Abstract
Slowing and loss of muscle power are major factors limiting physical performance but little is known about the molecular mechanisms involved. The slowing might be a consequence of slow detachment of cross bridges and, if this were the case, then a reduction in the ATP cost of an isometric contraction would be expected as the muscle fatigued. The human anterior tibialis muscle was stimulated repeatedly under ischaemic conditions at 50 Hz for 1.6 s with a 50% duty cycle and muscle metabolites measured by 31P magnetic resonance spectroscopy. Over the course of 20 contractions the half-time of relaxation increased from 36.5 ± 0.09 ms (mean ±s.e.m.) to 113 ± 17 ms and isometric force was reduced to 63 ± 3% of the initial value. ATP turnover was determined from the change in high energy phosphates and lactate production, the latter estimated from the change of intracellular pH. ATP turnover over the first three contractions was 2.45 ± 0.09 mm s−1 and decreased to 1.8 ± 0.06 mm s−1 over the last five tetani. However, when this latter value was normalised for the decrease in isometric force, it became 2.56 ± 0.3 mm s−1, which is the same as the turnover of the fresh muscle. The data suggest that the rate of cross bridge detachment is unaffected by fatigue and are consistent the suggestion that it is the rate of attachment which is slowed rather than the rate of detachment. The present results focus attention on stages in the cross bridge cycle concerned with attachment and the transition from low to high force states that may be influenced by metabolic changes in the fatiguing muscle.
Metabolically demanding exercise that results in fatigue produces shifts in contractile characteristics of the working muscles that are similar to the differences between fast and slow muscle fibres and this analogy could provide an insight into the change of kinetic properties that occurs during fatigue. One feature of fast and slow muscles in the fresh state is that they have different economies of ATP turnover during isometric contractions, economy being defined as the ATP turnover divided by the isometric force generated. The difference in economy is reported to be about threefold in isolated animal muscles (Goldspink, 1978; Crow & Kushmerick, 1982) and three- to fourfold for human and rat muscle fibres (Söderlund et al. 1992; Bottinelli et al. 1994; Szentesi et al. 2001). While there are differences in the costs of calcium transport, etc., the major part of the differences in ATP costs between different fibre types can be ascribed to differences in cross bridge turnover. If, therefore, fatigue is analogous to a transition from a faster to a slower fibre type, the slowing of contractile properties might be associated with a decrease in ATP costs and an increase in economy of the muscle.
Edwards et al. (1975) first suggested that slow relaxation of fatigued muscle might be a consequence of slow cross bridge detachment and presented data on an isolated mouse soleus preparation that indicated a reduction in ATP turnover as the muscle fatigued and slowed. Crow & Kushmerick (1982) found no evidence of such a reduction in the soleus muscle although the experimental protocols differed in the two studies. However the latter authors did find that the economy of the faster EDL increased over 15 s of stimulation. Subsequently they (Crow & Kushmerick, 1983) showed that the change in ATP turnover in the EDL correlated with changes in the maximum velocity of unloaded shortening, although values were measured at only two time points, 3 and 9 s. Other studies have been less successful in demonstrating a decrease in the energy costs of force generation during contractions that would be expected to result in substantial contractile slowing. In one of the first MRS studies of skeletal muscle, Dawson et al. (1980) found no change in the economy of frog muscle as it fatigued and, likewise, Hultman & Sjöstrom (1983) found no change in a biopsy study of human muscle stimulated at 20 Hz over 50 s. In another biopsy study of human muscle Chasiotis et al. (1987) found a tendency for economy to decrease during intermittent stimulation while for continuous stimulation there was a slight increase in economy in the last stage. However, while the difference in energy cost between the two protocols was significant in the latter stages, the changes with time within each protocol were not significant. Westra et al. (1988) compared isometric force and ATP costs in electrically stimulated rat quadriceps and found biphasic changes with time, first an increase in economy and then a decrease. More recently, Giannesini et al. (2001) measured the ATP costs of stimulating the rat gastrocnemius muscle at a range of frequencies from 0.8 to 7.6 Hz and related this to the force of the evoked twitches. With protocols that induced a loss of force, the ATP costs declined more than force and the apparent economy increased, but there was no correlation between the extent of fatigue and change in economy and, even with the protocols that produced no loss of force, there was still an improvement in economy with time. There is therefore some confusion as to whether fatigue-induced slowing of contractile properties is accompanied by a reduction in ATP costs of the contraction.
High intensity exercise is associated with a number of changes in contractile properties, the most evident being an increased time for relaxation from an isometric contraction (Edwards et al. 1975; Curtin, 1986, 1988; Cady et al. 1989b) and a change in the force–velocity relationship, including a decrease in the velocity of unloaded shortening (Crow & Kushmerick, 1983; de Haan et al. 1989; de Ruiter et al. 1999, 2000). While most attention has focused on changes in the maximum velocity of shortening, we have shown that the time course of the change in relaxation rate differed from that of the slowing of shortening velocity with the change in the latter being a relatively late event during fatigue and playing a minor role in the characteristic loss of power (Jones et al. 2006). What was notable and unexpected in that study was the fact that the curvature of the force–velocity relationship increased substantially with fatigue and this not only accounted for most of the loss of power but it also correlated with the change in relaxation rate. The change in curvature is difficult to explain in terms of slower cross bridge detachment and the authors suggested that it was the apparent rate of cross bridge attachment which decreased, possibly as a consequence of increased levels of inorganic phosphate.
A decrease in the rate of cross bridge detachment should be evident as an increase in the economy of maintaining isometric force since a slowing of the rate of detachment would both increase force and decrease ATP turnover. On the other hand, a decrease in the rate of attachment would not be expected to affect economy since there would be concomitant decreases in both force and ATP turnover. Consequently we have undertaken a study of ATP turnover in electrically stimulated human anterior tibialis (AT) muscle and related this to changes in the rate of relaxation from an isometric tetanus, using this as an indication of contractile slowing that results in a loss of muscle power. Muscle metabolites were measured by 31P magnetic resonance spectroscopy (MRS) and the ATP turnover determined and expressed as a function of force, giving a measure of economy for the isometric contractions.
Methods
Subjects
Four healthy subjects (one female) aged 26–49, were studied after giving their informed consent to the experimental protocol, which conformed to the standards set by the latest revision of the Declaration of Helsinki and was approved by the University College Hospital Ethics Committee.
Force recording and electrical stimulation
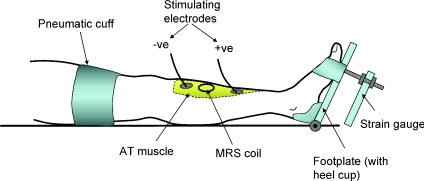
Subjects lay supine with one leg extended in the bore of the MRS magnet (see below for details) and securely strapped to a Perspex, wood and aluminium cradle that positioned the belly of the AT within the uniform field of the magnet (see Constantin-Teodosiu et al. 1997; Fig. 1). The heel rested on a small cup fixed to a hinged wooden plate and the foot and toes were firmly strapped to the upper part of the plate that was attached to an aluminium strain gauge. A screw arrangement between footplate and transducer allowed adjustment of the angle of the foot plate and thus optimal muscle length. The MRS coil was positioned over the belly of the AT against the skin and adhesive ECG electrodes, used for stimulating the muscle, were placed just proximal and distal to the coil. The stimulating current was set 20% above that required to produce maximal tetanic force. Stimulation parameters were 100 ms duration square wave pulses at 50 Hz and a current of ∼50 mA.
Figure 1. Diagram of the experimental arrangement for stimulating and measurement of metabolites from the anterior tibialis muscle.
Subjects lay with their leg in the bore of the magnet and the belly of the muscle positioned within the uniform magnetic field.
Tetanic force at the end of each 1.6 s contraction was measured. The relaxation rate was taken as the half-time (t0.5), in seconds, of the exponential phase (usually from 50 to 25% tetanic force). The speed of relaxation was also expressed as a rate constant, calculated as 0.693/t0.5.
Fatiguing procedure
The muscle was first rendered ischaemic by means of a sphygmomanometer cuff placed around the thigh and inflated to 200 mmHg. A period of 30 s elapsed between cuff inflation and the start of stimulation to deplete intramuscular stores of O2. The ischaemic muscle was then stimulated with 1.6 s contractions with a 1.6 s recovery interval between: stimulation was continued for 3, 10, 15 or 20 contractions on separate occasions. At the end of the stimulation period the electrode leads and those to the strain gauge were rapidly removed and muscle metabolites determined whilst the muscle remained ischaemic.
Metabolite measurements
31P MRS spectra were collected before and after the ischaemic exercise using methods similar to those previously described by Cady et al. (1989a). Briefly, relative concentrations of muscle metabolites were measured using a spectrometer equipped with a 1.9 T superconducting magnet and a 21 cm clear bore. A 2.1 cm two-turn coil was tuned for 31P (32.5 MHz) and 1H (80.3 MHz) at the centre of the magnetic field. Radio frequency pulses giving a 90 deg flip angle at the coil centre were used with an interpulse interval of 2.256 s. A small sphygmomanometer cuff was placed under the calf and was used to adjust the position of the muscle in relation to the coil, keeping it in firm contact with the skin. The position of the coil and whether it had moved could usually be judged at the end of the experiment from the position and depth of the indentation made in the skin.
Each composite spectrum comprised 128 free induction decays (total collection time, 4.8 min) which were subjected to Fourier transformation, phasing and baseline correction. The areas for the resonance peaks of phosphomonoester (PM), inorganic phosphate (Pi), phosphocreatine (PC), phosphodiester (PD) and βATP were calculated by integration of the area between pre-selected gates. The total MRS visible phosphorus was taken to be the sum of PM + Pi+ PD + PC + (3 ×βATP) and the individual compounds were expressed as a fraction of the total. Individual metabolite peaks were corrected for partial saturation using factors derived from comparisons of the data obtained with the 2.256 s interval with that obtained with a 20.56 s interval.
Although the MRS collection time was 4.8 min there was always some delay in repositioning the leg and shimming the magnet so the time elapsed between the end of stimulation and the end of the collection was between 7 and 10 min.
βATP/total P for the resting muscles was 0.125 ± 0.007. The total P was calculated assuming the ATP concentration to be 8.2 mm in the intracellular water (Cady et al. 1989a) giving a total P of 65.9 ± 3.7 mm. This value was then used to estimate the absolute metabolite concentrations for the post-exercise measurements, making the assumption that the total visible P signal remained constant.
Intracellular pH was estimated from the chemical shift of the Pi peak from the PC peak:
where δ is the chemical shift (ppm) of the Pi peak relative to PC.
Lactate production was calculated by assuming that it was equivalent to the protons required to produce the observed change of pH, making further assumptions about the concentrations and dissociation constants of the intracellular buffers. These buffers were taken to be: (a) protein-bound histidine, 56 mm, pK= 6.8 (Fürst et al. 1970); (b) bicarbonate, 10 mm, pK= 6.1 (Sahlin et al. 1977); (c) carnosine, 7 mm, pK= 6.8 (Mannion et al. 1992); and (d) phosphate, added to the computation according to the phosphate measured by MRS (pK= 6.73). The non-phosphate buffer capacity came to 34.5 slykes, and this increased to approximately 50 slykes when Pi reached its highest levels in the fatigued muscles. The free intracellular ADP concentration was calculated from the concentrations of ATP, PC and H+ assuming the total creatine and phosphocreatine remained constant throughout the exercise at 42.5 mm:
The equilibrium constant (Keq) for the reaction was taken to be 1.66 × 10−9m−1.
The concentration of the monobasic phosphate was estimated from the total Pi and pH: [H2PO4−]= ([H+][Pi])/(Kp, +[H+]).
The equilibrium constant Kp was taken to be 1.86 × 10−7m−1.
ATP cost was estimated from changes (Δ; post-contraction − pre-contraction) in PC, ATP and LA concentrations:
Data handling
Four measurements were made for each subject so for each there were four determinations of resting metabolite levels and one at the end of 3, 10, 15 and 20 tetani. The four resting measurements for each subject were averaged to provide a single value which was then used to determine the change as a result of stimulation. The force records yielded four values for force and relaxation for each subject for fresh muscle and for the first three tetani, four values for the next five, three for the next five, and so on. As with the metabolites, values for each subject were averaged at each time point to give a single value to be combined with the data from the other subjects. ATP turnover is reported as mm s−1, this being millimoles ATP per litre intracellular water per second. Values are given as means and standard errors of the mean unless stated otherwise.
Results
The stimulation protocol was well tolerated by the subjects, the only problem being the ischaemic interval at the end of stimulation, which became a little tedious while adjustments were being made and MRS data collected. However the only ill effects were attacks of pins and needles once the blood returned to the limb.
Changes in force and contractile properties with fatigue
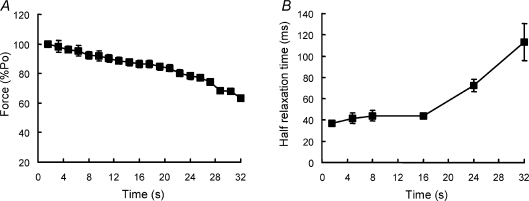
Peak force (expressed as a percentage of the value of the first tetanus) for the series of 20 contractions is shown in Fig. 2A. There was a suggestion of a biphasic response with a fairly steady decline over the first 20 s of stimulation (12–13 tetani) with a somewhat faster decline thereafter. At the end of the protocol tetanic force was reduced to 63 ± 3% of the initial value.
Figure 2. Change in contractile properties with fatigue.
The ischaemic muscle was stimulated repeatedly at 50 Hz for 1.6 s with 1.6 s rest. Force (A) is expressed as a percentage of the initial isometric contraction (Po) and the rate of relaxation (B) is expressed as the half-time of force decay.
Relaxation half-time (Fig. 2B) changed relatively little over the first 15 s of stimulation (9–10 tetani) but then increased rapidly during the second half of the protocol. Values for the fresh muscle were 36.5 ± 0.9 ms and these increased to 113 ± 17 ms by the end of the protocol, just over a threefold increase. Expressed as rate constants, the values declined from 19 ± 0.5 s−1 in the fresh muscle to 6.7 ± 1.2 s−1 at the end the fatiguing series. The point of inflection for the relaxation–time relationship corresponded roughly with the time at which force began to decline more rapidly (compare Fig. 2A and B).
Muscle metabolites
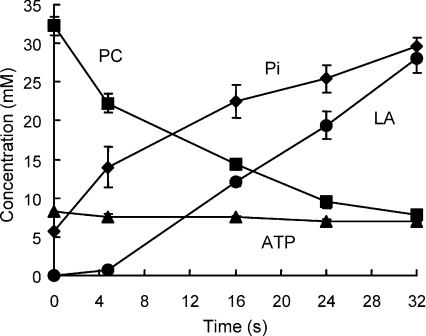
Muscle metabolites measured at rest and after 3, 10, 15 and 20 fatiguing tetani are given in Table 1 and shown in Fig. 3. Values are reported as concentrations in the intracellular water and are normalised to 8.2 mm in the fresh muscle and, thereafter, to a constant total phosphate (see Methods).
Table 1.
Muscle metabolites
| Time (s) | 0 | 4.8 | 16 | 24 | 32 |
|---|---|---|---|---|---|
| ATP | 8.2 ± 0.0 | 7.6 ± 0.8 | 7.6 ± 0.2 | 6.9 ± 0.7 | 7.0 ± 0.4 |
| PC | 32.3 ± 2.4 | 22.2 ± 2.4 | 14.4 ± 1.4 | 9.5 ± 1.6 | 7.8 ± 1.6 |
| Pi | 5.6 ± 0.2 | 14.0 ± 5.3 | 22.4 ± 4.3 | 25.4 ± 3.6 | 29.6 ± 2.2 |
| ME | 3.3 ± 1.1 | 3.6 ± 0.6 | 6.9 ± 1.5 | 9.4 ± 2.2 | 8.4 ± 1.2 |
| pH | 7.01 ± 0.02 | 7.09 ± 0.02 | 6.90 ± 0.02 | 6.78 ± 0.05 | 6.65 ± 0.05 |
| Lactate | 0 | 0.7 ± 0.9 | 12.1 ± 1.1 | 19.4 ± 3.6 | 28.0 ± 3.6 |
The anterior tibilalis was stimulated for the times indicated and metabolites measured in the ischaemic muscle by 31P MRS. Values are given as mmoles per litre intracellular water, assuming an ATP concentration of 8.2 mm in the resting muscle (0 time stimulation) and a constant total MRS visible P thereafter. ME, phospho-monoesters. Data are given as means and s.d.
Figure 3. Changes in ATP, phosphocreatine (PC), inorganic phosphate (Pi) and lactate (LA) estimated by 31P MRS, during the course of the fatiguing series of contractions illustrated in Fig. 2.
Concentrations are given as mmoles per litre intracellular water, assuming an ATP concentration of 8.2 mm in the resting muscle.
ATP levels were unaffected for the first 10 contractions but thereafter declined slightly, falling to 7.0 ± 0.2 mm from the initial value of 8.2 mm. Phosphocreatine declined rapidly from a starting value of 32.3 ± 1.2 mm to a final value of 7.8 ± 0.8 mm (ΔPC –24.5) and this was matched by a stochiometric rise in Pi from 5.6 ± 1 mm to 29.6 ± 1.1 mm (ΔPi 24.0). There was a rise in phospho-monoesters, due to accumulation of AMP or IMP as a consequence of the loss of ATP, but this peak would also include sugar phosphates and other glycolytic intermediates.
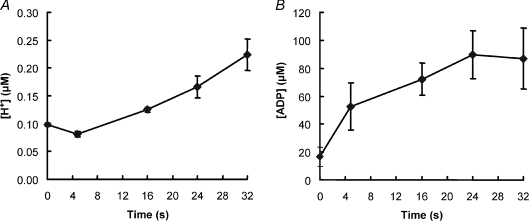
Intracellular changes in hydrogen ion are shown in Fig. 4A, and the corresponding pH values in Table 1. In the first 5 s there was a small alkalosis as a consequence of PC breakdown, after which pH declined in a linear fashion. Resting muscle pH was 7.01 ± 0.01 which declined to 6.65 ± 0.03 by the last contraction.
Figure 4. Changes in intracellular pH (A) and ADP (B) with fatigue.
Experimental details as described for Fig. 3.
Lactate accumulation was calculated from the changes of intracellular hydrogen ion concentration (Fig. 4A) and the estimated buffer capacity of the muscle and it can be seen that there was virtually no evidence of glycolysis during the first 5 s of contraction (3 tetani: Fig. 3), and thereafter lactate increased in a linear fashion to reach 28.0 ± 1.8 mm by the last contraction.
ADP concentrations were estimated from the levels of ATP, PC and H+, assuming that the total creatine remained constant, and the values are given in Fig. 4B. After an initial increase the concentration appeared to stabilise at around 80 μm.
Muscle metabolites and relaxation
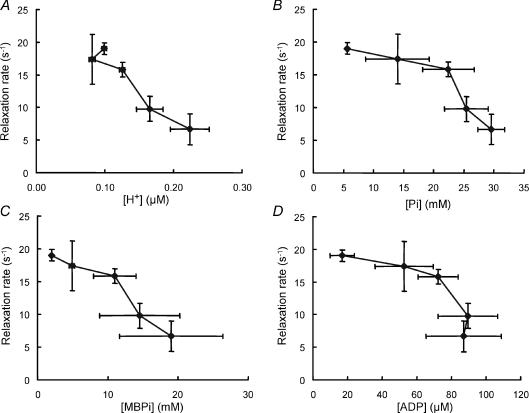
There were no simple relationships between change in any of the metabolites and the slowing of relaxation, largely due to the fact that the major change in contractile speed occurred relatively late in the series of contractions while the major changes in metabolites occurred early in the process. The changes in total ATP were small and although levels continued to fall throughout the series there was no increased loss towards the end of the series that might have correlated with the slowing of relaxation. After an initial fall, hydrogen ion concentration rose steadily with increasing duration of stimulation and, at the higher concentrations, there was a linear relationship between relaxation and hydrogen ion concentration (Fig. 5A), but this certainly did not apply over the whole pH range. The relationship between inorganic phosphate (Pi) and rate of relaxation (Fig. 5B) was also biphasic with small changes in relaxation over the period when Pi was changing rapidly and more rapid slowing of relaxation in the latter stages. Inorganic phosphate exists as the dibasic (HPO42−) and the monobasic form (H2PO4−), with the proportion of the monobasic form increasing as the intracellular H+ concentration increases. The relaxation rate showed a more linear relationship with the monobasic phosphate (Fig. 5C) than with the total Pi, although the uncertainty becomes greater since the estimates of monobasic phosphate combine the errors involved in measuring both Pi and H+. ADP might affect cross bridge function in the fatiguing muscle but it appears unlikely that there is any causal relationship between ADP and rate of relaxation (Fig. 5) since ADP levels were constant at the time when relaxation rates were changing rapidly.
Figure 5. Muscle metabolite concentrations and relaxation.
Relaxation rate in relation to: A, hydrogen ion concentration; B, inorganic phosphate (Pi); C, ADP; and D, monobasic phosphate (MBPi) after different periods of stimulation. Experimental details as described for Fig. 3. Data given as means ±s.d.
ATP turnover
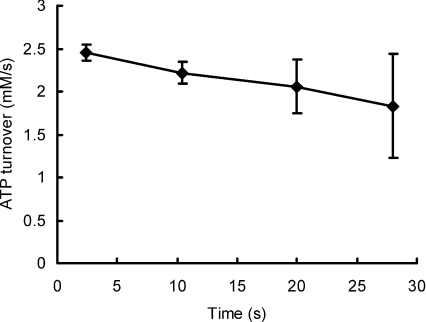
ATP turnover was calculated for each subject on the basis of change in ATP, PC and 1.5 × lactate. The change in total ATP turnover at the end of each period of stimulation (0, 4.8, 8, 16, 24 s) was subtracted from the values calculated for the next longest period (4.8, 8, 16, 24, 32 s respectively) and the results plotted as a mean value at the average time between these two times (2.4, 10.4, 20 and 28 s) in Fig. 6. The initial ATP turnover was 2.45 ± 0.09 mm s−1 during the first three contractions and this declined to 1.8 ± 0.6 mm s−1 over the last 5 tetani. However it should be noted that during this time the tetanic force also declined (Fig. 2) and, if the values for ATP turnover are normalised for loss of force (Fig. 7), no decrease in turnover was evident over the course of the fatiguing contractions, the corrected turnover for the last five contractions being adjusted to 2.56 ± 0.8 mm s−1.
Figure 6. ATP turnover.
Turnover was calculated from the data shown in Fig. 3 as the change in high energy phosphates plus the ATP from glycolysis estimated from lactate accumulation. Values are plotted against the average time over which the estimates were made.
Figure 7. Economy of force production.
Economy of isometric force production during the fatiguing series of contractions estimated by normalising the data shown in Fig. 6 by the force data in Fig. 2A.
Discussion
The main purpose of this study was to determine whether there is a change in the economy of an isometric contraction as the muscle fatigues and its contractile properties become slower. A slow relaxation has been associated with a loss of power and this might suggest a slower rate of cross bridge detachment that would result in an improved economy. However, the observation is that the economy was unchanged, a result that is consistent with the suggestion (Jones et al. 2006) that it is the rate of cross bridge attachment that changes with fatigue.
Unlike in many animals, the human AT is a relatively slow muscle, probably because it plays an opposing role to the soleus muscle in maintaining an upright posture. Resting metabolite levels and contractile properties, however, were very similar to those of the hand muscles, the first dorsal interosseous (FDI) (Cady et al. 1989a) and adductor pollicis (Newham et al. 1995). The stimulated rate of ATP turnover found in the present work of approximately 2.5 mm s−1 was similar to that reported by Sacco et al. (1994), but about half the rate found for the adductor pollicis during a 5 s continuous tetanus (Newham et al. 1995).
The time course and extent of force loss were similar to those seen with other human muscles fatigued in a similar fashion. The relaxation phase at the end of each tetanus slowed with the onset of fatigue, the half-relaxation time being three times the value in the fresh muscle. The extent of the change was somewhat less than the fourfold change reported by de Ruiter et al. (2000) and Jones et al. (2006) for the adductor pollicis, but the difference is probably explicable in terms of the differing stimulation protocols, since the shortening contractions used in the previous studies would have been more energetically demanding than the isometric contractions used in the present work.
The measurement of metabolites by in vivo31P MRS makes a number of assumptions and has some practical limitations. The raw MRS data give values for the phosphorous metabolites as ratios and in converting these to absolute concentrations we have made two assumptions. The first is that the intracellular ATP concentration is 8.2 mm in the fresh muscle, while the second is that the total MRS visible phosphate remains constant throughout the series of fatiguing contractions. The first assumption will affect the absolute value of the ATP turnover but will not affect the conclusion that the rate of turnover does not change with duration of activity. If the total visible P was to decrease this would have the effect of reducing the apparent change in PC and ATP while accentuating changes in Pi. Lactate production is the major determinant of ATP turnover in the latter stages of the exercise and phosphate is a significant component of the total buffer capacity. Consequently overestimating phosphate will overestimate the lactate production. However, any such overestimate would almost exactly be balanced by an underestimation of PC utilisation. In any case, it is unlikely that there is a significant loss of total visible P since changes in PC and Pi were almost exactly equal and opposite. MRS is a valuable technique in that it allows repeated measurements to be made on the same muscle but has the disadvantage of being relatively insensitive so that a number of spectra have to be averaged. In the system we used it was necessary to collect spectra over 4.8 min to obtain sufficient resolution of the major peaks. The technique is also sensitive to small changes in the position of the muscle in relation to the volume of the homogeneous field of the magnet. Consequently it was not possible to make concurrent force and metabolite measurements and we adopted the technique of stimulating the muscle and then measuring metabolites whilst the muscle was maintained under ischaemic conditions. The assumption here is that no further metabolic change occurs during the time from the end of stimulation to collection of the final spectrum, which could be up to 10 min. However resting metabolic rates of skeletal muscle are very low compared to the rates during contraction. We have found that the resting ATP turnover for the adductor pollicis is of the order of 0.01 mms−1 compared to 5.5 mms−1 during a tetanus (Turner, Jones & Newham, unpublished observations). It might be argued that the resting rate would be significantly greater following a period of activity, but Crowther et al. (2002a,b); have shown that glycolysis turns off rapidly at the end of contractile activity. In either case, any resting metabolism will make an equal contribution to all our measurements and since the rates of turnover were calculated by subtracting one value from another, a constant additional component will not contribute to the difference and thus the ATP turnover reported. One further assumption is that the muscle was working entirely anaerobically. Thirty seconds was allowed between making the muscle ischaemic and the start of the experiment, but this may not have been sufficient time to completely deplete O2 stores in the muscle and would mean a higher ATP turnover than was calculated. However, it is likely that any residual oxygen would be used rapidly at the start of the contractile activity (Harris et al. 1975) and any uncertainty on this point should not affect conclusions about the economy of the longer contractions when the major changes in contractile properties occurred.
Although cross bridge activity may be the major process using ATP, it is also required for calcium pumping and maintaining electochemical gradients. The energy requirements of these latter processes might be expected to remain relatively constant, especially if the ATP turnover is corrected for a decrease in force, assuming this is largely the result of a decrease in calcium release. The proportion of the total ATP consumed by cross bridge activity is not known for the human AT muscle but it is likely to be 30–40%, as for other muscle preparations (Homsher & Kean, 1978; Crow & Kushmerick, 1983; Szentesi et al. 2001). If, then, the rate of cross bridge ATP turnover were to be halved, a reduction of perhaps 30% of the total turnover might be expected. The data shown in Fig. 6 suggest a 20% decrease in turnover, but no evidence of a decrease is seen if a correction is made for force loss (Fig. 7).
We have not been able to measure the force–velocity relationships of the AT muscle since this is a technically demanding measurement to make on human muscle in situ, especially within the NMR magnet, but it is reasonable to infer that the curvature of the relationship would have changed in a similar way to that seen with the adductor pollicis in pervious studies where the slowing of relaxation correlated well with changes in curvature (Fig. 4C in Jones et al. 2006). Using the same argument, it also seems likely that in the present experiments Vmax might have changed relatively little since change in shortening velocity is a smaller and later feature of the fatigue process (Jones et al. 2006).
The slowing of contractile properties with fatigue has been interpreted as an indication of slow cross bridge detachment (de Haan et al. 1989; de Ruiter et al. 1999, 2000). However, the subsequent observation that changes in relaxation and loss of power were associated with an increased curvature of the force–velocity relationship (halving of a/Fo, see Jones et al. 2006) has changed that view. In a two-state model of cross bridge action (Huxley, 1957), a/Fo is determined by (f+g1)/g2, where f is the rate constant for attachment, g1 that for detachment within the positive working range of the cross bridge and g2 the rate constant for detachment once the cross bridge has passed the neutral position. The latter constant (g2) determines Vmax. In the absence of a major change in Vmax, and thus g2, a decrease in a/Fo implies a decrease in (f+g1). Values for f are generally assumed to be three- to fourfold higher than for g1 to allow a reasonable proportion of cross bridges to be attached during an isometric contraction, and consequently it is impossible to account for a twofold change in (f+g1), as required to explain the reported decrease in a/Fo with fatigue, by changing g1 alone. It was argued, therefore, that to account for the changes in curvature of the force–velocity relationship with fatigue a major change in f is required, implying a decrease in the rates of cross bridge attachment rather than the previously held view that it was the rate of detachment that changed. In the cross bridge model of Huxley (1957), and in more recent models of muscle during slow shortening or in the isometric state (e.g. Piazzesi & Lombardi, 1995), each cross bridge cycle is associated with the hydrolysis of one ATP, so the ATP turnover per unit force in the isometric state (economy) gives a measure of g1, the rate constant for detachment. Our present observation of a lack of change in economy over the course of a series of fatiguing contractions (Fig. 7) indicates that g1 does not change and, by implication, any decrease in a/Fo is due to a decrease in f, the rate constant for attachment.
There is then the question of whether a decrease in the rate constant for cross bridge attachment accounts for the slowing of relaxation from an isometric tetanus. Slow relaxation could be due to a change in cross bridge kinetics or slow Ca2+ removal from troponin into the sarcoplasmic reticulum. Although evidence from the use of mammalian preparations indicates that slowing with fatigue is primarily due to a change in cross bridge kinetics (Westerblad & Allen, 1993; Westerblad et al. 1997) this has not been established for human muscle working at body temperature. Assuming, for the moment, that cross bridge action does determine the rate of relaxation, it is still not immediately obvious how a decrease in attachment might slow the rate at which force decays. In single fibres the relaxation phase is characterised by an initial, relatively slow, linear phase of force decline followed by a shoulder and then a more rapid exponential decay of force. The shoulder, and subsequent loss of force, is associated with relative movements of different elements of the fibre, generally with the end sarcomeres being stretched (Huxley & Simmons, 1970). Curtin & Edman (1989) showed that these differential movements are reduced in the fatigued state and this was associated with an increased resistance of the fibre to stretch. Subsequently they confirmed the increased resistance to stretch and showed that muscle stiffness was increased relative to force in the fatigued state and suggest that this may act to stabilise the fibre. Human muscle working in situ does not show the two phases of relaxation but it does become more resistant to stretch when fatigued (de Ruiter et al. 2000). In a two-state cross bridge model the rate constant for attachment must include a number of steps, from the actual attachment through one or more intermediary steps to the formation of the high force state. Consequently an apparent decrease in the constant f could be as a result of an increased population of cross bridges that are attached but in low force intermediate states. This population would add resistance to stretch but reduce force during shortening and so could stabilise sarcomere lengths and prevent stronger sarcomeres extending weaker ones during relaxation, thereby slowing the decay of force. An accumulation of cross bridges in the low force state would also have the effect of reducing curvature and thus power output of the fatigued muscle. Thus a decrease in the apparent rate of cross bridge attachment could explain both slowing of relaxation and loss of power.
An increase in the concentration of inorganic phosphate is one way in which the proportion of attached cross bridges in the low force state could increase and consequently a clear correlation might be seen between accumulation of Pi and slowing of contractile properties. While the data shown in Fig. 5B do not rule this out, they are not convincing evidence of a causal relationship. An increase in H+ might also affect cross bridge kinetics and, again, the data in Fig. 5C are inconclusive. The combination of Pi and H+ leads to an increase in monobasic phosphate and the data in Fig. 5D do suggest a stronger, possibly causal, relationship with contractile properties. Nevertheless, this cannot be the whole explanation since relaxation becomes slowed in patients with myophosphorylase deficiency in the absence of any increase in H+ or monobasic Pi (Cady et al. 1989b). It is possible that relationships between changes in metabolite levels and contractile function are obscured by compartmentation of metabolites either within or between fibres.
The slowed contractile speed and loss of muscle power seen with fatigue are major limitations to activity in sport and everyday life yet very little is known about the molecular basis of these changes in function. The present results add weight to the suggestion that it is a reduction in the apparent rate of cross bridge attachment leading to an accumulation of cross bridges in low force states that underlies these changes. Attention thus focuses on factors that affect the stages in the cross bridge cycle concerned with attachment and the transition from low to high force states as causes of contractile slowing in fatigued muscles.
Glossary
Abbreviations
- AT
anterior tibialis
- MRS
magnetic resonance spectroscopy
- PC
phosphocreatine
- PD
phosphodiester
- PM
phosphomonoester
Author contributions
The authors contributed equally to the design of the study, the experimental work, subsequent analysis and drafting of the manuscript. The experimental work was carried out in the Department of Medicine, University College London.
References
- Bottinelli R, Canepari M, Reggiani C, Stienen GJ. Myofibrillar ATPase activity during isometric contraction and isomyosin composition in rat single skinned muscle fibres. J Physiol. 1994;481:663–675. doi: 10.1113/jphysiol.1994.sp020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady EB, Jones DA, Lynn J, Newham DJ. Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol. 1989a;418:311–325. doi: 10.1113/jphysiol.1989.sp017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady EB, Elshove H, Jones DA, Moll A. The metabolic causes of slow relaxation in fatigued human skeletal muscle. J Physiol. 1989b;418:327–337. doi: 10.1113/jphysiol.1989.sp017843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasiotis D, Bergstrom M, Hultman E. ATP utilization and force during intermittent and continuous muscle contractions. J Appl Physiol. 1987;63:167–174. doi: 10.1152/jappl.1987.63.1.167. [DOI] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Greenhaff PL, McIntyre DB, Round JM, Jones DA. Anaerobic energy production in human skeletal muscle in intense contraction: a comparison of 31P magnetic resonance spectroscopy and biochemical techniques. Exp Physiol. 1997;82:593–601. doi: 10.1113/expphysiol.1997.sp004049. [DOI] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982;79:147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Correlated reduction of velocity of shortening and the rate of energy utilization in mouse fast twitch muscle during a continuous tetanus. J Gen Physiol. 1983;82:703–720. doi: 10.1085/jgp.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther GJ, Carey MF, Kemper WF, Conley KE. Control of glycolysis in contracting muscle. I. Turning it on. Am J Physiol Endocrinol Metab. 2002a;282:E67–73. doi: 10.1152/ajpendo.2002.282.1.E67. [DOI] [PubMed] [Google Scholar]
- Crowther GJ, Carey MF, Kemper WF, Conley KE. Control of glycolysis in contracting muscle. II. Turning it off. Am J Physiol Endocrinol Metab. 2002b;282:E74–79. doi: 10.1152/ajpendo.2002.282.1.E74. [DOI] [PubMed] [Google Scholar]
- Curtin NA. Effects of carbon dioxide and tetanus duration on relaxation of frog skeletal muscle. J Muscle Res Cell Motil. 1986;7:269–275. doi: 10.1007/BF01753560. [DOI] [PubMed] [Google Scholar]
- Curtin NA. Intracellular pH and relaxation of frog muscle. Adv Exp Med Biol. 1988;226:657–669. [PubMed] [Google Scholar]
- Curtin NA, Edman KA. Effects of fatigue and reduced intracellular pH onsegment dynamics in ‘isometric’ relaxation of frog muscle fibres. J Physiol. 1989;413:159–174. doi: 10.1113/jphysiol.1989.sp017647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, Wilkie DG. Mechanical relaxation rate and metabolism studies in fatiguing muscle by phosphorous nuclear magnetic resonance. J Physiol. 1980;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan A, Jones DA, Sargeant AJ. Changes in velocity of shortening, power output and relaxation rate during fatigue of rat medial gastrocnemius muscle. Pflügers Arch. 1989;413:422–428. doi: 10.1007/BF00584493. [DOI] [PubMed] [Google Scholar]
- de Ruiter CJ, Didden WJM, Jones DA, De Haan A. The force–velocity relationship of human adductor pollicis muscle during stretch and the effects of fatigue. J Physiol. 2000;526:671–681. doi: 10.1111/j.1469-7793.2000.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter CJ, Jones DA, Sargeant AJ, de Haan A. The measurement of force/velocity relationships of fresh and fatigued human adductor pollicis muscle. Eur J Appl Physiol. 1999;80:386–393. doi: 10.1007/s004210050608. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol. 1975;251:287–301. doi: 10.1113/jphysiol.1975.sp011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P, Jonsson A, Josephson B, Vinnars E. Distribution in muscle and liver vein protein of 15N administered as ammonium acetate in man. J Appl Physiol. 1970;29:307–312. doi: 10.1152/jappl.1970.29.3.307. [DOI] [PubMed] [Google Scholar]
- Giannesini B, Izquierdo M, Le Fur Y, Cozzone PJ, Bendahan D. In vivo reduction in ATP cost of contraction is not related to fatigue levels in stimulated rat gastrocnemius muscle. J Physiol. 2001;536:905–915. doi: 10.1111/j.1469-7793.2001.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G. Energy turnover during contraction of different types of muscle. In: Asmussen E, Jorgensen C, editors. Biomechanics VI-A. International Series on Biomechanics. 2B. Baltimore: University Park Press; 1978. pp. 27–39. [Google Scholar]
- Harris RC, Hultman E, Kaiser L, Nordesjo L-O. The effect of circulatory occlusion on isometric exercise capacity and energy metabolism of the quadriceps femoris muscle in man. Scand J Clin Lab Investig. 1975;35:87–95. [PubMed] [Google Scholar]
- Homsher E, Kean CJ. Skeletal muscle energetics and metabolism. Annu Rev Physiol. 1978;40:93–131. doi: 10.1146/annurev.ph.40.030178.000521. [DOI] [PubMed] [Google Scholar]
- Hultman E, Sjöstrom H. Energy metabolism and contraction force of human skeletal muscle in situ during electrical stimulation. J Physiol. 1983;345:525–532. doi: 10.1113/jphysiol.1983.sp014994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF. Muscle structure and theories of contraction. Progr Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley AF, Simmons RM. Rapid ‘give’ and the tension ‘shoulder’ in the relaxation of frog muscle fibres. J Physiol. 1970;210:32P. [PubMed] [Google Scholar]
- Jones DA, de Ruiter CJ, de Haan A. Change in contractile properties of human muscle in relationship to the loss of power and slowing of relaxation seen with fatigue. J Physiol. 2006;576:913–922. doi: 10.1113/jphysiol.2006.116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion AF, Jakeman PM, Dunnett PM, Harris RC, Willian PL. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Eur J Appl Physiol. 1992;64:47–50. doi: 10.1007/BF00376439. [DOI] [PubMed] [Google Scholar]
- Newham DJ, Jones DA, Turner DL, McIntyre D. The metabolic costs of different types of contractile activity of the human adductor pollicis muscle. J Physiol. 1995;488:815–820. doi: 10.1113/jphysiol.1995.sp021013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G, Lombardi V. A cross bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys J. 1995;68:1966–1979. doi: 10.1016/S0006-3495(95)80374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco P, McIntyre DB, Jones DA. Effects of length and stimulation frequency on fatigue of the human anterior tibialis muscle. J Appl Physiol. 1994;77:1148–1154. doi: 10.1152/jappl.1994.77.3.1148. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Alvestrand A, Bergström J, Hultman E. Intracellular pH and bicarbonate metabolism as determined in biopsy samples from the human quadriceps muscle of man at rest. Clin Sci Mol Med. 1977;53:459–466. doi: 10.1042/cs0530459. [DOI] [PubMed] [Google Scholar]
- Söderlund K, Greenhaff PL, Hultman E. Energy metabolism in type I and II human muscle fibres during short term electrical stimulation at high frequencies. Acta Physiol Scand. 1992;144:15–22. doi: 10.1111/j.1748-1716.1992.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Szentesi P, Zaremba R, van Mechelen W, Stienen GJM. ATP utilization for calcium uptake and force production in different types of human skeletal muscle fibres. J Physiol. 2001;531:393–403. doi: 10.1111/j.1469-7793.2001.0393i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol. 1993;468:729–740. doi: 10.1113/jphysiol.1993.sp019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J, Allen DG. Slowed relaxation in fatigued skeletal muscle fibers of Xenopus and mouse: contribution of [Ca2+]i and cross-bridges. J Gen Physiol. 1997;109:385–399. doi: 10.1085/jgp.109.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra HG, de Haan A, van Doorn JE, de Haan EJ. Anaerobic chemical changes and mechanical output during isometric tetani of rat skeletal muscle in situ. Pflügers Arch. 1988;412:121–127. doi: 10.1007/BF00583740. [DOI] [PubMed] [Google Scholar]