubMed] [Google Scholar]
- 41.Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cambiasso MJ, Carrer HF. Nongenomic mechanism mediates estradiol stimulation of axon growth in male rat hypothalamic neurons in vitro. J Neurosci Res. 2001;66:475–481. doi: 10.1002/jnr.1238. [DOI] [PubMed] [Google Scholar]
- 43.Cambiasso MJ, Colombo JA, Carrer HF. Differential effect of oestradiol and astroglia-conditioned media on the growth of hypothalamic neurons from male and female rat brains. Eur J Neurosci. 2000;12:2291–2298. doi: 10.1046/j.1460-9568.2000.00120.x. [DOI] [PubMed] [Google Scholar]
- 44.Camu F, Shi L, Vanlersberghe C. The role of COX-2 inhibitors in pain modulation. Drugs. 2003;63 Suppl 1:1–7. doi: 10.2165/00003495-200363001-00002. [DOI] [PubMed] [Google Scholar]
- 45.Carrer HF, Cambiasso MJ, Gorosito S. Effects of estrogen on neuronal growth and differentiation. J Steroid Biochem Mol Biol. 2005;93:319–323. doi: 10.1016/j.jsbmb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Castle DJ, Abel K, Takei N, Murray RM. Gender differences in schizophrenia: hormonal effect or subtypes. Schizophr Bull. 1995;21:1–12. doi: 10.1093/schbul/21.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Cheney DL, Uzunov D, Costa E, Guidotti A. Gas chromatographic-mass framentographic quantitation of 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. J Neurosci. 1995;15:4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 49.Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Collado D, Aguilar E. Further evidence that prolactin secretion in adult female rats is differently modified after neonatal estrogenization or androgenization: responses to methysergide, quipazine, pizotifen. Physiol Behav. 1993;53:161–165. doi: 10.1016/0031-9384(93)90025-b. [DOI] [PubMed] [Google Scholar]
- 51.Coolen LM, Peters HJ, Veening JG. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- 52.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cushing BS, Wynne-Edwards KE. Estrogen receptor-alpha distribution in male rodents is associated with social organization. J Comp Neurol. 2006;494:595–605. doi: 10.1002/cne.20826. [DOI] [PubMed] [Google Scholar]
- 54.De Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, van de Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res Bull. 1989;23:91–96. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- 55.De Vries GJ, al-Shamma HA, Zhou L. The sexually dimorphic vasopressin innervation of the brain as a model for steroid modulation of neuropeptide transmission. Ann NY Acad Sci. 1994;743:95–120. doi: 10.1111/j.1749-6632.1994.tb55789.x. [DOI] [PubMed] [Google Scholar]
- 56.DeFranco DB. Navigating steroid hormone receptors through the nuclear compartment. Mol Endocrinol. 2002;16:1449–1455. doi: 10.1210/mend.16.7.0880. [DOI] [PubMed] [Google Scholar]
- 57.Delpire E. Cation-chloride cotransporters in neuronal communication. News Physiol Sci. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- 58.Diaz DR, Fleming DE, Rhees RW. The hormone-sensitive early postnatal periods for sexual differentiation of feminine behavior and luteinizing hormone secretion in male and female rats. Dev Brain Res. 1995;86:227–232. doi: 10.1016/0165-3806(95)00029-d. [DOI] [PubMed] [Google Scholar]
- 59.Dohler KD, Hancke JL, Srivastava SS, Hofmann C, Shryne JE, Gorski RA. Participation of estrogens in female sexual differentiation of the brain: neuroanatomical, neuroendocrine and behavioral evidence. Prog Brain Res. 1984;61:99–117. doi: 10.1016/S0079-6123(08)64430-1. [DOI] [PubMed] [Google Scholar]
- 60.DonCarlos LL. Developmental profile and regulation of estrogen receptor (ER) mRNA expression in the preoptic area of prenatal rats. Dev Brain Res. 1996;20:224–233. doi: 10.1016/0165-3806(96)00067-3. [DOI] [PubMed] [Google Scholar]
- 61.DonCarlos LL, Handa RJ. Developmental profile of estrogen receptor mRNA in the preoptic area of male and female neonatal rats. Brain Res Dev. 1994;79:283–289. doi: 10.1016/0165-3806(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 62.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropinreleasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 63.Ehrhardt AA, Meyer-Bahlburg HF, Rosen LR, Feldman JF, Veridiano NP, Elkin EJ, McEwen BS. The development of gender-related behavior in females following prenatal exposure to diethylstilbestrol (DES) Horm Behav. 1989;23:526–541. doi: 10.1016/0018-506x(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 64.Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci. 2004;24:7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faber KA, Hughes CL., Jr The effect of neonatal exposure to diethylstilbestrol, genistein, zearalenone on pituitary responsiveness and sexually dimorphic nucleus volume in the castrated adult rat. Biol Reprod. 1991;45:649–653. doi: 10.1095/biolreprod45.4.649. [DOI] [PubMed] [Google Scholar]
- 66.Ferguson SA. Effects on brain and behavior caused by developmental exposure to endocrine disrupters with estrogenic effects. Neurotoxicol Teratol. 2002;24:1–3. doi: 10.1016/s0892-0362(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 67.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Fotopoulos S, Pavlou K, Skouteli H, Papassotiriou I, Lipsou N, Xanthou M. Early markers of brain damage in premature low-birth-weight neonates who suffered from perinatal asphyxia and/or infection. Biol Neonate. 2001;79:213–218. doi: 10.1159/000047094. [DOI] [PubMed] [Google Scholar]
- 69.Fraser DD, Mudrick-Donnon LA, MacVicar BA. Astrocytic GABA receptors. Glia. 1994;11:83–93. doi: 10.1002/glia.440110203. [DOI] [PubMed] [Google Scholar]
- 70.Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 71.Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F. Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology. 2004;29:475–485. doi: 10.1016/s0306-4530(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 72.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 73.Gahr M. Developmental changes in the distribution of oestrogen receptor mRNA expressing cells in the forebrain of female, male and masculinized female zebra finches. Neuroreport. 1996;7:2469–2473. doi: 10.1097/00001756-199611040-00013. [DOI] [PubMed] [Google Scholar]
- 74.Galanopoulou AS. GABA receptors as broadcasters of sexually differentiating signals in the brain. Epilepsia. 2005;46 Suppl 5:107–112. doi: 10.1111/j.1528-1167.2005.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Segura LM, Chowen JA, Duenas M, Torres-Aleman I, Naftolin F. Gonadal steroids as promoters of neuro-glial plasticity. Psychoneuroendocrinology. 1994;19:445–453. doi: 10.1016/0306-4530(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Segura LM, Sanz A, Mendez P. Cross-talk between IGF-I and estradiol in the brain: focus on neuroprotection. Neuroendocrinology. 2006;84:275–279. doi: 10.1159/000097485. [DOI] [PubMed] [Google Scholar]
- 77.George FW, Ojeda SR. Changes in aromatase activity in the rat brain during embryonic, neonatal, infantile development. Endocrinology. 1982;111:522–529. doi: 10.1210/endo-111-2-522. [DOI] [PubMed] [Google Scholar]
- 78.Gerlach JL, McEwen BS, Toran-Allerand CD, Friedman WJ. Perinatal development of estrogen receptors in mouse brain assessed by radioautography, nuclear isolation and receptor assay. Brain Res. 1983;313:7–18. doi: 10.1016/0165-3806(83)90197-9. [DOI] [PubMed] [Google Scholar]
- 79.Giovannini MG, Scali C, Prosperi C, Bellucci A, Pepeu G, Casamenti F. Experimental brain inflammation and neurodegen-eration as model of Alzheimer’s disease: protective effects of selective COX-2 inhibitors. Int J Immunopathol Pharmacol. 2003;16:31–40. [PubMed] [Google Scholar]
- 80.Girault I, Bieche I, Lidereau R. Role of estrogen receptor alpha transcriptional coregulators in tamoxifen resistance in breast cancer. Maturitas. 2006;54:342–351. doi: 10.1016/j.maturitas.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Girault JA, Costa A, Derkinderen P, Studler JM, Toutant M. FAK and PYK2/CAKbeta in the nervous system: a link between neuronal activity, plasticity and survival? Trends Neurosci. 1999;22:257–263. doi: 10.1016/s0166-2236(98)01358-7. [DOI] [PubMed] [Google Scholar]
- 82.Grossman SD, Hsieh-Wilson LC, Allen PB, Nairn AC, Greengard P. The actin-binding domain of spinophilin is necessary and sufficient for targeting to dendritic spines. Neuromol Med. 2002;2:61–69. doi: 10.1385/NMM:2:1:61. [DOI] [PubMed] [Google Scholar]
- 83.Gu Q, Korach KS, Moss RL. Rapid action of 17beta-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 84.Handa RJ, Rodriguez EW, Fox CA, Jacobson CD. Characterization and distribution of estrogen receptors in the diencephalon of the gray short-tailed opossum. Brain Res. 1991;539:6–10. doi: 10.1016/0006-8993(91)90680-t. [DOI] [PubMed] [Google Scholar]
- 85.Harder DR, Coulson PB. Estrogen receptors and effects of estrogen on membrane electrical properties of coronary vascular smooth muscle. J Cell Physiol. 1979;100:375–382. doi: 10.1002/jcp.1041000218. [DOI] [PubMed] [Google Scholar]
- 86.Harlan RE, Gorski RA. Effects of postpubertal ovarian steroids on reproductive function and sexual differentiation of lightly an-drogenized rats. Endocrinology. 1978;102:1716–1724. doi: 10.1210/endo-102-6-1716. [DOI] [PubMed] [Google Scholar]
- 87.Heil SH. Activational and organizational actions of gonadal hormones and the sex-specific effects of prolactin on food intake by rats. Dev Psychobiol. 1999;35:61–67. [PubMed] [Google Scholar]
- 88.Herbison AE, Pape JR, Simonian SX, Skynner MJ, Sim JA. Molecular and cellular properties of GnRH neurons revealed through transgenics in the mouse. Mol Cell Endocrinol. 2001;185:185–194. doi: 10.1016/s0303-7207(01)00618-9. [DOI] [PubMed] [Google Scholar]
- 89.Hewitt SC, Deroo BJ, Korach KS. Signal transduction. A new mediator for an old hormone? Science. 2005;307:1572–1573. doi: 10.1126/science.1110345. [DOI] [PubMed] [Google Scholar]
- 90.Hilton GD, Bambrick LL, Thompson SM, McCarthy MM. Estradiol modulation of kainic acid-induced calcium elevation in neonatal hippocampal neurons. Endocrinology. 2006;147:1246–1255. doi: 10.1210/en.2005-1258. [DOI] [PubMed] [Google Scholar]
- 91.Hilton GD, Ndubuizu A, Nunez JL, McCarthy MM. Simultaneous glutamate and GABA(A) receptor agonist administration increases calbindin levels and prevents hippocampal damage induced by either agent alone in a model of perinatal brain injury. Brain Res. 2005;159:99–111. doi: 10.1016/j.devbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Hilton GD, Ndubuizu AN, McCarthy MM. Neuroprotective effects of estradiol in newborn female rat hippocampus. Dev Brain Res. 2004;150:191–198. doi: 10.1016/j.devbrainres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Hilton GD, Nunez JL, Bambrick L, Thompson SM, McCarthy MM. Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca2+ from intracellular stores and is prevented by estradiol. Eur J Neurosci. 2006;24:3008–3016. doi: 10.1111/j.1460-9568.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hilton GD, Nunez JL, McCarthy MM. Sex differences in response to kainic acid and estradiol in the hippocampus of newborn rats. Neuroscience. 2003;117:383–391. doi: 10.1016/s0306-4522(02)00716-9. [DOI] [PubMed] [Google Scholar]
- 95.Hoffmann C. COX-2 in brain and spinal cord implications for therapeutic use. Curr Med Chem. 2000;7:1113–1120. doi: 10.2174/0929867003374282. [DOI] [PubMed] [Google Scholar]
- 96.Hojo Y, Hattori T, Enami TAF. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochrome P45017 alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2003;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- 98.Houtsmuller EJ, Brand T, De Jonge FH, Joosten RN, van den Poll N, Slob AK. SDN-POA volume, sexual behavior, partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56:535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 99.Hull EM, Lorrain DS, Du J, Matuszewich L, Bitran D, Nishita JK, Scaletta LL. Organizational and activational effects of dopamine on male sexual behavior. In: Ellis L, Ebertz L, editors. Males, Females and Behavior: Toward Biological Understanding. Greenwood Press; 1998. pp. 79–96. [Google Scholar]
- 100.Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- 101.Hutchinson TH, Pickford DB. Ecological risk assessment and testing for endocrine disruption in the aquatic environment. Toxicology. 2002;181–182:383–387. doi: 10.1016/s0300-483x(02)00471-7. [DOI] [PubMed] [Google Scholar]
- 102.Ibanez C, Guennoun R, Liere P, Eychenne B. Developmental expression of genes involved in neurosteroidgenesis: 3beta-hydroxysteroid dehydrogenase/isomerase in the rat brain. Endocrinology. 2003;144:2902–2911. doi: 10.1210/en.2002-0073. [DOI] [PubMed] [Google Scholar]
- 103.Ibanez MA, Gu G, Simerly RB. Target-dependent sexual differentiation of a limbic-hypothalamic neural pathway. J Neurosci. 2001;21:5652–5659. doi: 10.1523/JNEUROSCI.21-15-05652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ivanova T, Beyer C. Ontogenetic expression and sex differences of aromatase and estrogen receptor-alpha/beta mRNA in the mouse hippocampus. Cell Tissue Res. 2000;300:231–237. doi: 10.1007/s004410000199. [DOI] [PubMed] [Google Scholar]
- 105.Jacobson CD, Gorski RA. Neurogenesis of the sexually dimorphic nucleus of the preoptic area in the rat. J Comp Neurol. 1981;196:519–529. doi: 10.1002/cne.901960313. [DOI] [PubMed] [Google Scholar]
- 106.Jansson JO, Ekberg S, Isaksson OG, Eden S. Influence of gonadal steroids on age-and sex-related secretory patterns of growth hormone in the rat. Endocrinology. 1984;114:1287–1294. doi: 10.1210/endo-114-4-1287. [DOI] [PubMed] [Google Scholar]
- 107.Katz DL, Frankenburg FR, Benowitz LI, Gilbert JM. Psychosis and prenatal exposure to diethylstilbestrol. J Nerv Ment Dis. 1987;175:306–308. [PubMed] [Google Scholar]
- 108.Kimoto T, Tsurugizawa T. Neurosteroid synthesis by cytochrome P450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142 doi: 10.1210/endo.142.8.8327. 3578-3389. [DOI] [PubMed] [Google Scholar]
- 109.Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26:465–478. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- 111.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 112.Kudwa AE, Bodo C, Gustafsson JA, Rissman EF. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc Natl Acad Sci USA. 2005;102:4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lauber ME, Lichtensteiger W. Pre- and postnatal ontogeny of aromatase cytochrome p450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology. 1994;135:1661–1668. doi: 10.1210/endo.135.4.7925130. [DOI] [PubMed] [Google Scholar]
- 114.Leinekugel X, Tseeb V, Ben-Ari Y, Bregestovski P. Synaptic GABAA activation induces Ca2+rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol. 1995;487:319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- 116.Lephart ED. Dimorphic expression of calbindin-D[28k] in the medial basal hypothalamus from perinatal male and female rats. Dev Brain Res. 1996;96:281–284. doi: 10.1016/0165-3806(96)00100-9. [DOI] [PubMed] [Google Scholar]
- 117.Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lish JD, Ehrhardt AA, Meyer-Bahlburg HF, Rosen LR, Gruen RS, Veridiano NP. Gender-related behavior development in females exposed to diethylstilbestrol (DES) in utero: an attempted replication. J Am Acad Child Adolesc Psychiatry. 1991;30:29–37. doi: 10.1097/00004583-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 119.Lu H, Nishi M, Matsuda K, Kawata M. Estrogen reduces the neurite growth of serotonergic cells expressing estrogen receptors. Neurosci Res. 2004;50:23–28. doi: 10.1016/j.neures.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 120.MacLusky NJ, Lieberburg I, McEwen BS. The development of estrogen receptor systems in the rat brain: perinatal development. Brain Res. 1979;178:129–142. doi: 10.1016/0006-8993(79)90093-3. [DOI] [PubMed] [Google Scholar]
- 121.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 122.MacLusky NJ, Walters MJ, Clark AS, Toran-Allerand CD. Aromatase in the cerebral cortex, hippocampus, mid-brain: ontogeny and developmental implications. Mol Cell Neurosci. 1994;5:691–698. doi: 10.1006/mcne.1994.1083. [DOI] [PubMed] [Google Scholar]
- 123.Mahesh VB, Brann DW. Regulatory role of excitatory amino acids in reproduction. Endocrine. 2005;28:271–280. doi: 10.1385/ENDO:28:3:271. [DOI] [PubMed] [Google Scholar]
- 124.Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matagne V, Rasier G, Lebrethon MC, Gerard A, Bourguignon JP. Estradiol stimulation of pulsatile gonadotropin-releasing hormone secretion in vitro: correlation with perinatal exposure to sex steroids and induction of sexual precocity in vivo. Endocrinology. 2004;145:2775–2783. doi: 10.1210/en.2003-1259. [DOI] [PubMed] [Google Scholar]
- 126.Matsumoto A, Arai Y. Male-female differences in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinolgy. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- 127.Matsumoto A, Arai Y. Sexual dimorphism in “wiring pattern” in the hypothalamic arcuate nucleus and its modification by neonatal hormonal environment. Brain Res. 1980;19:238–242. doi: 10.1016/0006-8993(80)91173-7. [DOI] [PubMed] [Google Scholar]
- 128.McCarthy MM, Auger AP, Perrot-Sinal TS. Getting excited about GABA and sex differences in the brain. Trends Neurosci. 2002;25:307–312. doi: 10.1016/s0166-2236(02)02182-3. [DOI] [PubMed] [Google Scholar]
- 129.McCarthy MM, Besmer HR, Jacobs SC, Keiden GMO, Gibbs RB. Influence of maternal grooming, sex and age on FOS immunoreactivity in the preoptic area of neonatal rats: Implications for sexual differentiation. Dev Neurosci. 1997;19:488–496. doi: 10.1159/000111246. [DOI] [PubMed] [Google Scholar]
- 129a.McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 130.McCarthy MM, Perrot-Sinal TS, Auger AP, Sickel MJ. In: Excitatory GABA as a Mediator of Steroid-Induced Brain Sexual Differentiation. Handa RJ, Terasawa E, Hayashi S, Kawata M, editors. Boca Raton, FL: CRC; 2000. pp. 323–345. [Google Scholar]
- 131.McCarthy MM, Schlenker EH, Pfaff DW. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor mRNA on sexual differentiation of rat brain. Endocrinology. 1993;133:433–439. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- 132.McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci USA. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 134.Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. Physiology of Reproduction. New York: Raven; 1994. pp. 3–106. [Google Scholar]
- 135.Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 136.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 137.Michael RP, Bonsall RW, Rees HD. Sites at which testosterone may act as an estrogen in the brain of the male primate. Neuroendocrinology. 1987;46:511–521. doi: 10.1159/000124874. [DOI] [PubMed] [Google Scholar]
- 138.Miranda RC, Toran-Allerand CD. Developmental expression of estrogen receptor mRNA in the rat cerebral cortex: a nonisotopic in situ hybridization histochemistry study. Cereb Cortex. 1992;2:1–15. doi: 10.1093/cercor/2.1.1. [DOI] [PubMed] [Google Scholar]
- 139.Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neurprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144:306–312. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- 140.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Horm Behav. 1996;30:553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- 142.Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Dev Brain Res. 2002;139:151–158. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- 143.Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 144.Mong JA, Nunez JL, McCarthy MM. GABA mediates steroidinduced astrocyte differentiation in the neonatal rat hypothalamus. J Neuroendocrinol. 2002;14:1–16. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- 145.Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 146.Newbold RR. Gender-related behavior in women exposed prenatally to diethylstilbestrol. Environ Health Perspect. 1993;101:208–213. doi: 10.1289/ehp.93101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nilsson M, Eriksson PS, Ronnback L, Hansson E. GABA induces Ca2+ transients in astrocytes. Neuroscience. 1993;54:605–614. doi: 10.1016/0306-4522(93)90232-5. [DOI] [PubMed] [Google Scholar]
- 148.Nopoulos P, Flaum M, Andreasen NC. Sex differences in brain morphology in schizophrenia. Am J Psychiatry. 1997;154:1648–1654. doi: 10.1176/ajp.154.12.1648. [DOI] [PubMed] [Google Scholar]
- 149.Norstedt G, Palmiter R. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell. 1984;36:805–812. doi: 10.1016/0092-8674(84)90030-8. [DOI] [PubMed] [Google Scholar]
- 150.Nunez JL, Alt J, McCarthy MM. A new model for prenatal brain damage. I. GABAA receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nunez JL, Alt J, McCarthy MM. A new model for prenatal brain damage. II. Long-term deficits in hippocampal cell number and hippocampal dependent behavior following neonatal GABAA receptor activation. Exp Neurol. 2003;181:270–280. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nunez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of gamma-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21:3251–3261. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- 153.Nunez JL, McCarthy MM. Cell death in the rat hippocampus in a model of prenatal brain injury: time course and expression of death related proteins. Neuroscience. 2004;129:393–402. doi: 10.1016/j.neuroscience.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 154.Nunez JL, McCarthy MM. Estradiol exacerbates hippocampal damage in a model of preterm brain injury. Endocrinology. 2003;144:2350–2359. doi: 10.1210/en.2002-220840. [DOI] [PubMed] [Google Scholar]
- 155.Nurhidayat Tsukamoto Y, Sigit K, Sasaki F. Sex differentiation of growth hormone-releasing hormone and somatostatin neurons in the mouse hypothalamus: an immunohistochemical and morphological study. Brain Res. 1999;821:309–321. doi: 10.1016/s0006-8993(99)01081-1. [DOI] [PubMed] [Google Scholar]
- 156.Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc Natl Acad Sci USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Olesen KM, Auger AP. Sex differences in Fos protein expression in the neonatal rat brain. J Neuroendocrinol. 2005;17:255–261. doi: 10.1111/j.1365-2826.2005.01302.x. [DOI] [PubMed] [Google Scholar]
- 159.Park JJ, Baum MJ, Paredes RG, Tobet SA. Neurogenesis and cell migration into the sexually dimorphic preoptic area/anterior hypothalamus of the fetal ferret. J Neurobiol. 1996;30:315–328. doi: 10.1002/(SICI)1097-4695(199607)30:3<315::AID-NEU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 160.Patchev AV, Gotz F, Rohde W. Differential role of estrogen receptor isoforms in sex-specific brain organization. FASEB J. 2004;18:1568–1570. doi: 10.1096/fj.04-1959fje. [DOI] [PubMed] [Google Scholar]
- 161.Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 162.Perrot-Sinal TS, Sinal CJ, Reader JC, Speert DB, McCarthy MM. Sex difference in the chloride cotransporters NKCC1 and KCC2, in the developing hypothalamus. J Neuroendocrinol. 2007;19:1–7. doi: 10.1111/j.1365-2826.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 163.Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 164.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 165.Pietras RJ, Szego CM. Estrogen receptors in uterine plasma membrane. J Steroid Biochem. 1979:1471–1483. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- 166.Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Herbert SC, Delpire E. Expression of the Na-K-2Cl cotransporter BSC2 in the nervous system. Am J Physiol Cell Physiol. 1997;272:C173–C183. doi: 10.1152/ajpcell.1997.272.1.C173. [DOI] [PubMed] [Google Scholar]
- 167.Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 168.Polston EK, Gu G, Simerly RB. Neurons in the principle nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience. 2004;123:793–803. doi: 10.1016/j.neuroscience.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 169.Polston EK, Simerly RB. Sex-specific patterns of galanin, cholecystokinin, substance P expression in neurons of the principal bed nucleus of the stria terminalis are differentially reflected within three efferent preoptic pathways in the juvenile rat. J Comp Neurol. 2003;465:551–559. doi: 10.1002/cne.10841. [DOI] [PubMed] [Google Scholar]
- 170.Pozzo-Miller LD, Aoki A. Stereological analysis of the hypothalamic ventromedial nucleus. II. Hormone induced changes in the synaptogenic pattern. Dev Brain Res. 1991;61:189–196. doi: 10.1016/0165-3806(91)90131-2. [DOI] [PubMed] [Google Scholar]
- 171.Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- 172.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: a G proteincoupled receptor for estrogen. Mol Cell Endocrinol. In press doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002;143:3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- 174.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 175.Reddy VV, Rajan R, Daly MJ. Estrogen metabolism in neural tissues of six-day-old rats. Brain Res. 1980;197:443–452. doi: 10.1016/0006-8993(80)91129-4. [DOI] [PubMed] [Google Scholar]
- 176.Ren XR, Ming GL, Xie Y, Hong Y, Sun DM, Zhao ZQ, Feng Z, Wang Q, Shim S, Chen ZF, Song HJ, Mei L, Xiong WC. Focal adhesion kinase in netrin-1 signaling. Nat Neurosci. 2004;7:1204–1212. doi: 10.1038/nn1330. [DOI] [PubMed] [Google Scholar]
- 177.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 178.Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990;21:781–786. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- 179.Rhoda J, Corbier P, Roffi J. Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization of testosterone to 17β-estradiol. Endocrinology. 1984;114:1754–1760. doi: 10.1210/endo-114-5-1754. [DOI] [PubMed] [Google Scholar]
- 180.Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 182.Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- 183.Robel P, Baulieu EE. Neurosteroids: biosynthesis and function. Crit Rev Neurobiol. 1995;9:383–394. [PubMed] [Google Scholar]
- 184.Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- 185.Roselli CE, Resko JA. Aromatase activity in the rat brain: hormonal regulation and sex differences. J Steroid Biochem Mol Biol. 1993;44:499–508. doi: 10.1016/0960-0760(93)90254-t. [DOI] [PubMed] [Google Scholar]
- 186.Sa SI, Madeira MD. Estrogen modulates the sexually dimorphic synaptic connectivity of the ventromedial nucleus. J Comp Neurol. 2005;484:68–79. doi: 10.1002/cne.20451. [DOI] [PubMed] [Google Scholar]
- 187.Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. J Neurobiol. 1999;41:221–229. [PubMed] [Google Scholar]
- 188.Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci USA. 1992;89:7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJM. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;3:1–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 190.Schwartz-Bloom RD, Sah R. γ-Aminobutyric acid(A) neurotransmission and cerebral ischemia. J Neurochem. 2001;77:353–371. doi: 10.1046/j.1471-4159.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- 192.Shughrue PJ, Stumpf WE, MacLusky NJ, Zielinski JE, Hochberg RB. Developmental changes in estrogen receptors in mouse cerebral cortex between birth and postweaning: studied by autoradiography with 11 beta-methoxy-16 alpha-[125I]iodoestradiol. Endocrinology. 1990;126:1112–1124. doi: 10.1210/endo-126-2-1112. [DOI] [PubMed] [Google Scholar]
- 193.Sickel MJ, McCarthy MM. Calbindin D28-K immunoreactivity is a marker for a subdivision of the sexual dimorphic nucleus of the preoptic area in the rat: developmental profile and gonadal steroid modulation. J Neurobiol. 2000;12:397–402. doi: 10.1046/j.1365-2826.2000.00474.x. [DOI] [PubMed] [Google Scholar]
- 194.Simerly RB, Swanson LW, Gorski RA. Reversal of the sexually dimorphic distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus by treatment with perinatal androgen. Brain Res. 1985;340:91–98. doi: 10.1016/0006-8993(85)90777-2. [DOI] [PubMed] [Google Scholar]
- 195.Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci USA. 1997;94:14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 199.Speert DB, Konkle ATM, Zup SL, Schwarz JA, Shiroor C, Taylor M. Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology. In press doi: 10.1210/en.2006-0845. [DOI] [PubMed] [Google Scholar]
- 200.Stefan M, Murray R. Schizophrenia: developmental disturbance of brain and mind? Acta Paediatr Suppl. 1997;422:112–116. doi: 10.1111/j.1651-2227.1997.tb18358.x. [DOI] [PubMed] [Google Scholar]
- 201.Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- 202.Stromstedt M, Waterman MR. Messenger RNAs encoding ste-roidogenic enzymes are expressed in rodent brain. Brain Res. 1995;34:75–88. doi: 10.1016/0169-328x(95)00140-n. [DOI] [PubMed] [Google Scholar]
- 203.Stuart E, Lephart ED. Dimorphic expression of medial basal hypothalamic-preoptic area calbindin-D(28K) mRNA during perinatal development and adult distribution of calbindin-D(28K) mRNA in Sprague-Dawley rats. Mol Brain Res. 1999;73:60–67. doi: 10.1016/s0169-328x(99)00236-3. [DOI] [PubMed] [Google Scholar]
- 204.Suzuki M, Bannai M, Matsumuro M, Furuhata Y, Ikemura R, Kuranaga E, Kaneda Y, Nishihara M, Takahashi M. Suppression of copulatory behavior by intracerebroventricular infusion of antisense oligodeoxynucleotide of granulin in neonatal male rats. Physiol Behav. 2000;68:707–713. doi: 10.1016/s0031-9384(99)00241-3. [DOI] [PubMed] [Google Scholar]
- 205.Suzuki M, Nishiahara M. Granulin precursor gene: a sex steroidinducible gene involved in sexual differentiation of the rat brain. Mol Genet Metab. 2002;75:31–37. doi: 10.1006/mgme.2001.3274. [DOI] [PubMed] [Google Scholar]
- 206.Suzuki M, Yonezawa T, Fujioka H, Matuamuro M, Nishihara M. Induction of granulin precursor gene expression by estrogen treatment in neonatal rat hypothalamus. Neurosci Lett. 2001;297:199–202. doi: 10.1016/s0304-3940(00)01699-2. [DOI] [PubMed] [Google Scholar]
- 207.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 208.Thornberg E, Thiringer K, Odeback A, Milsom I. Birth asphyxia: incidence, clinical course and outcome in a Swedish population. Acta Paediatr. 1995;84:927–932. doi: 10.1111/j.1651-2227.1995.tb13794.x. [DOI] [PubMed] [Google Scholar]
- 209.Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm Behav. 2005;48:512–521. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 210.Todd BJ, Schwarz JM, Mong JA, McCarthy MM. Glutamate AMPA/kainate receptors, not GABA(A) receptors, mediate estradiolinduced sex differences in the hypothalamus. Dev Neurobiol. 2007;67:304–315. doi: 10.1002/dneu.20337. [DOI] [PubMed] [Google Scholar]
- 212.Toran-Allerand CD. Coexistence of alpha-fetoprotein, albumin and transferrin immunoreactivity in neurones of the developing mouse brain. Nature. 1980;286:733–735. doi: 10.1038/286733a0. [DOI] [PubMed] [Google Scholar]
- 213.Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, 17beta-estradiol. Ann NY Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 214.Toran-Allerand CD. Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 215.Toran-Allerand CD. Neuronal uptake of alpha-fetoprotein (AFP) synthesized and secreted by hepatocytes in liver/brain co-cultures. Neurosci Lett. 1987;83:35–40. doi: 10.1016/0304-3940(87)90212-6. [DOI] [PubMed] [Google Scholar]
- 216.Toran-Allerand CD. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: implications for sexual differentiation. Brain Res. 1976;106:407–412. doi: 10.1016/0006-8993(76)91038-6. [DOI] [PubMed] [Google Scholar]
- 217.Toran-Allerand CD. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro. II. Morphological correlates and hormonal specificity. Brain Res. 1980;189:413–427. doi: 10.1016/0006-8993(80)90101-8. [DOI] [PubMed] [Google Scholar]
- 218.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ESJ, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Toran-Allerand CD, Hashimoto K, Greenough WT, Saltarelli M. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro. III. Effects of estrogen on dendritic differentiation. Brain Res. 1983;283:97–101. doi: 10.1016/0165-3806(83)90085-8. [DOI] [PubMed] [Google Scholar]
- 220.Toran-Allerand CD, Singh M, Setalo G., Jr Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 221.Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17Alpha-estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–3850. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- 222.Tsai LY, Beisler JM. The development of sex differences in infantile autism. Br J Psychiatry. 1983;142:373–378. doi: 10.1192/bjp.142.4.373. [DOI] [PubMed] [Google Scholar]
- 223.Turner CE. Paxillin is a major phosphotyrosine-containing protein during embryonic development. J Cell Biol. 1991;115:201–207. doi: 10.1083/jcb.115.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Uht RM, Anderson CM, Webb P, Kushner PJ. Transcriptional activities of estrogen and glucocorticoid receptors are functionally integrated at the AP-1 response element. Endocrinology. 1997;138:2900–2908. doi: 10.1210/endo.138.7.5244. [DOI] [PubMed] [Google Scholar]
- 226.Vreeburg JT, van der Vaart PD, van der Shoot P. Prevention of central defeminization but not masculinization in male rats by inhibition neonatally of oestrogen biosynthesis. J Endocrinol. 1977;74:375–382. doi: 10.1677/joe.0.0740375. [DOI] [PubMed] [Google Scholar]
- 227.Wagner C, Morrell JI. Distribution and steroid hormone regulation of aromatase MRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurology. 1995;370:71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 228.Wagner C, Morrell JI. Neuroanatomical distribution of aromatase MRNA in the rat brain: indications of regional regulation. J Steroid Biochem Mol Biol. 1997;61:3–6. [PubMed] [Google Scholar]
- 229.Wagner CK. The many faces of progesterone: a role in adult and developing male brain. Front Neuroendocrinol. 2006;27:340–359. doi: 10.1016/j.yfrne.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 230.Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ. Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor alpha expression. J Neurobiol. 2001;47:176–182. doi: 10.1002/neu.1025. [DOI] [PubMed] [Google Scholar]
- 231.Wagner CK, Xu J, Pfau JL, Quadros PS, De Vries GJ, Arnold AP. Neonatal mice possessing an Sry transgene show a masculinized pattern of progesterone receptor expression in the brain independent of sex chromosome status. Endocrinology. 2004;145:1046–1049. doi: 10.1210/en.2003-1219. [DOI] [PubMed] [Google Scholar]
- 232.Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 233.Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff D, editor. Hormones Brain and Behavior. London, UK: Academic; 2002. pp. 385–424. [Google Scholar]
- 234.Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci USA. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Warner M, Gustafsson JA. Nongenomic effects of estrogen: why all the uncertainty? Steroids. 2006;71:91–95. doi: 10.1016/j.steroids.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 236.Webb P, Nguyen P, Kushner PJ. Differential SERM effects on corepressor binding dictate ERalpha activity in vivo. J Biol Chem. 2003;278:6912–6920. doi: 10.1074/jbc.M208501200. [DOI] [PubMed] [Google Scholar]
- 237.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses and neonatal offspring. Endocrinology. 1980;106:306–313. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 238.Whalen RE. Hormone-induced changes in the organization of sexual behavior in the male rat. J Comp Physiol Psychol. 1964;57:175–182. doi: 10.1037/h0041247. [DOI] [PubMed] [Google Scholar]
- 239.Whalen RE, Olsen KL. Role of aromatization in sexual differentiation: effects of prenatal ATD treatment and neonatal castration. Horm Behav. 1981;15:107–122. doi: 10.1016/0018-506x(81)90022-2. [DOI] [PubMed] [Google Scholar]
- 240.Willoughby KN, Sarkar AJ, Boyadjieva NI, Sarkar DK. Neonatally administered tert-octylphenol affects onset of puberty and reproductive development in female rats. Endocrine. 2005;26:161–168. doi: 10.1385/ENDO:26:2:161. [DOI] [PubMed] [Google Scholar]
- 241.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wizemann TM, Pardu ML. Exploring the Biological Contributions to Human Health. Does Sex Matter? Washington, DC: National Academy of Sciences; 2001. [PubMed] [Google Scholar]
- 243.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 244.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src /ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 246.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 247.Zhou J, Pfaff DW, Chen G. Sex differences in estrogenic regulation of neuronal activity in neonatal cultures of ventromedial nucleus of the hypothalamus. Proc Natl Acad Sci USA. 2005;102:14907–14912. doi: 10.1073/pnas.0507440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zwain IH, Yen SS. Dehydroepiandrosterone: biosynthesis and metabolism in the brain. Endocrinology. 1999;140:880–887. doi: 10.1210/endo.140.2.6528. [DOI] [PubMed] [Google Scholar]