Abstract
Histological studies have demonstrated that polycystic ovaries (PCO) contain increased numbers of preantral follicles with a specific increase in primary follicles. Polycystic ovary syndrome is associated with hyperandrogenism and pre- and postnatal androgenisation of primates increases the pool of growing follicles producing changes resembling PCO. In vitro studies could test the hypothesis that androgens alter early folliculogenesis, but conventional culture techniques for small follicles are generally unsuitable in non-rodent species. Our objective was to develop and use a method to investigate the effects of testosterone on early folliculogenesis. We adapted an in ovo technique in which lamb cortical ovarian fragments were grafted onto the chorioallantoic membrane of fertilised chick eggs. Optimal experimental conditions for vascularisation and survival of tissue were determined and the model then used to investigate the effects of testosterone on follicle growth. Eggs were inoculated with testosterone at the time of implantation of the ovarian tissue, which was retrieved 5 days later. Tissue was sectioned and follicles staged and counted. There was no wholesale initiation of primordial follicle growth over the 5-day in ovo culture. Importantly, the proportion of primordial, primary and secondary follicles remained similar to those in unimplanted tissue. Testosterone increased the number of primary follicles by 50% compared with controls, an effect that was largely due to a reduction in atresia. In conclusion, incubation of ovarian cortex with testosterone reproduces the changes in early folliculogenesis reported in histological studies of PCO.
Introduction
Polycystic ovaries (PCO) are present in ∼20% of the female population of reproductive age (Polson et al. 1988, Farquhar et al. 1994, Michelmore et al. 1999). These ovaries are enlarged and diagnosed by the increased number of follicles or cysts at the antral stage (Adams et al. 1985). It is becoming clear, however, that the increase in follicle number actually occurs during preantral folliculogenesis. Until recently, there was only one publication that quantified this increase and that reported an approximate doubling of each of the growing stages of follicle and twice as many atretic follicles in PCO as in normal ovaries (Hughesdon 1982). Recently, two further studies have been added significantly to this area. The first study, utilising ovarian biopsies, found approximately six times as many preantral follicles in PCO as in normal ovaries and a particular increase in follicles at the primary stage (Webber et al. 2003). The findings of the second study counting follicles in fixed slices of whole ovary confirmed that ‘stockpiling’ of follicles occurred at the primary stage (Maciel et al. 2004).
The cause of this specific increase in primary follicles is unknown; however, there are a number of lines of evidence pointing to the raised androgens that are a diagnostic feature of polycystic ovary syndrome (PCOS; Gilling-Smith et al. 1994, Nelson et al. 1999) as the most likely agent. In primates, in vivo androgen treatment induced multiple ovarian cysts and increased the number of small follicles (Vendola et al. 1998), whereas in primates and sheep, prenatal androgenisation also produced offspring with ovaries containing multiple antral follicles (Abbott et al. 2002). Intrinsic androgen overproduction in humans from causes such as congenital adrenal hyperplasia induces PCO (Padmanabhan et al. 1998, Merke et al. 2002) and laboratory animals treated with androgens develop cystic ovaries (Beloosesky et al. 2004, Tamura et al. 2005). Finally, an association between serum androgen concentration and ovarian follicle numbers in humans has also been demonstrated (Franks et al. 1995, Battaglia et al. 2000). Together, these findings provide good indirect evidence that androgens cause changes in folliculogenesis that are characteristic of PCO.
To date, there have been no studies directly investigating the effects of androgens on primate follicles grown in culture, largely due to the methodological problems of wholesale activation of primordial follicles and high rates of atresia. In 2002, Fortune et al. described the implantation of foetal bovine ovarian cortical pieces ‘under’ the chorioallantoic membrane (CAM) of chick embryos (Cushman et al. 2002). They reported that this in ovo culture procedure did not result in wholesale initiation of primordial follicular growth. The aim of our study was to adapt this technique to examine the hypothesis that testosterone is responsible for the specific increase in primary follicles seen in PCO. We report the conditions required to permit follicle survival in pieces of prepubertal lamb ovarian cortex grafted onto the CAM of fertilised chicken eggs, the fate of follicles grafted in this way and the effects of in ovo testosterone exposure on follicle growth and survival.
Results
Factors affecting embryonic chick survival and vascularisation of implanted ovarian tissue
In establishing this method, conditions were optimised for embryonic chick survival and hence vascularisation of implanted ovarian tissue. Initial experiments determined that important determinants were the length of the pre-incubation period and the time for which the egg was out of the incubator i.e. during the windowing and implanting procedures. Survival dropped from 60% at 2 days of storage to less than 20% at 17 days. The highest survival (more than 50%) was seen when the total operative procedure was less than 3 min and this fell to 20% when 7 min or more were taken.
Embryonic survival and tissue vascularisation in eggs containing surviving embryonic chicks using ‘dropped’ versus ‘wedged’ techniques were 63% (n=30) and 50% (n=20) vs 50% (n=14) and 86% (n=7) respectively. There were no significant differences in these parameters (χ2); however, wedged tissue proved easier to retrieve and was therefore adopted. Non-vascularised tissue was generally located away from the CAM region (albumen, yolk sac, etc). Tissue that had become encapsulated by the CAM contained obvious blood vessels (Fig. 1A–E). The optimised procedure resulted in a mean embryo survival per experiment of 52% and of these 46% contained well-vascularised tissue (n=100 eggs).
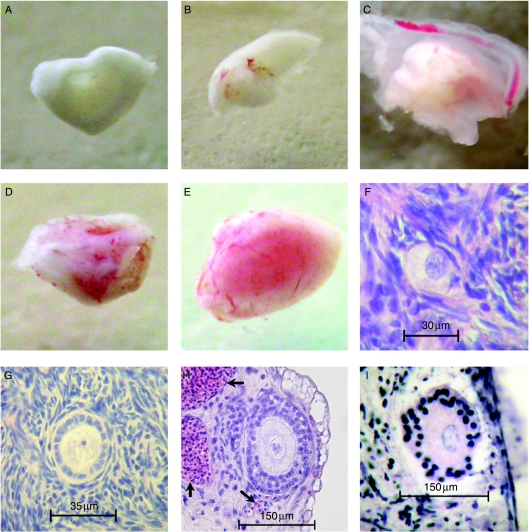
Figure 1.
(A–E) Variation in macroscopic vascularisation of tissue recovered 5 days after implantation. Figures in parentheses indicate the frequency with which each degree of vascularisation was found. (A) Non-vascularised tissue usually retrieved from the albumen or yolk or on the shell or vitelline membranes or amnion (24%). (B) Poorly vascularised tissue encapsulated by the CAM with obvious blood vessels but ovarian tissue pale in colour (18%). (C) Partly-vascularised tissue that was pink in isolated areas (22%). (D) Well-vascularised tissue that was mostly pink (28%) and (E) very well-vascularised tissue that was pink throughout (8%). (F–I) Histological sections of a primordial (F), primary (G) and secondary (H) follicles in lamb ovarian cortical tissue cultured using the CAM technique. Nucleated chick red blood cells (arrows) are clearly seen infiltrating the ovarian tissue. An atretic follicle is illustrated in (I).
Folliculogenesis in ovarian cortical fragments cultured in ovo for 5 days
Healthy follicles at all stages were seen in vascularised tissue although secondary follicles were rare (Fig. 1F–I). In the histological sections, nucleated chick blood cells could be seen infiltrating the lamb tissue. The percentage of primordial, primary and secondary follicles in cortical fragments implanted onto the CAM for 5 days was 69, 14.5 and 1.6 respectively and this was similar to the unimplanted baseline fragments in which the corresponding percentages were 76, 14.6 and 0.8 respectively. In total, 840 follicles (both healthy and atretic) were counted. Thus, the mean percentage of primordial follicles dropped by 10.3%, the mean percentage of primary follicles was unchanged and there was a doubling of secondary follicles in tissue cultured in ovo. These differences were not statistically significant. The percentage of atretic follicles in well-vascularised cortical tissue was double that in unimplanted tissue; 15% vs 8.4% (P=0.002).
Effects of testosterone
Testosterone was found to disperse rapidly throughout the compartments of the egg and although not evenly distributed large amounts were available in the CAM throughout the 5 days of experimentation. For the experiment on the effects of testosterone on follicle growth, of the 100 pieces implanted from two lambs, 24 vascularised pieces were retrieved and 14 of these were considered very well or well vascularised and processed further. Final numbers were three for each of the implanted control and testosterone-treated groups and four for each of baseline unimplanted, FSH- and testosterone and FSH-treated groups. There were no significant differences in vascularisation rates between different treatments (Mann–Whitney test).
A total of 2773 follicles were imaged; 275 follicles were classed as ungradable, in that they were of insufficient quality to permit accurate visualisation of follicular features. The number of tissue pieces and number and percentage of follicles of each stage are shown in Table 1. The number of follicles of each stage is the total number in all tissue pieces within a treatment group. The respective percentage is the weighted mean of the percentage data calculated from each individual tissue piece.
Table 1.
Numbers of embryos and well-vascularised pieces of tissue retrieved and used for comparison. In the first two columns, the figures in parentheses show the percentages. The last four columns show the total number of follicles found of each stage for each treatment. Numbers in parentheses show straight and weighted percentages.
| Treatment | Number of surviving embryos (%) | Number of tissue pieces well vascularised (%) | Number of primordial follicles (%/weighted %) | Number of primary follicles (%/weighted %) | Number of secondary follicles (%/weighted %) | Number of atretic follicles (%/weighted %) |
|---|---|---|---|---|---|---|
| Unimplanted | 401 (74/76) | 77 (15/15) | 4 (1/1) | 44 (11/8) | ||
| Implanted control | 13 (52) | 3 (50) | 266 (68/69) | 56 (15/15) | 6 (2/2) | 58 (15/15) |
| Testosterone | 13 (52) | 3 (60) | 575 (56/73) | 158 (22/20) | 6 (1/1) | 44 (22/6) |
| Testosterone+FSH | 14 (56) | 4 (67) | 302 (59/67) | 114 (30/25) | 5 (2/1) | 30 (10/7) |
| FSH | 12 (48) | 4 (57) | 62 (230/65) | 76 (23/22) | 6 (4/2) | 40 (13/11) |
Each of the hormone treatments caused a selective increase in the proportion of primary follicles. The proportion of primary follicles was 50% higher in the testosterone-treated group than in the implanted control: 21 vs 14%, P<0.01 (Fig. 2). The addition of FSH produced similar results (48% increase P=0.01) and the addition of testosterone and FSH together had an even greater effect with a 74% increase (P≤0.001). There was no change in the proportion of secondary follicles in tissue exposed to FSH, but there was a reduction in follicles of this stage in both groups exposed to testosterone. This difference was not significant, however, because of the wide range. Interestingly, there was a significant fall in the percentage of atretic follicles in both groups in which testosterone was added: 15% to 5.6 and 6.7% for testosterone alone and testosterone with FSH respectively. Both reductions were highly significant (P<0.001). Conversely, there was no significant change in the rate of follicle atresia in those pieces exposed to FSH alone.
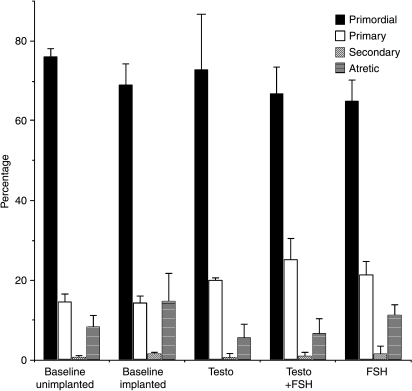
Figure 2.
The proportion of atretic and healthy follicles of different stages in each group of baseline or implanted pieces of tissue (weighted mean±s.e.m.). There was a significant decrease in the proportion of primordial follicles and an increase in atresia when tissue was implanted (P=0.014 and 0.002 respectively). The proportion of primary follicles was increased in those pieces exposed to androgen in the absence (P=0.019) or presence (P<0.001) of FSH. There was a highly significant corresponding reduction in atresia in each case (P<0.001). FSH also increased primary follicle numbers (P=0.013), but there was no change in the proportion of atretic follicles with this hormone.
Discussion
The first use of the CAM technique may have been as early as 1911, but it had clearly been used by several investigators by the 1920s and 1930s (Burnet 1933). It has been used by embryologists for many years (Rawles 1952) and more recently was adopted to study the factors involved in angiogenesis (Ribatti et al. 1996, Brooks et al. 1999, Storgard et al. 2005). There have been two reports describing the use of the CAM technique for studying early folliculogenesis in foetal bovine tissue (Fortune et al. 2000, Cushman et al. 2002). The authors did not, however, provide a detailed analysis of the optimal conditions required for successful vascularisation or data regarding success rates of this technique. Our interest in mechanisms controlling early folliculogenesis, particularly in relation to PCO, led us to investigate the potential use of this technique in non-foetal tissue. Our initial studies were carried out in human cortex but we found, similar to others (Schmidt et al. 2003), that the density of follicles in typical patients undergoing oophorectomy was too low to be of practical use and we changed to using lamb ovaries.
In excess of 500 eggs were implanted to establish the technique and from the early stages it was apparent that cortical fragment vascularisation was completely dependent upon chick embryo survival with both the duration of the pre-incubation storage and the operative procedure being important determinants. Increased embryonic death in relation to the duration of egg exposure to the air is likely to be due to dehydration and a lethal drop in temperature.
In vascularised tissue, there was evidence of extensive invasion or anastomosis by chick vessels, even after only 5 days. Cushman et al. (2002) also reported that, when successful, vascularisation was evident within 2 days of implantation of foetal bovine cortical slices and was extensive after 10 days. In contrast to these experiments, their pieces of implanted ovarian cortex were very small, i.e., 0.5 mm3. We inserted larger pieces because vascularisation was equally as good and these were more readily retrieved and visualised.
A number of approaches have been used to attempt to culture both follicles and whole ovaries and although there has been some success with murine tissue (Blandau et al. 1965, Eppig & O'Brien 1996), bovine and primate cortical slices have proven more difficult. There were two main problems making these methods unsuitable to address our hypothesis. The first was the synchronous activation of the majority of resting (primordial) follicles, which was mirrored by a massive increase in primary follicles (Fortune et al. 2000) within the first day. This is very different from the more gradual activation of primordial to primary follicles occurring in vivo. Similarly, wholesale activation of primordial follicles was reported in cortical slices of adult bovine (Braw-Tal & Yossefi 1997) and human ovarian cortical slices (Hovatta et al. 1997) and the techniques are addressed by Fortune (2003). In our experiments with the CAM method, there was no wholesale transition of primordial to primary follicles and the proportion of primordial, primary and secondary follicles remained largely unchanged with culture as was found by Cushman et al. (2002). Not surprisingly, we did see an increase in the proportion of atretic follicles when tissue was implanted.
The second problem with previous culture methods was the large number of follicles that became atretic. It is not uncommon for half of cultured follicles to be lost due to atresia using such techniques (Wandji et al. 1996). We were concerned that rapid initiation and a high rate of atresia may mask changes produced by androgens and therefore, although laborious, we considered the CAM method the most suitable to address our hypothesis. The rate of atresia that we have seen in our tissue, even using our strict criteria, was even lower than that reported by other groups using this technique (Wandji et al. 1996, Cushman et al. 2002), but this may be partly due to the fact that other studies were performed using foetal tissue in which the basal rate of follicle death is likely to be higher.
Using this technique, we have demonstrated that exposure to testosterone selectively increased the proportion of follicles at the primary stage. In other words, we have reproduced the findings of studies counting follicles in biopsies and slices of PCO. Our data add considerable weight to the hypothesis that androgens cause the alteration in early follicle development seen in PCO. Our data indicate that the most likely explanation for this is that testosterone reduced the number of follicles undergoing atresia. These data contrast with those of both Hughesdon (1982) and Webber et al. (2003) who found no change in the proportion of atretic follicles in cortical biopsies or slices from PCO. In a more recent study, however, it was discovered that cultured follicles from PCO survived to a much greater extent than those from normal ovaries and this was due to reduced atresia (Webber et al. 2007). Our data suggest a possible mechanism for this effect. It remains to be determined whether androgens are also having a direct effect on follicle growth or are modulating the levels of local or endocrine factors. To date, neither androgen nor FSH receptors have been demonstrated in primordial follicles of sheep or human follicles, but in sheep androgen receptor protein was present in type-2 follicles, equivalent to our primary and primary–secondary transitional follicles (Juengel et al. 2006) and in isolated human follicles, 18% of transitional follicles and 43% of primary follicles expressed testosterone receptors (Rice et al. 2007) suggesting that there might be a direct effect on atresia by androgen in at least this proportion of follicles. It is possible that exposure to androgens could increase the number of androgen receptor-positive follicles, but we have not yet determined this.
Our data show that follicle-stimulating hormone (FSH) also altered the proportion of primary follicles. While many studies have demonstrated preantral folliculogenesis in the absence of gonadotrophins, FSH receptors are present on early follicles and FSH may therefore be exerting an effect (Oktay et al. 1997, Rice et al. 2007), although the percentage of follicles expressing this receptor is very low. Interestingly, there was no significant effect of FSH on follicle atresia in our experiments and the increase in primary follicles appears to come from increased initiation as there was a fall in primordial follicles, although this was not significant. This supports the findings of an earlier paper in which FSH was found to promote preantral follicle growth in rats without having an effect on apoptosis (McGee et al. 1997).
Very few of the fundamental genes involved in early folliculogenesis have been identified; nevertheless some abnormalities have been shown in PCO. GDF9, for example, is implicated in early follicle growth and whereas in normal human ovaries GDF9 was detected in 32% of oocytes in primordial follicles and 96% of primary follicles, in PCO, GDF9 mRNA was not detected in primordial follicles and was present in less than 12% of primary follicles (Teixeira Filho et al. 2002). The effects of androgens on GDF9 expression are, however, unknown but this promises to be a fruitful area for future research.
Although higher in the serum of women with PCOS (Cook et al. 2002, Laven et al. 2004), the foetal differentiative factor AMH was present at a lower frequency in primordial and transitional follicles in these ovaries on immunostaining (Stubbs et al. 2005). AMH-knockout mice have increased follicle initiation and follicles progress rapidly through the early stages of folliculogenesis (Durlinger et al. 1999). Low levels of AMH in PCO could therefore produce the same effect. The effects of androgens on the production of this factor are, however, also unknown.
One disadvantage of the CAM method is that factors produced by the embryo itself may effect the growth of follicles in the implanted cortex. For example, Fortune et al. elegantly demonstrated that chick AMH inhibited follicle growth in this model by showing that, unlike their early experiments, growth did occur in tissue grafted from mice null mutant for the AMH type II receptor (Gigli et al. 2005). In our experiment, it was assumed that if implanted tissue was indeed exposed to chick AMH that this would be likely to affect all treatment groups equally.
In conclusion, we have shown that the method first adopted by Fortune et al. (2000) for investigating folliculogenesis in fragments of bovine embryo ovaries can be applied to lamb ovarian tissue. In addition, our studies have carefully defined the conditions under which successful vascularisation of cortical tissue occurs. Unlike classical methods of in vitro culture, this method did not result in wholesale activation of primordial to primary follicles. Exposure of the tissue in this model to testosterone, with or without FSH, caused a selective increase in the proportion of primary follicles reproducing the findings from key histological studies in PCO. Androgens are implicated in many of the manifestations of PCOS; reprogramming the HPO axis, the tendency to central adiposity and the insulin resistance. Now there is very good evidence that they are also responsible for the earliest follicular abnormalities seen in PCO. Elucidating the underlying cause of excess androgen production by these ovaries would appear to be of primary importance.
Materials and Methods
Development and characterisation of the CAM method
Fertilised eggs from White Leghorn chickens (Henry Stewart and Co. Ltd, Louth, Lincolnshire, UK) were kept at 12 °C for 0–17 days until required. Preliminary experiments investigated the effect of the length of time of pre-incubation on chick embryonic survival. Prior to implantation of ovarian tissue, eggs were transferred for 5 days to an incubator at 37–38 °C and relative humidity of 55–60% to permit foetal growth and development of the CAM. Both human and lamb cortical tissues were used while establishing the technique. Human ovarian tissue was collected with ethical approval and informed consent from five patients ranging in age from 36 to 46 years. The human tissue contained too few follicles to be experimentally useful. Lamb ovarian tissue from animals less than 1-year old was obtained from a local abattoir. Ovarian cortical fragments of ∼1×2×8 mm were dissected from lamb or human ovaries within 1 h of arrival in the laboratory. For baseline data, two fragments were fixed immediately in 4% paraformaldehyde.
The embryo, air sac and CAM were visualised through the eggshell using a fiber-optic light (‘candling’); the CAM and the air sac marked with a pencil and a small drill used to excise a triangular piece of shell of 8×8×8 mm over the CAM (‘windowing’). A 5 mm slit was made in the underlying shell membrane through which the ovarian tissue was implanted. Although Cushman et al. (2002) reported insertion of tissue beneath the CAM, we found that this was impossible to perform without puncturing the CAM with resultant death of the embryo. A preliminary series of experiments determined optimum conditions. Lamb ovarian fragments were either dropped onto the CAM or fixed more firmly by wedging the tissue in the junction between the shell membrane and the CAM. The window was sealed with transparent adhesive tape and the eggs returned to the incubator. The effect of the time taken to candle, window the egg and implant the tissue on survival of the embryo was also assessed.
After 5 days the tissue was retrieved. Tissue encapsulated by the CAM and containing blood vessels was graded for vascularity from poorly vascularised, which appeared pale, to very well vascularised, in which the entire fragment was pink. After dehydration and wax embedding, serial sections 5–6 μm were cut and stained with haematoxylin and eosin, visualised with a Zeiss microscope and the images captured with a Nikon DXM1200 digital camera (Nikon, Kingston-upon-Thames, UK) and LUCIA image analysis software (Nikon, version 2.62). Only follicles with a visible nucleolus were included to avoid duplicate counting.
Classifying follicular development and atresia
Primordial, primary and secondary follicles were classified according to the method of Wright et al. (1999). Atretic follicles were identified by the presence of one or more pyknotic nuclei of the granulosa cells and/or the nucleus of the oocyte. It became clear in the early stages that tissue that was poorly vascularised contain high proportions of atretic follicles and therefore only well- and very well-vascularised tissues were processed in further experiments.
Effects of testosterone treatment
Initially, the distribution of testosterone within the compartments of the egg was assessed by the addition of 10 μl tritiated testosterone (Amersham Biosciences, 9 MBq/ml) to each of 20 eggs. At 24 h intervals for the next 5 days, four eggs were broken into separate dishes and from each 250 μl of each of the following were collected: yolk, albumen, CAM cavity fluid, CAM, vitelline membrane and embryo. Tissues were macerated, spun and the supernatant aspirated. These were added to 4 ml scintillation fluid and the activity counted (LS 6000IC, Beckman Coulter, Buckinghamshire, UK).
For the testosterone treatment experiments, 50 eggs were implanted with pieces of ovarian cortex from one lamb. This experiment was performed twice. Follicles in tissue from five groups were counted: baseline unimplanted tissue and four groups of implanted tissue; control, testosterone treated, FSH treated and tissue exposed to both testosterone and FSH together. Testosterone at a final concentration in the egg (48 ml volume) of 10−7 M (Sigma Chemical Co.) and/or FSH at 0.04 IU/ml (8 ng/ml; purified human pituitary, Endocrine Services, Bidford-On Avon, UK) were added in 10 μl medium through the slit in the shell membrane at the time of implantation. For control implanted tissue, 10 μl medium alone was added. The window was sealed with Sellotape and the eggs returned to the incubator for a further 5 days after which the tissue was retrieved and processed as outlined above.
Statistical analysis
To assess the rate of atresia and the degree of follicle initiation, 840 follicles were counted. In the testosterone experiments, 2773 follicles were counted. The former represents all the healthy and atretic follicles counted in serial sections of four well-/very well-vascularised pieces of tissue and four baseline controls. For the analysis of the testosterone-treated tissue, transitional follicles were designated as either primordial or primary according to whether they had more or less than 50% cuboidal cells. Due to the wide range of follicle numbers within each cortical section, these data are more meaningfully analysed by proportion rather than absolute number. To adjust for the wide range of numbers of follicles in each tissue piece, weighted averages were calculated. We analysed the differences between the proportions using binomial regression allowing for overdispersion, using the blogit command with robust standard errors in Stata 9 (StataCorp LP, College Station, TX, USA). Kruskal–Wallis, Mann–Whitney, and χ2-tests were used for other analyses as indicated in the text.
Declaration of interest
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Funding
This research was supported by St George's Hospital Charitable Foundation, The Wellcome Trust and Eli Lilly.
References
- Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome – a hypothesis. Journal of Endocrinology. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, Morris DV, Price J, Jacobs HS. Multifollicular ovaries: clinical endocrine features and response to pulsatile gonadotrophin releasing hormone. Lancet. 1985;2:1375–1379. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- Battaglia C, Regnani G, Artini PG, Giulini S, Genazzani AD, Genazzani AR, Volpe A. Polycystic ovary syndrome: a new ultrasonographic and color Doppler pattern. Gynecological Endocrinology. 2000;14:417–424. doi: 10.3109/09513590009167713. [DOI] [PubMed] [Google Scholar]
- Beloosesky R, Gold R, Almog B, Sasson R, Dantes A, Land-Bracha A, Hirsh L, Itskovitz-Eldor J, Lessing JB, Homburg R, et al. Induction of polycystic ovary by testosterone in immature female rats: modulation of apoptosis and attenuation of glucose/insulin ratio. International Journal of Molecular Medicine. 2004;14:207–215. doi: 10.3892/ijmm.14.2.207. [DOI] [PubMed] [Google Scholar]
- Blandau RJ, Warrick E, Rumery RE. In vitro cultivation of embryo mouse ovaries. Fertility and Sterility. 1965;16:705–715. doi: 10.1016/s0015-0282(16)35761-2. [DOI] [PubMed] [Google Scholar]
- Braw-Tal R, Yossefi S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. Journal of Reproduction and Fertility. 1997;109:165–171. doi: 10.1530/jrf.0.1090165. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Cheresh DA. Use of the 10-day-old chick embryo model for studying angiogenesis. Methods in Molecular Biology. 1999;129:257–269. doi: 10.1385/1-59259-249-X:257. [DOI] [PubMed] [Google Scholar]
- Burnet FM. A virus disease of the canary of the fowl-pox group. Journal of Pathology and Bacteriology. 1933;37:107–122. [Google Scholar]
- Cook CL, Siow Y, Brenner AG, Fallatt ME. Relationship between serum Mullerian inhibiting substance and other reproductive hormones in untreated women in PCOS and normal women. Fertility and Sterility. 2002;77:141–146. doi: 10.1016/s0015-0282(01)02944-2. [DOI] [PubMed] [Google Scholar]
- Cushman RA, Wahl CM, Fortune JE. Bovine ovarian cortical pieces grafted to chick embryonic membranes: a model for studies on the activation of primordial follicles. Human Reproduction. 2002;17:48–54. doi: 10.1093/humrep/17.1.48. [DOI] [PubMed] [Google Scholar]
- Durlinger ALL, Kramer P, Karels B, de Jong FH, Uilenbroek JTJ, Grootegoed JA, Themmen JPN. Control of primordial follicle recruitment by anti-Mullerian hormone in mouse ovary. Endocrinology. 1999;12:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biology of Reproduction. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Birdsall M, Manning P, Mitchell JM. Transabdominal versus transvaginal ultrasound in the diagnosis of polycystic ovaries in a population of randomly selected women. Ultrasound in Obstetrics and Gynecology. 1994;4:54–59. doi: 10.1046/j.1469-0705.1994.04010054.x. [DOI] [PubMed] [Google Scholar]
- Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Animal Reproduction Science. 2003;78:135–163. doi: 10.1016/s0378-4320(03)00088-5. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Molecular and Cellular Endocrinology. 2000;163:53–60. doi: 10.1016/s0303-7207(99)00240-3. [DOI] [PubMed] [Google Scholar]
- Franks S, Willis D, Mason HD, Gilling-Smith C. Comparative androgen production from theca cells of normal women and women with polycystic ovaries. In: Chang RJ, editor. Polycystic Ovary Syndrome. Springer-Verlag; New York: 1995. pp. 334–352. [Google Scholar]
- Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti-Müllerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Molecular Reproduction and Development. 2005;71:480–488. doi: 10.1002/mrd.20338. [DOI] [PubMed] [Google Scholar]
- Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated theca cells from polycystic ovaries. Journal of Clinical Endocrinology and Metabolism. 1994;79:1158–1165. doi: 10.1210/jcem.79.4.7962289. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Abir R. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Human Reproduction. 1997;12:1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called ‘hyperthecosis’. Obstetrical and Gynecological Survey. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Heath D, Quirke LD, McNatty KP. Oestrogen receptor α and β, androgen receptor and progesterone receptor mRNA and protein localisation within the developing ovary and in small growing follicles of sheep. Reproduction. 2006;131:81–92. doi: 10.1530/rep.1.00704. [DOI] [PubMed] [Google Scholar]
- Laven JSE, Mulders AGMGJ, Visser JA, Themmen AP, De Jong FH, Frauser BCJM. Anti-Mullerian hormone serum concentrations in normovulatory and anovulatory women of reproductive age. Journal of Clinical Endocrinology and Metabolism. 2004;89:318–323. doi: 10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, Erickson GF. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2004;89:5321–5327. doi: 10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- McGee EA, Perlas E, LaPolt PS, Tsafriri A, Hsueh AJ. Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biology of Reproduction. 1997;57:990–998. doi: 10.1095/biolreprod57.5.990. [DOI] [PubMed] [Google Scholar]
- Merke DP, Bornstein SR, Avila NA, Chrousos GP. NIH conference. Future directions in the study and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Annals of Internal Medicine. 2002;136:320–334. doi: 10.7326/0003-4819-136-4-200202190-00012. [DOI] [PubMed] [Google Scholar]
- Michelmore KF, Balen AH, Dunger DB, Vessey MP. Polycystic ovaries and associated clinical and biochemical features in young women. Clinical Endocrinology. 1999;51:779–786. doi: 10.1046/j.1365-2265.1999.00886.x. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss JF, III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Molecular Endocrinology. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- Oktay K, Briggs D, Gosden RG. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. Journal of Clinical Endocrinology and Metabolism. 1997;82:3748–3751. doi: 10.1210/jcem.82.11.4346. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans N, Taylor JA, Robinson JE. Prenatal exposure to androgens leads to the development of cystic ovaries in the sheep. Biology of Reproduction. 1998;56(Supplement 1):194. [Google Scholar]
- Polson DW, Wadsworth J, Adams J, Franks S. Polycystic ovaries: a common finding in normal women. Lancet. 1988;1:870–872. doi: 10.1016/s0140-6736(88)91612-1. [DOI] [PubMed] [Google Scholar]
- Rawles M. Transplantation of normal embryonic tissue in the chick embryo in biological research. Annals of the New York Academy of Sciences. 1952;55:302. doi: 10.1111/j.1749-6632.1952.tb26546.x. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Roncali L, Dammacco F. The chick embryo chorioallantoic membrane as a model for in vivo research on angiogenesis. International Journal of Developmental Biology. 1996;40:1189–1197. [PubMed] [Google Scholar]
- Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of androgen receptor (AR), follicle-stimulating hormone receptor (FSHR) and anti-Müllerian hormone type II receptor (AMHRII) in single, isolated, human preantral follicles: relevance to polycystic ovaries (PCO) Journal of Clinical Endocrinology and Metabolism. 2007;923:1034–1040. doi: 10.1210/jc.2006-1697. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Byskov AG, Nyboe Andersen A, Muller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Human Reproduction. 2003;18:1158–1164. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- Storgard C, Mikolon D, Stupack DG. Angiogenesis assays in the chick CAM. Methods in Molecular Biology. 2005;294:123–136. doi: 10.1385/1-59259-860-9:123. [DOI] [PubMed] [Google Scholar]
- Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, Themmen AP, Visser JA, Groome NP, Franks S. Anti-Mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. Journal of Clinical Endocrinology and Metabolism. 2005;90:5536–5543. doi: 10.1210/jc.2005-0907. [DOI] [PubMed] [Google Scholar]
- Tamura N, Kurabayashi T, Nagata H, Matsushita H, Yahata T, Tanaka K. Effects of testosterone on cancellous bone, marrow adipocytes, and ovarian phenotype in a young female rat model of polycystic ovary syndrome. Fertility and Sterility. 2005;84(Supplement 2):1277–1284. doi: 10.1016/j.fertnstert.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Teixeira Filho FL, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, Shimasaki S, Erickson GF. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2002;87:1337–1344. doi: 10.1210/jcem.87.3.8316. [DOI] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. Journal of Clinical Investigation. 1998;1010:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandji S-A, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biology of Reproduction. 1996;55:942–948. doi: 10.1095/biolreprod55.5.942. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs SA, Stark J, Margara RA, Trew GH, Lavery SA, Hardy K, Franks S. Prolonged survival in culture of preantral follicles from polycystic ovaries. Journal of Clinical Endocrinology and Metabolism. 2007;92:1975–1978. doi: 10.1210/jc.2006-1422. [DOI] [PubMed] [Google Scholar]
- Wright CS, Hovatta O, Margara R, Trew G, Winston RM, Franks S, Hardy K. Effects of follicle-stimulating hormone and serum substitution on the in vitro growth of human ovarian follicles. Human Reproduction. 1999;14:1555–1562. doi: 10.1093/humrep/14.6.1555. [DOI] [PubMed] [Google Scholar]