Abstract
Increased glucose transporter GLUT4 expression in skeletal muscle is an important benefit of regular exercise, resulting in improved insulin sensitivity and glucose tolerance. The Ca2+–calmodulin-dependent kinase II (CaMKII), calcineurin and AMPK pathways have been implicated in GLUT4 gene regulation based on pharmacological evidence. Here, we have used a more specific genetic approach to establish the relative role of the three pathways in fast and slow muscles. Plasmids coding for protein inhibitors of CaMKII or calcineurin were co-transfected in vivo with a GLUT4 enhancer-reporter construct either in normal mice or in mice expressing a kinase dead (KD) AMPK mutant. GLUT4 reporter activity was not inhibited in the slow soleus muscle by blocking either CaMKII or calcineurin alone, but was inhibited by blocking both pathways. GLUT4 reporter activity was likewise unchanged in the soleus of KD-AMPK mice, but was significantly reduced by incapacitation of either CaMKII or calcineurin in these mice. On the other hand, in the fast tibialis anterior (TA) muscle, calcineurin appears to exert a prominent role in the control of GLUT4 reporter activity, independent of CaMKII and AMPK. The results point to a muscle type-specific and redundant regulation of GLUT4 enhancer based on the interplay of multiple signalling pathways, all of which are known to affect myocyte enhancing factor 2 (MEF2) transcriptional activity, a point of convergence of different pathways on muscle gene regulation.
The glucose transporter GLUT4 is responsible for glucose uptake induced by insulin and contractile activity in skeletal muscle, and its level of expression is a major determinant of the capacity for glucose uptake by skeletal muscle fibres. Overexpression of GLUT4 in skeletal muscle promotes glucose uptake and counteracts insulin resistance in diabetic mice (Ren et al. 1995; Leturque et al. 1996; Tsao et al. 1996). Exercise increases muscle GLUT4 gene transcription (Neufer & Dohm, 1993) and GLUT4 protein levels in skeletal muscle (Ren et al. 1994). This effect is thus an important health benefit of regular exercise and the identification of the signalling pathways controlling GLUT4 expression has obvious clinical relevance. In fact, the increase in skeletal muscle GLUT4 expression is probably one of the most important adaptations to regular exercise resulting in increased insulin action (Frøsig et al. 2007).
Previous studies have suggested a role of the calcineurin, Ca2+–calmodulin-dependent-kinase (CaMK) and AMP-activated protein kinase (AMPK) pathways in controlling GLUT4 gene transcription in skeletal muscle. However, most experiments were done in cultured muscle cells, which may not reflect the response of adult muscle fibres in vivo, and used pharmacological approaches, the specificity of which are generally limited. For example, evidence for a role of AMPK on GLUT4 expression is based on chronic treatment of L6 myotubes (Ojuka et al. 2002) or rats (Holmes et al. 1999) with the AMPK activator drug AICAR. However, AICAR has effects independent of AMPK, for example on the synthesis of some inositol phosphates (Choi et al. 2008). The involvement of CaMKII is likewise based on the finding that the CaMK inhibitor KN-93 abrogates the caffeine-induced increase of GLUT4 in L6 myotubes (Ojuka et al. 2002) and reduces, but does not abolish, the increase in GLUT4 mRNA and protein induced by exercise in vivo (Smith et al. 2007). However, KN-93 inhibits all CaMK isoforms and thus does not allow identification of the specific CaMK involved and, in addition, has non-specific inhibitory effects on voltage-dependent K+ channels and L-type calcium channels (Ledoux et al. 1999; Gao et al. 2006). A role of calcineurin was suggested by the finding that GLUT4 expression is increased in transgenic mice overexpressing activated calcineurin (Ryder et al. 2003). However, interpretation of this result is complicated by possible non-physiological consequences of overexpression of a constitutively active mutant. Another study reported that the calcineurin inhibitor cyclosporin A does not affect exercise-induced increase in muscle GLUT4 (Garcia-Roves et al. 2005), but cyclosporin A itself has additional effects, for example it inhibits the mitochondrial permeability transition pore (Crompton et al. 1988).
Here, we used a specific genetic approach in vivo to establish the relative role of the CaMKII, calcineurin and AMPK pathways on GLUT4 gene transcription in fast and slow mouse muscles. To monitor GLUT4 transcriptional activity, we used a GLUT4 enhancer, linked to a minimal promoter controlling luciferase expression, the activity of which reflects the response of the whole GLUT4 promoter both in cultured muscle cells and in skeletal muscle in vivo (Moreno et al. 2003). A plasmid containing the GLUT4 enhancer-luciferase construct was co-transfected in adult muscles with plasmids encoding the CaMKII-specific peptide inhibitor KIIN and the calcineurin-specific inhibitor Cain. The experiments were performed either in normal mice or in mice expressing a dominant negative AMPK mutant. The results show that (i) the three signalling pathways redundantly control the GLUT4 enhancer and (ii) the relative role of these pathways is in part dependent on muscle type.
Methods
Animals and in vivo transfection
All experimental protocols were reviewed and supervised by the Veterinary Service and Animal Care Committee of the University of Padova, in accordance with the D.Lgs. 116/92.
Experiments were carried out in C57B1/6 mice (25–30 g). C57B1/6 mice overexpressing a kinase-dead Lys45Arg mutant AMPK α2, driven by the heart- and skeletal muscle-specific creatine kinase promoter, a gift of M. J. Birnbaum (Pennsylvania School of Medicine), have been described previously (Mu et al. 2001). A total of 57 wild-type (wt) and 28 mutant mice were used. All mice were anaesthetized by intraperitoneal injection of a mixture of Zoletil 100 (a combination of zolazapam and tiletamine, 1:1, 10 mg kg−1, Laboratoire Virbac) and Xilor (xylazine 2%, 0.06 ml kg−1, Bio98 Srl, Milan, Italy). TA and soleus muscles were surgically exposed and injected with plasmid DNA (total 50 μg DNA in saline) using a custom-modified microsyringe. Control plasmids were injected into the contralateral leg of the same animal receiving the test plasmids. Injection was followed by electroporation with stainless steel electrodes connected to an ECM830 BTX porator (Genetronics, San Diego, CA, USA) with the following settings: 5 pulses of 20 ms each and 200 ms interval, the voltage was adjusted according to the thickness of the muscle (220 V cm−1). Mice were killed by cervical dislocation 7 days post-transfection; muscles were removed frozen in liquid nitrogen-cooled isopentane and stored at −80°C.
Plasmids
A plasmid containing GLUT4enh-LUC, a reporter harbouring the GLUT4 enhancer sequence −502/−420 (Moreno et al. 2003) linked to a minimal herpes virus thymidine kinase (TK) promoter and driving the expression of firefly (Photinus pyralis) luciferase (LUC) was a gift of A. Zorzano (Institute for Research in Biomedicine, Barcelona, Spain). To normalize for transfection efficiency, a plasmid expressing the luciferase from Renilla reniformis under the control of a minimal promoter was co-transfected (Promega). A plasmid coding for the CaMKII inhibitory peptide KIIN (Chang et al. 1998) was obtained from Dr T. Soderling (Oregon Health and Sciences University, Portland, OR, USA). The plasmids coding for the calcineurin inhibitor Cain have been previously described (Serrano et al. 2001). The activity of nuclear factor of activated T cells (NFAT) transcription factors was monitored with an NFAT reporter, which consists of nine tandem NFAT-binding sites from the interleukin 4 gene fused to a basal α-myosin heavy chain (α-MyHC) promoter and linked to luciferase (McCullagh et al. 2004). The activity of cAMP-responsive element-binding protein (CREB) transcription factor was monitored with a CREB reporter (pCRE-LUC, Clontech), which consists of two copies of the CRE-binding sequence fused to a TATA-like promoter region from the herpes simplex virus thymidine kinase promoter and linked to luciferase.
Preparation of homogenates and luciferase assay
A commercially available dual luciferase assay system was used (E1960, Promega). Briefly, muscles were crushed in liquid nitrogen with mortar and pestle, lysed in 1× passive lysis buffer containing protease inhibitors (11-836-145-001, Complete, Roche) and centrifuged for 30 min at 11 000 g in a refrigerated microcentrifuge. The supernatant was assayed for both firefly and Renilla luciferase activity using a standard luminometer. Values are reported as firefly divided by Renilla luciferase activity levels.
Data analysis
The results of each transfection experiment represent the mean of at least four different muscles. All data are expressed as the mean ±s.e.m. Descriptive statistics and parametric tests were performed using KyPlot (freeware). Comparisons were made using Student's paired t test, with P < 0.05 being considered statistically significant.
Results
To analyse the role of CaMKII on GLUT4 enhancer in vivo we used a loss of function approach based on an endogenous inhibitory protein of CaMKII, KIIN, which was originally identified in brain by yeast two-hybrid screening. KIIN has been shown to be a potent and specific inhibitor of CaMKII, but not of other multifunctional CaMKs or kinases such as protein kinase A, protein kinase C (PKC) or mitogen-activated protein kinases (MAPKs) (Chang et al. 1998). The specific mechanism of action of KIIN and derived peptides has been determined (Vest et al. 2007) and its effectiveness in intact skeletal muscle fibres has been validated by direct injection of the peptide in isolated mouse flexor digitorum brevis fibres (Tavi et al. 2003). We further validated this inhibitor in vivo by showing that the activity of a CREB reporter, reflecting the activity of the transcription factor CREB, a well-known target of CaMKII (Wheeler et al. 2008), is inhibited by co-transfection with the plasmid coding for KIIN (Fig. 1A). However, when the slow-twitch soleus and fast-twitch TA muscles were co-transfected with the GLUT4 enhancer (−502 to −420 bp) fused to luciferase (GLUT4enh-LUC), no significant effect of KIIN was observed (Fig. 2).
Figure 1. Validation in vivo of KIIN as inhibitor of CaMKII and of Cain as inhibitor of calcineurin.
The activity of a CREB reporter, reflecting the activity of the transcription factor CREB, a well-known target of CaMKII, is inhibited by co-transfection with the plasmid coding for KIIN (A). The activity of an NFAT reporter, reflecting the activity of the transcription factor NFAT, a major target of calcineurin, is inhibited by co-transfection with the plasmid coding for Cain (B). Data are mean ±s.e.m. (n= 4 in A; n= 5 in B). *,**Significantly different from control vector group (P < 0.05 and 0.01 respectively).
Figure 2. CaMKII inhibitor KIIN has no effect on GLUT4 enhancer activity in transfected adult skeletal muscles.
Adult mouse soleus and tibialis anterior (TA) muscles were transfected with either KIIN or an empty vector together with the GLUT4 enhancer upstream of luciferase. Luciferase activity is expressed as a percentage of that measured in muscles injected with empty vector alone. Data are mean ±s.e.m. (A, n= 23; B, n= 24).
Next, we asked whether calcineurin is necessary for GLUT4 enhancer activity by transfecting Cain, a potent calcineurin inhibitor (Lai et al. 1998) that we have previously validated in rat skeletal muscle in vivo (Serrano et al. 2001). As shown in Fig. 1B, the activity of an NFAT reporter, a major target of calcineurin, is inhibited also in mouse skeletal muscle by co-transfection with a plasmid coding for Cain. When co-transfected together with GLUT4enh-LUC, Cain strongly reduced GLUT4 enhancer activity in TA whereas in soleus no significant difference was observed between Cain- and vector-transfected muscles (Fig. 3).
Figure 3. The calcineurin inhibitor Cain inhibits GLUT4 enhancer activity in soleus but not in TA.
GLUT4 reporter activity is not affected by cotransfection with Cain in soleus muscle (A) but is significantly inhibited in TA (B). Luciferase activity is expressed as a percentage of that measured in muscles injected with empty vector alone. Data are mean ±s.e.m. (A, n= 20; B, n= 21). **Significantly different from control vector group (P < 0.01).
The calcineurin target, NFAT, and another CaMKII target, MEF2, are known to act synergistically in many cell systems. We therefore asked whether these two signalling pathways interact in the control of GLUT4 enhancer activity in fast and slow muscle. To this aim, we co-transfected KIIN and Cain in the same muscle and compared their combined effects with the expression of one inhibitor only. Interestingly, a strong inhibition of GLUT4 reporter activity could be observed under these conditions in soleus, whereas neither KIIN nor Cain alone had an effect (Fig. 4). On the other hand, the inhibition observed in TA is quantitatively similar to that observed with Cain alone. These results indicate that in TA calcineurin plays a more prominent role in GLUT4 enhancer regulation, whereas in soleus the two pathways appear to be redundant, so that the block of one pathway can be compensated by the other.
Figure 4. Simultaneous block of CaMKII and calcineurin inhibits GLUT4 enhancer activity both in soleus and TA.
The two signalling pathways were inhibited by co-injection of KIIN and Cain together with GLUT4 enhancer in soleus (A) and TA (B). Luciferase activity is expressed as a percentage of that measured in muscles injected with empty vector alone. Data are mean ±s.e.m. (n= 13 in A and B). **Significantly different from control vector group (P < 0.01).
In both muscles, however, the inhibition of GLUT4 enhancer activity observed with combined KIIN and Cain is not complete, suggesting that other signalling pathways contribute to the regulation of GLUT4 gene transcription in skeletal muscle. Therefore we assessed the role of the AMPK pathway, since AMPK is a fuel-sensing enzyme that promotes utilization of fatty acids and glucose in response to activity (Richter & Ruderman, 2009) and because chronic activation of AMPK with AICAR increases GLUT4 protein expression (Holmes et al. 1999). To this aim, we used transgenic mice overexpressing a catalytically inactive (kinase dead) form of the AMPK α2 subunit (KD-AMPK) that acts as a dominant negative mutant. These mice lack essentially all AMPK activity in skeletal muscle (Mu et al. 2001; Jensen et al. 2007). Surprisingly, however, the level of GLUT4 mRNA was shown to be normal in KD-AMPK mice, both under resting and exercised conditions (Holmes et al. 2004), an observation in agreement with findings made in other AMPK transgenic mouse models (Jorgensen et al. 2007; Rockl et al. 2007). In agreement with this, GLUT4enh-LUC activity was essentially identical between KD-AMPK mice and wt littermates under our experimental conditions (Fig. 5).
Figure 5. Transgenic mice with dominant negative AMPK (KD-AMPK) have levels of GLUT4 enhancer activity indistinguishable from wild-type littermates.
GLUT4 enhancer activity was compared in adult soleus (A) and TA (B) muscles from KD-AMPK mice and wt littermates, transfected with an empty vector. Luciferase activity is expressed as a percentage of that measured in wt muscles. Data are mean ±s.e.m. (n= 9 in A and 8 in B).
This finding does not preclude the involvement of AMPK in the control of GLUT4 enhancer activity, because other signals could compensate for the lack of AMPK in these mice. To address this issue, we investigated the possibility that CaMKII and calcineurin cooperate with AMPK in the regulation of GLUT4. We thus inhibited CaMKII and calcineurin in KD-AMPK mice and measured GLUT4enh-LUC activity in soleus and TA. The results are shown in Fig. 6. In the absence of AMPK activity, CaMKII becomes rate-limiting in soleus, since a significant inhibition of GLUT4enh-LUC activity is observed with KIIN in KD-AMPK but not in wt mice (Fig. 6A, compare with Fig. 2A). This effect is muscle type-specific, since TA is not significantly affected by KIIN in mutant mice (Fig. 6B). Calcineurin also becomes rate-limiting for GLUT4 enhancer activity in soleus from AMPK-deficient mice (Fig. 6C), in striking contrast with the observation in wt mice (Fig. 3A), whereas the inhibition in TA is quantitatively similar to that seen in wt mice (Fig. 6D, compare with Fig. 3B), hinting again at a muscle type-dependent relationship between these signalling pathways. The greatest inhibition of GLUT4enh-LUC is observed when KIIN and Cain are co-transfected in the KD-AMPK mice in both fast and slow muscles (Fig. 6E and F).
Figure 6. Effect of calcineurin and CaMKII inhibition on GLUT4 enhancer activity in KD-AMPK mice.
The effects of KIIN (A and B), Cain (C and D) and of the two inhibitors combined (E and F) were assessed in KD-AMPK mice. Soleus (A, C and E) and TA (B, D and F) were transfected and analysed for luciferase expression. Luciferase activity is expressed as a percentage of that measured in muscles injected with empty vector alone. Data are mean ±s.e.m. (n= 14 for KIIN, 8 for Cain and 5 for KIIN + Cain). * and **, significantly different from control vector group (P < 0.05 and 0.01, respectively).
Altogether, these results point to a muscle type-specific regulation of GLUT4 enhancer activity based on differential usage of the CaMKII, calcineurin and AMPK signalling pathways. In soleus, GLUT4 enhancer is controlled by all three pathways, which can compensate for the lack of one, but not two of them, as indicated by the comparison of Fig. 2A with Fig. 6A and Fig. 3A with Fig. 6C. In TA, on the other hand, calcineurin signalling exerts a dominant effect on GLUT4 enhancer, seemingly independent of AMPK and CaMKII (compare Figs 3B and 6F).
Discussion
A major result of this study is that three signalling pathways, CaMKII, calcineurin and AMPK, act jointly on GLUT4 enhancer activity in fully differentiated mouse skeletal muscles. The redundant regulation is clearly illustrated by the effect of KIIN or Cain on GLUT4 enhancer in soleus muscle. Incapacitation of a single pathway is without effect on the response of the enhancer, and might thus lead to the false conclusion that this pathway is not important; it is only when two pathways are both inhibited that a transcriptional inhibition is observed. It appears that these two Ca2+-dependent pathways are both involved in GLUT4 enhancer regulation but can reciprocally compensate for the block of a single pathway. Combinatorial interaction between calcineurin and CaMK pathways in muscle gene regulation was previously reported in cultured muscle cells: activated calcineurin or CaMKIV had limited effect on transcription of a myoglobin promoter and an MEF2 reporter when expressed separately in C2C12 muscle cells, but evoked a greater response in combination (Wu et al. 2000). However, no detectable CaMKIV protein is expressed in murine skeletal muscle and indeed no change in skeletal muscle is found in CaMKIV null mice both at rest and in response to exercise (Akimoto et al. 2004). CaMKII appears to be the major, if not the only CaMK isoform expressed in skeletal muscle (Rose et al. 2006). A similar synergistic inhibition is observed in soleus muscle with AMPK: the dominant negative AMPK mutant has no significant effect on GLUT4 enhancer, however a decreased activity of the enhancer is observed when either CaMKII or calcineurin is blocked in this mutant, the greatest effect being found when all three pathways are incapacitated. Thus one must envisage the regulation of the GLUT4 gene, and probably of other genes involved in muscle metabolism, as the result of the concomitant action of multiple interacting pathways, whose role cannot be analysed in isolation, but only in the context of complex regulatory networks.
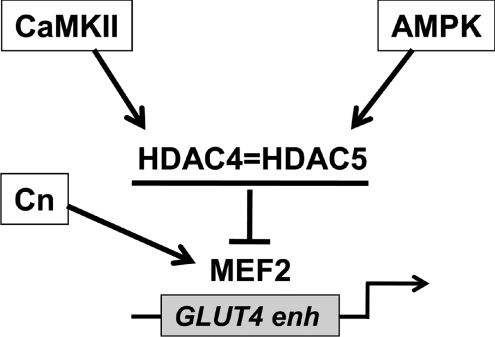
How can one explain the redundancy of these three signalling pathways on GLUT4 enhancer activity? One possibility is that all three pathways exert their effect mainly by acting on MEF2 transcriptional activity, which represents a point of convergence of multiple pathways on muscle gene regulation. The GLUT4 enhancer contains a conserved MEF2 binding site, which is critical for its transcriptional activity, since disruption of this MEF2-binding site abrogates GLUT4 promoter expression in transgenic mice (Thai et al. 1998). We have previously shown that the integrity of this site is also required following transfection in vivo in adult murine muscles (Moreno et al. 2003). Indeed, disruption of the MEF2-binding site caused a marked reduction of the activity of the enhancer both in fast and slow muscles, similar to that described here after blocking all three signalling pathways. As schematically represented in Fig. 7, all three pathways have been shown to control MEF2 transcriptional activity in muscle cells. Calcineurin can activate MEF2 either directly by MEF2 dephosphorylation (Wu et al. 2001) or indirectly by NFAT dephosphorylation and subsequent interaction between activated NFAT and MEF2 by protein–protein interaction via MEF2 binding sites (Youn et al. 2000). A direct effect of NFAT on the enhancer is unlikely due to lack of a consensus binding site for NFAT as judged by sequence analysis. CaMKs phosphorylate class IIa histone deacetylases (HDAC4, −5, −7 and −9) thus creating docking sites for the 14-3-3 chaperone protein, which binds phospho-HDACs and causes their translocation from nucleus to cytoplasm, thus relieving the inhibition by HDACs on MEF2 transcriptional activity (Grozinger & Schreiber, 2000; McKinsey et al. 2000). Cultured adult muscle fibres show activity-dependent translocation of HDAC4-GFP from nucleus to cytoplasm, which is abolished by the CaMK inhibitor KN−62 and is accompanied by activation of MEF2, as determined by a MEF2 promoter linked to luciferase (Liu et al. 2005). Among the CaMK isoforms, CaMKII interacts selectively with HDAC4 and controls the phosphorylation and nuclear export of HDAC4 (Backs et al. 2006). On the other hand AMPK has been reported to phosphorylate HDAC5 thus promoting its association with 14-3-3 proteins and concomitant activation of GLUT4 gene expression (McGee et al. 2008). The CaMKII–HDAC4 and AMPK–HDAC5 pathways are interdependent since HDAC4 can bind to and hetero-oligomerize with HDAC5 and, in this way, CaMKII bound to HDAC4 can transphosphorylate HDAC5 and promote its nuclear export (Backs et al. 2008).
Figure 7. Scheme of the regulation of GLUT4 transcription in skeletal muscle by the calcineurin (Cn), CaMKII and AMPK pathways via MEF2.
The ‘=’ symbol between HDAC4 and HDAC5 indicates the possibility of heterodimerization between the two HDACs (see text for details).
In conclusion, MEF2 transcription factors integrate diverse Ca2+-dependent and -independent signalling pathways in the control of GLUT4 enhancer activity. Both the existence of a common transcriptional target and of potential interactions between the different signalling pathways makes the control system redundant and may explain why the deficiency of any single pathway has no obvious consequence because of compensatory activity by other pathways. This effect may vary between fast and slow muscles possibly due to the different role of various pathways in relation to fibre-type composition. Available evidence indicates that the calcineurin–NFAT pathway is more active in slow than in fast muscles (McCullagh et al. 2004; Tothova et al. 2006), and HDAC4 and -5 are enriched in fast muscles (Potthoff et al. 2007). This interpretation fits with the observation of higher GLUT4 protein expression in slow versus fast muscle fibres in humans (Daugaard et al. 2000). One is tempted to speculate that the higher level of activity of all three pathways might account for the capacity of slow muscles to better compensate the incapacitation of any single pathway. On the other hand, the AMPK γ3 subunit is selectively expressed in fast glycolytic muscles and upregulation of GLUT4 gene expression by the AMPK activator AICAR is limited to fast glycolytic muscles (see Long & Zierath, 2008). Thus, at the moment it is difficult to interpret the different response of fast and slow muscles described in this study on the basis of present knowledge of the muscle fibre-type dependence of calcineurin, CaMK and AMPK signalling.
Finally, two limitations of this study should be mentioned. First, the GLUT4 enhancer used in our experiments, though certainly critical for GLUT4 gene expression, is not the only regulatory sequence of the gene. Another important regulatory domain is located upstream of the MEF2 binding enhancer (Charron et al. 1999). This domain binds a transcription factor, GLUT4 enhancer factor (GEF), required for transcriptional regulation of the human GLUT4 promoter in transgenic mice (Oshel et al. 2000). GEF and MEF2A appear to form a complex on the GLUT4 promoter that allows for recruitment of transcriptional co-regulators, including HDAC5, to control GLUT4 promoter activity (Knight et al. 2003; Sparling et al. 2008).
A second point to consider is that other signalling pathways are known to control MEF2 transcriptional activity and thus modulate GLUT4 promoter activity. For example, protein kinase D, which is activated through phosphorylation by PKC, when overexpressed in fast muscles of transgenic mice potently stimulates the transcriptional activity of MEF2 (Kim et al. 2008). MEF2 transcription factors are also controlled by the mitogen-activated protein kinase (MAPK) p38 (see Han & Molkentin, 2000), which is a strong inducer of GLUT4 in cardiomyocytes (Montessuit et al. 2004). Finally, it was recently shown that GLUT4 gene expression is controlled not only by HDACs but also by the antagonistic activity of histone acetyl-transferases, which are dependent on the generation of acetyl-CoA by the enzyme ATP-citrate lyase (ACL) (Wellen et al. 2009). It was thus suggested that ACL activity is required to link growth factor-induced increases in nutrient metabolism to the generation of histone acetylation and gene expression. It will be of interest to define the possible interaction of these additional pathways on GLUT4 gene regulation and to establish their role in mediating the effect of exercise. The approaches described here can provide a foundation for these future studies.
Acknowledgments
This study was supported by grants from the European Commission, Agenzia Spaziale Italiana, Copenhagen Muscle Research Centre, Danish Medical and Natural Science Research Councils, Danish Diabetes Association, the Novo-Nordisk Research and Lundbeck Foundations, Association Francaise Myopathies and EXGENESIS. We thank Dr M. J. Birnbaum (Howard Hughes Medical Institute and Department of Medicine, University of Pennsylvania School of Medicine, USA) for the KD-AMPK founder mice, Dr A. Zorzano (Institute for Research in Biomedicine, Barcelona, Spain) for the GLUT4 enhancer, Dr S. Snyder (Department of Neuroscience, Johns Hopkins University, USA) for Cain plasmid and Dr T. Soderling (Vollum Institute, Oregon Health and Sciences University, USA) for the KIIN construct. We also thank Anne Picard for technical assistance.
Glossary
Abbreviations
- ACL
ATP-citrate lyase
- AMP
adenosine mono-phosphate
- AMPK
AMP-activated protein kinase
- Cain
calcineurin inhibitor
- CaMKII
Ca2+–calmodulin-dependent-kinase II
- CREB
cAMP-responsive element-binding protein
- GLUT4
glucose transporter 4
- HDAC
histone deacetylase
- KD-AMPK, kinase dead AMPK; KIIN
Ca2+–calmodulin-dependent kinase II inhibitory protein
- LUC
firefly luciferase; MAPK, mitogen-activated protein kinase
- MEF2, myocyte enhancing factor 2; NFAT
nuclear factor of activated T cells
- PKC
protein kinase C
- TA
tibialis anterior; wt, wild-type
Author contributions
E.A.R. and S.S. designed and coordinated the project together with M.M. and T.E.J. Most of the experiments were performed by M.M., M.C. and T.E.J. M.G. contributed to the initial experiments with the CaMKII inhibitor. S.S. wrote the paper with critical input from E.A.R., M.M. and T.E.J. All authors read and approved the manuscript for publication.
Author's present address
M. Garcia: Inserm U839, Université Pierre et Marie Curie-Paris 6, France.
References
- Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol. 2004;287:C1311–C1319. doi: 10.1152/ajpcell.00248.2004. [DOI] [PubMed] [Google Scholar]
- Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol. 2008;28:3437–3445. doi: 10.1128/MCB.01611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron MJ, Katz EB, Olson AL. GLUT4 gene regulation and manipulation. J Biol Chem. 1999;274:3253–3256. doi: 10.1074/jbc.274.6.3253. [DOI] [PubMed] [Google Scholar]
- Choi K, Mollapour E, Choi JH, Shears SB. Cellular energetic status supervises the synthesis of bis-diphosphoinositol tetrakisphosphate independently of AMP-activated protein kinase. Mol Pharmacol. 2008;74:527–536. doi: 10.1124/mol.107.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA. Fibre type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes. 2000;49:1092–1095. doi: 10.2337/diabetes.49.7.1092. [DOI] [PubMed] [Google Scholar]
- Frøsig C, Rose AJ, Treebak JT, Kiens B, Richter EA, Wojtaszewski JF. Effects of endurance exercise training on insulin signalling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS160. Diabetes. 2007;56:2093–2102. doi: 10.2337/db06-1698. [DOI] [PubMed] [Google Scholar]
- Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun. 2006;345:1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Garcia-Roves PM, Jones TE, Otani K, Han DH, Holloszy JO. Calcineurin does not mediate exercise-induced increase in muscle GLUT4. Diabetes. 2005;54:624–628. doi: 10.2337/diabetes.54.3.624. [DOI] [PubMed] [Google Scholar]
- Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci U S A. 2000;97:7835–7840. doi: 10.1073/pnas.140199597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Molkentin JD. Regulation of MEF2 by p38 MAPK and its implication in cardiomyocyte biology. Trends Cardiovasc Med. 2000;10:19–22. doi: 10.1016/s1050-1738(00)00039-6. [DOI] [PubMed] [Google Scholar]
- Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- Holmes BF, Lang DB, Birnbaum MJ, Mu J, Dohm GL. AMP kinase is not required for the GLUT4 response to exercise and denervation in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E739–E743. doi: 10.1152/ajpendo.00080.2004. [DOI] [PubMed] [Google Scholar]
- Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007;292:E1308–E1317. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPKα2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- Kim MS, Fielitz J, McAnally J, Shelton JM, Lemon DD, McKinsey TA, Richardson JA, Bassel-Duby R, Olson EN. Protein kinase D1 stimulates MEF2 activity in skeletal muscle and enhances muscle performance. Mol Cell Biol. 2008;28:3600–3609. doi: 10.1128/MCB.00189-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JB, Eyster CA, Griesel BA, Olson AL. Regulation of the human GLUT4 gene promoter: interaction between a transcriptional activator and myocyte enhancer factor 2A. Proc Natl Acad Sci U S A. 2003;100:14725–14730. doi: 10.1073/pnas.2432756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MM, Burnett PE, Wolosker H, Blackshaw S, Snyder SH. Cain, a novel physiologic protein inhibitor of calcineurin. J Biol Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Chartier D, Leblanc N. Inhibitors of calmodulin-dependent protein kinase are nonspecific blockers of voltage-dependent K+ channels in vascular myocytes. J Pharmacol Exp Ther. 1999;290:1165–1174. [PubMed] [Google Scholar]
- Leturque A, Loizeau M, Vaulont S, Salminen M, Girard J. Improvement of insulin action in diabetic transgenic mice selectively overexpressing GLUT4 in skeletal muscle. Diabetes. 1996;45:23–27. doi: 10.2337/diab.45.1.23. [DOI] [PubMed] [Google Scholar]
- Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol. 2005;168:887–897. doi: 10.1083/jcb.200408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long YC, Zierath JR. Influence of AMP-activated protein kinase and calcineurin on metabolic networks in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E545–E552. doi: 10.1152/ajpendo.90259.2008. [DOI] [PubMed] [Google Scholar]
- McCullagh KJ, Calabria E, Pallafacchina G, Ciciliot S, Serrano AL, Argentini C, Kalhovde JM, Lomo T, Schiaffino S. NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc Natl Acad Sci U S A. 2004;101:10590–10595. doi: 10.1073/pnas.0308035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SL, van Denderen BJ, Howlett KF, Mollica J, Schertzer JD, Kemp BE, Hargreaves M. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57:860–867. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montessuit C, Rosenblatt-Velin N, Papageorgiou I, Campos L, Pellieux C, Palma T, Lerch R. Regulation of glucose transporter expression in cardiac myocytes: p38 MAPK is a strong inducer of GLUT4. Cardiovasc Res. 2004;64:94–104. doi: 10.1016/j.cardiores.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Moreno H, Serrano AL, Santalucia T, Guma A, Canto C, Brand NJ, Palacin M, Schiaffino S, Zorzano A. Differential regulation of the muscle-specific GLUT4 enhancer in regenerating and adult skeletal muscle. J Biol Chem. 2003;278:40557–40564. doi: 10.1074/jbc.M306609200. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Neufer PD, Dohm GL. Exercise induces a transient increase in transcription of the GLUT-4 gene in skeletal muscle. Am J Physiol Cell Physiol. 1993;265:C1597–C1603. doi: 10.1152/ajpcell.1993.265.6.C1597. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+ Am J Physiol Endocrinol Metab. 2002;282:E1008–E1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- Oshel KM, Knight JB, Cao KT, Thai MV, Olson AL. Identification of a 30-base pair regulatory element and novel DNA binding protein that regulates the human GLUT4 promoter in transgenic mice. J Biol Chem. 2000;275:23666–23673. doi: 10.1074/jbc.M001452200. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibres. J Clin Invest. 2007;117:2459–2467. doi: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JM, Marshall BA, Mueckler MM, McCaleb M, Amatruda JM, Shulman GI. Overexpression of Glut4 protein in muscle increases basal and insulin-stimulated whole body glucose disposal in conscious mice. J Clin Invest. 1995;95:429–432. doi: 10.1172/JCI117673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JM, Semenkovich CF, Gulve EA, Gao J, Holloszy JO. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem. 1994;269:14396–14401. [PubMed] [Google Scholar]
- Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418:261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fibre type shift. Diabetes. 2007;56:2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol. 2006;574:889–903. doi: 10.1113/jphysiol.2006.111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder JW, Bassel-Duby R, Olson EN, Zierath JR. Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. J Biol Chem. 2003;278:44298–44304. doi: 10.1074/jbc.M304510200. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lomo T, Schiaffino S. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibres but not muscle growth. Proc Natl Acad Sci U S A. 2001;98:13108–13113. doi: 10.1073/pnas.231148598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Collins M, Grobler LA, Magee CJ, Ojuka EO. Exercise and CaMK activation both increase the binding of MEF2A to the Glut4 promoter in skeletal muscle in vivo. Am J Physiol Endocrinol Metab. 2007;292:E413–E420. doi: 10.1152/ajpendo.00142.2006. [DOI] [PubMed] [Google Scholar]
- Sparling DP, Griesel BA, Weems J, Olson AL. GLUT4 enhancer factor (GEF) interacts with MEF2A and HDAC5 to regulate the GLUT4 promoter in adipocytes. J Biol Chem. 2008;283:7429–7437. doi: 10.1074/jbc.M800481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavi P, Allen DG, Niemela P, Vuolteenaho O, Weckstrom M, Westerblad H. Calmodulin kinase modulates Ca2+ release in mouse skeletal muscle. J Physiol. 2003;551:5–12. doi: 10.1113/jphysiol.2003.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai MV, Guruswamy S, Cao KT, Pessin JE, Olson AL. Myocyte enhancer factor 2 (MEF2)-binding site is required for GLUT4 gene expression in transgenic mice. Regulation of MEF2 DNA binding activity in insulin-deficient diabetes. J Biol Chem. 1998;273:14285–14292. doi: 10.1074/jbc.273.23.14285. [DOI] [PubMed] [Google Scholar]
- Tothova J, Blaauw B, Pallafacchina G, Rudolf R, Argentini C, Reggiani C, Schiaffino S. NFATc1 nucleocytoplasmic shuttling is controlled by nerve activity in skeletal muscle. J Cell Sci. 2006;119:1604–1611. doi: 10.1242/jcs.02875. [DOI] [PubMed] [Google Scholar]
- Tsao TS, Burcelin R, Katz EB, Huang L, Charron MJ. Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. Diabetes. 1996;45:28–36. doi: 10.2337/diab.45.1.28. [DOI] [PubMed] [Google Scholar]
- Vest RS, Davies KD, O’Leary H, Port JD, Bayer KU. Dual mechanism of a natural CaMKII inhibitor. Mol Biol Cell. 2007;18:5024–5033. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol. 2008;183:849–863. doi: 10.1083/jcb.200805048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton JM, Chin ER, Simard AR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fibre type. EMBO J. 2000;19:1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 2001;20:6414–6423. doi: 10.1093/emboj/20.22.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn HD, Chatila TA, Liu JO. Integration of calcineurin and MEF2 signals by the coactivator p300 during T-cell apoptosis. EMBO J. 2000;19:4323–4331. doi: 10.1093/emboj/19.16.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]