Abstract
Selenium-Binding Protein1 (SBP1) gene expression was studied in Arabidopsis (Arabidopsis thaliana) seedlings challenged with several stresses, including cadmium (Cd), selenium {selenate [Se(VI)] and selenite [Se(IV)]}, copper (Cu), zinc (Zn), and hydrogen peroxide (H2O2) using transgenic lines expressing the luciferase (LUC) reporter gene under the control of the SBP1 promoter. In roots and shoots of SBP1∷LUC lines, LUC activity increased in response to Cd, Se(VI), Cu, and H2O2 but not in response to Se(IV) or Zn. The pattern of expression of SBP1 was similar to that of PRH43, which encodes the 5′-Adenylylphosphosulfate Reductase2, a marker for the induction of the sulfur assimilation pathway, suggesting that an enhanced sulfur demand triggers SBP1 up-regulation. Correlated to these results, SBP1 promoter showed enhanced activity in response to sulfur starvation. The sulfur starvation induction of SBP1 was abolished by feeding the plants with glutathione (GSH) and was enhanced when seedlings were treated simultaneously with buthionine sulfoxide, which inhibits GSH synthesis, indicating that GSH level participates in the regulation of SBP1 expression. Changes in total GSH level were observed in seedlings challenged with Cd, Se(VI), and H2O2. Accordingly, cad2-1 seedlings, affected in GSH synthesis, were more sensitive than wild-type plants to these three stresses. Moreover, wild-type and cad2-1 seedlings overexpressing SBP1 showed a significant enhanced tolerance to Se(VI) and H2O2 in addition to the previously described resistance to Cd, highlighting that SBP1 expression decreases sensitivity to stress requiring GSH for tolerance. These results are discussed with regard to the potential regulation and function of SBP1 in plants.
The highly conserved sequences of selenium-binding proteins (SBPs) among diverse species and kingdoms suggest that SBPs share a fundamental biological role (Agalou et al., 2005). SBP1 was first characterized in mouse liver as a 75selenium (Se)-binding protein in experiments designed to identify selenoproteins (Bansal et al., 1990). In mammals, Se is an essential nutrient. It is incorporated in the selenoamino acid Se-Cys, which is required for the translation of several proteins involved in cell defense or hormone regulation (Behne and Kyriakopoulos, 2001; Papp et al., 2007), and can as well be bound by binding proteins such as SBP1 (Bansal et al., 1990). Although a physiological function has not yet been assigned to SBP1, its involvement in detoxification mechanisms is largely suggested. Down-regulation of SBP1 expression is correlated with rapid tumor development in many organs (Kim et al., 2006; Stammer et al., 2008), and recently, SBP1 was characterized as a biomarker for schizophrenia (Glatt et al., 2005). Moreover, its homolog SBP2 is suggested to play a protective role as a scavenger of toxic electrophiles or oxidant species (Lanfear et al., 1993; Cohen et al., 1997; Mattow et al., 2006). Other functions, such as intra-Golgi protein transport, have been assigned to mammalian SBP (Porat et al., 2000).
To date, Se has not been demonstrated to be essential in land plants, but a Se-containing glutathione (GSH) peroxidase has been isolated from Chlamydomonas reinhardtii (Fu et al., 2002). Except at low concentration, where it can have a positive effect on plant growth, Se is highly toxic. Se toxicity results from its chemical similarity with sulfur (S), leading to nonspecific replacement of S by Se in proteins and other S compounds (White et al., 2004; Sors et al., 2005). Se is taken up by roots from the soil mostly in inorganic forms {selenate [Se(VI)] or selenite [Se(IV)]} and then converted to organic forms that can accumulate in plant tissues or be volatilized in the atmosphere (Terry et al., 2000; Ellis and Salt, 2003). Mechanisms of Se tolerance in plants could be achieved by the conversion of selenoamino acids into their methylated forms, nonincorporable into proteins, or by compartmentalization into vacuoles in organic or inorganic forms (Lauchli, 1993; Nakamuro et al., 2000). SBP may participate in Se tolerance, as Arabidopsis (Arabidopsis thaliana) plants overexpressing SBP1 have increased resistance to Se(IV) (Agalou et al., 2005).
In a previous study, we showed that SBP1 protein accumulated in response to cadmium (Cd) in Arabidopsis cultured cells and plants (Sarry et al., 2006; Dutilleul et al., 2008). SBP1 overexpression led to enhanced tolerance to Cd in Arabidopsis, suggesting that SBP1 may represent a new detoxification mechanism that plants use to face heavy metal toxicity, possibly through direct binding to the metal (Dutilleul et al., 2008). Besides its involvement in response to metal and metalloid stresses, data suggest other functions. For example, in Lotus japonicus, SBP1 was reported to be involved in nodule formation and function (Flemetakis et al., 2002). In rice (Oryza sativa), overexpression of SBP1 led to enhanced tolerance against different pathogens (Sawada et al., 2004).
In the Arabidopsis genome, three SBP genes are present. SBP1 appeared to be the most expressed gene in healthy plants and in response to stress (Dutilleul et al., 2008). We showed that SBP1 was ubiquitously expressed in nonstressed conditions, notably in actively growing tissues and during seed development, highlighting the dynamic regulation of SBP1 expression during development as well (Dutilleul et al., 2008). To get a better understanding of SBP1 protein function in plants, the main goal of this article was to analyze SBP1 promoter activity in response to different stresses and to highlight signals that may regulate its expression. Expression analysis using luciferase (LUC) imaging showed that SBP1 and PRH43, which encodes 5′-Adenylylphosphosulfate Reductase2, a marker for the induction of the S assimilation pathway, were similarly regulated by the different stresses and highly induced in response to S starvation (−S). In addition, SBP1-overexpressing plants showed increased tolerance to stress affecting the GSH level. These results are discussed together with the potential function of SBP1 in plants.
RESULTS
Comparative Effects of Cd, Se, Copper, Zinc, and Hydrogen Peroxide on Arabidopsis Seedlings
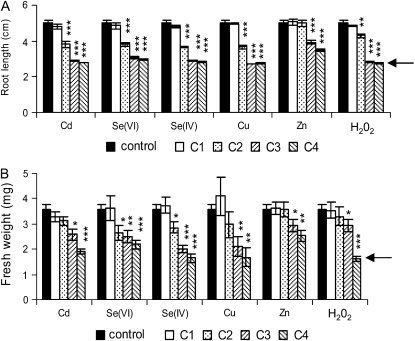
Before investigating SBP1 promoter activity in response to these different stresses, the toxicity of each treatment was monitored by measuring root elongation and fresh weight of Arabidopsis seedlings after 72 h of exposure (Fig. 1). Seedlings were challenged with different concentrations of Cd, Se, supplied as Se(VI) or Se(IV), zinc (Zn), copper (Cu), and hydrogen peroxide (H2O2). Root growth started to be significantly inhibited (P < 0.001) at 50 μm for Cd, Se(VI), Se(IV), and Cu, at 250 μm for Zn, and at 5 mm for H2O2 (Fig. 1A). Increased sensitivity was observed with increasing toxic compound concentrations (Fig. 1A) and was already noticed after 24 h of exposure (data not shown). The EC50, which represents for each toxic compound the concentration that reduces to 50% the growth compared with the control seedlings, was, in our experimental conditions, approximately 30, 50, 60, 90, and 300 μm for Cu, Se(IV), Cd, Se(VI), and Zn, respectively, and 8 mm for H2O2. Fresh weight was less affected than root growth (Fig. 1B), and as reported for root elongation, Zn showed less toxicity than the other treatments (Fig. 1B). Fresh weight started to be significantly inhibited at 250 μm for Cd (P < 0.01), at 50 μm for both Se(VI) and Se(IV) (P < 0.05), at 150 μm for Cu (P < 0.01), at 250 μm for Zn (P < 0.05), and at 10 mm for H2O2 (P < 0.05). The EC50 was approximately 90, 150, 180, 240, and 420 μm for Cu, Se(IV), Cd, Se(VI), and Zn, respectively, and 15 mm for H2O2. The toxicity of the different treatments, therefore, was Cu > Se(IV) > Cd > Se(VI) > Zn > H2O2. After 72 h of exposure, seedlings were still green at all concentrations (Supplemental Fig. S1), while death of the seedlings was clearly observed after 8 d for Cu (150 μm), Cd (250 μm), and Se(IV) (250 μm; data not shown).
Figure 1.
Toxicity of Cd, Se(VI), Se(IV), Cu, Zn, and H2O2 on Arabidopsis seedlings. Seven-day-old seedlings were transferred on different media, and the toxicity of each treatment was assayed by measuring root length (A) and fresh weight (B) after 72 h of exposure. C1, C2, C3, and C4 correspond to 5, 50, 250, and 500 μm for Cd, Se(VI), Se(IV), and Zn, to 5, 50, 150, and 250 μm for Cu, and to 1, 5, 10, and 40 mm for H2O2, respectively. The arrows indicate the root length and the fresh weight of the seedlings when the stresses were applied (2.7 ± 0.15 cm and 1.6 ± 0.1 mg, respectively). Data represent means ± se of at least three independent experiments. Each experiment was performed on at least eight seedlings. Asterisks indicate significant differences from seedlings grown in control conditions (* P < 0.05, ** P < 0.01, *** P < 0.001) evaluated by Student's t test.
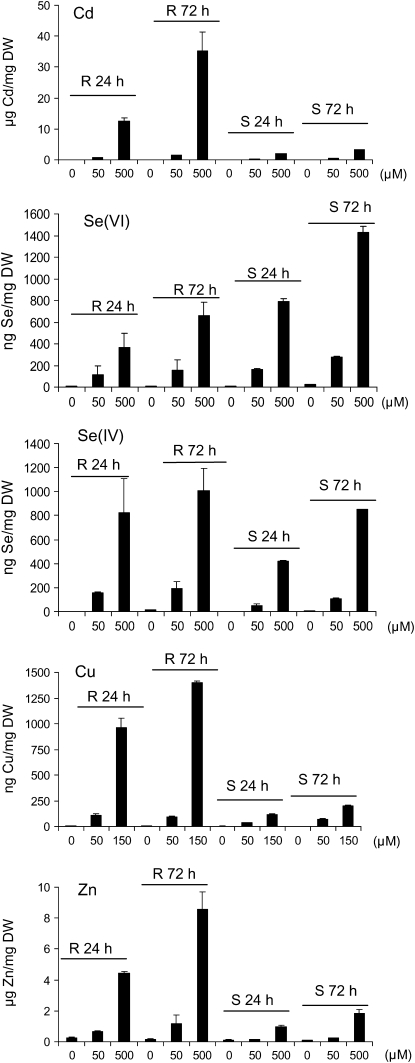
The level of accumulation of the different metals and metalloids was analyzed in roots and shoots of seedlings after 24 and 72 h of exposure by inductively coupled plasma mass spectrometry (ICP-MS; Fig. 2). For the five toxic compounds, the level of accumulation in roots and shoots was dose dependent and significantly increased at 72 h for the highest dose. Accumulation of Cd, Cu, and Zn per mg dry weight was higher in roots than in shoots (Fig. 2); in our growth conditions, the shoot-to-root ratio per seedling was 0.8, 1.2, and 1.8 for the highest dose of Cd, Cu, and Zn, respectively, at 72 h. In contrast, the accumulation of Se per mg dry weight in shoots was comparable to that in roots (Fig. 2), and the shoot-to-root ratio per seedling for Se(VI) and Se(IV) was 7.5 and 18, respectively, at 72 h. In addition, the level of accumulation of the different metals and metalloids per mg dry weight in the shoot was Cd ≥ Zn > Se(VI) > Se(IV) > Cu.
Figure 2.
Metal content in roots (R) and shoots (S) of Arabidopsis seedlings exposed to different concentrations of Cd, Se(VI), Se(IV), Cu, and Zn for 24 and 72 h. Seven-day-old wild-type seedlings grown vertically on half-strength Murashige and Skoog solid medium were transferred to different concentrations of toxic compound and sampled for metal/metalloid content by ICP-MS. Data show representative results from experiments performed in duplicate for roots and triplicate for shoots. A minimum of 60 seedlings were pooled per measurement. In control seedlings, Cu content was around 6 ng mg−1 dry weight (DW) in roots and 3 ng mg−1 dry weight in shoots; Zn content was around 0.2 μg mg−1 dry weight in roots and 0.1 μg mg−1 dry weight in shoots.
SBP1 Expression in Response to Cd, Se, Cu, Zn, and H2O2
Arabidopsis transgenic lines expressing the LUC reporter gene under the control of the SBP1 promoter (i.e. SBP1∷LUC seedlings; Dutilleul et al., 2008) were challenged in parallel with 35S∷LUC lines to study the effect of the different stresses on SBP1 promoter activity (Figs. 3 and 4).
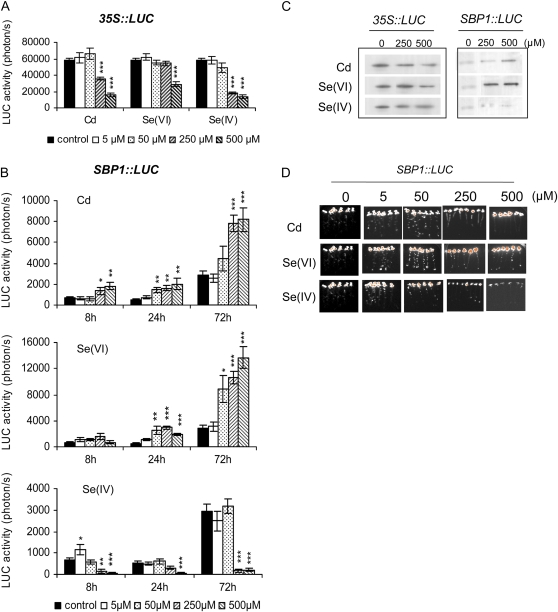
Figure 3.
Effects of Cd, Se(VI), and Se(IV) on LUC activity and accumulation in Arabidopsis 35S∷LUC and SBP1∷LUC seedlings. A and B, Seven-day-old 35S∷LUC and SBP1∷LUC seedlings were transferred on medium containing Cd, Se(VI), Se(IV), or no toxic compound (control) for 72 h. LUC activity was recorded in parallel on 35S∷LUC (A) and SBP1∷LUC (B) seedlings for 2 and 5 min, respectively, using a CCD camera after 72 h (A) and 8, 24, and 72 h (B). A typical experiment performed on eight seedlings is shown. Asterisks indicate significant differences from seedlings grown in control conditions (* P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001) evaluated by Student's t test. C, Western-blot analyses showing the accumulation of LUC protein after 72 h of exposure of SBP1∷LUC and 35S∷LUC seedlings to Cd, Se(VI), or Se(IV). Ten micrograms of protein was loaded per lane. D, LUC bioluminescence images recorded on SBP1∷LUC lines after 72 h of exposure to different concentrations of Cd, Se(VI), and Se(IV). Orange represents the highest bioluminescence intensity. Color images correspond to the seedlings shown in Supplemental Figure S1.
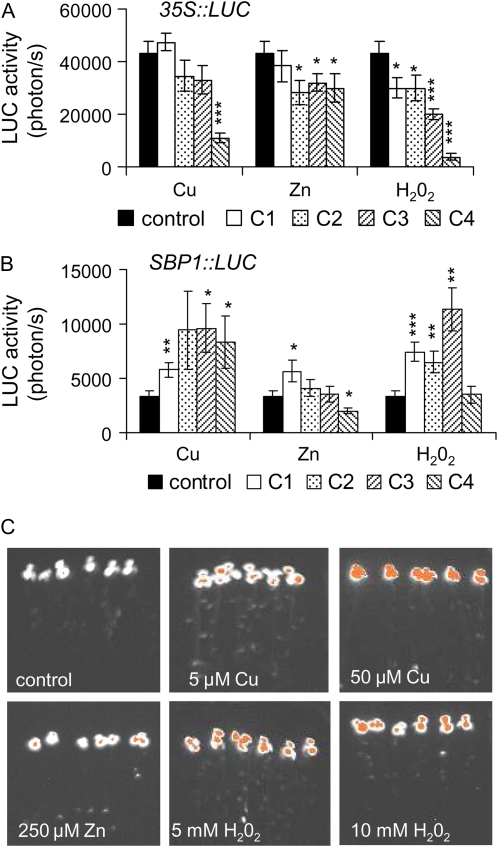
Figure 4.
Effects of Cu, Zn, and H2O2 on 35S∷LUC and SBP1∷LUC seedlings. A and B, Seven-day-old 35S∷LUC (A) and SBP1∷LUC (B) seedlings were transferred on medium containing Cu, Zn, H2O2, or no toxic compound (control) for 72 h. LUC activity was recorded in parallel on 35S∷LUC and SBP1∷LUC seedlings for 2 and 5 min, respectively, using a CCD camera after 72 h of exposure to the toxin. C1, C2, C3, and C4 correspond to 5, 50, 150, and 250 μm for Cu, to 5, 50, 250, and 500 μm for Zn, and to 1, 5, 10, and 40 mm for H2O2. A typical experiment performed on eight seedlings is shown. Asterisks indicate significant differences from seedlings grown in control conditions (* P < 0.05, ** P < 0.01, *** P < 0.001) evaluated by Student's t test. C, LUC bioluminescence images recorded on SBP1∷LUC seedlings after 72 h of exposure to Cu, Zn, or H2O2. Orange represents the highest bioluminescence intensity. Color images correspond to the seedlings shown in Supplemental Figure S1.
Globally, LUC activity in 35S∷LUC lines challenged with Cd, Se(VI), and Se(IV) for 72 h decreased in a dose-dependent manner (Fig. 3A) in correlation with the loss of fresh weight per seedling (Fig. 1B). In contrast to these results, a net increase in LUC activity was observed in SBP1∷LUC seedlings treated with Cd (2- to 4-fold at 250 and 500 μm) and Se(VI) (2- to 6-fold starting at 50 μm; Fig. 3B). Opposite to Se(VI), only a slight and transitory induction was observed at 8 h and 5 μm Se(IV), and LUC activity decreased similarly in both SBP1- and 35S∷LUC lines (Fig. 3B). Similar results were obtained at 48 h (data not shown).
Western-blot analyses using antibodies raised against LUC protein showed that at 72 h, the LUC protein accumulated in SBP1∷LUC lines in response to Cd and Se(VI) but not in response to Se(IV) (Fig. 3C), while no change or a slight decrease in LUC protein was observed in 35S∷LUC seedlings, demonstrating that even in conditions of high toxicity, LUC protein was stable (Fig. 3C). LUC bioluminescence recorded at 72 h on SBP1∷LUC lines showed that in roots, LUC activity was enhanced at 5 and 50 μm for Cd and Se(VI) treatments, while a slight induction was observed with Se(IV) at 5 μm (Fig. 3D).
Figure 4 shows LUC activity recorded on 35S∷LUC and SBP1∷LUC seedlings after 72 h of exposure to Cu, Zn, and H2O2. As reported in response to Cd and Se (Fig. 3A), Cu, Zn, and H2O2 triggered a decrease in LUC activity in 35S∷LUC lines (Fig. 4A) similar to the drop of fresh weight (Fig. 1B). However, the SBP1 promoter showed enhanced activity (≥2-fold) in response to Cu (starting at 5 μm) and H2O2 (starting at 1 mm), while Zn showed no marked effect (Fig. 4B). Similar results were obtained at 24 h, although with a lower level of induction by H2O2 (data not shown). LUC bioluminescence recorded on SBP1∷LUC lines showed a clear induction of SBP1 in roots at 5 μm Cu and 5 mm H2O2 at 72 h (Fig. 4C).
These data indicated that SBP1 promoter activity is highly enhanced in response to Cd, Cu, Se(VI), and H2O2 stresses but not in response to Se(IV) and Zn. The lack of responsiveness of SBP1 promoter to Se(IV) and Zn was not due to a lack of accumulation of the toxic compound in the tissues. Zn-treated seedlings (500 μm) showed a 20-fold increase in leaf Zn content at 72 h, and Se(IV) contents reached 850 ng mg−1 dry weight in Se(IV)-treated seedlings (500 μm) after 72 h (Fig. 2).
Western-blot analysis using antibodies raised against purified SBP1 showed that Cd, Se(VI), Cu, and H2O2 treatments also triggered SBP1 protein accumulation (Fig. 5). No accumulation was observed in response to Se(IV) or Zn.
Figure 5.
Effects of Cd, Se(VI), Se(IV), Cu, Zn, and H2O2 on SBP1 protein accumulation in wild-type seedlings. Seven-day-old wild-type seedlings were transferred on medium containing Cd, Se(VI), Se(IV), Cu, Zn, or H2O2 at the indicated concentrations (μm for Cd, Se, Cu, and Zn and mm for H2O2) or no toxic compound (control) for 72 h. Total proteins were extracted from seedlings, and western-blot analyses were performed on 10 μg of proteins using antibodies raised against recombinant SBP1 (Dutilleul et al., 2008).
Comparison Analysis of SBP1 Expression with Other Cd-Inducible Genes in Response to Cd, Se, Cu, Zn, and H2O2
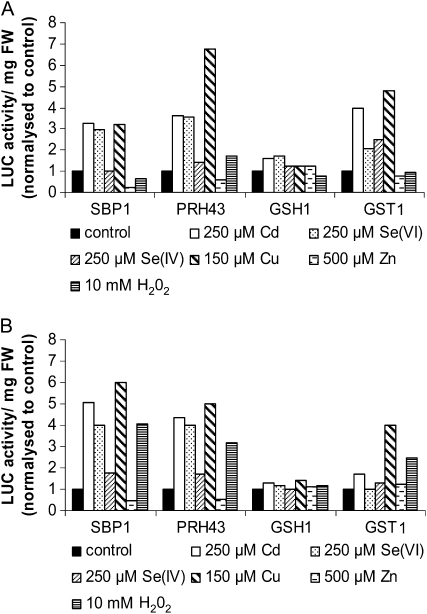
The pattern of expression of the SBP1 gene in response to the different stresses was compared with three other genes involved in Arabidopsis response to Cd stress: PRH43, also named APR2, which encodes 5′-Adenylylphosphosulfate Reductase2 involved in the S assimilation pathway; GSH1, the γ-glutamyl-cysteine synthetase involved in the first step of GSH synthesis; and GST1, a GSH-S-transferase implicated in detoxification mechanisms. The expression levels of these three genes were previously shown to be highly (PRH43 and GST1) and moderately (GSH1) induced by Cd (Herbette et al., 2006; Sarry et al., 2006). These lines were challenged by the different stresses at a single concentration and for two times of exposure (Fig. 6). As expected, LUC activity in SBP1, PRH43, and GST1∷LUC seedlings showed greater than 2-fold induction in response to Cd, while less induction was obtained in GSH1∷LUC seedlings (Fig. 6). Interestingly, LUC activity in SBP1 and PRH43∷LUC lines showed a very similar profile [e.g. induction by Cd, Se(VI), Cu, and H2O2, and no induction by Se(IV) and Zn]. In contrast, GST1 showed less induction by Se(VI) compared with SBP1 and a strong induction with Se(IV) at 24 h (Fig. 6). No significant regulation was observed for GSH1. PRH43 is suggested to be important for the flux control of sulfate reduction, and its transcripts are accumulating abundantly under −S conditions (Gutierrez-Marcos et al., 1996; Vauclare et al., 2002). These results suggest that stresses enhancing the S assimilation pathway are inducers of SBP1 expression.
Figure 6.
Comparative effects of Cd, Se(VI), Se(IV), Cu, Zn, and H2O2 on SBP1, PRH43, GSH1, and GST1 promoter activity using LUC imaging. Seven-day-old LUC seedlings were transferred on medium containing 250 μm Cd, 250 μm Se(VI), 250 μm Se(IV), 150 μm Cu, 500 μm Zn, 10 mm H2O2, or no toxic compound (control) for 72 h. LUC activity was recorded after 24 h (A) and 72 h (B) of treatment for 2 to 5 min using a CCD camera. To compare the effect of the different toxic compounds on the different LUC lines, LUC activity produced by a total of eight seedlings was recorded and expressed per mg fresh weight (FW). All of the lines showed different basal levels of LUC activity; therefore, LUC activity in treated conditions was normalized to control conditions, where the level of expression was set at 1. For each promoter construct, two independent lines were tested and showed similar results.
Effect of S Starvation and GSH Level on SBP1 Promoter Activity
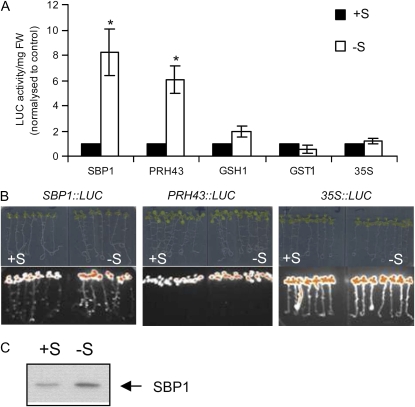
We investigated the impact of −S on SBP1∷LUC expression in parallel with PRH43∷LUC, GSH1∷LUC, GST1∷LUC, and 35S∷LUC lines. Seven-day-old seedlings grown on a complete medium were transferred on a complete or S-deprived medium and LUC activity was measured at 72 h (Fig. 7A). Both SBP1 and PRH43 promoter activities were highly induced in response to −S (Fig. 7, A and B), while no induction was observed for GSH1 and GST1. Similar results were observed at 24 h (data not shown). Western-blot analysis using antibodies raised against SBP1 showed that enhanced SBP1 promoter activity in −S conditions led to SBP1 protein accumulation (Fig. 7C). In response to −S, no significant drop of fresh weight was observed (data not shown), which correlated well with the stability of LUC activity recorded on 35S∷LUC lines at 72 h (Fig. 7A). The impact of the −S treatment on plant metabolism was studied by analyzing the tissue GSH content, which is considered as a S storage metabolite. −S provoked a significant drop of GSH of 20% to 40% at 72 h in the shoots, and this phenomenon was emphasized with time (data not shown).
Figure 7.
Effects of −S on SBP1 promoter activity. A, Comparative effects of −S on SBP1, PRH43, GSH1, GST1, and 35S promoter activity using LUC imaging at 72 h. To compare the effects of −S treatment on the different LUC lines, LUC activity produced by a total of eight seedlings was recorded and expressed per mg fresh weight (FW). All of the lines showed different basal levels of LUC activity; therefore, LUC activity in treated conditions was normalized to control conditions, where the level of expression was set at 1. Data show means of three independent experiments ± se. Asterisks indicate significant differences from seedlings grown in control conditions (* P < 0.05) evaluated by Student's t test. B, Images of the SBP1∷LUC, PRH43∷LUC, and 35S∷LUC seedlings treated in A and the corresponding bioluminescence images. The highest pixels are highlighted in orange. C, Western blot showing SBP1 protein accumulation in response to −S. Proteins were extracted from total seedlings exposed to −S treatment for 72 h, and western-blot analyses were performed on 10 μg of protein using antibodies raised against recombinant SBP1 (Dutilleul et al., 2008).
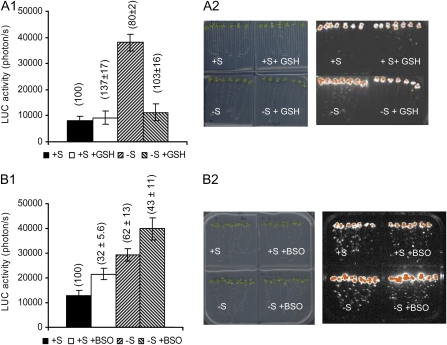
Detailed kinetic studies further showed a similar pattern of induction between SBP1 and PRH43 in response to −S (data not shown). We identified the presence of many copies of the GAGAC motif in the SBP1 promoter (at positions −68, −217, −549, and −1,038; the complementary sequence was identified at positions −400, −637, and −796), earlier identified as being a −S cis-regulatory response element (Maruyama-Nakashita et al., 2005), and a single copy in the PRH43 promoter identified at position – 23. The activity of promoters containing such motifs was proposed to be repressed by GSH (Maruyama-Nakashita et al., 2005). When GSH was added to the medium, no significant changes in LUC activity was observed in SBP1∷LUC lines (P = 0.7; Fig. 8A), and similar results were observed in 35S∷LUC lines (data not shown), indicating that in normal growth conditions the addition of GSH did not clearly impact SBP1 expression. However, when GSH was added in combination with a −S treatment, the −S induction of SBP1 (about 4-fold; P < 0.0001) was completely abolished (Fig. 8A), suggesting that a GSH drop is necessary for −S induction of SBP1. Adding the GSH synthesis inhibitor buthionine sulfoxide (BSO) triggered about 40% reduction of GSH level compared with control seedlings. The 35S∷LUC lines showed stable LUC activity per seedling, and no detectable change in fresh weight was noticed (data not shown). Adding BSO triggered a slight induction of SBP1 expression (1.5-fold; P < 0.05; Fig. 8B), indicating that a GSH drop alone is not sufficient to highly induce SBP1 expression. BSO in combination with a −S treatment triggered an increase in SBP1 promoter activity (P < 0.05) compared with both treatments alone (BSO or −S; Fig. 8B), suggesting that lowering GSH level in response to −S increases SBP1 expression. It is important to note that the results presented in Figure 8, A and B, correspond to two sets of independent experiments in which the LUC activity in response to −S was lower in Figure 8B than in Figure 8A. Together, these results suggested that in response to −S, GSH level participates in the regulation of SBP1 expression.
Figure 8.
Effects of modifying the GSH level on SBP1 promoter activity in response to −S. A1, LUC activity was recorded on SBP1∷LUC seedlings transferred on plates containing or not GSH (0.3 mm) in combination or not with a −S treatment at 72 h (A1). Data show a typical experiment performed on eight seedlings. GSH contents in the seedlings (expressed in percentage of the control) are given in parentheses. A2, Seedlings and the corresponding bioluminescence images at 72 h, with the highest bioluminescence intensity represented in orange. B1, LUC activity recorded on SBP1∷LUC seedlings transferred on plates containing or not BSO (0.1 mm) in combination or not with a −S treatment at 72 h. Data show a typical experiment performed on eight seedlings. GSH contents in the seedlings (expressed in percentage of the control) are given in parentheses. B2, Seedlings and the corresponding bioluminescence images at 72 h, with the highest bioluminescence intensity represented in orange.
GSH Level and Function in Response to Cd, Se, Cu, Zn, and H2O2
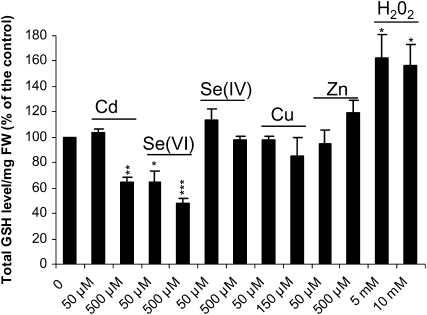
A possible link between enhanced S demand, GSH level, and SBP1 expression was suggested, so we investigated how GSH levels were affected by the different stresses. In response to 500 μm Cd and both 50 and 500 μm Se(VI), the shoot GSH content was significantly decreased after 24 h of stress exposure (Fig. 9). In contrast, a marked increase resulted from 5 and 10 mm H2O2 treatment, and no change was observed in response to Se(IV), Cu, and Zn at the two studied concentrations.
Figure 9.
Effects of Cd, Se(VI), Se(IV), Cu, Zn, and H2O2 on plant GSH content. Seven-day-old wild-type seedlings grown vertically on half-strength Murashige and Skoog solid medium were transferred onto different concentrations of toxic compounds and sampled for total GSH measurement after 24 h of exposure. Data show means of three replicates ± sd. Each replicate was performed on at least 100 seedlings. Experiments were reproduced twice and showed similar results. In the control seedlings, GSH level was 0.23 ± 0.01 nmol GSH mg−1 fresh weight (FW). Asterisks indicate significant differences from seedlings grown in control conditions (* P < 0.05, ** P < 0.01, *** P < 0.001) evaluated by Student's t test. The reduced form of GSH that represented 92% ± 4% of total GSH in control conditions was unchanged in the presence of 150 μm Cu and decreased to 74% ± 8%, 83% ± 6%, and 50% ± 4% in response to 500 μm Se(IV), 500 μm Zn, and 10 mm H2O2, respectively. The oxidized form of GSH was not detected in the presence of Cd and Se(VI) (500 μm).
Because the two forms of Se showed differential impacts on SBP1 expression (Fig. 3) and on GSH level (Fig. 9), we further performed a detailed kinetic study of changes in the GSH pool in Se(VI)- and Se(IV)-treated seedlings in comparison with Cd-treated seedlings (Supplemental Table S1). Short (2-h) Se(VI) exposure strongly reduced GSH content starting at 250 μm (reduction of 40% compared with control). In contrast, a slight decrease in GSH content (<15%) was obtained in the presence of Se(IV) that triggered GSH oxidation (Supplemental Table S1).
Because GSH plays an important role in stress tolerance, notably through detoxification (Howden et al., 1995a; Clemens, 2006; Rausch et al., 2007) and regulation of stress-related gene expression (Ball et al., 2004), the sensitivities of the wild type and the cad2-1 mutant that show reduced level of GSH (Howden et al., 1995a) were compared in response to the different stresses (Fig. 10; Supplemental Figs. S2 and S3). In the case of Cd, cad2-1 seedlings are more sensitive than wild-type plants because GSH is required for phytochelatin synthesis (Howden et al., 1995a, 1995b; Supplemental Fig. S2). Only a very slight enhanced sensitivity to Cu was observed in cad2-1 compared with wild-type plants (Supplemental Figs. S2 and S3), consistent with previous reports (Cobbett and Goldsbrough, 2002). The wild type and cad2-1 showed similar responses in the presence of Se(IV) and Zn (Supplemental Figs. S2 and S3). Interestingly, cad2-1 seedlings showed enhanced sensitivity to Se(VI) and H2O2 compared with wild-type plants (Fig. 10; Supplemental Fig. S2). For example, root growth was already twice more inhibited at 25 μm Se(VI) in cad2-1 than in the wild-type plants, and even stronger sensitivity was observed for higher concentrations (Supplemental Fig. S2). Fresh weight was about two times more affected in cad2-1 than in the wild type at all concentrations tested (Fig. 10; Supplemental Fig. S2). We checked that wild-type and cad2-1 seedlings accumulated similar amounts of Se and that cad1-3 seedlings that lack phytochelatin only (and not GSH) were not hypersensitive to Se(VI) (data not shown). Similarly, root growth was twice more inhibited at 0.25 mm H2O2 in the cad2-1 mutant than in the wild type (Fig. 10; Supplemental Fig. S2).
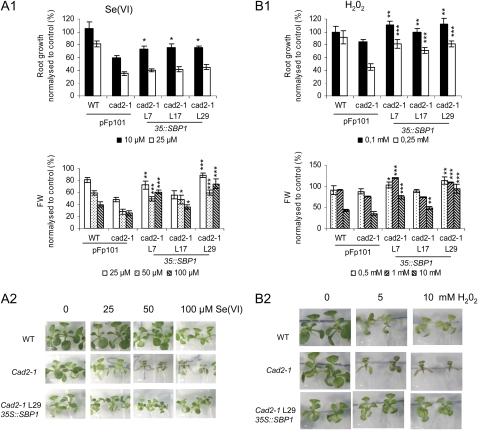
Figure 10.
Effects of SPB1 overexpression on Se(VI) and H2O2 sensitivity of cad2.1 seedlings. Four-day-old seedlings were transferred on medium containing either Se(VI) (A) or H2O2 (B) at the indicated concentrations. Toxicity of each treatment was investigated by measuring root growth and fresh weight (FW; A1 and B1) after 6 d of exposure. A typical experiment performed on 16 seedlings is shown. The wild type (WT) and cad2-1 were transformed with the empty vector (pFp101) for a control. L7, L17, and L29 correspond to three independent lines expressing the 35S∷SBP1 constructs in the cad2-1 background (Dutilleul et al., 2008). Wild-type and cad2-1 untransformed seedlings showed similar sensitivity to the different stresses as the wild type and cad2-1 transformed with the empty vector pFp101. A2 and B2 show the phenotypes of cad2-1 35S∷SBP1 L29 on Se(VI) and H2O2, respectively. Asterisks indicate significant differences between 35S∷SBP1 lines and the cad2-1 mutant (* P < 0.05, ** P < 0.01, *** P < 0.001) evaluated by Student's t test.
These results indicated that GSH is important for reducing sensitivity to Se(VI) and H2O2.
Consequences of Plant SBP1 Overexpression in Response to Se, Cu, Zn, and H2O2
To determine if SBP1 overexpression would impact plant sensitivity to Se, Cu, Zn, and H2O2 in addition to its earlier described function in response to Cd (Dutilleul et al., 2008), we used previously described Arabidopsis seedlings overexpressing SBP1 (Dutilleul et al., 2008). These lines were generated in the wild-type Columbia background and in the mutant cad2-1 that showed a reduced level of GSH (Howden et al., 1995a). For each stress, root growth and fresh weight were monitored in both backgrounds transformed with the empty vector (pFp101) and in two or three independent lines expressing 35S∷SBP1 in the wild-type or cad2-1 background, respectively (Fig. 10; Supplemental Figs. S3 and S4).
When challenged with the different stresses, apart from being more tolerant to Cd (Dutilleul et al., 2008), SBP1-overexpressing seedlings were markedly more resistant to Se(VI) and H2O2, and this was more obvious in the cad2-1 background (Fig. 10; Supplemental Figs. S3 and S4). SBP1 overexpression reduced root growth inhibition from 40% to 25% in the presence of 10 μm Se(VI) (P < 0.05; Fig. 10). For lower Se(VI) concentration, cad2-1 35S∷SBP1 line 29 showed no inhibition at all, although root growth of control plants was reduced by 20% (data not shown). In shoots, at 50 and 100 μm Se(VI), the three lines showed significantly reduced loss of fresh weight (P < 0.001 for lines 7 and 29, P < 0.05 for line 17) compared with cad2-1 pFp101. At these concentrations, the hypersensitivity of cad2-1 was restored back to wild-type sensitivity in the three independent cad2-1 35S∷SBP1 lines (Fig. 10A1). In roots at 0.1 mm H2O2, the three independent cad2-1 35S∷SBP1 lines showed no more growth inhibition compared with cad2-1 pFp101 (P < 0.01) and strongly increased root growth at 0.25 mm H2O2 (P < 0.001). The hypersensitivity of the cad2-1 seedlings was almost abolished by SBP1 overexpression up to 0.25 mm H2O2. Enhanced tolerance to H2O2 was as well observed up to 10 mm based on fresh weight (Fig. 10). The shoot phenotype of cad2-1 35S∷SBP1 line 29 is shown in response to Se(VI) (Fig. 10A1) and H2O2 (Fig. 10A2). Similar results were obtained in the wild-type background (Supplemental Fig. S4). The results obtained in response to Se(IV), Cu, and Zn were not homogeneous among the several independent SBP1-overexpressing lines but indicated a slightly enhanced sensitivity to Se(IV) and Cu and a slightly enhanced tolerance to Zn (Supplemental Fig. S3). Agalou et al. (2005) reported that wild-type 35S∷SBP1 lines showed enhanced tolerance to Se(IV). The discrepancy with our results may come from the time of exposure to the toxic compound, which was more than 3 weeks in their experiment and up to 6 d in ours. It is interesting that wild-type and cad2-1 seedlings showed similar loss of fresh weight in response to −S and that accumulation of SBP1 did not reduce this phenotype in cad2-1 35S∷SBP1 lines (data not shown). These results indicated that SBP1 accumulation enhanced tolerance to Se(VI) and H2O2 that both required GSH for tolerance.
DISCUSSION
The physiological function of SBP in mammals and plants has not been established. Arabidopsis SBP1 protein accumulates in response to Cd stress and potentially represents a novel factor for metal detoxification (Sarry et al., 2006; Dutilleul et al., 2008). To better understand the possible function(s) of SBP1 in plants, this work evaluates the different stresses that regulate SBP1 expression and identifies some of the signals that participate in the regulation of its expression. A relevant purpose was also to determine the impact of the two predominant environmental forms of Se available to plants, Se(VI) and Se(IV). To this aim, we investigated SBP1 promoter activity using the LUC reporter gene that shows many advantages compared with GUS and GFP: the measurement process is not destructive, and the high turnover of the protein allows time-course studies (Welsh et al., 2005; Southern et al., 2006). In addition, Arabidopsis lines overexpressing SBP1 were tested for their sensitivity in response to the different stresses.
GSH-Enhanced Demand Is Probably One of the Elements Triggering SBP1 Up-Regulation
Many stresses, but not all, were inducers of SBP1 expression and triggered SBP1 protein accumulation. Cd, Se when provided as Se(VI), Cu, and H2O2 activated SBP1 expression, while Se provided as Se(IV) and Zn had no effect. The lack of responsiveness of the SBP1 promoter to Se(IV) and Zn was not due to a lack of toxicity of the treatment or the lack of accumulation of the compounds in tissues. The specific induction pattern of SBP1 similar to PRH43, a gene encoding an enzyme involved in the S assimilation pathway, led us to hypothesize that stresses provoking an increase in S demand were inducers of SBP1. This hypothesis was reinforced by the fact that SBP1 expression was highly induced in response to −S treatment. Correlated with this point, we identified the presence of several copies of the GAGAC motif, a −S response element (Maruyama-Nakashita et al., 2005), in the SBP1 promoter. This element was proposed to be in part responsible for the −S induction of several genes, notably SULTR1;1, encoding a high-affinity sulfate transporter (Maruyama-Nakashita et al., 2005). The expression of sulfate transporters and enzymes of the S assimilation pathway is regulated by signals reflecting the nutritional status of the plant. Among them, GSH has been suggested to play a role.
GSH main functions include the regulation of cell redox state, storage and transport of reduced S in plants (Noctor and Foyer, 1998a), and detoxification mechanisms (Howden et al., 1995a, 1995b; Clemens, 2006; Rausch et al., 2007). More recently, a crucial role for GSH in disease resistance was suggested (Parisy et al., 2007; Schlaeppi et al., 2008). GSH is also involved in controlling stress-related gene expression (Ball et al., 2004; Maruyama-Nakashita et al., 2005). In addition, GSH level regulates the sulfate assimilation pathway, notably by controlling the uptake and expression of enzymes involved in the first assimilation steps (Lee and Leustek, 1999; Vauclare et al., 2002). In response to −S, SBP1 up-regulation was abolished by feeding the plants with GSH and enhanced by reducing GSH level with BSO treatment, highlighting a role for the nutritional status of the plant and GSH level in regulating SBP1 expression. However, adding GSH or BSO alone did not clearly impact SBP1 expression. Correlated to these results, no change in SBP1 transcript accumulation was observed in the mutant cad2-1 (data not shown), which shows 10% of total GSH compared with wild-type plants (Howden et al., 1995a; Ball et al., 2004). This suggested that the level of GSH alone is not sufficient to regulate SBP1 expression and that additional signals are required.
Among the stresses that induced SBP1 expression, the level of GSH evaluated after 24 h of exposure was affected in response to Cd, Se(VI), and H2O2. SBP1 induction in response to Cd and Se(VI) could be triggered by the observed GSH drop, together with additional signal as mentioned above. In response to H2O2, the GSH level increased as reported previously (May and Leaver, 1993; Xiang and Oliver, 1998). This could suggest that the induction of SBP1 would involve additional signaling pathways probably independent of the GAGAC motif. The slim1 mutant, recently characterized, showed impairment in the expression of several −S-responsive genes (Maruyama-Nakashita et al., 2006) that would not necessarily contain the GAGAC motif in their promoters. In addition to GSH, O-acetylserine was also proposed to act as a regulator of the S pathway (Lee and Leustek, 1999; Koprivova et al., 2000; Hirai et al., 2003). O-Acetylserine did not activate SBP1 promoter activity in shoots (data not shown) and several other promoters containing the GAGAC motif (Maruyama-Nakashita et al., 2005). In addition, the signaling pathway that acts on SBP1 gene expression through the GAGAC motif may be activated by another “enhanced S demand signal” different from GSH. We cannot exclude that an earlier GSH drop may happen and be the primary signal. The enhanced S demand observed in response to Cd, Se(VI), and H2O2 was well correlated with the changes observed in GSH level in response to these three stresses and might be explained by the use of GSH in tolerance (see below). As described by Rausch et al. (2007) and the references therein, any increase in the GSH-based tolerance mechanism could at least transiently decrease the cytosolic GSH content and impinge on the cellular GSH/oxidized GSH redox potential. Therefore, in addition to a GSH drop, the redox poise of the cell may be involved in SBP1 expression. In the case of Cu, changes in GSH level for phytochelatin production, which are known to be produced in vivo (Cobbett and Goldsbrough, 2002), may happen but not be detected at the whole plant level, because the production of GSH satisfies the demand in this case.
Differential Impact of Se(VI) and Se(IV) on SBP1 Expression, Plant GSH Content, and cad2-1 Sensitivity
Se(VI) and Se(IV) have differential impacts on the expression of SBP1 first but also on the different genes analyzed in this study, providing more information about the cell response to Se. Both forms of Se are metabolized by plants (Terry et al., 2000; Sors et al., 2005; Li et al., 2008). Se is assimilated through the S assimilation pathway, and Se(VI) enters the plant cells using the high-affinity sulfate transporters while Se(IV) competes with phosphate uptake. Reduction of Se(VI) to Se(IV), involving ATP sulfurylase and APS reductase, is suggested to be a rate-limiting step in plants, explaining why Se(VI) is less readily metabolized than Se(IV). Se(IV) can be reduced nonenzymatically via GSH and is more readily converted into selenoamino acid than Se(VI). Recently published data showed that both Se(VI) and Se(IV) induced the expression of genes related to S metabolism in Arabidopsis after 1 week of treatment (Tamaoki et al., 2008; Van Hoewyk et al., 2008). In our study, the activation of PRH43 (encoding an APS reductase isoform) promoter was observed with Se(VI) but not Se(IV), demonstrating that at least some enzymes of the S assimilation pathway are more specifically induced in the early response to Se(VI). Moreover, a detailed kinetic study of changes in the GSH pool in Se(VI)- and Se(IV)-treated seedlings showed that very early Se(VI) triggered a GSH drop (Supplemental Table S1) that correlated with a recent report (Van Hoewyk et al., 2008). Surprisingly, in response to Se(VI), cellular sulfate content increased compared with control conditions (White et al., 2004; Van Hoewyk et al., 2008). In addition, the level of GSH dropped earlier in response to Se(VI) than in response to −S (data not shown), indicating that this drop was not due to a sulfate starvation phenotype driven by Se(VI). This raises a question: why does the GSH level drop in response to Se(VI)? Van Hoewyk et al. (2008) proposed that the GSH level would decrease in an effort to conserve more important primary S compounds such as S-containing proteins. We observed a strong sensitivity of cad2-1 to Se(VI) compared with wild-type seedlings, and they both showed similar loss of fresh weight in response to −S (data not shown), suggesting that GSH is important in tolerance to Se(VI), although the underlying mechanism is not understood. On the contrary, very early, Se(IV) triggered GSH oxidation (Supplemental Table S1), indicating that Se(IV) triggers oxidative stress. This is in good agreement with our result showing that Se(IV) but not Se(VI) induced GST expression, whose role is linked to cell defense, and correlated well with recently published microarray analysis in response to Se(IV) (Poggi et al., 2008). As mentioned above, the induction of SBP1 with Se(VI) but not Se(IV) could reflect their differential impact on GSH level.
Hypothesis Regarding the Cellular Function of SBP1
The above results strongly suggested that SBP1 expression is tightly linked to S metabolism and that SBP1 would be required for adaptation to a S-starved environment and/or an internal S demand. When challenged by the different stresses, the previously described sbp1 null mutant showed similar growth inhibition when compared with wild-type plants (data not shown), as was reported for Cd (Dutilleul et al., 2008). This lack of phenotype was explained by the overexpression of one of the SBP1 homologs, the SBP2 gene, in sbp1 (Dutilleul et al., 2008). However, Arabidopsis seedlings overexpressing SBP1 clearly showed enhanced tolerance to Se(VI) and H2O2, in addition to the previously described function in Cd response (Dutilleul et al., 2008). It is important to note that SBP1 overexpression in cad2-1 seedlings did not affect GSH content (data not shown). Interestingly, a common point was observed between Se(VI) and H2O2: strong reduction of GSH level enhanced sensitivity of the plants. These results indicated that SBP1 and GSH might share similar functions, and this was well correlated with the proposed enhancement of SBP1 expression in response to cellular S and GSH demand. In mammals, GSH is used in the major acetaminophen-detoxification process, and hSBP2, the human homolog of SBP1, becomes the target of this drug when GSH level becomes too low (Cohen et al., 1997; Mattow et al., 2006). In this context, the authors proposed that hSBP2 protein may play a protective role by sequestering and/or inactivating reactive electrophile or by serving as an intracellular sensor of such electrophiles (Cohen et al., 1997). The fact that SBP1 overexpression clearly enhanced tolerance in wild-type and cad2-1 seedlings in response to H2O2 is in favor of SBP1 having antioxidant properties, although the underlying mechanisms remain to be identified. Similarly, why SBP1 overexpression triggered enhanced tolerance to Se(VI) and why cad2-1 shows enhanced sensitivity compared with wild-type plants still need to be elucidated. Dutilleul et al. (2008) reported the ability of SBP1 to bind Cd, highlighting that some additional functions of SBP1 may be related to metal homeostasis. In this work, wild-type and cad2-1 35S∷SBP1 showed slightly enhanced tolerance to Zn and reduced tolerance to Cu and Se(IV), which could reflect the ability of SBP1 to interact with these compounds.
CONCLUSION
In Arabidopsis, three SBP genes (SBP1–SBP3) are present. The SBP1 coding sequence shares 85% and 69% identity with SBP2 and SBP3, respectively. The −S-responsive element, the GAGAC motif, is present in the promoter region of all SBPs (data not shown). In the sbp1 mutant, SBP2 overexpression prevented the discovery of any visible phenotype. Recently, the new technique of artificial microRNA was shown to be very effective at silencing gene families (Schwab et al., 2006; Ossowski et al., 2008). We believe that such an approach, together with promoter mutation analysis, will provide better understanding of SBP1 regulation and function in plants.
MATERIALS AND METHODS
Plant Material
All experiments were performed using Arabidopsis (Arabidopsis thaliana) wild type in the Columbia background. Transgenic lines overexpressing the LUC gene under the control of SBP1, PRH43, GST1, GSH1, or the 35S promoter (PROMO∷LUC lines) were generated as described below in “Promoter Cloning and Plasmid Constructions for Expression in Plants.” T-DNA insertion lines in SBP1 (N647322) were obtained at the Salk Institute from the Nottingham Arabidopsis Stock Center and were described previously (Dutilleul et al., 2008). Arabidopsis cad2.1 mutants, affected in GSH synthesis (Howden et al., 1995a), were kindly provided by C. Cobbett (University of Melbourne). Wild-type and cad2-1 seedlings overexpressing SBP1 were previously described (Dutilleul et al., 2008).
Plant Growth Conditions and Stress Application
Arabidopsis seeds were sterilized, stratified for 4 d at 4°C, and sown on basic half-strength Murashige and Skoog medium (M0404; Sigma) supplemented with 5g L−1 Suc, 0.5 g L−1 MES (pH 5.7), and 8 g L−1 agar type A. Plates were then placed in a controlled-environment growth chamber, in long-day conditions (16 h), at 56% humidity and 21°C (day) or 20°C (night). Irradiance was set at 120 μE m−2 s−1. Plants were grown vertically to allow LUC imaging to be performed on both roots and shoots. Seven-day-old seedlings were transferred on half-strength Murashige and Skoog medium with or without Cd (CdNO3), Se(VI) (Na2SeO4), Se(IV) (Na2SeO3), Cu (CuSO4), Zn (ZnSO4), H2O2, and BSO (Sigma). Root length and fresh weight were measured as indicators of toxicity after 24 and 72 h of exposure. For the −S experiment, the Murashige and Skoog medium was prepared manually. MgSO4, ZnSO4, MnSO4, and FeSO4 were replaced by MgCl2, ZnCl2, MnCl2, and FeCl3, respectively. Seedlings were grown for 7 d on 500 μm S-containing medium and then transferred on 5 μm S-containing medium (−S). To compare the sensitivity of wild-type and cad2-1 seedlings untransformed or transformed with the empty vector pFp101 or expressing the 35S∷SBP1 construct, in response to different stresses, 4-d-old seedlings were transferred on different concentrations of the toxic compound and root growth and fresh weight were measured after 6 d of exposure.
Promoter Cloning and Plasmid Construction for Expression in Plants
SBP1 (At4g14030), PRH43 (At1g62180), GST1 (At1g02930), and GSH1 (At4g23100) promoter regions were amplified using specific primers designed with BamHI restriction sites at the end, from genomic DNA isolated from Arabidopsis ecotype Columbia (for primer sequences, gene function, and Munich Information Center for Protein Sequences codes, see Supplemental Table S2). PCR was performed using Pfu polymerase for 28 cycles, and the PCR product was cloned into the pGEM-T Easy vector (Promega). Cloned promoter sequences were checked (Genome Express). A BamHI fragment containing the cauliflower mosaic virus 35S promoter was isolated from the 35S∷GFP vector (Kost et al., 1998). BamHI DNA fragments containing SBP1, PRH43, GST1, GSH1, or 35S promoters were introduced into the pATM-Domega plasmid, kindly provided by Andrew Millar (Department of Biological Science, University of Warwick), which contains the LUC reporter gene (Welsh et al., 2005). The resulting SmaI cassettes containing promoter sequence, LUC gene, and terminator sequence were further cloned into pFP100 vector (Bensmihen et al., 2004) to allow GFP selection of Arabidopsis transformants. Resulting expression vectors (promoter∷LUC constructs) were introduced in the Agrobacterium tumefaciens C58 strain by electroporation. Arabidopsis flowers were then transformed following the protocol described by Clough and Bent (1998). For each promoter∷LUC construct, a minimum of three independent lines were generated and analyzed.
LUC Imaging
LUC imaging was performed as described by Welsh et al. (2005) using a CCD camera (Princeton Instrument) linked to a dark box. Data were analyzed using Metavue software (Bio-Rad). Plants were sprayed with a fresh solution of 1 mm luciferin (Promega) in Triton X-100 (0.02%, v/v) and kept for 5 min in the dark chamber before recording luminescence. LUC activity was then recorded for 2 to 10 min. For each experiment, a minimum of eight seedlings per line were analyzed for each condition. More than three independent experiments were performed per treatment.
Protein Extraction and Western-Blot Analysis
Proteins were extracted from Arabidopsis tissues in 100 mm Tris buffer (pH 7.5) supplemented with an anti-protease cocktail (Roche). After centrifugation, protein concentration in the supernatant was determined using the Bio-Rad protein assay reagent. Five to 10 μg of total soluble proteins was separated on a 10% to 12% acrylamide gel and transferred to a nitrocellulose membrane. Western-blot analyses were performed using monoclonal anti-LUC antibody (Sigma) and polyclonal antibodies raised against His-tagged SBP1 protein (Dutilleul et al., 2008) further purified by affinity using GST-tagged SBP1 at a 1:5,000 dilution.
Cd, Se, Cu, and Zn Measurements in Plant Tissues
For Cd, Zn, and Cu measurements, shoots and roots of treated and untreated plants were dried for 3 d at 50°C and mineralized in 3 mL of 65% HNO3 (Suprapur; Merck) and 1 mL of 30% HCl (Suprapur; Merck) for 3 h at 85°C. After complete evaporation of the mixture, residual material was dissolved in 1% HNO3. For Se measurements, mineralization was performed in closed containers to avoid evaporation. Metal(loid) concentrations in the extract were then determined using by ICP-MS (HP4500 ChemStation ICP-MS device; Yokogawa Analytical Systems).
GSH Content Measurements
GSH contents were measured using the enzymatic recycling assay, which involves the NADPH-driven GSH-dependent reduction of 5,5-dithiobis 2-nitrobenzoic acid at 412 nm as described (Noctor and Foyer, 1998b), and by HPLC analysis using a method adapted from Kreft et al. (2003) as described by Loizeau et al. (2008).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phenotypes of Arabidopsis seedlings after 72 h of exposure to Cd, Se [Se(VI) or Se(IV)], Cu, Zn, and H2O2
Supplemental Figure S2. Comparative toxicity of Cd, Se [Se(VI) or Se(IV)], Cu, Zn, and H2O2 on wild-type and cad2.1 seedlings.
Supplemental Figure S3. Comparative toxicity of Se(IV), Cu, and Zn on Arabidopsis wild-type, cad2.1, and cad2-1 35S∷SBP1 lines.
Supplemental Figure S4. Comparative toxicity of Se(VI) and H2O2 on Arabidopsis wild-type and 35S∷SPB1 lines.
Supplemental Table S1. GSH contents and redox states in Arabidopsis seedlings exposed to Cd, Se(VI), and Se(IV).
Supplemental Table S2. List of primers used for promoter cloning in the different LUC lines
Supplementary Material
Acknowledgments
We thank François Parcy (CEA) for advice, the editor and reviewers for their helpful comments on the manuscript, Enrique De La Cruz (Yale University) for careful reading of the manuscript, and Yec'han Laizet (Josef Fourier University) and Stephane Ravanel (CEA) for technical assistance performing LUC imaging and HPLC measurements, respectively. We are also grateful to François Tardif (CEA) for providing the ICP-MS facilities.
This work was supported by the “Programme Inter-Organismes CEA CNRS INRA INSERM de Toxicologie Nucléaire Environmentale.”
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Véronique Hugouvieux (veronique.hugouvieux@cea.fr).
The online version of this article contains Web-only data.
References
- Agalou A, Roussis A, Spaink HP (2005) The Arabidopsis selenium-binding protein confers tolerance to toxic levels of selenium. Funct Plant Biol 32: 881–890 [DOI] [PubMed] [Google Scholar]
- Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia-Carranza J, Reynolds H, et al (2004) Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16: 2448–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal MP, Mukhopadhyay T, Scott J, Cook RG, Mukhopadhyay R, Medina D (1990) DNA sequencing of a mouse liver protein that binds selenium: implications for selenium's mechanism of action in cancer prevention. Carcinogenesis 11: 2071–2073 [DOI] [PubMed] [Google Scholar]
- Behne D, Kyriakopoulos A (2001) Mammalian selenium-containing proteins. Annu Rev Nutr 21: 453–473 [DOI] [PubMed] [Google Scholar]
- Bensmihen S, To A, Lambert G, Kroj T, Giraudat J, Parcy F (2004) Analysis of an activated ABI5 allele using a new selection method for transgenic Arabidopsis seeds. FEBS Lett 561: 127–131 [DOI] [PubMed] [Google Scholar]
- Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88: 1707–1719 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA (1997) Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol 143: 1–12 [DOI] [PubMed] [Google Scholar]
- Dutilleul C, Jourdain A, Bourguignon J, Hugouvieux V (2008) The Arabidopsis putative selenium-binding protein family: expression study and characterization of SBP1 as a potential new player in cadmium detoxification processes. Plant Physiol 147: 239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DR, Salt DE (2003) Plants, selenium and human health. Curr Opin Plant Biol 6: 273–279 [DOI] [PubMed] [Google Scholar]
- Flemetakis E, Agalou A, Kavroulakis N, Dimou M, Martsikovskaya A, Slater A, Spaink HP, Roussis A, Katinakis P (2002) Lotus japonicus gene Ljsbp is highly conserved among plants and animals and encodes a homologue to the mammalian selenium-binding proteins. Mol Plant Microbe Interact 15: 313–322 [DOI] [PubMed] [Google Scholar]
- Fu LH, Wang XF, Eyal Y, She YM, Donald LJ, Standing KG, Ben-Hayyim G (2002) A selenoprotein in the plant kingdom: mass spectrometry confirms that an opal codon (UGA) encodes selenocysteine in Chlamydomonas reinhardtii gluththione peroxidase. J Biol Chem 277: 25983–25991 [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Everall IP, Kremen WS, Corbeil J, Sasik R, Khanlou N, Han M, Liew CC, Tsuang MT (2005) Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc Natl Acad Sci USA 102: 15533–15538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Marcos JF, Roberts MA, Campbell EI, Wray JL (1996) Three members of a novel small gene-family from Arabidopsis thaliana able to complement functionally an Escherichia coli mutant defective in PAPS reductase activity encode proteins with a thioredoxin-like domain and “APS reductase” activity. Proc Natl Acad Sci USA 93: 13377–13382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbette S, Taconnat L, Hugouvieux V, Piette L, Magniette ML, Cuine S, Auroy P, Richaud P, Forestier C, Bourguignon J, et al (2006) Genome-wide transcriptome profiling of the early cadmium response of Arabidopsis roots and shoots. Biochimie 88: 1751–1765 [DOI] [PubMed] [Google Scholar]
- Hirai MY, Fujiwara T, Awazuhara M, Kimura T, Noji M, Saito K (2003) Global expression profiling of sulfur-starved Arabidopsis by DNA macroarray reveals the role of O-acetyl-l-serine as a general regulator of gene expression in response to sulfur nutrition. Plant J 33: 651–663 [DOI] [PubMed] [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS (1995. a) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Andersen CR, Cobbett CS (1995. b) Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol 107: 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kang HJ, You KT, Kim SH, Lee KY, Kim TI, Kim C, Song SY, Kim HJ, Lee C (2006) Suppression of human selenium-binding protein 1 is a late event in colorectal carcinogenesis and is associated with poor survival. Proteomics 6: 3466–3476 [DOI] [PubMed] [Google Scholar]
- Koprivova A, Suter M, den Camp RO, Brunold C, Kopriva S (2000) Regulation of sulfate assimilation by nitrogen in Arabidopsis. Plant Physiol 122: 737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua NH (1998) A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J 16: 393–401 [DOI] [PubMed] [Google Scholar]
- Kreft O, Hoefgen R, Hesse H (2003) Functional analysis of cystathionine gamma-synthase in genetically engineered potato plants. Plant Physiol 131: 1843–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear J, Fleming J, Walker M, Harrison P (1993) Different patterns of regulation of the genes encoding the closely related 56-kDa selenium-binding and acetaminophen-binding proteins in normal tissues and during carcinogenesis. Carcinogenesis 14: 335–340 [DOI] [PubMed] [Google Scholar]
- Lauchli A (1993) Selenium in plants: uptake, functions, and environmental toxicity. Bot Acta 106: 455–468 [Google Scholar]
- Lee SM, Leustek T (1999) The affect of cadmium on sulfate assimilation enzymes in Brassica juncea. Plant Sci 141: 201–207 [Google Scholar]
- Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178: 92–102 [DOI] [PubMed] [Google Scholar]
- Loizeau K, De Brouwer V, Gambonnet B, Yu A, Renou JP, Van Der Straeten D, Lambert WE, Rebeille F, Ravanel S (2008) A genome-wide and metabolic analysis determined the adaptive response of Arabidopsis cells to folate depletion induced by methotrexate. Plant Physiol 145: 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Nakamura Y, Tohge T, Saito K, Takahashi H (2006) Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell 18: 3235–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Nakamura Y, Watanabe-Takahashi A, Inoue E, Yamaya T, Takahashi H (2005) Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsis roots. Plant J 42: 305–314 [DOI] [PubMed] [Google Scholar]
- Mattow J, Demuth I, Haeselbarth G, Jungblut PR, Klose J (2006) Selenium-binding protein 2, the major hepatic target for acetaminophen, shows sex differences in protein abundance. Electrophoresis 27: 1683–1691 [DOI] [PubMed] [Google Scholar]
- May MJ, Leaver CJ (1993) Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol 103: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamuro K, Okuno T, Hasegawa T (2000) Metabolism of selenoamino acids and contribution of selenium methylation to their toxicity. J Health Sci 46: 418–421 [Google Scholar]
- Noctor G, Foyer CH (1998. a) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998. b) Simultaneous measurement of foliar glutathione, gamma-glutamylcysteine, and amino acids by high-performance liquid chromatography: comparison with two other assay methods for glutathione. Anal Biochem 264: 98–110 [DOI] [PubMed] [Google Scholar]
- Ossowski S, Schwab R, Weigel D (2008) Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J 53: 674–690 [DOI] [PubMed] [Google Scholar]
- Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9: 775–806 [DOI] [PubMed] [Google Scholar]
- Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F (2007) Identification of PAD2 as a gamma-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J 49: 159–172 [DOI] [PubMed] [Google Scholar]
- Poggi V, Del Vescovo V, Di Sanza C, Negri R, Hochkoeppler A (2008) Selenite transiently represses transcription of photosynthesis-related genes in potato leaves. Photosynth Res 95: 63–71 [DOI] [PubMed] [Google Scholar]
- Porat A, Sagiv Y, Elazar Z (2000) A 56-kDa selenium-binding protein participates in intra-Golgi protein transport. J Biol Chem 275: 14457–14465 [DOI] [PubMed] [Google Scholar]
- Rausch T, Gromes R, Liedschulte V, Muller I, Bogs J, Galovic V, Wachter A (2007) Novel insight into the regulation of GSH biosynthesis in higher plants. Plant Biol (Stuttg) 9: 565–572 [DOI] [PubMed] [Google Scholar]
- Sarry JE, Kuhn L, Ducruix C, Lafaye A, Junot C, Hugouvieux V, Jourdain A, Bastien O, Fievet JB, Vailhen D, et al (2006) The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics 6: 2180–2198 [DOI] [PubMed] [Google Scholar]
- Sawada K, Hasegawa M, Tokuda L, Kameyama J, Kodama O, Kohchi T, Yoshida K, Shinmyo A (2004) Enhanced resistance to blast fungus and bacterial blight in transgenic rice constitutively expressing OsSBP, a rice homologue of mammalian selenium-binding proteins. Biosci Biotechnol Biochem 68: 873–880 [DOI] [PubMed] [Google Scholar]
- Schlaeppi K, Bodenhausen N, Buchala A, Mauch F, Reymond P (2008) The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J 55: 774–786 [DOI] [PubMed] [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D (2006) Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86: 373–389 [DOI] [PubMed] [Google Scholar]
- Southern MM, Brown PE, Hall A (2006) Luciferases as reporter genes. Methods Mol Biol 323: 293–305 [DOI] [PubMed] [Google Scholar]
- Stammer K, Edassery SL, Barua A, Bitterman P, Bahr JM, Hales DB, Luborsky JL (2008) Selenium-binding protein 1 expression in ovaries and ovarian tumors in the laying hen, a spontaneous model of human ovarian cancer. Gynecol Oncol 109: 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki M, Freeman JL, Pilon-Smits EA (2008) Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis. Plant Physiol 146: 1219–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N, Zayed AM, De Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51: 401–432 [DOI] [PubMed] [Google Scholar]
- Van Hoewyk D, Takahashi H, Inoue E, Hess A, Tamaoki M, Pilon-Smits EA (2008) Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol Plant 132: 236–253 [DOI] [PubMed] [Google Scholar]
- Vauclare P, Kopriva S, Fell D, Suter M, Sticher L, von Ballmoos P, Krahenbuhl U, den Camp RO, Brunold C (2002) Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5′-phosphosulphate reductase is more susceptible than ATP sulphurylase to negative control by thiols. Plant J 31: 729–740 [DOI] [PubMed] [Google Scholar]
- Welsh DK, Imaizumi T, Kay SA (2005) Real-time reporting of circadian-regulated gene expression by luciferase imaging in plants and mammalian cells. Methods Enzymol 393: 269–288 [DOI] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, et al (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55: 1927–1937 [DOI] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10: 1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.